Abstract
Light-mediated chloroplast movements are common in plants. When leaves of Alocasia brisbanensis (F.M. Bailey) Domin are exposed to dim light, mesophyll chloroplasts spread along the periclinal walls normal to the light, maximizing absorbance. Under high light, the chloroplasts move to anticlinal walls. It has been proposed that movement to the high-light position shortens the diffusion path for CO2 from the intercellular air spaces to the chloroplasts, thus reducing CO2 limitation of photosynthesis. To test this hypothesis, we used pulsed photoacoustics to measure oxygen diffusion times as a proxy for CO2 diffusion in leaf cells. We found no evidence that chloroplast movement to the high-light position enhanced gas diffusion. Times for oxygen diffusion were not shorter in leaves pretreated with white light, which induced chloroplast movement to the high-light position, compared with leaves pretreated with 500 to 700 nm light, which did not induce movement. From the oxygen diffusion time and the diffusion distance from chloroplasts to the intercellular gas space, we calculated an oxygen permeability of 2.25 × 10–6 cm2 s–1 for leaf cells at 20°C. When leaf temperature was varied from 5°C to 40°C, the permeability for oxygen increased between 5°C and 20°C but changed little between 20°C and 40°C, indicating changes in viscosity or other physical parameters of leaf cells above 20°C. Resistance for CO2 estimated from oxygen permeability was in good agreement with published values, validating photoacoustics as another way of assessing internal resistances to CO2 diffusion.
Light-mediated chloroplast movements in the leaves of some plants are so striking that they create patterns visible to the naked eye. They have attracted the attention of plant physiologists for more than a century. Chloroplast movements of all types have been the subject of numerous reviews (Britz, 1979; Haupt and Scheuerlein, 1990; Wada et al., 1993; Yatsuhashi, 1996; Haupt, 1999; Wada and Kagawa, 2001; Kagawa and Wada, 2002). In leaves, chloroplasts spread along the periclinal walls of mesophyll cells (the face position) in low light, whereas in high light, they move toward the anticlinal walls (the profile position), effectively forming cylinders of chloroplasts in palisade tissue. In darkness, the chloroplasts are generally in an intermediate position, although this varies among species (Inoue and Shibata, 1974) and depends on the growth environment (Trojan and Gabrys, 1996). Because of the optical sieve effect (Britz and Briggs, 1987), these marked chloroplast movements alter light absorption (Zurzycki, 1961), which, in many leaves, gives rise to the visible color changes that have attracted attention for so long.
Chloroplast movements are widespread in algae, mosses, ferns, and seed plants. Among seed plants, they are common in both monocots and dicots, and they occur in plants with widely differing leaf anatomy, from submerged aquatic plants (Zurzycki and Lelatko, 1969) to sclerophyllous evergreens (Del Hierro et al., 2000). Chloroplast movements in seed plants with multilayered leaves, the subject of the present study, have received less attention than movements in structurally simpler systems such as algae or fern gametophytes because microscopic observation is more difficult in leaves. However, in many leaves, changes in light absorption have been used to follow chloroplast movements because a decrease in light absorption accompanies movement to the profile position (Lelatko, 1970; Inoue and Shibata, 1974; Gabrys and Walczak, 1980; Brugnoli and Björkman, 1992). These absorption changes have been correlated with chloroplast movements observed in fixed tissue using microscopic techniques (Zurzycki, 1961; Trojan and Gabrys, 1996; Gorton et al., 1999). In addition, microscopy and microbeam irradiation have been used to study light-induced movement of individual chloroplasts in vacuum-infiltrated leaves of Arabidopsis (Kagawa and Wada, 2000).
Chloroplast movements in leaves are controlled by blue/UV photoreceptors in the phototropin family. Chloroplast movement to the face position under low light is mediated by phot1 and phot2, whereas movement to the profile position in high light appears to be mediated by phot2 alone (Briggs et al., 2001; Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001; Kagawa, 2003). Chloroplast movements in higher plants require actin and are inhibited by actin antagonists such as m-maleimidobenzoic acid N-hydroxysuccinimide ester and cytochalasin-D (Malec et al., 1996; Gorton et al., 1999). Calcium participates in signal transduction for high- and low-light chloroplast movements, with internal stores of calcium likely to be more important than calcium flux across the plasmalemma (Tlalka and Gabrys, 1993; Tlalka and Fricker, 1999; Stoelzle et al., 2003).
Intracellular motility, including chloroplast movements, requires ATP-driven motor proteins and is energetically expensive (Slesak and Gabrys, 1996). There has been considerable speculation about the adaptive value of light-mediated chloroplast movements. Under light-limiting conditions, photosynthesis can be increased as chloroplasts move to the low-light position along periclinal walls, where they are perpendicular to the incident light and absorption is maximized (Zurzycki, 1955; Lechowski, 1974; Brugnoli and Björkman, 1992). Under high-light conditions, when chloroplasts move to anticlinal walls, a photosynthetic gain has been more difficult to demonstrate (Lechowski, 1974). Movement to the high-light position can provide some protection from photodamage (Zurzycki, 1957; Brugnoli and Björkman, 1992; Park et al., 1996; Jeong et al., 2002; Kasahara et al., 2002). In addition, movement to the high-light position allows light to penetrate further into the leaf and may relieve light limitation to photosynthesis in deeper cell layers (Brugnoli and Björkman, 1992; Terashima and Hikosaka, 1995).
An alternative hypothesis focuses not on light utilization, but on CO2 uptake. Considering that the low-light position increases light absorption when light is limiting, chloroplast movement to the high-light position might increase CO2 uptake when light is abundant and CO2 has become limiting. To enter a leaf, CO2 must diffuse through stomata, a path governed largely by stomatal resistance (rs). Within the leaf, CO2 must diffuse from the substomatal cavity to the site of carboxylation within the chloroplasts. Resistance to CO2 diffusion from the substomatal cavity to the sites of carboxylation, known as transfer resistance (rw), is largely dependent on leaf anatomy. rw has two parts, resistance to CO2 diffusion in the gas phase from the substomatal cavity to the mesophyll wall (rias) and resistance to CO2 diffusion in the liquid phase from the film of water on the mesophyll cell wall to the site of carboxylation in the chloroplast stroma (rliq).
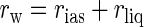 |
(1) |
(Evans and Von Caemmerer, 1996)
Usually rliq is much larger than rias, and whereas chloroplast movement is unlikely to influence rs or rias, it could enhance gas diffusion in the liquid phase. Gas diffusion in the liquid phase is influenced by two factors, both of which might potentially be altered by chloroplast movement: the surface area of chloroplasts exposed to intracellular air spaces (Sc) and the resistance to diffusion per unit area of exposed chloroplast surface (r'liq; see “Discussion”).
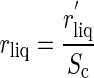 |
(2) |
It is possible to assess rliq using a pulsed photoacoustic technique, an experimental approach we have used in this study.
