Abstract
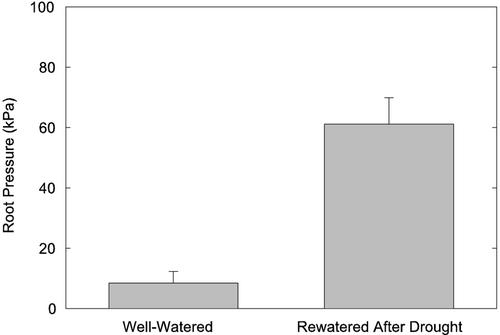
We investigated the role of xylem cavitation, plant hydraulic conductance, and root pressure in the response of rice (Oryza sativa) gas exchange to water stress. In the field (Philippines), the percentage loss of xylem conductivity (PLC) from cavitation exceeded 60% in leaves even in watered controls. The PLC versus leaf water potential relationship indicated diurnal refilling of cavitated xylem. The leaf water potential causing 50 PLC (P50) was –1.6 MPa and did not differ between upland versus lowland rice varieties. Greenhouse-grown varieties (Utah) were more resistant to cavitation with a 50 PLC of –1.9 MPa but also showed no difference between varieties. Six-day droughts caused concomitant reductions in leaf-specific photosynthetic rate, leaf diffusive conductance, and soil-leaf hydraulic conductance that were associated with cavitation-inducing water potentials and the disappearance of nightly root pressure. The return of root pressure after drought was associated with the complete recovery of leaf diffusive conductance, leaf-specific photosynthetic rate, and soil-leaf hydraulic conductance. Root pressure after the 6-d drought (61.2 ± 8.8 kPa) was stimulated 7-fold compared with well-watered plants before drought (8.5 ± 3.8 kPa). The results indicate: (a) that xylem cavitation plays a major role in the reduction of plant hydraulic conductance during drought, and (b) that rice can readily reverse cavitation, possibly aided by nocturnal root pressure.
Upland (aerobically grown) rice (Oryza sativa) and rain-fed lowland (periodically flooded) rice, which combined account for about one-half of the world's rice, are subjected to unpredictable periods of drought (Chaudhary and Rao, 1982). Rice even suffers from water stress when grown in permanently flooded paddies (Ishihara and Saito, 1987; Jiang et al., 1988; Hirasawa et al., 1992, 1996). Much effort has been put into research that addresses critical yield and productivity constraints in relation to water stress (Wade et al., 1999; Zeigler, 1999). However, progress in the development of drought-resistant cultivars has been slow (Cooper et al., 1999; Fukai et al., 1999). One aspect that has been largely overlooked is the hydraulic conductance of rice and its drought-induced reduction due to xylem cavitation. Work on woody plants has demonstrated an important hydraulic limitation on stomatal conductance and photosynthesis (Hubbard et al., 2001), a factor that could be equally important for herbaceous crop plants.
Recently, Miyamoto et al. (2001) found the hydraulic conductivity of rice roots to be substantially lower than that of other herbaceous roots. This low root conductivity, combined with a relatively high transpiration rate (Tanguilig et al., 1987), could explain the steep drops in leaf water potential and leaf gas exchange found in flooded rice studies. Decreased xylem water potential carries the inherent danger of xylem cavitation, which can substantially reduce the hydraulic conductivity of plants (Tyree et al., 1986; Sperry et al., 1993). Reduced xylem conductivity amplifies the water stress by causing a greater drop in water potential; therefore, plants must regulate their stomata to avoid critically low water potential and catastrophic xylem failure (Tyree and Sperry, 1988; Jones and Sutherland, 1991). Photosynthetic sensitivity to water stress may be largely determined by hydraulically constrained stomatal conductances. However, nothing is known of how vulnerable rice xylem is to cavitation.
Equally important as xylem cavitation is the ability of the plant to refill cavitated xylem conduits and restore lost xylem hydraulic conductance. Root pressure is common in rice, as observed by copious guttation from leaf margins. Root pressure is known to refill cavitated xylem conduits (Miller, 1985; Tyree et al., 1986; Sperry et al., 1987, 1988; Pickard, 1989; Cochard et al., 1994; Hacke and Sauter, 1996; Ewers et al., 1997), and it may be crucial in restoring daily losses in xylem conductivity in rice. However, there are also reports that embolized xylem can refill without root pressure, when the xylem pressure is substantially negative (Salleo et al., 1996; Holbrook and Zwieniecki, 1999; Tyree et al., 1999; Zwieniecki et al., 2000, 2001; Hacke and Sperry, 2003). If refilling mechanisms exist in rice, whether by root pressure or additional means, they will be important for the recovery of productivity after a drought.
In this paper, basic studies on the vulnerability of rice xylem to cavitation are reported, including field and laboratory measurements of daily cavitation and embolism. Controlled droughts and gas exchange measurements examined the relationship between reduction of gas exchange and the loss of hydraulic conductivity due to xylem cavitation. Rewatering experiments examined the association between drought recovery and the presence and magnitude of nocturnal root pressure and the refilling of cavitated xylem.
RESULTS
Vulnerability Curves
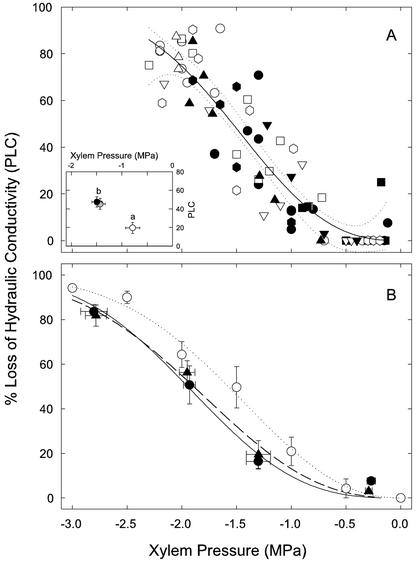
In the field, rice was vulnerable to cavitation, with leaves reaching as high as 90% loss of xylem conductivity (PLC) at minimum leaf water potential (Ψleaf) of ≈ –2 MPa (Fig. 1A). In both watered control and droughted plants, PLC above 50 was common. Droughted plants (white symbols) experienced only modestly more negative Ψleaf, perhaps because of an accidental flooding of the drought treatment. In fact, the predawn leaf water potentials of droughted plants (–0.50 ± 0.08 MPa) were not different from watered controls (–0.36 ± 0.07 MPa). As it happened, a severe drought treatment was not necessary to construct the vulnerability curve because the xylem was very susceptible to cavitation. Weibull curves fit to individual varieties showed no significant differences in 50 PLC (P50), despite the wide range in varietal characteristics (Table I). The P50 for the pooled curve was –1.6 MPa.
