Abstract
Terpenoids are characteristic constitutive and inducible defense chemicals of conifers. The biochemical regulation of terpene formation, accumulation, and release from conifer needles was studied in Norway spruce [Picea abies L. (Karst)] saplings using methyl jasmonate (MeJA) to induce defensive responses without inflicting physical damage to terpene storage structures. MeJA treatment caused a 2-fold increase in monoterpene and sesquiterpene accumulation in needles without changes in terpene composition, much less than the 10- and 40-fold increases in monoterpenes and diterpenes, respectively, observed in wood tissue after MeJA treatment (D. Martin, D. Tholl, J. Gershenzon, J. Bohlmann [2002] Plant Physiol 129: 1003–1018). At the same time, MeJA triggered a 5-fold increase in total terpene emission from foliage, with a shift in composition to a blend dominated by oxygenated monoterpenes (e.g. linalool) and sesquiterpenes [e.g. (E)-β-farnesene] that also included methyl salicylate. The rate of linalool emission increased more than 100-fold and that of sesquiterpenes increased more than 30-fold. Emission of these compounds followed a pronounced diurnal rhythm with the maximum amount released during the light period. The major MeJA-induced volatile terpenes appear to be synthesized de novo after treatment, rather than being released from stored terpene pools, because they are almost completely absent from needle oleoresin and are the major products of terpene synthase activity measured after MeJA treatment. Based on precedents in other species, the induced emission of terpenes from Norway spruce foliage may have ecological and physiological significance.
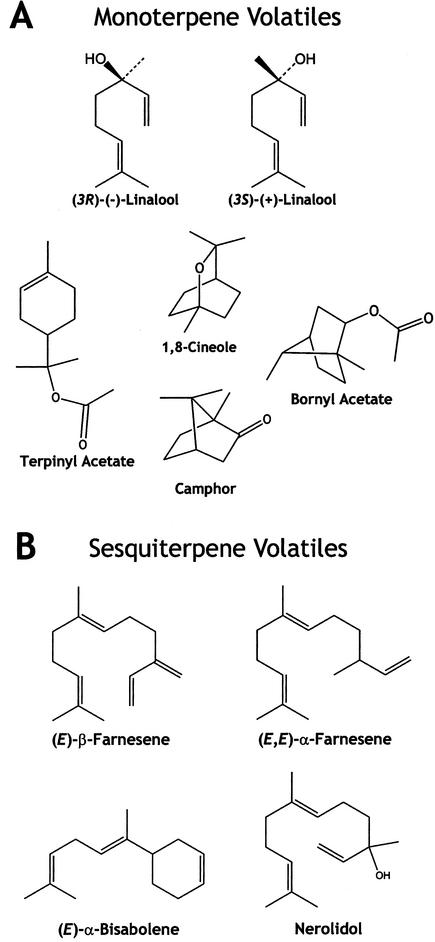
Terpenes contribute to the characteristic defense chemistry of conifers in many forms. Monoterpenes (C10) and diterpenes (C20) are major components of conifer oleroresins (Bohlmann and Croteau, 1999; Seybold et al., 2000; Trapp and Croteau, 2001), whereas sesquiterpenes (C15), though less abundant in resin, have enormous structural diversity (Steele et al., 1998b; Fig. 1). Terpene-containing oleoresin is mobilized to wound and infection sites and serves to protect conifers against herbivore and pathogen invasion because of its toxicity and deterrency (Phillips and Croteau, 1999). After exposure to the atmosphere, the volatile mono- and sesquiterpenes evaporate, whereas the diterpene acids polymerize, sealing the wound.
Figure 1.
Structures of monoterpenes (A) and sesquiterpenes (B) emitted by MeJA-treated Norway spruce saplings.
Oleoresin terpenes are products of constitutive and inducible pathways that have been characterized in some detail at the biochemical and molecular level in grand fir (Abies grandis; Lewinsohn et al., 1991; Bohlmann et al., 1998a; Steele et al., 1998b; Bohlmann and Croteau, 1999) and in Norway spruce (Picea abies; Fischbach et al., 2000; Martin et al., 2002; Fäldt et al., 2003). Much of this work has focused on the terpene synthases (TPSs), which catalyze the formation of the basic terpene carbon skeletons from acyclic phosphorylated intermediates (Bohlmann et al., 1998b). These enzymes convert geranyl diphosphate (GPP; C10), farnesyl diphosphate (FPP; C15), and geranylgeranyl diphosphate (GGPP; C20) to monoterpenes, sesquiterpenes, and diterpenes, respectively.
Research on conifer oleoresin biosynthesis to date has focused primarily on induced resin biosynthesis in the stem tissues. For example, it was shown recently that treatment of Norway spruce with methyl jasmonate (MeJA) induces a complex, traumatic oleoresin response including de novo differentiation of traumatic resin ducts in the developing xylem, increased accumulation of monoterpenes and diterpenes, and induced enzyme activities and gene expression of monoterpene synthases and diterpene synthases (Martin et al., 2002; Fäldt et al., 2003). In this regard, MeJA-induced resinosis in Norway spruce stems closely resembles responses to stemboring insects and pathogens in conifers (Alfaro et al., 2002; Franceschi et al., 2002; Martin et al., 2002).
In Norway spruce and other conifers, terpenes are accumulated not only in stems but also in needles (Schönwitz et al., 1987; Schönwitz et al., 1990a; Persson et al., 1996). However, much less is known about the biochemical regulation of terpene formation and accumulation in conifer needles. Simulated herbivory on needles was shown to increase monoterpene synthase activities in the foliage of different conifer species but had no effect on monoterpene accumulation (Litvak and Monson, 1998). In Norway spruce, monoterpene synthase activities have been characterized in the needles (Fischbach et al., 2000), and a gene encoding (+)-3-carene synthase was cloned from a cDNA library prepared from young shoots and foliage (Fäldt et al., 2003).
Besides their storage of terpenes, conifer needles also release a profusion of volatile terpenes into the environment, a topic studied by numerous researchers seeking to inventory the volatile organic compounds of the atmosphere (Kesselmeier and Staudt, 1999). Emission is influenced by physical factors, such as light, temperature, and biotic stress. Terpenes are also emitted from the foliage of a variety of angiosperm species, and much recent research has focused on emission in response to damage by insects or other arthropods. Terpenes serve as airborne signals that deter herbivores, attract predators and parasites of herbivores, or possibly act in plant-to-plant signaling (Turlings et al., 1990; Dicke and Vet, 1999; Paré and Tumlinson, 1999; Arimura et al., 2002; Kessler and Baldwin, 2002, Pichersky and Gershenzon, 2002) Herbivore-induced volatiles from gymnosperm foliage have also been reported. Egg deposition by the pine sawfly (Diprion pini) on Scots pine (Pinus sylvestris) needles triggers the release of volatiles, most likely monoterpenes and sesquiterpenes, which attract an egg parasitoid (Hilker et al., 2002). Knowledge of the regulation of volatile terpene formation is essential in understanding the mechanism of these responses and manipulating them in ecological studies.
The present study examines the biochemical regulation of terpene formation, accumulation, and release in the needles of Norway spruce. Based on the success of previous work with stems of this species (Martin et al., 2002), MeJA was chosen as a means to induce terpene-based responses. Unlike mechanical wounding, which has been used in several previous studies to trigger defense responses in conifers (Litvak and Monson, 1998; Steele et al., 1998b), MeJA does not inflict physical damage on terpene storage sites and does not destroy the tissues that are potentially involved in terpene biosynthesis, terpene accumulation, or terpene emission. This is of particular importance for attempts to characterize volatile emission in conifers that constitutively accumulate large quantities of low-Mr terpenes in resin canals or resin cells in needles. After application of MeJA, we monitored changes in the accumulation and emission of terpenes and the activity of TPSs.
