Abstract
Background
Thrombolysis for acute stroke is beneficial in selected patients. Because clinical trials generally exclude patients with pre-existing disability, this subgroup of patients has not been studied. We examined the outcomes after thrombolysis of patients with and without disability before their stroke.
Methods
We prospectively followed 112 consecutive patients with acute ischemic stroke who were given intravenous thrombolysis treatment according to published protocols. Three-month outcomes of the patients with pre-existing disability (defined as a prestroke score of 2 or more on the modified Rankin scale [MRS]) were compared with those of patients without pre-existing disability (defined as a prestroke MRS score of 0 or 1) and with those of 168 patients similarly treated in the National Institute of Neurological Disorders and Stroke trial.
Results
At 3 months after the stroke, patients with pre-existing disability (21% of the 112) had a higher mortality rate than those without (33% v. 14%) (odds ratio 3.2, 95% confidence interval 1.0–10.1) and worse function (median MRS score 3 v. 2, p = 0.03). However, there was little difference between the 2 groups in neurologic impairment among the survivors (median score on the National Institutes of Health stroke scale 4 v. 2, p = 0.41) or in the total proportion of those with an MRS score of 0 or 1 or, for those with a prestroke score greater than 1, a return to the prestroke score (42% v. 41%, p = 0.87).
Interpretation
Although the true effectiveness of thrombolysis for acute stroke in patients with pre-existing disability is not known, treated patients appear able to return to their prestroke level of function as often as patients without pre-existing disability, despite a significantly higher mortality rate.
The trial of the National Institute of Neurological Disorders and Stroke (NINDS) proved intravenous treatment with tissue plasminogen activator (tPA) to be effective and safe for acute ischemic stroke.1 However, the outcomes in patients with prior disability have not been examined.2 We report outcomes in this group as compared with patients without prior disability and patients in the NINDS trial.
Methods
The acquisition of patient data was part of a larger Canada-wide survey of intravenous tPA use.3 The protocol was not formally approved by the Ethics Review Board at the University of Western Ontario. The chair, Dr. Bessie Borwein, felt that approval was not required because the study only observed outcomes after a standard treatment intervention at this institution. Patients presenting with acute ischemic stroke in London, Ont., were given thrombolysis treatment according to the NINDS trial protocol,1 with 1 exception: patients with initial computed tomography (CT) evidence of involvement of more than one-third of the territory of the middle cerebral artery were excluded because they were possibly at higher risk of hemorrhage and less likely to benefit from thrombolysis treatment.4 Other trials had deviated from the NINDS protocol in the same way.5,6
All patients were followed for 3 months. They were assigned a score on the modified Rankin scale (MRS)7 for their functional disability (Box 1) before the stroke and at 3 months after the stroke. In addition, they were assigned a score on the National Institutes of Health stroke scale (NIHSS)8 for their neurologic impairment at baseline (just before the administration of tPA) and 24 hours and 3 months thereafter. In general, an NIHSS score of less than 8 reflects a mild stroke, 8–15 a moderate to severe stroke and more than 15 a severe stroke. The MRS and the NIHSS have acceptable validity and reliability.7,8,9 All patients underwent follow-up CT or magnetic resonance imaging at 24 hours and thereafter when clinically indicated. Intracerebral hemorrhage was considered symptomatic when accompanied by a 1-point increase in the level-of-consciousness score or a 4-point or greater increase in the NIHSS score.
Box 1.
