Abstract
Phototropism and hypocotyl growth inhibition are modulated by the coaction of different blue-light photoreceptors and their signaling pathways. How seedlings integrate the activities of the different blue-light photoreceptors to coordinate these hypocotyl growth responses is still unclear. We have used time-lapse imaging and a nontraditional mathematical approach to conduct a detailed examination of phototropism in wild-type Arabidopsis and various blue-light photoreceptor mutants. Our results indicate that high fluence rates of blue light (100 μmol m–2 s–1) attenuate phototropism through the coaction of the phototropin and cryptochrome blue-light photoreceptors. In contrast, we also demonstrate that phototropins and cryptochromes function together to enhance phototropism under low fluence rates (<1.0 μmol m–2 s–1) of blue light. Based on our results, we hypothesize that phototropins and cryptochromes regulate phototropism by coordinating the balance between stimulation and inhibition of growth of the hypocotyl depending on the fluence rate of blue light.
When growing in the dark, etiolated seedlings are heterotrophic and rely upon a limited supply of stored reserves for energy and carbon. Upon exposure to light, photomorphogenesis switches the seedling into a photoautotrophic developmental form. To improve light interception, seedlings will rapidly elongate and bend toward directional light sources through phototropism. Blue light inhibits hypocotyl elongation during photomorphogenesis, whereas blue-light-induced phototropism can be dependent upon growth promotion and inhibition to develop a differential growth gradient. For example, during second positive phototropism in Arabidopsis, Orbovic and Poff (1993) showed that the net growth rate is transiently inhibited on the “illuminated” flank of the hypocotyl and transiently stimulated on the “shaded” flank of the hypocotyl. Moreover, there must be a close coordination between growth promotion and inhibition on the flanks of the hypocotyl for normal phototropism to prevent the hypocotyl from curling around upon itself (Silk, 1984). To understand how these growth responses are coordinated by light during phototropism, it will be important determine how the multitude of photosensory signaling networks interact to control the dynamics of growth.
Arabidopsis has three known families of signaling photoreceptors: two blue-light/UV-A photoreceptor families, phototropins (phot1 and phot2; Christie et al., 1998; Kagawa et al., 2001) and cryptochromes (cry1 and cry2; Ahmad and Cashmore, 1993; Guo et al., 1998), and the red/far-red photoreceptor family of phytochromes (phyA through phyE; Clack et al., 1994). Coaction within and between these groups of photoreceptors affects photomorphogenesis, flowering time, circadian rhythms, phototropism, growth inhibition, and other responses, but the specifics of photoreceptor coaction are not well understood (Casal, 2000).
A series of studies on the growth kinetics of hypocotyl elongation in Arabidopsis (Cho and Spalding, 1996; Parks et al., 1998; Folta and Spalding, 2001a, 2001b) led Parks et al. (2001) to propose that high-intensity blue light induces three phases of growth inhibition. During the first phase of growth inhibition, phot1 activity rapidly decreases the growth rate. During the second phase, a subsequent escape from the initial state of growth inhibition is prevented by membrane depolarization by anion channels, which are activated through the coaction of phot1, cry1, cry2, and phyA. The final and long-term phase of blue-light-dependent growth inhibition is dependent upon cry1 activity and nuclear regulation of photomorphogenesis (Parks et al., 2001).
Recent reports on phototropism in Arabidopsis indicate that light quantity has an important affect upon the photoreceptors and down-stream effectors involved in phototropism (Lasceve et al., 1999; Liscum and Stowe-Evans, 2000; Sakai et al., 2000; Sakai et al., 2001). Under low fluence rates of light, phot1 is the principle photoreceptor regulating phototropism, whereas under high fluence rates, phototropism is regulated redundantly by phot1 and phot2 (Sakai et al., 2001). Cryptochromes are not required for low-light induction of phototropism (Lasceve et al., 1999) or for the induction of high-light phototropism (Sakai et al., 2000), but cryptochromes may have some modifying or indirect affect on phototropism because first-positive phototropism is attenuated in the cry mutant plants (Lasceve et al., 1999).
The traditional technique of measuring the angle of curvature at the hypocotyl tip is a valuable heuristic measurement, but it is subjective, labor intensive, and may not detect minor kinetic, mechanical, or morphological differences in phototropism (Silk, 1984; Firn, 1994). Therefore, we developed a simple mathematical technique based on Silk's (1984) work on stem curvature to analyze the kinetics and mechanics of phototropism. The analysis of mathematical curvature is a more comprehensive measurement of phototropism than angle of curvature measurements used in previous studies because it provides a description of the shape of the phototropic hypocotyl by approximating the amount of differential growth (Silk, 1984). Unlike traditional angle of curvature measurements taken at the hypocotyl tip, curvature measurements can also be used to identify the position of greatest differential growth (i.e., the vertex).
