Abstract
The ATP/ADP and NADP/NADPH ratios have been measured in whole-cell extract of the green alga Chlamydomonas reinhardtii, to understand their availability for CO2 assimilation by the Calvin cycle in vivo. Measurements were performed during the dark-light transition of both aerobic and anaerobic cells, under illumination with saturating or low light intensity. Two different patterns of behavior were observed: (a) In anaerobic cells, during the lag preceding O2 evolution, ATP was synthesized without changes in the NADP/NADPH ratio, consistently with the operation of cyclic electron flow. (b) In aerobiosis, illumination increased the ATP/ADP ratio independently of the intensity used, whereas the amount of NADPH was decreased at limiting photon flux and regained the dark-adapted level under saturating photon flux. Moreover, under these conditions, the addition of low concentrations of uncouplers stimulated photosynthetic O2 evolution. These observations suggest that the photosynthetic generation of reducing equivalents rather than the rate of ATP formation limits the photosynthetic assimilation of CO2 in C. reinhardtii cells. This situation is peculiar to C. reinhardtii, because neither NADPH nor ATP limited this process in plant leaves, as shown by their increase upon illumination in barley (Hordeum vulgare) leaves, independent of light intensity. Experiments are presented and were designed to evaluate the contribution of different physiological processes that might increase the photosynthetic ATP/NADPH ratio—the Mehler reaction, respiratory ATP supply following the transfer of reducing equivalents via the malate/oxaloacetate shuttle, and cyclic electron flow around PSI—to this metabolic situation.
The assimilation of CO2 in oxygenic photosynthesis depends upon the generation of NADPH and ATP by the light-driven electron transport from water to NADP. This process uses the two photochemical reactions catalyzed by photosystem II (PSII) and photosystem I (PSI) in series and also comprises the cytochrome b6f complex, which is reduced by PSII via plastoquinol and oxidized by PSI via plastocyanin. It is coupled to the synthesis of ATP via the generation of an electrochemical proton gradient across the photosynthetic membranes. The stoichiometry of one ATP per NADPH (or one ATP per two electrons; for review, see Witt, 1979) has been established in thylakoids isolated from higher plants, and more recent experiments have confirmed this stoichiometry (see, e.g. table I in Berry and Rumberg, 1999). Higher values have been calculated theoretically, combining reported values of H+/electron and H+/ATP ratios. However, such calculations are based on the still controversial values of H+/electron ratios (see Berry and Rumberg, 1999; Sacksteder et al., 2000). So, the measurements with isolated thylakoids consistently show lower ATP per two electrons ratios than that required for CO2 assimilation. A fraction of the light-driven electron transport must therefore be involved in the generation of ATP, without using NADP as the terminal electron acceptor.
Different pathways have been proposed to perform such a role: (a) The first one is the Mehler reaction (Egneus et al., 1975), i.e. the reduction of molecular oxygen by photosynthetic electron transfer at the level of the reducing side of PSI. This process is rather inefficient unless electrons are transferred via the ascorbate free radical (AFR), which is formed in the reaction of ascorbate with O2– (the ascorbate-Mehler reaction or Mehler ascorbate peroxidase pathway; Miyake and Asada, 1992; Forti and Ehrenheim, 1993; Grace et al., 1995). This radical (AFR) efficiently competes for electrons with NADP at the reducing side of PSI (Forti and Ehrenheim, 1993), and its reduction is coupled to ATP synthesis with the same ATP/2e– stoichiometry as NADP reduction (Forti and Elli, 1995). Furthermore, the regulation of the diversion of electrons at the reducing end of PSI alternatively to NADP or to the AFR is provided by the redox state of the NADP/NADPH couple (Forti and Ehrenheim, 1993). In steady-state photosynthesis, the latter is determined by the availability of 1,3-diphosphoglycerate and therefore of ATP (for discussion, see Forti and Elli, 1995).
Alternatively, (b) the ATP needed in excess of NADPH could be provided by cyclic photophosphorylation, i.e. the synthesis of ATP coupled to the cyclic electron transport around PSI. This process does not reduce NADP, but results in ATP synthesis because of the generation of a ΔpH during plastoquinol oxidation by the cytochrome b6f complex. Finally, (c) the interaction between the photosynthetic and respiratory metabolism might result in the transfer of reducing equivalents from the chloroplast to the mitochondria, where their consumption by respiration may result in ATP synthesis. At least two shuttle systems enable such transfer: the “malate/oxaloacetate valve” (Scheibe, 1987; Backhausen et al., 1994), and the triose phosphate exchanger (Flugge and Heldt, 1991; for further discussion, see also Noctor and Foyer, 2000).
Because of the involvement of these additional processes in the photosynthetic activity in vivo, the energetics of CO2 fixation appears to be significantly modified with respect to the in vitro conditions. Consistent with the idea that the sole linear electron flow in isolated spinach (Spinacia oleracea) chloroplasts does not provide the ATP/NADPH stoichiometry required for CO2 assimilation, the steady-state level of ATP decreased drastically after a decrease of the light intensity that caused a 70% reduction of the O2 evolution rate, whereas NADPH remained fully reduced (Heber, 1973). This clearly indicates that its reoxidation is limited by ATP availability. Furthermore, an ATP/ADP ratio of approximately 0.25 was observed at steady state in intact chloroplasts performing high rates of CO2 assimilation (Portis et al., 1977). In contrast, it is well established that the effect of light is to increase the ATP concentration in both intact leaves and isolated protoplasts (e.g., see Santarius and Heber, 1965; Stitt et al., 1982; Furbank and Horton, 1987) under steady-state conditions.
In this work, we analyze in details the changes in the redox state of the NADPH/NADP couple and the ATP/ADP ratio in intact cells of Chlamydomonas reinhardtii—and in barley (Hordeum vulgare) leaves— during the dark-light transitions and at steady state under both saturating and limiting light intensities. We report that light induced ATP synthesis without changes in the redox state of NADP in C. reinhardtii under anaerobic conditions, consistent with the previously reported operation of cyclic flow around PSI (Finazzi et al., 1999, 2002). In aerobiosis, ATP rapidly increased upon illumination to an extent that was slightly larger at higher light intensities. On the contrary, the NADPH/NADP ratio substantially decreased under low light conditions, regaining the initial dark value when the light intensity became saturating. These changes were related to photosynthetic activity, as shown by their absence in a mutant incapable of photosynthetic CO2 assimilation (T60, devoid of Rubisco; Khrebtukova and Spreitzer, 1996) or in the wild type in the absence of CO2. A different pattern of responses to light was observed in barley leaves, where not only the ATP/ADP ratio but also the NADPH/NADP ratio was increased by illumination in a intensity-dependent way.
