Abstract
Salicylic acid (SA), a natural defensive signal chemical, and antimycin A, a cytochrome pathway inhibitor, induce resistance to Tobacco mosaic virus (TMV). Pharmacological evidence suggested signaling during resistance induction by both chemicals involved alternative oxidase (AOX), sole component of the alternative respiratory pathway (AP). Roles of the AP include regulation of intramitochondrial reactive oxygen species and maintenance of metabolic homeostasis. Transgenic tobacco (Nicotiana tabacum) with modified AP capacities (2- to 3-fold increased or decreased) showed no alteration in phenotype with respect to basal susceptibility to TMV or the ability to display SA-induced resistance to systemic viral disease. However, in directly inoculated tissue, antimycin A-induced TMV resistance was inhibited in plants with increased AP capacities, whereas SA and antimycin A-induced resistance was transiently enhanced in plant lines with decreased AP capacities. We conclude that SA-induced TMV resistance results from activation of multiple mechanisms, a subset of which are inducible by antimycin A and influenced by AOX. Other antiviral factors, potentially including the SA-inducible RNA-dependent RNA polymerase, are regulated by AOX-independent mechanisms.
Salicylic acid (SA) is an important signal molecule in plants that is required for the induction of systemic acquired resistance (SAR) against a wide variety of pathogens, including fungi, bacteria, and viruses (for review, see Dempsey et al., 1999). Increased biosynthesis of SA and the induction of SAR often follow a hypersensitive response (HR), a form of resistance that is usually characterized by localized cell death at or around the initial point of pathogen entry (Heath, 2000). The ability to exhibit the HR is genetically determined and is highly pathogen specific (Dangl and Jones, 2001). In contrast, SAR is broad spectrum in nature and can even be induced in plants independently of a resistance gene-mediated HR if they are treated with SA or one of its derivatives or functional analogs (Dempsey et al., 1999; Murphy et al., 2001; Oostendorp et al., 2001).
Mechanistically, the enhanced resistance to pathogens exhibited by plants after the induction of SAR or after treatment with SA is due in part to the induction of pathogenesis-related (PR) proteins (Bowles, 1990; Hammerschmidt, 1999; van Loon and van Strien, 1999). Many PR proteins have antifungal or antibacterial properties (van Loon and van Strien, 1999), but none of the PR proteins examined so far appear to have antiviral activity (Cutt et al., 1989; Linthorst et al., 1989). Nevertheless, PR protein induction remains a useful molecular marker for the induction of SAR.
An important advance in our understanding of inducible antiviral mechanisms occurred recently when Chen and colleagues discovered that SA induces the expression of a tobacco (Nicotiana tabacum) gene, NtRdRp1, encoding an RNA-dependent RNA polymerase (RdRp; Xie et al., 2001). Host RdRp enzymes are important factors in the establishment of certain forms of RNA silencing, a homology-based RNA degradation system that can act as an antiviral mechanism (Baulcombe, 2001; Voinnet, 2001; Ahlquist, 2002). Antisense suppression of NtRdRp1 expression in transgenic tobacco resulted in plants that showed increased susceptibility to Tobacco mosaic virus (TMV), demonstrating that NtRdRp1 does possess antiviral activity, albeit not sufficient to confer complete resistance (Xie et al., 2001). However, when the same antisense plants were treated with SA, they were still able to display induced resistance to the virus (Xie et al., 2001). Although this experiment showed that NtRdRp1 alone cannot mediate SA-induced resistance to TMV, it does not rule out the possibility that NtRdRp1 activity and RNA silencing may, along with other factors, contribute to the overall phenomenon of SA-induced resistance to viruses.
In tobacco treated with SA the replication of Potato virus X and TMV, as well as the cell-to-cell movement of TMV, are inhibited in directly inoculated leaf tissue (Chivasa et al., 1997; Naylor et al., 1998; Murphy and Carr, 2002). However, replication and cell-to-cell movement of Cucumber mosaic virus (CMV) are not inhibited by SA but the chemical does inhibit the systemic movement of CMV through the phloem tissue (Naylor et al., 1998). The ability of CMV to evade the primary layers of SA-induced virus resistance is conferred by its 2b protein. This multifunctional protein influences virus movement and symptom development (Soards et al., 2002), but most importantly it can counter induction of RNA silencing (Béclin et al., 1998; Brigneti et al., 1998) and SA-induced resistance (Ji and Ding, 2001). The ability of the CMV 2b protein to act as a counter defense factor is dependent on its localization to the cell nucleus (Lucy et al., 2000; Mayers et al., 2000), where it affects expression of host genes including at least one SA-inducible gene: the mitochondrial alternative oxidase (AOX; Ji and Ding, 2001).
All plants possess AOX, which by itself constitutes a distinct branch of the cytochrome pathway (CYT) linking the oxidation of the ubiquinol/ubiquinone (UQ) pool directly to the reduction of oxygen to water. This branch is usually referred to as the alternative respiratory pathway (AP; Affourtit et al., 2001, 2002). AP activity is not coupled to ATP generation. Instead, it is thought to play a potentially crucial role in protecting all plant cells against the lethal effects of reactive oxygen species (ROS; Maxwell et al., 1999; Yip and Vanlerberghe, 2001), and in the maintenance of plant homeostasis under varying growth conditions (Affourtit et al., 2001, 2002; Sakano, 2001; Moore et al., 2002). AOX is a homodimeric protein and activity is regulated by the redox-sensitive formation or breakage of an intersubunit disulfide bridge (Rhoads et al., 1998). AOX activity and transcription of Aox mRNA can be stimulated by inhibitors of the CYT (antimycin A [AA] or cyanide), as well as by SA and the synthetic resistance-inducing chemical, 2,6-dichloroisonicotinic acid (Raskin et al., 1987; Rhoads and McIntosh, 1992; Chivasa and Carr, 1998; Chivasa et al., 1999).
While investigating the possible involvement of AOX in signaling during pathogen resistance induction in tobacco and Arabidopsis, we found that the defensive signal transduction pathway branches downstream of SA. One branch induces PR proteins and resistance to bacteria and fungi, whereas another triggers induction of resistance to viruses (Murphy et al., 1999; Wong et al., 2002). Initial evidence for this was based on pharmacological data. Specifically, resistance to viruses can be activated using AA and cyanide, or inhibited with salicylhydroxamic acid (SHAM, an AOX inhibitor), independently of the induction of PR gene expression (Chivasa et al., 1997; Chivasa and Carr, 1998). Subsequent experiments using Arabidopsis npr1/nim1 mutants confirmed the existence of this branch point downstream of SA (Kachroo et al., 2000; Wong et al., 2002).
The results of our pharmacological experiments were consistent with a role for AOX in the regulation of induced resistance to viruses. In addition, it was noted by ourselves and others (Lennon et al., 1997; Chivasa and Carr, 1998; Lacomme and Roby, 1999; Simons et al., 1999) that Aox gene expression and AOX protein accumulation are elevated in plant tissue expressing the HR, further suggesting an association between AOX and pathogen resistance. Although superficially compelling, such pharmacological and correlative data do not provide conclusive evidence for or against any role(s) for AOX in the induction of resistance to viruses.
