Abstract
The plant hormone auxin is a central regulator of plant development. In Arabidopsis, the PINOID (PID) protein serine/threonine kinase is a key component in the signaling of this phytohormone. To further investigate the biological function of PID, we performed a screen for PID-interacting proteins using the yeast two-hybrid system. Here, we show that PID interacts with two calcium-binding proteins: TOUCH3 (TCH3), a calmodulin-related protein, and PID-BINDING PROTEIN 1 (PBP1), a previously uncharacterized protein containing putative EF-hand calcium-binding motifs. The interaction between PID and the calcium-binding proteins is significant because it is calcium dependent and requires an intact PID protein. Furthermore, the expression of all three genes (PID, TCH3, and PBP1) is up-regulated by auxin. TCH3 and PBP1 are not targets for phosphorylation by PID, suggesting that these proteins act upstream of PID. PBP1 was found to stimulate the autophosphorylation activity of PID, and calcium influx and calmodulin inhibitors where found to enhance the activity of PID in vivo. Our results indicate that TCH3 and PBP1 interact with the PID protein kinase and regulate the activity of this protein in response to changes in calcium levels. This work provides the first molecular evidence for the involvement of calcium in auxin-regulated plant development.
The plant hormone auxin plays a central role in plant growth and development and has therefore been the subject of study for more than seven decades. Auxin is unique among the plant hormones in that it is actively transported in a polar fashion from its sites of biosynthesis. Polar auxin transport has been generally recognized as a major determinant underlying the action of this hormone, as shown by its involvement in developmental processes such as vascular differentiation and tropic growth (Luschnig et al., 1998; Mattson et al., 1999; Rashotte et al., 2000). The pin formed 1 (pin1) mutant of Arabidopsis is defective in polar auxin transport and develops pin-like inflorescences (Okada et al., 1991; Gälweiler et al., 1998). The PIN1 gene is part of a small gene family that encodes transporter-like membrane proteins. In accordance with their proposed function as efflux carriers in polar auxin transport, the cellular localization of these proteins was shown to be polar (Gälweiler et al., 1998; Müller et al., 1998). Recently, cycling of PIN-containing vesicles from endosomal compartments to the plasma membrane along the actin cytoskeleton was found to underlie the polar localization of PIN proteins (Geldner et al., 2001).
Loss-of-function pinoid (pid) mutants phenocopy pin mutants (Bennett et al., 1995), and phenotypic changes caused by ectopic expression of the PID protein kinase (Christensen et al., 2000) can be partially rescued by application of polar auxin transport inhibitors (Benjamins et al., 2001). Based on these observations, we proposed that PID is a positive regulator of polar auxin transport, although some aspects of PID activity can also be explained by feedback regulation on auxin signaling (Christensen et al., 2000; Benjamins et al., 2001). As protein kinases are signal transduction components per se, we refer to PID as a component in auxin signaling.
In 1973, dela Fuente and Leopold (dela Fuente and Leopold, 1973) suggested a role for calcium in the regulation of polar auxin transport. More than a decade later, Hasenstein et al. (1986) showed that calcium induces a transient inhibition of root elongation in a manner similar to treatment with low concentrations of auxin. The authors suggested that auxin action on root growth is mediated by an auxin-induced increase in the level of cytosolic free calcium ([Ca2+]cyt), which in turn induces growth responses. This hypothesis was verified in a later study in which auxin was shown to induce an increase in [Ca2+]cyt within minutes after its application (Gehring et al., 1990). Evidence for the role of calcium in polar auxin transport came from gravistimulation studies. After gravistimulation, [Ca2+]cyt peaks were found to coincide with the basipetal movement of auxin at the lower side of the root from the root tip toward the elongation zone (Lee et al., 1984). Moreover, roots were found to curve toward a calcium-containing agar block and away from a block containing the calcium-chelating agent EGTA (Lee et al., 1983). These observations indicate that the variations in [Ca2+]cyt during root gravitropic response are coupled to the direction of auxin transport, and they suggest that auxin transport is directed by local increases in [Ca2+]cyt, or that [Ca2+]cyt peaks are induced by increased auxin levels in cells at the lower side of the root tip. Recently, Plieth and Trewavas (2002) used transgenic seedlings expressing aequorin to show that [Ca2+]cyt is transiently increased in roots upon gravistimulation. However, evidence against a role for calcium in gravitropism has also been reported (Legue et al., 1997). This underlines the complications that are encountered in determining the exact role of calcium in a auxin-regulated processes, because calcium is involved in a large number of other cellular processes, such as ethylene action (Lau et al., 1977), stomatal opening (Wood et al., 2000), vesicle aggregation (dela Fuente and Parra, 1995), and plant defense (Lecourieux et al., 2002).
Here, we describe the interaction of PID with two different calcium-binding proteins (CBPs), one of which is TOUCH3 (TCH3), a calmodulin-related protein involved in touch response (Braam and Davis, 1990) and the other being PID-BINDING PROTEIN 1 (PBP1), which contains putative EF-hand calcium-binding motifs. Our data show that these interactions are specific and calcium dependent, thereby indicating a role for calcium in the regulation of PID activity.
