Abstract
We compared the structural, biochemical, and physiological characteristics involved in photorespiration of intergeneric hybrids differing in genome constitution (DtDtR, DtDtRR, and DtRR) between the C3-C4 intermediate species Diplotaxis tenuifolia (DtDt) and the C3 species radish (Raphanus sativus; RR). The bundle sheath (BS) cells in D. tenuifolia included many centripetally located chloroplasts and mitochondria, but those of radish had only a few chloroplasts and mitochondria. In the hybrids, the numbers of chloroplasts and mitochondria, the ratio of centripetally located organelles to total organelles, and the mitochondrial size in the BS cells increased with an increase in the constitution ratio of the Dt:R genome. The P-protein of glycine decarboxylase (GDC) was confined to the BS mitochondria in D. tenuifolia, whereas in radish, it accumulated more densely in the mesophyll than in the BS mitochondria. In the hybrids, more intense accumulation of GDC in the BS relative to the mesophyll mitochondria occurred with an increase in the Dt:R ratio. These structural and biochemical features in the hybrids were reflected in the gas exchange characteristics of leaves, such as the CO2 compensation point. Our data indicate that the leaf structure, the intercellular pattern of GDC expression, and the gas exchange characteristics of C3-C4 intermediate photosynthesis are inherited in the hybrids depending on the constitution ratio of the parent genomes. Our findings also demonstrate that the apparent reduced photorespiration in C3-C4 intermediate plants is mainly due to the structural differentiation of mitochondria and chloroplasts in the BS cells combined with the BS-dominant expression of GDC.
In ordinary air, Rubisco catalyzes bifunctional reactions, namely, the carboxylation and oxygenation of ribulose-1,5-bisphosphate. Glycolate-2-phosphate produced by the oxygenase activity of Rubisco is recycled into glycerate-3-phosphate via the photorespiratory pathway, and glycerate-3-phosphate is used for the regeneration of ribulose-1,5-bisphosphate via the C3 cycle (Leegood et al., 1995; Tolbert, 1997; Douce and Neuburger, 1999). This oxidative photosynthetic carbon cycle involves three subcellular compartments: chloroplasts, peroxisomes, and mitochondria. The CO2 released during photorespiration comes from Gly oxidation (Oliver, 1994; Douce et al., 2001). In C3 plants, the release of CO2 by photorespiration in ordinary air is estimated to be approximately 25% of photosynthetically fixed CO2 (Sharkey, 1988) and decreases the photosynthetic efficiency (Zelitch, 1992). However, some plants have evolved biochemical mechanisms, such as the C4 photosynthetic pathway, to suppress photorespiration.
The leaves of C3-C4 intermediate plants have anatomical and physiological characteristics between those of C3 and C4 plants (Edwards and Ku, 1987; Brown and Hattersley, 1989; Monson and Rawsthorne, 2000). The leaves of C3-C4 intermediate plants possess a Kranz-like anatomy, in which the bundle sheath (BS) cells include numerous chloroplasts and mitochondria. The gas exchange characteristics, such as the CO2 compensation point (Γ) and O2 inhibition of photosynthesis, of C3-C4 intermediate plants are intermediate between those of C3 and C4 plants (Edwards and Ku, 1987; Monson and Rawsthorne, 2000). It is thought that the reduction in photorespiratory CO2 loss results from the cellular compartmentalization of Gly decarboxylation (Hylton et al., 1988; Monson and Rawsthorne, 2000).
Together with Ser hydroxymethyltransferase, Gly decarboxylase (GDC) catalyzes the oxidative conversion of Gly to Ser, NH3, and CO2. GDC is a complex of four heterologous protein subunits—P, H, L, and T—and requires the concerted effects of these four proteins to complete its reactions (Oliver, 1994; Douce et al., 2001). The mitochondria of the BS cells of C3-C4 intermediate plants contain all of the proteins of GDC, but those of the mesophyll cells substantially lack at least the P-protein (Hylton et al., 1988; Morgan et al., 1993). This situation means that Gly generated in the mesophyll cells must be transported into the BS cells to be decarboxylated by GDC. In the BS cells, the mitochondria are surrounded by chloroplasts. Thus, a large part of the CO2 released from the mitochondria is recaptured by the chloroplasts before it escapes from the BS cells. The increased efficiency of CO2 recapture in C3-C4 intermediate plants may give rise to better photosynthetic performance at warm temperatures and improved water-use efficiency, as compared with C3 plants (McVetty et al., 1989; Monson and Rawsthorne, 2000). C3-C4 intermediate species have been found in several genera including Mollugo, Panicum, Moricandia, and Flaveria (Edwards and Ku, 1987; Monson and Rawsthorne, 2000). Furthermore, some C3-C4 intermediate species of Flaveria use a partially functioning C4 cycle as well as the Gly decarboxylation system to reduce photorespiration, suggesting an evolutionary link to C4 plants (Edwards and Ku, 1987).
Various attempts have been made to genetically improve the photosynthetic efficiency of C3 plants (Zelitch, 1992; Brown and Bouton, 1993; Leegood, 2002; Surridge, 2002). The transfer of C3-C4 intermediate characteristics into C3 plants may be the method of choice (Leegood, 2002), because the mechanism underlying C3-C4 intermediate photosynthesis is less complicated than that of C4 photosynthesis. Until now, artificial interspecific hybrids have been produced between C3 and C3-C4 intermediate species (Brown and Bouton, 1993). In early studies, hybrids were found to exhibit photosynthetic characteristics intermediate between the parents (Apel et al., 1984; Brown et al., 1985). The Brassicaceae include C3-C4 intermediate species in the genera Moricandia, Diplotaxis, and Brassica as well as C3 species such as oilseed (Brassica napus), cabbage (Brassica capitata), and radish (Raphanus sativus; Apel, 1996; Apel et al., 1997). Hybridization has been successfully carried out between C3-C4 intermediate species of Moricandia and C3 species of other cruciferous genera (Apel et al., 1984; Bang et al., 1996; O'Neill et al., 1996; Razmjoo et al., 1996; Rawsthorne et al., 1998; Yan et al., 1999). These hybrids provide not only valuable materials for the breeding of agronomically important crops but also unique opportunities to study the genetic mechanisms of C3-C4 intermediate photosynthesis.