Experiments are presented to evaluate the contribution of different physiological processes (the Mehler reaction, respiratory ATP supply via the malate/oxaloacetate shuttle, and cyclic electron flow around PSI) to the photosynthesis of C. reinhardtii. These experiments suggest that the latter process is the main contributor to the “extra” ATP generation in the case of this alga.
RESULTS AND DISCUSSION
ATP/ADP and NADPH/NADP Cellular Contents in C. reinhardtii Cells
Table I shows that the NADPH/NADP ratio in dark-adapted aerobic cells of C. reinhardtii was approximately 1.5 (in substantial agreement with a previous report; Rebéillé and Gans, 1988). The sum of NADP and NADPH was constant during a dark-light transition, indicating that the changes were only due to the oxidation-reduction of the coenzyme. On this basis, NADP was routinely determined enzymatically (see “Materials and Methods”) in the acid extracts of the cells (together with ATP and ADP), and the changes of NADPH were evaluated as the difference between the NADP concentration and the sum of the two species.
Table I.
Concentration of NADP and NADPH in C. reinhardtii cells
Cells containing 150 μg mL–1 Chl were incubated at 25°C in the minimum HS medium with the addition of 5 mm NaHCO3 and then frozen and treated as described in “Materials and Methods.” The respiration rate was 7.4 ± 0.8 μmol O2 mg–1 Chl h–1. The maximum rate of photosynthesis (after correction for respiration) was 159.0 ± 28.0 μmol O2 mg–1 Chl h–1 at 830 μE m–2 s–1. The numbers of experiments are indicated in parentheses.
| Dark, Aerobic | Low Light, Aerobic | |
|---|---|---|
| nmol mg-1 Chl | ||
| [NADP] | 1.75 ± 0.2 (9) | 2.74 ± 0.14 (8) |
| [NADPH] | 2.71 ± 0.26 (8) | 1.92 ± 0.25 (7) |
| [NADP] + [NADPH] | 4.46 ± 0.32 | 4.66 ± 0.28 |
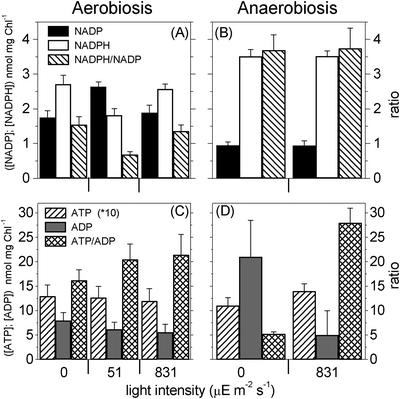
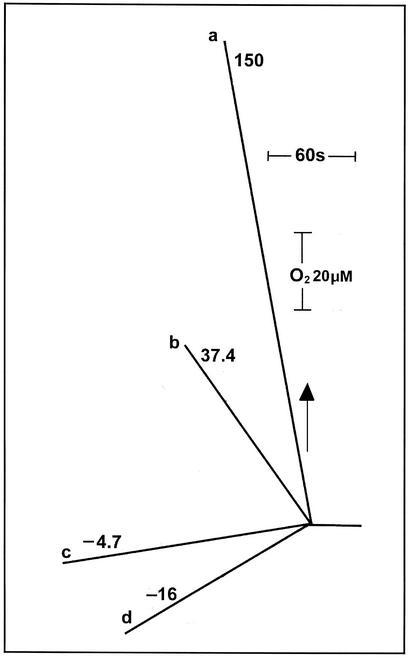
In the same samples, an ATP content of approximately 130 nmol mg–1 chlorophyll (Chl) was found, as shown in Figure 1C. Figure 1, A and C, refers to aerobic conditions. The ATP/ADP ratio dropped from approximately 15 (Fig. 1C) to approximately 5 when anaerobiosis was established (Fig. 1D), whereas the NADPH/NADP ratio rose from approximately 1.5 to 3.5 (Fig. 1B). Concomitantly, the maximum fluorescence emission yield (Fm) largely decreased (not shown), indicating the transition to state 2 (Wollman and Delepelaire, 1984). This latter condition is a particular state of the photosynthetic apparatus, where a substantial fraction of the major light-harvesting complex (LHCII) of PSII is functionally associated to PSI, in contrast to state 1, where all of the LHCII is connected to PSII (for review, see Allen, 1992; Wollman, 2001). This phenomenon, which is common to plants and algae, is regulated by the phosphorylation of the LHCII complex itself by a protein kinase (Allen et al., 1981; Horton and Black, 1981), which is reversibly activated by the redox state of the plastoquinone (PQ) pool (Allen et al., 1981).
Figure 1.
Changes in the NADP, NADPH, ATP, and ADP cellular contents in intact cells of C. reinhardtii wild type in both aerobic (A and C) and anaerobic (B and D) conditions. Algae were collected in the exponential growth phase and then resuspended in minimum high salt medium. They were maintained in suspension by gentle agitation in the presence of atmospheric oxygen concentration. Photosynthetic O2 evolution was 23.1 ± 7.0 μmol mg–1 Chl h–1 and 159.0 ± 28.0 μmol mg–1 Chl h–1at low and high light, respectively. Respiration was 7.4 ± 0.8 μmol O2 mg–1 Chl h–1.
Illumination of the cells under state 2 conditions, in the time lag preceding the start of O2 evolution (data not shown; however, see Finazzi et al., 1999), did not modify appreciably the concentration of NADP (Fig. 1B). On the other hand, ATP increased to its maximum level (Fig. 1D). The same result was obtained at both low and high actinic light intensities (not shown). This finding confirms our previous conclusion that the photosynthetic activity of C. reinhardtii involves only cyclic electron flow around PSI in state 2 conditions (Finazzi et al., 1999, 2002). Only photophosphorylation coupled to cyclic electron transport can explain ATP synthesis without changes in the redox state of NADP, because no contribution can be made by oxidative phosphorylation in anaerobiosis.