Using this experimental approach, we were able to detect significant differences in second-positive phototropism between wild-type and the different blue-light photoreceptor mutants under varying fluence rates of unilateral blue light. Our results indicate that all of the known blue-light photoreceptors are involved in the phototropic response in etiolated Arabidopsis hypocotyls. Specifically, we found that high fluence rates of blue light (100 μmol m–2 s–1) attenuated phototropism via phot1, phot2, cry1, and cry2 activity. At an intermediate fluence rate (10 μmol m–2 s–1), cry1 inhibited phot2-dependent phototropism in the absence of phot1. At low fluence rates (1 and 0.01 μmol m–2 s–1), phot1-dependent phototropism was enhanced by phot2 and the cryptochromes.
RESULTS
The Kinetics and Mechanics of Second-Positive Phototropism in Wild-Type Seedlings
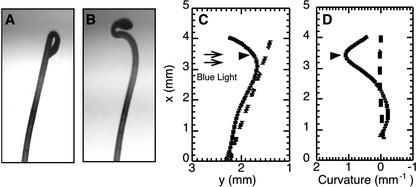
Because traditional angle of curvature measurements are inadequate for measuring the position and complexity of differential growth during phototropism (Firn, 1994), we developed a mathematical description of phototropism by using Silk's (1984) work on curvature (Fig. 1). Thirty x/y coordinates were collected from the length of a freestanding hypocotyl (Fig. 1, A and B) at each time point and the data were fit to a fourth order polynomial equation via nonlinear regression using Maple 6 (Fig. 1C, R > 0.98). With this polynomial equation, we could calculate the curvature along the hypocotyl using Equation 2 for curvature (Fig. 1D). After 240 min of unilateral blue light (0.1 μmol m–2 s–1), the initially straight hypocotyl had curved toward the light (Fig. 1, A and B) with the vertex located 3.45 mm along the x axis from the base of the hypocotyl (Fig. 1, C and D). By using this technique, we were able to find the position of the vertex and to measure the curvature along the hypocotyl. The vertex position also corresponds to the point of greatest growth differential across the hypocotyl (Silk, 1984).
Figure 1.
Mathematical descriptions of hypocotyl shape and curvature of a representative seedling at 0 and 240 min after the start of illumination with unilateral blue light (0.1 μmol m–2 s–1). A and B, Images of hypocotyl before (A) and after (B) phototropic stimulation. C, Plot of the mathematical description of phototropism using nonlinear regression (R > 0.98) to fit 30 x/y coordinates taken from the images of the hypocotyl shown in A and B. The x/y coordinates represent pixel coordinates from the time-lapse images (the axis are reversed to simplify the calculations). D, Plot of curvature (per millimeter) along the hypocotyl. Curvature is the reciprocal of the radius of curvature. The small black arrows indicate the light direction. The black arrowheads indicate the vertex position.
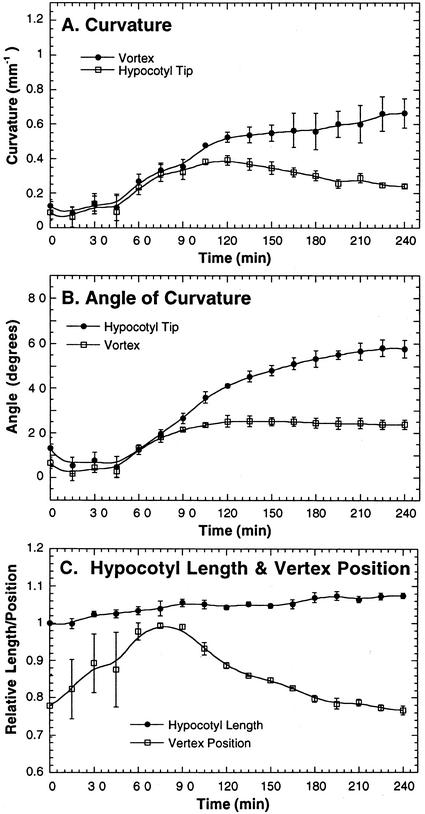
With time-lapse imaging and the analytical techniques described above, we measured the kinetics and mechanics of phototropism. The latent period before any phototropism response could be measured (Firn, 1994) was about 45 min under 0.01 μmol m–2 s–1 of blue light (Fig. 2, A and B). Once curvature began, it increased rapidly (Fig. 2, A and B). After approximately 100 min of exposure to blue light, the curvature started to decelerate. During this decelerating phase, vertex curvature continued to increase, whereas curvature at the tip decreased. Therefore, in the region between the vertex and tip, the convex side grew slower relative to the concave side of the hypocotyl to decrease the curvature at the tip. Moreover, curvature at the tip of the hypocotyl decreased without decreasing the overall angle of curvature during the deceleration phase because the vertex migrated down the length of the hypocotyl (Fig. 2C). This mathematical description of the phototropic process shows that the migration of the vertex is not caused by an increase in straight growth near the tip because the overall hypocotyl length changed less than the distance between the vertex and the tip.