Photoacoustic methods observe leaf photosynthesis as pressure waves caused by the conversion of absorbed light to heat and by the evolution of oxygen at photosystem II (for review, see Malkin, 1994; Herbert et al., 2000). Pulsed photoacoustics uses a short pulse of light to generate a single complex pressure wave to which heat and oxygen contribute (Canaani et al., 1988; Mauzerall, 1990). The thermal photoacoustic signal arises from conversion of absorbed light to heat in photosystems. This is followed by thermal diffusion of the heat to the internal gas space of the leaf, thermal expansion of the gas, and propagation of the signal as a pressure wave to the microphone detector (for review, see Buschmann and Prehn, 1990). The oxygen signal arises from oxygen evolution at photosystem II, chemical diffusion of the oxygen to the internal gas spaces of the leaf, and propagation of the signal to the microphone detector as a pressure wave. Thermal diffusion through cytoplasm and water is much faster than chemical diffusion, so the thermal signal precedes the oxygen signal to the detector, though the two waves overlap. The thermal and oxygen signals may be separated by deconvolution (Kolbowski et al., 1990; Tabrizi et al., 1998), but a simpler expedient is that of using background light to suppress the oxygen signal (Bults et al., 1982). Background light that saturates oxygen evolution abolishes the oxygen signal because the measuring pulse generates no further oxygen. The thermal signal remains, although it may be elevated by the loss of photochemical energy storage (Bults et al., 1982). Subtracting the photoacoustic signal obtained in the presence of saturating background light from that obtained in its absence yields a pure oxygen signal, reduced somewhat by the energy-storage effect. If the measuring pulse is of high intensity, the oxygen signal is maximized and energy storage is minimized, reducing the error of isolating the oxygen signal in this way to a few percent of the amplitude of the signal. The kinetics of the oxygen signal are not altered by the energy storage effect.
The main goal of this study was to use pulsed photoacoustics to test the hypothesis that chloroplast movement to the high-light position would decrease the time for oxygen diffusion from chloroplasts, consistent with enhanced CO2 diffusion to chloroplasts under high-light conditions. Our experimental plant, Alocasia brisbanensis (F.M. Bailey) Domin3, was a tropical understory plant with striking chloroplast movements that have been characterized in detail (Gorton et al., 1999). An additional goal of this study was to evaluate the pulsed photoacoustic technique as a method for estimating rliq, the main component of rw, which is difficult to measure by other methods (Evans and von Caemmerer, 1996).
RESULTS
Calculating the Oxygen Signal from the Microphone Signal
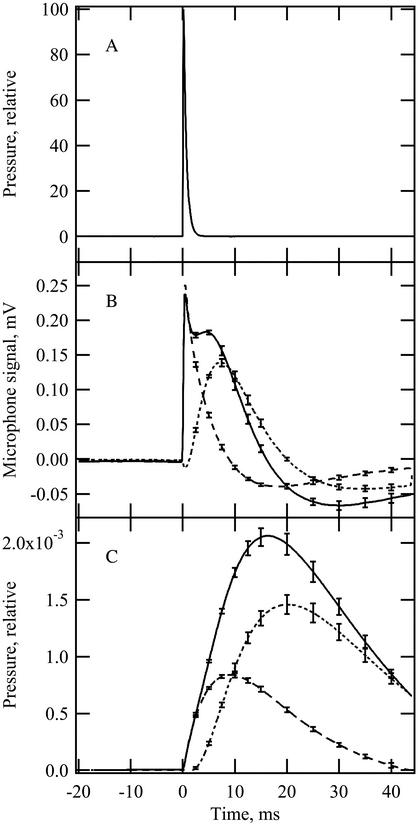
In a gas phase photoacoustic cell, irradiating a leaf sample with a short pulse of light produces a pressure wave that is detected by the microphone. The microphone signal (Fig. 1A) is the time derivative of the pressure wave (Mauzerall, 1990) and contains thermal and oxygen-evolution components. In our experiments, the two components were separated by applying continuous saturating background light such that measuring pulses caused no further oxygen evolution and the photoacoustic signal consisted of the thermal component only (Bults et al., 1982). The oxygen signal was determined by simply subtracting the total signal in the presence of background light from the total signal in the absence of background light (Fig. 1B). To measure diffusion times, the microphone signals were converted to their corresponding pressure waves (Fig. 1C).
Figure 1.
Measuring light pulse (A), microphone signals (B), and the calculated pressure waves for the photoacoustic thermal and oxygen signals (C) when a leaf disc was irradiated with the measuring light pulse. Signals in B and C were induced in the presence of continuous background light at 400 μmol photons m–2 s–1 (dashed line; thermal signal only) or in the absence of background light (solid line; thermal and oxygen signals together). The dotted lines (B and C) represent the calculated oxygen signal. Each sample was signal averaged for 64 flashes. Curves shown are averages for five samples at 20°C. Error bars represent se.
A strong light pulse was used to generate photoacoustic signals in all experiments. Using a strong light pulse saturated photosynthesis and produced the maximum oxygen signal with very low rates of photochemical energy storage because energy storage is proportional to quantum yield (for review, see Herbert et al., 2000). Under these conditions, adding background light to suppress the oxygen signal caused minimal changes in the thermal signal that result from energy storage and cause an error in calculation of the oxygen signal. The small negative excursion exhibited by the oxygen signal in Figure 1B shows the remaining error caused by energy storage in our experiments. This error decreased the calculated amplitude of the oxygen signal by a few percent, but did not influence its kinetics and was therefore ignored in all calculations.
Determining the Saturation Level for Background Irradiation
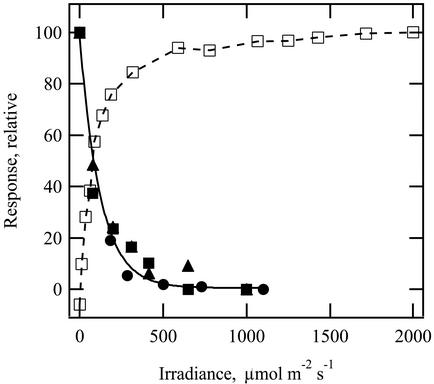
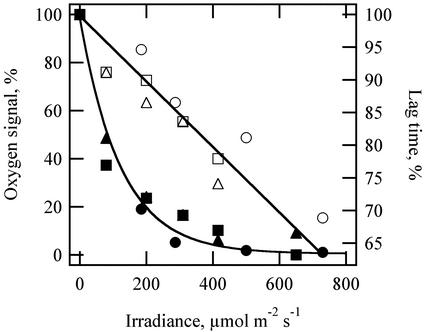
The photoacoustic oxygen signal, measured as the integrated area under the oxygen pressure curve, dropped to a negligible level when continuous background light was increased above 400 μmol m–2 s–1 (Fig. 2; black symbols). These data are in agreement with those for photosynthetic CO2 assimilation, which show saturation over a similar irradiance range (Fig. 2; white squares). Thus, a continuous background irradiance of 400 μmol m–2 s–1 was used to suppress the oxygen signal in all other experiments.
Figure 2.