RESULTS
MeJA Induces a 2-Fold Increase in Terpene Accumulation in Needles
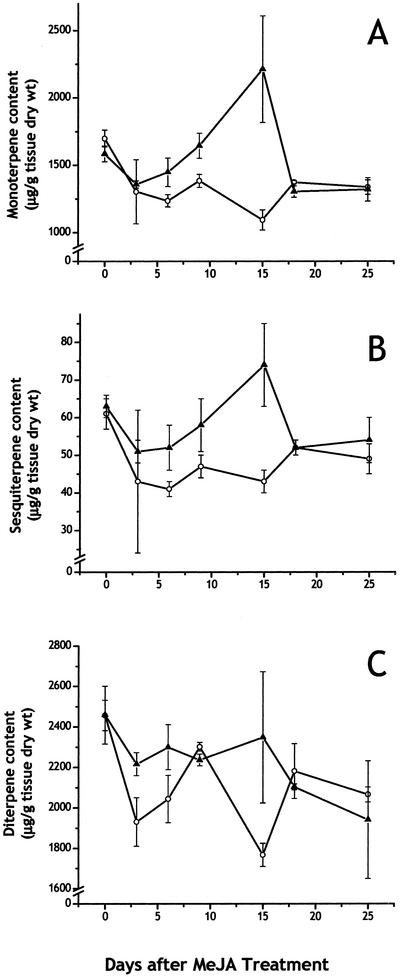
Based on recent reports that MeJA induces terpene biosynthesis in stem tissues of Norway spruce (Martin et al., 2002; Fäldt et al., 2003), we examined whether changes in terpene levels also occurred in needles of this species after MeJA treatment. Needles of young saplings (clone IA) harvested over a 25-d time course after treatment showed a transient, 2-fold increase in monoterpene and sesquiterpene accumulation compared with needles from untreated control saplings (Fig. 2, A and B). Maximum accumulation was observed 15 d after MeJA treatment with declines to control levels by 20 d. There was no significant increase in diterpene concentrations in treated saplings compared with untreated saplings over the time course of this experiment (Fig. 2C). The terpene composition of the needles was also not significantly affected by treatment (Table I). A total of 27 different monoterpenes, sesquiterpenes, and diterpenes were detected and most of them identified, of which the monoterpenes (bornyl acetate, α-pinene, camphene, and limonene) and the diterpenes (manool and dehydroabietate) were the most abundant.
Figure 2.
Time course of accumulation of monoterpenes (A), sesquiterpenes (B), and diterpenes (C) in needles of Norway spruce saplings (clone IA) after treatment with MeJA. Data are presented as the means with se of triplicate analyses from extracts of treated (▴) and control (○) trees.
Table I.
Composition of monoterpenes, sesquiterpenes, and diterpenes of Norway spruce needles of clone IA 15 d after treatment of trees with 10 mm MeJA in comparison with needles from untreated controls
Data are the means ± se of triplicate analyses.
| Terpene | Control | MeJA |
|---|---|---|
| μg g dry wt-1 | ||
| Monoterpenes | ||
| Tricyclene | 33.8 ± 1.8 | 64.4 ± 14.8 |
| α-Pinene | 165.9 ± 10.8 | 338.0 ± 62.0 |
| Camphene | 262.7 ± 17.6 | 542.1 ± 97.8 |
| β-Pinene | 36.7 ± 1.0 | 84.4 ± 17.8 |
| Sabinene | 0.0 ± 0.0 | 5.2 ± 3.4 |
| 3-Carene | 16.8 ± 0.2 | 22.4 ± 5.0 |
| Myrcene | 26.2 ± 1.6 | 52.1 ± 9.0 |
| Limonene | 54.9 ± 3.2 | 117.9 ± 20.0 |
| β-Phellandrene | 2.6 ± 2.6 | 5.6 ± 3.8 |
| 1,8-Cineole | 34.5 ± 1.2 | 77.8 ± 14.0 |
| Camphor | 5.7 ± 3.0 | 6.1 ± 4.0 |
| Bornyl acetate | 313.3 ± 27.0 | 624.3 ± 101.2 |
| Camphenilol | 58.8 ± 5.6 | 110.1 ± 19.0 |
| Terpinyl acetate | 7.4 ± 3.8 | 16.4 ± 2.4 |
| α-Terpineol | 14.4 ± 2.0 | 24.7 ± 3.2 |
| Borneol | 54.6 ± 10.0 | 106.1 ± 18.2 |
| Piperitone | 6.0 ± 3.0 | 15.4 ± 2.4 |
| Total monoterpenes | 1,094.5 ± 86.4 | 2,213.2 ± 372.6 |
| Sesquiterpenes | ||
| Longifolene | 3.7 ± 3.8 | 0 ± 0.0 |
| β-Caryophyllene | 21.3 ± 2.8 | 40.2 ± 7.2 |
| α-Humulene | 18.0 ± 2.2 | 34.3 ± 6.0 |
| Total sesquiterpenes | 43.1 ± 2.0 | 74.5 ± 6.6 |
| Diterpenes | ||
| Manool | 663.9 ± 35.8 | 820.7 ± 140.6 |
| Sandaracopimarate | 110.4 ± 3.6 | 140.8 ± 19.9 |
| Dehydroabietal | 22.6 ± 1.6 | 31.9 ± 1.1 |
| Dehydroabietate | 354.0 ± 13.6 | 546.3 ± 86.3 |
| Unknown 1 | 302.0 ± 12.4 | 346.4 ± 67.7 |
| Unknown 2 | 209.1 ± 12.9 | 325.5 ± 27.0 |
| Unknown 3 | 105.0 ± 11.7 | 136.4 ± 21.0 |
| Total diterpenes | 1767.0 ± 57.4 | 2348.0 ± 324.4 |
MeJA Induces a 5-Fold Increase in TPS Activity in Needles
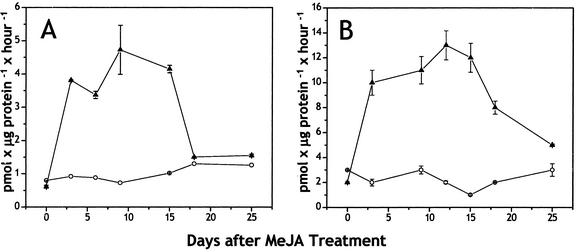
To determine if MeJA-induced terpene accumulation was due to de novo biosynthesis, we measured the activities of TPSs in cell-free extracts of needles of MeJA-treated and control saplings (clone IA) over a 25-d time course after treatment. Although the accumulation of monoterpenes and sesquiterpenes increased 2-fold in needles over this period in response to MeJA (Fig. 2), the activities of monoterpene synthases and sesquiterpene synthases increased over 5-fold, reaching a peak at d 10 or 12 and then declining precipitously after d 15 (Fig. 3). Diterpene synthase activity was not detectable in needle extracts from either treated or control trees, consistent with the lack of induction of diterpene accumulation in needles. The constitutive levels of diterpenes in mature needles (Fig. 2) could be due to enzyme activity in young needles and their accumulation and longevity in resin ducts.
Figure 3.
Time courses of monoterpene synthase (A) and sesquiterpene synthase (B) activities in the needles of Norway spruce saplings (clone IA) after treatment with MeJA. Data presented are the averages of duplicate assays from extracts of treated (▴) and control (○) trees. se are shown for treated tree samples. se for controls were in the range of 0.0002 pmol μg protein–1 h–1.
The Major TPS Products Are Linalool and (E)-β-Farnesene
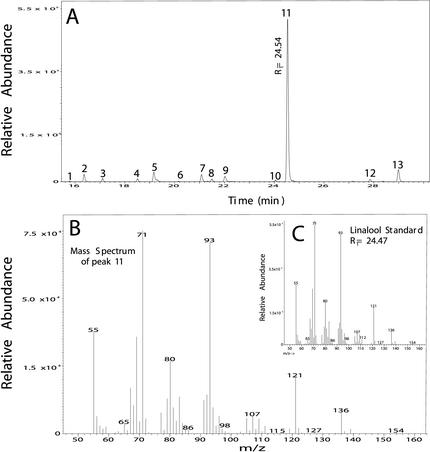
To investigate the discrepancy between the rates of TPS activity and terpene accumulation in needles after MeJA treatment, products of monoterpene synthase assays and sesquiterpene synthase assays were analyzed by gas chromatography (GC)-mass spectrometry (MS). Two clonal lines of different genetic background (IA and II) were used for these experiments thereby allowing us to address variation that might exist between these individual lines. In clone IA, the major product (55.4%) of the monoterpene synthase assays of MeJA-treated needles was the alcohol linalool (Table II), with an enantiomeric ratio of approximately 1:2 (3R:3S; Fig. 1). The terpene hydrocarbons, α-pinene (33.9%) and β-pinene (10.7%), were formed as additional products by monoterpene synthase activity in this clone (Table II). Linalool was also the major monoterpene product (72.9%) in enzyme assays with extracts of MeJA-treated needles of a second, unrelated Norway spruce clone (clone II; Fig. 4). In addition, enzyme assays with clone II also produced a number of minor monoterpene products (Fig. 4), each constituting 5% to 6% or less of the total product profile. Extracts of needles from control saplings of each clone (IA and II) showed only slight activity and any products formed were below the limits for identification by radio-GC. No detectable linalool synthase activity was present in extracts from control needles.