RESULTS
PID Interacts with CBPs
The yeast two-hybrid system was used to screen two Arabidopsis cDNA libraries for proteins that interact with the PID protein Ser/Thr kinase. Three independent transformation experiments, each yielding a saturating number of transformants, identified 25 positive clones that did not show autoactivation after retransformation with the empty pAS2-1 vector. These 25 positive clones represent three different genes. Here, we present the analysis of two of these genes, which encode CBPs (Table I). One of the CBP genes, TCH3 (At2g41100), was identified previously by Braam and Davis (1990) in a screen for genes that are up-regulated in response to mechanical stimuli such as wind and touch. TCH3 (324 amino acids) is designated a calmodulin-related protein because it has six EF-hand calcium-binding sites in contrast to the four EF-hand calcium-binding sites that are normally found in calmodulins (Braam, 1992). The second gene we identified, PINOID BINDING PROTEIN 1 (PBP1, At5g54490), encodes a previously uncharacterized protein of 127 amino acids with three possible EF-hand calcium-binding motifs. The sequence of two of these EF-hands does not completely match the canonical EF-hand consensus sequence, suggesting that these calcium-binding motifs may not be functional (Fig. 1). However, EF-hands are often found to work in pairs, and the function of the nonperfect EF-hands can be enhanced through the cooperative action of the perfect EF hand, resulting in high-affinity binding of calcium (Ikura, 1996).
Table I.
Interactors of PID identified by yeast two-hybrid screening
Number of clones obtained from three independent transformation experiments in which at least 1.0 × 106 transformants were generated.
| Interactor
|
Two-Hybrid cDNA
Librarya
|
|
|---|---|---|
| Green parts | Roots | |
| TCH3 | 2 | 7 |
| PBP1 | 2 | 13 |
See “Materials and Methods.”
Figure 1.
Protein sequence and calcium-binding motifs of PBP1. A, Protein sequence of PBP1. Putative EF-hands (underlined) were identified by scanning multiple databases (http://www.expasy.ch). B, Alignment of EF-hands present in PBP1 with the consensus sequence for this structural feature (Marsden et al., 1990).
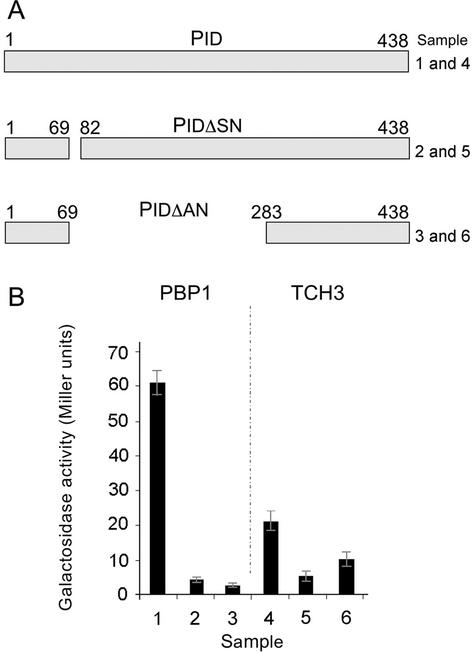
In the plating assays (data not shown) and the β-galactosidase activity assay, the interaction between PBP1 and PID was stronger than the interaction between TCH3 and PID (Fig. 2B). To determine the specificity of the interaction, two mutations were introduced into the PID coding region in the pAS-PID vector. One mutation results in a small deletion of amino acids 69 to 82, whereas the other mutation deletes most of the catalytic domain of the PID protein kinase (Fig. 2A, amino acids 82–283). Both deletions significantly reduced the interaction between PID and PBP1 or TCH3 (Fig. 2B). The deletions in PID cannot be used to map the interaction sites because they may affect the general protein structure of PID and lead to conformational changes that make binding of the interactors less efficient. However, the results do imply specificity of interaction between the protein kinase and the CBPs.
Figure 2.
PID interacts with PBP1 and TCH3 in yeast. Yeast cells containing pACT-PBP1 or pACT-TCH3 and pAS2-1-PID (lanes 1 and 4), pAS2-1-PIDΔSN (lanes 2 and 5), or pAS2-1-PIDΔAN (lanes 3 and 6). A, Two in-frame deletions of PID were used, one that deletes amino acids 69 to 82 and another that deletes residues 82 to 283 (lane 6). B, α-Galactosidase assays using the above-mentioned combinations.
