Abstract
Field-grown Miscanthus × giganteus maintains high photosynthetic quantum yields and biomass productivity in cool temperate climates. It is related to maize (Zea mays) and uses the same NADP-malic enzyme C4 pathway. This study tests the hypothesis that M. × giganteus, in contrast to maize, forms photosynthetically competent leaves at low temperatures with altered amounts of pyruvate orthophosphate dikinase (PPDK) and Rubisco or altered properties of PPDK. Both species were grown at 25°C/20°C or 14°C/11°C (day/night), and leaf photosynthesis was measured from 5°C to 38°C. Protein and steady-state transcript levels for Rubisco, PPDK, and phosphoenolpyruvate carboxylase were assessed and the sequence of C4-PPDK from M. × giganteus was compared with other C4 species. Low temperature growth had no effect on photosynthesis in M. × giganteus, but decreased rates by 80% at all measurement temperatures in maize. Amounts and expression of phosphoenolpyruvate carboxylase were affected little by growth temperature in either species. However, PPDK and Rubisco large subunit decreased >50% and >30%, respectively, in cold-grown maize, whereas these levels remained unaffected by temperature in M. × giganteus. Differences in protein content in maize were not explained by differences in steady-state transcript levels. Several different M. × giganteus C4-PPDK cDNA sequences were found, but putative translated protein sequences did not show conservation of amino acids contributing to cold stability in Flaveria brownii C4-PPDK. The maintenance of PPDK and Rubisco large subunit amounts in M. × giganteus is consistent with the hypothesis that these proteins are critical to maintaining high rates of C4 photosynthesis at low temperature.
The C4 photosynthetic pathway is considered to have the highest theoretical efficiency and potential productivity of all forms of higher plant photosynthesis because it largely eliminates the competing process of photorespiration (for review, see Long, 1999; Sage, 1999). However, this efficiency is normally realized only in high-light, humid, and warm environments (Long, 1999). Although C4 plants include some of our most productive crop species (e.g. maize [Zea mays], sorghum [Sorghum bicolor], and sugarcane [Saccharum officinarum]), early season growth and the extent of their growing range are limited by poor performance at low temperatures. Cold sensitivity is of particular importance to maize, where low temperature effects are the major limitation on production toward the northern edge of its current range of cultivation (Miedema et al., 1987; Greaves, 1996). Specifically, the expression of key photosynthetic enzymes and photosynthetic rates are reduced in maize grown at 14°C (Nie and Baker, 1991; Nie et al., 1992; Nie et al., 1995). It is unclear whether this cold-induced dysfunction derives from direct effects of temperature on gene expression, indirect effects on photosynthesis through the accumulation of damaging active oxygen species under photoinhibitory conditions, or a combination of these changes (Long et al., 1994).
The rhizomatous perennial grass Miscanthus × giganteus (Greef and Deuter ex Hodkinson and Renvoize; Hodkinson and Renvoize, 2001) is from the same taxonomic group as sugarcane (Saccharum officinarum), sorghum (Sorghum bicolor), and maize (Z. mays) and uses the same C4 photosynthetic pathway (NADP-ME form). In contrast to maize, field observations of M. × giganteus show that it maintains high quantum yields of CO2 assimilation under the chilling conditions (i.e. below 12°C) of the early growing season in southern England (Beale et al., 1996). M. × giganteus may also differ from other low-temperature tolerant C4 genera such as Muhlenbergia and Flaveria in its ability to achieve, in a cool temperate climate, efficiencies of energy conversion into biomass equivalent to those recorded for other C4 species in warm climates (Beale and Long, 1995). Thus, these field studies of M. × giganteus suggest that it could have an exceptional ability to maintain high photosynthetic rates while growing in a cool climate. However, because temperatures are continually fluctuating in the field, these findings do not preclude the possibility that these photosynthetically competent leaves were formed during brief periods of warm temperatures. If M. × giganteus, when grown in continuously controlled low temperature, can be shown to produce leaves with a high photosynthetic capacity, then this species will provide a resource for understanding how its agronomically important relatives might be altered to similarly achieve high photosynthetic capacity with growth at low temperature.
Using Flaveria and Amaranthus transgenically modified to express altered levels of enzymes of photosynthetic carbon metabolism, Rubisco, pyruvate orthophospate dikinase (PPDK), and phosphoenolpyruvate carboxylase (PEPc) have been shown to exert metabolic control over light-saturated C4 photosynthesis (Matsuoka et al., 2001). At low intercellular CO2 concentrations, control resides with PEPc, but at higher concentrations, including those typically found in photosynthesizing C4 leaves, regeneration of PEP is normally limiting. Rubisco and PPDK appear to share control of PEP regeneration (Furbank et al., 1997). Thus, chilling sensitivity in C4 species is expected to depend critically on the sensitivity of these key enzymes to cold temperatures.
In maize, photosynthetic efficiency and biomass accumulation are highly correlated with PPDK activity, but not with Rubisco (Sugiyama and Hirayama, 1983; Ward, 1987). Other studies have shown a correlation of photosynthesis with Rubisco and PPDK (Baer and Schrader, 1985; Usuda et al., 1985). A key role for PPDK in controlling C4 photosynthesis at low temperature is suggested from low extracted activities, which are often only just sufficient to support in vivo rates of photosynthesis (for review, see Long, 1983), and cold lability of the protein (Sugiyama, 1973; Shirahashi et al., 1978; Sugiyama et al., 1979). The C4 photosynthetic isoform of PPDK is low-temperature labile, with a sharp transition in activation energy requirement around 10°C (Shirahashi et al., 1978; Du et al., 1999a). The low temperature lability of PPDK appears to be dependent on subunit dissociation; the dimeric form is required for enzymatic activity (Shirahashi et al., 1978; Ohta et al., 1996). Specific amino acid residues have been associated with cold stability in PPDK from the cold-adapted C4 plant, Flaveria brownii (Ohta et al., 1996).
In contrast to the effects of temperature on PPDK, there appears to be no correlation between thermal properties of PEPc (Hamel and Simon, 1999) and other C4 enzymes with differences in cold sensitivity (Du et al., 1999a). However, various studies have demonstrated reduced activity of PPDK (Du et al., 1999b), Rubisco (Kingston-Smith et al., 1997; Pittermann and Sage, 2000, 2001), and PEPc (Kingston-Smith et al., 1997; Chinthapalli et al., 2003) upon chilling. Also, quantities of Rubisco that may be in excess of requirements at higher temperatures may become limiting at lower temperatures (Pittermann and Sage, 2000). Although these data suggest that PPDK is key to C4 photosynthesis at a low temperature, they also suggest that other C4 enzymes, particularly Rubisco, may play a role in cold tolerance of different species. Leaf photosynthetic rates at and below 20°C were very similar to maximum extractable activities of Rubisco in the cold-tolerant Bouteloua gracilis and in Amaranthus retroflexus (Pittermann and Sage, 2000; Sage, 2002).