Here, we report the anatomical and biochemical features of the leaves of hybrids produced between the C3-C4 intermediate Diplotaxis tenuifolia and the C3 species radish and that differ in their genome constitution. The data obtained clearly show that these anatomical and biochemical characteristics are correctly inherited from the parents by the hybrids according to the genome constitution. Our results also demonstrate that the apparent reduction of photorespiration in C3-C4 intermediate plants is caused by the anatomical differentiation of leaf cells combined with the intercellular location of GDC accumulation.
RESULTS
Gross Morphology
The gross morphology of the hybrids and the method for producing them have been reported in detail by Bang et al. (2000). In brief, D. tenuifolia (DtDt) and radish (RR) were used as the female parent and pollen parent, respectively. Three types of hybrids differing in genome constitution (DtDtR, DtDtRR, and DtRR) were produced through ovary culture followed by embryo culture (Bang et al., 2000). The leaf shape differs between the parents: D. tenuifolia has crenate leaves, and radish has lacerate leaves. The leaf shape of the DtDtRR hybrid was intermediate between those of the parents, whereas those of the DtDtR hybrid were more like those of D. tenuifolia, and those of the DtRR hybrid more resembled those of radish. The flowers of D. tenuifolia and radish had yellow and white petals, respectively. The petals of the three hybrids showed different levels of intermediate color depending on the Dt:R genome constitution.
Internal Leaf Structure
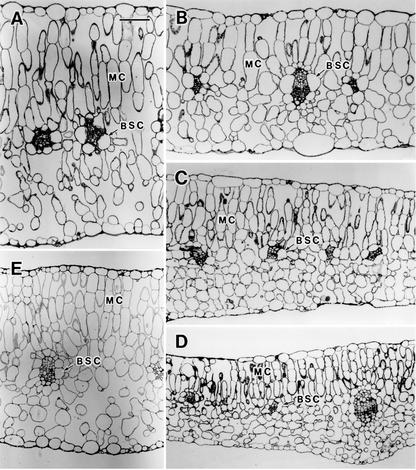
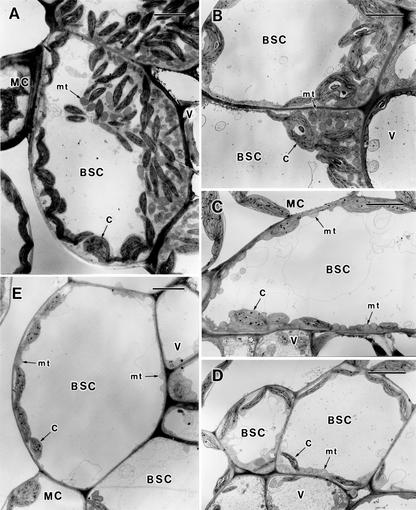
The leaves of the C3-C4 intermediate species D. tenuifolia contained large BS cells that included numerous organelles surrounding the vascular bundle (Fig. 1A). Their mitochondria were centripetally located, and most were adjacent to the inner walls of BS cells and were closely overlaid by numerous chloroplasts (Fig. 2A). The adaxial mesophyll cells exhibited an elongated shape, whereas the abaxial mesophyll cells were somewhat similar to spongy parenchyma. The abaxial mesophyll cells adjacent to BS cells were somewhat radially arranged. The leaves of radish showed a typical C3 anatomy (Fig. 1E). Although the BS cells of radish also surrounded the vascular bundle, they contained only a few chloroplasts and mitochondria (Fig. 2E). The mesophyll was clearly differentiated into palisade and spongy parenchyma between the adaxial and abaxial sides, respectively.
Figure 1.
Leaf anatomy of D. tenuifolia (A), the hybrids (B, DtDtR; C, DtDtRR; D, DtRR), and radish (E). BSC, BS cell; MC, mesophyll cell. Bar, 100 μm.
Figure 2.
Ultrastructure of bundle sheath cells of D. tenuifolia (A), the hybrids (B, DtDtR; C, DtDtRR; D, DtRR), and radish (E). BSC, BS cell; V, vascular bundle; C, chloroplast; mt, mitochondrion. Bar = 5 μm.
The internal structures of the leaves of the hybrid plants showed various intermediate anatomies between those of the parents (Fig. 1, B–D). This diversity was particularly evident in the structure of the BS cells. The BS cells of the DtDtR hybrid included numerous chloroplasts and mitochondria in the centripetal position; thus this configuration approached that of the BS cells of D. tenuifolia (Fig. 2B). The BS cells of the DtRR hybrid were similar to those of radish but included several chloroplasts and mitochondria in the centripetal position (Fig. 2D). The pattern of the BS cells of the DtDtRR hybrid was intermediate between the other two hybrids (Fig. 2C). In addition, the shape and arrangement of mesophyll cells showed a gradation from the C3-C4 intermediate to the C3 parent.
The interveinal distance was shorter in D. tenuifolia than in radish, and the values for the three hybrids were intermediate between those of the parents (Table I). The photosynthetic cells were larger in D. tenuifolia than in radish (Table I). In the DtDtR hybrid, the size of the mesophyll cells was intermediate to those of the parents, but the size of the BS cells was almost the same as that of the C4 parent. In the DtDtRR and DtRR hybrids, all types of photosynthetic cells, except for the BS cells of DtDtRR, were smaller than those in the C3 parent (Table I).
Table I.
Interveinal distance and size of photosynthetic cells in leaves of D. tenuifolia, radish, and their hybrids
MC, Mesophyll cells; BSC, BS cells. Values are given as the mean ± sd of 30 to 40 measurements for interveinal distance and 40 measurements for cell size.
| Plant and Genotype
|
Interveinal Distance
|
Cell Size
|
||
|---|---|---|---|---|
| Adaxial MC | Abaxial MC | BSC | ||
| μm | ||||
| D. tenuifolia (DtDt) | 238±52 | 116±24 | 78±19 | 40±9 |
| DtDtR hybrid | 245±66 | 83±17 | 61±10 | 39±10 |
| DtDtRR hybrid | 311±91 | 60±12 | 44±6 | 31±7 |
| DtRR hybrid | 243±93 | 49±13 | 36±11 | 19±5 |
| Radish (RR) | 340±138 | 67±21 | 56±11 | 27±5 |
Quantification of Organelles Involved in Photosynthesis and Photorespiration
The number of chloroplasts per BS cell in D. tenuifolia was about five times higher than that in radish (Table II), and the values for the hybrids were intermediate between the parents and showed a gradation. In D. tenuifolia, 56% of chloroplasts in the BS cells were centripetally located, but the remaining chloroplasts were centrifugal. In radish, only 15% of chloroplasts were centripetal (Table II). In addition, the number of chloroplasts per mesophyll cell was higher in D. tenuifolia than in radish, whereas the values for the hybrids were similar to that for radish. The number of mitochondria per BS cell differed greatly (six times) between the two parents (Table II), and the values for the hybrids again showed a gradation. In D. tenuifolia, all mitochondria in the BS cells were centripetally located. In the DtDtR and DtDtRR hybrids, almost all mitochondria were centripetal, as were 80% of those in the BS cells of radish (Table II). The number of mitochondria per mesophyll cell did not differ markedly among the plants, except that the DtRR hybrid had few. As a result, the concentration of mitochondria in the BS cells increased with an increase in the Dt:R genome constitution ratio. However, we did not observe such a trend in the number of peroxisomes (Table II). In all plants, the number of peroxisomes per cell was lower in BS cells than in mesophyll cells. The intracellular location of peroxisomes in the BS cells varied among the plants (Table II).