Interaction of TCH3 and PBP1 with PID Is Calcium Dependent
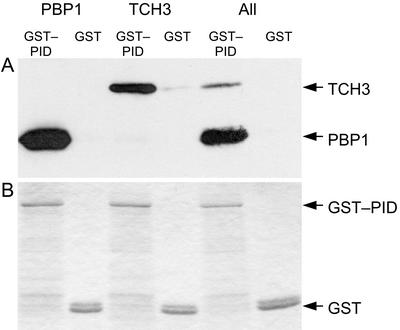
To confirm the interactions detected in the yeast two-hybrid system, we performed in vitro pull-down assays with glutathione S-transferase (GST):PID. In these experiments, His-tagged versions of TCH3 and PBP1 interacted specifically with PID; even both proteins were present in the same extract (Fig. 3). The stronger interaction between PBP1 and PID observed in the yeast two-hybrid system was also observed in the in vitro pull-down assays with GST:PID. However, the stronger signal observed in the pull-down assays is partly due to the fact that the production of His-tagged PBP1 in E. coli is more efficient than that of the His-tagged TCH3 protein.
Figure 3.
In vitro interaction of PBP1 and TCH3 with PID. A, Western-blot analysis with anti-His antibodies detects His-tagged PBP1 and TCH3 after pull down with GST:PID from the soluble fraction of Escherichia coli extracts. GST alone is used as a control for the specificity of binding. B, Loading differences visualized by Coomassie Blue staining of GST:PID and GST. The His-tagged-interacting proteins are not sufficiently abundant to be visualized by this staining method.
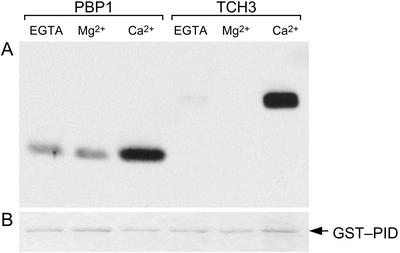
When the 10 mm calcium chloride in the binding buffer was replaced by 10 mm EGTA or 10 mm magnesium chloride, no HIS:TCH3 signal was detected with the anti-HIS antibody on the resulting western blot (Fig. 4). For PBP1, the binding to GST:PID was significantly reduced in the absence of calcium, but was not completely abolished (Fig. 4). These findings indicate that TCH3 binding to PID is calcium dependent, whereas the interaction between PID and PBP1 is enhanced by calcium.
Figure 4.
Calcium dependency of the in vitro interaction between PBP1 or TCH3 and PID. A, Protein gel-blot analysis with anti-His antibodies to detect pull down of His-tagged PBP1 or TCH3 with GST:PID from E. coli extracts in the presence or absence of calcium. CaCl2 (10 mm) in the binding buffer was exchanged with 10 mm MgCl2 or 10 mm EGTA. B, Loading differences visualized by Coomassie Blue staining of a parallel gel containing the same samples as used in A.
PID, TCH3, and PBP1 Are Auxin-Regulated Genes
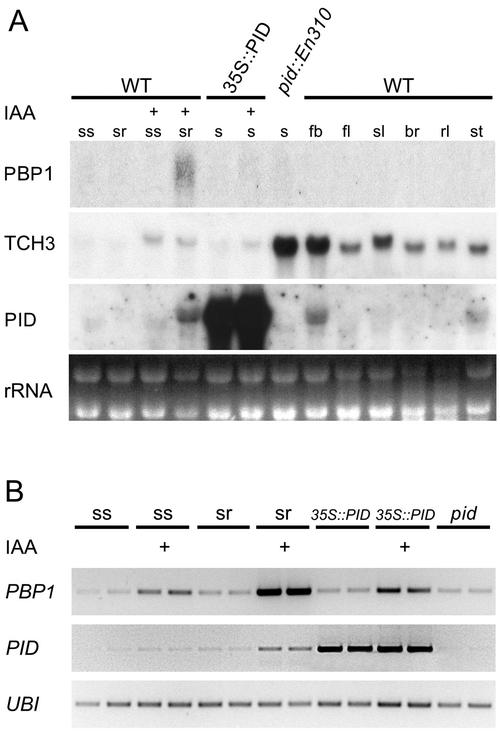
One criterion for a possible functional interaction between two proteins is that they colocalize in the same cells and/or tissues. TCH3 was previously shown to accumulate in cells or tissues that are exposed to mechanical strain, such as attachment points of secondary and cauline leaves to the stem, but the protein can also be found in xylem cells (Antosiewicz et al., 1995). The TCH3 transcript level was shown to be up-regulated by light, heat shock, and auxin treatment (Braam and Davis, 1990; Braam, 1992; Sistrunk et al., 1994; Antosiewicz et al., 1995). Expression analysis of the TCH3 gene confirmed that the gene is auxin inducible and is expressed throughout the plant (Fig. 5; Antosiewicz et al., 1995). PID transcription levels are also up-regulated by external auxin application, and the expression pattern of PID seems to overlap with that of TCH3 in the vasculature of the root and in flower buds (Antosiewicz et al., 1995; Benjamins et al., 2001). The highest levels of TCH3 expression were observed in flower buds where the PID mRNA is also most abundant. Interestingly, TCH3 expression was strongly up-regulated in seedlings of the pid::En310 loss-of-function mutant, and the auxin inducibility of TCH3 was slightly reduced in the 35S::PID overexpression background. These results suggest that in seedlings, there is feedback regulation on TCH3 expression by PID.
Figure 5.