Effect of varying the continuous background irradiance on amplitude of the oxygen pressure wave (black symbols with exponential fit). Black symbols are a scatter plot of three replicate experiments, all at 20°C. For each, the highest irradiance was 1,000 to 1,100 μmol m–2 s–1. White squares represent a photosynthetic light curve for CO2 assimilation obtained at 20°C to 22°C using conventional gas exchange techniques. Ambient CO2 concentration was 375 ± 3 μL L–1. Data are normalized for comparison.
Microscopic Observations of Chloroplast Movements
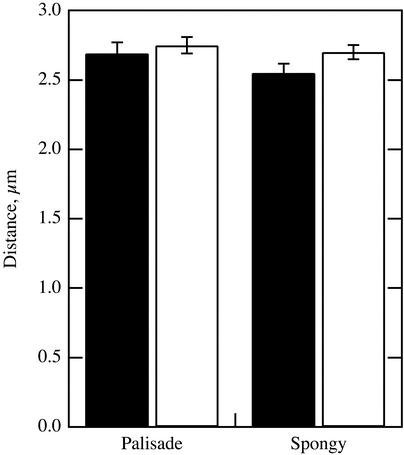
Distances from chloroplast centers to the nearest air space were measured microscopically in sections of fixed leaf tissue with chloroplasts in the low- and high-light positions (Fig. 3). The measurements were made in palisade and spongy mesophyll cells. In neither cell type was there a significant difference in the mean distance for the low- and high-light chloroplast positions (t test, P > 0.05).
Figure 3.
Diffusion distance measured microscopically between the centers of chloroplasts and the nearest air space. Measurements were made on thin sections from leaves with chloroplasts in the low-light position (black bars) and in the high-light position (white bars). Data are shown for chloroplasts in palisade and spongy mesophyll. n = 57–64. Error bars represent se.
Propagation of Thermal and Oxygen Pressure Waves from Chloroplasts to the Microphone
Movement of the thermal and oxygen signals from the photosystems in which they originate to the liquid-gas interface at the surfaces of leaf mesophyll cells depends on diffusion. The time required for diffusion can be modeled by a standard equation derived from Fick's second law:
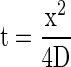 |
(3) |
This is a one-dimensional diffusion model treating the diffusion of a planar front of a substance along one axis, appropriate to represent diffusion between chloroplasts and the neighboring air space. D is the diffusion coefficient and x is the distance at which the concentration of the substance is 1/e (37%) of its concentration at the origin at time t.
Diffusion equations like Equation 3 are relevant in pulsed photoacoustic work (Mauzerall, 1990). For example, the lag time (t) between the light pulse and photoacoustic detection of the thermal pressure wave is determined by a thermal diffusion coefficient (Dth) and the distance (x) that the heat travels from the chloroplast to the intercellular air space, from which point the signal propagates as a pressure wave through the gas phase to the microphone. Equation 3 also holds for gas diffusion within a given medium, but when gases must diffuse between media, for example, between the liquid phase and the gas phase inside a leaf, the rate of diffusion will also depend on the solubility of the gas within the media. Therefore, for oxygen, Equation 3 becomes:
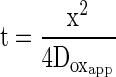 |
(4) |
Doxapp is an apparent diffusion coefficient for oxygen, and it is determined by the diffusion coefficients for oxygen in all the components of the liquid phase (including wall, plasma membrane, cytosol, and chloroplast) and by partition coefficients reflecting the ratio of the concentration in the various components of the liquid phase to the concentration in the gas phase (Nobel, 1999).
Distance “x” as well as diffusion coefficients Dth and Doxapp may be measured or estimated. Thus, it is possible to calculate the theoretical lag time between the light pulse and onset of the thermal pressure wave. Using the thermal diffusion coefficient for water, 1.44 × 10–3 cm2 s–1, which should be close to the thermal diffusion coefficient for the cytosol (Poulet et al., 1983), and the measured distance between the center of the chloroplasts and the intercellular air space in A. brisbanensis of 2.75 μm (Fig. 3), the predicted delay between the flash and observation of the thermal signal is 0.013 ms. This value is much shorter than the 50% rise time of 2 ms observed in our experiments (Fig. 1C), indicating that most of the observed delay results from propagation of the thermal acoustic signal through the gas phase to the microphone and the time required for the microphone response, both of which can vary in photoacoustic cells of different design. This delay would be the same for thermal and oxygen signals, in which case the thermal wave can be used as an internal reference for timing of oxygen diffusion. Thus, the time for the migration of oxygen from the chloroplasts to the intercellular air spaces can be approximated by the lag between the 50% rise times of the thermal and oxygen pressure waves (Mauzerall, 1990). This can be expressed as:
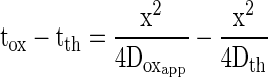 |
(5) |
and
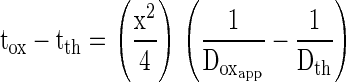 |
(6) |
where tox is the 50% rise time for oxygen pressure wave, tth is the 50% rise time for thermal pressure wave, Doxapp is the apparent diffusion coefficient for oxygen, and Dth is the thermal diffusion coefficient.
Empirical Measurement of the Apparent Diffusion Coefficient for Oxygen in Plant Cells
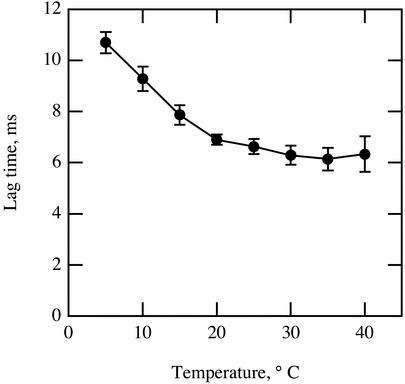
If their water content is high, biological materials often exhibit thermal diffusion properties similar to those of pure water (Bhavaraju et al., 2001). Given that the chloroplast, cytosol, and apoplastic solution are composed mostly of water, the value for Dth should be close to that of pure water and not strongly affected by temperature. On the other hand, chemical diffusion of oxygen and other small molecules within a liquid medium is affected by temperature directly and through effects on solubility and viscosity. For A. brisbanensis, as temperature increased from 5°C to 40°C, the lag time between the thermal and oxygen pressure waves decreased from 11 to 6 ms, with most change occurring between 5°C and 20°C (Fig. 4).
Figure 4.
Effect of temperature on the lag between the half-rise time for the thermal pressure wave and that for the oxygen pressure wave. n = 5 for each point. Error bars represent se.