Table II.
Numbers of organelles per cell profile in photosynthetic cells and ratios of centripetally located organelles in the BS cells in leaves of D. tenuifolia, radish, and their hybrids
MC, Mesophyll cells; BSC, BS cells; Cp, percentage of centripetally located organelles in the BS cells. Numbers of organelles per cell profile are given as means ± SD of 20 measurements.
| Plant and Genotype | Chloroplasts
|
Mitochondria
|
Peroxisomes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adaxial MC | Abaxial MC | BSC | Cp | Adaxial MC | Abaxial MC | BSC | Cp | Adaxial MC | Abaxial MC | BSC | Cp | |
| % | % | % | ||||||||||
| D. tenuifolia (DtDt) | 38.9 ± 7.9 | 31.6 ± 5.2 | 32.4 ± 8.1 | 56.2 | 22.3 ± 5.9 | 13.6 ± 5.0 | 60.4 ± 21.2 | 100.0 | 3.9 ± 2.8 | 1.8 ± 1.0 | 0.5 ± 0.9 | 20.0 |
| Hybrid (DtDtR) | 21.6 ± 4.6 | 20.1 ± 4.3 | 16.9 ± 8.0 | 47.3 | 22.7 ± 7.5 | 14.9 ± 4.4 | 27.0 ± 10.9 | 99.6 | 3.4 ± 1.5 | 2.7 ± 1.3 | 2.0 ± 2.6 | 60.0 |
| Hybrid (DtDtRR) | 19.8 ± 4.3 | 16.6 ± 4.3 | 9.5 ± 1.9 | 35.5 | 22.0 ± 7.3 | 16.2 ± 5.0 | 21.9 ± 9.0 | 98.2 | 3.0 ± 1.8 | 3.1 ± 3.2 | 0.8 ± 0.7 | 50.0 |
| Hybrid (DtRR) | 15.5 ± 3.7 | 11.9 ± 2.9 | 8.8 ± 2.2 | 34.1 | 15.9 ± 6.7 | 7.9 ± 3.7 | 12.5 ± 4.6 | 80.0 | 1.6 ± 1.0 | 0.7 ± 0.8 | 0.8 ± 1.3 | 62.5 |
| Radish (RR) | 20.0 ± 5.9 | 16.5 ± 4.5 | 6.0 ± 2.2 | 15.0 | 20.4 ± 7.7 | 15.9 ± 5.1 | 9.5 ± 4.0 | 80.0 | 2.7 ± 2.1 | 1.9 ± 1.6 | 1.5 ± 1.2 | 25.0 |
In all plants, the chloroplasts in the BS cells generally were smaller than those in the mesophyll cells (Table III). The size of chloroplasts in the various types of photosynthetic cells varied greatly among plants. Except for those in radish, the mitochondria in the BS cells were larger than those in the mesophyll cells (Table III). The size of mitochondria in the BS cells tended to increase as the constitution ratio of the Dt to R genome increased. In D. tenuifolia, the peroxisomes in the BS cells were larger than those in the mesophyll cells, whereas in radish, the opposite trend occurred (Table III). The peroxisomal sizes of the BS cells and mesophyll cells did not vary notably among the three hybrids.
Table III.
Sizes of organelles in photosynthetic cells in leaves of D. tenuifolia, radish, and their hybrids
MC, Mesophyll cells; BSC, BS cells. Values are given as the mean ± sd of 40 measurements for chloroplasts and mitochondria and 10 measurements for peroxisomes. The long axes and the diameters were measured for chloroplasts and for mitochondria and peroxisomes, respectively.
| Plant and Genotype
|
Chloroplasts
|
Mitochondria
|
Peroxisomes
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Adaxial MC | Abaxial MC | BSC | Adaxial MC | Abaxial MC | BSC | Adaxial MC | Abaxial MC | BSC | |
| μm | |||||||||
| D. tenuifolia (DtDt) | 4.8 ± 0.9 | 5.5 ± 1.0 | 4.8 ± 1.2 | 0.57 ± 0.12 | 0.58 ± 0.13 | 0.73 ± 0.19 | 0.79 ± 0.24 | 0.71 ± 0.21 | 0.91 ± 0.22 |
| DtDtR hybrid | 7.2 ± 1.5 | 6.3 ± 1.2 | 5.3 ± 0.9 | 0.51 ± 0.08 | 0.43 ± 0.06 | 0.65 ± 0.17 | 1.21 ± 0.24 | 1.10 ± 0.19 | 1.14 ± 0.22 |
| DtDtRR hybrid | 6.2 ± 1.2 | 6.3 ± 1.1 | 4.8 ± 0.8 | 0.46 ± 0.07 | 0.46 ± 0.09 | 0.68 ± 0.14 | 1.12 ± 0.17 | 1.20 ± 0.24 | 0.97 ± 0.14 |
| DtRR hybrid | 6.5 ± 1.3 | 6.1 ± 0.9 | 4.6 ± 1.2 | 0.50 ± 0.09 | 0.52 ± 0.10 | 0.61 ± 0.13 | 1.12 ± 0.20 | 0.87 ± 0.17 | 1.03 ± 0.09 |
| Radish (RR) | 6.2 ± 1.5 | 5.7 ± 1.2 | 4.3 ± 1.0 | 0.64 ± 0.11 | 0.65 ± 0.12 | 0.54 ± 0.09 | 0.91 ± 0.23 | 0.87 ± 0.14 | 0.65 ± 0.20 |
Immunogold Localization of the P-Protein of GDC
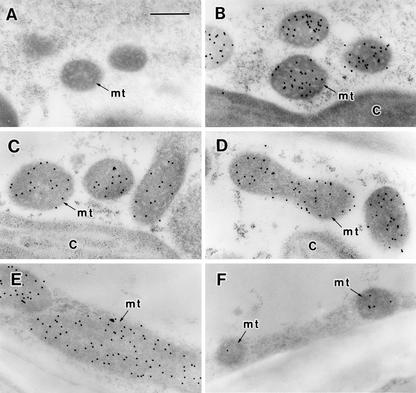
In D. tenuifolia, the BS mitochondria (Fig. 3B) but not the mesophyll mitochondria (Fig. 3A) showed intense labeling for the P-protein of GDC. The ratio of the labeling density for the BS mitochondria to that of the mesophyll mitochondria was 28.0 (Table IV). In radish, the mesophyll mitochondria (Fig. 3E) showed more intense labeling than did the BS mitochondria (Fig. 3F), and the ratio of labeling density for the BS:mesophyll mitochondria was very low (0.4; Table IV). Although marked labeling of the P-protein of GDC occurred in mesophyll as well as BS mitochondria of all three hybrids, there was a gradation in the cellular pattern of the labeling (Fig. 3, C and D). The labeling density of the mesophyll mitochondria decreased and that of the BS mitochondria increased as the constitution ratio of the Dt to R genome increased; for example, the ratio of labeling density for the BS:mesophyll mitochondria ranged from 1.5 in the DtRR hybrid to 2.7 in DtDtRR to 4.0 in DtDtR (Table IV).