Expression analysis of PBP1, TCH3, and PID. Expression pattern of both interactors was compared with the pattern of PID gene expression. A, Total RNA (10 μg) was isolated from 7-d-old seedling shoots (ss) and roots (sr), as well as from intact 35S::PID#21 and pid::En310 mutant seedlings (s), untreated or treated for 5 h with 5 μm indole-3-acetic acid (IAA) (+), and from different plant organs: flower buds (fb), flowers (fl), siliques (sl), bracts (br), rosette leaves (rl), and stems (st). The observed differences in running characteristics of the TCH3 transcript between samples is likely to be due to the different RNA isolation methods used. B, Reverse transcriptase (RT)-PCR analysis of PBP1 and PID expression in seedling tissues. The UBIQUITIN (UBI) gene was used as a quantitative control. Abbreviations and IAA treatments are compatible with those mentioned for A. Two independent parallel samples were taken to exclude quantitative errors.
RNA-blot analysis showed that the PBP1 transcript was not detectable in wild-type tissues, and was not detectable in the 35S::PID gain-of-function and pid loss-of-function mutants. PBP1 mRNA was only weakly detectable in roots of Arabidopsis seedlings after IAA treatment (Fig. 5A). RT-PCR analysis again detected the highest PBP1 expression in auxin-treated seedling roots, but a slight increase in expression was also detectable in seedling shoots after auxin treatment (Fig. 5B). PBP1 expression was unchanged in 35S::PID and pid mutant backgrounds, implicating that PBP1 expression is not dependent on PID. These results indicate that PBP1 expression, as with PID and TCH3 expression, is responsive to auxin.
TCH3 and PBP1 Are Not Phosphorylation Targets of PID
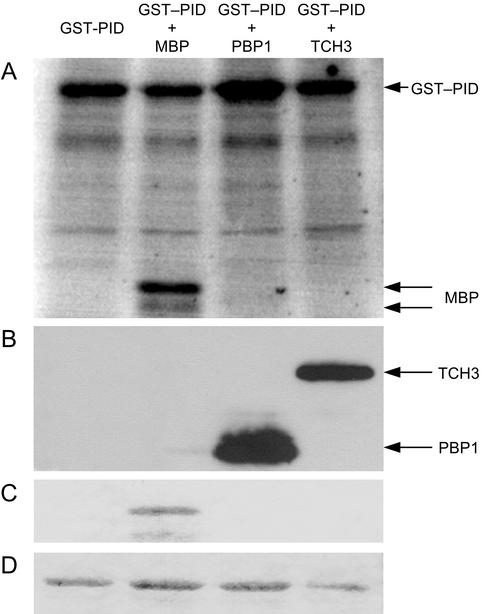
In vitro kinase assays using GST:PID and His-tagged TCH3 and PBP1 were performed to determine whether TCH3 and PBP1 are targets for phosphorylation by PID. Christensen et al. (2000) previously demonstrated that a GST:PID fusion protein shows autophosphorylation activity in vitro. However, the trans-phosphorylation capacity of PID has not yet been demonstrated. Therefore, we began by testing the capacity of PID for trans-phosphorylation using myelin basic protein (MBP) as an artificial substrate. GST:PID phosphorylates MBP, showing that the GST: PID protein is also active as a protein kinase (Fig. 6). Next, we determined whether PID phosphorylates TCH3 and PBP1 by coincubating GST:PID with the His-tagged versions of TCH3 or PBP1 in the presence of calcium. GST:PID did not phosphorylate TCH3 or PBP1, whereas autophosphorylation of GST:PID was still detectable. Interestingly, autophosphorylation of GST:PID was reproducibly enhanced in the presence of PBP1 (Fig. 6). Therefore, our findings suggest that TCH3 and PBP1 are not direct downstream targets of PID phosphorylation. Moreover, the increase in autophosphorylation of GST:PID in the presence of PBP1 suggests that this CBP is an upstream regulator of PID activity.
Figure 6.
Phosphorylation activity of GST:PID. A, Autophosphorylation of GST:PID (first lane), and phosphorylation of MBP (second lane). No phosphorylation of PBP1 or TCH3 was observed (third and fourth lanes, respectively). B, Parallel nonlabeled protein samples were run on a gel and were checked for the presence of the two His-tagged-interacting proteins by western blotting using anti-His antibodies. C and D, A second protein gel stained with Coomassie Blue detects MBP (C) and shows equal loading of the GST:PID fusion protein (D).
Calcium Negatively Regulates PID Activity in Vivo
Seedlings of 35S::PID lines show agravitropic growth of the hypocotyl and the root, and a few days after germination, the primary root tip loses its meristematic identity and eventually collapses (Benjamins et al., 2001). If calcium acts as a second messenger in PID signaling in vivo, then it would be expected that the effects of PID overexpression are influenced by inhibitors of the calcium signaling pathway. Therefore, we used the 35S::PID root collapse phenotype as a bioassay to monitor the effect of such inhibitors on PID activity.