We hypothesize that a potential mechanism for cold tolerance in M. × giganteus may be the maintenance of high levels of Rubisco and PPDK and/or a more cold-tolerant form of the latter. Previous studies have shown that 14°C is close to the limit of growth for maize leaves, and that leaves formed at this temperature have only a fraction of the photosynthetic capacity of leaves formed at warm temperatures, e.g. 25°C (Miedema et al., 1987; Nie et al., 1993). Therefore, we chose 14°C and 25°C as our growth temperatures for comparing the performance of M. × giganteus and maize. Three questions were addressed: Can the ability to form photosynthetically efficient leaves in M. × giganteus at a low temperature implied from field studies, and in contrast to maize, be demonstrated under controlled conditions? How are amounts of PPDK, PEPc, and Rubisco affected by growth at a low temperature in the two species? Given the potential role of PPDK in cold tolerance of C4 photosynthesis, are there obvious sequence differences in M. × giganteus PPDK that could be responsible for conferring cold tolerance to this enzyme? To address these questions, we measured C4 photosynthesis over a range of temperatures in maize and M. × giganteus grown at 14°C and 25°C. We also compared mRNA expression and protein accumulation for PPDK, PEPc, and Rubisco from the two species grown at the two temperatures. Furthermore, we cloned and sequenced M. × giganteus cDNAs from the C4-specific isoform of PPDK and compared their putative translated protein sequences with C4-PPDK from related species and from the cold-adapted F. brownii.
RESULTS
Photosynthetic CO2 Uptake
Growth temperature had very little effect on the temperature response of light-saturated photosynthesis in M. × giganteus. Cold- and warm-grown M. × giganteus leaves maintained virtually identical rates of CO2 uptake across a range of measurement temperatures (Fig. 1). In contrast, cold-grown maize exhibited an approximately 80% reduction in photosynthetic rate across all measurement temperatures in comparison with warm-grown plants. When grown at 25°C, photosynthetic rates were similar in both species. The temperature optimum of photosynthesis was between 30°C and 35°C for both species.
Figure 1.
Temperature response of photosynthetic CO2 uptake per unit leaf area for M. × giganteus and maize grown at 25°C/20°C or 14°C/11°C day/night temperatures. Error bars (±1 se) of the mean (n = 8–15) are shown, except when smaller than the symbol size.
Protein Accumulation of Key C4 Photosynthetic Enzymes
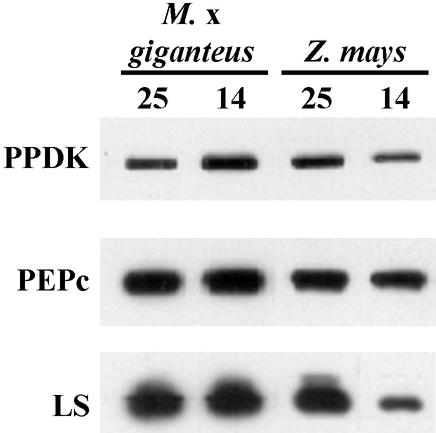
Total soluble protein content per unit leaf area was significantly reduced by 17% in cold-grown maize leaves relative to warm-grown leaves (Table I). There was no significant difference with temperature in M. × giganteus leaves, where total protein per unit leaf area was similar to values in warm-grown maize leaves. To determine if the differences in photosynthesis corresponded to changes in the amounts of three potentially rate-limiting enzymes, we used western blots (Fig. 2) to examine amounts of photosynthetic proteins extracted from leaves. The largest significant change was a 57% decrease in PPDK with growth at a cold temperature in maize. There were also significant decreases in PEPc (10%) and LS (39%) with growth at a cold temperature in maize. By contrast, amounts of these proteins did not differ significantly with temperature in M. × giganteus (Table I; Fig. 2). Although not measured directly, there was a visible reduction in chlorophyll in cold-grown maize.
Table I.
Protein content
Means and ses of C4 photosynthetic protein amounts (from western blots) for three replicate leaf samples of M. × giganteus and maize plants grown and measured at 25°C/20°C or 14°C/11°C day/night temperatures. Within each replicate, values were standardized to amount of protein in 25°C maize leaves. The bottom row contains values of total soluble protein (grams per square millimeter) extracted from four replicate leaf samples for each species and temperature. Percentage of change is relative to 25°C within each species.
|
Miscanthus × giganteus
|
Maize
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 25°C/14°C | 14°C/11°C | Percentage of change | P value | 25°C/14°C | 14°C/11°C | Percentage of change | P value | |
| PPDK | 0.68 (0.172) | 0.87 (0.064) | +28% | 0.191 | 1.00 (N/A) | 0.43 (0.186) | -57% | 0.032 |
| PEPc | 0.91 (0.007) | 0.90 (0.016) | -1% | 0.500 | 1.00 (N/A) | 0.90 (0.053) | -10% | 0.032 |
| LS | 0.98 (0.016) | 1.01 (0.014) | +3% | 0.191 | 1.00 (N/A) | 0.61 (0.192) | -39% | 0.032 |
| Total | 3.73 (0.043) | 3.82 (0.035) | +2% | 0.131 | 3.79 (0.037) | 3.12 (0.041) | -17% | 0.015 |
Figure 2.
Sample western blot of PPDK, PEPc, and the large subunit of Rubisco (LS) extracted from M. × giganteus and maize leaves grown at 25°C/20°C or 14°C/11°C day/night temperatures. Samples were loaded on an equal leaf area basis.
mRNA Expression of Key C4 Photosynthetic Enzymes
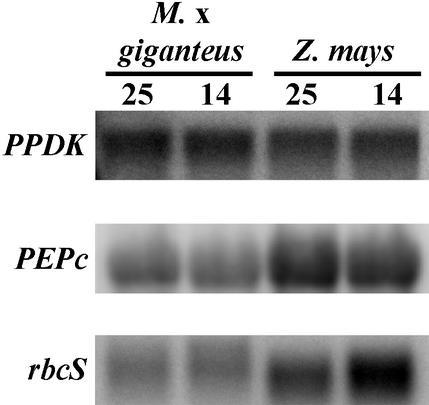
The large differences in protein accumulation observed between maize and M. × giganteus grown at cold temperatures could be mediated by transcriptional or posttranscriptional mechanisms. Steady-state levels of C4-PPDK mRNA assayed by northern-blot analysis did not change in response to low temperature growth in either species (Fig. 3). Similarly, PEPc mRNA expression was not responsive to cold temperature treatments. However, there was a large increase in the level of transcript encoding the rbcS in cold-treated maize seedlings, and possibly for M. × giganteus as well. The likely cause of fainter bands for PEPc and rbcS from M. × giganteus relative to maize is that the probes used for these transcripts were maize specific, whereas the C4-PPDK probe was M. × giganteus specific. To assay C4-PPDK transcript levels for a greater number of replicates, reverse transcriptase (RT)-PCR was used to quantify mRNA levels. There was no significant difference in steady-state amounts of PPDK mRNA with growth temperature for either species (P > 0.10), confirming the results obtained by northern analysis.