The chloroplast stroma contains 250 to 500 mg mL–1 dissolved proteins (Jensen and Bahr, 1977), which would increase viscosity. For this reason, the oxygen diffusion coefficient for the path from the chloroplast to the outer cell wall should be significantly lower than for a similar path through pure water. Given the physical complexity of the chloroplast, cytosol, cell membrane, and cell wall, it would be difficult to measure the physical constants needed to calculate a value for the overall oxygen diffusion coefficient and partition coefficient from first principles. On the other hand, an empirically derived value for an overall apparent oxygen diffusion coefficient is easily calculated from photoacoustic measurements of the oxygen evolution lag time and the measured distance between the chloroplasts and intercellular air space. From Equation 6, the apparent oxygen diffusion coefficient is:
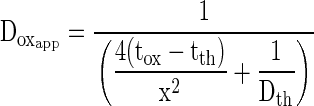 |
(7) |
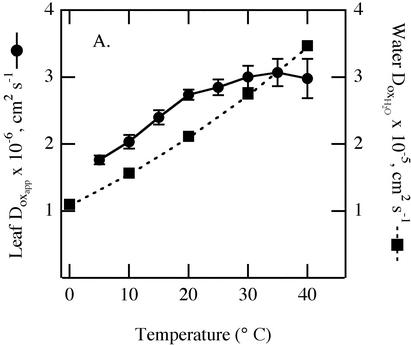
Using the thermal diffusion coefficient for water and a measured distance of 2.75 μm for the migration path of oxygen from the chloroplast to the intercellular air space, experimentally derived values for Doxapp were calculated for different temperatures (Fig. 5). Calculated values for Dox in pure water (Wilke-Chang equation, Poling et al., 2001) are about an order of magnitude smaller and are plotted on the same graph for comparison. Apparent oxygen diffusion coefficients for the leaf samples ranged from 1.8 to 3.0 × 10–6 cm2 s–1and were lowest at 5°C. Values increased with temperature up to 20°C but, unlike for water, the temperature effect became weak in leaf samples above 20°C.
Figure 5.
Effect of temperature on apparent oxygen diffusion coefficients in leaf samples calculated from data in Figure 4 using Equation 7 (•). The oxygen diffusion coefficient of pure water are shown for comparison (▪). y axis scales for Doxapp for the leaf and DoxH2O for water differ by 10-fold.
The Effect of Temperature on Oxygen Lag Time
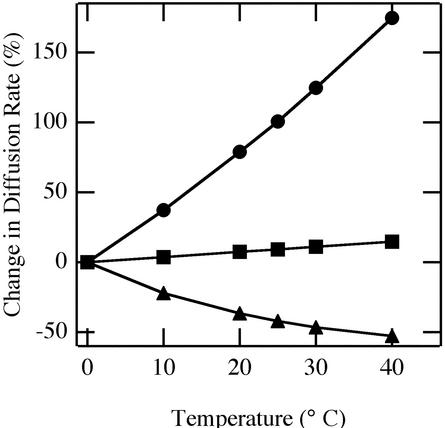
We can ask what the most important cause of the temperature dependence of Doxapp might be. Candidates include the direct effect of temperature on molecular motion, temperature effects on viscosity, and temperature effects on oxygen solubility. Diffusion is directly proportional to temperature (°K) and solubility, but is inversely proportional to viscosity. Effects of temperature on oxygen solubility in water (Battino and Clever, 1966) and on viscosity of water (Perry, 1950) are known, so we can estimate the contributions to all three factors on oxygen diffusion rate in water. Temperature-mediated viscosity changes would have the greatest effect on oxygen diffusion in pure water (Fig. 6). We can expect a similar predominance of viscosity in leaf cells where, in contrast to water, temperature effects caused a nonproportional effect on diffusion rate.
Figure 6.
Calculated direct and indirect effects of temperature on oxygen diffusion rate in water. Data are expressed as percentage change from the rate at 0°C. The direct effect of temperature on diffusion (squares) as well as indirect effects via changes in water viscosity (circles) and oxygen solubility in water (triangles) are shown.
The Effect of Constant Background Irradiance on Oxygen Lag Time
The intensity of background irradiance had an unexpected effect on oxygen diffusion. As the intensity of the background light was increased to the saturation level for oxygen evolution, the amplitude of the oxygen signal decreased predictably, but the lag time between the thermal pressure wave and the oxygen pressure wave also decreased (Fig. 7). In three separate experiments, the lag times between thermal and oxygen waves decreased by 20% to 25% as background irradiance increased from 0 to saturating irradiances above 400 μmol m–2 s–1.
Figure 7.
Effect of background light intensity on amplitude and lag time for the photoacoustic oxygen signal. Amplitudes for the oxygen signal are from Figure 2 (black symbols). Lag times between the thermal signal and the oxygen signal for the same three replicate experiments are also shown (white symbols). Data are normalized for comparison; maximum lag times varied between 7.1 and 8.4 ms for these experiments.
Chloroplast Movement, Stomatal Conductance, and Oxygen Diffusion
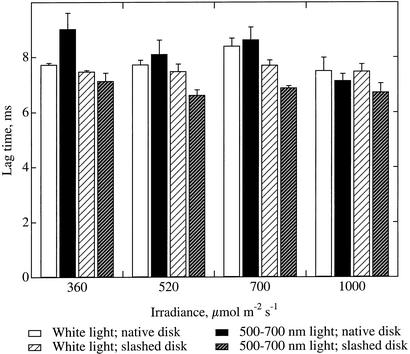
Chloroplasts move under bright white light but not under equivalent irradiances of 500 to 700 nm light (Gorton et al., 1999). Pretreating leaf samples with different irradiances of white light or 500 to 700 light allowed comparison of samples with chloroplasts in the profile or face positions, respectively. We found no consistent decrease in the diffusion times for oxygen between samples with chloroplasts in the profile position (Fig. 8, white bars) and samples with chloroplasts in the face position (Fig. 8, black bars).
Figure 8.
Effect of chloroplast movement on the lag time between the thermal and oxygen pressure waves. Chloroplasts were in the high-light position under white light but in the low-light position under 500 to 700 nm light. Data are shown for native discs and for discs with the lower epidermis slashed to eliminate possible stomatal limitations. n = 3; error bars represent se.
Stomatal opening is promoted by blue light and is mediated by the same phototropin photoreceptors as chloroplast movement (Zeiger and Field, 1982; Sakai et al., 2001). It was possible that leaf samples exposed to 500 to 700 nm light might have lower stomatal conductance than those receiving white, which could affect the photoacoustic oxygen signal. One report suggests that stomatal conductance has no effect on the photoacoustic oxygen signal in continuously modulated measurements (Bults et al., 1982), but stomatal conductance was not rigorously determined in those experiments. Light-induced changes in stomatal conductance could confound possible differences caused by simultaneous chloroplast movement. To evaluate this possibility, we made measurements on intact leaf discs, and then made multiple incisions through the lower epidermis to eliminate any stomatal limitation to gas diffusion, and repeated the measurements. Slashing the lower epidermis in this manner had no effect on the lag time for oxygen diffusion from leaf discs that had been under white light (t test, all irradiances taken together, P > 0.1, n = 12). Slashing decreased the lag time for leaf discs that had been under 500 to 700 nm light by an average of 17% (all irradiances together, P < 0.001, n = 12). Slashed discs as a group showed slightly longer lag times in the white light-treated group than in the 500 to 700 nm light-treated group (all irradiances together, P < 0.0001, n = 12), confirming that chloroplast movement induced by white light did not shorten diffusion times for oxygen.