In aerobic conditions again, large variations of the NADPH/NADP and ATP/ADP ratios were also observed upon illumination. When a light (51 μE m–2 s–1) that was limiting the rate of electron transport to 15% of its maximum was provided, a decrease of the NADPH/NADP ratio to a value of approximately 0.7 was observed (Fig. 1A). At the same time, the ATP/ADP ratio increased with respect to the dark level, which was already very high (Fig. 1C). At saturating intensity (831 μE m–2 s–1), the NADPH/NADP ratio largely increased, recovering its dark value, whereas the ATP/ADP ratio again slightly increased with respect to the level measured at low light (Fig. 1C). These observations strongly suggest that it is the rate of NADP reduction rather than that of ATP synthesis that sets the velocity of carbon assimilation in C. reinhardtii when light intensity is limiting the rate of photosynthesis. It should be noticed, however, that the figures presented above refer to estimations of the nucleotide pools size in the entire cell. This raises the question as to the real significance of the observed changes: Although variations in the NADPH/NADP pools should reflect essentially photosynthetic activity, a misinterpretation of photosynthetic contribution to changes in the ATP content cannot be ruled out a priori, because of the bias induced by the turnover of the other cellular pools. Two pieces of evidences, however, indicate that the photosynthetic contribution is largely prevailing under illumination: (a) The overall photosynthetic ATP metabolism can be estimated (from the photosynthetic oxygen evolution activity at saturating light saturation) as 160*3 = 480 μmol mg–1 Chl h–1 or approximately 130 nmol mg–1 Chl s–1. This indicates that the overall ATP cellular content is consumed by photosynthesis in 1 s. In addition, (b) under anaerobic conditions (state 2, no CO2 assimilation), where the dark ATP content is decreased by less than a factor of 2 (Fig. 1) and the respiratory activity is prevented, illumination rapidly restored maximum level of ATP concentration, again suggesting that most of the observed changes involve chloroplast activity. This idea is also reinforced by previous estimation of the different cellular ATP pools in vascular plants, where the chloroplast one represents at least 50% of the total amount (Stitt et al., 1982).
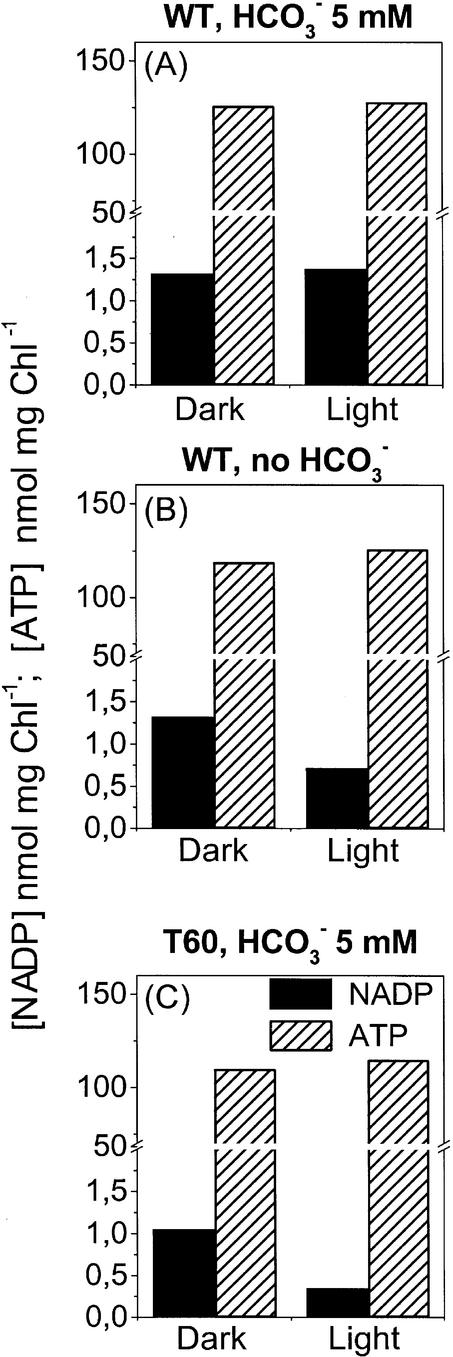
To establish whether the reoxidation of NADPH upon illumination was due to its use for carbon assimilation, we measured the changes of NADP concentration during illumination under conditions where CO2 assimilation was prevented. This was obtained by omitting bicarbonate from the reaction medium adjusted to pH 6.0 and letting photosynthesis proceed until all available CO2 had been consumed. At this stage, oxygen evolution was strongly reduced (to approximately 5% of the initial value) and the compensation point of O2 evolution and uptake by respiration was attained. After 10 more min of illumination with saturating light, the cells were analyzed for NADP and ATP content, as above. Figure 2 shows that under CO2 limitation, the NADP concentration decreased to the same value observed in anaerobic conditions (Fig. 2B), whereas in the presence of bicarbonate, this decrease was not observed (Fig. 2A). The ATP concentration, however, remained high in both samples. As a complementary approach, NADP and ATP concentrations were measured in a mutant deficient in the Rubisco enzyme (T60; Khrebtukova and Spreitzer, 1996). In this mutant, the rate of photosynthesis is reduced to less than 5% of the control rate (data not shown). Accordingly, illumination caused a relevant NADP reduction, seen as the decrease of the oxidized coenzyme (Fig. 2C), whereas the ATP level did not change.
Figure 2.
Changes in the NADP and ATP cellular content in whole cells of C. reinhardtii wild type in the presence (A) and absence (B) of CO2 and in the T60 mutant (C). Light intensity was 830 μE m–2 s–1; pH of the medium was 6.0. Other conditions are as in Figure 1. All data are in nanomoles per milligram of Chl.
Effect of Ionophores on Oxygen Evolution in C. reinhardtii
To further confirm the idea that the NADPH generation rate limits
photosynthesis in C. reinhardtii under low light intensity, we have
measured the effect of low concentrations of ionophores on the light-driven
oxygen evolution at low light. We reasoned that stimulation of photosynthetic
O2 evolution would be expected if NADPH generation is really the
limiting factor, because the partial uncoupling of electron transport should
accelerate the rate of NADPH formation. This would occur via the partial
removal of the “photosynthetic control”, i.e. the kinetic
inhibition exerted by the
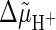 on electron transport (Bendall,
1982). On the contrary, the opposite might be expected if ATP is
limiting, the efficiency of its synthesis being decreased by the uncoupler. We
chose the ionophore carbonylcyanide-p-(trifluoromethoxy)
phenylhydrazone (FCCP), which has been already shown to be able to decrease
the cellular ATP content and to increase the NADPH concentration
(Bulté et al.,
1990).
on electron transport (Bendall,
1982). On the contrary, the opposite might be expected if ATP is
limiting, the efficiency of its synthesis being decreased by the uncoupler. We
chose the ionophore carbonylcyanide-p-(trifluoromethoxy)
phenylhydrazone (FCCP), which has been already shown to be able to decrease
the cellular ATP content and to increase the NADPH concentration
(Bulté et al.,
1990).