Figure 1.
A, Vulnerability curve showing PLC in leaf xylem versus Ψleaf in field-grown rice. Even watered plants (solid symbols, n = 33) experienced up to 90 PLC. The drought treatment (white symbols, n = 41) was mild, perhaps because of accidental flooding; even so, nearly the complete vulnerability curve was obtained. Symbols represent different varieties: Bala (circles, n = 21), Moroberekan (squares, n = 17), Azucena (down triangles, n = 11), IR62266 (up triangles, n = 10), and Lemont (hexagons, n = 15). Weibull curves fit to the data of each variety were not different; therefore, data were pooled and fit to a single Weibull function (solid line). Insert, Same data grouped by measurement time (white symbol, 6–8; gray symbol, 9–11; and black symbol, 13–16). Letters above symbols indicate significant differences. Means and se, n = 22–29, P < 0.05, lsd test. B, Vulnerability curves of leaf (solid symbols) and stem (white symbol) xylem in greenhouse-grown rice of variety Azucena (circles) and IR64 (triangles). Means and se, n = 4–8 (leaves), n = 4 (stems). Lines represent a Weibull fit to the leaf data (solid and dashed lines) and to the stem data (dotted line). Vulnerability curves of the leaves were not different between the two varieties.
Table I.
Rice varieties
Asterisked names were used in the field experiments at the International Rice Research Institute. Plants of the japonica subspecies generally have lower tillering than indica subspecies and often have deeper root distribution (Lafitte et al., 2001). Improved cultivars are generally shorter with greater tiller number per plant and higher grain yield under favorable conditions than traditional varieties. Upland cultivars that were selected for non-flooded (aerobic) conditions generally have less of a yield reduction under drought stress than lowland types selected for cultivation in flooded conditions for at least part of the growth cycle.
| Variety Name | Description |
|---|---|
| Moroberekan* | Traditional japonica upland cultivar; origin: Ivory Coast |
| Azucena* | Traditional japonica upland cultivar; origin: Philippines |
| IR 60080-46A | Improved japonica upland cultivar; origin: Philippines |
| IR64 | Improved indica lowland cultivar; origin: Philippines |
| IR 62266-42-6-2* | Improved indica lowland cultivar; origin: Southeast Asia |
| Vandana | Improved indica upland cultivar, origin: Eastern India |
| Bala* | Improved indica upland cultivar; origin: Eastern India |
| Lemont* | Improved temperate japonica cultivar; origin: Southern United States |
The field PLC measurements indicated diurnal refilling of embolized xylem. The PLC measurements were made over several successive days and at times of day ranging from early morning to midafternoon. Afternoon values of low leaf Ψ and high PLC were followed the next morning by high leaf Ψ and low PLC (Fig. 1, insert). In the absence of cavitation reversal, we would have seen high afternoon PLC at early morning leaf Ψ.
The centrifuge curves made on leaves of Utah-grown Azucena and IR64 varieties had a similar shape as the field curves, but with a lower P50 of –1.9 MPa (Fig. 1B, solid symbols). Again, no differences were seen between varieties, which represented both japonica and indica backgrounds adapted to upland versus lowland conditions, respectively (Table I). Stem curves could only be done on Azucena because the fragile nodal diaphragm in IR64 fractured during flushing, prohibiting maximum hydraulic conductivity (Kmax) measurements. The Azucena stem curve was more vulnerable than the leaves (Fig. 1B, white symbols, P50: –1.6 MPa).
Photosynthetic Gas Exchange and Drought-Rewatering Experiments
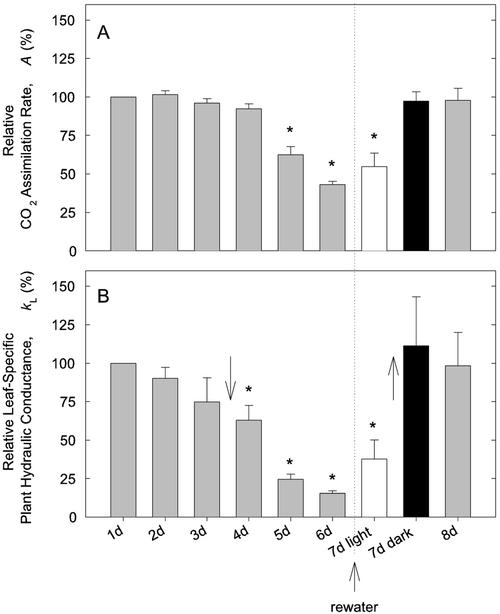
Figure 2 shows A and kL during the drought and rewatering cycle relative to initial values before the drought. These changes were not significantly different between the two cultivars Azucena and IR62266; therefore, the pooled results are shown. During the first 3 d of successive drought, A and kL remained nearly unchanged (Fig. 2). Days 5 and 6 saw large reductions in A to 43.1% ± 2.1% of its initial value. The kL also declined to 15.4% ± 1.6% of the initial value. During the drought, Ψleaf fell to –2.56 ± 0.23 MPa on d 6 with no difference between varieties (mean is pooled data). From the laboratory vulnerability curves (Fig. 1B), this Ψleaf would have caused 74.5 ± 7.7 PLC, assuming all varieties had the same vulnerability to cavitation as the data indicate (Fig. 1). Additional reduction in whole-plant kL could have come from cavitation in stems and roots and soil drying.
Figure 2.