DISCUSSION
Chloroplast Movement and Gas Diffusion
We expected that if chloroplast movement to the high-light, profile position decreased gas diffusion time, then the lag time for oxygen diffusion would be shorter for leaf discs treated with white light than for leaf discs treated with 500 to 700 nm light. We also expected that this difference would be greatest at higher irradiances, which produce the greatest chloroplast movement (Gorton et al., 1999). Contrary to our expectations, the lag time for oxygen diffusion was not significantly shorter for white-light treated discs than for discs treated with 500 to 700 nm light under any conditions. When there was a significant difference in lag time for discs treated with white light versus 500 to 700 nm light, as for the slashed discs, it was in the opposite direction from that predicted by the hypothesis (Fig. 8). Thus, there was no evidence that chloroplast movement to the high-light position caused a reduction in the time for oxygen diffusion from leaves. If oxygen diffusion from leaf tissue can be used as a proxy for CO2 diffusion to the site of carboxylation (Mauzerall, 1990 and further discussion below), we conclude that chloroplast movements do not significantly improve internal CO2 diffusion rates in A. brisbanensis. This conclusion is consistent with microscopic observations of chloroplasts in profile and face positions (Fig. 3). These observations showed no significant difference in the measured distances between the centers of chloroplasts and the nearest gas space, although the resolution of the microscopic measurements was insufficient to detect small movements that could affect gas diffusion.
Diffusion in the gas phase is 104 times faster than in the liquid phase, so liquid-phase resistances predominate in leaves (Evans, 1999) even though the path length for liquid-phase diffusion is shorter. Diffusion in the liquid phase is the parameter observed by pulsed photoacoustics. It is influenced by the composition and thickness of the cell wall, cell membrane, cytoplasm, and chloroplast, factors that affect r'liq, as well as by Sc (Laisk et al., 1970; Evans et al., 1994; Evans, 1999; Terashima et al., 2001). Chloroplast movement to the high-light position could enhance gas diffusion by allowing chloroplasts to be more closely appressed to the wall, which would decrease r'liq. Movement could also cause chloroplasts to become more flattened or to move from areas adjacent to neighboring cells to areas adjacent to intercellular spaces—both of which would increase Sc as well as decreasing the path length for gas diffusion and decreasing r'liq. However, changes in r'liq or Sc would alter the oxygen lag time, and no such alterations were observed in our photoacoustic data.
ses for oxygen lag times were on the order of 0.5 ms (Fig. 4). We can use this value to estimate the detection limit for measurement of diffusion distance with the pulsed photoacoustic technique. Using Equation 7 and our empirically derived apparent diffusion coefficient for oxygen at 20°C, the 0.5-ms detection limit for changes in oxygen lag time corresponds to a detection limit of less than 0.2 μm for changes in oxygen diffusion distance caused by chloroplast movement.
The thermal and oxygen pressure waves must propagate from the internal gas spaces of the leaf to the microphone detector via the cut edges of the sample, via open stomata, or both. Changes in the path of these pressure waves would affect the thermal and oxygen signals equally, not alter the lag between them. Thus, although stomatal closure may damp photoacoustic signals as they propagate from cell surfaces in a leaf to the detector, it should not alter the lag time between oxygen and thermal signals. However, there was evidence of stomatal influence on the lag time for oxygen diffusion from leaf discs. Discs treated with 500 to 700 nm light had significantly shorter oxygen lag times after they were slashed with a razor blade than in their native, unslashed condition (Fig. 8). This trend was not apparent for discs treated with white light, consistent with the greater rs known for leaves exposed to light without the blue portion of the spectrum (Zeiger and Field, 1982). Partial closure of stomata may have caused the layer of water on leaf cell surfaces to be thicker in the native, 500 to 700 nm-treated discs, lengthening the diffusion path. Partial infiltration of water into the cut edges of leaf discs would have had a similar effect. We noted that if the edges of the leaf discs became moist during light treatments, the apparent lag time for oxygen diffusion slowed and became less reproducible (data not shown). Additional experiments will be necessary to define how stomata can influence the photoacoustic oxygen signal differently from the photoacoustic thermal signal, as appears to have occurred in our experiments.
The lag times for oxygen diffusion were significantly shorter for discs treated with 500 to 700 nm light than for those that had been exposed to white light when possible stomatal effects were removed by slashing the lower epidermis. If this difference was caused by chloroplast movement, then chloroplast movement to the high-light position must interfere with gas diffusion rather than facilitate it. However, there are alternative explanations. For example, the white and 500 to 700 nm pretreatment irradiation may have caused down-regulation or photoinhibition of different populations of chloroplasts, such that different populations contributed to the oxygen signal during subsequent photoacoustic measurements.
Apparent Diffusion Coefficients
Because we measured the diffusion path from the center of the chloroplasts to the intercellular air space microscopically, we were able to calculate overall values for diffusion of oxygen along that path. These calculated values for Doxapp represent the entire pathway of oxygen diffusion from the site of oxygen evolution to the intercellular air space, including the cell wall, plasmalemma, cytoplasm, chloroplast envelope, and chloroplast stroma (Evans et al., 1994). Our measured value for Doxapp at 20°C of 2.74 × 10–6 cm2 s–1 is close to the 3.7 × 10–6 cm2 s–1 value calculated for tobacco (Nicotiana tabacum) leaves using modulated photoacoustics where it was assumed that the diffusion distance between the chloroplasts and intercellular air spaces was 1 μm (Poulet et al., 1983). Our measured value for Doxapp also falls within a broader range of values reported for animal tissues (Dutta and Popel, 1995).
Our Doxapp values were about an order of magnitude lower than the corresponding values for oxygen diffusion in pure water at any given temperature. This difference could be attributed to lower solubility of oxygen in the liquid phase of the leaf tissue relative to water, but is more likely attributable to greater viscosity of the plant's liquid phase. Oxygen solubility in tissue can actually be greater than in water. For example, oxygen solubility in frog sartorius muscle is 1.24 times higher than that in water at 22.8°C, a difference attributable to the much greater solubility of oxygen in lipid relative to water (Dutta and Popel, 1995). Thus, the most likely cause of the difference between our Doxapp and the Dox for water is the greater viscosity of leaf cells. This difference is not surprising given the high concentrations of dissolved proteins in the chloroplast stroma and the potential resistances imposed by cell walls. In addition to viscosity, collision with solid cellular components can slow diffusion through cells and tissues (Kao et al., 1993).