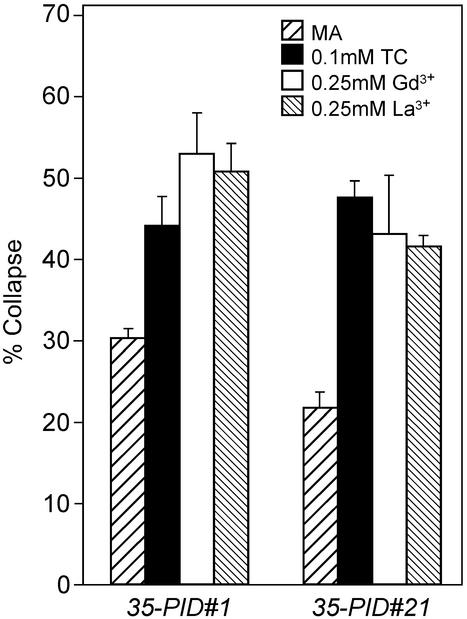
Wild-type seeds, and seeds of two overexpression lines, 35S::PID#1 and 35S::PID#21, were germinated on medium with the calmodulin-inhibitor tetracain (TC) and the calcium channel blockers, GdCl3 and LaCl3, and 3 d after germination, the number of seedlings with collapsed roots was counted. Germination of 35S::PID seeds on medium containing 0.1 mm TC or different concentrations of the calcium channel blockers LaCl3 and GdCl3 (0.10–0.25 mm) significantly increased the percentage of collapsed root tips (Fig. 7). The concentrations used did not inhibit growth of wild-type (Colombia [Col-0]) seedlings (data not shown), whereas higher concentrations of TC (>0.25 mm) or of the calcium channel blockers (0.50 mm or higher) did inhibit wild-type root growth. Germination on medium lacking calcium could not be tested because this severely affects the growth rate of roots of (wild-type) seedlings. These results indicate that the application of calcium signaling inhibitors can, in a narrow concentration window, significantly enhance the effect of PID activity in the 35S::PID background (t-test: P > 0.05). The data imply that PID activity is negatively regulated by calcium and/or calmodulins. This function may be performed by one of the PID-interacting CBPs. However, we cannot exclude that the compounds used here interfere with pathways that act in parallel with PID signaling, and, therefore, we consider these data as a first indication of the functionality of the PID-CBP interaction. Future research, including in vivo pull-down experiments and double mutant studies, will further confirm the role of the CBPs in PID action.
Figure 7.
Effect of calcium influx and calmodulin inhibitors on root tips of two 35S::PID lines (5). Seedlings of 35S::PID lines 1 and 21 were grown on normal MA medium and MA medium containing 0.1 mm TC (calmodulin inhibitor), 0.25 mm GdCl3, or 0.25 mm LaCl3 (calcium influx inhibitors). The timing of collapse of the primary root meristem was determined by counting the number of seedlings with collapsed root meristems 3 d after germination. The experiments were performed three times for line 1 and two times for line 21. The averages of the experiments are shown ± the se of the mean.
DISCUSSION
The protein Ser/Thr kinase PID was previously shown to be a component in auxin signaling (Christensen et al., 2000; Benjamins et al., 2001). To obtain more insight into the signaling pathways that involve PID, we characterized proteins that interact with PID in a yeast two-hybrid system.
Two of the proteins identified as PID interactors are CBPs, one of which is the calmodulin-related protein encoded by the touch-responsive gene TCH3 (Braam and Davis, 1990). The other protein, PBP1, contains three putative EF-hand calcium-binding sites. The significance of the interaction of these CBPs with PID was supported by the repeated identification of cDNAs corresponding to these CBPs in the two-hybrid screens, by the calcium dependency of the interactions in in vitro pull-down assays, and by the fact that the interaction in the yeast two-hybrid system only occurred with the intact version of the PID protein. Moreover, PID, TCH3, and PBP1 are all up-regulated by treatment with auxin and are expressed in overlapping tissues, indicating that the spatial and temporal distribution of the proteins in the plant could permit the interaction to occur in vivo.
PID belongs to the plant-specific ACG group VIII family of protein Ser/Thr kinases (Christensen et al., 2000; Benjamins et al., 2001), of which only a few members have been studied in detail. Two of these ACG group VIII kinases, NPH1/PHOT1 and NPL1/PHOT2 act as UV-A/blue light receptors in directing phototropic growth and chloroplast movement, respectively (Christie et al., 1998; Jarillo et al., 2001). Baum et al. (1999) showed that photostimulation of NPH1/PHOT1 induces a rapid transient increase in [Ca2+]cyt. This observation and the interaction of PID with two CBPs make it tempting to speculate that PID activity may also induce a rapid transient increase in [Ca2+]cyt. Auxin treatment is known to induce an elevation of [Ca2+]cyt (Gehring et al., 1990), and PID could be involved in inducing this initial calcium peak. However, the relatively slow increase in PID mRNA after auxin treatment (Benjamins et al., 2001) suggests that this calcium peak can only occur in cells where PID is already present, and that the PID proteins that are produced in response to auxin then cause a second wave of increased [Ca2+]cyt.
