Abstract
Although several investigations have demonstrated a plasma membrane (PM)-bound peroxidase activity in plants, this study is the first, to our knowledge, to purify and characterize the enzymes responsible. Proteins were extracted from highly enriched and thoroughly washed PMs. Washing and solubilization procedures indicated that the enzymes were tightly bound to the membrane. At least two distinct peroxidase activities could be separated by cation exchange chromatography (pmPOX1 and pmPOX2). Prosthetic groups were identified in fractions with peroxidase activity by absorption spectra, and the corresponding protein bands were identified by heme staining. The activities of the peroxidase enzymes responded different to various substrates and effectors and had different thermal stabilities and pH and temperature optima. Because the enzymes were localized at the PM and were not effected by p-chloromercuribenzoate, they were probably class III peroxidases. Additional size exclusion chromatography of pmPOX1 revealed a single activity peak with a molecular mass of 70 kD for the native enzyme, whereas pmPOX2 had two activity peaks (155 and 40 kD). Further analysis of these fractions by a modified sodium dodecyl sulfate-polyacrylamide gel electrophoresis in combination with heme staining confirmed the estimated molecular masses of the size exclusion chromatography.
Peroxidases (EC 1.11.1.7, etc.) belong to a large family of enzymes that are ubiquitous in fungi, plants, and vertebrates. These proteins usually contain a ferriprotoporphyrin IX prosthetic group and oxidize several substrates in the presence of hydrogen peroxide (H2O2; Penel et al., 1992; Vianello et al., 1997). In higher plants, the number of isoenzymes may be extremely high, up to 40 genes corresponding to isoperoxidases for each plant, and several other isoforms can be generated by posttranscriptional and posttranslational modifications (Welinder et al., 1996; De Marco et al., 1999).
Although many soluble intracellular and extracellular peroxidases have been characterized in detail (for refs., see Gaspar et al., 1982; Hiraga et al., 2001; Shigeoka et al., 2002), less is known about membrane-bound enzymes, in particular the peroxidases of plant plasma membranes (PMs). Evidence for a PM-bound peroxidase activity in higher plants has been demonstrated frequently. Lin (1982) reported an increased oxygen consumption by intact corn (Zea mays) root protoplasts in the presence of extracellular NADH. Pantoja and Willmer (1988) obtained similar results using guard cell protoplasts from Commelina communis in the presence of NAD(P) H. PMs isolated from several species and plant parts showed NAD(P) H oxidase activities, which were comparable with a peroxidase (Mφller and Bérczi, 1986; Askerlund et al., 1987; Vianello et al., 1990, 1997; De Marco et al., 1995; Zancani et al., 1995; Sagi and Fluhr, 2001). Because the application of detergents did not significantly affect the activity observed and because activity could be detected with intact protoplasts, peroxidase activity has been suggested to be located at the apoplastic surface of the PM.
The NADH oxidation by PM from cauliflower (Brassica oleracea) could be stimulated by phenolic substances or inhibited by typical effectors of peroxidases like catalase, superoxide dismutase, cyanide, or azide (Askerlund et al., 1987). In PM-enriched fractions of Arabidopsis and Chinese cabbage (Brassica campestris L. subsp. pekinensis) seedlings, oxidation of Trp was reported in the presence of H2O2 (Ludwig-Müller et al., 1990; Ludwig-Müller and Hilgenberg, 1992). PM isolated from soybean (Glycine max) roots showed a peroxidase activity in the presence of substrates like o-dianisidine, guaiacol, and ascorbate (Vianello et al., 1997). The oxidation of ascorbate could be strongly stimulated by phenolic acids, like caffeic and ferulic acid. Guaiacol or o-dianisidine oxidation rates were increased by CaCl2 and inhibited by potassium cyanide and azide. When proteins solubilized by SDS from non-washed PM were separated by SDS-PAGE, two bands (38 and 45 kD) could be detected by heme staining. Peroxidase activity of these bands was not demonstrated, and only one, less intensive band remained after partial washing of the membrane vesicles (Vianello et al., 1997).
In addition to these experiments, antibodies specific for apoplastic peroxidases were used to detect PM-bound peroxidases by immunogold labeling and electron microscopy in situ (Hu et al., 1989; Penel and Castillo, 1991; Crevecoeur et al., 1997). However, Askerlund et al. (1987) demonstrated that the presence of peroxidases in PM preparations depends largely on the final PM washing procedure, which decreases the level of peroxidases significantly. A PM-bound peroxidase has not yet been isolated and characterized from highly purified and properly washed PM (Bérczi and Mφller, 2000).
In the present work, we demonstrate the occurrence of at least two distinct peroxidase activities (pmPOX1 and 2) in corn root PM. A purification protocol for the isolation of these enzymes was developed, and the properties of the partially purified proteins were investigated by comparing them with soluble peroxidase activities.
RESULTS AND DISCUSSION
Binding to the PM
To check if peroxidase activities were loosely bound to the PM or entrapped inside the vesicles, different washing procedures were carried out. Independent of the salt concentrations used a maximum of 40% of the activity could be washed off in the presence of 1 mm EDTA and 0.01% (w/v) Triton X-100, i.e. 79% ± 7.2% (n = 2) of the activity remained in the PM at 150 mm KCl and 60% ± 1.9% (n = 4) at 500 mm KCl, respectively. Using 1 mm EGTA instead of EDTA did not change this result. A combination of 150 mm KCl, 1 mm EDTA, 0.01% (w/v) Triton X-100, and 0.1% (w/v) CHAPS (i.e. a detergent:protein ratio of 6:1 [w:w]) removed 62% ± 0.4% (n = 2) of the peroxidase activity from the PM.
Due to the fact that neither physiological or high salt concentrations in the presence of detergent and EDTA or EGTA nor high detergent concentrations were able to remove the activity completely from the PM, we conclude that these enzymes are probably tightly bound to the PM. Salts should have minimal effects on the micellar size of Triton X-100, whereas effects on the zwitterionic detergent CHAPS cannot be excluded. Thus, the presence of higher salt concentrations could change the critical micellar concentration of CHAPS, thereby increasing the proportion of washed off peroxidase activity as a result of partial solubilization.
However, because peroxidase activity remains in the low detergent phase after Triton X-114 solubilization and temperature-induced phase separation (data not shown), the peroxidases were probably not strongly hydrophobic. Independently of the detergent to protein ratio used, none of the detergents tested (Triton X-114, Triton X-100, CHAPS, or octylglucopy-ranoside) could solubilize the activity completely from the PM. The mechanism of the binding to the PM is unknown, but sequence analysis of intracellular peroxidases indicated that transmembrane domains may exist in plant peroxidases (Bunkelmann and Trelease, 1996; Jespersen et al., 1997; Nito et al., 2001).