It is likely that decreased viscosity is the main factor contributing to the increase of Doxapp with temperature (Fig. 6). Solubility effects operate in the opposite direction. Direct effects of temperature are in the observed direction but cannot explain the bulk of the change. Our experimental temperatures increased by only 13%, from 278°K to 303°K, yet the lag times for oxygen diffusion decreased almost 50%, from 10.7 to 6.3 ms. Values of Doxapp leveled off at temperatures above 20°C, unlike Dox for pure water. This result might be caused by nonproportional interactions between temperature and the soluble proteins of the stroma, ionic strength, pH, or membrane fluidity, all of which can influence the bulk viscosity of the medium and thereby its conductance to oxygen diffusion. Permeability to oxygen in animal tissues also changes with temperature in a complex way that probably depends on enhanced oxygen solubility in membranes, phase transitions, and the three-dimensional structure of the membrane systems (Dutta and Popel, 1995). Recently, the effect of temperature on CO2 diffusion in leaf cells was also shown to differ from the known effect of temperature on CO2 diffusion in water (Bernacchi et al., 2002), consistent with our results for oxygen diffusion (Fig. 5).
Photoacoustic Measurements of rliq for CO2 Diffusion
Photoacoustics allows for a rapid assessment of factors that might influence gas diffusion in the liquid phase, in this case, chloroplast movement. One can also estimate rliq for CO2 diffusion from the Doxapp derived from photoacoustic data. Our Doxapp is 2.85 × 10–6 cm2 s–1 at 25°C. This value incorporates the actual diffusion coefficients and the partition coefficients that depend on solubility of the gas in the various components of the liquid phase. To find the equivalent value for CO2, we must consider the differential solubility of O2 and CO2 as well as their molecular weights. In a given medium, CO2 diffusion is a bit slower than oxygen diffusion because it is a larger molecule, but in water, the overriding factor is that CO2 solubility is so much greater than that of oxygen. Based on these theoretical considerations, overall permeability to CO2 is 21.1 times permeability for oxygen in the liquid phase of leaf tissue (Farquhar, 1983). A similar factor of about 20 describes the difference between CO2 and O2 permeability in a variety of animal tissue systems, as well (Dejours, 1975; Patton et al., 1989). Thus, the corresponding value for DCO2app is 21.1(2.85 × 10–6) = 60.2 × 10–6 cm2 s–1.
One can convert this diffusion coefficient and known diffusion distance to resistance:
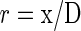 |
(8) |
We assume this resistance is entirely rliq, and that the lag between thermal and oxygen signals is solely due to the slower diffusion of oxygen than heat through the liquid phase. Given our measured diffusion distance of 2.75 μm and DCO2app of 60.2 × 10–6 cm2 s–1, Equation 8 gives a value of 4.6 s cm–1 for rliqCO2.
One can compare this value with an estimate of resistance for CO2 diffusion from known photosynthetic rates. Conductance and photosynthesis are related according to the following empirically derived relationship:
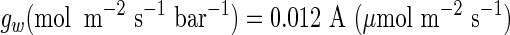 |
(9) |
(Evans and Von Caemmerer, 1996)
where gw is the transfer conductance from substomatal cavity to site of carboxylation and A is photosynthetic capacity measured at 1,000 μmol m–2 s–1, 350 μL L–1 Ca, and a temperature of 25°C. For our A. brisbanensis, A = 6.7 μmol m–2 s–1 (an average from five photosynthetic light curves on different days and with different plants). Calculating gw according to Equation 9, converting to resistance (rw = 1/gw) and changing units (Evans et al., 1994) gives rw = 5.0 s cm–1.
The photoacoustic technique assesses only resistance to diffusion in the liquid phase (rliq), whereas the estimate of resistance based on photosynthetic rate includes any internal resistance in the air space (rias) as well. A. brisbanensis is a loosely organized leaf tissue, therefore rias is likely to be small and rliq should approximate rw. We have a value of rliqCO2 of 4.6 s cm–1 determined from photoacoustics and a value for rwCO2 of 5.0 s cm–1. The two estimates are remarkably close, thus supporting the photoacoustic technique for assessing CO2 diffusion (and estimating rliqCO2) using oxygen as a proxy.
Other methods to assess rw for CO2 involve gas exchange in conjunction with isotopic techniques or chlorophyll fluorescence, and a variety of empirical and mathematical techniques have been used to distinguish rias and rliq, the two components of rw (Evans et al., 1994; Evans and von Caemmerer, 1996). Pulsed photoacoustics provides a rapid and simple addition to these approaches. Although photoacoustics does not provide information about rias, by using the timing of the thermal pressure wave as an internal standard, one can quickly and easily determine the timing of the oxygen pressure wave, which is related to rliq.
Observing Viscosity and Other Physical Parameters in Living Cells
Pulsed photoacoustics provides a new window on the physical properties of living plant cells. For example, a surprising finding in our study was the decrease in lag time for oxygen diffusion that occurred when continuous background irradiation was increased (Fig. 7). Increasing the background light may decrease the diffusion pathway by causing swelling of the chloroplasts and increased compression between the vacuole and cell wall, or perhaps by a cytoskeletal response causing the chloroplasts to flatten more tightly against the wall, exposing more surface area. Another possibility is that a strong electrochemical potential across the thylakoid membranes might decrease the viscosity within the chloroplast or increase the gas permeability of the membranes. Further exploitation of photoacoustics to study the effect of light and other environmental factors on oxygen diffusion will improve the view of the physiology in leaf cells and how it relates to their complex physical properties.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Alocasia brisbanensis (F.M. Bailey) Domin plants were grown from plants that were a gift from Dr. Robert Pearcy (University of California, Davis, CA). Plants were grown in a greenhouse under subdued daylight (100–400 μmol m–2 s–1) and the ambient photoperiod. Temperature ranged from 15°C to 20°C. Young fully expanded leaves were used for experiments.
Xenon Flash-Photoacoustic System
The pulsed photoacoustic detection system consisted of a xenon flash lamp (model FX 201; EG&G/Perkin Elmer, Santa Clara, CA) with associated charger (model PS-550AC; EG&G/Perkin Elmer), capacitor (3.0 μF 3.5 kV; CSI Technologies, East Dundee, IL), and trigger module (FYD-602; EG&G/Perkin Elmer). The 50% flash duration time was 0.5 ms, measured with a high-speed (1-ns rise time) photodetector (model DET 210; Thorlabs, Newton, NJ) fitted with a 550-nm interference filter (S40–550; Corion, Franklin, MA; Fig. 1A). The frequency of the flash-lamp discharge was controlled by a programmable waveform generator (model 154; Wavetek, Everett, WA) that sent impulses at selected intervals to the flash-lamp-control electronics. The xenon flash was routed through a Plexiglas rod (50 cm × 1.2 cm diameter) to a branched fiber optic cable assembly and then to a photoacoustic cell constructed as described previously (Fork and Herbert, 1991) except that the gas-permeable cell wall was replaced with a temperature control system consisting of a 2-mm-thick borosilicate window appressed to a double-cascade thermoelectric plate (Melcor, Trenton, NJ) controlled by a 5 Amp temperature controller (model LFI-3551; Wavelength Electronics, Bozeman, MT). By placing a leaf sample against the borosilicate window, it was possible to subject it to different temperatures during experiments after the sample was sealed in the photoacoustic cell. To minimize stray vibrational noise, the photoacoustic system was mounted on a floating optical table (Kinetics Systems, Chandler Heights, AZ). The detector in the photoacoustic cell consisted of a small microphone (type 1785; Knowles Electronics, Itasca, IL). Photoacoustic signals from the microphone were amplified 100-fold by a preamplifier (model 560; Stanford Research Systems, Sunnyvale, CA) with low- and high-pass filters set at 0.1 Hz and 3 kHz and were recorded with Scope (version 3.3.5) and a MacLab 2e recording system (AD Instruments, Milford, MA), which collected data points every 25 μs.