Figure 2.
Wild-type phototropic response during the first 240 min of unilateral blue light. Etiolated seedlings were incubated for 70 h at 23°C in the dark before blue-light treatment (450 ± 50 nm; 0.01 μmol m–2 s–1). Images of the hypocotyls were taken at 15-min intervals with an infrared-sensitive camera. A, Curvature (per millimeter) at the apex and vertex. B, Degrees of curvature at the apex of the hypocotyl and the vertex. C, The ratio of hypocotyl length at a particular time point to the initial hypocotyl length, and the ratio of the position of the vertex to the total hypocotyl length at each time point. Error bars represent se.
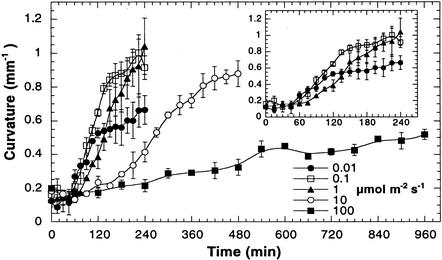
Although phot1 and phot2 have been shown to control low- and high-light-induced phototropic responses, no significant kinetic or morphological differences between the phototropic responses induced by low and high fluence rates have been reported in wild-type seedlings (Sakai et al., 2000, 2001). To determine if there are fluence rate-dependent differences in phototropism, we measured the kinetics of phototropism over a wide range of fluence rates of unilateral blue light (Fig. 3). The magnitude of the response was significantly greater under 0.1 than 0.01 μmol m–2 s–1 (Fig. 3, inset), but they both resulted in similar latent periods (approximately 45 min). However, at 1.0 μmol m–2 s–1, the latent period was about 30 min longer than at the lower fluence rates. Hypocotyls exposed to 10.0 μmol m–2 s–1 had an even longer latent period and also showed a slower rate of curvature, but this did not decrease the final magnitude of the curvature after 8 h of continuous illumination. Irradiation with 100 μmol m–2 s–1 of unilateral blue light greatly extended the latent period and decreased the rate and magnitude of curvature. We did not include points within the apical hook in our analysis of phototropic curvature of the hypocotyl to avoid complications with apical hook opening that may occur during the illumination period. From these results, we propose that the reduction in the phototropic response under higher fluence rates is caused by inhibition of elongation mediated through the activities of the blue-light photoreceptors.
Figure 3.
Fluence rate-dependent attenuation of wild-type phototropic curvature. At fluence rates above 1 μmol m–2 s–1, the lag time required for phototropic curvature increased and the magnitude of curvature was attenuated. The inset shows the time period for the low-fluence-rate phototropic responses. Error bars represent se.