Leaf-specific assimilation rate (A; A) and soil-leaf hydraulic conductance (kL; B) relative to d 1 during a soil drought/rewatering cycle. Well-watered rice plants (n = 6, varieties Azucena and IR62266) were subjected to 6 d of increasing soil drought. The vertical dotted line indicates the time of rewatering after the 6th d. After rewatering, lights were either turned off overnight (black bar, n = 3) or kept on (white bar, n = 3). Arrows in B, Cession of guttation (downward arrow) and its reappearance (upward arrow). Asterisked means are different from d 1 values (P < 0.05, lsd test). Absolute A values on d 1 for Azucena and IR62266 were 27.6 ± 3.3 μmol s–1 m–2 and 14.4 ± 0.8 μmol s–1 m–2, respectively. Absolute kL values were 10.2 ± 3.4 and 4.7 ± 1.3 mmol s–1 s–2 MPa–1, respectively.
Plants that were rewatered and given a dark night (Fig. 2, 7 d dark, black bars) completely recovered their predrought A and kL by the next day. In contrast, plants rewatered and given a light night (Fig. 2, 7 d light, white bars) showed no significant rebound in A or kL. The fact that kL remained low despite saturated soil indicated that the bulk of the loss of kL during drought was in the plant rather than the soil component of the pathway. When these plants were given an additional dark night (Fig. 2, 8 d bars), the kL and A rebounded to predrought values, indicating the restoration of plant hydraulic conductance in the interim.
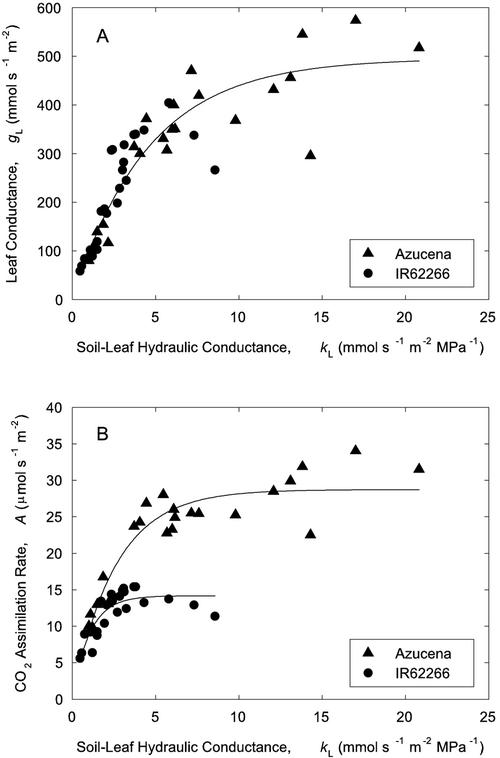
Absolute values of leaf diffusive conductance (gL) and kL throughout the experiment, whether before or after the drought, were closely related across both varieties (Fig. 3A). The relationship between the two parameters followed a saturation curve, with a linearly increasing region followed by a plateau. The same shape was seen for A versus kL (Fig. 3B), suggesting that A was limited by stomatal closure in the linear region and by maximum photosynthetic capacity in the saturated portion. However, Azucena saturated at a greater maximum A than IR62266, perhaps because of less self-shading in the more open Azucena canopy.
Figure 3.
A, gL and kL during a soil drought/rewatering cycle for IR62266 (circles) and Azucena (triangles). Data points from the same experiment as in Figure 2. Within each variety, gL saturated at a similar value with increasing kL. An exponential rise to a maximum function [y = a(1 –bx)] was fitted to the pooled data (a = 495.8, b = 0.80, R2 = 0.85, P < 0.001). B, Relationship between A and kL for the same varieties and experiment. Within each variety, A saturated with increasing kL. However, maximum A and kL were greater for Azucena than IR62266. An exponential rise to a maximum function [y = a(1 –bx)] was fitted to the data of each variety. Azucena, a = 28.73, b = 0.68, R2 = 0.86, P < 0.001; IR62266, a = 14.17, b = 0.38, R2 = 0.73, P < 0.001.
Root Pressure and Recovery of Photosynthesis after Drought
Based on observations of guttation, root pressure during the gas exchange experiments ceased after d 3 of the drought regardless of variety (Fig. 2B, down arrow), corresponding with a significant reduction in kL the following days. Root pressure returned in rewatered plants, but only if the night was dark (Fig. 2B, up arrow). The return of root pressure was associated with the rebound in A and kL. Rewatering by itself had no effect on A or kL, despite a water-saturated soil root system (Fig. 2, 7 d of light, white bars). We observed a dramatic increase in the amount of guttation water after droughted plants were rewatered compared with the well-watered plants in the beginning of the experiment.
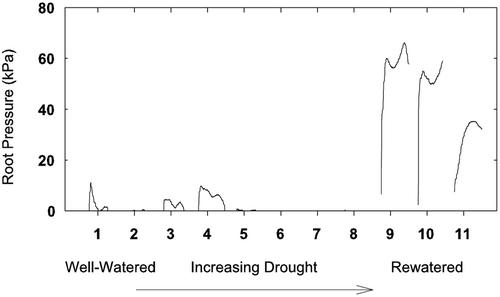
Guttation observations on Azucena and IR6266 varieties were consistent with continuous root pressure measurements of Bala and Vandana varieties subjected to a similar drought and rewatering cycle. Figure 4 shows the root pressures of a representative plant (Bala) during 11 consecutive nights. At the beginning, root pressures were below 20 kPa and showed a bimodal time course with a first, higher peak before midnight and a second, lower peak shortly before sunrise (Fig. 4). During the early drought stages, root pressures were similar with peak pressures reaching approximately 8 to 10 kPa. During the second one-half of the drought, root pressure disappeared completely. After rewatering, nightly root pressures increased almost 10-fold, consistent with our observations of enhanced guttation after the drought.
Figure 4.
Root pressure of a representative rice plant (variety Bala) during 1 week of increasing soil drought. The plant was rewatered in the evening of d 8. Numbers on the x axis indicate midnight.