In Arabidopsis, three genes encoding membrane-bound ascorbate peroxidases were found (Jespersen et al., 1997). One of the corresponding proteins is probably bound to microbodies by a C-terminal transmembrane domain like membrane-bound intracellular peroxidases of other plant species (Bunkelmann and Trelease, 1996; Nito et al., 2001). However, sequence analysis of these peroxidases revealed that they are class I peroxidases, which implies they cannot occur in the PM.
Purification
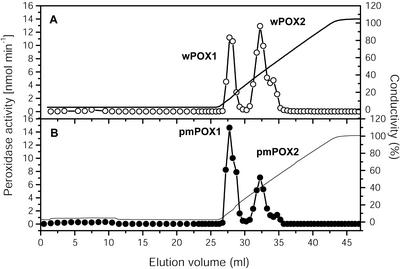
Solubilization by CHAPS yields about 30% ± 1% (n = 2) of the activity and increased up to 73% ± 4% (n = 5) in the presence of the dipole aminocaproic acid. Two activity peaks (pmPOX1 and pmPOX2) could be separated by cation exchange chromatography (Fig. 1). Peroxidase activities were eluted at 115 and 395 mm KCl. The total activity was divided into 59% ± 3% and 41% ± 2% (n = 4) for pmPOX1 and pmPOX2, respectively. Starting from washed PM (specific activity 401 ± 52 nmol min–1 mg protein–1; n = 5), a 24.0- and 8.8-fold purification for peak fractions of pmPOX1 and pmPOX2 with an overall yield of 31.4% was achieved. To compare the properties of pmPOX with soluble peroxidases, activities of the washing fluid of the PM (wPOX) were concentrated and separated by the same protocol (Fig. 1). The elution profile obtained was similar to that from the PM-bound POX. The total activity was divided into 30% and 70% for wPOX1 and wPOX2, respectively.
Figure 1.
Elution profiles of POX after cation exchange chromatography. Enzyme activities isolated from corn root PM were separated on a Uno S1 column. Bound proteins were eluted by a KCl gradient from0to1 m. The flow rate was 1 mL min–1, and fractions of 1.0 and 0.5 mL were collected. A, Separation of POX activities from washing fluid of PM (wPOX; ○). B, Elution profile of PM-bound peroxidase activities (pmPOX; •). Enzyme activities were measured in the presence of 8.26 mm guaiacol and 8.8 mm H2O2.
Relative Molecular Mass
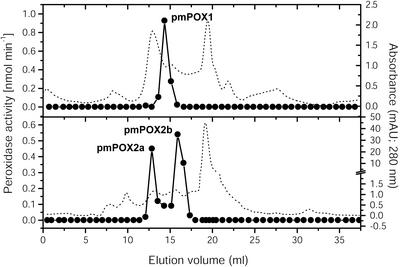
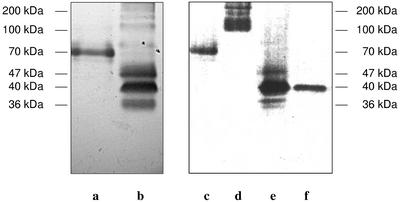
As shown in Figure 2, pmPOX1 displayed a single peak after size exclusion chromatography. By modified SDS-PAGE and heme staining, a protein band with an apparent molecular mass of 70 kD could be identified (Fig. 3). However, pmPOX2 was clearly separated into two peaks after size exclusion chromatography (pmPOX2a and pmPOX2b; Fig. 2). In comparison with peak fractions eluted during the cation exchange chromatography, analysis of pmPOX2b showed a significant increase in intensity of a 40-kD band after heme staining (Fig. 3). pmPOX2a exhibited a protein band between 100 and 170 kD. Due to the modifications of the SDS-PAGE, these are molecular masses of whole enzymes, i.e. oligomers were not separated into subunits.
Figure 2.
Elution profiles of PM-bound POX after size exclusion chromatography. Peak fractions collected from several Uno S runs were combined, concentrated, and applied onto a Superdex 200 column. Proteins were separated by a flow rate of 0.5 mL min–1. The fraction size was automatically adjusted between 0.75 and 0.5 mL depending on increase or decrease in A280 (dotted line) Enzyme activities were measured as described in Figure 1. PM-bound peroxidase activities could be separated into three peaks.
Figure 3.
Heme staining of pmPOX fractions after modified SDS-PAGE. Electrophoresis was performed using a low concentrated SDS-PAGE, i.e. 0.1% (w/v) SDS in all solutions and gels without dithiothreitol or mercaptoethanol. Thus, the oligomers were not separated into their subunits. Heme-containing protein bands were visualized by their reaction with the peroxidase substrates tetramethylbenzidine (TMB) and H2O2 as described in “Materials and Methods.” Left, pmPOX1 (a) and pmPOX2 (b) are shown after cation exchange chromatography. Further purification of these fractions by size exclusion is presented on the right: pmPOX1 (c), pmPOX2a (d), and pmPOX2b (e). In addition, f shows pmPOX2b treated with 25 mm dithiothreitol. Bars indicate the corresponding molecular masses. After size exclusion chromatography, the PM-bound enzymes had apparent molecular masses of 70 and 40 kD for pmPOX1 and pmPOX2b, whereas pmPOX2a exhibited a broad protein band between 100 and 170 kD.
Molecular masses were also calculated by elution volumes of the size exclusion purification step in comparison with marker proteins. The native enzymes revealed apparent molecular masses of 70, 155, and 38 kD for pmPOX1, pmPOX2a, and pmPOX2b, respectively, confirming results obtained by gel electrophoresis and suggesting the presence of three distinct peroxidases at the plant PM. On the other hand, the separation of pmPOX2 into two peroxidase peaks by size exclusion chromatography could be due to proteins that were not fully solubilized and remained as aggregates (i.e. protein detergent or protein aggregates). However, the data obtained by SDS-PAGE excluded this hypothesis. Known class III peroxidases revealed molecular masses in a range of 28 to 60 kD (Hiraga et al., 2001) and a 70- or a 155-kD protein have not been described for soluble peroxidases from higher plants.
In PM isolated from soybean roots, 38- and 45-kD bands were identified by SDS-PAGE and heme staining (Vianello et al., 1997), masses comparable with that found for pmPOX2b (Fig. 3). However, both bands decreased in intensity after partial washing of the PM vesicles with NaCl, and several apoplastic peroxidases with these molecular masses were identified in different plant species (Hendriks et al., 1991; Melo et al., 1996; De Marco et al., 1999; Blee et al., 2001). The molecular masses of pmPOX1 and pmPOX2a were different compared with the protein bands identified in soybean PM. However, this could be due to the different material.
