Abstract
Studies of the desiccation tolerance of the seedlings of five tropical trees were made on potted plants growing in a greenhouse. Pots were watered to field capacity and then dehydrated for 3 to 9 weeks to reach various visual wilting stages, from slightly wilted to dead. Saturated root hydraulic conductance was measured with a high-pressure flowmeter, and whole-stem hydraulic conductance was measured by a vacuum chamber method. Leaf punches (5.6-mm diameter) were harvested for measurement of leaf water potential by a thermocouple psychrometer method and for measurement of fresh and dry weight. In a parallel study, the same five species were studied in a field experiment in the understory of a tropical forest, where these species frequently germinate. Control seedlings were maintained in irrigated plots during a dry season, and experimental plants were grown in similar plots with rain exclusion shelters. Every 2 to 4 weeks, the seedlings were scored for wilt state and survivorship. After a 22-week drought, the dry plots were irrigated for several weeks to verify visual symptoms of death. The field trials were used to rank drought performance of species, and the greenhouse desiccation studies were used to determine the conditions of moribund plants. Our conclusion is that the desiccation tolerance of moribund plants correlated with field assessment of drought-performance for the five species (r2 > 0.94).
Spatial and temporal variation in the composition of tropical forest plant communities has fascinated researchers for a long time, and understanding the causes of this variation remains a prominent topic of study. The factors that best correlate with the distribution of individual species and with diversity gradients in tropical forests are the annual amount and seasonality of rainfall (e.g. Gentry, 1988; Condit, 1998; Veenendaal and Swaine, 1998; Bongers et al., 1999). Wet forests typically have many more species than dry forests, and most species occur only over a limited range of rainfall. Tropical forests also undergo dramatic temporal changes in species abundance and distribution, on scales of decades as well as thousands of years (e.g. Flenley, 1998). Again, these shifts in species composition seem to be associated with changes in moisture availability. Because tropical forest plants experience drought and water stress (Tobin et al., 1999; Walsh and Newbery, 1999; Nakagawa et al., 2000), moisture availability in tropical forest soils may be one of the main factors influencing plant growth (Chandrashekar et al., 1998; Ito et al., 2000; Poorter and Hayashida-Oliver, 2000), mortality (Condit et al., 1995; Veenendaal et al., 1995), and habitat associations (Sollins, 1998; Webb and Peart, 2000). Thus, although tropical rainforests receive substantial rainfall, the available data suggest that water availability is the most important determinant of species' distributions.
To understand how rainfall and seasonality affect the spatial and temporal changes in tropical forest composition and diversity, we need to understand the physiological mechanisms by which rhizosphere water availability affects tropical rainforest plants. We have to be able to link the mechanisms of drought resistance in plants to plant survival, which in turn affects species distributions and ultimately forest diversity. We hypothesize that differential drought resistance of plant species, leading to differential survival during drought, is an important cause of plant distributions associated with rainfall, topography, and soil type in the wet tropics. The seedling stage is the most critical because seedlings have shallow roots and limited access to soil water and because seedlings are a key stage in the life history of woody plants.
Mechanisms of drought resistance can be divided into two classes: desiccation delay and desiccation tolerance. Desiccation delay involves traits that increase access to water and reduce water loss. These include deep roots, early stomatal closure, low cuticular conductance, water storage in plant organs, osmotic adjustments, and leaf shedding. Desiccation tolerance is promoted by physiological traits that permit continued water transport, gas exchange, or cell survival at low water content (w) and low water potentials (ψ), increased resistance of xylem to embolism, and ability of cells (especially meristems) to remain alive at low w or ψ.
Although species differences in drought resistance has been hypothesized to explain differences in species distribution in rainfall gradients, we know of no published systematic studies attempting to measure drought resistance in terms of survivorship and to see if drought survival can be explained by either desiccation tolerance or desiccation delay.
This paper focuses on desiccation tolerance properties and relates them to survivorship in the field. In field experiments, we compared performance of watered plants with plants in rainout shelters, and we monitored wilt state and survivorship over a 22-week period coincident with the natural dry season in central Panama. In parallel greenhouse experiments, we correlated visual symptoms of wilt with changes in leaf water content (w), leaf water potential (ψ), and whole-stem hydraulic conductance (i.e. the whole shoot with leaves removed [kws]).
We also quantified desiccation tolerance, how dry plants can become and still survive after rewatering, and compared this with measurements of drought performance under field conditions. We argue that to understand the role of the commonly studied mechanisms of drought resistance, one must quantify drought performance and desiccation tolerance. Unless we can quantify whole-plant responses that integrate complex, interacting mechanisms (e.g. desiccation tolerance and drought performance), our understanding of specific mechanisms will be reduced to the limited insight of comparative physiology (see “Discussion”).
RESULTS
The dry season of 2000 to 2001 started and ended about 3 weeks later than normal (based on 71 years of weather records) but had average precipitation for a dry season. The rainout shelters were relatively ineffective at keeping out moisture once the rainy season returned, but during the dry season gravimetric water contents average about 20% below that of the wet plots.