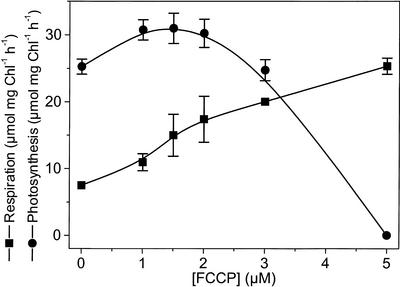
Figure 3 shows that when this compound was added at low concentrations, a stimulation of photosynthesis was observed, together with the expected stimulation of respiration. This strongly confirms the limitation of CO2 assimilation by the rate of NADP reduction rather than by that of ATP formation in C. reinhardtii. At higher concentrations, the ionophore inhibited photosynthesis (the effect being almost complete at the concentration of 5 μm), as expected because of the drastic inhibition of ATP synthesis.
Figure 3.
Effect of the ionophore FCCP on the rate of oxygen uptake and evolution by whole cells of C. reinhardtii. Chl concentration was 150 μg mL–1. Light intensity was adjusted as to give 20% to 25% of the maximum rate of photosynthesis. Figures refer to seven independent experiments. Other conditions are as in Figure 1.
ATP/ADP and NADPH/NADP Cellular Contents during a Dark-Light Transition in Barley Leaves
The remarkable difference existing between the results obtained in intact chloroplasts isolated from a number of vascular plants and those obtained here calls for an explanation. The differences observed might depend on the fact that in intact cells the photosynthetic metabolism occurs together with all of the other cellular activities, whereas in isolated mature chloroplasts photosynthesis, it is the only physiological process going on.
We have therefore decided to compare the observations made above for C. reinhardtii with the changes of ATP and NADP in vascular plants. We have estimated the level of these metabolites in intact barley leaves, either upon dark adaptation or in steady-state photosynthesis under low or high (14 times higher) light intensity. Figure 4 shows that illumination was accompanied by a decrease of the NADP content (indicating an increase of the NADPH concentration), which was essentially light independent, at variance with what observed in Figure 1 in the case of C. reinhardtii. An increase of the ATP/ADP ratio was also observed, consistent with the results obtained in Figure 1 and with previous data from plant leaves and isolated protoplasts (e.g., see Santarius and Heber, 1965; Stitt et al., 1982; Furbank and Horton, 1987).
Figure 4.
Changes of ATP, ADP, and NADP upon illumination of barley leaves. Barley leaves, freshly collected from the orchard, were incubated at room temperature either in the dark or in low- and high-light conditions. After 3 min, liquid nitrogen was poured on them, and the frozen leaves were ground to a fine powder. They were then extracted with TCA, and the extracts were treated as described in “Materials and Methods.”
Thus, it appears that the situation observed in the case of plants is somewhat intermediate between that of chloroplasts, because the same pattern of NADPH is observed in the two systems, and that of C. reinhardtii, because no ATP decrease is observed. We note however, that Prinsley and coworkers (1986) have shown that the ATP/ADP ratio measured in spinach leaves discs (which was about 1.2 at high light intensity) decreased transiently during the transition from high to low light intensity and then recovered the initial value when the uptake of CO2 stabilizes at a lower steady rate. This might suggest that the situation measured in plant leaves is essentially the same as the one observed in the case of chloroplasts, where ATP synthesis limits the assimilation of CO2. In leaves, however, other metabolic phenomena, having a slower activation time than photosynthesis, might provide the required ATP/NADPH ratio at steady state. The mechanism proposed to explain this phenomenon implies that reducing equivalents generated in the chloroplast during illumination can be oxidized in the mitochondria—mostly thanks to the malate/oxaloacetate shuttle system (Scheibe, 1987)—and that the ATP synthesized in this organelle can then be transferred back to the chloroplast where it is used for CO2 assimilation. This phenomenon has been already described in the case in barley protoplasts (Kroemer et al., 1988) and has been ascribed to the rapid equilibration both of oxaloacetate/malate and of ATP between the different cell compartments (for a review, see Hoefnagel et al., 1998; Noctor and Foyer, 2000; see also Dutilleul et al., 2003).
Contribution of Mitochondrial ATP Synthesis and of the Mehler-Ascorbate Reaction to Chloroplast ATP Metabolism
To understand whether the same interaction between chloroplastic and mitochondrial metabolism might explain the finding that in C. reinhardtii, ATP is produced in excess with respect to NADPH, we have measured the efficiency of oxaloacetate to act as an electron acceptor. For this experiment, cells were illuminated in the absence of CO2 (at the CO2 compensation point, where no O2 changes are observed), and the ability of different compounds to restore O2 evolution was tested.
We found that oxaloacetate addition induced a restoration of activity, which was approximately 25% of the maximum rate observed upon addition of bicarbonate (Fig. 5). This result suggests a possible contribution of mitochondrial metabolism to photosynthetic CO2 assimilation through the malate-oxaloacetate shuttle system, as already proposed in the case of plants. The occurrence of such a phenomenon in the case of C. reinhardtii is consistent with the findings of Lemaire and coworkers (1988), who isolated a mutant lacking an active chloroplast ATP synthase but still capable to grow phototrophically in inorganic medium, even if at about 25% of the maximum rate. In the frame of this hypothesis, the drastic decrease of NADPH observed under conditions where light intensity is limiting photosynthesis (as in Fig. 1) could be ascribed to its use for oxaloacetate reduction in the chloroplast and the oxidation of malate so generated in the mitochondrion (running at a maximum rate of around 10 μmol O2 mg–1 Chl h–1; see legend of Fig. 1). In the improbable hypothesis that all of the ATP made by mitochondria would be transferred to the chloroplast, the latter would be provided with ATP at a rate barely sufficient to account for the observed photosynthetic rate of 23 μmol mg–1 Chl h–1, because it is well known that the oxidation of substrates (in this case malate) via the Krebs cycle in the mitochondria can form a maximum of five ATP per CO2 emitted. Such a mechanism could not, however, contribute a relevant fraction of the ATP needed for CO2 assimilation at saturating light, running at a rate of over 150 μmol mg–1 Chl h–1.
Figure 5.
Ascorbate- and oxaloacetate-driven oxygen consumption and evolution in C. reinhardtii intact cells. The cells (150 μg Chl mL–1) were illuminated in the oxygraph cell at pH 6.0, without the addition of bicarbonate, until the CO2 present in the buffer was exhausted and the CO2 compensation point was attained. The changes of O2 concentration were recorded upon the addition (arrow) of 5 mm NaHCO3 (a); 2 mm oxaloacetate (b); 0.4 mm ascorbate (c); and 4 mm ascorbate (d). Figures on the traces represent the specific activity in micromoles per milligram of Chl per hour.