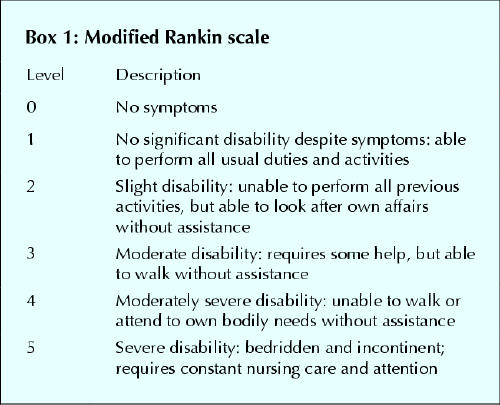
Patients were stratified into 2 groups: those without pre-existing disability (defined as an MRS score of 0 or 1 before the stroke [group 1]) and those with pre-existing disability (defined as an MRS score before the stroke of 2 or more [group 2]). Baseline characteristics and outcomes at 24 hours and at 3 months were compared in these 2 groups and in patients given intravenous tPA treatment in part 2 of the NINDS trial.1 A good outcome at 24 hours was defined as an improvement of 4 or more points from baseline in the NIHSS score. A good or favourable outcome at 3 months was defined as an NIHSS score of 0 or 1, an MRS score of 0 or 1, or, for patients with an MRS score greater than 1 before the stroke, a return to the prestroke MRS score.
A 2-tailed t-test or Wilcoxon 2-sample test was used to compare means and medians, respectively. Yates χ2 or Fisher's exact test was used to compare proportions between groups. Results are presented before and after adjustment for age, initial NIHSS score, and the presence of hypertension, atrial fibrillation and previous stroke. An α level greater than 0.05 was considered significant.
Results
We prospectively studied 112 consecutive patients: 88 in group 1 and 24 in group 2. NIHSS scores at 24 hours were not recorded for 3 patients: 2 in group 1 and 1 in group 2. The results of 3-month follow-up visits were obtained for 109 patients; the remaining 3 patients were assessed by telephone for MRS scores but not for NIHSS scores.
In baseline characteristics (Table 1) groups 1 and 2 were comparable except that group 2 had a higher mean age (p = 0.05) and higher proportions with hypertension (p = 0.03) and previous stroke (p = 0.02). Group 2 patients also were older (p = 0.03) and more had atrial fibrillation (p = 0.04) and previous stroke (p < 0.01) than treated patients in part 2 of the NINDS trial.
Table 1
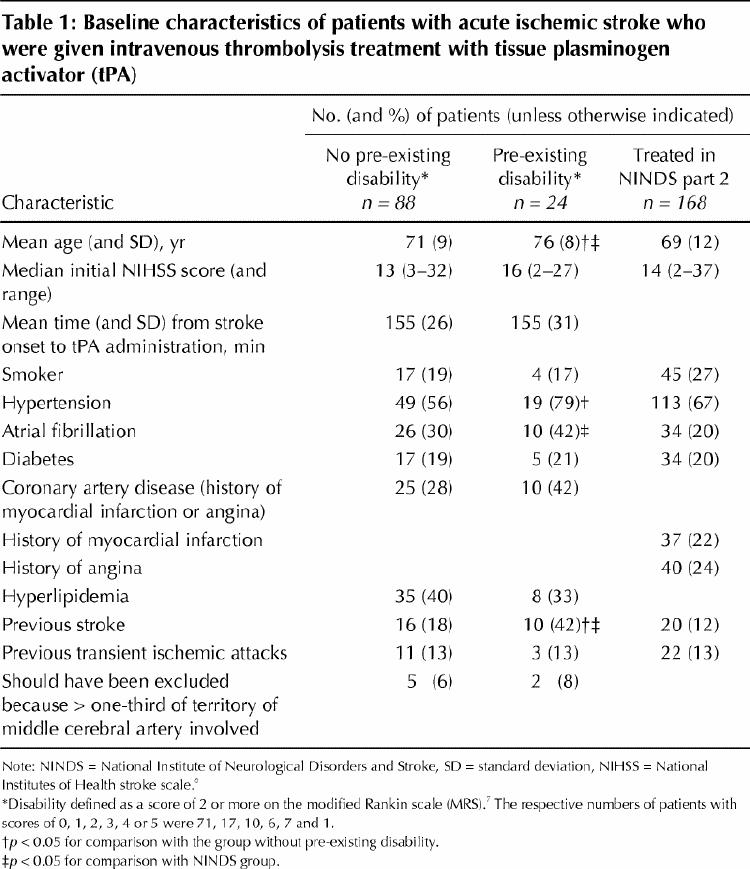
The 3-month mortality rate was higher in group 2 (33%) than in group 1 (14%) (unadjusted odds ratio [OR] 3.2, 95% confidence interval [CI] 1.0–10.1). Symptomatic intracerebral hemorrhage occurred in 1 patient (1.1%) in group 1 and 2 patients (8.3%) in group 2, and all were fatal; the patients were 79, 81 and 81 years old and their initial NIHSS scores 13, 22 and 27, respectively. Fewer group 1 patients than patients in part 2 of the NINDS trial had symptomatic intracerebral hemorrhages (p = 0.04).