Prosthetic Groups
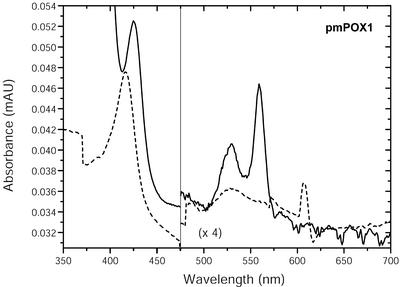
UV/visible absorption spectra of pmPOX1 and pmPOX2 were almost identical and typical for heme-containing proteins (e.g. Converso and Fernandez, 1995; Kvaratskhelia et al., 1997). Both pmPOXs exhibited a Soret peak at 416 nm (Fig. 4). In addition to these, the oxidized enzymes showed α- and β-absorption bands at 607 and 528 nm, respectively. The Soret peak and the α-band shifted to 425 and 559 nm when the proteins were reduced by sodium dithionite. The A416/A280 values, which are a criterion of purity and heme content, were 0.5 and 0.2 for pmPOX1 and pmPOX2, suggesting that the enzymes were not purified to homogeneity, which was also shown by SDS-PAGE. Thus, the α-absorption band at 607 nm cannot be definitely ascribed to the heme group of the peroxidase. The spectra of both pmPOXs more closely resemble those of guaiacol rather than ascorbate peroxidases (Chen and Asada, 1989; Converso and Fernandez, 1995; Kvaratskhelia et al., 1997). Peroxidase staining of the isolated proteins suggests a relatively strong binding of the heme groups to the enzymes. Only pmPOX2b could be detected by heme staining after treatment with dithiothreitol and revealed the same molecular mass as without reducing compounds (Fig. 3). Thus, pmPOX2b was identified as a monomer. pmPOX1 and pmPOX2a did not reveal any visible band after the same treatment (data not shown). Conformational changes due to the cleavage of disulfide bridges within the molecules possibly resulted in a release of the heme groups.
Figure 4.
Absorption spectra of partially purified pmPOX1. Samples (1.1 mg protein mL–1) containing the native enzyme (dashed line) were measured in 50 mm sodium phosphate buffer (pH 7.0) with buffer as reference. Ferric enzymes were reduced by the addition of approximately 1.5 mm dithionite (straight line). The spectra were measured at 50 nm min–1. n = 2 independent preparations showing identical results. The spectra indicate the presence of heme groups as the prosthetic group.
pH Optimum and Kinetic Studies
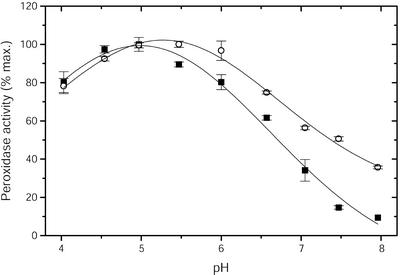
The properties of POX, which were separated by cation exchange chromatography, were further characterized. As shown in Figure 5, the highest activity with guaiacol as a substrate was observed between pH 4.5 and 5.5 for pmPOX1, whereas pmPOX2 exhibited a pH optimum in the range of 5.0 to 6.0. With guaiacol as substrate, acidic pH optima have often been reported for the apoplastic peroxidases of several plant species (Hendriks et al., 1991; Melo et al., 1996; Nair and Showalter, 1996). Variations in pH optima could represent efficient regulatory means in vivo to shift optimal conditions from one isoenzyme to another and thereby favor different processes (De Marco et al., 1999).
Figure 5.
Dependence of the guaiacol peroxidase activity of partially purified pmPOX on pH. The rates of guaiacol oxidation were determined under the standard assay conditions except that 25 mm sodium acetate (pH 4.0–5.0), MES (pH 5.5–6.5), or HEPES (pH 7.0–8.0) buffers were used. Data presented are average values ± sd of n = 3 experiments. ▪, pmPOX1; ○, pmPOX2.
The Kms of both PM-bound peroxidase activities for guaiacol were comparable (12.2 mm for pmPOX1 and 14.3 mm for pmPOX2, calculated by Eadie-Hofstee plots). Km values in a millimolar range are typical for peroxidases with artificial substrates like guaiacol. For instance, soluble peroxidases from kiwifruit (Actinidia deliciosa) and tomato (Lycopersicon esculentum) fruits had Km values of 7.4 and 10 mm, respectively (Soda et al., 1991).
Temperature Optima and Thermal Stability
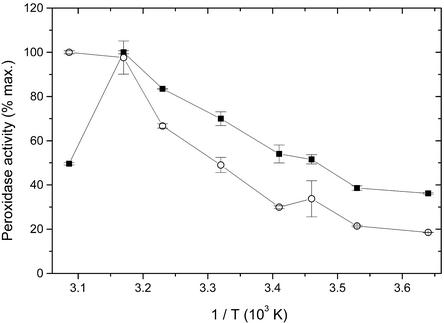
At low temperatures the enzyme activity of pmPOX2 was about 2-fold lower compared with pmPOX1 (Fig. 6). The activity of both protein fractions increased with higher temperatures. Although the activity of pmPOX2 more or less continuously increased in the range of 2°C to 51°C, pmPOX1 showed a maximum of activity at 43°C and decreased dramatically afterward.
Figure 6.
Dependence of the guaiacol peroxidase activity of purified pmPOX on temperature (Arrhenius plot). The rates of guaiacol oxidation were determined under the standard assay conditions except for temperature. Data presented are average values ± sd of n = 3 experiments. ▪, pmPOX1; ○, pmPOX2.
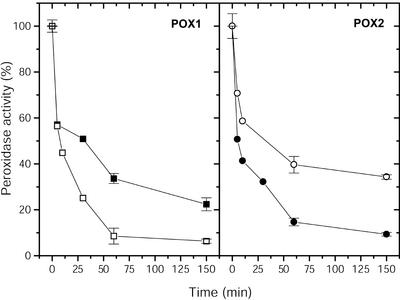
In a second set of experiments, the thermal stability of soluble and PM-bound peroxidases was investigated (Fig. 7). All enzymes lost between 40% and 50% of their activities within 5 min at 50°C. During an incubation time of 3 h, the guaiacol peroxidase activities decreased exponentially to values between 5.7% and 34.3%. After 3 h, pmPOX1 showed twice the activity of pmPOX2. Most peroxidases from plants and animals seemed to have high temperature optima and show high thermal stabilities (Bakardjieva et al., 1996; Madhavan and Naidu, 2000). Apoplastic, cytosolic, and soluble peroxidases of several plant tissues showed temperature optima between 30°C and 60°C, the most between 50°C and 60°C (Soda et al., 1991; Bakardjieva et al., 1996; Nair and Showalter, 1996; Bernards et al., 1999; Loukili et al., 1999). Due to the fact that pmPOX1 had a lower temperature optimum than pmPOX2, the latter enzyme seemed to be more stable (Fig. 6). However, for longer treatments of higher temperatures, pmPOX1 revealed a higher thermal stability (Fig. 7).