Figure 3.
Immunogold localization of the P-protein of GDC in D. tenuifolia (A and B), the DtDtRR hybrid (C and D), and radish (E and F). A, C, and E, Mesophyll cells. B, D, and F, BS cells. C, Chloroplast; mt, mitochondrion. Bar = 0.5 μm.
Table IV.
Immunogold labeling of the P-protein of GDC in photosynthetic cells of D. tenuifolia, radish, and their hybrids
MC, Mesophyll cells, BSC, BS cells. Number of gold particles per square micrometer is given as the mean ± sd. The number of mitochondrial or cell profiles examined is given in parentheses.
| Plant and Genotype
|
Number of Gold Particles
|
Ratio (BSC:MC)
|
|||
|---|---|---|---|---|---|
| MC
|
BSC
|
||||
| Mitochondria | Cytosol + Others | Mitochondria | Cytosol + Others | ||
| μm-2 | |||||
| D. tenuifolia (DtDt) | 3.5 ± 5.3 (29) | 0.2 ± 0.2 (12) | 98.1 ± 17.7 (49) | 0.3 ± 0.1 (10) | 28.0 |
| DtDtR hybrid | 18.1 ± 9.4 (54) | 0.2 ± 0.2 (17) | 73.1 ± 18.2 (62) | 0.1 ± 0.1 (14) | 4.0 |
| DtDtRR hybrid | 23.5 ± 14.4 (54) | 0.2 ± 0.2 (13) | 64.2 ± 16.1 (58) | 0.3 ± 0.4 (11) | 2.7 |
| DtRR hybrid | 40.2 ± 10.9 (51) | 0.1 ± 0.1 (13) | 60.0 ± 15.2 (50) | 0.1 ± 0.2 (14) | 1.5 |
| Radish (RR) | 75.2 ± 23.3 (37) | 0.1 ± 0.1 (12) | 32.0 ± 14.4 (43) | 0.3 ± 0.5 (18) | 0.4 |
Activities of Photosynthetic Enzymes
The photosynthetic carbon metabolism of D. tenuifolia has not yet been characterized fully, although the leaf anatomy and CO2 gas exchange characteristics have been reported (Apel, 1996; Apel et al., 1997). Therefore, we compared the activities of the C3 and C4 photosynthetic enzymes in leaves of this C3-C4 intermediate species with those of the C3 parent radish and the DtDtRR hybrid (Table V). The activity of the key C4 enzyme phosphoenolpyruvate (PEP) carboxylase was higher in D. tenuifolia than in radish. That of pyruvate, Pi dikinase, was very low in both species. Regarding the three types of decarboxylating enzymes, the activities of NADP- and NAD-malic enzymes were higher in D. tenuifolia than in radish, whereas PEP carboxykinase activity was undetectable in both species. Rubisco showed high activity in both species. In the DtDtRR plants, the activities of PEP carboxylase and NADP-malic enzyme were intermediate between those of both parents, whereas the activities of the other enzymes generally were lower than in the C3 species.
Table V.
Activities of C3 and C4 photosynthetic enzymes in leaves of D. tenuifolia, radish, and their DtDtRR hybrid
Values are given as the mean ± sd of three or four experiments. ND, Not detectable.
| Plant and Genotype | Rubisco | PEP Carboxylase | Pyruvate,Pi Dikinase | NADP-Malic Enzyme | NAD-Malic Enzyme | PEP Carboxykinase |
|---|---|---|---|---|---|---|
| μmol mg Chl-1 h-1 | ||||||
| D. tenuifolia (DtDt) | 1,820 ± 225 | 106.9 ± 20.9 | 2.3 ± 0.1 | 77.7 ± 10.4 | 30.3 ± 2.5 | ND |
| DtDtRR hybrid | 1,277 ± 319 | 64.2 ± 6.3 | 1.4 ± 0.6 | 51.8 ± 11.4 | 14.5 ± 8.4 | ND |
| Radish (RR) | 1,398 ± 63 | 38.8 ± 3.5 | 1.4 ± 0.5 | 31.6 ± 3.4 | 18.2 ± 3.6 | ND |
Gas Exchange Characteristics
The photosynthetic rate was higher in D. tenuifolia than in radish (Table VI). The rates of the DtDtRR and DtRR hybrids were intermediate between those of the parents, and that of DtDtR was somewhat higher than that of D. tenuifolia. Under high PPFD, D. tenuifolia showed a Γ value typical of C3-C4 intermediate plants, whereas that of radish was within the range for C3 plants (Table VI). The Γ values of the DtDtR and DtDtRR hybrids were intermediate between those of the parents, whereas that of DtRR was the same as that of the C3 parent. It is known that the Γ of C3-C4 intermediate plants increases as the PPFD is reduced, but that of C3 plants is unaffected (Holaday et al., 1982). When measured at low PPFD, the Γ values of all plants examined were somewhat increased (Table VI). However, the magnitude of the increase was larger in D. tenuifolia and the DtDtR hybrid than in the other plants.