By the same experimental approach, we found that the addition of ascorbate did not restore O2 evolution but rather induced a slow O2 uptake, indicating that ascorbate, or rather the free radical produced by its oxidation (Miyake and Asada, 1992; Forti and Ehrenheim, 1993; Forti and Elli, 1995), is a poor terminal electron acceptor in the chloroplast of C. reinhardtii. This result and the finding that the properties of the ascorbate peroxidase—the key enzyme of the Mehler-ascorbate system (Takeda et al., 1997)—of C. reinhardtii are very different from those of higher plants strongly suggest that the role of the ascorbate-Mehler reaction in this alga is likely the protection against the generation of reactive oxygen species (Asada, 2000) rather than the contribution to the energy requirements of photosynthesis.
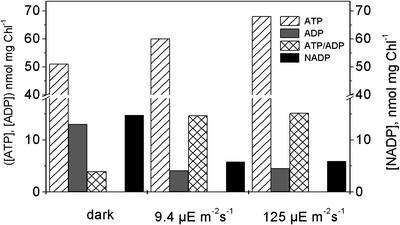
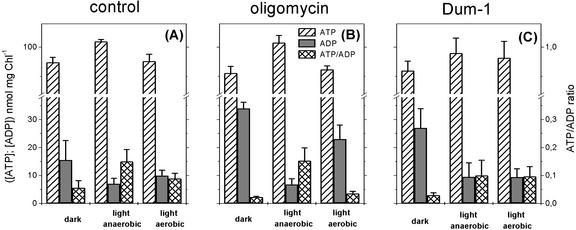
To estimate the contribution of the mitochondrial metabolism to photosynthesis in C. reinhardtii, we have measured the effect of oligomycin on oxygen evolution and on the ATP/ADP ratios measured at both low and high light intensities. This compound specifically inhibits the mitochondrial ATP synthase complex without affecting that of the chloroplasts (Kroemer et al., 1988). We reasoned that under conditions where no ATP synthesis in mitochondria is associated with the oxidation of the malate generated in the chloroplast, the limitation of CO2 fixation by NADPH availability should be replaced by the limitation imposed by ATP availability, as reported in the case of isolated chloroplasts (Heber, 1973). Figure 6 shows that, whereas oligomycin decreased the ATP/ADP ratio in the dark (as expected because of inhibition of mitochondrial oxidative phosphorylation), the ratio increased upon illumination of low intensity, essentially as in the untreated samples (Fig. 6, compare B and A). Moreover, the addition of oligomycin did not result in any inhibition of the rate of oxygen evolution (Fig. 6, see legend) at any light intensity (not shown), whereas inhibition has been reported in higher plants protoplasts (Kroemer et al., 1988) and leaves (Kroemer and Heldt, 1992). The inhibitor did not affect respiration either (Fig. 6, legend). This latter observation can be easily understood because C. reinhardtii is endowed with the pathway of respiration alternative to the mitochondrial cytochrome oxidase pathway (Peltier and Thibault, 1985), which is insensitive to the inhibitors of the latter such as mixothiazol and cyanide and is not under “respiratory control” by ΔpH. As an independent approach, we measured the same parameters in the Dum-1 mutant, which is defective in mitochondrial electron transport (Matagne et al., 1989). In this mutant, again, the ATP/ADP ratio was decreased in the dark and was increased upon illumination as in the wild type (Fig. 6C).
Figure 6.
Effect of oligomycin and of the mitochondrial mutation Dum-1 on cellular ATP and ADP concentrations in C. reinhardtii cells. Conditions are as in Figure 1. The Chl concentration was 150 μg mL–1. Oligomycin was 10 μg mL–1. Light intensity was 24 μE m–2 s–1, producing an activity of 17.9, 18.2, and 18.7 μmol O2 mg–1 Chl h–1, respectively, in the control, oligomycin-treated, and DUM samples and a respiration of 10.3, 10.2, and 10.5, respectively. At saturating light intensity, the photosynthetic rate was, respectively, 127, 134, and 143 μmol mg–1 Chl h–1 in the control, in the presence of oligomycin, and in the Dum-1 mutant. The data are the average of four experiments ± se. A, Wild type, untreated sample; B, wild type, oligomycin-treated sample; and C, Dum-1 mutant, untreated.
This suggests that the contribution of respiration to the generation of the ATP required to assimilate CO2 is rather limited in the case of C. reinhardtii. This latter conclusion is also substantiated by the comparison of the effects of oligomycin (and of the Dum-1 mutation) on the ATP levels during illumination under anaerobic and aerobic conditions. Although ATP synthesis takes place without consumption for CO2 fixation in the first condition (the cells are in state 2, and no photosynthetic O2 evolution is observed in this state; Finazzi et al., 1999), both synthesis and consumption take place simultaneously in the second one (state 1, active photosynthesis). Consequently, a decrease in the ATP levels is observed in the latter condition, which probably reflects the balance between its generation and use at steady state. If the mitochondrial ATP contribution to steady-state photosynthesis were relevant, large differences would be expected between aerobic and anaerobic cells upon addition of oligomycin. On the contrary, the decrease observed in oligomicin-treated algae (approximately 20%) is not so dramatic when compared with that observed in untreated cells (approximately 15%). In our opinion, this confines the ATP contribution to photosynthesis by mitochondria to a rather minor role.
Contribution of Cyclic Electron Flow to the ATP Generation in C. reinhardtii Cells
The results presented above indicate that the photosynthetic electron transport per se is able to produce the ATP and NADPH in the ratio of more than 1.5 in C. reinhardtii. Both the ascorbate-Mehler reaction and mitochondrial metabolism seem to play a minor role in the generation of the extra ATP required to promote CO2 fixation.
Therefore, a contribution from cyclic electron flow seems to be a better candidate, because it has been already shown to be extremely efficient in C. reinhardtii, at least under state 2 conditions (Finazzi et al., 1999, 2002; Fig. 1). State 2, however, represents a peculiar situation of the photosynthetic apparatus, where approximately 80% of the LHCII becomes associated with PSI (Delosme et al., 1996), and the cytochrome b6f concentration in its proximity is increased (Vallon et al., 1991). In state 1 conditions, on the contrary, no evidence for the occurrence of cyclic flow has been obtained (Finazzi et al., 1999), raising the question of the real contribution of cyclic flow to ATP photosynthetic generation also in the case of C. reinhardtii.