Figure 3.
Northern blots of PPDK, PEPc, and the small subunit of Rubisco (rbcS) mRNA from M. × giganteus and maize leaves grown at 25°C/20°C or 14°C/11°C day/night temperatures.
Cloning and Characterization of C4-PPDK cDNAs from M. × giganteus
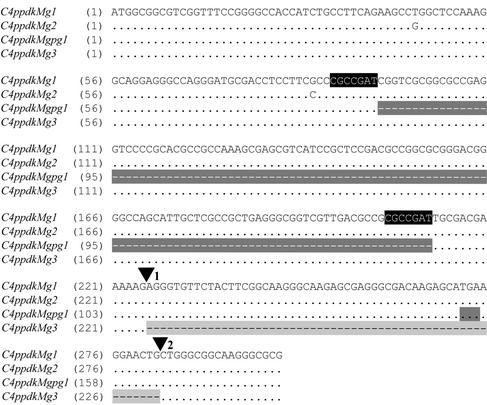
To determine if there are obvious sequence differences in M. × giganteus C4-PPDK that could be responsible for conferring cold tolerance to this enzyme, we cloned cDNA fragments from M. × giganteus PPDK genes via RT-PCR. We initially cloned cDNA fragments from the 5′ end of the gene corresponding to the region specific for C4-PPDK from maize and sugarcane. In maize, there are two genetic sequences for PPDK. Chloroplastic PPDK (C4ppdkZm1) and one of the cytosolic PPDKs (cyppdkZm1) are differentially transcribed from the same gene, and thus differ only in that C4ppdkZm1 contains a transit peptide sequence that is located within an intron of cyppdkZm1 (Sheen, 1999). The second cytosolic PPDK (cyppdkZm2) originates from a separate gene that lacks the transit peptide, but is otherwise highly homologous to cyppdkZm1. Therefore, these versions show major differences only within the first few hundred basepairs. Five versions of M. × giganteus C4-PPDK mRNA transcript were found, identified by variation within the sequence at the 5′ end of the transcript (Fig. 4). Only the first 300 bp from four of these variants are shown, which contain the majority of the differences among the transcripts. C4ppdkMg1 (GenBank accession no. AY262272) and C4ppdkMg2 (GenBank accession no. AY262273) differ by only six single bp changes throughout the first 623 bp, which was the longest sequenced section in this region. These nucleotide substitutions result in only one amino acid change (A to P) at position 29 (Fig. 5, asterisk). C4ppdkMgpg1 (GenBank accession no. AY262275) is a pseudogene; it contains an 118-bp deletion (Fig. 4, dark gray shading) that results in a frame shift of the translated sequence that generates a stop codon 60 nucleotides after the deletion (Fig. 4, black text on dark gray background). There is a 7-bp repeat (Fig. 4, white text on black background) that flanks this deletion. Two versions of C4ppdkMgpg were found, with differences completely analogous to those between C4ppdkMg1 and C4ppdkMg2 (C4ppdkMgpg2, GenBank accession no. AY262276, not shown). C4ppdkMg3 (GenBank accession no. AY262274) is a rare transcript (only two of the 22 clones sequenced) containing a 57-bp deletion that is precisely homologous to the second exon of C4ppdkZm1 (Sheen, 1991). Thus, C4ppdkMg3 probably represents an alternatively spliced transcript of C4ppdkMg1 where the 57-bp second exon was spliced out along with the first two introns. This alternative splicing event maintains the open reading frame, resulting in an internal deletion of 19 amino acids (Fig. 5, black text on light gray background). Because M. × giganteus is a triploid hybrid, it is not unexpected to find what appear to be at least two different versions of expressed C4-PPDK genes.
Figure 4.
Partial nucleotide sequence comparison of the 5′-coding regions for four versions of chloroplastic C4-PPDK cDNA found in M. × giganteus. Nucleotides identical to C4ppdkMg1 are indicated by dots. Missing nucleotides are indicated by a dash and shading. ▾, The splice acceptor sites for the first two introns in C4-PPDK, as determined by homology with maize. The 7-bp repeat in C4ppdkMg1 and C4ppdkMg2 is shown in white text on a black background. The stop codon generated by the frame-shift deletion in C4ppdkMgpg1 is identified in black text on a dark gray background.
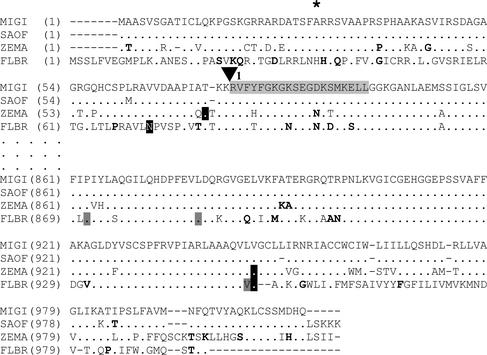
Figure 5.
Partial putative protein sequence comparison of chloroplastic C4-PPDK from M. × giganteus (MIGI), sugarcane (SAOF), maize (ZEMA), and F. brownii (FLBR). Protein sequences were deduced by translation of cDNA: GenBank accession numbers AY262272 (MIGI), AF194026 (SAOF), J03901 (ZEMA), and U08399 (SAOF). Vertical ellipsis marks represent a break in the pictured sequence. Gaps in sequence alignment are indicated by a dash. Residues identical and similar to M. × giganteus are indicated by dots or bolding, respectively. ▾, The splice acceptor site for the first intron in C4-PPDK, as determined by homology with maize. The asterisk identifies the amino acid position at which C4ppdkMg1 and C4ppdkMg2 differ in the region picture. The region of deletion in C4ppdkMg3 is indicated by black text on a light gray background. The N terminus and C terminus of ZEMA (Matsuoka, 1995) and FLBR (Usami et al., 1995) are indicated by white text on a black background. Note that FLBR is a C3/C4 intermediate dicot with a PPDK that is reported to be cold stable (Usami et al., 1995), whereas the remaining species are C4 grasses. The three residues reported to be responsible for conferring cold tolerance to FLBR PPDK (Ohta et al., 1996) are indicated by black text on a dark gray background.
Overlapping fragments from the remainder of M. × giganteus PPDK cDNAs, which would be expected to be common among plastidial and cytosolic isoforms, were also obtained by RT-PCR using primers targeting nucleotide sequences conserved among PPDK sequences from maize and sugarcane. The nucleotide sequence compiled from all overlapping segments of M. × giganteus PPDK cDNAs is 3,027 bp long, with a translated putative protein sequence of 1,009 amino acids (partially shown, Fig. 5). Within the 2,404 bp at the 3′ end of the sequence, there were 12 sites with nucleotide differences that could be classified as belonging to two groups of sequences. These differences translated into only three amino acid changes at positions 220, 304, and 418: M, T, and T in C4ppdkMg1, and L, I, and A, in C4ppdkMg2 (not shown). The sequence data indicate that at least two versions of C4-PPDK transcript exist in M. × giganteus that are translatable into protein.