To test the hypothesis that AOX plays a role in signaling during the induction of virus resistance, we produced a number of independent transgenic tobacco lines with altered Aox gene expression and AP capacity. Consistent with recently published work from another group (Ordog et al., 2002), we found that altering Aox gene expression in TMV-susceptible tobacco had no clear cut effects on SA-induced resistance to systemic infection with TMV. Nonetheless, further, more detailed investigations revealed that the modification of Aox gene expression did affect chemically induced resistance to TMV in the directly inoculated leaf tissue. However, there were differential effects on SA- and antimycin A-induced resistance. Together with other data, these findings indicate that SA-induced resistance to TMV results from the activation of multiple antiviral mechanisms, a subset of which are affected by AOX.
RESULTS
Construction and Characterization of Transgenic Tobacco Plants with Altered Respiratory Characteristics
Transgenic tobacco plants with modified Aox gene expression levels have been reported previously (Vanlerberghe et al., 1994), but were not available for distribution, making it necessary for us to generate new lines. Agrobacterium tumefaciens-mediated transformation of cv Xanthi tobacco (a TMV-susceptible variety) was used to produce transgenic tobacco plants harboring the Bright Yellow tobacco Aox1a cDNA in sense or antisense orientation fused to an enhanced Cauliflower mosaic virus 35S promoter for constitutive expression of the transgene. Primary transformants and plants of subsequent generations were selected on hygromycin, and analysis of Aox gene expression and protein accumulation was carried out by northern and western blotting, respectively (Fig. 1 and data not shown).
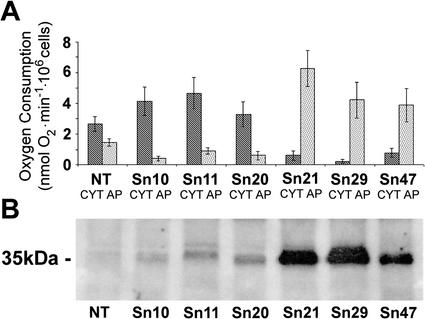
Figure 1.
Characterization of alternative respiratory capacity and AOX protein levels in transgenic tobacco lines. A, Cells were isolated from leaves of nontransgenic (NT) cv Xanthi and T2 generation Aox-transgenic tobacco plants (lines Sn10, Sn11, Sn20, Sn21, Sn29, and Sn47). These cells were used for the measurement of CYT capacity (black bars) and alternative pathway (AP) capacity (gray bars) using an oxygen electrode. CYT is defined as oxygen consumption sensitive to a final concentration of 20 μM antimycin A, whereas AP corresponds to oxygen uptake insensitive to 20 μM AA but sensitive to 2 mm SHAM. Steady-state oxygen consumption levels were measured before the successive additions of AA and SHAM. The rates of CYT and AP oxygen consumption presented have had residual rates (oxygen uptake in the presence of both inhibitors) subtracted from all values. Data is expressed as the mean ± se (n = 12). B, Immunoblot analysis of levels of AOX protein isolated from nontransgenic (NT) cv Xanthi and T2 generation sense Aox-transgenic tobacco lines (Sn10, Sn11, Sn20, Sn21, Sn29, and Sn47). Proteins were denatured in the presence of 0.1 m dithiothreitol and were separated on a 15% (w/v) acrylamide SDS-PAGE gel and subjected to immunoblot analysis using an anti-AOX monoclonal antibody. Antibody binding was detected using anti-mouse immuno-globulin (Ig) G conjugated to horseradish peroxidase (HRP) and a chemiluminescent substrate. Each lane was loaded with a total of 20 μg of protein. The apparent Mr (in kilodaltons) of the reduced form of the AOX protein is indicated. Prestained Mr markers were not visible on the x-ray film.
The respiratory characteristics of T1 and T2 generation transgenic plants were examined to assess whether transformation with the Aox cDNA constructs resulted in an effect on their AP capacities. AP capacity is a measure of the maximum potential activity of AOX (Moore and Siedow, 1991) and is relatively straightforward to assay. In contrast, direct measurements of in vivo AOX activity require specialized mass spectrometry equipment to determine the relative uptake of the 16O and 18O isotopes of oxygen during respiration (Robinson et al., 1992). AP capacity was assessed using cells released from leaves by digestion with macerase. Unlike protoplasts, which are obtained by digestion of leaf tissue with macerase plus cellulase, these cells retain their walls. Retention of the cell wall means that cells can safely be subjected to stirring in the oxygen electrode. Additionally, this uniform suspension of cells can take up chemicals and exchange gases much more efficiently than tissue slices. The method also avoids the need to generate cell suspension cultures for each line to be analyzed. In any case, cultured plant cells probably do not accurately represent the physiology of leaf tissue and have to be maintained in the presence of plant growth regulators, some of which are known to trigger defense gene expression (Antoniw et al., 1981).
Oxygen consumption by freshly prepared leaf cells was measured in the presence or absence of inhibitors of the CYT or the AP, or combinations of both types of inhibitor. The inhibitor studies revealed the proportion of oxygen consumption attributable to AOX versus cytochrome c oxidase (Fig. 1A). As a control, oxygen consumption in the absence or presence of the uncoupler carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP) was also measured to check that the cells' mitochondria were intact. In all experiments, FCCP treatment greatly increased overall oxygen consumption (i.e. induced uncoupling of respiration). Thus, cells prepared by this method had fully functional mitochondria in which electron flow through the CYT was coupled to proton translocation across the inner mitochondrial membrane (data not shown).
Initial results with antisense Aox transgenics at the T0 generation (primary transformants) were promising in that experiments using detached leaves indicated that SA-induced Aox transcript accumulation was inhibited. Unfortunately, T1 generation antisense plants did not inherit this phenotype and, furthermore, these plants showed wild-type, or even increased, AP capacities (data not shown). Therefore, these lines were not used for further experimentation. Fortunately, T1 and T2 generation plants from three independent lines resulting from transformation with the sense Aox construct had AP capacities that were significantly lower than the AP capacity of untransformed tobacco (Fig. 1A, lines Sn10, Sn11, and Sn20). Initially, it was thought that this resulted from cosuppression of Aox gene expression, an effect seen previously in studies of Aox transformants (Vanlerberghe et al., 1994). However, AOX protein levels in these lines were similar to, or slightly higher than, that seen in untransformed tobacco, making this explanation less likely (Fig. 1B). Speculatively, the decreased AP capacity seen in these lines may be explainable by a dominant-negative mutation-like mechanism (Herskowitz, 1987). That is, the level of expression of the Aox transgene may have been insufficient to cause a significant increase in AOX protein accumulation and in AP capacity. However, the expression of low levels of Bright Yellow tobacco AOX 1a expressed in these transformed cv Xanthi plants may be enough to interfere with the formation of active (noncovalent) dimers of the native AOX protein and promote formation of less active heterodimers of Bright Yellow and cv Xanthi AOX polypeptides. In any case, the lowered AP capacities seen in plants of lines Sn10, Sn11, and Sn20 were comparable with those in the antisense Aox transgenic tobacco lines produced by Vanlerberghe et al. (1994).
Three other transgenic lines harboring the sense Aox gene sequence exhibited clearly increased levels of AOX protein accumulation and had enhanced AP capacities (Fig. 1, lines Sn21, Sn29, and Sn47). Cells from these three lines showed a greater than 2-fold increase in AP capacity compared with cells from untransformed tobacco (Fig. 1A).