Figure 7.
Thermal stability of guaiacol peroxidase activities. Soluble and PM-bound POX were incubated at 50°C at different time slices. Data presented are average values ± sd of n = 2 experiments. ▪, pmPOX1; •, pmPOX2; □, wPOX1; ○, wPOX2.
Effector Studies
As shown in Table I, classical peroxidase inhibitors like potassium cyanide or sodium azide caused a complete loss of the peroxidase activities or decreased the rates more than 90%. These results were consistent with the presence of heme groups as prosthetic groups.
Table I.
Guaiacol-dependent activity in the absence or presence of typical peroxidase effectors
Peroxidase activity was measured with the partially purified enzymes after cation exchange chromatography in the presence of 8.26 mm guaiacol and 8.8 mm H2O2 at pH 7.0 as described in “Materials and Methods.” Data are given as mean ± sd (n).
| Substance
|
Concentration
|
Peroxidase Activity
|
|||
|---|---|---|---|---|---|
| pmPOX1 | pmPOX2 | wPOX1 | wPOX2 | ||
| μmol min-1 mg protein-1 | |||||
| Control | 5.2 ± 0.1 (3) | 1.6 ± 0.1 (3) | 12.0 ± 0.3 (3) | 16.8 ± 0.1 (3) | |
| (% of control) | |||||
| Control | 100.0 ± 1.5 (3) | 100.0 ± 4.9 (3) | 100.0 ± 2.3 (3) | 100.0 ± 0.3 (3) | |
| KCN | 1 mM | n.d.a (3) | n.d. (3) | 1.2 ± 1.6 (3) | 0.6 ± 0.8 (3) |
| Azide | 1 mM | 10.6 ± 0.4 (3)b | 2.9 ± 0.6 (3)b | 0.2 ± 0.3 (2)b | 7.4 ± 0.9 (2)b |
| p-Chloromercuribenzoate (pCMB) | 50 μM | 112.5 ± 7.1 (3) | 111.1 ± 5.7 (3) | 99.7 ± 9.6 (3) | 96.4 ± 4.6 (3) |
| 200 μM | 105.7 ± 2.3 (3) | 102.2 ± 5.0 (3) | 101.4 ± 2.0 (3) | 102.1 ± 8.3 (3) | |
| 1 mM | 110.2 ± 4.3 (3) | 106.9 ± 0.6 (3) | 54.5 ± 10.9 (3) | 77.2 ± 9.1 (3) | |
n.d., Not detectable. b pH 5.0.
The localization of the enzymes at the plant PM suggests that they may be part of the secretory pathway. According to Welinder et al. (1996), pCMB, a sulfhydryl inhibitor, is often used to distinguish between class I and class III peroxidases. As shown in Table I, this inhibitor did not effect the PM-bound activities of pmPOX1 or pmPOX2, indicating that SH groups did not participate in the active center or maintenance of the conformation of the isoenzymes. Thus, the PM-bound peroxidases were probably class III peroxidases. In contrast to the pmPOX, wPOX1 and wPOX2 were slightly inhibited in the presence of 1 mm pCMB.
Both PM-bound peroxidase activities were decreased by distinct concentrations of the lectins concanavalin A (Con A) and wheat germ agglutinin (WGA; Table II), whereas the Ulex europaeus agglutinin (UEA1) was without significant effect (data not shown). Inhibition of wPOX1 and wPOX2 was weak and occurred only at higher concentrations of Con A and WGA (Table II). The effects of lectins indicate glycosylation of the enzymes. These results are consistent with the finding of Vianello et al. (1997) that treatment of soybean roots with tunicamycin, an inhibitor of glycoprotein synthesis, reduced the guaiacol peroxidase activity of unwashed PM vesicles by 40%. Due to the possible glycosylation, which was also indicated by diffuse protein bands in SDS gels (Fig. 3), the real molecular masses of all identified proteins may be different from the calculated values. However, the structures of the proteins have to be further elucidated.
Table II.
The effects of lectins on guaiacol-dependent peroxidase activity of POX
Guaiacol-dependent peroxidase activity was measured at pH 5.0 after cation exchange chromatography. Fractions were measured in absence or presence of different lectins. Pre-incubation was for 3 min. Data are given as mean ± sd (n). For control rates, see Table I.
| Substance | Concentration | pmPOX1 | pmPOX2 | wPOX1 | wPOX2 |
|---|---|---|---|---|---|
| μg mL-1 | % of control | ||||
| Control | 100.0 ± 1.5 (3) | 100.0 ± 4.9 (3) | 100.0 ± 2.3 (3) | 100.0 ± 0.3 (3) | |
| Con A | 1 | 79.8 ± 3.3 (4) | 79.8 ± 2.0 (4) | 97.9 ± 5.2 (3) | 98.4 ± 3.6 (3) |
| 5 | n.m.a | 78.1 ± 2.8 (3) | 111.5 ± 1.9 (3) | 90.5 ± 4.1 (3) | |
| WGA | 1 | 78.2 ± 2.4 (3) | 85.6 ± 1.9 (4) | 106.1 ± 9.0 (3) | 90.4 ± 1.9 (3) |
| 5 | 81.9 ± 4.9 (3) | n.m. | 88.8 ± 2.6 (3) | 83.6 ± 3.2 (3) | |
n.m., Not measured.
Ca2+ reduced the activity of pmPOX2 and wPOX2. Mn2+ had no effect on pmPOX1 or pmPOX2 (Table III). In contrast to the PM-bound enzymes, many peroxidases exhibit increased activities after treatment with Ca2+ or Mn2+ (Gaspar et al., 1982; Van Huystee et al., 1996; Greppin et al., 1999). Calcium probably maintains the conformation of the proteins, whereas Mn2+ could be involved in regulatory processes (Van Huystee et al., 1996). However, Loukili et al. (1999) characterized plant peroxidases that were not influenced by these ions. Furthermore, Mn2+ was not detectable in PM from corn roots (Lüthje et al., 1995). On the other hand, unwashed PM from soybean roots showed a 42% increase of activity in the presence of CaCl2 (Vianello et al., 1997). Possibly, this increase was caused by soluble peroxidases that were loosely attached to the PM or due to the different plant material.
Table III.