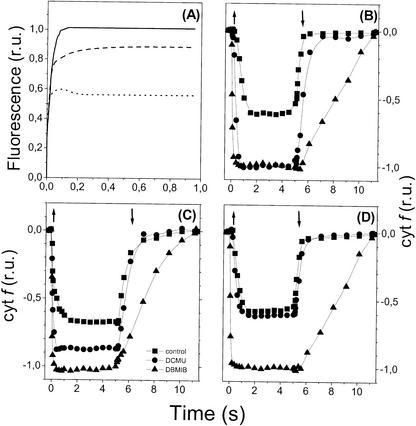
We have already recognized, however (Finazzi et al., 2002), that both states 1 and 2 represent extreme conditions, as required to fully oxidize or reduce the PQ pool in the dark. Under more physiological conditions, as the one employed in Figure 1, an intermediate state between states 1 and 2 might exist, where both linear and cyclic flow might contribute simultaneously to photosynthesis, thus explaining the lack of limitation of photosynthetic CO2 assimilation by ATP observed in Figure 1. The existence of this intermediate state is suggested by the finding that the Fm, measured in the presence of the PSII inhibitor 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) of C. reinhardtii cultures is usually not identical to those of state 1 but intermediate between that of states 1 and 2 (F.-A. Wollman, personal communication).
To verify this possibility, we have measured the effect of DCMU and of the cytochrome b6f inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB) on electron flow through the cytochrome b6f complex in the case of cells treated as in Figure 1, i.e. incubation under dim light in a weakly agitated flask. As a consequence of this treatment, the redox state of the PQ pool (which controls the activation of the kinase responsible for state transitions; for review, see Wollman, 2001) is expected to be more reduced than in state 1 (where it is mostly oxidized because of the strong agitation of the cells in the dark) and more oxidized than in state 2 (where it is completely reduced by anaerobiosis). This should lead to a partial activation of the kinase molecules and give rise to the intermediate level hypothesized above.
Figure 7A shows that this is the case, because the Fm measured in algae incubated under the same conditions of Figure 1 was actually intermediate between that of states 1 and 2. As expected, the consequences of DCMU addition on the electron flow through cytochrome f were also intermediate. In state 1, this compound completely inhibited electron flow (Fig. 7B), while being without effect in state 2 (Fig. 7D), as already established previously (Finazzi et al., 1999). In the conditions explored here (Fig. 7C), its inhibition was equal to 80% of that of DBMIB (Fig. 7C). This was not due to an incomplete block by DCMU of the photochemical activity of PSII in these conditions, as indicated by the observation that the rise of fluorescence to the maximum value was essentially the same as in state 1 conditions (Fig. 7A). This result indicates that part of the reducing equivalents used to reduce cytochrome f under the conditions employed in Figure 1 were not generated at the level of PSII and so probably originated from PSI.
Figure 7.
Cytochrome f redox changes and fluorescence emission measurements in C. reinhardtii cells. A, Fluorescence induction measurements in the presence of 10 μm DCMU. Continuous line, State 1 conditions (algae were kept in darkness under continuous vigorous agitation in air to prevent reduction of the PQ pool). Dotted line, State 2 conditions, obtained through dark incubation of algae in argon. Dashed lines, same conditions as in Figure 1. Absorbance variations, indicating cytochrome f redox changes. B, State 1; C, same conditions as in Figure 1; D, state 2. Light intensity was 1,000 μE m–2 s–1. Squares, No inhibitors; circles, 10 μm DCMU; and triangles, 2 μm DBMIB. Upward and downward arrows indicate the switch on and off of the actinic light, respectively. A decrease of absorbance corresponds to oxidation of cytochrome f. Traces were normalized on the amplitude of a PSI-driven charge separation signal measured at 515 nm.
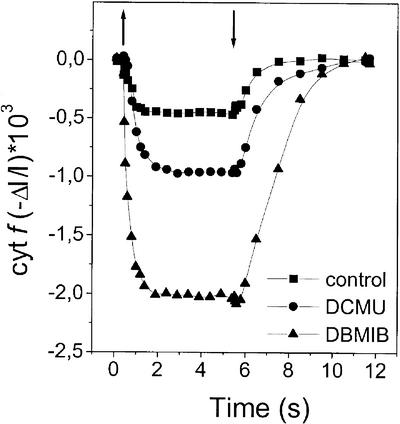
This finding can be interpreted in two ways: (a) Cyclic and linear electron transport coexist in the same chloroplast; or (b) two populations of cells are present at the same time in the experimental system, one in state 1 and the other in state 2 conditions. To discriminate between these two hypotheses, we have repeated the same measurement at lower light intensity. We reasoned that, if the rate of cyclic flow is lower than that of the linear one, then competition between the two might influence the relative fraction of the cytochrome complexes involved in cyclic flow according to the first hypothesis, but not according to the other, where no contact between the chains operating in the linear and cyclic electron flow mode is expected. The results of these experiments are reported in Figure 8 and clearly indicate that the extent of inhibition by DCMU was largely decreased at low light intensity, suggesting that cyclic and linear flow are not confined to different cell populations.
Figure 8.
Influence of DCMU on cytochrome f redox changes under limiting light intensity. Same conditions and symbols as in Figure 7C, except that light intensity was 100 μE m–2 s–1.
CONCLUSIONS
The data presented here are consistent with the well established concept of “assimilatory power” (for review, see Giersh et al., 1980; Dietz and Heber, 1984; Noctor and Foyer, 2000), which states that it is the balance between the rate of generation and consumption of reducing equivalents and ATP that sets the rate of CO2 assimilation. We show however that in aerobic cells of the green alga C. reinhardtii, the rate of NADPH generation is a crucial factor in the assimilation of CO2 under steady-state, light-limited conditions. This is indicated by the decrease of the NADPH concentration (Fig. 1), which is strictly dependent on CO2 assimilation (Fig. 2). The stimulation of photosynthetic O2 evolution in vivo observed upon addition of low concentrations of the uncoupler FCCP under low light intensity (Fig. 3) is also consistent with this idea, suggesting that the increase in the rate of NADP photoreduction at the expense of lower coupling of ATP formation stimulates photosynthesis. In contrast to this, it is well known that uncouplers inhibit O2 evolution in isolated chloroplasts when CO2 is the only final electron acceptor supplied.
We interpret this finding as the consequence of the fact that linear and cyclic electron flow operate at the same time in chloroplasts in C. reinhardtii, thus contributing to the increase of the amount of ATP at the expense of the NADPH content. The very high efficiency of cyclic flow observed in C. reinhardtii (Figs. 7 and 8; see also Finazzi et al., 1999), which might contribute up to 50% of the overall electron flow, is probably responsible for the strong limitation in the rate of NADPH generation, which is not observed in higher plants (see e.g. Fig. 4). Consistent with such a difference, the question as to the coexistence of cyclic and linear electron flow processes in the case of C3 plants under steady-state photosynthetic conditions is still under debate, despite the fact that one of the components of the “cyclic electron flow chain” has been recently characterized (Munekage et al., 2002).