Transgenic Plants with Altered Alternative Respiratory Capacity Display Normal TMV-Induced Systemic Disease Development and SA-Induced Delay in the Onset of Systemic Disease
Experiments were carried out to determine whether transgenic plants with altered respiratory characteristics exhibited any unusual responses to infection with TMV or any change in SA-induced resistance to TMV-induced disease symptoms. In these experiments, untransformed cv Xanthi plants, in parallel with T1 or T2 generation plants of transgenic lines with increased (Sn21, Sn29, and Sn47) or decreased (Sn10, Sn11, and Sn20) AP capacity, were sprayed with 1 mm SA or water for 3 to 5 d before inoculation of one lower leaf on each plant with TMV (2 μg mL–1). Depending upon the experiment, there were 14 or 21 plants of each line (including nontransgenic cv Xanthi) for each treatment. After inoculation with TMV, plants were monitored daily for the appearance of systemic disease symptoms (vein clearing, chlorosis, stunting, and mosaic) in the uninoculated leaves.
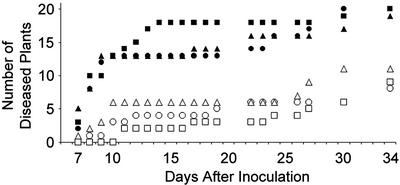
In eight separate experiments, we observed no consistent evidence that the transgenic plants showed altered systemic disease responses to TMV or that they differed from nontransgenic plants in their ability to exhibit SA-induced resistance to the virus. Figure 2 shows an example of data taken from one such experiment using nontransgenic cv Xanthi tobacco plants and plants belonging to lines Sn47 and Sn10, which possess increased and decreased AP capacities, respectively. It can be seen that the kinetics of disease development in all three groups of plants are substantially the same and that all three groups of plants showed similar SA-induced delays in disease development (Fig. 2). The results show that modification of the AP capacity of tobacco plants does not affect their basal resistance or susceptibility to TMV, and it does not have any obvious effect on the ability of plants to respond to SA. The results are broadly consistent with data obtained by Ordog et al. (2002).
Figure 2.
Development of TMV-induced systemic disease symptoms on nontransgenic tobacco and transgenic tobacco with modified alternative respiratory capacities. In this experiment, the plants used were nontransgenic cv Xanthi tobacco (circles), together with transgenic lines Sn10 (squares) and Sn47 (triangles) and there were 21 plants per group. Lines Sn10 and Sn47 exhibit decreased and increased AP capacities, respectively (see Fig. 1). Plants were treated normally (black symbols) or sprayed with 1 mm SA (white symbols) once daily for 5 d before inoculation with TMV. Inoculation was carried out on one lower leaf per plant with a suspension of 2 μg mL–1 TMV in water using carborundum to increase the efficiency of inoculation. The plants were observed at regular intervals over a period of 34 d and plants were recorded as being “diseased” upon the appearance of visible systemic symptoms (vein clearing, chlorosis, or mosaic) on noninoculated leaves.
Alterations in Alternative Respiratory Capacity Modify the Induction of Resistance to TMV by AA and SA in Directly Inoculated Leaf Tissue
SA and AA primarily inhibit replication and local movement of TMV in directly inoculated tissue, rather than systemic movement (Chivasa et al., 1997; Chivasa and Carr, 1998; Murphy and Carr, 2002). Therefore, we investigated TMV accumulation in directly inoculated tissue using western-blot analysis to detect the viral coat protein (CP) in chemically treated leaves. In previous work with tobacco, we found that spraying plants or simply floating leaf tissue on solutions of AA are not effective means of inducing Aox gene induction or resistance to TMV, and that tissue must be infiltrated with the solution using a syringe (Chivasa and Carr, 1998). While optimizing this procedure in nontransgenic tobacco leaves, we noted that resistance to TMV was induced immediately after infiltration not only with solutions of antimycin A, but also with solutions of SA. Therefore, for the experiments in the present study, we infiltrated resistance-inducing chemical or control solutions into leaves immediately before inoculation with TMV to ensure that all the cells in the tissue received a uniform and synchronized dose of SA or antimycin A. In these experiments, resistance is characterized by a delay in the onset of detectable virus accumulation or in a reduced level of accumulation.
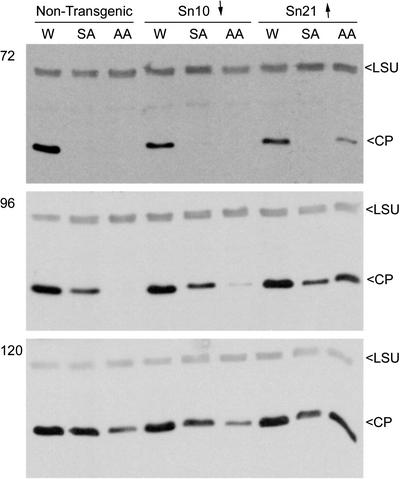
We found that 25 μm AA was an effective inducer of resistance to TMV in nontransgenic tissue and tissues of transgenic plants with diminished AP capacity (Fig. 3). However, antimycin A-induced resistance to TMV was inhibited in leaf tissue from transgenic plants with an enhanced AP capacity. Figure 3 shows the accumulation of TMV CP over time in directly inoculated leaves from nontransgenic plants and plants of line Sn21 (enhanced AP capacity) and line Sn10 (decreased AP capacity). It can be seen that the virus accumulated to a detectable level in 25 μm antimycin A-treated Sn21 tissue earlier than in similarly treated tissues from nontransgenic or Sn10 tobacco leaf, which showed the expected delay in virus accumulation (Fig. 3). Consistent with the earlier experiments that showed that modification of alternative respiratory capacity had no effect on SA-induced resistance to systemic disease induction (Fig. 2), there was no apparent effect on SA-induced resistance to virus accumulation in either of the transgenic lines (Fig. 3). The same effect on antimycin A-induced resistance was also seen in the other transgenic lines with enhanced alternative respiratory capacities (lines Sn29 and Sn47; data not shown).
Figure 3.
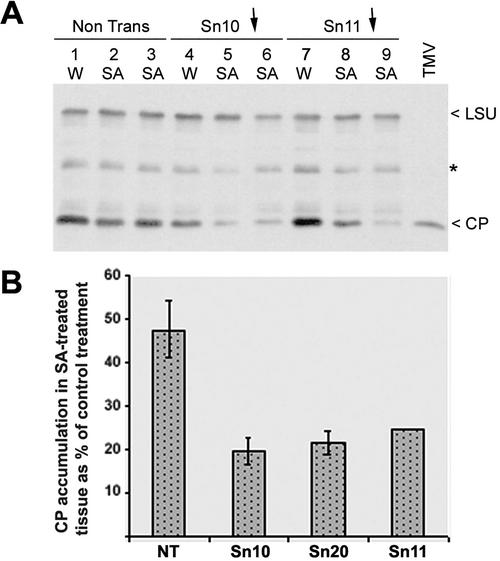
Differential effects of altered alternative respiratory pathway capacity on SA- and antimycin A-induced resistance to TMV in directly inoculated leaf tissue. Leaves of nontransgenic cv Xanthi tobacco plants or plants of the transgenic lines Sn10 and Sn21 were infiltrated with 0.5 mm SA, 25 μm AA, or a control solution of water (W) containing 0.05% (v/v) ethanol. The control solution contained an amount of ethanol equivalent to that used to dissolve the SA and AA before dilution with water. Within 10 min of infiltration, the leaves were inoculated with a suspension of TMV strain U1 at a concentration of 10 μg mL–1. At various times after infiltration and inoculation (72, 96, and 120 h), four leaf discs (12 mm in diameter) were punched out of the leaves of each plant and used for extraction of total soluble proteins. The proteins were analyzed by immunoblotting using, simultaneously, polyclonal rabbit antisera against the TMV CP and the large subunit (LSU) of ribulose 1,5-bisphosphate carboxylase (RuBPCase). The LSU band was used as an indicator of equal loading of protein. Binding of the primary antibodies was detected by probing the immunoblots with anti-rabbit IgG conjugated to HRP and visualizing the CP and LSU bands on x-ray film using a chemiluminescent HRP substrate. The transgenic lines Sn10 and Sn21 have, compared with nontransgenic cv Xanthi, decreased (↓) and increased (↑) AP capacities (refer to Fig. 1).