The translated putative protein sequences of C4ppdkMg1 and C4ppdkMg2 from M. × giganteus were 97%, 89%, and 71% homologous to sugarcane, maize and F. brownii, respectively. The N terminus and C terminus amino acids of maize (Matsuoka, 1995) and F. brownii (Usami et al., 1995) PPDK have been determined (Fig. 5, white text on black background). These amino acids appear conserved in M. × giganteus PPDK, with the exception of the N terminus of F. brownii, which differs from that of the other species shown. The active site of the enzyme, which is highly conserved within PPDKs in general (Matsuoka, 1995), was also completely conserved in M. × giganteus PPDK (not shown). Two of the three residues reported to be responsible for conferring cold tolerance in F. brownii (Ohta et al., 1996) were also conserved among M. × giganteus, maize, and F. brownii (Fig. 5, black text on dark gray background).
DISCUSSION
Although we now know a number of C4 species that survive cold temperature, M. × giganteus appears exceptional in its ability not only to survive, but also achieve high efficiencies of conversion of absorbed light into biomass and high productivities (Long, 1999). Here, we show that this may be explained by a remarkable capacity to form leaves in cool conditions with a very similar capacity for photosynthesis to those formed under warm conditions. This is in complete contrast to maize. Our data for maize confirm earlier studies (e.g. Baker et al., 1990; Nie et al., 1992; Kingston-Smith et al., 1997) that show that cold-grown leaves have only a fraction of the photosynthetic capacity of leaves formed in warm temperatures. Leaves of M. × giganteus grown at 14°C show a light-saturated photosynthetic rate of 10 μmol m–2 s–1 when measured at 10°C, compared with 1.5 μmol m–2 s–1 for maize (Fig. 1). This is consistent with previous field studies that have shown that even under the cool conditions of southeastern England (mean July temperature of 16°C), this species is able to achieve quantum yields of photosynthesis equivalent to C4 crops growing under warm conditions (Beale et al., 1996). M. × giganteus grown at low temperatures, however, has similar rates of photosynthesis when measured at warm temperatures (25°C–35°C) to maize grown at warm temperatures (Fig. 1). Thus, low-temperature tolerance in M. × giganteus is not at the expense of photosynthetic capacity under warm conditions (Fig. 1). When measured at 10°C, leaves of M. × giganteus grown at 14°C/11°C have only a slightly higher light-saturated rate of CO2 uptake to leaves of maize grown at 25°C/20°C. The difference then is not in potential photosynthetic rates at low leaf temperatures, but in the ability to realize high rates of photosynthesis when grown at low temperatures.
These high rates of photosynthesis in cold-grown M. × giganteus correspond to its maintenance of high levels of total soluble protein, particularly PPDK and LS, in contrast to maize (Table I; Fig. 2). Three photosynthetic enzymes serve as the major control points of the C4 pathway under conditions of high light: Rubisco, PPDK, and PEPc (Matsuoka et al., 2001). Western-blot analysis indicates that the largest and most significant changes were in amounts of PPDK, which decreased markedly in maize at low temperatures while exhibiting a consistent, although nonsignificant, increase in M. × giganteus (Table I; Fig. 2). Cold-grown M. × giganteus also maintained high levels of PEPc and LS in contrast to significant decreases in both with cold in maize; however, relative differences between the two species in the response of PEPc to temperature were small compared with the large differences seen in the response of PPDK and LS. This supports the hypothesis that PPDK and Rubisco play critical roles in enhancing cold tolerance in M. × giganteus.
For maize, the reductions in amounts of photosynthetic proteins (Table I) cannot completely account for the approximate 80% reduction in photosynthesis seen (Fig. 1). Although the amount of available enzyme sets the upper limit on enzyme activity levels, it may not reflect the in vivo activity of the enzyme. The greater effect of cold temperature on the chloroplastic proteins PPDK and LS, in contrast to the cytosolic enzyme PEPc, may be related to the production of reactive oxygen species within the chloroplasts of maize (Wise, 1995), which could be responsible for further reductions in enzyme activity. Additionally, decreased photosynthesis with cold in maize may also be due, in part, to decreases in accumulation of a number of thylakoid proteins (Nie and Baker, 1991) as well as direct effects on chloroplast and thylakoid structure (Kratsch and Wise, 2000).
Changes in amounts of extracted protein might result from differences in mRNA transcription, mRNA stability, mRNA translation efficiency, or differences in protein stability. Northern-blot analysis indicated that steady-state levels of PEPc transcript did not differ with temperature for M. × giganteus or maize (Fig. 3). This is consistent with the western blots, which show that amounts of this enzyme are unaffected by growth temperature in M. × giganteus, whereas they decrease only a small amount in maize (Table I). Kingston-Smith et al. (1999) and Chinthapalli et al. (2003) also found no change in amounts of PEPc in response to cold temperatures. These data suggest that relative cold tolerance of these species is not effected by differences in amounts of PEPc. However, a recent report suggests that enzyme activity of PEPc within C4 species is relatively more sensitive to cold temperatures than PEPc from C3 species (Chinthapalli et al., 2003).
In contrast, rbcS, which is nuclear encoded, appears to have increased steady-state amounts of transcript in cold-grown relative to warm-grown maize (Fig. 3), whereas LS protein amounts exhibit a significant decrease (Table I; Fig. 2). For M. × giganteus, amounts of LS protein did not differ with temperature. Changes in M. × giganteus rbcS may be similar to maize, but were more difficult to assess due to fainter bands. Studies indicate that growth at cold temperatures disrupts the coordination of nuclear and chloroplast gene expression in maize (Nie and Baker, 1991; Bredenkamp et al., 1992). For thylakoid proteins, this disruption was not the result of differences in protein synthesis with temperature. Instead, Nie and Baker (1991) hypothesize that it may be related to reduced stability of chloroplast-encoded proteins in the cold. If an accumulation of rbcS is not matched by an accumulation of LS, the subunit stoichiometry of Rubisco protein would be controlled by a rapid degradation of unassembled small subunits (Rodermel, 1999), resulting in a reduction in the amount of the final assembled protein despite an apparent increase in rbcS transcript. Alternatively, Kingston-Smith et al. (1999) saw an increase in the amounts of breakdown products of Rubisco at 14°C relative to 20°C in maize, which suggests an increase in Rubisco degradation or a decrease in the processing of degradation products at low temperatures in maize. These lines of evidence suggest that the amount of functional Rubisco protein is reduced in cold-grown maize in contrast to M. × giganteus.