In these plants, Forti and Parisi (1963) have shown evidence for cyclic electron flow only in non-physiological conditions that abolish photosynthesis. It has been shown that cyclic electron flow might be induced by CO2 depletion (Harbinson and Foyer, 1991), drought (Katona et al., 1992), and other stress conditions (for review, see Bendall and Manasse, 1995). More recently, cyclic electron flow has been observed in dark-adapted leaves at the onset of illumination (Joliot and Joliot, 2002). Occurrence of cyclic electron transport in C3 plants in steady-state physiological conditions has been recently proposed on the basis of measurements of PSI-dependent energy storage by photoacoustic methods (Joet et al., 2002). Nevertheless, the rates of P700 reduction through the cyclic pathway reported were very low, the t1/2 being at best of the order of 400 ms: much too low to account for a relevant role in photosynthesis. A substantial fraction of the extra ATP generated in higher plants in the light is likely due to the contribution of ATP by mitochondria through the malate-oxaloacetate shuttle (Kroemer et al., 1988; Dutilleul et al., 2003) or the Mehler-ascorbate system (Forti and Elli, 1995; Asada, 2000).
It appears therefore that different metabolic choices have been performed by photosynthetic organisms to achieve the correct balancing between generation of reducing equivalents and ATP, and thus maintain sufficient ATP and NADPH levels for CO2 assimilation during illumination, at variance with isolated chloroplast.
MATERIALS AND METHODS
Strains and Culture Conditions
Chlamydomonas reinhardtii wild type (from strain 137 C) and T60 (devoid of Rubisco; Khrebtukova and Spreitzer, 1996) were provided by the Laboratoire de Physiologie Cellulaire et Moléculaire du Chloroplaste, at the Institut de Biologie Pligico-Chemique (Paris). Cells were grown at 24°C in acetate-supplemented medium (Harris, 1989) under approximately 60 μE m–2 s–1 continuous white light. They were harvested during exponential growth (approximately 2 × 106 cells mL–1), and resuspended in minimal medium (Harris, 1989).
Measurements of Oxygen Evolution and Uptake and of Fluorescence
Photosynthesis and respiration were measured as O2 exchanges in the presence of 5 mm NaHCO3 (unless otherwise indicated) using a Clark-type electrode (Radiometer, Copenhagen) in a homemade cell, at 25°C. Illumination was provided by a halogen lamp, which was filtered through a heat filter. Light intensity was adjusted, as needed, by neutral density filters. Fluorescence emission was measured in the same cell employed to measure oxygen exchanges using a PAM fluorimeter (Walz, Effeltrich, Germany). For measurements, algae were resuspended at 120 to 150 μg Chl mL–1. Fluorescence induction kinetics were measured in a homemade apparatus, where emission was excited at 590 nm, and were measured in the near infrared region. Chl concentration was determined by measuring the A680 of the cell culture in a spectrophotometer equipped with a scatter attachment, on the basis of a calibration curve constructed after extraction of the Chl with 80% (w/v) acetone (Finazzi et al., 1999).
Determination of ATP, ADP, NADP, and NADPH
An aliquot of the cell suspension used to measure oxygen exchanges and fluorescence emission was used to measure ATP and ADP in trichloroacetic acid extracts as previously described (Finazzi et al., 1999). Oxygen contents in the solution was monitored just before sample preparation with the same electrode described before. The same extracts were used for the determination of NADP, whereas NADPH was completely destroyed in the acid extract, as confirmed upon addition of known amounts of it. For the determination of NADPH, the cell suspension was frozen in the light or in the dark and then treated with 0.5 volume of NaOH (0.5 m, 90°C), and the very basic extract was heated at 95°C for 5 min. During the treatment, NADP was completely destroyed (as shown by the lack of recovery of any NADP added to the cells at the time of quenching), and ATP was also largely hydrolyzed. The extracts were rapidly cooled and centrifuged in the cold, and the clear supernatants were neutralized and buffered at pH 8.0.
NADP and NADPH were measured at pH 8.0 by enzymatic cycling, using Glc-6-phosphate as the electron donor and 2,6-dichlorophenolindophenol as the acceptor in the presence of Glc-6-phosphate dehydrogenase and ferredoxin-NADP reductase used here as an NADPH-specific diaphorase. The rate of 2,6-dichlorophenolindophenol reduction was measured in a dual wavelength spectrophotometer, as the absorbance change at 605–500 nm. A calibration was done in each sample upon addition of a known amount of NADP.
Pure ferredoxin-NADP reductase was prepared as previously described (Forti, 1971), and the other enzymes were purchased from Sigma-Aldrich (St Louis).
Spectroscopic Measurements
Algae were resuspended in a minimal medium, with the addition of 20% (w/v) Ficoll to prevent cell sedimentation. Chl concentration was approximately 70 μg Chl mL–1. Spectroscopic measurements were performed at room temperature, using a homemade spectrophotometer (Joliot et al., 1981). Actinic light was provided by a SDL diode laser (SDL, Planor, TX; 500 mW, emission peak at 690 nm). Light-induced absorption changes were measured as absorption of flash monochromatic light at discrete times. Cytochrome f redox changes were calculated as the difference between the absorption at 554 nm and a baseline drawn between 545 and 573 nm (Finazzi et al., 1997) and were corrected for the contribution of the electrochromic signal (5% of the signal observed at 515 nm; Finazzi et al., 1999). State 1 was obtained through dark incubation of the cells under strong agitation, whereas state 2 was induced through anaerobiosis (Bulté et al., 1990).
Acknowledgments
We gratefully acknowledge the skillful technical assistance of Sandro Zangrossi.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018861.
This work was supported by the Consiglio Nazionale delle Ricerche of Italy.