Phototropin and Cryptochrome Activity Attenuates High-Light Phototropism
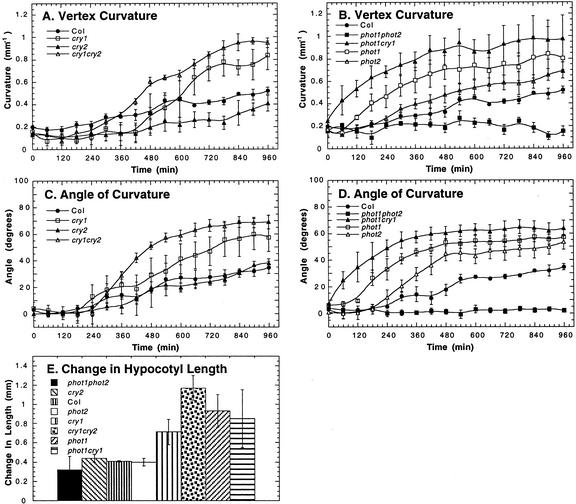
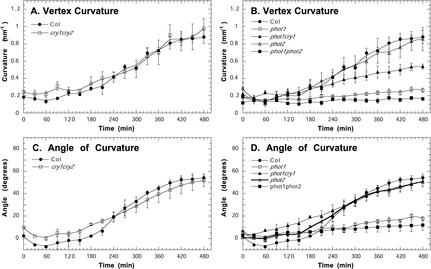
To test the hypothesis that high fluence rates interfere with phototropic curvature of the hypocotyl through the action of blue-light photoreceptors, we measured the kinetics of phototropism in the blue-light photoreceptor mutants under 100 μmol m–2 s–1 of blue light (Fig. 4). We observed enhanced phototropic responses in the phot1, phot2, and cry1 single mutants, and the phot1cry1 and cry1cry2 double mutants, indicating that phototropin and cryptochrome activity attenuates phototropism at high light levels. Although the cry2 mutant did not escape from the attenuating effect of high light, cry2 has an additive affect on attenuation because the cry1cry2 double mutants had a shorter latent period than the cry1 single mutant (Fig. 4, A and C). Phot1 seems to be the primary photoreceptor involved in the high-light-induced attenuation because the phot1 single and phot1cry1 double mutant showed a greater enhancement of the high-light phototropic response than the cryptochrome mutants (Fig. 4, A–D). In addition, phot1 and cry1 appear to function synergistically to attenuate phototropism because phot1cry1 double mutants had a shorter latent period than did the phot1 or cry1 single mutants. Consistent with previously published results (Sakai et al., 2001), phototropism was not observed in phot1phot2 double mutants (Fig. 4, B and D). In correspondence with an enhanced high-light phototropic response, the phot1, phot1cry1, cry1, and cry1cry2 mutants were longer than wild type after 960 min of 100 μmol m–2 s–1 unilateral blue light (Fig. 4E).
Figure 4.
Attenuation of phototropic curvature by phot1, phot2, cry1, and cry2 under 100.0 μmol m–2 s–1 of blue light. Measurements of phototropism in the Arabidopsis blue-light photoreceptor mutants are as follows: A and B, vertex curvature; C and D, angle of curvature at the hypocotyl tip; and E, the change in hypocotyl length after 960 min of 100.0 μmol m–2 s–1 of blue light. Error bars represent se.
We also investigated whether the extended latent period seen in wild-type seedlings at intermediate fluence rates of unilateral blue light (10.0 μmol m–2 s–1) was controlled by any of the known blue-light photoreceptors (Fig. 5). At this fluence rate, we could not detect a difference in phototropism between wild-type, cry1cry2, and phot2 seedlings (Fig. 5). Therefore, the cryptochromes and phot2 are probably not causing the extended latent period at moderate fluence rates. However, our data shows that cry1 may inhibit phot2 activity because phot1cry1 seedlings were phototropic even though phot1 single mutants did not respond at this light intensity (Fig. 5, B and D). Interestingly, angle of curvature measurements (Fig. 5D) indicate that phot1cry1 double mutants have the same phototropic response as wild-type seedlings, but reduced vertex curvature measurements (Fig. 5B) indicate that the bend in the phot1cry1 double mutants was more gradual than wild type.
Figure 5.
Cry1 inhibits of phot2-dependent phototropism at 10 μmol m–2 s–1 in the absence of phot1. A and B, Vertex curvature. C and D, Angle of curvature at the hypocotyl tip. Error bars represent se.
Phot2, cry1, and cry2 Activity Enhances Low-Light Phototropism
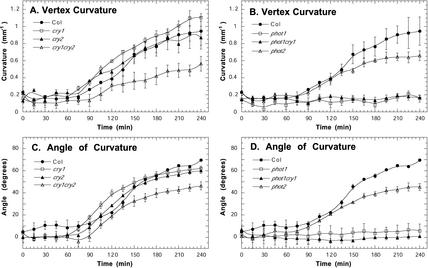
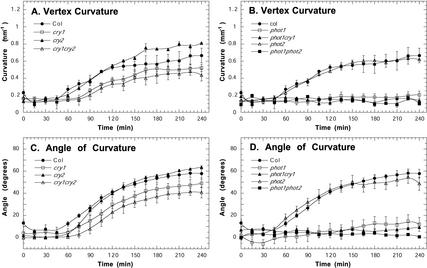
We also examined the kinetics of phototropism under low fluence rates (0.01 and 1.0 μmol m–2 s–1) of unilateral blue light (Figs. 6 and 7) over 4 h, which is sufficient time for complete phototropic curvature to develop in wild-type Arabidopsis. Indicating that the cryptochromes are acting redundantly at 1.0 μmol m–2 s–1 of blue light, cry1 and cry2 single mutants showed a wild-type response, but the cry1cry2 seedlings had a slower rate of curvature compared with wild-type seedlings (Fig. 6, A and C). At 0.01 μmol m–2 s–1, cry1 mutants and cry1cry2 double mutants had a similar reduction in curvature, but the late stage of phototropism was slightly enhanced in cry2 mutants in comparison with wild type (Fig. 7, A and C). As expected from other reports (Liscum and Briggs, 1995; Sakai et al., 2001), we did not detect any phototropism under low light conditions in phot1 single mutants, phot1phot2 double mutants, and phot1cry1 double mutants (Figs. 6, B and D, and 7, B and D). However, in contrast to previously reported results (Sakai et al., 2001), our results indicate that phot2 may enhance phototropism under low-light conditions because hypocotyl curvature was reduced in phot2 mutants at 1 μmol m–2 s–1 (Fig. 6, B and D).