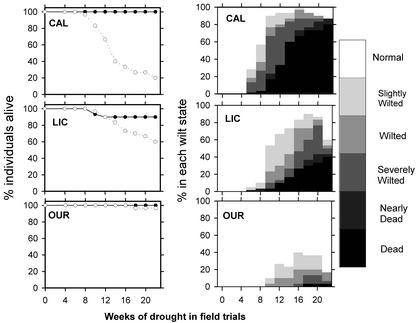
Examples of wilting states are shown in Figure 1 from greenhouse experiments, and these are representative of wilting states in field experiments. Survival values and wilting states on the field census dates are shown in Figure 2. Even slight wilting was rare in the wet plots; therefore, wilting states are given only for the dry plots. Mortality in the dry plots was significantly higher than in the wet plots after 22 weeks for C. longifolium, V. surinamensis, and L. platypus (ANOVA P < 0.02) but not significantly different for O. lucens and Dipteryx panamensis (ANOVA P > 0.5), both of which were thought to be tolerant of the average drought in Barro Colorado Island, Panama.
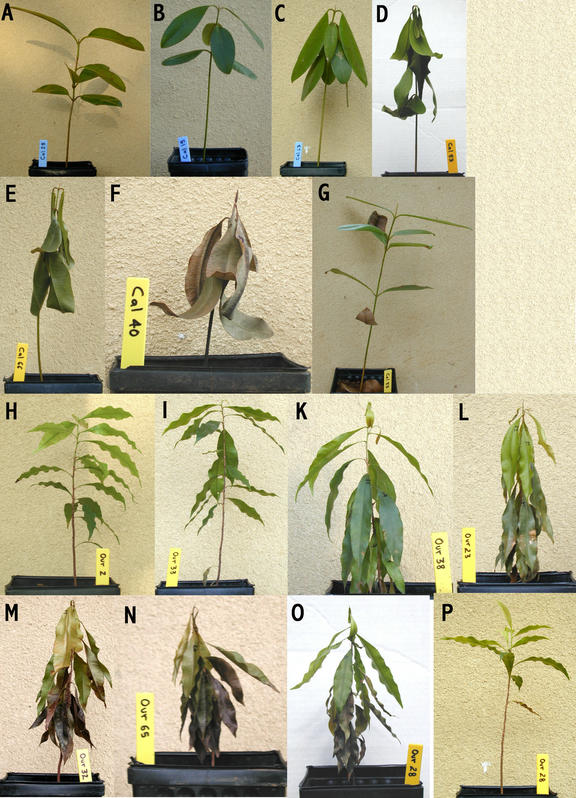
Figure 1.
Wilt state of Calophyllum longifolium (A–F) and Ouratea lucens (H–O) are shown for representative plants. Wilt stages are: normal (A and H), slightly wilted (B and I), wilted (C and K), severely wilted (D and L), nearly dead (E and M), and dead (F and N). Also shown are plants before and 60 d after rewatering for CAL53 (severely wilted → alive, D and G) and OUR28 (nearly dead → alive, O and P).
Figure 2.
Shown are field survey results of survival and wilt state for C. longifolium (CAL), Licania platypus (LIC), and O. lucens (OUR). Black and white symbols, Survival in wet and dry plots, respectively. Results for CAL were very similar to those of Virola surinamensis, and OUR were similar to Dipterix panamensis (DIP). The recovery of wilt states of living plants after week 18 was due to resumption of rain events and lateral movement of water to the rain-out shelters from the wetter regions, perhaps by lateral hydraulic lift facilitated by the mature trees.
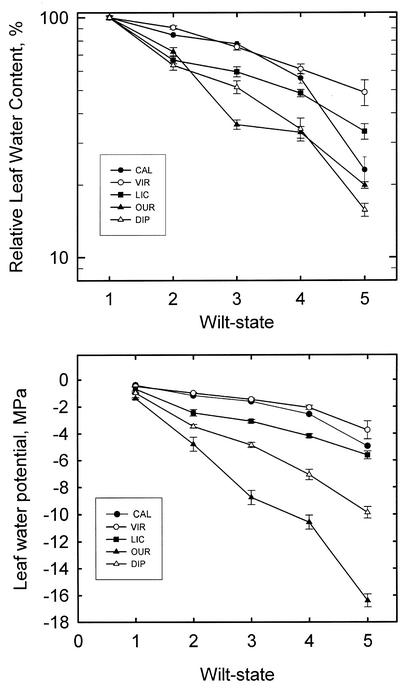
In greenhouse experiments, in contrast, all species were desiccated until death was noted. The survival figures for plants rewatered from the last three wilt states are shown in Table I. Moribund plants might be defined as plants in the driest wilt state with 50% mortality; however, we believe that replication of rewatered plants was not sufficient to determine the moribund wilt state with statistical certainty. However, even if we could determine the moribund wilt state, we are unlikely to get accurate values of leaf water potential (ψ) and water content (w) because most leaves are dead. In all species except O. lucens, the w of necrotic (discolored) tissue was significantly less than green tissue (data not shown) for plants in any given wilt state. In the “nearly dead” state, buds may be alive, but nearly all leaves are necrotic; hence, few samples of living leaf tissue can be found. A biologically significant variable for desiccation tolerance would be the lowest values of w and ψ associated with living leaf tissue. Values of w and ψ versus wilt state are shown in Figure 3.
Table I.