There were no differences in the categorized outcomes (Fig. 1) between all treated London patients or between group 1 patients and those in the NINDS trial. Although more patients in group 1 than in group 2 had the good outcome of a 3-month MRS score of 0 or 1 (41% v. 8%), there was little difference between the 2 groups in the proportions of patients with a good NIHSS score at 24 hours or at 3 months or in those with a favourable outcome at 3 months, defined as either a 3-month MRS score of 0 or 1 or, for patients with an MRS score greater than 1 before the stroke, a return to the prestroke MRS score (Table 2, Fig. 2). Using an MRS score of 3 or more to define pre-existing disability produced similar results, except that the mortality rate was then 50% in those with prior disability and 13% in those without (OR 6.5, 95% CI 1.7–25.6).
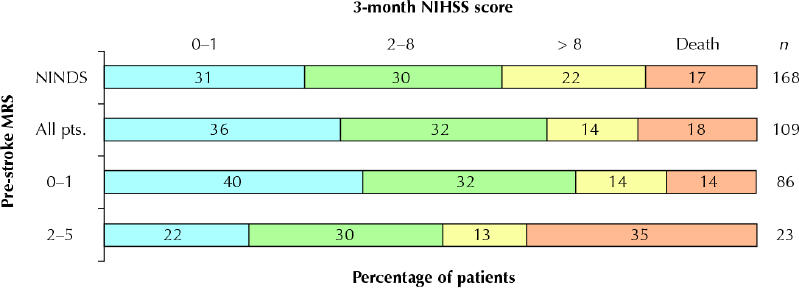
Fig. 1: Unadjusted proportions of patients with acute ischemic stroke who were given intravenous thrombolysis treatment with tissue plasminogen activator, by scores on the modified Rankin scale (MRS)7 and the National Institutes of Health stroke scale (NIHSS)8 before the stroke and at 3 months after the stroke. NINDS = patients so treated in part 2 of the trial of the National Institute of Neurological Disorders and Stroke.1 All pts. = all London, Ont., patients so treated. Three patients had 3-month MRS scores (but not NIHSS scores) assessed by telephone.
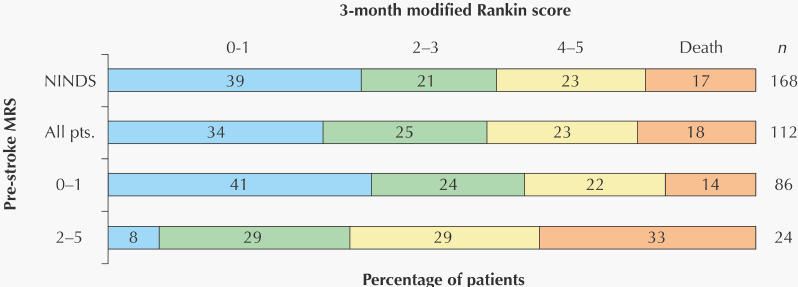
Fig. 1: Unadjusted proportions of patients with acute ischemic stroke who were given intravenous thrombolysis treatment with tissue plasminogen activator, by scores on the modified Rankin scale (MRS)7 and the National Institutes of Health stroke scale (NIHSS)8 before the stroke and at 3 months after the stroke. NINDS = patients so treated in part 2 of the trial of the National Institute of Neurological Disorders and Stroke.1 All pts. = all London, Ont., patients so treated. Three patients had 3-month MRS scores (but not NIHSS scores) assessed by telephone.