Guaiacol-dependent activity in presence of salts, solvents, or detergents
Guaiacol-dependent peroxidase activity was measured at pH 5.0 after cation exchange chromatography. Data are given as mean ± sd (n).
| Substance | Concentration | pmPOX1 | pmPOX2 | wPOX1 | wPOX2 |
|---|---|---|---|---|---|
| % of control | |||||
| Control | 100.0 ± 1.5 (3) | 100.0 ± 4.9 (3) | 100.0 ± 2.3 (3) | 100.0 ± 0.3 (3) | |
| CaCl2 | 500 μM | 108.2 ± 7.4 (4) | 85.1 ± 1.7 (3) | 95.0 ± 0.4 (2) | 88.5 ± 4.8 (2) |
| MnCl2 | 100 μM | 105.3 ± 1.2 (3) | 107.4 ± 3.4 (3) | 94.4 ± 5.4 (3) | 89.1 ± 1.3 (3) |
| 500 μM | 102.3 ± 3.6 (3) | 101.6 ± 2.4 (3) | 99.0 ± 3.5 (3) | 86.7 ± 0.5 (3) | |
| Dimethyl sulfoxide (DMSO) | 2% (v/v) | 104.9 ± 8.6 (3) | 77.0 ± 2.3 (3) | 75.6 ± 4.9 (2) | 78.0 ± 1.2 (2) |
| Triton X-100 | 0.02% (w/v) | 98.2 ± 5.1 (3) | 81.3 ± 6.7 (2) | n.m. | n.m. |
| Triton X-114 | 0.02% (w/v) | 119.5 ± 3.5 (2) | 119.5 ± 5.0 (2) | n.m. | n.m. |
DMSO had no effect at 0.5% (v/v), the final concentration of DMSO used in experiments with phenolic compounds as effectors (Table III). PM showed 90.4% ± 0.3% (n = 3) peroxidase activity in the presence of 2% (w/v) DMSO. pmPOX1 was not effected by this concentration, whereas pmPOX2 and the washed off peroxidase activities were inhibited. Detergents like Triton X-100 and Triton X-114 induced a decrease or increase of the enzyme activities.
The activities of cell wall-bound and apoplastic peroxidases have often been reported to be stimulated by phenolic substances, like ferulic acid and coumaric acid (Mäder and Füssl, 1982; Lobarzewski et al., 1996; De Marco et al., 1999). As shown in Table IV, activities of the partially purified peroxidases increased to 220% and 400% of the control in the presence of ferulic acid as a substrate, whereas coumaric acid had no effect on pmPOX2 and wPOX2, and pmPOX1 and wPOX1 were slightly decreased. The phenolic compound propyl gallate inhibited the guaiacol-dependent peroxidase activity of all fractions. In the presence of IAA, the activity of pmPOX2 decreased slightly, whereas all other activities were not effected. The inhibitory effects of propyl gallate and IAA suggest a competition between the substrates, which was further indicated by their substrate specificity.
Table IV.
Guaiacol-dependent peroxidase activity in presence of different substrates
Guaiacol-dependent peroxidase activity of the partially purified enzymes was measured at pH 5.0. Substrates were added to the assay at concentrations as indicated. Data are given as mean ± sd (n).
| Substance | Concentration | pmPOX1 | pmPOX2 | wPOX1 | wPOX2 |
|---|---|---|---|---|---|
| μM | % of control | ||||
| Control | 100.0 ± 1.5 (3) | 100.0 ± 4.9 (3) | 100.0 ± 2.3 (3) | 100.0 ± 0.3 (3) | |
| Coumaric acid | 100 | 86.7 ± 2.4 (2) | 111.0 ± 0.1 (2) | 86.9 ± 2.8 (2) | 101.9 ± 2.1 (2) |
| Ferulic acid | 100 | 221.5 ± 8.2 (3) | 402.0 ± 7.1 (2) | 372.7 ± 3.9 (2) | 264.1 ± 5.8 (2) |
| Propyl gallate | 500 | 2.4 ± 0.8 (3) | 5.0 ± 0.1 (3) | 7.2 ± 0.2 (2) | 5.7 ± 0.1 (2) |
| Indole-3-acetic acid (IAA) | 10 | 98.5 ± 0.5 (3) | 83.2 ± 0.2 (3) | 102.7 ± 4.8 (3) | 99.1 ± 6.0 (3) |
Substrate Specificity
Artificial electron donors were used by pmPOX1 in the following order: o-dianisidine > guaiacol > TMB » 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS; Table V). In contrast to pmPOX1, pmPOX2 showed a higher affinity for TMB than for guaiacol. Both pmPOXs oxidized natural substrates like phenolic acids and alcohols in the following order: coniferyl alcohol > ferulic acid > coumaric acid. Hydroxycinnamyl alcohol species are used by apoplastic peroxidases to participate in lignin polymerization, whereas hydroxycinnamic acids could be incorporated into suberin (for refs., see Hiraga et al., 2001).
Table V.
Substrate specifity of soluble and pmPOX
Enzyme activities were measured in presence of 8.8 mm H2O2 and given concentrations of common peroxidase substrates at pH 5.0. Data are given as mean ± sd (n). Guaiacol oxidation rates are shown in Table I.
| Substrate | Concentration | Relative Activity
|
|||
|---|---|---|---|---|---|
| pmPOX1 | pmPOX2 | wPOX1 | wPOX2 | ||
| mM | % | ||||
| Guaiacol | 8.26 | 100.0 ± 1.5 (3) | 100.0 ± 4.9 (3) | 100.0 ± 2.3 (3) | 100.0 ± 0.3 (3) |
| ABTS | 0.36 | 1.8 ± 0.1 (3) | 10.9 ± 0.3 (3) | 1.0 ± 0.4 (3) | 7.6 ± 0.4 (5) |
| o-Dianisidine | 0.127 | 133.1 ± 6.1 (4) | 206.9 ± 10.7 (3) | 124.9 ± 9.9 (3) | 231.0 ± 4.6 (3) |
| TMB | 0.083 | 86.0 ± 4.4 (3) | 179.6 ± 8.5 (3) | 85.6 ± 3.5 (3) | 194.6 ± 9.4 (4) |
| Ascorbate | 0.5 | n.d. (4)a | n.d. (4)a | n.d. (3)a | 20.6 ± 0.9 (3)a |
| Coniferyl alcohol | 0.1 | 225.9 ± 4.6 (3) | 288.3 ± 9.2 (3) | 235.3 ± 16.4 (3) | 177.9 ± 2.3 (3) |
| Coumaric acid | 0.1 | 9.5 ± 0.2 (3) | 4.3 ± 0.1 (3) | n.d. (3) | 38.8 ± 1.6 (3) |
| Ferulic acid | 0.1 | 71.1 ± 2.4 (3) | 30.1 ± 1.5 (3) | 121.0 ± 16.4 (3) | 59.2 ± 3.3 (3) |
| IAA | 0.2 | 60.3 ± 3.4 (4) | 56.1 ± 4.3 (4) | 14.2 ± 0.8 (3) | n.d. (3) |
pH 7.0
In vitro IAA oxidation by peroxidases has been reported several times (Converso and Fernandez, 1995; Gazaryan and Lagrimini, 1996). This plant hormone was used by pmPOX1, pmPOX2, and wPOX1, whereas the auxin was not oxidized by wPOX2 (Table V).