The increase in root pressure before versus after a 7-d drought period is summarized in Figure 5 for all before and after measurements—both the gas exchange plants (Azucena and IR62266 measured only before and after the drought) and continuous time course plants (Bala and Vandana). Before drought, plants exhibited a maximum root pressure of 8.5 ± 3.8 kPa (mean ± se). After drought, the maximum root pressures of the same plants increased 7-fold to 61.2 ± 8.8 kPa. There were no obvious differences between the four varieties in the magnitude of root pressure, but the sample size was not sufficient for statistical evaluation.
Figure 5.
Root pressures of well-watered rice plants before and after a 7-d drought period. Means and se, n = 11 plants, P < 0.001.
DISCUSSION
The results indicate an important role of xylem cavitation in the rice water stress response. Leaf xylem of field-grown rice experienced over 60 PLC under well-watered aerobic conditions and as much as 90 PLC under a mild drought (Fig. 1A). Cavitation contributed significantly to the drop in whole-path kL during drought—the 85% drop in kL (Fig. 2B) was associated with a predicted 75 PLC in the leaf xylem. Additional cavitation doubtless occurred in the root system, for which we have no vulnerability data. We expect that root xylem will be even more vulnerable, based on studies in other species (Kolb and Sperry, 1999; Hacke et al., 2000).
Perhaps compensating for its vulnerability to cavitation, rice appears to be very capable of reversing cavitation on a daily basis in the field (Fig. 1A), and after controlled droughts (Fig. 2B). Our results indicate a strong association between root pressure and cavitation reversal in that droughted plants rewatered without a dark night showed no root pressure and no significant increase in kL, whereas plants rewatered with a dark night showed root pressure and completely recovered kL (Fig. 2B). Nocturnal root pressure was commonly observed in the field-grown plants (V. Stiller, personal observation). However, our results do not rule out cavitation reversal without root pressure. The increase in kL in the rewatered light-night plants (Fig. 2B, 7 d of light), though statistically insignificant, leaves open the possibility that some refilling occurred even when the transpiration stream was under negative pressure. However, a slight increase in kL would be expected given that the soil component of the pathway would be restored to full conductance by rewatering.
The substantial increase in root pressure that we observed after drought (Figs. 4 and 5) suggests an adaptive response. High levels of embolism may demand high root pressures to dissolve the gas and restore conduction. Maximum post-drought pressures were as high as 115 kPa. This is comparable with values found in bamboo (Rhipidocladum racemiflorum; Cochard et al., 1994) and grapevine (Vitis labrusca and Vitis riparia; Sperry et al., 1987) but less than values reported by Miller (1985) for maize (Zea mays; 400 kPa). Drought-induced increases of root pressure have been previously reported for rhodes-grass (Chloris gayana Kunth.; Ogata et al., 1985) and maize (Bakh-tenko and Yakushkina, 1995; Zhang et al., 1995). The fact that root pressures remained elevated for several days after rewatering (Fig. 4), even after plants had resumed transpiration, suggests the pressure response was not caused merely by passive accumulation of ions during reduced transpiration under drought. The release of ions during metaxylem maturation in roots has been suggested as contributing to root pressure (McCully et al., 1987), and it is possible that a resumption of root growth after drought is involved.
The cavitation in rice xylem will indirectly limit gas exchange because of the relationship between kL and A (Fig. 3B). Similar kL relationships with A (and gL) have been observed in other species studies (Meinzer and Grantz, 1990; Sperry et al., 1993; Meinzer et al., 1995; Saliendra et al., 1995; Hubbard et al., 2001). Reduced kL limits A by lowering Ψleaf during transpiration and by inducing a drop in gL via stomatal closure and leaf rolling. Although the drop in gL counters the drop in Ψleaf, it also reduces CO2 uptake. The reduction in A is probably mostly due to CO2 limitation rather than a direct effect of low Ψleaf on photosynthetic biochemistry (Kaiser, 1987). Although the reduction in gL inhibits A, the adaptive significance in terms of preserving water conduction is clear from the vulnerability curves in Figure 1. If gL had not been reduced by over 4-fold as kL dropped during drought (Fig. 2A), Ψleaf would have dropped well below the observed minimum of –2.56 ± 0.23 MPa on d 6 of the drought. How far Ψleaf would have dropped can be estimated from Equation 1:
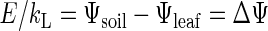 |
(1) |
where E ∝ gL because of the constant evaporative gradient. If gL were not reduced by 4-fold, ΔΨ would be increased by 4-fold, yielding a Ψleaf below –4 MPa for d 6. Such low Ψleaf would have completely cavitated the leaf xylem (Fig. 1B) and air dried the foliage. We did not assess the relative importance of stomatal closure versus leaf rolling in the gL response, but both cultivars exhibited leaf rolling by the end of the drought.
In this study, we only report steady-state “midday” values of gas exchange and hydraulic conductance, with a time course of days during a drought and rewatering cycle. However, the field data (Fig. 1A, insert) indicate large diurnal changes in cavitation and, hence, whole-path kL during a 24-h period that may explain some diurnal variation in A as well as day-to-day changes. The Ψleaf of well-watered rice can swing widely from near zero at night to below –1.5 MPa at midday, depending on the day and the cultivar (V. Stiller, personal observation). As Figure 1A indicates, this will necessarily be associated with large increases in xylem cavitation from morning to midday, influencing kL and gas exchange (i.e. Fig. 3). The diurnal variation in PLC is consistent with reports of reduced net photosynthesis and leaf conductance in the afternoon compared with values before noon (Ishihara et al., 1981; Dingkuhn et al., 1989). The high vulnerability to xylem cavitation could also explain why even paddy-grown rice can display symptoms of water shortage (Ishihara and Saito, 1987; Jiang et al., 1988; Hirasawa et al., 1992, 1996). Rice's ability to reverse cavitation is very significant because without diurnal refilling, rice productivity would drop dramatically because 1 d's cavitation would permanently reduce the plant kL.