Table II.
Products of terpene synthase activities in needles of two Norway spruce clones (IA and II) after MeJA treatment
Assays were performed on pooled extracts harvested 3 to 15 d after treatment and analyzed by radio-GC. Product identification was confirmed by GC of authentic standards and GC-MS. Assay products with needles from untreated saplings were below the level required for identification of individual components. Products of phosphohydrolase activity seen in controls assays with buffer only are not listed.
| Assay Product
|
Total Products
|
|
|---|---|---|
| Clone IA | Clone IC | |
| % | ||
| Monoterpene synthase assays | ||
| Tricyclene | - | 0.2 |
| α-Pinene | 33.9 | 3.3 |
| Camphene | - | 1.3 |
| β-Pinene | 10.7 | 1.3 |
| Myrcene | - | 5.0 |
| 3-Carene | - | 0.2 |
| Limonene | - | 3.6 |
| (E)-β-ocimene | - | 1.2 |
| (Z)-β-ocimene | - | 3.0 |
| γ-Terpinene | - | 0.7 |
| Linalool | 55.4 | 72.9 |
| Borneol | - | 1.1 |
| α-Terpineol | - | 6.1 |
| Sesquiterpene synthase assays | ||
| Unknown 1 | - | 2.9 |
| β-Caryophyllene | - | 2.1 |
| (E)-β -farnesene | 100.0 | 17.2 |
| α-Humulene | - | 2.1 |
| (E)-bergomatene | - | 1.2 |
| (Z)-α-bisabolene | - | 0.5 |
| (E,E)-α-farnesene | - | 8.1 |
| (E)-γ-bisabolene | - | 0.6 |
| γ-Elemene | - | 0.4 |
| (E)-α-bisabolene | - | 30.3 |
| Nerolidol | - | 33.8 |
| Unknown 2 | - | 0.2 |
| Unknown 3 | - | 0.5 |
Figure 4.
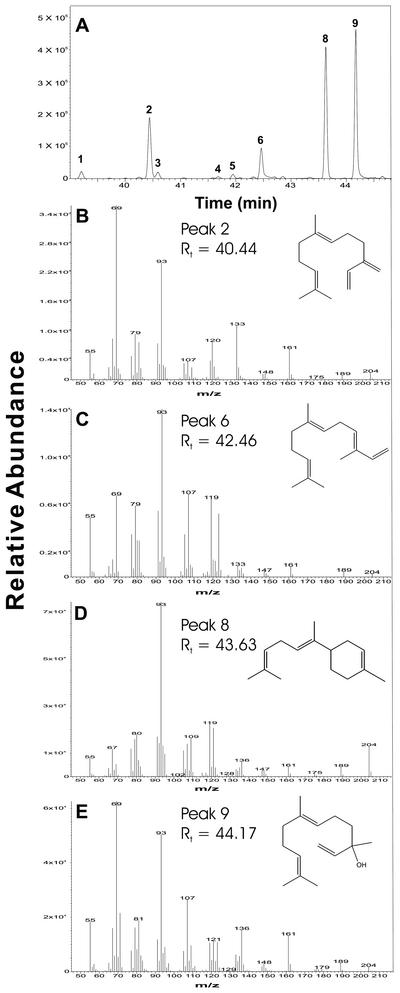
Identification of linalool as the major product of induced monoterpene synthase activity in clone II. Enzyme activity was assayed in extracts of needles harvested 30 h after MeJA treatment. GC-MS total ion chromatograph of monoterpene synthase assay products (A) showing 13 monoterpene products: 1, tricyclene (0.2%); 2, α-pinene (3.3%); 3, camphene (1.3%; 4, β-pinene (1.3%); 5, myrcene (5%); 6, 3-carene (0.2%); 7, limonene (3.6%); 8, (E)-β-ocimene (1.2%); 9, (Z)-β-ocimene (3%); 10, γ-terpinene (0.7%); 11, linalool (72.9%); 12, borneol (1.1%); and 13, α-terpineol (6.1%). Retention time and mass spectrum of peak 11 (B) match that of an authentic linalool standard (C).
The only product of the sesquiterpene synthase assays of MeJA-treated needles of clone IA was (E)-β-farnesene (Table II) as analyzed by radio-GC. Sesquiterpene synthase assays with extracts of MeJA-treated needles from clone II also produced (E)-β-farnesene (17.2%) as one of four major products. Additional major sesquiterpene synthase enzyme products of clone II were (E,E)-α-farnesene (8.1%), (E)-α-bisabolene (30.3%), and nerolidol (33.8%), together with five minor sesquiterpene hydrocarbons (Fig. 5). Each of the minor constituents contributed less than 3% of total sesquiterpene synthase products. Extract of needles from control untreated saplings showed low levels of sesquiterpene synthase enzyme activity, but product formation was below the limits for identification of individual sesquiterpene synthase products.
Figure 5.
Identification of major products of induced sesquiterpene synthase activity in clone II. Enzyme activity was assayed in extracts of needles harvested 30 h after MeJA treatment. GC-MS total ion chromatograph showing nine sesquiterpenes produced (A). Major products (peaks 2, 6, 8, and 9) were identified by comparison of retention time and mass spectra with authentic standards as (E)-β-farnesene (17.2%; B), (E,E)-α-farnesene (8.1%; C), (E)-α-bisabolene (30.3%; D), and nerolidol (33.8%; E).
MeJA Induces Increases in Terpene Emissions and Drastic Changes in the Composition of Terpene Emissions
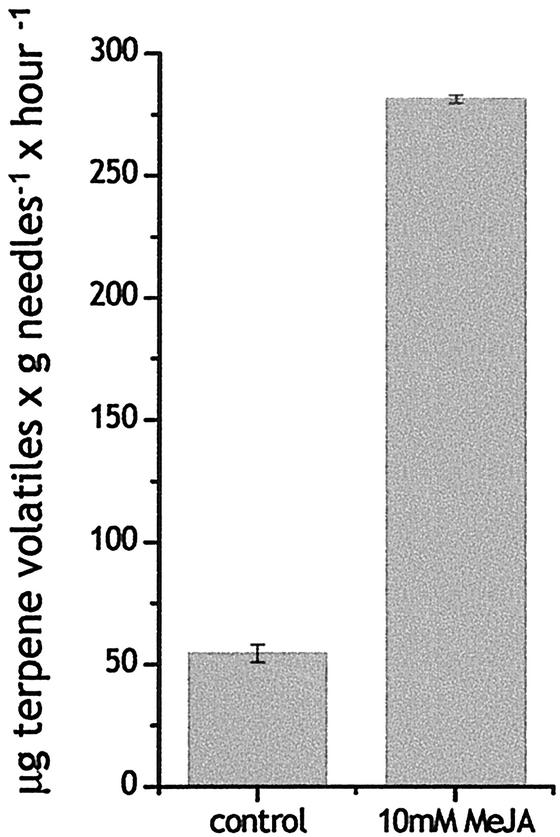
Because the major products of the induced needle monoterpene synthase and sesquiterpene synthase enzyme activities (Table II) in clone IA were not observed to accumulate in the needles (Table I), we analyzed volatile terpene emissions from Norway spruce saplings after MeJA treatment to access whether significant amounts of these products were released. Because of limited numbers of trees of the identical genetic background, we used a related (similar chemotype) clone IB for volatile analysis. Headspace collections revealed that the rate of terpene emission after MeJA treatment was approximately 5-fold that of unsprayed control saplings, 281.4 versus 54.3 μg g needle fresh weight h–1, averaged over a collection period of 4 d after treatment for two replicates of each treatment with two saplings in each (Fig. 6).
Figure 6.
Emission rates of total monoterpenes and sesquiterpenes from Norway spruce in control and MeJA-treated trees. Total volatiles were measured over a period of 93 h from two independent sets of treated and control trees of clone IB. Data represented are the mean quantities of volatiles emitted per hour and gram fresh weight needles. se bars are shown.