Based on the in vitro phosphorylation assays, we conclude that TCH3 and PBP1 are not downstream targets of phosphorylation by PID, but rather, they act as upstream regulators of PID activity. TCH3, PBP1, and PID are auxin-responsive genes, and although we do not know the exact timing of auxin-induced TCH3 and PBP1 expression, this observation suggests that the respective gene products are present in cells with relatively high auxin levels. PBP1 is expressed at a low level, but its interaction with PID is relatively strong, even in the absence of calcium. PBP1 appears to enhance the autophosphorylating activity of PID in vitro. These results suggest that PBP1 acts as a cofactor to positively regulate PID activity in specific tissues; however, it is not known if autophosphorylation activates the PID protein kinase. TCH3 is expressed at a much higher level than PBP1. The observation that the TCH3-PID interaction is completely dependent on the presence of calcium, and that a calmodulin inhibitor enhances PID activity, suggests that TCH3 negatively regulates PID activity. TCH3 expression is elevated in the pid loss-of-function background, implying that in seedlings, PID regulates its own activity through feedback control of TCH3 expression.
Our observations suggest a fine-tuned mechanism underlying the control of PID activity, including transcriptional control by auxin of the three genes (PID, PBP1, and TCH3), feedback regulation, autophosphorylation, and the interference of the two CBPs with the activity of the kinase. The identification of TCH3 as a PID-interacting protein also suggests a mechanism linking touch responses and calcium to auxin transport. TCH3 is proposed to be involved in tissue reinforcement and cell expansion through interference with vesicular transport (Braam et al., 1997). Considering the involvement of vesicular trafficking along actin filaments in the localization of auxin efflux carriers, this could indicate a role for TCH3 and PID in this process and thereby in auxin transport (Geldner et al., 2001).
Calcium and auxin have been proposed as regulators of the same cellular processes, including the establishment of cell polarity, growth, and vesicular transport along actin filaments and secretion (dela Fuente and Parra, 1995; Chen et al., 1999; Hepler et al., 2001; Vissenberg et al., 2001). In 1973, dela Fuente and Leopold (dela Fuente and Leopold, 1973) showed that treatment of sunflower stem sections with EGTA (a calcium chelator) reduced auxin transport and that this transport was restored after washing with calcium. In later reports, a relationship between auxin transport and the location of calcium peaks during root gravitropism was suggested. Later, Evans and coworkers (Evans et al., 1992; Young and Evans, 1996) found evidence for the involvement of calcium in gravistimulated movement of auxin in root tips. Although this stimulation can be indirect, it indicates that there is a link between changes in calcium peaks and auxin transport. The authors suggest that gravity-induced calcium asymmetry is important in establishing auxin asymmetry during the root gravitropic response. Other reports corroborate the long-thought crosstalk between auxin and calcium signaling. For example, Yang and Poovaiah (2000) identified a small auxin upregulated RNA protein as a calmodulin-binding protein, whereas Okamoto et al. (1995) described the isolation of an auxin-regulated calmodulin gene from mung bean (Vigna radiata).
In conclusion, the identification of the PID-interacting proteins TCH3 and PBP1 has brought us closer to the dissection of the biological function of this protein kinase in auxin-mediated plant growth and development. The fact that PID, a component in auxin signaling, exhibits calcium-dependent interactions with CBPs reveals for the first time to our knowledge how the auxin response may be coupled to the second messenger calcium. Further functional and expression analysis of TCH3 and PBP1 is needed to determine the dynamics of the interaction with PID and the exact role of these interactors in the PID signaling pathway.
MATERIALS AND METHODS
Two-Hybrid Interaction
The Matchmaker yeast two-hybrid system (Clontech, Palo Alto, CA) was used to screen two Arabidopsis cDNA libraries fused to the GAL4-activation domain (pACT) with a PID:GAL4-DNA-binding domain (pAS2-1) fusion. One cDNA library was constructed using mRNA isolated from green parts of 6-week-old flowering Arabidopsis plants. The second cDNA library was constructed using a 1:1 ratio of mRNA from auxin-treated (1 μm 1-naphthaleacetic acid for 24 h) and wild-type roots of 10-d-old Arabidopsis seedlings. The yeast strain PJ69-4a (James et al., 1996) containing pAS2-1-PID was transformed with each of the pACT cDNA libraries (Gietz et al., 1992), and was screened for His and adenine auxotrophy. Growth rates of transformants were compared with controls (the wild-type strain and the wild-type strain containing only pAS2-1-PID). When screening for adenine auxotrophy, reddening of the colonies was used as an indication for the strength of interaction. Screens were performed at 20°C and 30°C. α-Galactosidase assays in liquid yeast cultures were performed as described (Meijer et al., 2000).
RNA Purification, Northern-Blot, and RT-PCR Analysis
Total RNA was purified using the RNeasy kit (Qiagen, Valencia, CA) or using the method described by Jaakola et al. (2001). Northern-blot analysis was performed using approximately 10 μg of RNA per sample as previously described (Memelink et al., 1994).