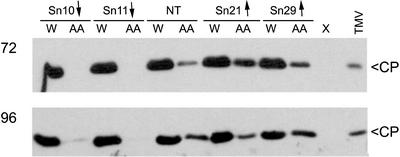
In subsequent experiments, a lower concentration of AA (12.5 μm) was infiltrated into the leaves on nontransgenic and transgenic plants before inoculation with TMV. Infiltration of AA at this concentration was less effective at inducing resistance to TMV in the leaves of nontransgenic cv Xanthi plants or in leaves of transgenic plants with an enhanced AP capacity (Fig. 4). Surprisingly, this diminished concentration of AA was able to induce resistance to TMV in leaves of transgenic plants that had a decreased AP capacity. This can be seen in Figure 4, which shows that accumulation of TMV is still inhibited in lines Sn10 and Sn11, both of which have decreased AP capacities.
Figure 4.
Transgenic tobacco lines with decreased alternative respiratory capacities show increased sensitivity to the resistance-inducing effect of AA. Leaves of nontransgenic (NT) cv Xanthi tobacco plants or plants of the transgenic lines Sn10 and Sn11 (both with decreased alternative respiratory capacity, indicated by ↓), as well as lines Sn21 and Sn29 (both with increased alternative respiratory capacity, indicated by ↑), were infiltrated with 12.5 μm AA or a control solution of water (W) containing 0.05% (v/v) ethanol. Within 10 min of infiltration, the leaves were inoculated with a suspension of TMV strain U1 at a concentration of 11 μg mL–1. At various times after infiltration and inoculation (72 and 96 h), four leaf discs (12 mm in diameter) were punched out of the leaves of each plant and were used for extraction of total soluble proteins. Equal amounts of protein were analyzed by immunoblotting using polyclonal anti-TMV CP serum. Lanes labeled X and TMV were, respectively, empty or loaded with pure TMV as a marker for CP. Equal loading of proteins was checked by staining with a solution of 0.1% (w/v) Ponceau red (data not shown).
Because transgenic lines with decreased alternative respiratory capacities appeared to be more sensitive to the resistance-inducing activity of antimycin A, we decided to see if the same was true for SA-induced resistance. First, preliminary experiments were carried out to investigate the dose-response characteristics of SA-induced resistance to TMV in nontransgenic leaves infiltrated with a single dose of SA immediately before inoculation (data not shown). In nontransgenic leaf tissue, infiltration with SA at a concentration of 0.25 mm or greater resulted in complete inhibition of TMV CP accumulation for at least 72 h after infiltration and inoculation (for example, see Fig. 3). In contrast, SA at concentrations below 0.2 mm did not completely inhibit the accumulation of the virus in nontransgenic tissue (Fig. 5A). However, although 0.2 mm SA did not induce complete resistance in tissues of plants belonging to transgenic lines with lowered alternative respiratory capacities, there was a greater reduction in TMV CP accumulation in these plants (Fig. 5A). Densitometry was used to quantify the relative levels of TMV CP accumulation in nontransgenic and transgenic plant tissues treated with 0.2 mm SA at 72 h after infiltration and inoculation. It was found that for all three transgenic lines with decreased alternative respiratory capacities, 0.2 mm SA treatment consistently decreased TMV CP accumulation by approximately 76% or more, whereas the SA-induced decrease in TMV CP accumulation in nontransgenic tissue was consistently around 50% (Fig. 5B). The results show that plants with decreased alternative respiratory capacity are more sensitive to the virus resistance-inducing activity of SA in directly inoculated leaves, although the extent of this effect would be unlikely to affect the eventual outcome of systemic TMV infection.
Figure 5.
Transgenic tobacco lines with decreased alternative respiratory capacities show increased sensitivity to the resistance-inducing effect of SA. A, Immunoblot analysis of TMV CP accumulation (72 h after inoculation) in directly inoculated leaves of nontransgenic cv Xanthi (plants 1–3) and plants belonging to the transgenic lines Sn10 (plants 4–6) and Sn11 (plants 7–9), both of which have decreased alternative respiratory capacities (indicated by ↓). At the concentration of SA used (0.2 mm), TMV CP has already reached readily detectable levels in the SA-treated nontransgenic and transgenic plants (compare with data in Fig. 3 in which 0.5 mm SA was used). However, the accumulation of TMV CP in the inoculated leaves of SA-treated plants belonging to lines Sn10 (plants 5 and 6) or Sn11 (plants 8 and 9) is less than that seen in the SA-treated nontransgenic plants. Infiltration with SA or the water control solution (W), inoculation, and protein extractions were carried out as described in the Figure 3 legend. Proteins were analyzed by immunoblot analysis using anti-TMV CP simultaneously with anti-LSU (to monitor for equal protein loading). The positions of TMV CP and RuBPCase LSU are indicated to the right of the blot. On this blot, an additional artifactual band (possibly a breakdown product of LSU) was present (indicated by an asterisk). TMV, Lane was loaded with pure TMV as a marker for CP. B, Densitometric analysis of TMV CP accumulation in nontransgenic (NT) and three transgenic plant lines with decreased AP capacity (Sn10, Sn11, and Sn20). Data is expressed as TMV CP accumulation in virus-inoculated tissues treated with 0.2 mm SA as a proportion of the accumulation occurring in tissue treated with the control solution. Data refers to protein extracted at 72 h postinoculation from two separate experiments (NT, n = 8; Sn10, n = 6; Sn20, n = 3, and Sn11, n = 2). Data expressed as the mean ± se.
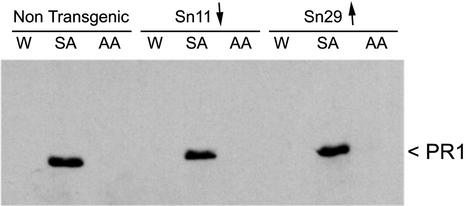
The induction of PR-1 protein accumulation in the transgenic lines was analyzed by western blotting using anti-PR1 serum. PR protein accumulation was similar in SA-treated Aox-transgenic and untransformed plants (Fig. 6), indicating that AOX has no influence on PR protein gene expression. Interestingly, Maxwell et al. (1999) noted that PR1 gene expression was enhanced in a transgenic tobacco line with a decreased AP capacity. However, that work was carried out using exponentially growing suspension cell cultures in which the tobacco cells may have been subjected to additional stresses or chemicals (such as plant growth regulators) that could have caused the induction of PR1 genes (Antoniw et al., 1981).
Figure 6.
SA-induced induction of the SAR marker protein PR1 is not affected by genetic modification of alternative respiratory capacity. Leaves of nontransgenic cv Xanthi tobacco plants or plants of the transgenic lines Sn11 (decreased AP capacity indicated by ↓) and Sn29 (increased AP capacity indicated by ↑) were infiltrated with 0.5 mm SA, 25 μm AA, or a control solution of water (W) containing 0.05% (v/v) ethanol. Equal amounts of total soluble proteins extracted from leaf tissues 96 h after infiltration were subjected to immunoblot analysis using rabbit polyclonal anti-PR1 serum. In all three types of plant, infiltration with the SA solution is the only treatment that induced PR1 accumulation.