We were surprised to find no differences in vulnerability to cavitation between upland and lowland rice varieties (Fig. 1), although there was a difference between greenhouse versus field-grown plants (Fig. 1, A versus B) that was possibly an acclimation to different ambient humidity. The lack of varietal differences in cavitation resistance—at least in leaf xylem—means that this factor does not explain observed differences in drought sensitivity between varieties (Lafitte and Courtois, 2002). However, cultivars did differ in their maximum kL (Fig. 3) and in the range of Ψleaf they developed (Fig. 1A)—presumably because of differences in rooting depth and mechanisms of gL regulation (rolling versus stomatal control). Such differences will result in different kL trajectories under identical climatic conditions, with hypothetical consequences for A and yield. Azucena and IR62266 did differ in their maximum A (Fig. 2B), but we did not assess the basis for this difference, other than to note the possibility of greater self-shading in IR62266.
Several studies have shown reduced biomass and grain yield as a result of drought (O'Toole, 1982; Jearakongman et al., 1995; Fukai et al., 1999), and largest reductions in yield were observed when drought occurred during the reproductive phase (O'Toole, 1982; Tsuda et al., 1994b; Boonjung and Fukai, 1996; Saxena et al., 1996). Most authors explain this observation with reduced photosynthesis during drought (Fukoshima et al., 1985; Dingkuhn et al., 1989) and/or with drought-induced damage to the developing rice panicles. The mechanism of this panicle damage, however, is poorly understood. Interestingly, guttation water has been shown to keep rice panicles hydrated during booting stage (Tsuda et al., 1994a). Our results suggest that the drought-induced lack of root pressure would allow panicles to dehydrate, which could cause sterility. Several authors suggest that “root activity” and rooting depth play an important role in drought resistance of rice (Ishihara et al., 1981; Ishihara and Kuroda, 1986; Hirasawa et al., 1987; Tsuno and Toryu, 1987; Yamauchi et al., 1988; Jiang et al., 1994; Kobata et al., 1996). We agree with these conclusions, suggesting more specifically the importance of root pressure and cavitation dynamics as important factors in the rice water stress response.
MATERIALS AND METHODS
Plant Material
Field studies were conducted March through May 2001 at IRRI (Los Banos, Philippines; latitude 14°13′ north, longitude 121°15′ east). Rice (Oryza sativa) plants (Table I) were grown from IRRI seed in a silty clay loam (Typic Hapludol) on an aerobic upland field (latitude 14°13′ north, longitude 121°15′ east). Five rice varieties were grown, representing the indica and japonica subspecies and adaptation to upland versus lowland conditions (Table I). Plots were 3 m long and 0.5 m wide and randomly distributed within watered control versus droughted blocks so that two plots per variety were in each watering treatment. During initial plant growth, all plots were drip irrigated to field capacity three times per week. At 56 d after planting, irrigation was stopped for the droughted blocks, and irrigation continued as before for the watered control blocks. Plants for the native embolism study were harvested during the following 60 d. Average daytime temperature during this period was approximately 28°C, and the relative humidity was about 80%. Significant rainfall occurred on d 81 (47 mm) and 95 (42 mm), which temporarily relieved the drought stress in nonirrigated plants. Predawn leaf water potential of the rice plants was measured once per week to assess the soil water availability.
Greenhouse and laboratory measurements were conducted at the University of Utah (Salt Lake City). Varieties (Table I) were grown from seed in a greenhouse in 4.5-L pots under natural light. Soil used was fritted clay (Balcones Mineral Corp., Flatonia, TX). During growth, the pots were kept in water-filled trays (5-cm water level), and plants were frequently watered. Plants were grown for 60 to 90 d at 22°C and approximately 60% relative humidity until early flowering, at which stage experiments began.
Cavitation “Vulnerability Curves”
“Vulnerability curves” show the relationship between xylem pressure and PLC.
Field Curves
In the field at IRRI, we measured PLC in leaves as a function of Ψleaf in the watered control and droughted treatments for cultivars listed in Table I. In this way, we assessed the extent of cavitation as Ψleaf dropped during the day and to lower values in the droughted versus well-watered plants. This also allowed us to assess the presence of diurnal refilling because in the absence of cavitation reversal, PLC will remain constant at its highest level caused by minimum Ψleaf and, thus, show no relationship with less negative Ψleaf. The PLC was measured at comparable developmental stages from early flowering through to early grain filling.
Measurements were made on the youngest mature leaf of two adjacent tillers on each plant. The leaf of one tiller (for Ψleaf measurement) was harvested in air and transported in a dark, moist bag to the lab. The Ψleaf was measured approximately 20 min after sampling with a Scholander type pressure chamber (Soilmoisture Equipment Corp., Santa Barbara, CA). The second tiller (for PLC measurement) was harvested under water and was kept submerged during the transport to the lab. The youngest mature leaf was removed under water with approximately 1 to 2 cm of the leaf sheath attached and 2 to 3 cm of its apex removed. Such leaf segments usually lacked continuous aerenchyma ducts. The hydraulic conductivity (Knative, volume flow rate per pressure gradient) of the leaf blade was measured. For this, we attached the leaf at the ligule with a rubber gasket to a tubing system that was connected to an electronic balance (BA210S, Sartorius, Goettingen, Germany). The leaf blade was submerged in a water-filled container that could be raised and lowered approximately 30 cm to create the 3.0-kPa pressure head for the K measurement. After lowering the container, we allowed 5 to 10 min for the plastic tubing to equilibrate with the changed water pressure and for flow rates to reach steady state before measuring K. Each K measurement was repeated three times, and the mean was calculated. After measuring Knative, the leaves were flushed at 100 kPa for 60 min with deionized and filtered (0.2 μm) water to reverse any native embolism. Flushing was done with the cut, apical end submerged under water. If large air bubbles emerged from the cut surface during flushing, the leaf was discarded because this indicated a continuous aerenchyma duct. After flushing, Kmax was measured using the same protocol and pressure used for Knative. The percentage that Knative was below Kmax gave the segment's PLC due to reversible xylem embolism.