Experiments with Excised Leaf Tissue
Eight to 12 h before experiments, A. brisbanensis plants were placed in a darkened room to allow the chloroplasts to move to their low-light position along the periclinal cell walls (Gorton et al., 1999). For experiments, rectangular pieces of leaf about 4 cm by 5 cm were excised with a razor blade and placed on moistened paper towels under 360, 520, 700, or 1,000 μmol m–2s–1 of white or light 500 to 700 nm (yellow and red) light for 1 to 2 h. White light was obtained from metal halide lamps (MH400/C/U; Philips, Eindhoven, The Netherlands) and was filtered through 2 cm of flowing water and a hot mirror (OCLI, Santa Rosa, CA) to remove excess heat. Irradiance was adjusted by placing the leaf discs under metal-coated neutral-density filters. Chloroplasts moved to the high-light position along the anticlinal cell walls in leaf discs treated with white light, but not in discs treated with the same irradiance of 500 to 700 nm light, which was created with a filter combination previously described (Gorton et al., 1999).
Leaf discs (8-mm diameter) were taken with a cork borer from the light-treated leaf tissue immediately before inserting them into the cuvette for measurement. Keeping leaf discs on moistened paper towel resulted in minor infiltration at the edges of the disc, and this infiltration distorted the photoacoustic oxygen signals. Cutting discs from larger pieces of leaf tissue immediately before measurement prevented this problem.
For photoacoustic measurements, a leaf disc was placed in the photoacoustic cell such that the abaxial (lower) leaf surface faced the microphone detector. Saturating flashes of xenon light were supplied at 2 Hz and 64 sequential traces of the photoacoustic signal were averaged and recorded. To separate the oxygen component from the microphone signal, which contains thermal and oxygen components, the thermal component was measured in isolation by suppressing the oxygen evolution signal with saturating white background light (400 μmol m–2 s–1 except where noted), obtained from a type EKE halogen projector lamp operated by a regulated DC power supply. Subtraction of values in the presence of background light from values in its absence yielded the oxygen signal.
The propagation of photoacoustic signals out of the leaf mesophyll to the air in the photoacoustic cell could be influenced by stomatal opening. To bypass a potential stomatal constraint on the transmission of signals out of the leaf, in some experiments, numerous shallow incisions (25–35) were made through the abaxial epidermis with a razor blade to expose the mesophyll directly to the air in the photoacoustic cell.
Most experiments were done with the temperature of the photoacoustic cell set to 20°C. To ascertain how the thermal and oxygen photoacoustic signals were influenced by leaf temperature, measurements were made from 5°C to 40°C. A leaf disc was placed in the photoacoustic cell and measurements were made at 20°C. The temperature was increased in 5°C increments and the measurements were repeated, progressing to 40°C. Similar measurements were then made starting at 20°C and progressing to 5°C. Temperature equilibrium at each new temperature was reached within 1 min, permitting rapid progression through the temperature series.
Microscopy
To measure chloroplast position before and after movement, leaf samples were fixed and embedded for microscopy. Leaf discs (0.5-cm diameter) were vacuum infiltrated and fixed for 1 h in 2.5% (v/v) gluteraldehyde, pH 6.9. They were then dehydrated in a 10% (v/v) step series of ethanol and were embedded in LR White resin (London Resin Company, Reading, UK). Thin sections, 5 μm thick, were cut on glass knives and stained in 1% (w/v) toluidine blue O in 0.1% (w/v) Na2CO3. Distances were measured using a digital camera (SensiCam; Cooke Corporation, Auburn Hills, MI) and image analysis software (IPLab; Scanalytics, Fairfax, VA) calibrated with a stage micrometer.
Photosynthetic Gas Exchange Measurements
Photosynthesis measurements were made with a gas exchange system (LI-6200; LI-COR, Lincoln, NE) equipped with a one-quarter-liter chamber. Light was provided by a 1,000-W mixed metal halide lamp (ET-SU-1000-MH-CH; Energy Technics, York, PA). Light was passed through 4 cm of flowing water and a hot mirror to reduce heating of the samples.
Acknowledgments
We are grateful to Dr. Robert Pearcy for plant material and to Dr. William Williams, Dr. John Evans, and Dr. Susanne von Caemmerer for many helpful discussions and valuable suggestions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019612.
This work was funded by the National Science Foundation (grant nos. IBN–0075847 and DBI–9724499).
Alocasia brisbanensis (F.M. Bailey) Domin is the currently accepted name of the only Alocasia species endemic to the Australian mainland (Hay and Wise, 1991). The species has previously been known as Alocasia macrorrhiza (L.) G. Don.