Survival data in greenhouse desiccation experiment
Plants in the three driest wilt states were rewatered and assessed for new growth after 2 months. In all species, some individuals survived the nearly dead state, but few were alive when scored dead. Species codes as in Table II.
| Species
|
Severely Wilted
|
Nearly Dead
|
Dead
|
|||
|---|---|---|---|---|---|---|
| Alive | Dead | Alive | Dead | Alive | Dead | |
| CAL | 7 | 0 | 1 | 6 | 0 | 8 |
| VIR | 5 | 1 | 2 | 6 | 0 | 4 |
| LIC | 2 | 0 | 4 | 0 | 1 | 8 |
| OUR | 7 | 1 | 3 | 5 | 0 | 8 |
| DIP | 5 | 1 | 2 | 6 | 0 | 4 |
Figure 3.
Shown are mean andse values of relative leaf water content (A) and water potential (B) versus wilt state where 1 = normal, 2 = slightly wilted, 3 = wilted, 4 = severely wilted, and 5 = nearly dead. Means are based only on leaf disc samples from leaf areas thought to be alive. For wilt state 5, there were few leaves to sample (n = 6–10) for all other states (n = 60–90). Species code as in Table I.
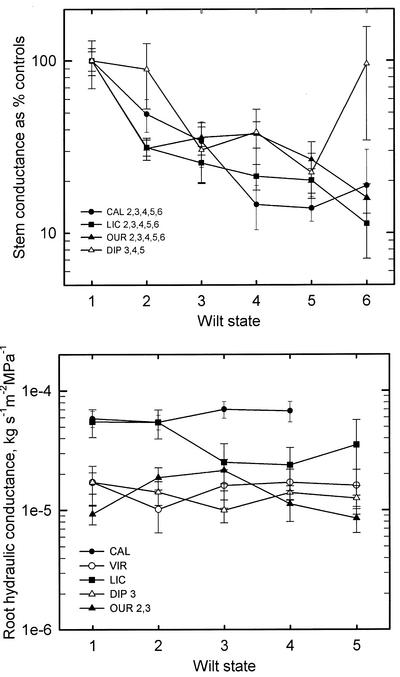
The hydraulic conductance of whole stems (without leaves) generally fell with wilt state in C. longifolium, L. platypus, O. lucens, and D. panamensis (Fig. 4A; see Table II for abbreviations). V. surinamensis stem conductances were discarded because they were much lower than the other species, apparently because of a pink latex that exudes from cut petioles. The whole-stem hydraulic conductances are expressed as percentage change from unstressed controls (percentage native conductance), and the conductance of controls is given in the caption of Figure 4. In all species, whole-shoot conductance declined with wilt state except for occasional increases in the dead state, which also corresponded with stem splitting. The hydraulic conductance of roots (approximately 0.5 h after rehydration) was generally not significantly different (Fig. 4B). Root hydraulic conductance (scaled to leaf area at the start of the experiment) of O. lucens increased with wilt states 2 and 3 for possible reasons to be discussed below.
Figure 4.
Shown are hydraulic properties of whole stems without leaves (A) and roots (B) versus wilt states. Numbers of x axis and species codes are as in Figure 3. In A, whole-stem conductance is given as percentage of the control conductance of unstressed plants, i.e. what might be called percentage native conductance. Control conductances ±se (n = 5–9) were: CAL, 1.70 ± 0.21 × 10–4; LIC, 1.60 ± 0.15 × 10–4; OUR, 2.97 ± 0.37 × 10–5; and DIP, 3.74 ± 0.82 × 10–5 kg s–1 m–2 MPa–1. VIR data are excluded because values were low (approximately 2 × 10–6) due to latex plugging of xylem. In B, root conductances, measured in saturated soil, are normalized to initial leaf surface area, i.e. the area of leaves before death and necrosis of leaves in the later wilt states. Note that OUR is unusual in exhibiting a significant increase in root conductance in the slightly wilted and wilted states. Significance tests were by ANOVA; numbers after each species code indicate which wilt states are significantly different from controls (wilt state = 1); ANOVA significant differences in A generally had P < 0.01 and P < 0.05 for B. Non-significance means P > 0.05.
Table II.
Species used in this study
Heights and leaf areas below apply to plant sizes for the field studies. Plants for desiccation studies in the greenhouse were larger.