Figure 6.
Phototropins and cryptochromes enhance phototropic curvature under 1.0 μmol m–2 s–1 of blue light. A and B, Vertex curvature, C and D, Angle of curvature at the hypocotyl tip. Error bars represent se.
Figure 7.
Phototropins and cryptochromes modulate phototropic curvature under 0.01 μmol m–2 s–1 of blue light. A and B, Vertex curvature (per millimeter). C and D, Angle of curvature at the hypocotyl tip (degrees). Error bars represent se.
DISCUSSION
Phototropism research has rarely taken into account the temporal or spatial complexity of the response and has largely relied on end-point measurements after curvature has developed (Firn, 1994). Mathematical curvature has been measured to measure apical hook opening and phototropism (Silk and Erickson, 1978; Silk, 1984; Cosgrove, 1985). Using elemental curvature to measure phototropism, Cosgrove (1985) found that curvature initiated simultaneously along the length of the cucumber hypocotyl rather than originating from the tip and migrating down. Unfortunately, Arabidopsis hypocotyls lack morphological features that can be used as elemental markers, and the small size of Arabidopsis seedlings complicates the task of putting elemental markers such as beads onto etiolated hypocotyls in the dark. To overcome this difficultly, we analyzed localized differential growth using a mathematical description of the Arabidopsis hypocotyl. Our measurements in Arabidopsis indicate that the position of the vertex of curvature migrates down the hypocotyl (Fig. 2). However, migration of the vertex does not rule out the possibility that curvature initiates simultaneously along the hypocotyl. Vertex migration essentially moves the fulcrum down the hypocotyl so that less differential growth at a particular location is required to produce a larger angle of curvature at the tip of the hypocotyl. Vertex migration also permits the autotropic response, which occurs when the “illuminated” side begins to grow faster relative to the “shaded” side of the hypocotyl. This reversal of the differential growth gradient above the vertex could be caused by increasing growth on the illuminated side of the hypocotyl, increasing growth inhibition on the shaded side, or a combination of both processes. This aspect of the phototropic response keeps the hypocotyl from curling back around during vertex migration (Silk, 1984; Firn, 1994).
Our analysis of phototropism reaffirms the hypothesis that phototropism is caused by a complex interplay of growth promotion and growth inhibition because although curvature increased at the vertex, it simultaneously decreased at the tip of the hypocotyl during the deceleration phase (Fig. 2). In theory, slight changes in the balance between hypocotyl growth inhibition and promotion should have an effect upon phototropic curvature in Arabidopsis because the diameter of Arabidopsis hypocotyls is small (Silk, 1984; Orbovic and Poff, 1993; Gendreau et al., 1997). Therefore, light conditions and mutants that affect hypocotyl growth may have an effect upon the kinetics or mechanics of phototropism.