Taken together, the results of our experiments examining the effects of chemical treatment on TMV accumulation in directly inoculated tissues suggest that altering Aox gene expression and the AP capacity can affect chemically induced resistance to the virus. Importantly, altering the AP capacity has differential effects on SA-induced resistance compared with antimycin A-induced resistance.
Inducible RdRP Gene Expression Is Triggered by SA But Not by Antimycin A
In transgenic plants with an enhanced alternative respiratory capacity, the induction of resistance to TMV by AA is inhibited, but resistance induced by SA appears to be unaffected (Fig. 3). Why is this? One possibility is that SA and AA induce resistance to TMV by completely separate signaling pathways, only one of which is influenced by AOX and AP capacity. However, the finding that antimycin A- and SA-induced resistance are to some degree enhanced in transgenic plants with decreased alternative respiratory capacity argues against this. A second possibility is that SA may influence more than one resistance-inducing signal pathway. If this were the case, it is possible that if only one of these mechanisms was regulated by AOX, then its inhibition in transgenic plants with altered alternative respiratory capacity would be masked by the induction of one or more additional SA-induced resistance mechanisms via AOX-independent signaling.
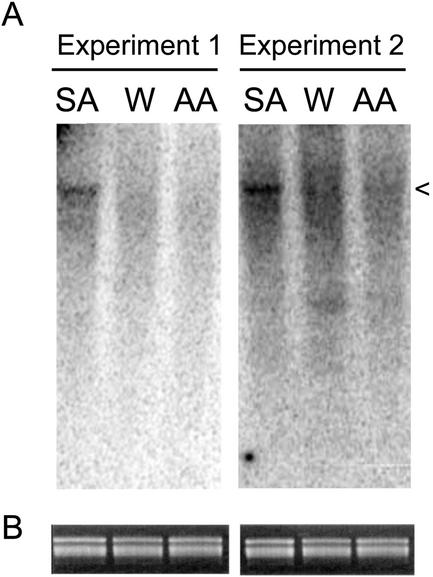
To investigate this possibility, we examined the induction of the gene for NtRdRp1, an SA-inducible enzyme that is known to have antiviral activity but which is dispensable for SA-induced resistance (Xie et al., 2001). In particular, we wanted to know if it was induced by AA as well as by SA. Primer extension was used to analyze steady-state levels of NtRdRp1 transcripts in untreated, SA-treated, and antimycin A-treated tobacco leaf tissue because this is a specific and quantitative method for transcript analysis (Fig. 7). As expected, SA induced an increase in steady-state NtRdRp1 transcript accumulation. However, AA did not. This indicates that although NtRdRp1 could play a role in SA-induced resistance, it cannot participate in antimycin A-induced resistance. This result lends credence to the possibility that resistance to TMV resulting from the induction of additional antiviral factors, like NtRdRp1, may be masking any effect that alterations in AP capacity may have on SA-induced resistance. It is also consistent with the idea that the induction of antiviral resistance by SA is controlled by more than one signaling pathway and is effected by more than one mechanism.
Figure 7.
NtRdRp1 transcript accumulation is induced by SA but not by AA. In two separate experiments, nontransgenic cv Xanthi tobacco tissue was treated with 0.5 mm SA, 25 μm AA, or a control solution of water (W) containing 0.05% (v/v) isopropanol. After 24 h of treatment, total RNA was extracted and was used as substrate for primer extension with a 32P-labeled oligonucleotide complementary to the NtRdRp1 transcript. Reaction products were analyzed by PAGE and were visualized by phosphorimaging (A). The expected primer extension product for NtRdRp1 (indicated by <) was only present at an increased level in reactions using template RNA extracted from SA-treated tissue. B, Sections (containing the rRNA bands) of ethidium bromide-stained agarose gels loaded with the template RNAs to demonstrate that equal amounts of template RNA were used in each reaction.
DISCUSSION
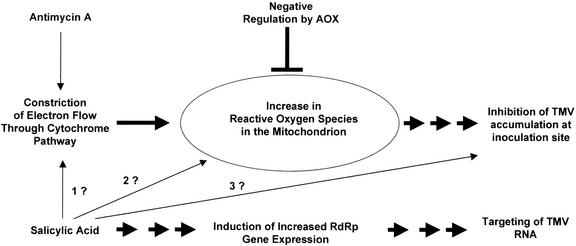
We found that genetic modification of Aox gene expression and AP capacity did not alter the overall susceptibility of plants to TMV-induced systemic disease or their ability to resist the systemic spread of the virus after treatment with SA. To this extent, our conclusions are in agreement with those of Ordog et al. (2002) who studied the accumulation of TMV CP in noninoculated leaves of Aox-transgenic tobacco plants with or without hydroponic administration of SA. However, we extended our investigation to examine antimycin A-induced resistance because previous studies showed that this chemical, like SA, can induce resistance to TMV in tobacco and to a closely related tobamovirus (Turnip vein clearing virus) in Arabidopsis (Chivasa and Carr, 1998; Wong et al., 2002). In addition, we carried out further studies of the effect of SA on TMV accumulation in directly inoculated tissue because, in tobacco, SA affects local TMV replication and cell-to-cell movement, rather than systemic spread (Chivasa et al., 1997; Murphy and Carr, 2002). These additional experiments showed that alteration of Aox gene expression and AP capacity in transgenic tobacco did, in fact, affect the induction of resistance to TMV by AA and SA. However, we found that altering AP capacity had differential effects on the induction of resistance by the two chemicals. Therefore, it may be premature to conclude, as Ordog et al. (2002) recently have, that AOX does not play a role in the induction of virus resistance, but our results do indicate that the relationship between AOX and induced resistance to TMV is by no means simple. Therefore, it is likely that AOX is a regulator rather than a trigger of defensive signaling, and that SA may induce multiple resistance mechanisms, some of which are not regulated by AOX (Fig. 8).
Figure 8.
Model for the relationship between AOX and chemically induced resistance to TMV in tobacco. It is proposed that ROS generated in the mitochondrion may function as signals leading via subsequent, unknown steps to the induction of a subset of antiviral mechanisms (inhibition of replication and/or movement). Thus, nonlethal concentrations of AA (an inhibitor of complex III in the CYT) inhibits respiration by constricting electron flow through the CYT, leading to an increase in mitochondrial ROS levels (the putative signal). Engagement of AOX could negatively regulate ROS production by enhancing conversion of reduced to oxidized forms of UQ, modulating the signal. However, in transgenic plants with increased or decreased AOX capacity, signaling would be dampened or amplified, respectively. The situation with SA is more complex. SA may be able to increase mitochondrial ROS by inhibiting the activity of the respiratory chain (Xie and Chen, 1999), although the mechanism of inhibition is unclear (Arrow “1?”). It may also increase mitochondrial ROS levels by other mechanisms (Arrow “2?”). Hence, two possible routes, neither of which are mutually exclusive, leading from SA to ROS, are shown. Additionally, SA, but not AA, can increase expression of RdRp1 (which has been shown to enhance resistance to the virus; Xie et al., 2001). However, other AOX-independent mechanisms of TMV resistance induction are not ruled out by this study (Arrow “3?”).