| Species | Species Code | Family | Height | Leaf Area |
|---|---|---|---|---|
| mm | cm2 | |||
| C. longifolium Willd. | CAL | Clusiaceae | 307 ± 6 | 263.18 ± 7.85 |
| D. panamensis (Pittier) Record & Mell | DIP | Fabaceae | 280 ± 10 | 216.50 ± 15.10 |
| L. platypus (Hemsl) Fritsch | LIC | Chrysoblancaceae | 274 ± 5 | 164.42 ± 6.31 |
| O. lucens (Kunth) Engl. | OUR | Ochnaceae | 93 ± 2 | 27.49 ± 1.50 |
| V. surinamensis (Rol. ex Rottb.) Warb. | VIR | Myristicaceae | 143 ± 3 | 170.01 ± 6.24 |
DISCUSSION
For many years, researchers have studied drought-induced xylem dysfunction. Vulnerability curves relate how stem segment hydraulic conductance declines with stem water potential (ψstem), and these have been measured on >60 species of plants, e.g. see the literature survey of Tyree et al. (1994). More recently, a vacuum chamber technique has been used to measure vulnerability curves on whole shoots of arid zone species (Kolb et al., 1996). Many species have been compared in terms of the ψstem needed to induce 50% loss of hydraulic conductance (P50), and there has been a satisfying general correlation in which species preference to wet versus dry sites correlates with less negative to more negative values of P50, respectively. The presumption is that the plants tend to die when stems loose too much hydraulic conductance; hence, species with xylem more resistant to cavitation are presumed to be more drought resistant. However, to our knowledge, there are no studies of whole plants that demonstrate what values of w, ψ, or kwhole-stem correlate with plant death. There is, however, a growing body of evidence that loss of stem hydraulic conductance correlates with branch dieback in the shoots of some temperate plants (Rood et al., 2000; Davis et al., 2002; Sperry and Hacke, 2002). There have been studies of leaf tissue viability after desiccation (Peace and Mcdonald, 1980; Buckley et al., 1981; Loesch, 2001), but our field experience has revealed that many plants are still alive even after most or all leaf tissue is dead.
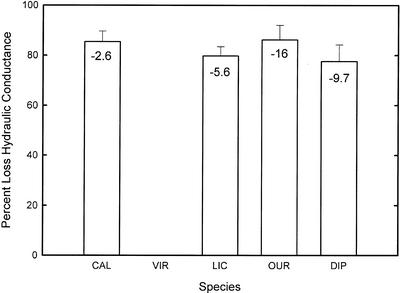
One objective of our work was to monitor loss of conductance of whole stems (without leaves) of seedlings as they approach death. Whole-shoot conductance fell to a critical percent of native state (controls) of about 15% to 20% (i.e. 80%–85% loss conductance) in severely wilted and nearly dead plants (Figs. 4 and 5). Plants with good drought performance fell to a critically low conductance at much more negative ψ values than C. longifolium, which has poor drought performance. Hence, our limited data set is consistent with the notion that vulnerability to cavitation determines desiccation tolerance and that the critical value of whole-stem conductance for survival of seedlings might be around 15% to 20% of unstressed plants (Tyree et al., 1994). In many species, the cavitation of xylem in roots might have a larger impact on desiccation tolerance than in shoots. More work is needed to determine if this is generally true.
Figure 5.
Bar graph showing the percentage loss hydraulic conductance (PLC) of whole stems (without leaves) that corresponds to the moribund state. The numbers in the bars are the water potential at the corresponding PLC. This supports the hypothesis that >80% loss of hydraulic conductance causes death and that resistance to cavitation is an important factor in desiccation tolerance.
The effect of drought on whole-root hydraulic conductance has been studied in Crassulacean acid metabolism plants (e.g., Nobel and Sanderson, 1984; North et al., 1992) but less commonly in non-Crassulacean acid metabolism species. The only example we know of is a study on seedlings of olive (Olea oleaster). Root conductance of irrigated seedlings was compared with root conductance of droughted seedlings measured immediately after rewatering the seedlings. LoGullo et al. (1998) found that drought reduced root conductance, and this reduction was correlated with morphological changes to the root in response to dry soil, i.e. the formation of corky layers and shedding of fine roots. Recovery of root conductance after a drought event correlated with the emergence of new fine roots.
Rather more work has been done on loss of conductance in woody root segments (Kolb et al., 1996; Tsuda and Tyree, 1997; Hacke et al., 2000; Sperry and Hacke, 2002), but it is difficult to extrapolate from root segments to whole roots without resorting to some kind of model. The hydraulic conductance of whole roots is likely to be limited more by fine root conductance than woody root conductance for reasons discussed in detail by Tyree and Zimmermann (2002); hence, an 80% loss of conductance observed in a large woody root segment is likely to have 3- to 5-fold lower impact on loss of whole-root conductance. Our technique for measuring root conductance is likely to dissolve most embolisms in the vessels of roots; hence, it measures only the effect of drought on morphological changes such as loss of fine roots and growth of cortex in thicker roots.
At one extreme, L. platypus roots behaved like olive seedlings, but at the other extreme, O. lucens increased root conductance >200% when in the wilted state versus controls (Fig. 4B). This increase in root conductance in response to drought might be a new mechanism of desiccation delay not reported previously. However, a more parsimonious explanation might be that O. lucens roots are damaged and become leaky when exposed to drought. The latter explanation would not be consistent with the unusually high desiccation tolerance of O. lucens (see below), but more work needs to be done to resolve the issue.
Quantifying Drought Performance and Drought Resistance
We introduced the classical definition of “drought resistance” in the introduction, where drought resistance is defined in terms of its possible physiological mechanisms. Although we have a notion of what drought resistance represents, we do not have any way of measuring it and this is a major conceptual obstacle that must be overcome if we ever hope to understand drought resistance at a mechanistic level. In contrast, we understand the mechanisms of electrical circuits in part because we have an instrument to measure electrical resistance; similarly, we understand the hydraulic architecture of plants because we have an instrument to measure hydraulic resistance (or conductance) of whole plants or plant parts. Without a quantitative measure of drought resistance, we cannot decide the relative importance of the various measures of desiccation tolerance and desiccation delay in determining drought resistance!