For RT-PCR purposes poly-A RNA was isolated from tissues of 7-d-old Arabidopsis (Col-0) seedlings using the QuickPick mRNA isolation kit (Bio-Nobile, Turku, Finland). Poly-A RNA was eluted in 10 μL, of which 9 μL was added to 2 μL of 0.1 m dithiothreitol, 4 μL of 5× first strand buffer, and 1 μL (200 units) of Superscript reverse transcriptase (Invitrogen, Carlsbad, CA) in a total volume of 20 μL. RT was performed for 50 min at 37°C and was then stopped by heating to 70°C for 15 min. The resulting cDNA samples were diluted eight times, and 2 μL was used as template in a PCR using the gene-specific primer pairs PBP1-RT-F1 (5′-CTCCTAAATCCTCAACAAGACC-3′) and PBP1-RT-R1 (5′-TGCCGGTAAAACTCTTCCT-3′) for PBP1; PID-RT-F1 (5′-GTTAGATCCGACGGTCACATT-3′) and PID-RT-R1 (5′GTAAGCGTACGAATGAGCGC-3′) for PID; and UBI5-F (5′-AACCCTTGAGGTTGAATCATC-3′) and UBI5-R (5′-GTCCTTCTTTCTGGTAAACGT-3′) for UBIQUITIN. PCR conditions were optimized for PBP1, and this PCR program (25 cycles of 30 sec at 95°C, 30 sec at 53°C, and 90 sec at 72°C) was used for all three genes.
Plant Lines and Growth Conditions
Seedlings of wild-type Col-0 and two 35S:PID lines (Col-0 background; Benjamins et al., 2001) were plated on MA medium (Masson and Paszkowski, 1992) containing 0, 0.1, 0.25, or 0.50 mm tetracain, LaCl3, or GdCl3. Plates were incubated vertically for 8 d at 21°C (16-h photoperiod, 3,000–4,000 lux). During this time, the development of the seedling root was monitored and scored for collapse of the primary root tip (Benjamins et al., 2001).
Protein Purification, in Vitro Interaction, and Phosphorylation Assays
The complete coding region of PID, excluding the start codon, was PCR amplified from the PID cDNA using the primer pairs PID-SalI-F1(5′-GG-SalI-TTACGAGAATCAGACGGTGAG-3′) and PID-XbaI-R1 (5′-CC-XbaI-CCGTAGAAAACGTTCAAAAGT-3′). The XbaI-site of the amplified product was blunted and the resulting fragment was ligated at the C terminus of GST in the SalI-site in the pGEX-KG vector (Guan and Dixon, 1991). The complete cDNAs (excluding the start codon) of TCH3 and PBP1 were PCR amplified using the primer pairs TCH3-SalI-F1 (5′-AA-SalI-GCGGATAAGCTCACTGA-3′) and TCH3-PstI-R1 (5′-GG-PstI-GAAGCGCTGTTATCTTGA-3′), and PBP1-SalI-F1 (5′-AA-SalI-GCATCTCCTAAATCCTCAAC-3′) and PBP1-PstI-R1 (5′-AA-PstI-AGAGTTTTACCGGCATTGA-3′, respectively. The PCR products were first cloned as SalI-PstI fragments into pBluescript-SK+ and subsequently as a SalI-SmaI fragments at the C terminus of the His-tag (10× His) in pET16b. Escherichia coli BL21-DE3 cells were used for heterologous expression of the recombinant proteins. The GST:PID fusion protein was purified by binding to a gluthathione-sepharose matrix (Amersham-Pharmacia, Piscataway, NJ) essentially as described in Christensen et al. (2000). E. coli cell lysates (in binding buffer (50 mm Tris-HCL, pH 6.8, 100 mm NaCl, 10 mm CaCl2, and 0.1% [w/v] Tween 20) expressing the His-tagged interactors, were incubated with the GST:PID-bound matrix for 1.5 h at 4°C. After several washes with binding buffer, SDS/PAGE sample buffer was added, the samples were boiled, and SDS-PAGE (12.5% [w/v] gel) analysis was performed. The proteins were blotted on a polyvinylidene difluonide membrane and the His-tagged interactors were detected using penta-His-antibodies (Qiagen). A duplicate SDS-polyacrylamide gel was Coomassie stained to check for loading differences.
After binding of the His-tagged interactors and washing of the matrix, a small fraction (10 μL) of the GST:PID-bound matrix was used for in vitro phosphorylation assays. Phosphorylation buffer (5×: 125 mm Tris-HCl, pH 7.5, 25 mm MgCl2, and 1 mm EDTA) and 1 μL of [α-32P]ATP (3,000 Ci mm–1) were added to the matrix fraction and the samples were incubated for 30 min at 30°C. For MBP phosphorylation, 1 μg of MBP (no. M1891; Sigma, St. Louis) was added to the GST:PID-bound matrix. SDS-sample buffer was added to quench the reaction and then SDS-PAGE was performed. The gel (12.5%, w/v) was washed for 2 h at room temperature in running buffer to remove unincorporated label and was then vacuum dried. Detection was performed using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Images were analyzed using ImageQuant software (Molecular Dynamics).