MeJA treatment also had a dramatic effect on the composition of volatile terpenes emitted by Norway spruce saplings measured at peak emission at around 24 to 30 h, or approximately 1 d, after treatment. During this peak emission, composition of volatiles collected over a period of 2 h (26.5–28.5 h after treatment) was analyzed (Table III). The rate of sesquiterpene emission was increased approximately 30-fold by MeJA 1 d after treatment, whereas that of monoterpenes was increased only 2.2-fold. Hence, sesquiterpenes, which made up only 19% of the terpene emission of untreated saplings, were 77% of the total terpene at peak emission after MeJA treatment. These compositional changes were largely maintained throughout the time course of up to 7 d. Among all terpenes emitted, the sesquiterpene (E)-β-farnesene became the most abundant compound after MeJA application (Table III). Its rate of emission increased over 100-fold, accounting for nearly three-quarters of the total sesquiterpenes. Among the monoterpenes, the oxygenated monoterpenes (linalool and 1,8-cineole) showed the greatest proportional increases after MeJA treatment and came to make up nearly 40% of the total monoterpenes. The prominence of linalool and (E)-β-farnesene emission after MeJA treatment in clone IB is consistent with them being the principal products of monoterpene and sesquiterpene synthase assays found in the similar clone IA.
Table III.
Composition of monoterpene and sesquiterpenes emitted from Norway spruce clone IB and collected over a period of 2 h between 26.5 and 28.5 h after treatment of trees with 10 mm MeJA in comparison with emission from untreated control saplings
| Terpene | Control | MeJA |
|---|---|---|
| ng h g needles fresh wt-1 | ||
| Monoterpenes | ||
| α-Pinene | 106.6 | 166.7 |
| Camphene | 28.1 | 40.7 |
| β-Pinene | 175.2 | 283.4 |
| Sabinene | 14.4 | 23.3 |
| 3-Carene | 16.0 | 8.4 |
| Myrcene | 40.6 | 51.9 |
| Limonene | 37.3 | 30.4 |
| β-Phellandrene | 31.2 | 18.1 |
| 1,8-Cineole | 20.4 | 101.6 |
| γ-Terpinene | 0.0 | 17.4 |
| Linalool | 6.5 | 313.6 |
| Bornyl acetate | 0.0 | 3.4 |
| Terpinen-4-ol | 0.0 | 4.1 |
| α-Terpinyl acetate | 4.4 | 4.6 |
| α-Terpineol | 6.0 | 19.7 |
| Borneol | 6.9 | 7.3 |
| Other monoterpenes | 8.3 | 26.0 |
| Total monoterpenes | 502.0 | 1120.7 |
| Sesquiterpenes | ||
| (Z)-α-bergamotene | 0.0 | 10.5 |
| (E)-β-farnesene | 26.7 | 2653.4 |
| (Z,E)-α -farnesene | 0.0 | 12.6 |
| (E,E)-α-farnesene | 29.4 | 34.2 |
| (E)-α-bisabolene | 62.9 | 972.3 |
| Other sesquiterpenes | 0.0 | 10.5 |
| Total sesquiterpenes | 118.9 | 3693.5 |
It was surprising to find that the major TPS assay products were volatilized rather than accumulating in the needles. To confirm this unexpected result, we investigated saplings of a second clone (clone II) from a completely different genetic background. After spraying with MeJA, the principal emitted monoterpenes, sampled by solid-phase microextraction (SPME), were β-pinene and linalool (Table IV), whereas the principal sesquiterpenes detected were (E)-β-farnesene, (E,E)-α-farnesene, and (E)-α-bisabolene. The major TPS assay products identified by GC-MS for this clone corresponded closely with the volatiles. Linalool was the dominant product formed in monoterpene synthase assays (Fig. 4), whereas (E)-β-farnesene, (E,E)-α-farnesene, (E)-α-bisabolene, and (E)-nerolidol were the dominant products formed in sesquiterpene synthase assays (Fig. 5). Thus, the biosynthetic machinery after MeJA induction appears to be devoted principally to the formation of emitted compounds as was shown with two independent Norway spruce clones.
Table IV.
Relative composition of terpene headspace volatiles emitted from saplings of Norway spruce (clone II) at different time points after treatment with 10 mm MeJA
| Compound | Total Terpenes
|
||
|---|---|---|---|
| 5.5 h after Treatment | 8.5 h after Treatment | 32.5 h after Treatment | |
| % | |||
| α-Pinene | 0 | 0 | 0 |
| Camphene | 0 | 0 | 2.1 |
| β-Pinene | 0 | 2.8 | 5.5 |
| β-Phellandrene | 0 | 0 | 0.9 |
| 1,8-Cineole | 0 | 0 | 2.0 |
| Linalool | 0 | 0 | 6.4 |
| Camphor | 13.7 | 2.1 | 0.8 |
| α-Terpineol | 0 | 0 | 1.1 |
| Bonyl acetate | 86.3 | 5.8 | 2.1 |
| (E)-β-farnesene | 0 | 38.1 | 26.0 |
| (Z,E)-α-farnesene | 0 | 0 | 1.4 |
| (E,E)-α-farnesene | 0 | 34.1 | 26.4 |
| (E)-α-bisabolene | 0 | 17.1 | 23.1 |
| Nerolidol | 0 | 0.0 | 2.1 |
Induced Terpene Emission Follows a Diurnal Rhythm
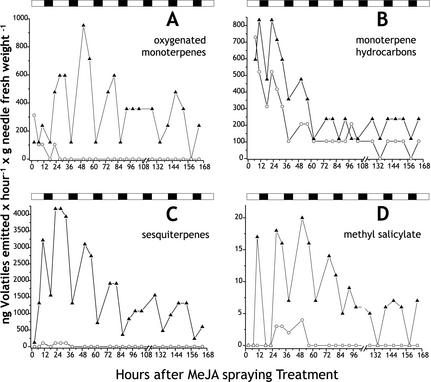
Over a time course of 7 d after MeJA treatment, the emission of induced volatiles of clone IB followed a day-night cycle with increased release rates during the light period and lower emission during the dark period (Fig. 7). Maximum levels of monoterpene and sesquiterpene emission were detected during the light period approximately 24 or 48 h after MeJA treatment (Fig. 7). Over the rest of the time course, the amplitude of the daytime maximum slowly declined. The more strongly induced classes of terpenes, the oxygenated monoterpenes and sesquiterpenes, followed a much more pronounced diurnal rhythm than the monoterpene olefins. When rhythmic emission profiles of individual oxygenated monoterpenes and sesquiterpenes were monitored, we found that few compounds such as terpinen-4-ol peaked very early within the first 12 h after treatment and then diminished rapidly during subsequent light periods, whereas other compounds such as linalool, 1,8-cineole, and (E)-β-farnesene were elevated during the light periods over the entire time course. In addition to the terpenes, MeSA was also found to be released after MeJA treatment in a diurnal rhythm at rates of less than 20 ng h g needle fresh weight–1 (Fig. 7D). This compound was not detected in most measurements with control saplings.
Figure 7.
Time course of diurnal emission of oxygenated monoterpenes (A), monoterpene hydrocarbons (B), sesquiterpenes (C), and methyl salicylate (MeSA; D) from Norway spruce saplings (clone IB) either treated with MeJA (▴) and or untreated (○). Volatiles were analyzed by GC-MS. Periods of light (white) and dark (hatched) phases are illustrated above each graph.
DISCUSSION
Induced Terpene Accumulation in Needles Is Much Lower Than in Wood
MeJA treatment of Norway spruce had profound effects on the synthesis, accumulation, and emission of terpenes in foliage that were quite different from the effects of MeJA on the synthesis and accumulation of terpenes in the stems of this species. The increases in terpene accumulation in foliage were much less dramatic than in stems. In saplings sprayed with a 10 mm solution of MeJA, the wood tissue of the stems showed a 12-fold increase in monoterpene concentration and a nearly 40-fold increase in diterpene concentration, peaking 15 to 25 d after treatment, but no change in sesquiterpene levels (Martin et al., 2002). In contrast, the same concentration of MeJA caused only a 2-fold increase in monoterpenes and sesquiterpenes in needles and no increase in diterpenes when measured 15 d after treatment. The increases in needles were of the same general magnitude as those seen in stem bark upon MeJA treatment (Martin et al., 2002). The large differences in the relative response of these organs can be attributed to the fact that induced terpene accumulation in wood tissue occurs in association with newly formed traumatic resin canals in the xylem (Franceschi et al., 2002; Martin et al., 2002). Bark and needles also possess resin canals, but de novo formation of these structures after treatment with MeJA has not been observed previously. Thus, these tissues do not have storage space for large quantities of additional terpene oleoresin.