The highest peroxidase activities were reached with coniferyl alcohol as substrate for both pmPOX. Because the accumulation of the enzymes was different, the specific activities of the soluble POX were apparently higher than the specific activities of the pmPOX.
The washed off peroxidase activities could not only be distinguished from the PM-bound POX by their different substrate specificities for phenolic compounds and IAA but also by their ability to oxidize ascorbate (Table V). Only wPOX2 revealed an ascorbate peroxidase activity, suggesting that intracellular or extracellular soluble peroxidases were attached to the PM during the isolation procedure and removed by washing of the membranes. Also, both pmPOXs did not show any ascorbate peroxidase activity in presence of twice the amounts of enzyme into the assay (data not shown). However, the ability to oxidize ascorbate may have been lost during the purification process, as has been described for several ascorbate peroxidases extracted in the absence of ascorbate (Chen and Asada, 1989; Amako et al., 1994). Other cytosolic ascorbate peroxidases are resistant to depletion of ascorbate (Mittler and Zilinskas, 1991; Koshiba, 1993). Vianello et al. (1997) reported ascorbate peroxidase activities at non-washed plant PM isolated in the absence of ascorbate from soybean roots, confirming the presented results.
In general, pmPOX1 and pmPOX2 showed more properties corresponding to apoplastic than to cytosolic peroxidases. On the other hand, a localization on the outside or inside of the plant PM cannot be concluded by these properties.
CONCLUDING REMARKS
The results of the present work demonstrate the presence of at least two distinct PM-bound peroxidase activities in corn roots. Although peroxidases are usually difficult to distinguish due to their similar characteristics (De Marco et al., 1999), both pmPOXs showed definitely distinct properties in dependence on substrate concentration, pH optima, temperature, and effectors. The biochemical characteristics of both activities are typical for class III peroxidases. Thus, it is the first time, to our knowledge, that enzymes of this class have been found with such high molecular masses in plants.
Until now, the physiological function of PM-bound POX is not clear, and several distinct functions have been postulated (Mφller and Bérczi, 1986; Askerlund et al., 1987; Pantoja and Willmer, 1988; Ludwig-Müller et al., 1990; Ludwig-Müller and Hilgenberg, 1992; De Marco et al., 1995; Zancani et al., 1995; Vianello et al., 1997). Due to the differences observed for pmPOX1 and pmPOX2, it is possible that these enzymes have distinct functions. Most of the possible functions like detoxifying or production of reactive oxygen species as signal mediators or antimicrobial agents at the interface cell wall/PM could be part of defense mechanisms against pathogen infection (Hiraga et al., 2001). A flavonoid-peroxidase reaction as a mechanism for H2O2 scavenging was demonstrated by Yamasaki et al. (1997). Thus, protection and membrane repair mechanisms of the PM may also be possible.
However, the location (cytoplasmic or apoplastic side of the PM), the binding properties to the PM, and the physiological function of PM-bound POX activities have to be further elucidated.
MATERIALS AND METHODS
PMs
PM have been prepared from 5-d-old corn (Zea mays L. cv Jet, Saatenunion, Hannover, Germany) roots by phase partitioning as described earlier (Lüthje et al., 1998). The final pellet was stored at –80°C until use.
Solubilization of Membrane Proteins
Isolated PM were washed according to Bérczi and Mφller (1998) with minor modifications. PM were incubated in 25 mm sodium acetate-HCl (pH 4.0), 500 mm KCl, 1 mm EDTA, and 0.01% (w/v) Triton X-100 for 30 min at 4°C under continuous stirring to remove peripheral and adsorbed soluble proteins. Washed membranes were pelleted at 105,000g for 45 min at 4°C, resuspended in acetate buffer (25 mm sodium acetate-HCl [pH 4.0] and 1 mm EDTA), and solubilized with CHAPS at a detergent:protein ratio of 30:1 (w/v) in the presence of 0.5 mm aminocaproic acid. After incubation for 1 h at 4°C, solubilized proteins were separated by ultracentrifugation (1 h at 105,000g and 4°C).
Protein Purification
Proteins were purified by a combination of cation exchange chromatography and size exclusion using an HPLC-System (AKTA, Amersham Pharmacia Biotech, Freiburg, Germany) with a 10-mL super-loop. All of the following purification steps were performed at 4°C. Solubilized enzymes were applied on an Uno S1 column (HR 5/5, Bio-Rad, Munich) equilibrated with 25 mm sodium acetate-HCl (pH 4.0), 1 mm EDTA, 1% (w/v) glycerol, and 1 mm CHAPS. After loading, the matrix was washed with 10 column volumes of sodium acetate buffer, and bound proteins were eluted by a continuous KCl gradient (0–1 m KCl in sodium acetate buffer, flow rate, 1 mL min–1; total volume, 13 column volumes), followed by 2 column volumes of 1 m KCl. Fractions of 1 and 0.5 mL were collected for the flow through and gradient, respectively. Peak fractions of several Uno S runs were combined and concentrated using Centricon YM-10 concentrators (Millipore, Bedford, MA). Concentrated fractions (500 μL) or calibration proteins (thyroglobulin [669 kD], ferritin [440 kD], catalase [232 kD], aldolase [158 kD], bovine serum albumin [68 kD], horseradish peroxidase [44 kD], and ribonuclease A [13.7 kD], Amersham Pharmacia Biotech) were applied on a Superdex 200 column (HR 10/30, Amersham Pharmacia Biotech) equilibrated with 4 column volumes of phosphate buffer (50 mm Na3PO4 [pH 7.0], 150 mm NaCl, 1 mm CHAPS, and 1 mm EDTA). Proteins were eluted by 1.5 column volumes of buffer. The flow rate was 0.5 mL min–1. The fraction size was automatically adjusted between 0.75 and 0.5 mL depending on the absorption (λ = 280 nm). Estimates of the molecular masses of native pmPOX were calculated using a semilogarithmic plot of the molecular mass values for the calibration proteins against the elution volumes.
SDS-PAGE
Successive steps of purification were monitored by SDS-PAGE, which were performed with 11% (w/v) polyacrylamide slab gels according to Laemmli (1970). Protein bands were visualized by the method of Merril et al. (1984) using a silver staining kit (Bio-Rad).
PAGE for heme staining was performed at room temperature by modified SDS-PAGE. The final concentration of SDS was 0.1% (w/v) in all solutions and gels (Trost et al., 2000). Concentrated samples (0.9–5.0 μg of protein) were diluted in loading buffer to final concentrations of 62.5 mm Tris-HCl, 0.1% (w/v) SDS, 10% (w/v) glycerol, and 0.002% (w/v) bromo-phenol blue without reducing compounds, and loaded onto the gels within 30 min without heating. Horseradish peroxidase as a positive control and each sample were loaded twice once on each one-half of the gel. The gels were cut in one-half after the run. One-half of the gel was used for silver staining, whereas the other one-half was stained in the presence of 6.3 mm TMB and 30 mm H2O2 (Thomas et al., 1976). Because the running characteristics of monomers inside the gels were not effected by the lower SDS concentration, molecular mass standards (Broad Range, Bio-Rad) were used according to Laemmli (1970).