Table VI.
Photosynthetic rate and effect of photosynthetic photon flux density (PPFD) on the CO2 compensation point (Γ) in leaves of D. tenuifolia, radish, and their hybrids
Values are given as the mean ± sd of four measurements. High and low PPFD are 1,000 and 300 μmol m–2 s–1, respectively.
| Plant and Genotype
|
Photosynthetic Rate
|
Γ
|
Ratio of Γ at High/Low PPFD
|
|
|---|---|---|---|---|
| High PPFD | Low PPFD | |||
| μmol m-2 s-1 | μmol mol-1 | |||
| D. tenuifolia (DtDt) | 20.9 ± 2.8 | 19.8 ± 3.2 | 42.4 ± 6.5 | 0.47 ± 0.04 |
| DtDtR hybrid | 22.6 ± 2.6 | 28.5 ± 3.3 | 38.0 ± 4.6 | 0.76 ± 0.09 |
| DtDtRR hybrid | 18.8 ± 3.3 | 36.9 ± 3.9 | 39.6 ± 3.8 | 0.93 ± 0.07 |
| DtRR hybrid | 18.6 ± 2.6 | 50.2 ± 1.6 | 55.9 ± 1.4 | 0.90 ± 0.01 |
| Radish (RR) | 16.7 ± 1.5 | 49.7 ± 2.7 | 53.2 ± 2.6 | 0.93 ± 0.02 |
DISCUSSION
D. tenuifolia was reported to be a C3-C4 intermediate species (Apel, 1996). Among the Brassicaceae, almost all species are C3 type; a few C3-C4 intermediate species have been documented, but no C4 species has been identified (Apel et al., 1997). Our enzyme data indicate that the activities of several C4 enzymes are somewhat higher in D. tenuifolia than in the C3 species radish. The same trend has been found in other C3-C4 intermediate species lacking a C4 cycle (Holaday et al., 1981; Edwards and Ku, 1987). However, the numbers of organelles in the BS cells of D. tenuifolia seem greater than those in Moricandia arvensis, a well-known C3-C4 intermediate species (Holaday et al., 1981; Beebe and Evert, 1992). In this regard, D. tenuifolia may be more useful than M. arvensis as the parent in hybridization studies of C3-C4 intermediacy.
Our study clearly demonstrated that the anatomical and biochemical characteristics of leaves of the parent plants are inherited correctly in the hybrids depending on the constitution ratio of the Dt:R genomes. The hybrids exhibited various levels of intermediacy and provide intriguing materials for dissecting the structural and biochemical features of photosynthesis of C3-C4 intermediate species. The BS cells in the DtDtR and DtDtRR hybrids were larger, and those of DtRR were smaller, than those in radish. However, the chloroplasts and mitochondria in the BS cells of all three hybrids were always larger than those of radish and showed a gradation according to the Dt:R genome constitution. These data suggest that the size of the BS cells may be regulated independently of the sizes of their chloroplasts and mitochondria. The numbers of chloroplasts and mitochondria per BS cell were higher in D. tenuifolia than in radish, and those in the hybrids were intermediate between the parents, showing again a gradation depending on the genome constitution. The peroxisomes of leaves are involved in the photorespiratory metabolism (Douce and Neuburger, 1999). In D. tenuifolia, the peroxisomes in the BS cells were larger than those in the mesophyll cells, whereas the reverse trend occurred in radish. In the hybrids, the two cell types did not differ notably. In C3-C4 intermediate species of Panicum, the BS cells contain more peroxisomes than do the mesophyll cells (Brown et al., 1983). In D. tenuifolia as well as the hybrid plants that we generated, however, the number of peroxisomes per mesophyll cell was always greater than that per BS cell. Why the cellular pattern of peroxisomes differs between the two groups is unknown. The reason may be related to differences due to evolutionary steps from C3 type to C3-C4 intermediacy.
In the BS cells of C3-C4 intermediate plants, the mitochondria are centripetally distributed and are externally surrounded by chloroplasts. This arrangement of organelles may be effective for the recapture of photorespired CO2 from mitochondria (Edwards and Ku, 1987; Monson and Rawsthorne, 2000). Similarly, all of the mitochondria in the BS cells of D. tenuifolia were located centripetally. The ratios of centripetally located mitochondria in the BS cells of the hybrids were between those of the parents. However, it is interesting to note that even in the C3 parent radish, 80% of mitochondria in the BS cells were located centripetally. This C3 species may represent a very early stage in the evolution of C3-C4 intermediate photosynthesis, although the structural and functional features of the BS cells in C3 plants remain to be clarified (Koroleva et al., 2000).
The location of chloroplasts within the BS cells showed a gradation similar to that found for the mitochondria, but the ratios of centripetally located chloroplasts were lower. The structural features of organelles in the BS cells of most C3-C4 intermediate species are similar to those in the BS cells of NAD-malic enzyme-type C4 plants (Brown and Hattersley, 1989). The BS cells of D. tenuifolia, however, had many centrifugal chloroplasts, whereas in NAD-malic enzyme-type C4 plants, all chloroplasts are located centripetally (Dengler and Nelson, 1999). Therefore, the different intracellular locations of the chloroplasts in the BS cells of C3-C4 intermediate plants, such as D. tenuifolia, may indicate distinct roles: Centripetal chloroplasts may recapture photorespired CO2, and centrifugal chloroplasts (like their mesophyll counterparts) may fix CO2 from the intercellular space. The centrifugal chloroplasts may also recapture CO2 leaking from the inner aggregation of organelles. In contrast, we noted no consistent trend regarding the intracellular location of the peroxisomes in the BS cells.
In C3-C4 intermediate plants, GDC activity is confined to the BS mitochondria, and this feature is responsible for the reduction of photorespiration, with the structural features of the BS cells (Hylton et al., 1988; Monson and Rawsthorne, 2000). Previous studies on hybridization between C3 and C3-C4 intermediate species have revealed that the Γ values of some hybrids are intermediate between those of the parents (Apel et al., 1984; Brown et al., 1985; O'Neill et al., 1996; Razmjoo et al., 1996; Rawsthorne et al., 1998; Yan et al., 1999). However, except for the study of Rawsthorne et al. (1998), none of these previous studies examined biochemical characteristics such as GDC localization. In the present study, we identified a gradation in the pattern of intercellular accumulation of the P-protein of GDC between the mesophyll and BS mitochondria, although the intercellular patterns of other subunits remain unknown. The gas exchange characteristics of leaves reflected these patterns of localization of the P-protein of GDC and the structural features of the leaf cells. Showing a gradation dependent on the Dt:R genome constitution, the Γ values of our hybrids generally were between those of the parents. We also noted light-dependent changes in Γ for the DtDtR hybrid as well as for D. tenuifolia.