In contrast to the changes in PPDK protein amounts seen in the western-blot analysis, northern-blot analysis and the semiquantitative RT-PCR indicated that steady-state levels of mRNA transcript for C4-PPDK were unaffected by growth temperature for M. × giganteus or maize (Fig. 3). This suggests that differences in PPDK protein amounts in response to cold temperature for the two species were more likely a result of differences in protein turnover. There could be differences in protein structure for M. × giganteus PPDK that increase its stability and longevity in leaves growing in the cold, in contrast to maize.
To determine if there are obvious sequence differences that could be responsible for conferring low temperature tolerance to M. × giganteus PPDK, we examined sequences of expressed C4-PPDK. M. × giganteus is an allotriploid species that contains the genomes of Miscanthus sinensis and Miscanthus sacchariflorus (Greef and Deuter, 1993; Linde-Laursen, 1993; Hernández et al., 2001). Two of the genomes should be from one parent and one from the other, although assumptions as to the specific origins of M. × giganteus have recently been brought into question (Hodkinson et al., 2002). Thus, it is not surprising that in M. × giganteus, we identified multiple versions of C4-PPDK transcript (Fig. 4). Two versions, C4ppdkMg1 and C4ppdkMg2, differ only by a few amino acids and are highly homologous to C4-PPDK from sugarcane and maize (Figs. 4 and 5). Two other versions, C4ppdkMgpg1 and C4ppdkMgpg2, are identical to C4ppdkMg1 and C4ppdkMg2, but contain a large deletion within the transit peptide region (Fig. 4). Because this deletion is out of frame and thus generates a stop codon, these are considered pseudogenes that would not be translated into functional proteins. Interestingly, there is a 7-bp repeat that flanks this region within the full-length versions (Fig. 4, white text on black background) that may be relevant to the mechanism that generated the deletions observed in C4ppdkMgpg1 and C4ppdkMgpg2.
The C4ppdkMg3 transcript is probably derived from an alternative splicing event of C4ppdkMg1 that removes the second exon along with the first two introns (Figs. 4 and 5). Although this deletion is in frame and could thus potentially produce a protein with an internal deletion of 19 amino acids in the chloroplast transit peptide sequence, it is unclear whether the smaller protein is functional in M. × giganteus. The deletion could interfere with plastid import and hence lead to accumulation of a cytosolic PPDK isoform; however, we did not observe two differentially migrating PPDK species in our western blots (Fig. 2), suggesting that any such additional isoform does not persist in the cytoplasm. Additionally, if such a cytosolic PPDK were present in M. × giganteus, it would have no effect on photosynthesis. If the polypeptide encoded by the C4ppdkMg3 transcript is imported into the plastid appropriately, it would be indistinguishable from PPDK produced from the fully spliced C4ppdkMg1 transcript once the transit peptides have been cleaved.
The putative protein sequences for the full-length C4-specific PPDKs from M. × giganteus were 97%, 88%, and 71% homologous to sugarcane, maize, and F. brownii, respectively (partially shown, Fig. 5). The high similarity to sugarcane is not surprising as Miscanthus species can form fertile hybrids with Saccharum, suggesting that the division into two genera is artificial (Chen et al., 1993; Sobral et al., 1994). F. brownii, although it has a cold-stable PPDK (Usami et al., 1995), is a C3/C4 dicot intermediate and thus its lower degree of homology is also to be expected. There are no obvious differences in the sequence of the active site or the protein size that might immediately imply functional differences (not shown). However, it is possible that individual amino acid changes, e.g. the 3% difference in homology between M. × giganteus and sugarcane, could confer cold tolerance to this enzyme. Using chimeric constructs and point mutations, Ohta et al. (1996) identified three amino acids that strongly influenced cold tolerance in F. brownii (Fig. 5, black text on dark gray background). Two of these are conserved in the noncold-tolerant sugarcane and maize and also in M. × giganteus, indicating that these amino acids are not responsible for generally conferring cold tolerance to PPDK in these grass species. The remaining residue is unique to F. brownii among these species, suggesting that a single amino acid in F. brownii might be primarily influential in conferring cold tolerance to the enzyme in that species. In F. brownii, cold tolerance of PPDK was associated with increased subunit association (reduced lability) and greater enzyme activity in the cold (Ohta et al., 1996).
In conclusion, this research supports the hypothesis that M. × giganteus is capable of forming leaves with high photosynthetic capacity at low temperatures in sharp contrast to maize. Although a key role for PPDK in controlling C4 photosynthesis at low temperatures has been suggested, there were no obvious differences in the sequence of M. × giganteus C4-PPDK relative to sugarcane and maize that could explain increased protein stability of this enzyme at low temperatures. In M. × giganteus, increased photosynthetic capacity corresponds to maintenance of amounts of PPDK and Rubisco in leaves grown at cool temperatures, whereas large significant decreases in these enzymes correspond to loss of photosynthetic capacity with growth at low temperature in maize.
MATERIALS AND METHODS
Plant Material
Miscanthus × giganteus clones were propagated from rhizomes in 1.2-liter pots in a 1:1:1 mix of soil:calcined clay:torpedo sand, and maize (Zea mays) genotype FR1064 (a commercial inbred line provided by Illinois Foundation Seeds, Tolono, IL) seeds were germinated in 0.3-liter pots in Sunshine Mix LC1 (SunGro Horticulture, Bellevue, WA). Plants were grown in controlled environment chambers (Conviron E15; Controlled Environments, Winnipeg, Manitoba, Canada) under 400 μmol m–2 s–1 photosynthetic photon flux density (PPFD), 70% relative humidity, and 25°C/20°C (warm) or 14°C/11°C (cold) day/night temperatures. Plants, with their associated treatments, were rotated between chambers biweekly to avoid confounding any undetected difference between the chambers with the treatments. Plants were kept well watered and fertilized once a week with a 20:20:20 (N:P:K) commercial fertilizer (Peter's Professional; The Scotts Co., Marysville, OH) at the recommended rate. All measurements were made on the youngest fully expanded leaf on a shoot with an emerged ligule and were confined to the second or third leaf formed. Ligule emergence was used as a marker of maturation and completion of expansion of the blade.
Gas Exchange Measurements
Photosynthetic rates were measured on intact leaves using an open gas exchange system (LI-6400; LI-COR, Lincoln, NE) equipped with a red/blue LED light source (6400-02B). To allow measurement over a wide range of temperatures, the chamber was modified by replacing the peltier external heat sink with a metal block containing water channels that were connected to a heating/cooling circulating water bath (Bernacchi et al., 2001). The concentration of CO2 was controlled within the sample cuvette at a constant rate of 360 μL L–1, and the leaf-to-air vapor pressure deficit was maintained below 3.0 kPa at all measurement temperatures. Variation in leaf-to-air vapor pressure deficit within this limit had little effect on mesophyll internal [CO2] in these C4 plants. The temperature response of photosynthesis was measured at 5°C, 10°C, 15°C, 20°C, 25°C, 30°C, 35°C, and 38°C leaf temperature on eight to 15 leaves at each temperature with each leaf measured at three to eight different temperatures. For each measurement, leaves were light and temperature acclimated in the gas exchange cuvette at 500 μmol m–2 s–1 PPFD and at the first measurement temperature until steady state (20–30 min). Subsequently, steady-state photosynthetic rates were measured at 1,000 μmol m–2 s–1 PPFD at each measurement temperature.