Enzyme Assays
Peroxidase activities were measured as oxidation of guaiacol (8.26 mm, ε = 26.6 mm–1 cm–1) in the presence of 8.8 mm H2O2 within 2 min. The assay (1 mL) contained 25 mm sodium acetate-HCl (pH 5.0) and 25 μL of fraction. PM vesicles (50 μg of protein) were measured in 25 mm sodium acetate (pH 5.0), 1 mm EDTA, and 0.01% (w/v) Triton X-100. The reaction was started by addition of guaiacol and followed spectrophotometrically (DU 7500, Beckmann, Munich) as the increase of absorption at 470 nm. Rates were corrected by chemical control experiments. The oxidation rates of other substrates were measured as increases or decreases in absorption using the same reaction mixture and assay conditions but with guaiacol replaced by ABTS (A405; ε = 36.8 mm–1 cm–1), coniferyl alcohol (A265; ε = 7.5 mm–1 cm–1), coumaric acid (A310; ε = 16.6 mm–1 cm–1), o-dianisidine (A460; ε = 30.0 mm–1 cm–1), ferulic acid (A310; ε = 16.6 mm–1 cm–1), IAA (A261; ε = 3.2 mm–1 cm–1), or tetramethyl-benzidine (A652; ε = 39.0 mm–1 cm–1). The peroxidase activity with ascorbate as the reducing substrate was determined in a reaction mixture containing 50 mm potassium phosphate (pH 7.0), 0.5 mm ascorbate, and 8.8 mm H2O2. Oxidation of ascorbate was followed by the decrease in A290 (ε = 2.8 mm–1 cm–1) within 1 min. The pH dependence of peroxidase activities was ascertained in 25 mm sodium acetate (pH 4.0–5.0), MES (pH 5.5–6.5), and HEPES (pH 7.0–8.0), respectively. Phenolic compounds were dissolved in 50% (v/v) DMSO, resulting in a final concentration of 0.5% (v/v) DMSO per assay. Absorption spectra were recorded in quartz cuvettes (1 cm) on a UV/Vis spectrophotometer (Uvikon 943, Kontron Instruments, Milano, Italy) with a scan speed of 50 nm min–1. Data presented were calculated with Microcal Origin (version 5.0, Additive GmbH, Friedrichsdorf/TS, Germany).
Acknowledgments
The authors appreciate support by Michael Böttger (University of Hamburg, Germany), helpful discussions with Alajos Bérczi (Academy of Sciences, Szeged, Hungary), and critical reading of the manuscript by Richard Becket (University of Natal, Scottsville, South Africa).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.020396.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. DFG Lu 668/1–2) and by the University of Hamburg (PhD student's grant no. HmbNFG to A.M.).
References
- Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35: 497–504 [Google Scholar]
- Askerlund P, Larsson C, Widell S, Mφller IM (1987) NAD(P) H oxidase and peroxidase activities in purified plasma membranes from cauliflower inflorescences. Physiol Plant 71: 9–19 [Google Scholar]
- Bakardjieva NT, Cristova NV, Cristov K (1996) Reaction of peroxidase from different plant species to increased temperatures and the effect of calcium and zinc ions. In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Proceedings of the IV International Symposium on Plant Peroxidases: Biochemistry and Physiology. University of Vienna, Austria, and University of Geneva, Switzerland, pp 345–351
- Bérczi A, Mφller IM (1998) Characterization and solubilization of residual redox activity in salt-washed and detergent-treated plasma membrane vesicles from spinach leaves. Protoplasma 205: 59–65 [Google Scholar]
- Bérczi A, Mφller IM (2000) Redox enzymes in the plant plasma membrane and their possible roles. Plant Cell Environ 23: 1287–1302 [Google Scholar]
- Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang X, Sabatino A, Plourde GL (1999) Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol 121: 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blee KA, Jupe SC, Richard G, Zimmerlin A, Davies DR, Bolwell GP (2001) Molecular identification and expression of the peroxidase responsible for the oxidative burst in French bean (Phaseolus vulgaris L.) and related members of the gene family. Plant Mol Biol 47: 607–620 [DOI] [PubMed] [Google Scholar]
- Bunkelmann J, Trelease RN (1996) Ascorbate peroxidase: a prominent membrane protein in oilseed glyoxysomes. Plant Physiol 110: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30: 987–998 [Google Scholar]
- Converso DA, Fernandez ME (1995) Peroxidase isozymes from wheat germ: purification and properties. Phytochemistry 40: 1341–1345 [Google Scholar]
- Crevecoeur M, Pinedo M, Greppin H, Penel C (1997) Peroxidase activity in shoot apical meristem from spinach. Acta Histochem 99: 177–186 [DOI] [PubMed] [Google Scholar]
- De Marco A, Guzzardi P, Jamet É (1999) Isolation of tobacco isoperoxidases accumulated in cell-suspension culture medium and characterization of activities related to cell wall metabolism. Plant Physiol 120: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco A, Pinton R, Fischer-Schliebs E, Varanini Z (1995) Possible interaction between peroxidase and NAD(P) H-dependent nitrate reductase activities of plasma membranes of corn roots. J Exp Bot 46: 1677–1683 [Google Scholar]
- Gaspar T, Penel C, Thorpe T, Greppin H, editors (1982) Peroxidases 1970–1980: A Survey of their Biochemical and Physiological Roles in Higher Plants. University of Geneva Press, Switzerland
- Gazaryan IG, Lagrimini LM (1996) Tobacco anionic peroxidase overexpressed in transgenic plants: aerobic oxidation of indole-3-acetic acid. Phytochemistry 42: 1271–1278 [Google Scholar]
- Greppin H, Wiater RG, Ginalska G, Lobarzewski J (1999) The cabbage peroxidase isoforms changes influenced by Ca2+ and Mg2+ ions. Plant Peroxidase Newslett 13: 129–135 [Google Scholar]
- Hendriks T, Wijsman HJ, Van Loon LC (1991) Petunia peroxidase a: isolation, purification and characteristics. Eur J Biochem 199: 139–146 [DOI] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42: 462–468 [DOI] [PubMed] [Google Scholar]
- Hu C, Smith R, Van Huystee R (1989) Biosynthesis and localization of peanut peroxidases: a comparison of the cationic and the anionic isozymes. Plant Physiol 135: 391–397 [Google Scholar]
- Jespersen HM, Kjærsgård IVH, φstergaard L, Welinder KG (1997) From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochem J 326: 305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T (1993) Cytosolic ascorbate peroxidase in seedlings and leaves of maize (Zea mays). Plant Cell Physiol 34: 713–721 [Google Scholar]