Our study suggests that both the structural features of leaves and the intercellular patterns of GDC accumulation are prerequisites for the reduction in Γ. The DtRR hybrid showed slight increases in the number and size of mitochondria and in the ratio of centripetally located chloroplasts in the BS cells but showed a reduction in GDC accumulation in mesophyll cells relative to that in BS cells, compared with those of the C3 parent radish. However, these structural and biochemical alterations are still insufficient for reducing Γ. Similar results were found in the hybrid between Moricandia nitens (C3-C4) and oilseed (C3), in which the ratio of labeling density for the P-protein of GDC in the BS:mesophyll mitochondria was 1.7 and the value of Γ was close to that of the C3 parent (Rawsthorne et al., 1998). However, we observed a reduction of Γ in the DtDtRR and DtDtR hybrids, in which the P-protein of GDC was expressed more intensely in the BS mitochondria than in the mesophyll mitochondria, as compared with the case of the DtRR hybrid, but the mesophyll mitochondria still accumulated noteworthy amounts of this protein. Our data suggest that complete suppression of expression of the P-protein of GDC in the mesophyll is not required to reduce Γ. To date, all C3-C4 intermediate species examined for GDC localization exhibit BS-specific expression of the P-protein of GDC (Hylton et al., 1988; Morgan et al., 1993; Ueno and Agarie, 1997; Rylott et al., 1998; Voznesenskaya et al., 2001). It would be intriguing to investigate whether C3-C4 intermediate species having the characteristics of the DtDtRR and DtDtR hybrids occur naturally.
If each parent gene contributes equally to the hybrid and if dominance is not involved, then the characteristics of the hybrids should be similar to the mid-parent mean (Brown and Bouton, 1993). It seems that in our hybrids, the expression of structural features and cell-specific GDC localization are modulated depending on the constitution ratio of the parent genomes. We cannot evaluate the possible influence of a cytoplasmic effect in the expression of C3-C4 intermediate characteristics, because we did not evaluate the reciprocal hybrids. Although the gene for the large subunit of Rubisco is maternally inherited (Hudson et al., 1990), there is little evidence that other characteristics are maternally influenced in hybrids between C3, C4, and C3-C4 intermediate species (Araus et al., 1990; Brown and Bouton, 1993). Although mitochondria contain proteins encoded in both the nuclear and mitochondrial genomes (McCabe et al., 2000), all four proteins of GDC are encoded by nuclear genes (Douce et al., 2001). In our hybrid plants, it appears that, irrespective of differences in expression levels, the structural and biochemical components of C3-C4 intermediacy were expressed coordinately. Dense accumulation of GDC in the BS cells relative to that in mesophyll cells occurred with increased partitioning of mitochondria into BS cells relative to mesophyll cells. However, previous studies on Moricandia species have suggested that the structural and biochemical components of C3-C4 intermediate characteristics are controlled by different mechanisms (Rawsthorne et al., 1998; Rylott et al., 1998). Furthermore, C4 photosynthesis may reflect a combination of independently inherited characteristics (Brown and Bouton, 1993; Ueno, 2001a).
A study of barley (Hordeum vulgare) mutants with reduced GDC activities showed that GDC has no control over CO2 assimilation under normal growth conditions, but appreciable control by GDC becomes apparent under conditions of enhanced photorespiration, such as low CO2 and high light (Wingler et al., 1997). In contrast, antisense reduction of the P-protein of GDC in potato (Solanum tuberosum) plants resulted in a reduction of photosynthetic and growth rates under ordinary air (Heineke et al., 2001). Superficially, photorespiration appears to be a wasteful process, but it may serve as an energy sink to prevent overreduction of the photosynthetic electron chain and photoinhibition (Osmond and Grace, 1995; Kozaki and Takeba, 1996; Wingler et al., 2000). In C3-C4 intermediate plants, considerable photorespiration occurs within leaf cells, even though apparent photorespiration is reduced (Edwards and Ku, 1987). The introduction of C3-C4 intermediate characteristic may be one possible option for improving the photosynthesis of C3 plants, although the molecular and genetic mechanisms responsible for the development of C3-C4 intermediate characteristics remain to be solved.
MATERIALS AND METHODS
Plant Material
Seeds of Diplotaxis tenuifolia (L.) DC. and radish (Raphanus sativus) were sown in 8-L pots filled with sufficiently fertilized field soil. We produced three types of hybrids differing in genome constitution by using D. tenuifolia (DtDt, 2n = 22) as the female parent and radish (RR, 2n = 18) as the pollen parent, as described elsewhere (Bang et al., 2000). The F1 plants were obtained through ovary culture followed by embryo culture. To induce amphidiploidy (DtDtRR, 2n = 40), colchicine was applied to the apical meristems of F1 seedlings. Backcrossing to parent plants followed by embryo rescue was performed to obtain the other progenies (DtDtR [2n = 31] and DtRR [2n = 29]). Plants were grown in a naturally illuminated greenhouse maintained at 25°C to 28°C/15°C to 18°C (day/night temperature) during the spring. They were watered daily. Fully expanded young leaves of plants were examined 2 to 3 months after planting.
Anatomical and Ultrastructural Studies
Samples from the midsections of leaves were fixed in 3% (v/v) glutaraldehyde in 50 mm sodium phosphate buffer (pH 6.8) at room temperature for 3 h. After being washed with water, they were hand-sectioned with a razor blade. We determined the mean interveinal distance in these sections from 30 to 40 measurements. Semithin sections were prepared from leaf samples that had been fixed with glutaraldehyde and osmium tetroxide and embedded in Suppur's resin, as described previously (Ueno, 1996). Semithin sections on glass slides were stained with toluidine blue O and were used to determine the sizes of the adaxial and abaxial mesophyll cells and BS cells (n = 40 cells). The sizes of the adaxial and abaxial mesophyll cells were represented by the length of the long axis. That of the BS cells was represented by the diameter of the cells. For BS cells that were not circular in cross-section, the size was estimated as the mean of the longest and shortest diameters.