Without a measure of drought resistance, we are confined to the limited insights gained by comparative physiology. Comparative physiology methods might reveal that species A has higher cuticular resistance than species B, and species A is less vulnerable to cavitation than species B and because species A grows in a dryer ecosystem than species B, therefore, we might think A is more drought resistant than B, and this difference might be explained by the cuticular resistance and the vulnerability to cavitation. The weakness of this kind of comparative study is that we usually take it as “given” that A is more drought resistant than B without experimental proof, and, hence, we cannot evaluate if the physiological traits reported even correlate with the superior growth or survival of A versus B. Furthermore, such comparative studies provide no information on the relative importance of traits of desiccation delay and desiccation tolerance.
Survival data similar to that in Figure 2 might ultimately provide measures of drought resistance or drought performance, which are different concepts as explained below. If we perform a pristine experiment on a number of species in which drought is the only controlled stress, then there will be a singular plant response to the single stress that will be manifested in terms of differences in growth or survival of drought-stressed plants versus the controls. Such differences in growth and survival might be used to measure drought resistance. On the other hand, if we perform an experiment with multiple stresses from, say, drought, pathogens, and insects, the plant could well have a very complex response. The stress response mechanisms might interact with each other such that the stress of drought might make an attack of a pathogen more serious or lethal, whereas the attack of the pathogen alone might reduce growth but not increase mortality (Schoeneweiss, 1986). Alternatively, some types of stress may actually precondition the plant to withstand drought better. In most field-based experiments and in many greenhouse experiments, we usually have control over one or more stresses but there may be other uncontrolled stresses changing the growth and survival responses, which we might call measures of drought performance.
In an ecological setting, measures of drought performance might be more important than measures of drought resistance. However, if the objective of an experiment is to evaluate the relative importance of the mechanisms of drought resistance (desiccation tolerance and desiccation delay), then single-stress experiments might be more appropriate.
How can we use survival as an initial measure of drought performance? One measure might be the differential survival at the end of the 22-week drought period in Figure 2, i.e. D*p1 = Sw – Sd, where Sw is the percentage survival of the irrigated plants and Sd is the percentage survival of droughted plants. However, we had some mortality in irrigated plants; hence, multiple stresses were present. In some cases, the non-drought stresses might act independently of drought and occur equally in both irrigated and dry plots. In this case, we might want a measure D*p2 = 100(Sw – Sd)/Sw evaluated at 22 weeks. A minor disadvantage of D*p1 and D*p2 is that plants with good drought performance (e.g. O. lucens in Fig. 2) have values of nearly 0% for D*p1 and D*p2, whereas we might want a better performer to have a bigger number. This is resolved by defining new parameters of drought performance as:
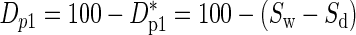 |
(1) |
or
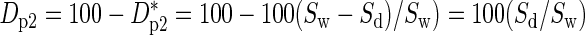 |
(2) |
The parameters defined in Equations 1 or 2 might be appropriate for drought performance in a time-limited measure of the effects of drought, but these measures are inherently limited because: (a) The values computed will change with the duration of the drought, and (b) it is not good at evaluating differences in species at the extremes of low and high drought performance. To be most useful, the survivorship data will have to be evaluated when as many species as possible in the trial lie between the two extremes of complete survival and complete mortality. A better measure might be based on time integrals of the survivorship curves in an experiment carried out over a time interval large enough to cause complete mortality of all species. In that case, plants with low drought performance will have a smaller integral value than plants with good drought performance, but these issues are beyond the scope of this paper. For now, we will use the values of Dp1 and Dp2 evaluated at 22 weeks (Fig. 2) to see if either measure of drought performance is related to some measure of desiccation tolerance.
Is Drought Performance Correlated with Desiccation Tolerance?
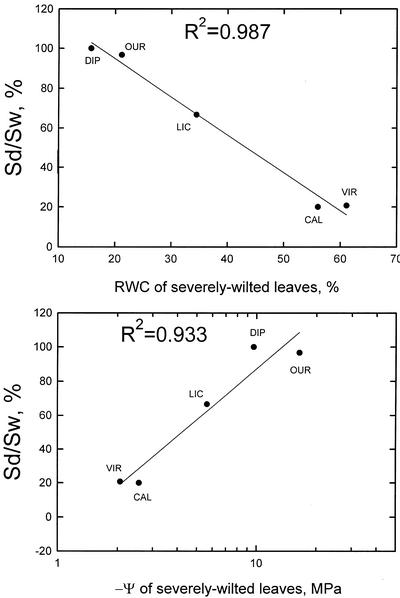
Two possible measures of desiccation tolerance are the leaf ψ or leaf relative water content (Rwc) just before death. In Figure 6, we plotted values of Dp2 versus the mean ψ or mean Rwc evaluated in severely wilted plants. The r2 values are >0.9, and a significant correlation is found. Values of Dp1 and Dp2 differed from each other only a few percent in the five species of our study; therefore, r2 values are also >0.9 for the Dp1 parameter. Other combinations of plots with Dp1 or Dp2 versus ψ and Rwc values for nearly dead plants also have r2 > 0.9 (data not show), but mean values of ψ and Rwc are based on quite small data sets in nearly dead plants because little living tissue was found for measurement of ψ and Rwc.