Protein Extraction and Western-Blot Analysis
Leaf tissue (25–30 cm2) was collected from a parallel sample of leaves and was frozen in liquid nitrogen before protein extraction. Leaf area was determined with an image scanner and digitizing software (Scan jet IICX, Areacalc; Hewlett Packard, Palo Alto, CA). Total soluble protein was extracted according to the method of Nie et al. (1993). An aliquot of the extract was used to determine protein concentration using the DC microplate protein assay (Bio-Rad Life Science Group, Hercules, CA), based on the method described by Lowry et al. (1951). The remaining supernatant was used for SDS-PAGE. Total leaf proteins were loaded on an equal leaf area basis and were separated by SDS-PAGE (10%–18% [w/v] acrylamide), and then blotted onto nitrocellulose (Trans-Blot; Bio-Rad Life Sciences Group) in transfer buffer (50 mol m–3 Tris base, 380 mol m–3 Gly, 0.1% [w/v] SDS, and 20% [v/v] methanol) at approximately 8°C overnight at 50 V. The nitrocellulose membranes were incubated with the appropriate primary polyclonal antibodies for 1.5 h after blocking for 2 h at room temperature with 6% (w/v) skimmed milk in phosphate-buffered saline (PBS) containing 0.0005% (v/v) Tween 20 (PBS-T; Sigma, Poole, Dorset, UK). Primary polyclonal antibodies to maize proteins were raised in rabbits and were provided for our use by the following: LS, Christine Raines (Department of Biological Sciences, University of Essex, Colchester, UK); PEPc, Richard C. Leegood (Department of Animal and Plant Sciences, University of Sheffield, Western Bank, UK); and PPDK, James N. Burnell (Department of Biochemistry and Molecular Biology, James Cook University, Townsville, Queensland, Australia). Although cytosolic (C3) versions of PEPc and PPDK exist in C4 plants, the C4 versions are more highly expressed in green leaves (Ku et al., 1996) and are therefore more predominantly represented in the western blots. After six washes with PBS-T, blots were incubated for 2 h at room temperature with a 1:5,000 dilution in PBS-T of sheep anti-rabbit secondary antibody conjugated to horseradish peroxidase (Serotec, Oxford, UK). After six PBS-T washes, the secondary antibodies were detected using enhanced chemiluminescence according to the manufacturer's directions (Amersham Life Science, Little Chalfont, UK) and were quantified with a computer-controlled, two-dimensional laser scanning densitometer (model 300A; Molecular Dynamics, Sunnyvale, CA). Proteins from three replicate leaf samples were extracted for each species and temperature. For each replicate sample, separate gels/blots were run/probed for each protein, and each contained all species/temperature combinations. To account for variability in blotting and probing within each replicate blot, amounts of extracted protein were normalized to the amount of the enzyme in 25°C maize leaves. Normalized date were compared between the two temperature treatments within each species with a Wilcoxon rank sum test (proc NPAR1WAY WILCOXON, SAS v8.02; SAS Institute, Cary, NC) and differences were reported at the P < 0.10 level. Probability values are reported for a one-tailed test because our initial hypothesis was that amounts of these enzymes would decrease with cold temperatures or remain the same, relative to warm temperatures.
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted from flash-frozen young green leaves using Tri-Reagent (Molecular Research Center, Cincinnati) or the RNeasy Plant Mini kit (Qiagen, Valencia, CA) according to the manufacturer's recommended protocols. RNA from the former technique was used for northern blotting, whereas RNA from the latter technique was used in all other applications (see below). For northern blots, 5 μg of total RNA was electrophoresed on denaturing formaldehyde gels and was blotted to a charged nylon membrane (Magnacharge; Osmonics, Westborough, MA) according to the manufacturer's recommended protocols. Blots were probed with radiolabeled cDNA probes. Maize probes were provided by Jen Sheen (Harvard Medical School, Boston; PEPc) and by Ray Zielinski (University of Illinois, Urbana, IL; rbcS). For C4-PPDK, a 391-bp fragment of M. × giganteus cDNA that is unique to the C4 isoform of PPDK was used. This fragment was also used in our semiquantitative RT-PCR assay and its development, as described in the next section. The isolated DNA fragments were labeled with 32-P by random priming (Feinberg and Vogelstein, 1984). Hybridizations and final washes in 0.1× SSC and 0.1% (w/v) SDS were conducted at 65°C according to the manufacturer's recommended protocol. Labeled membranes were exposed to a storage phosphor screen and were scanned using a variable mode imager (Typhoon 8600; Amersham Biosciences, Piscataway, NJ).
Semiquantitative RT-PCR of C4-PPDK
To provide an alternative quantification of transcript levels for leaf samples, semiquantitative RT-PCR was conducted. Total RNA was extracted from the same leaves used for the photosynthesis measurements described above. Sample size was five leaves (four for warm-grown M. × giganteus). The RNA was quantified using RiboGreen fluorescence (Molecular Probes, Eugene, OR). cDNA was synthesized from equal amounts of total RNA (3 μg) by reverse transcription with Superscript II (Invitrogen, Carlsbad, CA) using a poly-T primer according to the manufacturer's recommended protocol.
PCR primers were designed to sequences highly conserved among cDNAs for the C4-specific PPDK isoforms from maize (Sheen, 1991) and sugarcane (Saccharum officinarum; GenBank accession no. AF194026). Primers spanned two adjacent exons so that they would not amplify genomic PPDK DNA (Sheen, 1991). The sequence of the 3′ primer was 5′-CGCCCATGTACTCCTCCACGAACTGCAGGCCGTC-3′ and that of the 5′ primer was 5′-GATGCGACCTCCTTCGCCCGCCGATCGGTCGC-3′ (Operon Technologies, Alameda, CA). For PCR, initial denaturation at 94°C for 2 min was followed by 36 cycles of denaturation (94°C for 1 min), annealing (70°C for 1 min), and extension (72°C for 1 min), and then a final extension at 72°C for 7 min. Taq DNA Polymerase (Invitrogen) and 2 μL of a one-tenth dilution of cDNA from the original RT-PCR reaction were used for PCR. The primers were successful in amplifying a 391-bp fragment from M. × giganteus leaf cDNA with a sequence highly similar to the 5′-coding region of maize and sugarcane C4-PPDK genes.
The above PCR primers and the 391-bp M. × giganteus C4-PPDK fragment were then used to develop a semiquantitative RT-PCR assay to measure the amounts of C4-PPDK mRNA in warm- and cold-grown M. × giganteus and maize. Cleavage at two internal HindII sites followed by religation generated a 160-bp deletion in the 391-bp PCR fragment for use as a quantification standard (qs) that could be amplified competitively in the same reaction with the same primers, but produced a product that was distinguishable from amplified C4-PPDK cDNA by differential migration on an agarose gel.