Dark-grown seedlings have a rapid rate of elongation, and progressively higher intensities of overhead blue light stimulate greater hypocotyl growth inhibition (Liscum et al., 1992; Poppe et al., 1998; Ahmad et al., 2002). Because growth promotion is required along with growth inhibition during second positive phototropism in Arabidopsis (Orbovic and Poff, 1993), high fluence rates of light should have an inhibitory effect upon the kinetics of phototropism. Our results support this hypothesis because high fluence rates of light lengthen the latent period and decrease the rate of curvature (Fig. 3). The attenuation of high-light phototropism is mediated through the phototropins and cryptochromes (Fig. 4), which also mediate hypocotyl growth inhibition (Parks et al., 2001). For example, mutants lacking the photoreceptors that cause the strongest general growth inhibition under high blue light show the greatest enhancement of phototropism in response to high blue light (Fig. 4E). Moreover, the sequence of involvement of these blue-light photoreceptors during phototropism is similar to the sequence of changes leading to hypocotyl growth inhibition, where phot1 regulates the earlier stage, and the cryptochromes and phototropins regulate the later stage of the response (Folta and Spalding, 2001a). Furthermore, our results indicate that cry1 is required for attenuation of phototropic curvature in high-light conditions, whereas cry2 functions redundantly with cry1 but is not required (Fig. 4, A and C). The limited involvement of cry2 in the high-light attenuation response may be related to cry2 degradation under high fluence rates of blue light (Guo et al., 1999). These results suggest that the rapid phase of inhibition of hypocotyl growth mediated by phot1 (Folta and Spalding, 2001a) is responsible for the short-term inhibition of phototropism under high fluence rates, whereas cryptochrome maintenance of growth inhibition (Folta and Spalding, 2001a) results in the long-term attenuation of phototropin mediated high-light phototropism.
On first examination, our high-light phototropism results in wild-type seedlings appear to be inconsistent with reports that indicate that the final magnitude of high-light phototropism is similar to low-light phototropism (Sakai et al., 2000, 2001). However, there are important differences in experimental protocol in these studies. Sakai et al. (2000, 2001) grew their seedlings on vertical agar containing nutrient salts and did not examine the kinetics of the response. Electrolytes have been shown to increases the growth rate of cucumber (Cucumis sativus) hypocotyls and alter the phototropism response (Spalding and Cosgrove, 1993). By growing our seedlings without nutrient salts, we may have slowed the basal growth rate down, making it easier to distinguish between light-dependent growth inhibition and light-induced growth promotion. In addition, our measurements from time-lapse images did not include the apical hook region because we wanted to analyze hypocotyl phototropism without the confounding effects of light-induced hook opening and the light-dependent “solar tracking” response of the cotyledons that are among the many photomorphogenic responses that occur when seedlings are exposed to several hours of unilateral blue light. Our analysis of numerous time-lapse series of freestanding seedlings grown without an external source of electrolytes indicate that the end-point curvature measurements made by others after extended light treatments may be more indicative of events that occur in the apical hook region rather than hypocotyl curvature. Differentiating between hypocotyl curvature and the combined effect of light-induced hook opening and cotyledon orientation would be very difficult in nonkinetic studies of phototropism.
Under intermediate fluence rates, our results suggest that cry1 functionally inhibits phot2 because phot1 mutants were aphototropic, whereas phot1cry1 double mutants responded to moderate fluence rates (Fig. 5, B and D). Moreover, cry1 inhibition of phot2 was relieved by the action of phot1 because cry1cry2 double mutants and wild-type plants had a strong phototropic response under moderate fluence rates (Fig. 5, A and C). Therefore, at intermediate fluence rates, the phototropins appear to be acting synergistically to counter act the activity of the cryptochromes. The phototropins may be inhibiting the activity of the cryptochromes by promoting growth, which, at intermediate fluence rates, could counter act cryptochrome-mediated growth inhibition. Because we did not detect an enhancement of phototropism in the cryptochrome double mutants, we conclude that attenuation is not dependent upon the cryptochromes under moderate fluence rates. From these results, it appears that during phototropism, blue light inhibits hypocotyl elongation through the coaction of the phototropins and cryptochromes, and simultaneously promotes growth though the phototropins independently from the cryptochromes.
The previous explanation of phototropin and cryptochrome regulation of hypocotyl growth dynamics under moderate fluence rates can also apply to low-light phototropism. Several lines of evidence indicate that under low fluence rates, growth inhibition may be a limiting factor during phototropism in rapidly elongating etiolated seedlings. First, the phototropic response was greater in wild-type seedlings after exposure to 1.0 μmol m–2 s–1 than to 0.01 μmol m–2 s–1 of blue light (Fig. 3, inset). Second, cryptochrome activity enhanced phototropism under low fluence rates of light because phototropism was reduced in the cryptochrome double mutants under the low fluence rates (Figs. 6, A and C and 7, A and C). Third, because phot2 mutants also showed a decrease in phototropic curvature under 1.0 μmol m–2 s–1, it appears that phot2 may also participate in the growth inhibition response (Fig. 6, B and D). However, under 0.01 μmol m–2 s–1, we did not detect a phot2 affect upon phototropism (Fig. 7, B and D), indicating that phot2 activity probably is not important under very low fluence rates. Increased phot2 activity might also explain why wild-type seedlings responded greater to 1.0 μmol m–2 s–1 than 0.01 μmol m–2 s1 of blue light (Fig. 3). Unfortunately, we were not able to measure differences in overall hypocotyl growth between the genotypes under the intermediate and lower fluence rates (data not shown) because the resolution of our images was not sufficient to detect significant differences in hypocotyl growth during the shorter duration of the experiments at these fluence rates. High-resolution image analysis of blue light-regulated hypocotyl growth under low fluence rates of light will be necessary for understanding details of the growth dynamics during low-light phototropism (Parks et al., 1998).