Acknowledgments
We thank Johan Memelink and Bert van der Zaal for providing the green part and the auxin-induced root-specific two-hybrid cDNA libraries, respectively, Peter Hock and Tobias Sieberer for help with the figures and RT-PCR analysis, respectively, Dolf Weijers and Haico van Attikum for valuable discussions, and Christian Luschnig and Kim Boutilier for valuable comments on the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.019943.
References
- Antosiewicz DM, Polisensky DH, Braam J (1995) Cellular localization of the Ca2+ binding TCH3 protein of Arabidopsis. Plant J 8: 623–636 [DOI] [PubMed] [Google Scholar]
- Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 69: 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas PJJ, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520 [Google Scholar]
- Braam J (1992) Regulation of expression of calmodulin and calmodulin-related genes by environmental stimuli in plants. Cell Calcium 13: 457–463 [DOI] [PubMed] [Google Scholar]
- Braam J, Davis RW (1990) Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 60: 357–364 [DOI] [PubMed] [Google Scholar]
- Braam J, Sistrunk ML, Polisensky DH, Xu W, Purugganan MM, Antosiewicz DM, Campbell P, Johnson KA (1997) Plant responses to environmental stress: regulation and functions of the Arabidopsis TCH genes. Planta Suppl 203: S35–S41 [DOI] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- dela Fuente RK, Leopold AC (1973) A role for calcium in auxin transport. Plant Physiol 51: 845–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Fuente RK, Parra AV (1995) Vesicle aggregation by annexin I: role of a secondary membrane binding site. Biochemistry 34: 10393–10399 [DOI] [PubMed] [Google Scholar]
- Evans ML, Young LM, Hasenstein KH (1992) The role of calcium in the regulation of hormone transport in gravistimulated roots. Adv Space Res 12: 211–218 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA 87: 9645–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gietz D, St-Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 25: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE (1991) Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem 192: 262–267 [DOI] [PubMed] [Google Scholar]
- Hasenstein KH, Evans ML (1986) Calcium dependence of rapid auxin action in maize roots. Plant Physiol 81: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Ikura M (1996) Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci 21: 14–17 [PubMed] [Google Scholar]
- Jaakola L, Pirtila AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19: 201–203 [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144: 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410: 952–954 [DOI] [PubMed] [Google Scholar]
- Lau OL, John WW, Yang SF (1977) Effect of different cytokinins on ethylene production by mung bean hypocotyls in the presence of indole-3-acetic acid or the calcium iron. Physiol Plant 39: 1–3 [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Mulkey TJ, Evans ML (1983) Reversible loss of gravitropic sensitivity in maize roots after application of calcium chelators. Science 220: 1375–1376 [DOI] [PubMed] [Google Scholar]
- Lee JS, Mulkey TJ, Evans ML (1984) Inhibition of polar calcium movement and gravitropism in roots treated with auxin-transport inhibitors. Planta 160: 536–543 [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden BJ, Shaw GS, Sykes BD (1990) Calcium binding proteins: elucidating the contributions to calcium affinity from an analysis of species variants and peptide fragments. Biochem Cell Biol 68: 587–601 [DOI] [PubMed] [Google Scholar]
- Masson J, Paszkowski J (1992) The culture response of Arabidopsis thaliana protoplasts is determined by the growth conditions of donor plants. Plant J 2: 208–218 [Google Scholar]
- Mattson J, Sung ZR, Berleth T (1999) Responses of plant vascular system to auxin transport inhibition. Development 117: 2979–2991 [DOI] [PubMed] [Google Scholar]
- Meijer AH, Schouten J, Ouwerkerk PBF, Hoge JHC (2000) Yeast as a versatile tool in transcription factor research. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp E3 1–28
- Memelink J, Swords KMM, Staehelin LA, Hoge JHC (1994) Southern, northern and western blot analysis. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp F 1 –23
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement for the polar auxin transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Tanaka Y, Sakai S (1995) Molecular cloning and analysis of the cDNA for an auxin-regulated calmodulin gene. Plant Cell Physiol 36: 1531–1539 [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol 129: 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady S, Reed R, Ante S, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 120: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Braam J (1994) Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell 6: 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Feijo JA, Weisenseel MH, Verbelen JP (2001) Ion fluxes, auxin and the induction of elongation growth in Nicotiana tabacum cells. J Exp Bot 52: 2161–2167 [DOI] [PubMed] [Google Scholar]
- Wood NT, Allan AC, Haley A, Viry-Moussaid M, Trewavas AJ (2000) The characterization of differential calcium signaling in tobacco guard cells. Plant J 24: 335–344 [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW (2000) Molecular and biochemical evidence for the involvement of calcium/calmodulin in auxin action. J Biol Chem 275: 3137–3143 [DOI] [PubMed] [Google Scholar]
- Young LM, Evans ML (1996) Patterns of auxin and abscisic acid movement in the tips of gravistimulated primary roots of maize. Plant Growth Regul 20: 253–258 [DOI] [PubMed] [Google Scholar]