To quantify the amount of C4-PPDK cDNA in each sample, two replicates of a six-point titration curve were generated using equal amounts of the RT reaction and a range of known concentrations of the linearized qs for each PCR reaction. PCR reactions were carried out as described above with the following exceptions: 5 μL of a one-tenth dilution of cDNA from the original RT-PCR reaction was used, the annealing temperature was 68°C, and 28 cycles were performed. Equal amounts of PCR product were run out on an agarose gel for quantification. Amounts of PCR product were quantified from ethidium bromide-stained gels via densitometry with a digital camera (Kodak Digital Science Electrophoresis Documentation and Analysis System 120; Eastman-Kodak, Rochester, NY). The amounts of input C4-PPDK cDNA in the original sample were estimated according to Alvarez et al. (2000) and were compared statistically between the two temperatures within each species as above. We assume that the amount of cDNA is proportional to the original amount of mRNA in the sample and that the efficiency of the RT reaction is the same for each sample. However, because we cannot know the actual efficiency of the RT reaction, the assay was semiquantitative, allowing comparison of only relative copy numbers of input mRNA molecules. Preliminary tests found that the amplification efficiency of our qs was greater than that of our target. Under these conditions, any potential differences found in target template will be overestimated (Alvarez et al., 2000), decreasing the probability of making a Type II error.
Cloning and Sequencing of C4-PPDK cDNAs from M. × giganteus
Based upon the sequence of C4-PPDK cDNA from sugarcane (GenBank accession no. AF194026) and by comparison with the work of Sheen (1991), PCR primers were designed to amplify cDNA fragments from the entire chloroplast transit peptide region predicted to be specific to C4-PPDK of M. × giganteus. The sequence of the 5′ primer was 5′-AGAAGGATGGCGGCGTCGGTTTCC-3′ and that of the 3′ primer was 5′GTGTCGTAGTCGAAGCGCTCCCCG3′ (W.M. Keck Center for Comparative and Functional Genomics, University of Illinois, Urbana, IL). PCR reactions were carried out as described above, except with an annealing temperature of 65°C on 2 μL of the original RT-PCR reaction. The resulting fragments were cloned into the pCR2.1 TOPO vector (Invitrogen). Plasmid DNA was isolated (QIAprep Spin Miniprep kit; Qiagen) from positive colonies and inserts were sequenced using ABI Prism BigDye Terminators v 3.0 (Applied Biosystems, Foster City, CA) followed by electrophoresis on ABI 377 sequencers (W.M. Keck Center). Twelve clones of this fragment were sequenced and aligned using the ContigExpress assembly program within the VectorNTI Suite 7.0 software package (Informax, Bethesda, MD). Two other independent PCR reactions and transformations of slightly different length fragments in the same region generated another 10 clones. Thus, a sequence of 22 clones was used to derive a consensus for the 5′ end of the gene.
A similar approach was used to amplify and sequence the remaining portions of M. × giganteus PPDK cDNA in three additional overlapping segments. Five to 10 clones were sequenced and used to derive a consensus for each segment. In total, the entire M. × giganteus PPDK cDNA was amplified, cloned, and sequenced in four overlapping sections ranging from 566 to 930 bp long. Longer segments (over 700 bp) were sequenced in both directions to obtain the entire sequence. We determined that there were five versions of C4-PPDK mRNA transcript that contained minor sequence variations within the first approximate 1,700 bp, most within the first 300 bp. However, because these differences were not always within the overlapping regions, it was difficult to definitively compile the various sequences. To confirm the compilations, we cloned the first approximate 1,800 bp in one piece and chose representatives of the major variants to sequence. We then generated several clones of each representative with “primer islands” added into the plasmid insert (Primer Island Transposition kit; Applied Biosystems). We sequenced these in various directions using sequencing primers located on the plasmid and the primer island.
The translated putative protein sequence from M. × giganteus was compared with that of sugarcane (GenBank accession no. AF194026), maize (GenBank accession no. J03901), and F. brownii (GenBank accession no. U08399), a known cold-tolerant C3/C4 intermediate (Usami et al., 1995). Amino acid sequence alignments were performed using CLUSTALW within the VectorNTI Sute 7.0 software package.
Acknowledgments
We thank Illinois Foundation Seeds (Champaign, IL) for providing the maize FR1064 seeds. We also thank Dr. Jen Sheen for generously providing a clone of C4-PPDK from maize, which we used to design and test our RT-PCR assay, and Dr. Richard C. Leegood and Dr. James N. Burnell who provided the antibodies used in the western blotting. Thanks also go to Melissa Langosch for assistance with the PCR, cloning, and sequencing, and to members of the Moose and Long laboratories for stimulating discussions. Finally, we thank Dr. Donald R. Ort for reviewing and providing valuable comments on an early version of this manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021790.
This work was supported in part by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant nos. 2000–35100–9057 and 2002–35100–12424 to S.P.L. and S.P.M.).