Figure 6.
This figure shows the correlation of drought performance (survivorship) from field trials to measures of desiccation tolerance evaluated at the severely wilted state. Survivorship was evaluated at 22 weeks. Sd, Percentage survival in dry treatment; Sw, percentage survival in wet treatment. A, Drought performance from field trials is plotted versus relative leaf water content. B, Drought performance from field trials is plotted versus –ψ. See text for more details.
To our knowledge, our study is the first in which quantitative measures of drought performance are correlated with quantitative measures of desiccation tolerance. We tentatively conclude that most of the differences in drought performance of the five species in our study are explained by differences in desiccation tolerance. Much more work is needed to see if this relationship holds for more species. Also, more robust measures of drought performance must be sought. If the correlation is less strong with a regression involving 15 or 20 species, then residuals from the regression might serve as a basis for evaluating through a statistical model the impact of parameters that provide measures of desiccation delay.
MATERIALS AND METHODS
Five species from central Panama were selected for this study. Two were thought to be drought sensitive (Virola surinamensis and Calophyllum longifolium), two were thought to be drought resistant (Ouratea lucens and Dipteryx panamensis), and one was intermediate (Licania platypus; see Table II). Selection of species was based in part on suggestions of likely drought performance from field observations by plant ecologists who have worked at Barro Colorado Island, Smithsonian Tropical Research Institute, in central Panama.
Field Trials for Wilting States and Survivorship
Within a few days after collection, seeds were set out in seedling trays for germination in the greenhouse under moderately low light conditions (5%–10% full sunlight). They were later transplanted and grown in pots (of 0.3–1.0 l according to seedling size) in the greenhouse until transplanting. Seedlings were transplanted to plots in the forest from September through November 2000. For all seedlings, the dry season 2000/2001 was the first dry season they experienced. Due to time of fruiting and germination, the seedlings were between 9 and 2 months old at the start of the experiment. Our intention was to work with seedlings at the age naturally occurring at the onset of their first dry season. Seedling size varied between species, with an average seedling height of the species of 93 to 307 mm and average leaf areas of 27 to 263 cm2 (Table II).
The seedlings were exposed to two treatments, wet and dry, for 22 weeks in the dry season of 2000 to 2001 (December 18, 2000–June 12, 2001). Sixty plots (0.8 × 1.0 m) were established in the understory. Light conditions in the plots were assessed with hemispherical photographs (model Coolpix-950, Nikon, Melville, NY) and analyzed with Hemiview 2.1 (Delta-T Devices, Cambridge, UK). Plot positions were chosen along the established trails with a minimum distance of 10 m between plots, and all plots were situated near large trees to allow for root competition for water. All plots were caged with wire mesh (1.1- × 1.1-cm mesh width) to exclude vertebrate (and some invertebrate) herbivores and to minimize damage through leaf, twig, and branch fall. The species were randomly assigned to positions within the plots. One seedling of each species was transplanted to each plot, and their growth and survival followed during the course of the experiment. To ensure the same range of light conditions in both treatments, the plots were paired by light conditions as predicted by the Hemiview analysis, and one plot of each pair was randomly assigned to each treatment.
Dry plots were covered with rain-out shelters made from transparent plastic sheets to protect them from any dry season rains. Light intensities (photosynthetic photon flux density) decreased by approximately 9% due to the plastic cover (assessed by quantum sensor measurements, LI-COR, Lincoln, NE). Wet plots were watered regularly with water from the Lake Gatun. Initially, 15 mm (15 L m–2) of water was applied with watering cans three times a week, equivalent to 193 mm of monthly rain. Later in the dry season, the amount of water applied had to be increased because competition from neighboring plants decreased relative soil water content even in the wet plots. The amount of water applied was increased individually for the different plots according to occasional visible wilting of the seedlings. Censuses of gravimetric soil water content, seedling survival and plant wilting stage were initially conducted monthly, later biweekly throughout the experiment.
Greenhouse Trials for Desiccation Tolerance
Methods for studying desiccation tolerance were developed for L. platypus are described in detail elsewhere (Tyree et al., 2002). In brief, we did the following for all species.
Experiments were performed on potted seedlings 12 to 20 months old. All seedlings were grown under shade cloth to simulate normal growth conditions (5%–7% photosynthetically active radiation measured above the forest canopy). Plants were grown in unaltered forest soil collected from the site of our field trials above. Soils had high clay content.
Sixty to 80 plants were studied in each experiment. Pots were immersed in water for a few minutes to start all at field capacity then water was withheld. Measurements were made at six wilt states: normal, slightly wilted, wilted, severely wilted, nearly dead, and dead. Because we could not guarantee that mortality would correspond to visual wilt states, subsets of plants from the last three states were rewatered and observed for an additional 2 months.
The following measurements were performed on all the above nine categories: (a) water potential measured by thermocouple psychrometers on six to 10 leaf punches per plant, (b) fresh weight and dry weight of the samples used in the thermocouple psychrometers, (c) whole-stem hydraulic conductance of the shoots (leaves removed) measured by a vacuum chamber method, and (d) whole-root hydraulic conductance measured by a high-pressure flowmeter.