Table 2
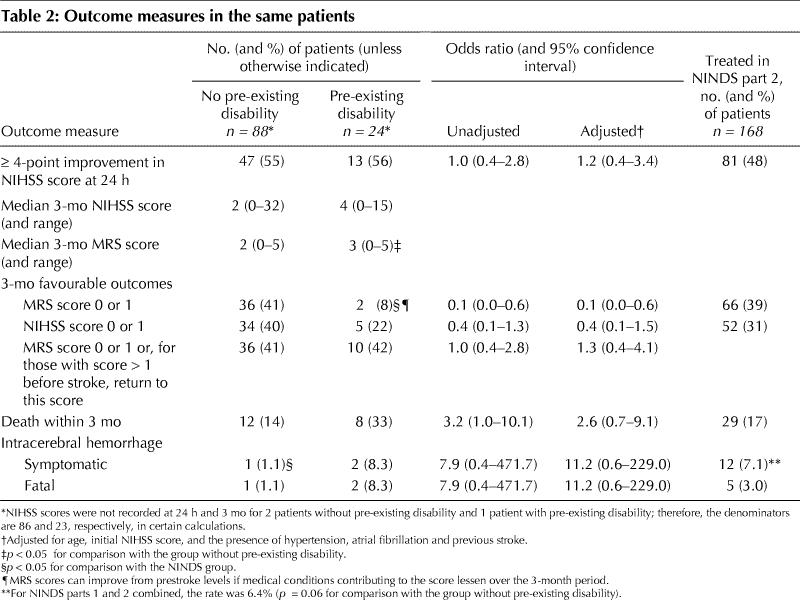
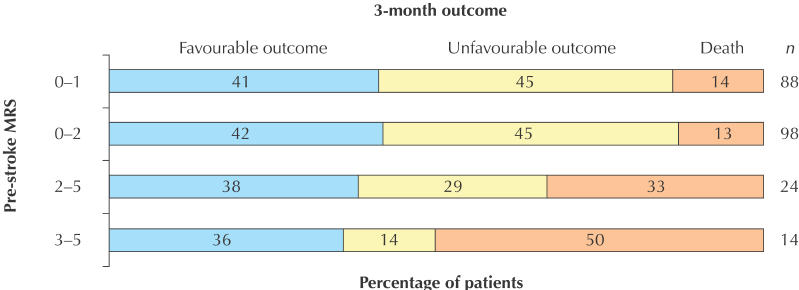
Fig. 2: Unadjusted proportions of the same patients by 3-month favourable or unfavourable outcome, a favourable outcome being defined as either an MRS score of 0 or 1, or, for patients with an MRS score greater than 1 before the stroke, a return to the prestroke MRS score.
Interpretation
Our study demonstrated that acute stroke patients with pre-existing disability who are treated with intravenously administered tPA have a poorer outcome, in terms of both survival and function, than those without pre-existing disability and those in the NINDS study. The mortality rate is high (33%) in those with pre-existing disability and especially high (50%) in those with a prestroke MRS score of 3–5. These findings are consistent with those from larger studies of stroke patients not treated with tPA that have shown pre-existing disability to be a predictor of outcome,10,11,12 with a 30-day relative risk of death of 2.2 (95% CI 1.2–4.1).12
In contrast, there was little difference between patients with or without pre-existing disability in the proportion at 3 months with a “favourable” NIHSS score or a return to a good prestroke MRS score.