The results presented in this paper are consistent with the hypothesis that our attempts, and those of Ordog and colleagues (2002), to assess the effect of AOX on SA-induced resistance to systemic infection with TMV were confounded by the induction of one or more resistance mechanisms that are inducible by SA but not by antimycin A. Our rationale for suggesting that NtRdRp1 is potentially responsible for masking any inhibitory effect of increased AOX on SA-induced resistance (as seen for antimycin A-induced resistance) is that this enzyme is SA-inducible and is known to impede TMV accumulation (Xie et al., 2001). Furthermore, our data (Fig. 7) shows that NtRdRp1 is not induced by AA (and therefore cannot be influenced by AOX), which would explain why the inhibitory effect of enhanced AP capacity on AA-induced resistance is apparent (Figs. 3 and 4). Our model suggests that SA can simultaneously induce multiple antiviral mechanisms regulated by more than one signaling process, as illustrated diagrammatically in Figure 8.
The results obtained with the Aox-transgenic plants suggest that AOX is regulating a signal or signals. If this is the case, what is the nature of these putative AOX-regulated signal or signals? AOX is increasingly being viewed as a potentially important homeostatic regulator, for example, by allowing for flexible and rapid adaptation of mitochondrial respiratory efficiency (Moore et al., 2002) or regulation of the redox status of electron transport components (Robson and Vanlerberghe, 2002). However, an important physiological role for AOX resides in the minimization of ROS levels in the mitochondria (Wagner and Moore, 1997; Maxwell et al., 1999; Yip and Vanlerberghe, 2001). Restriction of electron flow through the CYT by AA causes a build up of reducing power in the electron transport components, which in turn will enhance the rate of generation of ROS in the mitochondrion (Maxwell et al., 1999). It is known that ROS act as signals at a number of points in defensive signal transduction and that they can influence the expression of a wide range of plant genes (Bolwell and Wojtaszek, 1997; Desikan et al., 2001; Mittler, 2002; Neill et al., 2002). These may include those controlling certain aspects of induced resistance to TMV. However, the most intensively studied ROS production phenomenon is the oxidative burst that occurs at the cell periphery early on during a defense response, rather than ROS accumulation in the mitochondrion. Nonetheless, the level of ROS accumulation in mitochondria does influence plant nuclear gene expression (Maxwell et al., 2002).
SA can also enhance the production of ROS (for review, see Alvarez, 2000) possibly by inhibiting enzyme targets within the cytosol, such as catalase (Chen et al., 1993) and ascorbate peroxidase (Durner and Klessig, 1995), or a recently discovered carbonic anhydrase within the chloroplast (Slaymaker et al., 2002). There is also evidence indicating that SA can inhibit respiration in the mitochondrion within minutes of administration (Xie and Chen, 1999; A. Gilliland, unpublished data), an effect that might also increase ROS production within that organelle. The mechanism of respiratory inhibition by SA is not well understood, although it has been suggested to involve loss of cytochrome c from the mitochondria (Robson and Vanlerberghe, 2002). However, cytochrome c loss was only seen after incubation of cultured cells for 8 h in the presence of 0.5 mm SA (Robson and Vanlerberghe, 2002), a much longer time frame than that needed to see respiratory inhibition (Xie and Chen, 1999; A. Gilliland, unpublished data). Nonetheless, the increased sensitivity of transgenic plants with decreased AP pathway capacities to the resistance-inducing activity of SA (Fig. 5) could be explained by the impaired ability of these plants to dissipate ROS in the mitochondria. Whether these ROS are generated as a result of inhibition of respiration or as a result of other SA-induced mechanisms remains to be seen.
Thus, we hypothesize that the accumulation of ROS in the mitochondrion is most likely to be the resistance-inducing signal under the control of AOX (Fig. 8). In this model, an increased level of AOX (and, therefore, an increased level of alternative respiratory capacity) would damp ROS production and, therefore, signal generation. This would be consistent with the data shown in Figures 3 and 4. Conversely, the increased sensitivity to AA and SA seen in plants with decreased AP capacities (Figs. 4 and 5) can be explained by less effective dissipation of ROS due to less effective oxidation of the UQ pool.
Although this proposed mechanism might go some way to explaining how AOX could function in the induction of some aspects of resistance to TMV, it does leave some key questions unanswered. For example, if increasing the level of AOX in transgenic plants “damps” signaling by inhibiting ROS build up in the mitochondria, why is Aox gene expression transiently enhanced in plants treated with resistance-inducing chemicals (for example, see Wong et al., 2002)? Similarly, if decreasing the AP capacity in transgenic plants causes an increase in sensitivity to resistance-inducing chemicals, how does the suppression of Aox gene induction by CMV apparently help that particular virus evade SA-induced resistance in directly inoculated tissue, as observed by Ji and Ding (2001)? Both of these observations imply that increased expression of Aox is important for resistance to be successful. One possibility is that, after the induction of resistance, the transient increase in Aox gene expression acts to reset the signaling mechanism, whereas another possibility is that it is necessary for the re-establishment of normal mitochondrial homeostasis, an important function of AOX (Sakano, 2001; Moore et al., 2002). If this resetting does not occur, it may interfere with the further operation of the defensive signaling network and prejudice the plant's ability to respond to secondary pathogen challenges or other stresses.
It appears that although a relationship between AOX and chemically induced resistance to viruses exists, it is more complex than originally envisaged. Thus, increasing or decreasing AP capacity can, respectively, impede or enhance antimycin A-induced resistance to TMV. However, SA-induced resistance is only affected in the directly inoculated leaves of transgenic plants with decreased AP capacity. Furthermore, the alterations in AP capacity (at least to the extent achievable in transgenic plants) did not alter the overall response of the plants to systemic disease with or without prior SA treatment (this study and Ordog et al., 2002). Our interpretation of these results is that in the case of antimycin A-induced resistance, this chemical triggers the production of increased levels of ROS from the over-reduction of electron transport chain components, and these ROS can function as signals that are indirectly regulated by AOX. In the case of SA-induced resistance, increasing ROS in the mitochondria is only one of several potential signaling mechanisms that could be activated by this chemical. Therefore, we conclude that SA-induced resistance to TMV involves multiple resistance mechanisms, some of which are subject to regulation by AOX.
MATERIALS AND METHODS
Plant Growth Conditions
Seeds of tobacco (Nicotiana tabacum) cultivar Xanthi (nn genotype, TMV susceptible) and of the transgenic lines were germinated under sterile conditions on 1% (w/v) agar containing 1× Murashige and Skoog medium (Melford, Ipswich, UK). For germination of transgenic seed, the medium was supplemented with hygromycin B (Melford) to a concentration of 100 μg mL–1. After transfer to soil, all plants were maintained under greenhouse conditions with supplementary lighting in winter. Chemically treated and virus-inoculated plants were also maintained under greenhouse conditions.
Construction of Transgenic Plants
A cDNA encoding AOX1a from Bright Yellow tobacco (Vanlerberghe and McIntosh, 1994) was made available (gift of L. McIntosh, Michigan State University) and used for Agrobacterium tumefaciens-mediated transformation of cv Xanthi tobacco, a cultivar that is normally susceptible to infection by TMV. The cDNA was inserted in sense or antisense orientation behind the enhanced 35S promoter of Cauliflower mosaic virus of the expression cassette of pFF19 (Timmermans et al., 1990). The sense and antisense Aox gene expression cassettes were subcloned into the plant transformation vector pGPTV-HPT (Becker et al., 1992) to yield pDJSn and pDJAnt, respectively. pDJSn and pDJAnt were introduced into A. tumefaciens LBA4404 by electroporation (Shen and Forde, 1989) using a Gene Pulser apparatus (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. A. tumefaciens lines harboring pDJSn and pDJAnt were used for tobacco leaf disc transformation (Horsch et al., 1985). Primary transformants were selected on hygromycin B (Becker et al., 1992) before transfer to soil. Upon request, these plant lines will be made available in a timely manner for noncommercial research purposes subject to a Cambridge University materials transfer agreement.