Thermocouple psychrometers can measure ψ only down to –7 or –8 MPa. As leaf water content per disc falls below a critical value, wc, corresponding to ψ approximately –7 MPa, the psychrometer reads erroneously high (less negative) values ofψ. Water potential isotherms of –1/ψ versus leaf water content per disc (w) were used to establish a linear relationship between –1/ψ and w, and the resulting regression was used to estimate ψ whenever w was less than wc. In a previous paper (Tyree et al., 2002), water content per unit dry weight (wd) was used, but upon reanalysis of all species, w had a smaller coefficient of variation for each wilt state than did wd. Hence, with w a more reliable regression was obtained for estimation of ψ below the critical wc.
All plants were harvested within 1 h of sunrise at which point plants were scored for wilt state, photographed, and psychrometer samples were collected. Pots were then immersed in water to excise the shoots and leaves under water to avoid introducing extra embolisms. Root hydraulic conductance (Tyree et al., 1995, 1998) and whole-stem conductance were measured within 1 h and were normalized to initial leaf area on each plant, which was estimated from leaf length and width and previously determined regressions of leaf area to the product of length and width. Whole shoot and root conductances were measured as follows.
The entire pot and shoot were immersed in water and the shoot was excised 2 cm above the soil level with a new razor blade. The leaves were immediately excised with the razor blade, and the base of the shoot was connected under water to a compression fitting and peek tubing of the type supplied with a high-pressure flowmeter (Dynamax Inc., Houston). The peek tubing was passed through a tight-fitting hole of rubber stopper, sized to fit in the top of a 2-L vacuum flask. The whole shoot was sealed in the vacuum flask and the peek tubing was connected to an ultralow flowmeter (ULFM) described below. Water was sucked through the whole stem system by applying partial vacuum pressures in the sequence of 0, 23, 26, 70, 59, 35, 12, and 0 kPa. This sequence of partial pressure permitted us to check for hysteresis in flow versus applied suction pressure. Hysteresis rarely occurred, but when noted the data were discarded and the measurement was repeated.
Our method was very similar (except for physical scale) to that described by Kolb et al. (1996) and was selected as the method least likely to displace air in embolized vessels during measurement. The flow rates, however, were too small to measure very quickly using a digital balance, but this problem was solved with the ULFM (see Tyree et al. (2002) for a description of the ULFM. The slope of flow versus applied vacuum pressure gave the kws.
Whole-root conductances were measured on rewetted pots within 20 min of excising the shoots under water. The high-pressure flowmeter technique was identical to that reported by Tyree et al. (1998). In a previous study on severely dehydrated olive (Olea oleaster) seedlings, LoGullo et al. (1998) found a semipermanent loss of whole root conductance that lasted for days after soil was rewatered, so we expected to find similar effects in other species.
Pots were also regularly weighed to assess the rate of water loss in pots with plants present and with plants removed. The rate of dehydration was due primarily to water loss from the soil mass (> 80%), and the remainder was due to evaporation from the leaves (<20%; data not shown). Hence, the rates of dehydration were not related to plant size or species but rather due mostly to air humidity and temperature in the greenhouse.
Wilt states for L. platypus were published in photographs elsewhere (Tyree et al., 2002), and representative wilt states for C. longifolium and O. lucens are shown in Figure 1. “Generic” wilt states for both field and greenhouse experiments are: slightly wilted, leaves green but leaf angled slightly toward the ground compared with controls; wilted, leaves green with leaf angles near 45° and leaf blades have begun to fold (curl) inward parallel to midrib, very limited necrosis (gray-green to gray-brown); severely wilted, leaves green, most leaf angles near 90° from horizontal and extensive curling of leaves, more extensive necrotic zones (gray-green to gray-brown) mostly near leaf margins or leaf tips; nearly dead, most leaves necrotic, more extensive curling, leaf angles mostly near 90° from horizontal, some young leaves still green near the midrib or apical bud still green and pliable; and dead, necrosis on all leaves, extensive curling, leaf blades brittle, leaf angles mostly near 90° from horizontal. However, specific descriptions varied slightly between species. To ensure consistency within species, photographs were taken of every plant at the time of harvest and at the time of rewatering from the three driest states.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018937.
This work was supported by the Andrew Mellon Foundation, by the U.S. Forest Service, and by the University of Utah.