The relatively small increase in terpene accumulation in needles was not accompanied by any significant changes in terpene composition, unlike in stems. The major needle monoterpenes detected in this study were similar to those previously reported for Norway spruce (Schönwitz et al., 1987; Persson et al., 1993, 1996; Paule and Yazdani, 1992; Holubova et al., 2000) and correspond to compositional type 1b (high limonene, low myrcene, and high bornyl acetate) as described by Schönwitz et al. (1990a). The elevated levels of monoterpenes and sesquiterpenes after MeJA spraying peaked 15 d after treatment but declined to control levels rapidly after that, probably as a result of volatilization or catabolism. Seasonal declines of monoterpene levels in Norway spruce foliage have been noted previously (Schönwitz et al., 1990b).
Induced TPS Activity in Needles Reflects Terpene Volatilization, Not Accumulation
Monoterpene and sesquiterpene synthase activities in Norway spruce foliage jumped 5-fold after MeJA treatment. Increases in monoterpene synthase activity of a similar magnitude (up to 4-fold) have been measured in the foliage of other conifer species after real or simulated herbivory (Litvak and Monson, 1998). On the other hand, in conifer stems, much greater increases in induced monoterpene and diterpene synthase activity were recorded: 5- to 25-fold increases in monoterpene synthase activity in grand fir after mechanical wounding or fungal inoculation (Lewinsohn et al., 1991, 1993; Funk et al., 1994), an approximately 400-fold increase in diterpene synthase activity in grand fir after mechanical wounding (Funk et al., 1994), and 20- to 30-fold increases in monoterpene and diterpene synthase activities in Norway spruce after MeJA treatment (Martin et al., 2002). However, only low increases in sesquiterpene synthase activities have been recorded in stems of grand fir, in keeping with the minor occurrence of this class in stem tissue (Steele et al., 1998b).
Although the 5-fold increases in monoterpene and sesquiterpene synthase activities in Norway spruce foliage after MeJA treatment are sufficient to account for the 2-fold increase in monoterpene and sesquiterpene accumulation, the major products of MeJA-induced TPS activity in Norway spruce needles [linalool and (E)-β-farnesene in clone IA; and linalool, (E)-β-farnesene, (E,E)-α-farnesene, and (E)-α-bisabolene in clone II] did not match the monoterpenes or sesquiterpenes accumulating in these organs. Instead, they corresponded well to the major terpene volatiles emitted from needles of these plants after MeJA treatment. Treatment with MeJA led to a 5-fold increase in monoterpene and sesquiterpene emission and to a drastic shift in the composition of emitted terpenes, from predominantly monoterpenes to predominantly sesquiterpenes. Nearly all of the major terpenes emitted after induction, including the monoterpene, linalool, and the sesquiterpenes, (E)-β-farnesene, (E,E)-α-farnesene (clone II only), and (E)-α-bisabolene, were released only in trace amounts before MeJA treatment. The emission of volatile monoterpenes from Norway spruce has been documented in many previous studies (Janson, 1993; Kempf et al., 1996; Kesselmeier and Staudt, 1999), but prior reports of sesquiterpene emission come only from other conifer species, such as Scots pine (Heiden et al., 1999).
Terpenes Emitted after Induction Are Synthesized De Novo and Released in a Diurnal Rhythm
Two lines of evidence indicate that the terpenes emitted in abundance after MeJA treatment are products of de novo synthesis and not released from storage reservoirs. First, these substances represent the principal products of induced TPS activities in needles present after treatment, and, second, they are nearly completely absent from stored needle oleoresin. This conclusion is corroborated by 13CO2 feeding experiments with Norway spruce that showed that volatilized terpenes were rapidly labeled from newly assimilated photosynthate (Schürmann et al., 1993; Kesselmeier and Staudt, 1999).
Several previous studies of terpene volatilization also attempted to distinguish between volatilization from preformed pools of resin terpenes and de novo synthesis and release. In some cases, both processes may be occurring. For example, in grand fir, volatilization of monoterpenes after stem wounding was accompanied by increased monoterpene synthase activity suggesting de novo synthesis (Lewinsohn et al., 1993). However, unlike in Norway spruce, the composition of volatile monoterpenes was similar to that of the accumulated oleoresin, suggesting emission from stored pools. In addition, after wounding, the monoterpene content of stems declined with respect to unwounded controls.
In several other conifer species, wounding of needles also reduced the pool of accumulated terpenes while increasing both monoterpene emission and monoterpene synthase activity (Litvak and Monson, 1998). In such cases, terpene synthesis may help replace compounds lost by wounding. However, in the present study, no net loss of terpenes from storage pools of needles was observed after induction, but rather a 2-fold increase could be detected due to the use of MeJA that avoided inflicting physical damage on terpene storage sites. With MeJA treatment and radio-GC to identify TPS assay products, the induced TPS activity could be unequivocally associated not with the formation of terpenes accumulating in needles but with de novo synthesis of the induced sesquiterpene-rich mixture of terpene volatiles. Emission of such mixtures may also occur in other conifers after wounding or other stresses, but sesquiterpene emission has not always been measured in previous studies of terpene volatilization. The biosynthetic machinery for de novo volatile formation may be localized separately from the sites of accumulated oleoresin formation in needles, which are thought to be associated with specialized resin cells or the epithelial cells of resin canals (Bouvier et al., 2000).
The volatilization of terpenes from Norway spruce saplings in our study followed a diurnal cycle, with total emission greater during the light periods than the dark periods regardless of treatment. Similar diurnal rhythms have been reported in previous studies of Norway spruce monoterpene emission (Bufler and Wegmann, 1991; Janson, 1993; Kesselmeier and Staudt, 1999), with peak nocturnal release only 50% to 75% as great as that at midday. Curiously, we observed much stronger rhythmic patterns of emissions for the two classes of highly induced compounds, sesquiterpenes and oxygenated monoterpenes, than for monoterpene olefins. Only very low amounts of sesquiterpenes and oxygenated monoterpenes were released during the dark period. For oxygenated monoterpenes, there is a precedent for this behavior. In stone pine (Pinus pinea), emission of the oxygenated monoterpenes, linalool and 1,8-cineole, along with the olefin (E)-β-ocimene, occurred only in daylight, whereas emission of other monoterpene olefins occurred during both day and night periods (Staudt et al., 2000). Emission of oxygenated terpenes may be closely correlated with stomatal opening because of their relatively low gas/liquid phase partition coefficients (Niinemets et al., 2002).
The Biological Functions of Terpene Emission Are Unknown
The emission of volatile terpenes by Norway spruce seems likely to have an important physiological or ecological function in this species because the rate of release after MeJA treatment is 5-fold higher than before treatment with a vastly different composition dominated by sesquiterpene and oxygenated monoterpenes and a strong diurnal rhythm. The major components of the volatilized blend, linalool, (E)-β-farnesene, (E,E)-α-farnesene, and (E)-α-bisabolene, are commonly reported as volatiles from other plant species after herbivore damage, where they serve to attract predators or parasitoids of the herbivores or repel herbivores directly (Paré and Tumlinson, 1997; De Moraes et al., 2001; Hern and Dorn, 2001; Kessler and Baldwin, 2001; Pichersky and Gershenzon, 2002). Similar roles can be envisioned for these components in Norway spruce. The release of herbivore-induced monoterpenes or sesquiterpenes from other species shares additional characteristics with the emission described in this study. It is often triggered by treatment with jasmonic acid or MeJA (Hopke et al., 1994; Dicke et al., 1999; Gols et al., 1999; Koch et al., 1999; Halitschke et al., 2000; Kessler and Baldwin, 2001; Rodriguez-Saona et al., 2001; Schmelz et al., 2001) and found to occur with a distinct diurnal rhythm (Loughrin et al., 1994; De Moraes et al., 2001). Moreover, MeSA, a non-terpenoid detected as a volatile in this study, has also been found to be released after herbivore damage in other species, where it functions in the attraction of herbivore enemies (Dicke et al., 1999; Ozawa et al., 2000; Kessler and Baldwin, 2001).