Laboratory Measurements
At the University of Utah, vulnerability curves were measured on leaves of greenhouse-grown material of the Azucena (japonica, upland) and IR64 (indica, lowland) cultivars (Table I) using a modification of the centrifugal force method (Alder et al., 1997). One to two leaves from five plants of each variety were measured for each vulnerability curve. As for the field measurements, leaves were removed under water approximately 1 to 2 cm below the ligule, and 1 to 2 cm of the leaf apex was removed. Leaf blades were flushed at 100 kPa for 30 min with deionized and filtered (0.2 μm) water to reverse any native embolism. One 0.14-m long segment was then cut under water from the apical side of the blade (where aerenchyma ducts were generally noncontinuous). The blade was spun on a custom-built centrifuge rotor that generated a pressure gradient dropping from zero at the blade ends to a minimum negative pressure at the center. The leaf was spun for 3 min—sufficient to saturate the embolism response (Alder et al., 1997). After centrifugation, three 2-cm sections from the apical end of the blade were cut under water—each having been exposed to a different pressure during centrifugation, as calculated from the angular velocity, length of the blade, and position of the 2-cm section (Alder et al., 1997). The hydraulic conductivity of each 2-cm section was measured (Kspun), and sections were flushed at 100 kPa for 30 min with deionized and filtered (0.2 μm) water to reverse the embolism created by the centrifugation. After flushing, Kmax was measured, and the PLC induced by centrifugation was calculated. Blade sections found to contain continuous aerenchyma ducts were not used.
We measured vulnerability curves of stems using the same centrifugal method. However, because stems were more sturdy than leaves, we were able to measure complete curves on single stems—repeatedly measuring Kspun on the same stem after spinning to progressively more negative pressures as in the standard method (Alder et al., 1997). Basal stem segments (0.14 m) containing at least one node of four plants were harvested under water and flushed at 100 kPa for 30 min with deionized and filtered (0.2 μm) water to reverse any native embolism. After flushing, Kmax was measured. The flushed segments were exposed to progressively more negative pressures by spinning them in the centrifuge rotor (3 min at each pressure). The K was remeasured between each pressure, and the PLC (relative to Kmax) associated with each pressure was recorded. To estimate the pressure causing P50, we used a Weibull function (Neufeld et al., 1992) to fit the PLC versus xylem pressure (P) data:
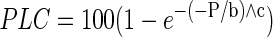 |
(2) |
where b and c are curve fitting parameters. We used the “solver” routine in Microsoft Excel 2000 (Microsoft, Redmond, WA) to find the parameter values giving the best fit to the data and then solved for the P50.
Photosynthetic Gas Exchange and Root Pressure during a Soil Drought and Rewatering Cycle
We measured A, transpiration (E), and leaf conductance to water vapor diffusion (gL) of six rice plants of the Azucena (japonica, upland) and IR62266 (indica, lowland) varieties (Table I) during a soil drought and rewatering cycle. The night before the gas exchange measurements, freshly watered plants were brought from the greenhouse to the lab and put into a 10-L outer pot that was filled with the same soil as the inner pots. The inner pot was fitted with a mesh bottom, insuring hydraulic contact with the soil in the outer pot. Adding this outer pot slowed down the soil-drying process and allowed soil sampling for water potential measurements. The shoots were sealed in a whole-plant cuvette for open gas exchange measurements described by Saliendra et al. (1995). This system measures CO2 uptake and water loss from the plant from the difference in CO2 and water vapor flux entering versus leaving the cuvette. The A was calculated from CO2 uptake and expressed per leaf area. The E was calculated from water vapor efflux and expressed per leaf area. The gL was calculated from E based on the leaf-air difference in water vapor mole fraction, which was kept near 20 mmol mol–1 throughout the measurement period. We did not attempt to calculate stomatal conductance because of the difficulty of determining the effect of leaf rolling on boundary layer conductance. Cuvette CO2 concentration was kept at 350 ± 2 μL L–1, relative humidity was maintained at 55% ± 2%, light levels were constant at 1,500 μmol photons m–2 s–1, and air temperature was near 30°C throughout 8 h of daily gas exchange measurements. Leaf temperatures were measured with thermocouples and were within 1.5° of ambient. Air was well mixed with fans. At night, the lights and cuvette fans were turned off, and there was no air flow through the cuvette. Nightly air temperature was near 25°C, and relative humidity was approximately 50% to 60%.
On the 1st d of measurement, plants were kept well watered for acclimation to cuvette conditions. On the 2nd d, watering ceased, and gas exchange measurements continued for another 5 d as the soil dried. Soil water potential (Ψsoil) and Ψleaf were measured daily after transpiration had stabilized, promoting steady-state flow conditions. Both Ψ measurements were made with an isopiestic psychrometer (Isopiestics Company, Lewes, DE), requiring soil samples to be extracted (from beneath the mesh bottom of the inner pot), and leaf discs were removed (with a leaf punch about 10 cm from the blade tip of the youngest mature leaf). Daily values of soil-to-leaf hydraulic conductance (kL, volume flow rate per leaf area per ΔΨ) were calculated as:
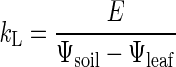 |
(3) |
Leaf areas (for expressing E, A, and gL on a leaf-specific basis) were measured after the experiment with an LI-3100 Area Meter (LI-COR Inc., Lincoln, NE). Equation 3 applies as long as the driving force for water uptake is solely hydrostatic pressure, as opposed to an additional contribution by an osmotic gradient across the root. Transpiration rates during the experiments were sufficiently high (>2 mmol s–1 m–2) even under drought conditions to eliminate the likelihood of significant osmotic water uptake (Fiscus, 1986). Dieback of older leaves that occurred during the drought experiment was noted daily, and changes in the estimated leaf area throughout the experiment were calculated accordingly.
In the evening of the 6th d, the soil in the inner pot was rewatered thoroughly and kept wet for the remainder of the experiment. In one group of plants, lights were turned off for the night to allow root pressure to occur. In the second group, the lights were kept on to inhibit root pressure.
The presence or absence of root pressure was determined by noting guttation from leaves. In addition, root pressure was measured throughout two nights during the experiment: immediately before the 1st d of gas exchange measurements and after the last day of measurements. For this, a single tiller was cut and attached to a pressure transducer (PX180-015GV, Omega Engineering Inc., Stamford, CT), and root pressures were recorded with a data logger (CR10, Campbell Scientific Inc., Logan, UT). Cut tillers tended to seal off after 1 d, making it impossible to monitor root pressure continuously without using up most of the tillers during the experiment. Transducers were always attached near the root-shoot junction where there was a minimum of aerenchyma to interfere with pressure build-up.