- Kvaratskhelia M, Winkel C, Thorneley RNF (1997) Purification and characterization of a novel class III peroxidase isoenzyme from tea leaves. Plant Physiol 114: 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lin W (1982) Responses of corn root protoplasts to exogenous reduced nicotinamide adenine dinucleotide: oxygen consumption, ion uptake and membrane potential. Proc Natl Acad Sci USA 79: 3773–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarzewski J, Brzyska M, Greppin H (1996) The fungal peroxidase kinetics with some phenolics hydrogen donors in relation to lignin degradation. In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Proceedings of the IV International Symposium on Plant Peroxidases: Biochemistry and Physiology. University of Vienna, Austria, and University of Geneva, Switzerland, pp 153–156
- Loukili A, Limam F, Ayadi A, Boyer N, Ouelhazi L (1999) Purification and characterization of a neutral peroxidase induced by rubbing tomato internodes. Physiol Plant 105: 24–31 [Google Scholar]
- Ludwig-Müller J, Hilgenberg W (1992) Tryptophan oxidizing enzyme and basic peroxidase isoenzymes in Arabidopsis thaliana (L.) Heynh.: are they identical? Plant Cell Physiol 33: 1115–1125 [Google Scholar]
- Ludwig-Müller J, Rausch T, Lang S, Hilgenberg W (1990) Plasma membrane bound high pI peroxidase isoenzymes convert tryptophan to indole-3-acetaldoxime. Phytochemistry 29: 1397–1400 [Google Scholar]
- Lüthje S, Niecke M, Böttger M (1995) Iron and copper in plasma membranes of maize (Zea mays L.) roots investigated by proton induced X-ray emission. Protoplasma 184: 145–150 [Google Scholar]
- Lüthje S, Van Gestelen P, Córdoba-Pedregosa MC, Gonzáles-Reyes JA, Asard H, Villalba JM, Böttger M (1998) Quinones in plant plasma membranes: a missing link? Protoplasma 205: 43–51 [Google Scholar]
- Mäder M, Füssl R (1982) Role of peroxidase in lignification of tobacco cells: II. Regulation by phenolic compounds. Plant Physiol 70: 1132–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan ND, Naidu KA (2000) Purification and partial characterization of peroxidase from human term placenta of non-smokers: metabolism of benzo(a) pyrene-7,8-dihydrodiol. Placenta 21: 501–509 [DOI] [PubMed] [Google Scholar]
- Melo NS, Calvete JJ, Thole HH, Welinder KG, Töpfer-Petersen E, Fevereiro PS (1996) Cationic peroxidases from Vaccinium myrtillus cell suspension cultures. Preliminary sequence results. In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Proceedings of the IV International Symposium on Plant Peroxidases: Biochemistry and Physiology. University of Vienna, Austria, and University of Geneva, Switzerland, pp 217–221
- Merril CR, Goldman D, Van Keuren ML (1984) Gel protein stains: silver stain. Methods Enzymol 104: 441–447 [DOI] [PubMed] [Google Scholar]
- Mittler R, Zilinskas A (1991) Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol 97: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mφller IM, Bérczi A (1986) Salicylhydroxamic acid-stimulated NADH oxidation by purified plasmalemma vesicles from wheat roots. Physiol Plant 68: 67–74 [Google Scholar]
- Nair AR, Showalter AM (1996) Purification and characterization of a wound-inducible cell wall cationic peroxidase from carrot roots. Biochem Biophys Res Commun 226: 254–260 [DOI] [PubMed] [Google Scholar]
- Nito K, Yamaguchi K, Kondo M, Hayashi M, Nishimura M (2001) Pumpkin peroxisomal ascorbate peroxidase is localized on peroxisomal membranes and unknown membranous structures. Plant Cell Physiol 42: 20–27 [DOI] [PubMed] [Google Scholar]
- Pantoja O, Willmer CM (1988) Redox activity and peroxidase activity associated with the plasma membrane of guard-cell protoplasts. Planta 174: 44–50 [DOI] [PubMed] [Google Scholar]
- Penel C, Castillo FJ (1991) Peroxidases of plant plasma membranes, apoplastic ascorbate, and relation of redox activities to plant pathology. In FL Crane, DJ Morré, H Loew, eds, Oxidoreduction at the Plasma Membrane, Vol II. CRC Press, Boca Raton, FL, pp 121–147 [Google Scholar]
- Penel C, Gaspar T, Greppin H (1992) Plant Peroxidases: 1980–1990: Topics and Detailed Literature on Molecular, Biochemical, and Physiological Aspects. University of Geneva, Switzerland
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53: 1305–1319 [PubMed] [Google Scholar]
- Soda I, Hasegawa T, Suzuki T, Ogura N (1991) Purification and some properties of peroxidase from kiwifruit. Agric Biol Chem 55: 1677–1678 [Google Scholar]
- Thomas PE, Ryan D, Levin W (1976) An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem 75: 168–176 [DOI] [PubMed] [Google Scholar]
- Trost P, Berczi A, Sparla F, Sponza G, Marzadori B, Asard H, Pupillo P (2000) Purification of cytochrome b-561 from bean hypocotyls plasma membrane: evidence for the presence of two heme centers. Biochim Biophys Acta 1468: 1–5 [DOI] [PubMed] [Google Scholar]
- Van Huystee RB, Rodriguez Marañón MJ, Wan L (1996) Peanut peroxidase, a trimetal glycoprotein. In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Proceedings of the IV International Symposium on Plant Peroxidases: Biochemistry and Physiology. University of Vienna, Austria, and University of Geneva, Switzerland, pp 42–44
- Vianello A, Zancani M, Macri F (1990) Hydrogen peroxide formation and iron ion oxidoreduction linked to NADH oxidation in radish plasmalemma vesicles. Biochim Biophys Acta 1023: 19–24 [DOI] [PubMed] [Google Scholar]
- Vianello A, Zancani M, Nagy G, Macri F (1997) Guaiacol peroxidase associated to soybean root plasma membranes oxidizes ascorbate. J Plant Physiol 150: 573–577 [Google Scholar]
- Welinder KG, Jespersen HM, Kjærsgård IVH, Ostergaard L, Abelskov AK, Hansen LN, Rasmussen SK (1996) What can we learn from Arabidopsis peroxidases? In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Proceedings of the IV International Symposium on Plant Peroxidases: Biochemistry and Physiology. University of Vienna, Austria, and University of Geneva, Switzerland, pp 173–178
- Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zancani M, Nagy G, Vianello A, Macri F (1995) Copper-inhibited NADH-dependent peroxidase activity of purified soya bean plasma membranes. Phytochemistry 40: 367–371 [Google Scholar]