References
- Alvarez M, Depino A, Podhajcer O, Pitossi F (2000) Bias in estimations of DNA content by competitive polymerase chain reaction. Anal Biochem 287: 87–94 [DOI] [PubMed] [Google Scholar]
- Baer GR, Schrader LE (1985) Relationships between carbon dioxide exchange rates and activities of pyruvate, orthophosphate dikinase and ribulose bisphospate carboxylase, chlorophyll concentration and cell volume in maize (Zea mays). Plant Physiol 77: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR, Nie G-Y, Ortizlopez A, Ort DR, Long SP (1990) Analysis of chill-induced depressions of photosynthesis in maize. In M Baltscheffsky, ed, Current Research in Photosynthesis, Vols. 1–4.Kluwer Academic Publishers, Dordrecht, The Netherlands, pp D565–D572 [Google Scholar]
- Beale CV, Bint DA, Long SP (1996) Leaf photosynthesis in the C4-grass Miscanthus × giganteus, growing in the cool temperate climate of southern England. J Exp Bot 47: 267–273 [Google Scholar]
- Beale CV, Long SP (1995) Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant Cell Environ 18: 641–650 [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24: 253–259 [Google Scholar]
- Bredenkamp GJ, Nie GY, Baker NR (1992) Perturbation of chloroplast development in maize by low growth temperature. Photosynthetica 27: 401–411 [Google Scholar]
- Chen YH, Chen C, Lo CC (1993) Studies on anatomy and morphology in Saccharum-Miscanthus nobilized hybrids: transmission of tillering, ratooning, adaptation and disease resistance from Miscanthus spp. J Agric Assoc China 164: 31–45 [Google Scholar]
- Chinthapalli B, Murmu J, Raghavendra AS (2003) Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. J Exp Bot 54: 707–714 [DOI] [PubMed] [Google Scholar]
- Du Y-C, Nose A, Wasano K (1999a) Thermal characteristics of C4 photosynthetic enzymes from leaves of three sugarcane species differing in cold sensitivity. Plant Cell Physiol 40: 298–304 [Google Scholar]
- Du Y-C, Nose A, Wasano K (1999b) Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant Cell Environ 22: 317–324 [Google Scholar]
- Feinberg AP, Vogelstein B (1984) ADDENDUM: a technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137: 266–277 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, Ashton AR (1997) Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Aust J Plant Physiol 24: 477–485 [Google Scholar]
- Greaves JA (1996) Improving suboptimal temperature tolerance in maize: the search for variation. J Exp Bot 47: 307–323 [Google Scholar]
- Greef JM, Deuter M (1993) Syntaxonomy of Miscanthus × giganteus GREEF et DEU. Angewandte Botanik 67: 87–90 [Google Scholar]
- Hamel N, Simon JP (1999) Molecular forms and kinetic properties of phosphoenolpyruvate carboxylase from barnyard grass (Echinochloa crusgalli L. Beauv.: Poaceae). Can J Bot 78: 619–628 [Google Scholar]
- Hernández P, Dorado G, Laurie DA, Martín A, Snape JW (2001) Microsatellites and RFLP probes from maize are efficient sources of molecular markers for the biomass energy crop Miscanthus. Theor Appl Genet 102: 616–622 [Google Scholar]
- Hodkinson TR, Chase MW, Takahashi C, Leitch IJ, Bennett MD, Renvoize SA (2002) The use of DNA sequencing (ITS and trnL-F), AFLP, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae). Am J Bot 89: 279–286 [DOI] [PubMed] [Google Scholar]
- Hodkinson TR, Renvoize SA (2001) Nomenclature of Miscanthus × giganteus. Kew Bulletin 56: 759–760 [Google Scholar]
- Kingston-Smith AH, Harbinson J, Foyer CH (1999) Acclimation of photosynthesis, H2O2 content and antioxidants in maize (Zea mays) grown at suboptimal temperatures. Plant Cell Environ 22: 1071–1083 [Google Scholar]
- Kingston-Smith AH, Harbinson J, WIlliams J, Foyer CH (1997) Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol 114: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant Cell Environ 23: 337–350 [Google Scholar]
- Ku MSB, Kano-Murakami Y, Matsuoka M (1996) Evolution and expression of C4 photosynthesis genes. Plant Physiol 111: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde-Laursen IB (1993) Cytogenetic analysis of Miscanthus “Giganteus,” an interspecific hybrid. Hereditas 119: 297–300 [Google Scholar]
- Long SP (1983) C4 photosynthesis at low temperatures. Plant Cell Environ 6: 345–363 [Google Scholar]
- Long SP (1999) Environmental responses. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 215–249
- Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45: 633–662 [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Matsuoka M (1995) The gene for pyruvate, orthophosphate dikinase in C4 plants: structure, regulation, and evolution. Plant Cell Physiol 36: 937–943 [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M (2001) Molecular engineering of C4 photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 52: 297–314 [DOI] [PubMed] [Google Scholar]
- Miedema P, Post J, Groot P (1987) The effects of low temperature on seedling growth in maize genotypes. In Agricultural Research Reports, Vol 926. Pudoc, Wageningen, The Netherlands, p 124 [Google Scholar]
- Nie G-Y, Baker NR (1991) Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiol 95: 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G-Y, Long SP, Baker NR (1992) The effects of development at suboptimal growth temperatures on photosynthetic capacity and susceptibility to chilling-dependent photoinhibition in Zea mays. Physiol Plant 85: 554–560 [Google Scholar]
- Nie G-Y, Robertson EJ, Fryer MJ, Leech RM, Baker NR (1995) Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant Cell Environ 18: 1–12 [Google Scholar]
- Nie G-Y, Tomasevic M, Baker NR (1993) Effects of ozone on the photosynthetic apparatus and leaf proteins during leaf development in wheat. Plant Cell Environ 16: 643–651 [Google Scholar]
- Ohta S, Usami S, Ueki J, Kumashiro T, Komari T, Burnell JN (1996) Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii pyruvate, orthophosphate dikinase. FEBS Lett 396: 152–156 [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sage R (2001) The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A. S. Hitchc. to long- and short-term chilling. J Exp Bot 52: 829–838 [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sage RF (2000) Photosynthetic performance at low temperature of Bouteloua gracilis Lag., a high-altitude C4 grass from the Rocky Mountains, USA. Plant Cell Environ 23: 811–823 [Google Scholar]
- Rodermel S (1999) Subunit control of Rubisco biosynthesis: a relic of an endosymbiotic past? Photosynth Res 59: 105–123 [Google Scholar]
- Sage RF (1999) Why C4 photosynthesis? In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 3–16
- Sage RF (2002) Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53: 609–620 [DOI] [PubMed] [Google Scholar]
- Sheen J (1991) Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell 3: 225–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1999) C4 gene expression. Annu Rev Plant Physiol Plant Mol Biol 50: 187–217 [DOI] [PubMed] [Google Scholar]
- Shirahashi K, Hayakawa S, Sugiyama T (1978) Cold lability of pyruvate, orthophosphate dikinase in the maize leaf. Plant Physiol 62: 826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral BWS, Braga DPV, Lahood ES, Keim P (1994) Phylogenetic analysis of chloroplast restriction enzyme site mutations in the Saccharinae Griseb subtribe of the Andropogoneae Dumort tribe. Theor Appl Genet 87: 843–853 [DOI] [PubMed] [Google Scholar]
- Sugiyama T (1973) Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry 12: 2862–2868 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Hirayama Y (1983) Correlation of the activities of phosphoenolpyruvate carboxylase and pyruvate, orthophosphate dikinase with biomass in maize seedlings. Plant Cell Physiol 24: 783–787 [Google Scholar]
- Sugiyama T, Schmitt MR, Ku SB, Edwards GE (1979) Differences in cold lability of pyruvate, Pi dikinase among C4 species. Plant Cell Physiol 20: 965–971 [Google Scholar]
- Usami S, Ohta S, Komari T, Burnell JN (1995) Cold stability of pyruvate, orthophosphate dikinase of Flaveria-brownii. Plant Mol Biol 27: 969–980 [DOI] [PubMed] [Google Scholar]
- Usuda H, Ku MSB, Edwards GE (1985) Rates of photosynthesis relative to activity of photosynthetic enzymes, chlorophyll, and soluble protein content among ten C4 species. Aust J Plant Physiol 11: 509–517 [Google Scholar]
- Ward DA (1987) The temperature acclimation of photosynthetic responses to CO2 in Zea mays and its relationship to the activities of photosynthetic enzymes and the CO2-concentrating mechanism of C4 photosynthesis. Plant Cell Environ 10: 407–411 [Google Scholar]
- Wise RR (1995) Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45: 79–97 [DOI] [PubMed] [Google Scholar]