References
- Battino R, Clever HL (1966) The solubility of gases in liquids. Chem Rev 66: 395–463 [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, Von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance: implications for the determination of RUBISCO enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavaraju NC, Cao H, Yuan DY, Valvano JW, Webster JG (2001) Measurement of directional thermal properties of biomaterials. IEEE Trans Biomed Eng 48: 261–267 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A et al. (2001) The phototropin family of photoreceptors. Plant Cell 13: 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz SJ (1979) Chloroplast and nuclear migration. In W Haupt, M Feinleib, eds, Encyclopedia of Plant Physiology, New Series, Vol 7. Springer-Verlag, New York, pp 170–205 [Google Scholar]
- Britz SJ, Briggs WR (1987) Chloroplast movement and light transmission in Ulva: the sieve effect in a light-scattering system. Acta Physiol Plant 9: 149–162 [Google Scholar]
- Brugnoli E, Björkman O (1992) Chloroplast movements in leaves: influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ΔpH and zeaxanthin formation. Photosynth Res 32: 23–35 [DOI] [PubMed] [Google Scholar]
- Bults G, Horwitz BA, Malkin S, Cahen D (1982) Photoacoustic measurements of photosynthetic activities in whole leaves: photochemistry and gas exchange. Biochim Biophys Acta 679: 452–465 [Google Scholar]
- Buschmann C, Prehn H (1990) Photoacoustic spectroscopy-photoacoustic and photothermal effects. In H-F Linskens, JF Jackson, eds, Modern Methods of Plant Analysis, Vol II. Springer-Verlag, New York, pp 148–180 [Google Scholar]
- Canaani O, Malkin S, Mauzerall D (1988) Pulsed photoacoustic detection of flash-induced oxygen evolution from intact leaves and its oscillations. Proc Natl Acad Sci USA 85: 4725–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejours P (1975) Principles of Comparative Respiratory Physiology. American Elsevier Publishing Company, New York
- Del Hierro AM, Kronberger W, Richter H (2000) Chloroplast movements in leaves of two evergreen sclerophyllous plants (Ilex aquifolium and Prunus laurocerasus). Plant Biosystems 134: 297–304 [Google Scholar]
- Dutta A, Popel AS (1995) A theoretical analysis of intracellular oxygen diffusion. J Theor Biol 176: 433–445 [DOI] [PubMed] [Google Scholar]
- Evans JR (1999) Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol 143: 93–104 [Google Scholar]
- Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21: 475–495 [Google Scholar]
- Farquhar GD (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10: 205–226 [Google Scholar]
- Fork DC, Herbert SK (1991) The application of photoacoustic techniques to studies of photosynthesis. Photosynth Res 27: 151–156 [DOI] [PubMed] [Google Scholar]
- Gabrys H, Walczak T (1980) Photometric study of chloroplast phototranslocations in leaves of land plants. Acta Physiol Plant 2: 281–290 [Google Scholar]
- Gorton HL, Williams WE, Vogelmann TC (1999) Chloroplast movement in Alocasia macrorrhiza. Physiol Plant 106: 421–428 [Google Scholar]
- Haupt W (1999) Chloroplast movement: from phenomenology to molecular biology. Prog Bot 60: 1–36 [Google Scholar]
- Haupt W, Scheuerlein R (1990) Chloroplast movement. Plant Cell Environ 13: 595–614 [Google Scholar]
- Hay A, Wise R (1991) The genus Alocasia (Araceae) in Australia. Blumea 35: 499–545 [Google Scholar]
- Herbert S, Han T, Vogelmann T (2000) New applications of photoacoustics to the study of photosynthesis. Photosyn Res 66: 13–31 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Shibata K (1974) Comparative examination of terrestrial plant leaves in terms of light-induced absorption changes due to chloroplast rearrangements. Plant Cell Physiol 15: 717–721 [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alaonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952–954 [DOI] [PubMed] [Google Scholar]
- Jensen RG, Bahr JT (1977) Ribulose 1, 5-bisphosphate carboxylase-oxygenase. Annu Rev Plant Physiol 28: 379–400 [Google Scholar]
- Jeong WJ, Park Y-I, Suh K, Raven JA, Yoo OJ, Liu JR (2002) A large population of small chloroplasts in tobacco leaf cells allows more effective chloroplast movement than a few enlarged chloroplasts. Plant Physiol 129: 112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T (2003) The phototropin family as photoreceptors for blue light-induced chloroplast relocation. J Plant Res 116: 75–80 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41: 84–93 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M (2002) Blue light-induced chloroplast relocation. Plant Cell Physiol 43: 367–371 [DOI] [PubMed] [Google Scholar]
- Kao HP, Abney JR, Verkman AS (1993) Determinants of the translational mobility of a small solute in cell cytoplasm. J Cell Biol 120: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kolbowski J, Reising H, Schreiber U (1990) Computer-controlled pulse modulation system for analysis of photoacoustic signals in the time domain. Photosyn Res 25: 309–316 [DOI] [PubMed] [Google Scholar]
- Laisk A, Oja V, Rahi M (1970) Diffusion resistance of leaves in connection with their anatomy. Fiziol Rast 17: 40–48 [Google Scholar]
- Lechowski Z (1974) Chloroplast arrangement as a factor of photosynthesis in multilayered leaves. Acta Soc Bot Pol 43: 531–540 [Google Scholar]
- Lelatko Z (1970) Some aspects of chloroplast movement in leaves of terrestrial plants. Acta Soc Bot Pol 39: 453–468 [Google Scholar]
- Malec P, Rinaldi RA, Gabrys H (1996) Light-induced chloroplast movements in Lemna trisulca: identification of the motile system. Plant Sci 120: 127–137 [Google Scholar]
- Malkin S (1994) The use and characteristics of the photoacoustic method in the study of photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45: 493–526 [Google Scholar]
- Mauzerall DC (1990) Determination of oxygen emission and uptake in leaves by pulsed, time resolved photoacoustics. Plant Physiol 94: 278–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel P (1999) Physicochemical and Environmental Plant Physiology, Ed 2. Academic Press, San Diego
- Park Y-I, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111: 867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R, eds (1989) Textbook of Physiology, Ed 21. W.B. Saunders Company, Philadelphia
- Perry JH, ed (1950) Chemical Engineers' Handbook, Ed 3. McGraw-Hill, New York
- Poling BE, Prausnitz JM, O'Connell JP (2001) The Properties of Gasses and Liquids, Ed 5. McGraw Hill, New York
- Poulet P, Cahen D, Malkin S (1983) Photoacoustic detection of photosynthetic oxygen evolution from leaves: quantitative analysis by phase and amplitude measurements. Biochim Biophys Acta 724: 433–446 [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesak I, Gabrys H (1996) Role of photosynthesis in the control of blue light-induced chloroplast movements: inhibitor study. Acta Physiol Plant 18: 135–145 [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi H, Schinner K, Spors J, Hansen U-P (1998) Deconvolution of the three components of the photoacoustic signal by curve fitting and the relationship of CO2 uptake to proton fluxes. Photosyn Res 57: 101–115 [Google Scholar]
- Terashima I, Hikosaka K (1995) Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ 18: 1111–1128 [Google Scholar]
- Terashima I, Miyazawa S-I, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114: 93–105 [Google Scholar]
- Tlalka M, Fricker M (1999) The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. Plant J 20: 461–473 [DOI] [PubMed] [Google Scholar]
- Tlalka M, Gabrys H (1993) Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta 189: 491–498 [Google Scholar]
- Trojan A, Gabrys H (1996) Chloroplast distribution in Arabidopsis depends on light conditions during growth. Plant Physiol 111: 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Grolig F, Haupt W (1993) Light-oriented chloroplast positioning: contribution to progress in photobiology. J Photochem Photobiol 17: 3–25 [Google Scholar]
- Wada M, Kagawa T (2001) Light-controlled chloroplast movement. In D-P Häder, M Lebert, eds, Photomovement, Vol 1. Elsevier, New York, pp 897–924 [Google Scholar]
- Yatsuhashi H (1996) Photoregulation systems for light-oriented chloroplast movement. J Plant Res 109: 139–146 [Google Scholar]
- Zeiger E, Field C (1982) Photocontrol of the functional coupling between photosynthesis and stomatal conductance in the intact leaf: blue light and PAR-dependent photosystems in guard cells. Plant Physiol 70: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzycki J (1955) Chloroplasts arrangement as a factor in photosynthesis. Acta Soc Bot Pol 24: 27–63 [Google Scholar]
- Zurzycki J (1957) The destructive effect of intense light on the photosynthetic apparatus. Acta Soc Bot Pol 26: 157–175 [Google Scholar]
- Zurzycki J (1961) The influence of chloroplast displacements on the optical properties of leaves. Acta Soc Bot Pol 30: 503–527 [Google Scholar]
- Zurzycki J, Lelatko Z (1969) Action dichroism in the chloroplasts rearrangements in various plant species. Acta Soc Bot Pol 38: 493–506 [Google Scholar]