Alternative Pathway Capacity Measurements and Characterization of the Transgenic Tobacco Lines
The AP capacity of plant lines was measured by obtaining cells (with cell walls still intact) from leaf strips using 0.5% (w/v) macerase (Macerozyme R-10; Yakult, Tokyo) in 0.7 m mannitol. This cell production procedure was based on the first step of a published protoplast purification method (Carr et al., 1994; Murphy and Carr, 2002). Cells were transferred to a Clarke-type oxygen electrode (Digital model 10; Rank Brothers, Cambridge, UK). Measurements of oxygen consumption were performed in the absence or presence of 20 μm AA or 1 mm cyanide (cytochrome path inhibitors); 2 mm SHAM or 10 μm n-propylgallate (AOX inhibitors); in the presence of both types of inhibitor, or with 0.2 μm FCCP. Measurements were carried out in the dark to prevent photosynthetic oxygen production. For each line examined, at least three sets of triplicate measurements were carried out to have statistically valid comparisons of AP capacity between nontransgenic and transgenic plant lines.
AOX protein and Aox transcript steady-state levels were assessed by western immunoblot and northern-blot analysis, respectively, using previously described methods (Chivasa and Carr, 1998; Chivasa et al., 1999). However, in this study, binding of the monoclonal anti-AOX antibody (Elthon et al., 1989) was detected using an anti-mouse secondary antibody conjugated to HRP and binding was visualized on x-ray film using a chemiluminescent HRP substrate (“western Lightning”; New England Nuclear, Boston).
Assessment of Plant Susceptibility to Systemic TMV-Induced Disease with and without Prior Treatment with SA
Groups (consisting of 14 or 21 individuals) of nontransgenic and transgenic plants were used for experiments at between 3 (in the summer) and 5 (in the winter) weeks after transfer to soil. Plants were between 5- and 7-weeks-old when used. The foliage was sprayed until runoff with 1 mm SA or water daily for 3 or 5 d before inoculation with TMV strain U1. TMV (2 μg mL–1 in water) was inoculated onto one lower leaf per plant using a cotton bud soaked in the virus suspension. To enhance the efficiency of infection, the leaves destined for inoculation with TMV were sprinkled with carborundum before application of the virus suspension. Thereafter, plants were checked daily for the appearance of TMV-induced systemic disease symptoms (chlorosis, vein clearing, and/or mosaic on upper, noninoculated leaves).
Detection of TMV Accumulation in Directly Inoculated, Chemically Treated and Untreated Tobacco Leaves
Expanded leaves of 5- to 7-week-old transgenic and nontransgenic tobacco plants were infiltrated with water or solutions of SA or AA (Sigma Chemical, St. Louis) using a 10-mL hypodermic syringe without a needle pressed against the lower surface of the leaf. The method is similar to that used for agroinfiltration (Schob et al., 1997). It should be noted that because AA was initially dissolved in a small volume of ethanol or isopropanol, a proportionate amount of solvent was added to the water and SA treatments. Ethanol was also used for the initial dissolution of SA. Immediately after infiltration, leaves were inoculated uniformly over their upper surfaces using a gauze pad soaked in TMV suspension (10 μg mL–1). Carborundum was sprinkled on leaf surfaces before inoculation to enhance the efficiency of infection. Note that the leaves were not detached from the plants during the course of the experiments.
At various times after infiltration and inoculation, samples were collected by punching out 12-mm discs of leaf tissue using a cork borer. For each treatment and line, between one and three plants were used and two leaves per plant were infiltrated and inoculated with TMV. Depending upon the experiment, between four and six discs were sampled per leaf. Leaf discs were pooled and soluble proteins were extracted for subsequent immunoblotting analysis of TMV CP accumulation using a rabbit polyclonal anti-TMV serum (Chivasa et al., 1997). In some cases, proteins were also analyzed by immunoblotting with a rabbit polyclonal anti-PR1 serum as previously described (Carr et al., 1987; Chivasa et al., 1997). Equal loading of gels with protein was checked by staining of immunoblots with 0.1% (w/v) Ponceau red and/or probing or reprobing of blots with a polyclonal rabbit antiserum raised against the LSU of RuBPCase (Berry et al., 1985; Carr et al., 1987). Primary antibody binding for anti-TMV CP and anti-LSU was detected with a secondary anti-rabbit IgG conjugated to HRP and visualized using chemiluminescence. In some experiments, band intensities were measured using a densitometer (Molecular Dynamics, Sunnyvale, CA) and ImageQuant 3.0 software (Amersham-Pharmacia, Chesham, UK). Experiments were carried out at least three times unless otherwise stated.
Detection of NtRdRp1 Gene Expression
Steady-state accumulation of the transcript for NtRdRp (Xie et al., 2001) was detected by primer extension (Calzone et al., 1987; Boorstein and Craig, 1989) with the oligonucleotide primer TCTTTACTTCCCAACACTGC end-labeled using γ-[32P] ATP and T4 polynucleotide kinase (Ambion, Austin, TX). After annealing the labeled primer to total RNA extracted from leaf tissue (Berry et al., 1985), the extension reaction was carried out using Avian myoblastosis virus reverse transcriptase. The reaction products were analyzed on an 8% (w/v) acrylamide sequencing gel and visualized using a Molecular Dynamics Typhoon 8600 phosphorimager system (Amersham-Pharmacia).
Acknowledgments
We thank Tom Elthon for monoclonal anti-AOX antibody, Mike Wilson for anti-TMV CP serum, and Jim Murray for pGPTV-HPT. We also thank Tony Moore and Steve Chivasa for useful discussions and advice, and Catherine Carr for plant care.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017640.
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) with additional support from the Leverhulme Trust. J.M.H. was supported by a BBSRC Studentship.