Ultrathin sections also were prepared from leaf samples embedded in Suppur's resin. These sections were placed on grids and stained with uranyl acetate and lead citrate. The chloroplasts, mitochondria, and peroxisomes per cell profile were counted for 20 cells of each type by using an electron microscope (model HU7000, Hitachi, Tokyo). The centripetal organelles (i.e. those located in the inner tangential walls and the inner half of the radial walls) of the BS cells were counted also. To determine the sizes of organelles, we measured the long axes of chloroplasts and the diameters of mitochondria and peroxisomes from electron micrographs at 4,000× for chloroplasts and 15,000× for mitochondria and peroxisomes. The values given are the means of 40 measurements for chloroplasts and mitochondria and of 10 measurements for peroxisomes.
Protein A-Immunogold Electron Microscopy of GDC
Small segments of leaves were fixed with 3% (v/v) glutaraldehyde in 50 mm sodium phosphate (pH 6.8), dehydrated through an ethanol series, and embedded in Lowicryl K4M resin (Chemische Werke Lowi GmbH, Waldkraiburg, Germany), as previously described (Ueno, 2001b). Ultrathin sections were immunolabeled with an antiserum to the P-protein of GDC and protein A-colloidal gold particles (EY Lab. Inc., San Mateo, CA) as described (Ueno, 2001b). For controls, the antiserum was replaced by nonimmune serum. The antiserum used was raised against the P-protein of GDC isolated from pea (Pisum sativum) leaf mitochondria and was kindly provided by Dr. D. J. Oliver (University of Idaho, Moscow, ID). This antiserum was the same as that used in our previous immunocytochemical studies (Ueno and Agarie, 1997; Ueno, 2001b). The cross-reactivity of the antiserum used was determined by western blotting of crude extracts of leaves after SDS-PAGE, as previously described (Ueno, 2001b). We confirmed that the antiserum used recognized the P-proteins of GDC from D. tenuifolia, radish, and their hybrids, generating strong single bands on the blots. For both immunolabeling and western blotting, the antiserum was used at a dilution of 1:500. The density of labeling was determined by counting the gold particles on electron micrographs at 25,000× magnification and calculating the number per unit area (square micrometers). Between 11 and 18 individual cells were examined on several immunolabeled sections.
Enzymatic Activities
Leaves (0.25 g) were ground by using a mortar and pestle containing 0.5 g of sea sand, 25 mg of polyvinylpyrrolidone, and 1 mL of grinding medium on ice. The grinding medium contained 50 mm HEPES-KOH (pH 7.5), 0.2 mm EDTA, 2.5 mm MgCl2, 2.5 mm MnCl2, 5 mm dithiothreitol, and 0.7% (w/v) bovine serum albumin. The homogenates were filtered through gauze, the filtrates were centrifuged at 10,000g for 5 min at 4°C, and the supernatants were used for the enzymatic assays. All enzymes were assayed spectrophotometrically as described by Ueno (1998).
Gas Exchange Measurements
The net photosynthetic rate was measured with an LI-6400 portable photosynthesis system (LI-COR, Lincoln, NE). Measurements were made at a photon flux density of 1,000 μmol m–2s–1, a leaf temperature of 25°C, and a CO2 concentration of 350 μL L–1. Light within the chamber was provided by a 6400-02 LED light source (LI-COR). The Γ value was determined by changing the CO2 concentration in the chamber (Apel, 1996). Measurements of Γ were made at photon flux densities of 300 and 1,000 μmol m–2 s–1; leaf temperature was maintained at 25°C.
Acknowledgments
We thank Dr. D. J. Oliver for his kind gift of the antiserum against the P-protein of GDC.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021329.
This study was supported in part by a grant-in-aid from the Ministry of Agriculture, Forestry, and Fisheries of Japan (BioDesign Project).
References
- Apel P (1996) Carbon metabolism type of Diplotaxis tenuifolia (L.) DC. (Brassicaceae). Photosynthetica 32: 237–243 [Google Scholar]
- Apel P, Bauwe H, Ohle H (1984) Hybrids between Brassica alboglabra and Moricandia arvensis and their photosynthetic properties. Biochem Physiol Pflanz 179: 793–797 [Google Scholar]
- Apel P, Horstmann C, Pfeffer M (1997) The Moricandia syndrome in species of the Brassicaceae: evolutionary aspects. Photosynthetica 33: 205–215 [Google Scholar]
- Araus JL, Brown RH, Bouton JH, Serret MD (1990) Leaf anatomical characteristics in Flaveria trinervia (C4), Flaveria brownii (C4-like), and their F1 hybrid. Photosynth Res 26: 49–57 [DOI] [PubMed] [Google Scholar]
- Bang SW, Kaneko Y, Matsuzawa Y (1996) Production of intergeneric hybrids between Raphanus and Moricandia. Plant Breed 115: 385–390 [Google Scholar]
- Bang SW, Mizuno Y, Kaneko Y, Matsuzawa Y (2000) Progenies of intergeneric hybrids between Diplotaxis tenuifolia (C3-C4 plant) and radish (C3 plant). Breed Res 2: 141 [Google Scholar]
- Beebe DU, Evert RF (1992) Photoassimilate pathway(s) and phloem loading in the leaf of Moricandia arvensis (L.) DC. (Brassicaceae). Int J Plant Sci 153: 61–77 [Google Scholar]
- Brown RH, Bouton JH (1993) Physiology and genetics of interspecific hybrids between photosynthetic types. Annu Rev Plant Physiol Plant Mol Biol 44: 435–456 [Google Scholar]
- Brown RH, Bouton JH, Evans PT, Malter HE, Rigsby LL (1985) Photosynthesis, morphology, leaf anatomy, and cytogenetics of hybrids between C3 and C3/C4 Panicum species. Plant Physiol 77: 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Bouton JH, Rigsby L, Rigler M (1983) Photosynthesis of grass species differing in carbon dioxide fixation pathways: VIII. Ultrastructural characteristics of Panicum species in the Laxa group. Plant Physiol 71: 425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Hattersley PW (1989) Leaf anatomy of C3-C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler NG, Nelson T (1999) Leaf structure and development in C4 plants. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 133–172
- Douce R, Bourguignon J, Neuburger M, Rebeille F (2001) The glycine decarboxylase system: a fascinating complex. Trends Plant Sci 6: 167–176 [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M (1999) Biochemical dissection of photorespiration. Curr Opinion Plant Biol 2: 214–222 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB (1987) Biochemistry of C3-C4 intermediates. In MD Hatch, NK Boardman, eds, The Biochemistry of Plants, Vol 10. Photosynthesis. Academic Press, San Diego, pp 275–325