References
- Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335 [DOI] [PubMed] [Google Scholar]
- Allen JF, Bennett J, Steinback KE, Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29 [Google Scholar]
- Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhausen JE, Kitzmann C, Scheibe R (1994) Competition between electron acceptors in photosynthesis: regulation of the malate valve during CO2 fixation and nitrite reduction. Photosynth Res 42: 75–86 [DOI] [PubMed] [Google Scholar]
- Bendall DS (1982) Photosynthetic cytochromes of oxygenic organisms. Biochim Biophys Acta 683: 119–151 [Google Scholar]
- Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229: 23–38 [Google Scholar]
- Berry S, Rumberg B (1999) Proton to electron stoichiometry in electron transport of spinach thylakoids. Biochim Biophys Acta 1410: 248–261 [DOI] [PubMed] [Google Scholar]
- Bulté L, Gans L, Rebéillé F, Wollman F-A (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim Biophys Acta 1020: 72–80 [Google Scholar]
- Delosme R, Olive J, Wollman F-A (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta 1273: 150–158 [Google Scholar]
- Dietz KL, Heber U (1984) Rate-limiting factors in leaf photosynthesis: 1. Carbon fluxes in the Calvin cycle. Biochim Biophys Acta 767: 432–443 [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory. Plant Physiol 131: 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egneus H, Heber U, Matthiesen U, Kirk M (1975) Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta 408: 252–268 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Buschlen S, de Vitry C, Rappaport F, Joliot P, Wollman F-A (1997) Function-directed mutagenesis of the cytochrome b6 f complex in Chlamydomonas reinhardtii: involvement of the cd loop of cytochrome b6 in quinol binding to the Q(o) site. Biochemistry 36: 2867–2874 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Furia A, Barbagallo RP, Forti G (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim Biophys Acta 1413: 117–129 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix J-D, Zito F, Forti G (2002) Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 31: 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge U-I, Heldt HW (1991) Metabolite translocation of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Bol 42: 129–144 [Google Scholar]
- Forti G, Parisi B (1963) Evidence for the occurrence of cyclic photophosphorylation in vivo. Biochim Biophys Acta 71: 1–6 [Google Scholar]
- Forti G (1971) NADPH-cytochrome f reductase from spinach. Methods Enzymol 23A: 447–451 [Google Scholar]
- Forti G, Ehrenheim AM (1993) The role of ascorbic acid in photosynthetic electron transport. Biochim Biophys Acta 1183: 408–412 [Google Scholar]
- Forti G, Elli G (1995) The function of ascorbic acid in photosynthetic phosphorylation. Plant Physiol 109: 1207–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Horton P (1987) Regulation of photosynthesis in isolated barley protoplasts: the contribution of cyclic photophosphorylation. Biochim Biophys Acta 894: 332–338 [Google Scholar]
- Giersh C, Heber U, Kobayashi Y, Inoue Y, Shibata K, Heldt HW (1980) Energy charge, phosphorylation potential and proton motive force in chloroplasts. Biochim Biophys Acta 590: 59–73 [DOI] [PubMed] [Google Scholar]
- Grace S, Pace R, Wydrzynski T (1995) Formation and decay of monodehydroascorbate radicals in illuminated thylakoids as determined by EPR spectroscopy. Biochim Biophys Acta 1229: 155–165 [Google Scholar]
- Harbinson J, Foyer CH (1991) Relationship between the efficiencies of photosystem I and II and stromal redox state in CO2-free air. Plant Physiol 97: 149–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E (1989) The Chlamydomonas Sourcebook. Academic Press, San Diego
- Heber U (1973) Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta 305: 140–152 [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin O, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta 1366: 235–255 [Google Scholar]
- Horton P, Black MT (1981) Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5′-triphosphate. Biochim Biophys Acta 635: 53–62 [DOI] [PubMed] [Google Scholar]
- Joet T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around Photosystem I in C3 plants: in vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128: 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P, Béal D, Frilley B (1981) Une nouvelle méthode spectrophotométrique destinée à l'étude des réactions photosynthétiques. J Chim Phys 77: 209–216 [Google Scholar]
- Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99: 10209–10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C, Wollman FA, Bennoun P (1988) Restoration of phototrophic growth in a mutant of Chlamydomonas reinhardtii in which the chloroplast atpB gene of the ATP synthase has a deletion: an example of mitochondria-dependent photosynthesis. Proc Natl Acad Sci USA 83: 1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona E, Neimanis S, Sconknecht G, Heber U (1992) Photosystem I-dependent cyclic electron-transport is important in controlling photosystem II activity in leaves under conditions of water stress. Photosynth Res 34: 449–464 [DOI] [PubMed] [Google Scholar]
- Khrebtukova I, Spreitzer RJ (1996) Elimination of the Chlamydomonas gene family that encodes the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA 93: 13689–13693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer S, Heldt HW (1992) On the role of mitochondrial oxidative phosphorylation in photosynthesis metabolism as studied by the effect of oligomycin on photosynthesis in protoplasts and leaves of barley (Hordeum vulgare). Plant Physiol 95: 1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer S, Stitt M, Heldt HW (1988) Mitochondrial oxidative phosphorylation participating in photosynthetic metabolism of a leaf cell. FEBS Lett 226: 352–356 [Google Scholar]
- Matagne RF, Michel-Wolwertz M-R, Munaut C, Duyckaerts C, Sluse F (1989) Induction and characterization of mitochondrial DNA mutants in Chlamydomonas reinhardtii. J Cell Biol 108: 1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K (1992) Thylakoid bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33: 541–553 [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (2000) Homeostasis of adenylate state during photosynthesis in a fluctuating environment. J Exp Bot 51: 347–356 [DOI] [PubMed] [Google Scholar]
- Peltier G, Thibault P (1985) O2 uptake in the light in Chlamydomonas. Plant Physiol 79: 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR, Chong J, Chon A, Mosbach A, Heldt HW (1977) Fructose and sedoheptulosebisphosphatase: the sites of a possible control of CO2 fixation by light dependent changes of the stromal Mg2+ concentration. Biochim Biophys Acta 461: 313–325 [DOI] [PubMed] [Google Scholar]
- Prinsley RT, Dietz K-J, Leegood RC (1986) Regulation of photosynthetic carbon assimilation in spinach leaves after a decrease in irradiance. Biochim Biophys Acta 849: 254–263 [Google Scholar]
- Rebéillé F, Gans P, (1988) Interaction between chloroplasts and mitochondria in microalgae: role of glycolysis. Plant Physiol 88: 973–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000) The proton to electron stoichiometry of steady-state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA 97: 14283–14288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius KA, Heber U (1965) Changes in the intracellular levels of ATP, ADP, AMP and Pi and regulation function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta 102: 39–54 [DOI] [PubMed] [Google Scholar]
- Scheibe R (1987) NADP+-malate dehydrogenase in C3 plants: regulation and role of light activated enzyme. Physiol Plant 71: 393–400 [Google Scholar]
- Stitt M, Ross MC, Lilley RM, Heldt HW (1982) Adenine nucleotide levels in the cytosol, chloroplasts and mitochondria of wheat leaf protoplasts. Plant Physiol 70: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Ishikawa T, Shigeoka S (1997) Metabolism of hydrogen peroxide by the scavenging system in Chlamydomonas reinhardtii. Physiol Plant 99: 49–55 [Google Scholar]
- Vallon O, Bulté L, Dainese P, Olive J, Bassi R, Wollman F-A (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA 88: 8262–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt HT (1979) Energy conversion in the functional membrane of photosynthesis: analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta 505: 355–427 [DOI] [PubMed] [Google Scholar]
- Wollman F-A (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F-A, Delepelaire P (1984) Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol 98: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]