In addition to (or instead of) an ecological role, the terpene volatiles of Norway spruce could have a physiological function that is fulfilled inside the plant before emission. Other plant terpene volatiles, such as the simple C5 terpene, isoprene, and various monoterpenes, are thought to have internal roles also, either serving to increase the thermal tolerance of photosynthesis (Delfine et al., 2000; Sharkey et al., 2000), or protecting against oxidative damage (Loreto and Velikova, 2001). To learn more about the roles of the volatile and the nonvolatile terpenes of Norway spruce, we are continuing to study the regulation of terpene biosynthesis in this species using molecular and biochemical approaches.
In summary, we have demonstrated that terpene metabolism in Norway spruce needles responds to MeJA induction very differently than terpene metabolism in the stems of this species. Although needles showed only relatively small increases in terpene accumulation compared with stems, MeJA triggered emission of a novel blend of volatiles that is rich in sesquiterpenes and linalool, synthesized de novo, and released in a diurnal rhythm.
MATERIALS AND METHODS
Plant Materials
Young saplings of three clonal lines of Norway spruce (Picea abies Karst) were employed in these investigations. Clones IA (no. 3166-728) and IB (no. 273-728) were propagated in 1998 from lateral branches of current and previous year growth at the Niedersächsische Forstliche Versuchsanstalt (Escherode, Germany). These clones represent similar terpenoid chemotypes. Fully regenerated, 2-year-old, rooted saplings were grown and maintained in growth chambers as previously described (Martin et al., 2002). Two-year-old trees of different genetic background, clone II (no. 924335 L11), were regenerated from somatic embryos obtained from the Canadian Forest Service (Laval, Canada).
Substrates, Standards, and Reagents
Reagents were from Sigma-Aldrich (Steinheim, Germany) or Roth (Karlsruhe, Germany). Terpene standards were from Sigma-Aldrich, Roth, Bedoukian Research (Danbury, CT), and Helix Biotech (Richmond, BC, Canada) and were of the highest purity available. All solvents were GC grade. The substrates, [1-3H]GPP (20 Ci mol–1) and [1-3H]GGPP, were from Biotrend (Köln, Germany). [1-3H]FPP (125 Ci mol–1) was the gift of Rodney Croteau (Washington State University, Pullman). Unlabeled GPP and FPP were from Echelon Research Laboratories Inc. (Salt Lake City).
MeJA Treatment of Saplings and Harvest of Tissues
Saplings were sprayed with a 10 mm solution of 95% pure (w/w) MeJA (Sigma-Aldrich) in distilled water as described previously (Martin et al., 2002). Saplings of approximately 40 to 50 cm in height with two whorls of branches were sprayed in a ventilated fume hood with 150 mL of 10 mm 95% pure (w/w) MeJA (Sigma-Aldrich) in distilled water as described previously (Martin et al., 2002). Spraying was carried out over a period of 30 min to obtain a complete and even coating after which plants were left in a fume hood for 1 to 2 h until foliage was dry. Control saplings were sprayed with water. For measurements of terpene content and TPS activities, four MeJA-treated saplings and four control saplings of clone IA were used at each time point. After the stems were cut off just above the ground, needles were removed from the stems and branches, frozen in liquid nitrogen, and stored at –80°C. Combined needles were used to make three replicate extracts for terpene analysis (0.1 g of needles per extract) and one extract for TPS assays (5 g of needles per extract).
Volatile Collection
Dynamic headspace sampling was carried out with an automated collection system built by Analytical Research Systems (Gainesville, FL) as described (Degenhardt and Gershenzon, 2000). The apparatus was installed in a controlled environment chamber set at 22°C constant temperature, 75% relative humidity, 350 μmol m–2 s–1 photosynthetically active radiation, and a 16-h photoperiod. For each experiment, a pair of saplings, one control and one MeJA treated, were placed in separate glass cylinders. Volatiles were collected only from the aerial portions of the saplings, which were 40 to 50 cm high with two whorls of branches. The lower 2 cm of the stem and pots, soil, and roots were excluded from the volatile collection cylinder using a separation plate that closed loosely around the stem (see Röse et al., 1996, for a detailed illustration of the system). A stream of purified moistened air (50% relative humidity) was passed over each plant at a rate of 5 L min–1. Although the majority of the air passed through the plate opening around the stem, thereby preventing influx of ambient air, a portion exited through one of a series of eight glass sampling tubes, arrayed around the base of the glass cylinder, each containing 75 mg of Super Q (80/100 mesh, Alltech Inc, Nicholasville, KY). Collection was controlled with an automated port control system that drew efflux air for 2 h at a rate of 1 L min–1 through individual glass sampling tubes and then switched to another tube four times in a 24-h period over the 165.5-h collection time course. Volatiles were eluted from sampling tubes with 0.5 mL of dichloromethane containing 12.5 μg mL–1 isobutylbenzene as internal standard and were analyzed by GC-MS.
The time course was repeated three times with a separate pair of treated and control saplings, each time with similar results. Results of two experiments are shown in Figure 6, and one experiment is shown in detail in Table III. Plants of another clone (IB) were used because insufficient material of clone IA was available. These two clones had nearly identical terpene profiles in their needles. For comparison with IB, an additional clone (II) was treated with MeJA, and its volatiles were monitored by SPME analysis over a 4-d period after treatment. Saplings were enclosed in an oven bag (LookTerinex Ltd., Bedford, UK) for a 30-min equilibration period. SPME fibers were inserted into the bag, left for 1 h, and then analyzed immediately by GC-MS.
Extraction of Terpenes from Needles
Extraction of terpenes was essentially as previously described (Martin et al., 2002) based on a protocol by Lewinsohn et al. (1993). All steps of this procedure were carried out in 2-mL vials (glass with a teflon-coated screw cap, Hewlett-Packard, Palo Alto, CA). Needle samples of approximately 0.1 g (dry weight) were submerged into 1.5 mL of tert-butyl methyl ether in a 2-mL vial containing 60 μg mL–1 isobutylbenzene as an internal standard. Needles were extracted over 14 h with constant shaking at room temperature, and the extract was transferred to a fresh vial and washed with 0.3 mL of 0.1 m (NH4)2CO3 (pH 8.0) to remove acidic impurities. The ether layer was then prepared for GC or GC-MS analysis by filtering through a Pasteur pipette column filled with 0.3 g of silica gel (Sigma 60 Å) overlaid with 0.2 g of anhydrous MgSO4. The column was then eluted with 1 mL of diethyl ether to release bound oxygenated terpenes, and both eluates were combined in a fresh vial. Finally, the sample was evaporated to an approximate volume of 100 μL and was stored at –20°C. The dry weights of each extracted tissue were determined after drying at 70°C for 20 h. Means and ses were calculated from three replicate extracts per time point per treatment.
Analysis of Monoterpenes and Sesquiterpenes
Super Q eluates from automated volatile collection and ethereal extracts from needles were analyzed using a Hewlett-Packard 6890 GC-MS system (70 eV) with a DB-WAX column (0.25 mm × 0.25 μm × 30 m, J&W Scientific, Folsom, CA). Split injections (1 μL) were made at a ratio of 5:1 with an injector temperature of 220°C. The instrument was programmed from an initial temperature of 40°C (3-min hold) and increased at a rate of 1.5°C min–1 until 45°C, 3°C min–1 until 80°C, 5°C min–1 until 180°C, followed by an additional ramp of 10°C min–1 up to 240°C (5-min hold). Helium was used at a constant flow of 1 mL min–1. For routine quantification, monoterpene and sesquiterpene analysis of ethereal extracts was also accomplished with a Hewlett-Packard 6890 GC equipped with a flame ionization detector (FID) fitted with a DB-WAX column as described above. The flow rate was 2 mL H2 min–1, and the FID was operated at 300°C. One microliter of extract was introduced into the injection port at 220°C and a split ratio of 5:1. The GC was programmed with an initial oven temperature of 40°C (3-min hold), a ramp of 3°C min–1 until 80°C, then a ramp of 5°C min–1 until 180°C, followed by a final ramp of 15°C min–1 until 240°C (5-min hold).