In conclusion, our kinetic and mechanical analysis of phototropism tentatively links coaction of four blue-light photoreceptors with specific growth responses during phototropism. Our results support recent ideas about hypocotyl growth being controlled by light through competing growth inhibition and promotion responses (Jensen et al., 1998; Parks et al., 2001). Therefore, phototropism is probably best considered as resulting from a complex coordination between the sensory systems that serve to modulate growth inhibition and growth promotion of the hypocotyl. Specifically, these results indicate that the coordination of growth stimulation and inhibition during phototropism involves the activity of the two phototropins, with the cryptochromes playing a significant secondary role by shifting the balance toward greater growth inhibition and, thus, adjusting the differential growth across the hypocotyl. These results, along with previous work showing that phyA and phyB are also important in phototropism (Parks et al., 1996; Janoudi et al., 1997), indicate that phototropism in natural settings most likely results from the orchestrated action of at least six light receptors.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Mutants of Arabidopsis in this study have been previously described: phot1-1 (Liscum and Briggs, 1995; Huala et al., 1997), phot2-1 (Kagawa et al., 2001; Kinoshita et al., 2001), cry2-1(Guo et al., 1998), and hy4-B104 (Bruggemann et al., 1996), hy4-B104cry2-1 double mutant (Lasceve et al., 1999), phot1-1hy4-B104 double mutant, and phot1-1phot2-1 double mutant. Columbia was the Arabidopsis wild-type ecotype used as a control in these experiments.
Arabidopsis seeds were incubated in 0.4 mL of purified water at 4°C for 3 d in the dark before planting. The seeds were then planted in a row along the top edge of a 3.5- × 6-cm rectangular piece of wet 3-mm chromatography paper (Whatman, Clifton, NJ). A second piece of wet chromatography paper was placed on top of the first piece, just below the row of seeds on the first piece. The layered filter papers were placed between two microscope slides with the top edge of the first filter paper lined up with the top edge of the two microscope slides and were fastened together with one-eight-inch binder clamps. The slide apparatus was placed into a custom-made Plexiglas trough filled with 15 mL of purified water with the bottom edges of the filter papers placed into the purified water. The trough was put into a magenta box containing wet paper towels to maintain saturating humidity. Germination was synchronized by 10 μmol m–2 s–1 of red light for 2 h, and then the seeds were incubated in the dark at 23°C for 70 h.
Phototropism Conditions
The trough containing the seedlings was removed from the incubator and was placed in a black box fitted with a blue interference filter (450 ± 50 nm) along one side and an infrared filter (RG 830) along an adjacent side. Light from a 150 W flood lamp (Quartziline; General Electric, Fairfield, CT) was filtered through a 1.0% (w/v) solution of CuSO4 · 7H2O in a 500-mL round-bottom flask, which served to collimate and focus the light through the blue interference filter port on the black box. The blue-light intensity inside the box was adjusted between 0.01 and 100 μmol m–2 s–1 by adjusting the bulb voltage, with a rheostat, over a range that did not affect the spectral quality of the filtered light. Time-lapse images of seedling hypocotyls were taken under physiological darkness through the infrared interference filter with an infrared-sensitive digital camera (Color Quickcam; Connectix, San Mateo, CA). Infrared illumination was provided by an infrared-light emitting diode (940 nm; Radio Shack, Fort Worth, TX) located inside the black box. The temperature inside the box was maintained between 23°C and 25°C. Each experiment was replicated at least three times with each replicate consisting of five to eight seedlings.