Limitations of this study include the relatively small number of patients and the lack of a control group against which to test the true effectiveness of intravenous treatment with tPA. Our CT exclusion criterion may have biased our results toward better outcomes and lower rates of intracerebral hemorrhage as compared with the NINDS trial.13 A potentially offsetting bias is the increased time until treatment in our patients as compared with those in the NINDS trial: only 5% of the London patients (6/112), compared with approximately 50% of the NINDS patients,1 were treated within 90 minutes. The higher rate of symptomatic intracerebral hemorrhage in patients with pre-existing disability may reflect the increased age and initial stroke severity in this group, because both factors increase risk in patients treated with intravenously administered tPA.14,15
Previous stroke likely accounts at least partly for the pre-existing disability in many patients. Other factors that limit mobility (arthritis, deconditioning, etc.) are nonspecifically reflected in the MRS score. Thus, patients may improve beyond their prestroke MRS status, as exemplified by the 2 patients in group 2 who had a 3-month MRS score of 0–1.
Despite shortcomings and limited data, our study has shown that patients with pre-existing disability may benefit from intravenous treatment with tPA. Further studies in this patient population are required.
Acknowledgments
We thank the Coordinated Stroke Strategy Program of the Heart and Stroke Foundation of Ontario, Chris O'Callaghan, Connie Frank, Mary McTaggart, Cheryl Mayer, Rose Freitas, David Spence, Andrew Kertesz and Bryan Young for help in the organization and delivery of patient care and the collection of data.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Foell and Silver initiated and designed the study. Dr. Foell wrote the manuscript and performed the data analysis. All authors contributed substantially to the acquisition of data, critically revised the manuscript and gave approval of the final manuscript.
Drs. Foell and Demaerschalk were supported by Heart and Stroke Foundation of Canada scholarships. Drs. Silver and Merino were supported by a Fisher Family/ Heart and Stroke Foundation scholarship.
Competing interests: Drs. Foell, Silver and Wong have received honoraria from Roche Canada (manufacturer of Activase in Canada) as speaker fees.
Correspondence to: Dr. R. Blaine Taylor Foell, 23 Dairy Lane, Huntsville ON P1H 1T4; fax 705 789-1922; rbtfoell@yahoo.ca
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7. [DOI] [PubMed]
- 2.The NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA stroke trial. Stroke 1997; 28:2119-25. [DOI] [PubMed]
- 3.Hill MD, Buchan AM. Methodology for the Canadian Activase for Stroke Effectiveness Study (CASES). CASES investigators. Can J Neurol Sci 2001; 28: 232-8. [DOI] [PubMed]
- 4.von Kummer R, Allen KL, Holle R, Bozzao L, Bastianello S, Manelfe C, et al. Acute stroke: usefulness of early findings before thrombolytic therapy. Neuroradiology 1997;205:327-33. [DOI] [PubMed]
- 5.Hacke W, Kaste M, Fieschi C, von Kummar R, Davalos A, Meier D, et al. Randomized double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II). Lancet 1998;352:1245-51. [DOI] [PubMed]
- 6.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. JAMA 1999;282:2019-26. [DOI] [PubMed]
- 7.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. [DOI] [PubMed]
- 8.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864-70. [DOI] [PubMed]
- 9.Wolfe CDA, Taub NA, Woodrow EJ, Burney PGJ. Assessment of scales of disability and handicap for stroke patients. Stroke 1991;22:1242-4. [DOI] [PubMed]
- 10.Anderson C. Baseline measures and outcome predictions. Neuroepidemiology 1994;13:283-9. [DOI] [PubMed]
- 11.Harwood RH, Gompertz P, Pound P, Ebrahim S. Determinants of handicap 1 and 3 years after a stroke. Disabil Rehabil 1997;19:205-11. [DOI] [PubMed]
- 12.Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry 1990;53:824-9. [DOI] [PMC free article] [PubMed]
- 13.Silver B, Demaerschalk B, Merino JG, Wong E, Tamayo A, Devasenapathy A, et al. Improved outcomes in stroke thrombolysis with pre-specified imaging criteria. Can J Neurol Sci 2001;28:113-9. [DOI] [PubMed]
- 14.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957-60. [DOI] [PubMed]
- 15.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109-18. [DOI] [PubMed]