A parallel drought experiment without gas exchange measurements allowed us to monitor root pressure throughout a 7-d drought. We measured root pressure each night during the drought and for several days after rewatering by attaching pressure transducers to a fresh tiller each evening. There were insufficient Azucena and IR62266 plants for these experiments. Instead, measurements were made on six plants, three each of the upland indica varieties, Bala and Vandana (Table I).
Statistics
Data were analyzed with the SPSS 8.0 statistics package for PC (SPSS Inc., Chicago, IL) using the 0.05 significance level. Comparisons of A, kL, PLC, root pressure, and Ψleaf (Figs. 1A, 2, 3, and 5) were made with a one-way ANOVA and the lsd test for a priori pair-wise comparisons (all daily means versus d 1, Figs. 2 and 3), or paired means before versus after rewatering (Fig. 5). For comparisons of P50s in different varieties, we used Tukey's honestly significant difference mean-separation test following a one-way ANOVA.
Acknowledgments
The authors thank anonymous reviewers for their thoughtful comments that helped us to improve the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019851.
This work was supported by the U.S. Agency for International Development (grant to the authors).
References
- Alder NN, Pockman WT, Sperry JS, Nuismer S (1997) Use of centrifugal force in the study of xylem cavitation. J Exp Bot 48: 665–674 [Google Scholar]
- Bakhtenko EY, Yakushkina NI (1995) Peculiarities of the effect of abscisic acid on water and ion transport in the process of root exudation. Fiziol Bio Kult Rast 27: 401–405 [Google Scholar]
- Boonjung H, Fukai S (1996) Effects of soil water deficit at different growth stages on rice growth and yield under upland conditions: II. Phenology, biomass production and yield. Field Crop Res 48: 47–55 [Google Scholar]
- Chaudhary D, Rao MJBK (1982) Breeding rice varieties for dryland and drought-prone areas in India. In Drought Resistance in Crops, with Emphasis on Rice. IRRI, Manila, Philippines, pp 265–272
- Cochard H, Ewers FW, Tyree MT (1994) Water relations of a tropical vine-like bamboo (Rhipidocladum racemiflorum): root pressures, vulnerability to cavitation and seasonal changes in embolism. J Exp Bot 45: 1085–1089 [Google Scholar]
- Cooper M, Fukai S, Wade LJ (1999) How can breeding contribute to more productive and sustainable rainfed lowland rice systems? Field Crop Res 64: 199–209 [Google Scholar]
- Dingkuhn M, Cruz RT, O'Toole JC, Doerffling K (1989) Net photosynthesis, water use efficiency, leaf water potential and leaf rolling as affected by water deficit in tropical upland rice. Aust J Agric Res 40: 1171–1182 [Google Scholar]
- Ewers FW, Cochard H, Tyree MT (1997) A survey of root pressures in vines of a tropical lowland forest. Oecologia 110: 191–196 [DOI] [PubMed] [Google Scholar]
- Fiscus EL (1986) Below ground costs: hydraulic conductance. In TJ Givnish, ed, On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, UK, pp 275–298
- Fukai S, Pantuwan G, Jongdee B, Cooper M (1999) Screening for drought resistance in rainfed lowland rice. Field Crop Res 64: 61–74 [Google Scholar]
- Fukoshima MT, Hinata K, Tsunoda S (1985) Varietal comparison on the responses of photosynthetic rate and leaf water balance at different soil moisture tensions in rice (Oryza sativa). Jpn J Breed 35: 109–117 [Google Scholar]
- Hacke U, Sauter JJ (1996) Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105: 435–439 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS (2003) Limits to xylem refilling under negative pressure in Laurus nobilis and Acer negundo. Plant Cell Environ 26: 303–311 [Google Scholar]
- Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schafer KVR, Oren R (2000) Influence of soil porosity on water use in Pinus taeda. Oecologia 124: 495–505 [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Araki T, Ishihara K (1987) The relationship between water uptake and transpiration rates in rice plants. Jpn J Crop Sci 56: 38–43 [Google Scholar]
- Hirasawa T, Tenmyo N, Suyuki M, Ishihara K (1996) Mechanism on occurrence of white head of rice plants at heading stage under high temperature, low humidity and high wind velocity conditions: factors increasing stem resistance to water flow at the panicle base. Jpn J Crop Sci 65: 129–130 [Google Scholar]
- Hirasawa T, Tsuchida M, Ishihara K (1992) Relationship between resistance to water transport and exudation rate and the effect of the resistance on the midday depression of stomatal aperture in rice plants. Jpn J Crop Sci 61: 145–152 [Google Scholar]
- Holbrook NM, Zwieniecki MA (1999) Embolism repair and xylem tension: do we need a miracle? Plant Physiol 120: 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RM, Stiller V, Ryan MG, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24: 113–121 [Google Scholar]
- Ishihara K, Hirasawa T, Iida O, Kimura M (1981) Diurnal transpiration rate, stomatal aperture, stomatal conductance, xylem water potential, and leaf water potential in rice plants under different growth conditions. Jpn J Crop Sci 50: 25–37 [Google Scholar]
- Ishihara K, Kuroda E (1986) Effects of air humidity on the photosynthetic rate in the leaf of the rice plant. Jpn J Crop Sci 55: 458–464 [Google Scholar]
- Ishihara K, Saito K (1987) Diurnal courses of photosynthesis transpiration and diffusive conductance in the single-leaf of the rice plants grown in the paddy field under submerged condition. Jpn J Crop Sci 56: 8–17 [Google Scholar]
- Jearakongman S, Rajatasereekul S, Naklang K, Romyen P, Fakai S, Skulkhu E, Jumpaket B, Nathabutr K (1995) Growth and grain yield of contrasting rice cultivars grown under different conditions of water availability. Field Crop Res 44: 139–150 [Google Scholar]
- Jiang CZ, Hirasawa T, Ishihara K (1988) Physiological and ecological characteristics of high yielding varieties in rice plants: II. Leaf photosynthetic rates. Jpn J Crop Sci 57: 139–145 [Google Scholar]