Analysis of Time-Lapse Images
Each time-lapse image series was compiled into a stack in NIH Image and was rotated 90° to simplify subsequent calculations. Cartesian coordinates were taken from each image at 30 points along the length of the imaged hypocotyl from the base of the hypocotyl to the cotyledon petiole-hypocotyl junction at each time point. The Maple 6 (Waterloo Maple, Waterloo, Canada) add-in for Excel (Microsoft, Redmond, WA) was used to calculate a fourth order polynomial equation describing the hypocotyl using a nonlinear regression fit of the x/y coordinates of the hypocotyl at each time point. The regression coefficient was calculated according to standard statistical methods and was usually between 0.98 and 1.0. The angle of curvature was determined by comparing the slope of the initial tangent with the slope of a tangent at a particular time point by equation 1, where mi is the slope of the initial tangent at the tip of the hypocotyl. The value obtained from Equation 1, γ, was converted from radians to degrees.
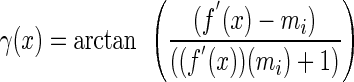 |
(1) |
The curvature values were calculated with Equation 2.
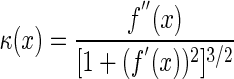 |
(2) |
The point of greatest curvature (the vertex) was found by differentiating Equation 2 and finding κmax, which is the position along the hypocotyl where κ′(x) = 0. Vertex curvature was calculated by substituting the x coordinate of the vertex position into Equation 2. Likewise, substituting the apical x coordinate into Equation 3 solved for the curvature near the hypocotyl tip. The length of the hypocotyl and arc length from the base of the hypocotyl to the vertex was determined by the following equation:
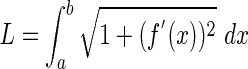 |
(3) |
We calculated the weighted means of each value because each replicate consisted of a different number of plants, and replicates with lower ses are more significant.
Acknowledgments
We thank Dr. Carolyn Yackel for discussing and encouraging the mathematics involved in this work, the UITS Center for Statistical and Mathematical Computing at Indiana University for technical assistance with Maple, and Stacy DeBlasio and Jack Mullen for their insightful discussions of this work.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018481.
This work was supported by the National Science Foundation (grant no. IBN–0080783) and by the Department of Energy (grant no. DE–FG02–01ER15223).
References
- Ahmad M, Cashmore AR (1993) Hy4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366: 162–166 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Galland P, Lardemer D (2002) Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol 129: 774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann E, Handwerger K, Essex C, Storz G (1996) Analysis of fast neutron-generated mutants at the Arabidopsis thaliana HY4 locus. Plant J 10: 755–760 [DOI] [PubMed] [Google Scholar]
- Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71: 1–11 [DOI] [PubMed] [Google Scholar]
- Cho MH, Spalding EP (1996) An anion channel in Arabidopsis hypocotyls activated by blue light. Proc Natl Acad Sci USA 93: 8134–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA (1994) The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol 25: 413–427 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1985) Kinetic separation of phototropism from blue-light inhibition of stem elongation. Photochem Photobiol 42: 745–751 [DOI] [PubMed] [Google Scholar]
- Firn R (1994) Phototropism. In RE Kendrick, GHM Kronenberg, eds, Photomorphogensis in Plants, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 659–681
- Folta KM, Spalding EP (2001a) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001b) Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J 28: 333–340 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hofte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Guo HW, Duong H, Ma N, Lin CT (1999) The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light-dependent post-transcriptional mechanism. Plant J 19: 279–287 [DOI] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Janoudi AK, Konjevic R, Whitelam G, Gordon W, Poff KL (1997) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plant 101: 278–282 [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Lasceve G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR (1999) Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol 120: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Stowe-Evans EL (2000) Phototropism: a “simple” physiological response modulated by multiple interacting photosensory-response pathways. Photochem Photobiol 72: 273–282 [DOI] [PubMed] [Google Scholar]
- Liscum E, Young JC, Poff KL, Hangarter RP (1992) Genetic separation of phototropism and blue-light inhibition of stem elongation. Plant Physiol 100: 267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbovic V, Poff KL (1993) Growth distribution during phototropism of Arabidopsis thaliana seedlings. Plant Physiol 103: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Cho MH, Spalding EP (1998) Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 118: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Folta KM, Spalding EP (2001) Photocontrol of stem growth. Curr Opin Plant Biol 4: 436–440 [DOI] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP (1996) Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Sweere U, Drumm-Herrel H, Schafer E (1998) The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J 16: 465–471 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2; a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WK (1984) Quantitative descriptions of development. Annu Rev Plant Physiol 35: 479–518 [Google Scholar]
- Silk WK, Erickson RO (1978) Kinematics of hypocotyl curvature. Am J Bot 65: 310–319 [Google Scholar]
- Spalding EP, Cosgrove DJ (1993) Influence of electrolytes on growth, phototropism, mutation and surface potential in etiolated cucumber seedlings. Plant Cell Environ 16: 445–451 [DOI] [PubMed] [Google Scholar]