References
- Affourtit C, Albury MSW, Crichton PG, Moore AL (2002) Exploring the molecular nature of alternative oxidase regulation and catalysis. FEBS Lett 510: 121–126 [DOI] [PubMed] [Google Scholar]
- Affourtit C, Krab K, Moore AL (2001) Control of plant mitochondrial respiration. BBA-Bioenergetics 1504: 58–69 [DOI] [PubMed] [Google Scholar]
- Ahlquist P (2002) RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296: 1270–1273 [DOI] [PubMed] [Google Scholar]
- Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44: 429–442 [DOI] [PubMed] [Google Scholar]
- Antoniw JF, Kueh JSH, Walkey DGA, White RF (1981) The presence of pathogenesis-related proteins in callus of xanthi-nc tobacco. Phytopathologische Zeit 101: 179–184 [Google Scholar]
- Baulcombe DC (2001) RNA silencing: diced defence. Nature 409: 295–296 [DOI] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195–1197 [DOI] [PubMed] [Google Scholar]
- Béclin C, Berthome R, Palauqui JC, Tepfer M, Vaucheret H (1998) Infection of tobacco or Arabidopsis plants by CMV counteracts systemic post-transcriptional silencing of nonviral (trans) genes. Virology 252: 313–317 [DOI] [PubMed] [Google Scholar]
- Berry JO, Nikolau BJ, Carr JP, Klessig DF (1985) Transcriptional and post-transcriptional regulation of ribulose-1,5-bisphosphate carboxylase gene expression in light and dark grown amaranth cotyledons. Mol Cell Biol 5: 2238–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiol Mol Plant Pathol 51: 347–366 [Google Scholar]
- Boorstein WR, Craig EA (1989) Primer extension analysis of RNA. Methods Enzymol 180: 347–369 [DOI] [PubMed] [Google Scholar]
- Bowles DJ (1990) Defense-related proteins in higher plants. Annu Rev Biochem 59: 873–907 [DOI] [PubMed] [Google Scholar]
- Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC (1998) Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17: 6739–6746 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Calzone FJ, Britten RJ, Davidson EH (1987) Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol 152: 611–632 [DOI] [PubMed] [Google Scholar]
- Carr JP, Dixon DC, Nikolau BJ, Voelkerding KV, Klessig DF (1987) Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol 7: 1580–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JP, Gal-On A, Palukaitis P, Zaitlin M (1994) Replicase-mediated resistance to cucumber mosaic virus in transgenic plants involves suppression of both virus replication in the inoculated leaves and long-distance movement. Virology 199: 439–447 [DOI] [PubMed] [Google Scholar]
- Chen ZX, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Chivasa S, Berry JO, ap Rees T, Carr JP (1999) Changes in gene expression during development and thermogenesis in Arum. Aust J Plant Physiol 26: 391–399 [Google Scholar]
- Chivasa S, Carr JP (1998) Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell 10: 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Naylor M, Carr JP (1997) Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell 9: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutt JR, Harpster MH, Dixon DC, Carr JP, Dunsmuir P, Klessig DF (1989) Disease response to tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology 173: 89–97 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18: 547–575 [Google Scholar]
- Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Klessig DF (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2, 6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA 92: 11312–11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal antibodies to the alternative oxidase of higher-plant mitochondria. Plant Physiol 89: 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55: 77–84 [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329: 219–222 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Ji LH, Ding SW (2001) The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant-Microbe Interact 6: 715–724 [DOI] [PubMed] [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomme C, Roby D (1999) Identification of new early markers of the hypersensitive response in Arabidopsis thaliana. FEBS Lett 459: 149–153 [DOI] [PubMed] [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN (1997) The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol 115: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst HJ, Meuwissen RL, Kauffmann S, Bol JF (1989) Constitutive expression of pathogenesis-related proteins PR-1, GRP, and PR-S in tobacco has no effect on virus infection. Plant Cell 1: 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy AP, Guo HS, Li WX, Ding SW (2000) Suppression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J 19: 1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Nickels R, McIntosh L (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J 29: 269–279 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers CN, Palukaitis P, Carr JP (2000) Subcellular distribution analysis of the cucumber mosaic virus 2b protein. J Gen Virol 81: 219–226 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Moore AL, Albury MS, Chrichton PG, Affourtit C (2002) Function of the Alternative Oxidase: Is it still a scavenger? Trends Plant Sci 7: 478–471 [DOI] [PubMed] [Google Scholar]
- Moore AL, Siedow JN (1991) The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochem Biophys Acta 1059: 121–140 [DOI] [PubMed] [Google Scholar]
- Murphy AM, Carr JP (2002) Salicylic acid has cell-specific effects on tobacco mosaic virus replication and cell-to-cell movement. Plant Physiol 128: 552–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Chivasa S, Singh DP, Carr JP (1999) Salicylic acid-induced resistance to viruses and other pathogens: a parting of the ways? Trends Plant Sci 4: 155–160 [DOI] [PubMed] [Google Scholar]
- Murphy AM, Gilliland A, Wong CE, West J, Singh DP, Carr JP (2001). Signal transduction in resistance to plant viruses. Eur J Plant Pathol 107: 121–128 [Google Scholar]
- Naylor M, Murphy AM, Berry JO, Carr JP (1998) Salicylic acid can induce resistance to plant virus movement. Mol Plant-Microbe Interact 11: 860–868 [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53: 1237–1247 [PubMed] [Google Scholar]
- Oostendorp M, Kunz W, Dietrich B, Staub T (2001) Induced disease resistance in plants by chemicals. Eur J Plant Pathol 107: 19–28 [Google Scholar]
- Ordog SH, Higgins VJ, Vanlerberghe GC (2002) Mitochondrial alternative oxidase is not a critical component of plant viral resistance but may play a role in the hypersensitive response. Plant Physiol 129: 1858–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Ehman A, Melander WR, Meeuse BJD (1987) Salicylic acid: a natural inducer of heat production in Arum lilies. Science 237: 1601–1602 [DOI] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L (1992) Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell 4: 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Sweet CR, Lennon AM, Rauch GS, Siedow JN (1998) Regulation of the cyanide-resistant alternative oxidase of plant mitochondria: identification of the cysteine residue involved in α-keto acid stimulation and intersubunit disulfide bond formation. J Biol Chem 273: 30750–30756 [DOI] [PubMed] [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Giles L, Osmond CB, Siedow JN, Berry JA (1992) Measurement of the engagement of cyanide-resistant respiration in the crassulacean acid metabolism plant Kalanchoe daigremontiana with the use of on-line oxygen isotope discrimination. Plant Physiol 100: 1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson CA, Vanlerberghe GC (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol 129: 1908–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano K (2001) Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defense reaction and secondary metabolism. Int Rev Cytol 206: 1–44 [DOI] [PubMed] [Google Scholar]
- Schob H, Kunc C, Meins F (1997) Silencing of transgenes introduced into leaves by agroinfiltration: a simple, rapid method for investigating sequence requirements for gene silencing. Mol Gen Genet 256: 581–585 [DOI] [PubMed] [Google Scholar]
- Shen W-J, Forde BG (1989) Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res 17: 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, van Loon LC, Lambers H (1999) Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv. tomato. Plant Physiol 120: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF (2002) The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive response. Proc Natl Acad Sci USA 99: 11640–11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soards AJ, Murphy AM, Palukaitis P, Carr JP (2002) Virulence and differential local and systemic spread of Cucumber mosaic virus in tobacco are affected by the CMV 2b protein. Mol Plant-Microbe Interact 15: 647–653 [DOI] [PubMed] [Google Scholar]
- Timmermans MCP, Maliga P, Vieira J, Messing JC (1990) The pFF plasmids: cassettes utilizing CaMV sequences for expression of foreign genes in plants. J Biotechnol 14: 333–344 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1994) Mitochondrial electron transport regulation of nuclear gene expression-studies with the alternative oxidase gene of tobacco. Plant Physiol 105: 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L (1994) Molecular-genetic alteration of plants respiration: silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol 106: 1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55: 85–97 [Google Scholar]
- Voinnet O (2001) RNA silencing as a plant immune system against viruses. Trends Genet 17: 449–459 [DOI] [PubMed] [Google Scholar]
- Wagner AM, Moore AL (1997) Structure and function of the plant alternative oxidase: its putative role in the oxygen defence mechanism. Biosci Rep 17: 319–333 [DOI] [PubMed] [Google Scholar]
- Wong CE, Carson RAJ, Carr JP (2002) Chemically induced virus resistance in Arabidopsis thaliana is independent of pathogenesis-related protein expression and the NPR1 gene. Mol Plant-Microbe Interact 15: 75–81 [DOI] [PubMed] [Google Scholar]
- Xie ZX, Chen ZX (1999) Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol 120: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZX, Fan BF, Chen CH, Chen ZX (2001) An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc Natl Acad Sci USA 98: 6516–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip JYH, Vanlerberghe GC (2001) Mitochondrial alternative oxidase acts to dampen the generation of active oxygen species during a period of rapid respiration induced to support a high rate of nutrient uptake. Physiol Planta 112: 327–333 [DOI] [PubMed] [Google Scholar]