For SPME analysis, the fiber was inserted directly into the injection port (200°C) of a GC-MS (6890 Agilent GC equipped with a 5973N quadrapole, 70 eV, Agilent, Palo Alto, CA), the program was immediately started, and the SPME fiber was removed after a period of 45 s. The GC was fitted with an HP-5 column (0.25 mm × 0.25 μm × 30 m, Hewlett-Packard), and the He carrier gas flowed at 0.7 mL min–1. The program had an initial oven temperature of 40°C (2-min hold), a ramp of 3°C min–1 until 160°C, then a ramp of 10°C min–1 until 200°C, followed by a final ramp of 20°C min–1 until 300°C (5-min hold). For analysis of chiral compounds, a Cyclodex-B (0.25 mm × 0.25 μm × 30 m, J&W Scientific) column was used with 1 μL of splitless injection. The oven was programmed with an initial temperature of 40°C (2-min hold), a ramp of 1°C min–1 until 80°C, a ramp of 5°C min–1 until 120°C, and a final ramp of 20°C min–1 until 200°C (5-min hold).
GC-FID- and GC-MS-generated peaks were integrated using Hewlett-Packard Chemstation software. Concentrations of monoterpenes and sesquiterpenes were calculated by comparing the integrated peak area with that of the internal standard isobutylbenzene. Identification of terpenes was based on comparison of retention times and mass spectra with authentic standards or with mass spectra in the Wiley library.
Enzyme Extraction
TPSs were extracted from needles essentially as previously described (Martin et al., 2002). Using an analytical grinding mill (A10, IKA WORKS, Cincinnati) or a mortar and pestle, 5 g of needles was ground to a fine powder in liquid nitrogen and combined with 50 mL of extraction buffer containing 50 mm MOPSO [3-(N-morpholino)-2-hydroxypropanesulfonic acid], pH 6.8, 5 mm ascorbic acid, 5 mm sodium bisulfite, 5 mm dithiothreitol, 10 mm MgCl2, 1 mm EDTA, 10% (v/v) glycerol, 1% (w/v) polyvinylpyrrolidone (Mr = 10,000), 4% (w/v) polyvinylpolypyrrolidone, 4% (w/v) Amberlite XAD-4, and 0.1% (v/v) Tween 20. The extract was allowed to shake at 4°C for 30 min and was then centrifuged at 10,000g for 30 min. The supernatant was then filtered through two layers of No. 1 filter paper (Whatman, Kent, UK), divided into 4-mL aliquots, frozen in liquid nitrogen, and kept at –80°C. Extracts were thawed only once before enzyme assay. Total protein concentration of each protein extract was determined with the Coomassie reagent and protocol of Bio-Rad Laboratories (Hercules, CA).
TPS Enzyme Assays
TPS activities were determined according to published procedures (Lewinsohn et al., 1991; Bohlmann et al., 1997; Lafever et al., 1994; Martin et al., 2002) with minor modifications. Before assaying enzyme activity, the frozen protein extracts were placed at 37°C until just thawed. The protein extracts were desalted in Bio-Rad Econo PacI0DG sizing columns preequilibrated with appropriate assay buffers. The mono-TPS assay buffer was 25 mm HEPES, pH 7.5, with 5 mm dithiothreitol, 10% (v/v) glycerol, 1 mm MnCl2, and 100 mm KCl. The sesqui-TPS assay buffer was 25 mm HEPES, pH 7.3, with 10 mm MgCl2, 10% (v/v) glycerol, and 10 mm dithiothreitol. The di-TPS assay buffer was 30 mm HEPES, pH 7.2, with 7.5 mm MgCl2, 20 μm MnCl2, 5% (v/v) glycerol, and 5 mm dithiothreitol. Enzyme activity was assessed with 1 mL of the desalted extracts with the addition of 10 μm GPP (with 1 μCi 3H-GPP) for mono-TPS assays, 7 μm FPP (1 μCi 3H-FPP) for sesqui-TPS assays, or 10 μm GGPP (0.5 μCi 3H-GGPP) for di-TPS assays. Monoterpene and sesquiterpene synthase enzyme assays with unlabeled substrates were done with 318 μm GPP or 157 μm FPP. All enzyme assays were done in duplicate, overlaid with 1 mL of pentane to collect released volatiles, and incubated at 30°C for 1.5 h. To stop all enzyme activity, the extracts were immediately frozen. After thawing, the aqueous assay fraction was rapidly extracted with the pentane fraction by vortexing, and separation of the aqueous and organic fractions was achieved by centrifugation at 2,500g for 2 min. The 1-mL pentane overlay was removed and filtered through a Pasteur pipette filled with 0.4 g of Silica gel (Sigma 60 Å) overlaid with 0.6 g of MgSO4 to remove nonspecific substrate hydrolysis products and to dry the pentane extract. Each enzyme assay was extracted with an additional two portions of pentane, vortexed, and centrifuged as before. These sequential extractions were also passed over the same column and pooled with the initial column eluent. Subsequently, the column was washed with pentane (2 × 1 mL), and the total volume was determined. The extracts were analyzed by liquid scintillation counting, 0.1 mL in 0.3 mL of Lipoluma (J.T. Baker, Deventer, The Netherlands), and GC. Pentane overlays were processed similarly for assays using non-labeled substrates, but the pentane was additionally washed with water to remove small organic acids (Peters et al., 2000).
Conditions for all enzyme assays, including pH optimum, incubation time, substrate concentration, and temperature optimum, were optimized for this system such that maximum activity was achieved in a linear range of product generation. In addition, the possibility that enzyme activities in induced tissues might have been inhibited by additional resin or phenolic substances was ruled out by experiments in which extracts from different stages of the time course were mixed together. In all cases, the resulting enzyme activity was additive, implying that additional compounds found in induced tissues had no effect on enzyme activity.
Analysis of TPS Assay Products
The extracts of enzyme assays were combined and evaporated to approximately 50 μL. From this, 2 μL was analyzed by GC-MS (6890 Agilent GC equipped with a 5973N quadrupole, 70 eV). The GC was fitted with an HP-5 column (0.25 mm × 0.25 μm × 30 m, Hewlett-Packard), the injection port was operated splitless at 200°C, and the He carrier gas flowed at 0.7 mL min–1. Both monoterpene and sesquiterpene synthase assays were analyzed by the same program. The oven temperature was programmed from 40°C (2-min hold) at 3°C min–1 until 160°C, then at 10°C min–1 until 200°C, followed by 20°C min–1 until 300°C (5-min hold). Chiral analysis of the monoterpene synthase assays was also carried out on GC-MS using a Cyclodex-B (0.25 mm × 0.25 μm × 30 m, J&W Scientific) column with 1-μL splitless injections. The oven temperature was programmed from 40°C (2-min hold) at 1°C min–1 until 80°C, then at 5°C min–1 until 120°C, followed by 20°C min–1 until 200°C (5-min hold). Products were identified by comparing retention times and mass spectra with those of authentic standards.
Acknowledgments
We thank Drs. Jürgen Schmidt and Armand Seguin for trees, Dr. Rodney Croteau for substrates, and Gazmed Zeneli, Tina Letsch, and Antje Thamm for excellent technical assistance. We would like to thank two anonymous reviewers and the editor for their insightful comments and suggestions that helped to improve an earlier version of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021196.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (funds to J.B.), by the Canadian Foundation for Innovation and the BC Knowledge and Development Funds (funds to J.B.), by the Human Sciences Frontier Program (funds to J.B.), by the Max Planck Society (funds to J.G. and fellowship to D.M.), and by the University of British Columbia (Walter C. Koerner Fellowship to D.M.).