- Heineke D, Bykova N, Garderstrom P, Bauwe H (2001) Metabolic response of potato plants to an antisense reduction of the P-protein of glycine decarboxylase. Planta 212: 880–887 [DOI] [PubMed] [Google Scholar]
- Holaday AS, Harrison AT, Chollet R (1982) Photosynthetic/photorespiratory CO2 exchange characteristics of the C3-C4 intermediate species Moricandia arvensis. Plant Sci Lett 27: 181–189 [Google Scholar]
- Holaday AS, Shieh Y-J, Lee KW, Chollet R (1981) Anatomical, ultrastructural and enzymic studies of leaves of Moricandia arvenesis, a C3-C4 intermediate species. Biochim Biophys Acta 637: 334–341 [Google Scholar]
- Hudson GS, Mahon JD, Anderson PA, Gibbs MJ, Badger MR, Andrews TJ, Whitfield PR (1990) Comparisons of rbcL genes for the large subunit of ribulose bisphosphate carboxylase from closely related C3 and C4 plant species. J Biol Chem 265: 808–814 [PubMed] [Google Scholar]
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW (1988) Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3-C4 intermediate species. Planta 175: 452–459 [DOI] [PubMed] [Google Scholar]
- Koroleva OA, Tomos AD, Farrar J, Roberts P, Pollock CJ (2000) Tissue distribution of primary metabolism between epidermal, mesophyll, and parenchymatous bundle sheath cells in barley leaves. Aust J Plant Physiol 27: 747–755 [Google Scholar]
- Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384: 557–560 [Google Scholar]
- Leegood RC (2002) C4 photosynthesis: principles of CO2 concentration and prospects for its induction into C3 plants. J Exp Bot 53: 581–590 [DOI] [PubMed] [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Hausler RE (1995) The regulation and control of photorespiration. J Exp Bot 46: 1397–1414 [Google Scholar]
- McCabe TC, Daley D, Whelan J (2000) Regulatory, developmental and tissue aspects of mitochondrial biogenesis in plants. Plant Biol 2: 121–135 [Google Scholar]
- McVetty PBE, Austin RB, Morgan CL (1989) A comparison of the growth, photosynthesis, stomatal conductance and water use efficiency of Moricandia and Brassica species. Ann Bot 64: 87–94 [Google Scholar]
- Monson RK, Rawsthorne S (2000) CO2 assimilation in C3-C4 intermediate plants. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publications, Dordrecht, The Netherlands, pp 533–550
- Morgan CL, Turner SR, Rawsthorne S (1993) Coordination of the cell-specific distribution of the four subunits of glycine decarboxylase and of serine hydroxymethyltransferase in leaves of C3-C4 intermediate species from different genera. Planta 190: 468–473 [Google Scholar]
- Oliver DJ (1994) The glycine decarboxylase complex from plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol 45: 323–337 [Google Scholar]
- O'Neill CM, Murata T, Morgan CL, Mathias RJ (1996) Expression of the C3-C4 intermediate character in somatic hybrids between Brassica napus and the C3-C4 species Moricandia arvensis. Theor Appl Genet 93: 1234–1241 [DOI] [PubMed] [Google Scholar]
- Osmond CB, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46: 1351–1362 [Google Scholar]
- Rawsthorne S, Morgan CL, O'Neill CM, Hylton CM, Jones DA, Frean ML (1998) Cellular expression pattern of the glycine decarboxylase P protein in leaves of an intergeneric hybrid between the C3-C4 intermediate species Moricandia nitens and the C3 species Brassica napus. Theor Appl Genet 96: 922–927 [Google Scholar]
- Razmjoo K, Toriyama K, Ishii R, Hinata K (1996) Photosynthetic properties of hybrids between Diplotaxis muralis DC, a C3 species, and Moricandia arvensis (L.) DC, a C3-C4 intermediate species in Brassicaceae. Genes Genet Syst 71: 189–192 [Google Scholar]
- Rylott EL, Metzlaff K, Rawsthorne S (1998) Developmental and environmental effects on the expression of the C3-C4 intermediate phenotype in Moricandia arvensis. Plant Physiol 118: 1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73: 147–152 [Google Scholar]
- Surridge C (2002) The rice squad. Nature 416: 576–578 [DOI] [PubMed] [Google Scholar]
- Tolbert NE (1997) The C2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48: 1–25 [DOI] [PubMed] [Google Scholar]
- Ueno O (1996) Structural characterization of photosynthetic cells in an amphibious sedge, Eleocharis vivipara, in relation to C3 and C4 metabolism. Planta 199: 382–393 [Google Scholar]
- Ueno O (1998) Induction of Kranz anatomy and C4-like biochemical characteristics in a submerged amphibious plant by abscisic acid. Plant Cell 10: 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O (2001a) Environmental regulation of C3 and C4 differentiation in the amphibious sedge Eleocharis vivipara. Plant Physiol 127: 1524–1532 [PMC free article] [PubMed] [Google Scholar]
- Ueno O (2001b) Ultrastructural localization of photosynthetic and photorespiratory enzymes in epidermal, mesophyll, bundle sheath and vascular bundle cells of the C4 dicot Amaranthus viridis. J Exp Bot 52: 1003–1013 [DOI] [PubMed] [Google Scholar]
- Ueno O, Agarie S (1997) The intercellular distribution of glycine decarboxylase in leaves of cassava in relation to the photosynthetic mode and leaf anatomy. Jpn J Crop Sci 66: 268–278 [Google Scholar]
- Voznesenskaya EV, Artyusheva EG, Franceschi VR, Pyankov VI, Kiirats O, Ku MSB, Edwards GE (2001) Salsola arbusculiformis, a C3-C4 intermediate in Salsoleae (Chenopodiaceae). Ann Bot 88: 337–348 [Google Scholar]
- Wingler A, Lea P, Leegood RC (1997) Control of photosynthesis in barley plants with reduced activities of glycine decarboxylase. Planta 202: 171–178 [Google Scholar]
- Wingler A, Lea P, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B 355: 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Tian Z, Huang B, Huang R, Meng J (1999) Production of somatic hybrids between Brassica oleracea and the C3-C4 intermediate species Moricandia arvensis. Theor Appl Genet 99: 1281–1286 [Google Scholar]
- Zelitch I (1992) Control of plant productivity by regulation of photorespiration. BioScience 42: 510–516 [Google Scholar]