- Jiang DA, Hirasawa T, Ishihara K (1994) Depression of photosynthesis in rice plant with low root activity following soluble starch application to the soil. Jpn J Crop Sci 63: 531–538 [Google Scholar]
- Jones HG, Sutherland R (1991) Stomatal control of xylem embolism. Plant Cell Environ 14: 607–612 [Google Scholar]
- Kaiser WM (1987) Effects of water deficit on photosynthetic capacity. Physiol Plant 71: 142–149 [Google Scholar]
- Kobata T, Okuno T, Yamamoto T (1996) Contributions of capacity for soil water extraction and water use efficiency to maintenance of dry matter production in rice subjected to drought. Jpn J Crop Sci 65: 652–662 [Google Scholar]
- Kolb KJ, Sperry JS (1999) Transport constraints on water use by the Great Basin shrub, Artemisia tridentata. Plant Cell Environ 22: 925–935 [Google Scholar]
- Lafitte HR, Champoux MC, McLaren G, O'Toole JC (2001) Rice root morphological traits are related to isozyme group and adaptation. Field Crop Res 71: 57–70 [Google Scholar]
- Lafitte HR, Courtois B (2002) Interpreting cultivar-by-environment interactions for yield in upland rice: assigning value to drought-adaptive traits. Crop Sci 42: 1409–1420 [Google Scholar]
- McCully ME, Canny MJ, Van Steveninck RFM (1987) Accumulation of potassium by differentiating metaxylem elements of maize roots. Physiol Plant 69: 73–80 [Google Scholar]
- Meinzer FC, Goldstein G, Jackson P, Holbrook NM, Gutierrez MV, Cavelier J (1995) Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties. Oecologia 101: 514–522 [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA (1990) Stomatal and hydraulic conductance in growing sugarcane: Stomatal adjustment to water transport capacity. Plant Cell Environ 13: 383–388 [Google Scholar]
- Miller DM (1985) Studies of root function in Zea mays: III. Xylem sap composition at maximum root pressure provides evidence of active transport into the xylem and a measurement of the reflection coefficient of the root. Plant Physiol 77: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52: 1835–1846 [DOI] [PubMed] [Google Scholar]
- Neufeld HS, Grantz DA, Meinzer FC, Goldstein G, Crisosto GM, Crisosto C (1992) Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiol 100: 1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S, Saneoka H, Matsumoto K (1985) Nutritional-physiological evaluation of drought resistance of warm season forage species: comparative studies on root development water and nutrient absorption of forage species at various soil moisture levels. J Jpn Soc Grass Sci 31: 263–271 [Google Scholar]
- O'Toole JC (1982) Adaptation of rice to drought-prone environments. In Drought Resistance in Crops, with Emphasis on Rice. IRRI, Manila, Philippines, pp 195–213
- Pickard WF (1989) How might a tracheary element which is embolized by day be healed by night? J Theor Biol 141: 259–280 [Google Scholar]
- Saliendra NZ, Sperry JS, Comstock JP (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196: 357–366 [Google Scholar]
- Salleo S, Lo Gullo MA, De Paoli D, Zippo M (1996) Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol 132: 47–56 [DOI] [PubMed] [Google Scholar]
- Saxena HK, Yadav RS, Parihar SKS, Singh HB, Singh GS (1996) Susceptibility and recovery potential of rice genotypes to drought at different growth stages. Ind J Plant Physiol 1: 198–202 [Google Scholar]
- Sperry JS, Alder NN, Eastlack SE (1993) The effect of reduced hydraulic conductance on stomatal conductance and xylem cavitation. J Exp Bot 44: 1075–1082 [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT (1988) Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum). Am J Bot 75: 1212–1218 [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT (1987) Spring filling of xylem vessels in wild grapevine. Plant Physiol 83: 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguilig VC, Yambao EB, O' Toole JC, De Datta SK (1987) Water stress effects on leaf elongation, leaf water potential transpiration and nutrient uptake of rice, maize and soybean. Plant Soil 103: 155–168 [Google Scholar]
- Tsuda M, Fujikawa T, Ikeda K (1994a) Diurnal change in water droplets adhering to rice panicles at the booting stage. Jpn J Crop Sci 63: 131–136 [Google Scholar]
- Tsuda M, Yamaguchi H, Takami S, Ikeda K (1994b) Effects of panicle water potential on water stress susceptibility in rice. Jpn J Crop Sci 63: 200–207 [Google Scholar]
- Tsuno Y, Toryu S (1987) The influence of the inhibition of root respiration on the photosynthetic activity in rice plant. Jpn J Crop Sci 56: 512–520 [Google Scholar]
- Tyree MT, Fiscus EL, Wullschleger SD, Dixon MA (1986) Detection of xylem cavitation in corn (Zea mays) under field conditions. Plant Physiol 82: 597–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R (1999) Refilling of embolized vessels in young stems of laurel: do we need a new paradigm? Plant Physiol 102: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS (1988) Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Physiol 88: 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade LJ, Fukai S, Samson BK, Ali A, Mazid MA (1999) Rainfed lowland rice: physical environment and cultivar requirements. Field Crop Res 64: 3–12 [Google Scholar]
- Yamauchi A, Kono Y, Tatsumi J (1988) Comparative growth analysis of upland rice and maize grown under different soil moisture conditions. Jpn J Crop Sci 57: 174–183 [Google Scholar]
- Zeigler RS (1999) The rainfed lowland rice research consortium: a multi-institutional approach for sustainable productivity increases in Asian rice-based systems. Exp Agr 35: 115–125 [Google Scholar]
- Zhang J, Zhang X, Liang J (1995) Exudation rate and hydraulic conductivity of maize roots are enhanced by soil drying and abscisic acid treatment. New Phytol 131: 329–336 [Google Scholar]
- Zwieniecki MA, Hutyra L, Thompson MV, Holbrook NM (2000) Dynamic changes in petiole specific conductivity in red maple (Acer rubrum L.), tulip tree (Liriodendron tulipifera L.) and northern fox grape (Vitis labrusca L.). Plant Cell Environ 23: 407–414 [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291: 1059–1062 [DOI] [PubMed] [Google Scholar]