References
- Alfaro RI, Borden JH, King JN, Tomlin ES, McIntosh RL, Bohlmann J (2002) Mechanisms of resistance in conifers against shoot infesting insects. In MR Wagner, KM Clancy, F Lieutier, TD Paine, eds, Mechanisms and Deployment of Resistance in Trees to Insects. Kluwer Academic Press, Dordrecht, The Netherlands, pp 101–126
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kühnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29: 87–98 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Crock J, Jetter R, Croteau R (1998a) Terpenoid-based defenses in conifers: cDNA cloning, characterization, and functional expression of wound-inducible (E)-α-bisabolene synthase from grand fir (Abies grandis). Proc Natl Acad Sci USA 95: 6756–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Croteau R (1999) Diversity and variability of terpenoid defenses in conifers: molecular genetics, biochemistry and evolution of the terpene synthase gene family in grand fir (Abies grandis). In DJ Chadwick, JA Goode, eds, Insect Plant Interactions and Induced Plant Defense. John Wiley and Sons Ltd., West Sussex, UK, pp 132–146 [DOI] [PubMed]
- Bohlmann J, Meyer-Gauen G, Croteau R (1998b) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95: 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Steele CL, Croteau R (1997) Monoterpene synthases from grand fir (Abies grandis). cDNA isolation, characterization, and functional expression of myrcene synthase, (–)-(4S)-limonene synthase, and (–)-(1S,5S)-pinene synthase. J Biol Chem 272: 21784–21792 [DOI] [PubMed] [Google Scholar]
- Bouvier F, Suire C, d'Harlingue A, Backhaus RA, Camara B (2000) Molecular cloning of geranyl diphosphate synthase and compartmentation of monoterpene synthesis in plant cells. Plant J 24: 241–252 [DOI] [PubMed] [Google Scholar]
- Bufler U, Wegmann K (1991) Diurnal variation of monoterpene concentrations in open-top chambers and in the Welzheim forest air, F.R.G. Atmosph Environ 25A: 251–256 [Google Scholar]
- Degenhardt J, Gershenzon J (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210: 815–822 [DOI] [PubMed] [Google Scholar]
- Delfine S, Csiky O, Seufert G, Loreto F (2000) Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol 146: 27–36 [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410: 577–580 [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907–1922 [Google Scholar]
- Dicke M, Vet L (1999) Plant-carnivore interactions: evolutionary and ecological consequences for plant, herbivore, and carnivore. In H Olff, V Brown, R Drent, eds, Herbivores: between Plant and Predators. Blackwell Science, Oxford, pp 483–520
- Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51: 119–133 [DOI] [PubMed] [Google Scholar]
- Fischbach RJ, Zimmer I, Steinbrecher R, Pfichner A, Schnitzler JP (2000) Monoterpene synthase activities in leaves of Picea abies (L.) Karst. and Quercus ilex L. Phytochemistry 54: 257–265 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89: 578–586 [DOI] [PubMed] [Google Scholar]
- Funk C, Lewinsohn E, Vogel BS, Steele CL, Croteau R (1994) Regulation of oleoresinosis in grand fir (Abies grandis): coordinate induction of monoterpene and diterpene cyclases and two cytochrome P450-dependent diterpenoid hydroxylases by stem wounding. Plant Physiol 106: 999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gols R, Posthumus MA, Dicke M (1999) Jasmonic acid induces the production of gerbera volatiles that attract the biological control agent Phytoseiulus persimilis. Entomol Exp Applic 93: 77–86 [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT (2000) Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124: 408–417 [DOI] [PubMed] [Google Scholar]
- Heiden AC, Hoffmann T, Kahl J, Kley D, Klockow D, Langebartels C, Mehlhorn H, Sandermann H Jr, Schraudner M, Schuh G et al. (1999) Emission of volatile organic compounds from ozone-exposed plants. Ecol Appl 9: 1160–1167 [Google Scholar]
- Hern A, Dorn S (2001) Induced emissions of apple fruit volatiles by the codling moth: changing patterns with different time periods after infestation and different larval instars. Phytochemistry 57: 409–416 [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varma M, Schrank K (2002) Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 205: 455–461 [DOI] [PubMed] [Google Scholar]
- Holubova V, Chvilickova I, Kuban V (2000) Comparison of procedures for isolation of monoterpene hydrocarbons from fresh needles of Picea abies and Picea omorica. Collect Czech Chem Commun 65: 1073–1081 [Google Scholar]
- Hopke J, Donath J, Blechert S, Boland W (1994) Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett 352: 146–150 [DOI] [PubMed] [Google Scholar]
- Janson RW (1993) Monoterpene emissions from Scots pine and Norwegian spruce. J Geophys Res 98: 2839–2850 [Google Scholar]
- Kempf K, Allwine E, Westberg H, Claiborn C, Lamb B (1996) Hydrocarbon emissions from spruce species using environmental chamber and branch enclosure methods. Atmosph Environ 30: 1381–1389 [Google Scholar]
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmosph Chem 33: 23–88 [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W (1999) Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol 121: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafever RE, Stofer-Vogel B, Croteau R (1994) Diterpenoid resin acid biosynthesis in conifers-enzymatic cyclization of geranylgeranyl pyrophosphate to abietadiene, the precursor of abietic acid. Arch Biochem Biophys 313: 139–149 [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R (1991) Defense-mechanisms of conifers: differences in constitutive and wound-induced monoterpene biosynthesis among species. Plant Physiol 96: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Muzika RM, Barton K, Croteau R (1993) Oleoresinosis in grand fir (Abies grandis) saplings and mature trees. Plant Physiol 101: 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak ME, Monson RK (1998) Patterns of induced and constitutive monoterpene production in conifer needles in relation to insect herbivory. Oecologia 114: 531–540 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Loughrin J, Manukian A, Heath R, Turlings T, Tumlinson J (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA 91: 11836–11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U, Reichstein M, Staudt M, Seufert G, Tenhunen JD (2002) Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol 130: 1371–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa R, Shimoda T, Kawaguchi M, Arimura G, Horiuchi J, Nishioka T, Takabayashi J (2000) Lotus japonicus infested with herbivorous mites emits volatile compounds that attract predatory mites. J Plant Res 113: 427–433 [Google Scholar]
- Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiology 114: 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–332 [PMC free article] [PubMed] [Google Scholar]
- Paule L, Yazdani R (1992) Geographical variation in monoterpene composition of foliar oleoresin in Swedish populations of Picea abies. Scand J For Res 7: 27–37 [Google Scholar]
- Persson M, Borg-Karlson A-K, Norin T (1993) Enantiomeric composition of six chiral monoterpene hydrocarbons in different tissues of Picea abies. Phytochemistry 33: 303–307 [Google Scholar]
- Persson M, Sjodin K, Borg Karlson AK, Norin T, Ekberg I (1996) Relative amounts and enantiomeric compositions of monoterpene hydrocarbons in xylem and needles of Picea abies. Phytochemistry 42: 1289–1297 [Google Scholar]
- Peters RJ, Flory JE, Jetter R, Ravn MM, Lee HJ, Coates RM, Croteau RB (2000) Abietadiene synthase from grand fir (Abies grandis): characterization and mechanism of action of the “pseudomature” recombinant enzyme. Biochemistry 39: 15592–15602 [DOI] [PubMed] [Google Scholar]
- Phillips MA, Croteau RB (1999) Resin-based defenses in conifers. Trends Plant Sci 4: 184–190 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Crafts-Brandner SJ, Paré PW, Henneberry TJ (2001) Exogenous methyl jasmonate induces volatile emissions in cotton plants. J Chem Ecol 27: 679–695 [DOI] [PubMed] [Google Scholar]
- Röse U, Manukian A, Heath RR, Tumlinson JH (1996) Volatile semio-chemicals released from undamaged cotton leaves—A systemic response of living plants to caterpillar damage. Plant Physiol 111: 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Schönwitz R, Merk L, Ziegler H (1987) Naturally occurring monoterpenoids in needles of Picea abies (L.) Karst. Trees 1: 89–93 [Google Scholar]
- Schönwitz R, Kloos M, Merk L, Ziegler H (1990a) Patterns of monoterpenes stored in the needles of Picea abies (L.) Karst. from several locations in mountainous regions of southern Germany. Trees Struct Funct 4: 27–33 [Google Scholar]
- Schönwitz R, Lohwasser K, Kloos M, Ziegler H (1990b) Seasonal variation in the monoterpenes in needles of Picea abies (L.) Karst. Trees 4: 34–40 [Google Scholar]
- Schürmann W, Ziegler H, Kotzias, Schönwitz, Steinbrecher R (1993) Emission of biosynthesized monoterpenes from needles of Norway spruce. Naturwissenschaften 80: 276–278 [Google Scholar]
- Seybold SJ, Bohlmann J, Raffa KF (2000) Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: evolutionary perspective and synthesis. Can Entomol 132: 697–753 [Google Scholar]
- Sharkey TD, Chen XY, Yeh S (2000) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Bertin N, Frenzel B, Seufert G (2000) Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees: implications for emission modeling. J Atmosph Chem 35: 77–99 [Google Scholar]
- Steele CL, Crock J, Bohlmann J, Croteau R (1998a) Sesquiterpene synthases from grand fir (Abies grandis): comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J Biol Chem 273: 2078–2089 [DOI] [PubMed] [Google Scholar]
- Steele CL, Katoh S, Bohlmann J, Croteau R (1998b) Regulation of oleoresinosis in grand fir (Abies grandis): differential transcriptional control of monoterpene, sesquiterpene, and diterpene synthase genes in response to wounding. Plant Physiol 116: 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Croteau R (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]