References
- Bongers F, Poorter L, Van Rompaey RSAR, Parren MPE (1999) Distribution of twelve moist forest canopy tree species in Liberia and Cote d'Ivoire: response curves to a climatic gradient. J Veg Sci 10: 371–382 [Google Scholar]
- Buckley RC, Corlett RT, Grubb PJ (1981) Are the xeromorphic trees of tropical upper montane rain forests drought-resistant? Biotropica 12: 124–136 [Google Scholar]
- Chandrashekar TR, Nazeer MA, Marattukalam JG, Prakash P, Annamalainathan K, Thomas J (1998) An analysis of growth and drought tolerance in rubber during the immature phase in a dry subhumid climate. Exp Agric 34: 287–300 [Google Scholar]
- Condit R (1998) Ecological implications of changes in drought patterns: shift in forest composition in Panama. Climate Change 39: 413–427 [Google Scholar]
- Condit R, Hubbell SP, Foster RB (1995) Mortality rates of 205 neotropical tree and shrub species and the impact of severe drought. Ecol Monogr 65: 419–439 [Google Scholar]
- Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams C (2002) Shoot dieback during prolonged drought in Ceanothus chaparral of California: a possible case of hydraulic failure. Am J Bot 89: 820–828 [DOI] [PubMed] [Google Scholar]
- Flenley JR (1998) Tropical forests under the climates of the last 30,000 years. Climate Change 39: 177–197 [Google Scholar]
- Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Missouri Bot Garden 75: 1–34 [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J (2000) Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic Appl Ecol 1: 31–41 [Google Scholar]
- Ito S, Nishiyama Y, Kustiawan W (2000) Responses of Dipterocarp seedlings to drought stress. In E Guhardja, M Fatawi, M Sutisna, T Mori, S Ohta, eds, Rainforest Ecosystems of East Kalimantan: El Niño, Drought, Fire and Human Impacts. Springer, Tokyo, pp 143–150
- Kolb KJ, Sperry JA, Lamont BB (1996) A method for measuring xylem hydraulic conductance and embolism in entire root and shoot systems J Exp Bot 47: 1805–1810 [Google Scholar]
- Loesch R (2001) Wasserhaushalt der Pflanzen. Quelle & Mayer, Wiebelsheim, Germany, p 391
- LoGullo MA, Nardini A, Salleo S, Tyree MT (1998) Changes in root hydraulic conductance (KR) of Olea oleaster seedlings following drought stress and irrigation. New Phytol 140: 25–31 [Google Scholar]
- Nakagawa M, Tanaka K, Nakashizuka T, Ohkubo T, Kato T, Maeda T, Sato K, Miguchi H, Nagamasu H, Ogino K et al. (2000) Impact of severe drought associated with the 1997–1998 El Niño in a tropical forest in Sarawak. J Trop Ecol 16: 355–367 [Google Scholar]
- Nobel PS, Sanderson J (1984) Rectifier-like activities of roots of two desert succulents. J Exp Bot 35: 727–737 [Google Scholar]
- North GB, Ewers FW, Nobel PS (1992) Main root-lateral root junctions of two desert succulents: changes in axial and radial components of hydraulic conductivity during drying. Am J Bot 79: 1039–1050 [Google Scholar]
- Peace WJH, Mcdonald FD (1980) An investigation of the leaf anatomy, foliar mineral levels, and water relations of trees of a Sarawak forest. Biotropica 13: 100–109 [Google Scholar]
- Poorter L, Hayashida-Oliver Y (2000) Effects of seasonal drought on gap and understory seedlings in a Bolivian moist forest. J Trop Ecol 16: 481–498 [Google Scholar]
- Rood SB, Patiño S, Coombs K, Tyree MT (2000) Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14: 248–257 [Google Scholar]
- Schoeneweiss D (1986) Water stress disposition to disease: an overview. In P Ayres, ed, Water, Fungi and Plants. Cambridge University Press, New York, pp 149–183
- Sollins P (1998) Factors influencing species composition in tropical lowland rainforest: does soil matter? Ecology 79: 23–30 [Google Scholar]
- Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Func Ecol 16: 367–378 [Google Scholar]
- Tobin MF, Lopez OR, Kursar TA (1999) Responses of tropical understory plants to a severe drought: tolerance and avoidance of water stress. Biotropica 31: 570–578 [Google Scholar]
- Tsuda M, Tyree MT (1997) Whole-plant hydraulic resistance and vulnerability segmentation in Acer saccharinum. Tree Physiol 17: 351–357 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? Int Assoc Wood Anatomists J (NS) 15: 335–360 [Google Scholar]
- Tyree MT, Patiño S, Bennink J, Alexander J (1995) Dynamic measurements of root hydraulic conductance using a high-pressure flowmeter for use in the laboratory or field. J Exp Bot 46: 83–94 [Google Scholar]
- Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA (2002) Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest tree seedling, Licania platypus (Hemsl.) Fritsch. J Exp Bot 53: 2239–2247 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Velez V, Dalling JW (1998) Growth dynamics of root and shoot hydraulic conductance in seedlings of five neotropical tree species: scaling to show possible adaptation to differing light regimes. Oecologia 114: 293–298 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap. Springer, Berlin
- Veenendaal EM, Swaine MD (1998) Limits to tree species distribution in lowland tropical rainforests. In DM Newbery, HHT Prins, N Brown, eds, Dynamics of Tropical Forest Communities: 37th Symposium of the British Ecological Society. Blackwell Science, Oxford, pp 163–191
- Veenendaal EM, Swaine MD, Agyeman VK, Blay D, Abebrese IK, Mullins CE (1995) Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. J Ecol 83: 83–90 [Google Scholar]
- Walsh RPD, Newbery DM (1999) The ecoclimatology of Danum, Sabah, in the context of the world's rainforest regions, with particular reference to dry periods and their impact. Phil Trans R Soc Lond B 354: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, Peart DR (2000) Habitat associations of trees and seedlings in a Bornean rain forest. J Ecol 88: 464–478 [Google Scholar]