Abstract
There is increasing evidence supporting a link between the endogenous opioid system and excessive alcohol consumption. Acute or light alcohol consumption stimulates the release of opioid peptides in brain regions that are associated with reward and reinforcement and that mediate, at least in part, the reinforcing effects of ethanol. However, chronic heavy alcohol consumption induces a central opioid deficiency, which may be perceived as opioid withdrawal and may promote alcohol consumption through the mechanisms of negative reinforcement. The role of genetic factors in alcohol dependency is well recognized, and there is evidence that the activity of the endogenous opioid system under basal conditions and in response to ethanol may play a role in determining an individual's predisposition to alcoholism. The effectiveness of opioid receptor antagonists in decreasing alcohol consumption in people with an alcohol dependency and in animal models lends further support to the view that the opioid system may regulate, either directly or through interactions with other neurotransmitters, alcohol consumption. A better understanding of the complex interactions between ethanol, the endogenous opioids and other neurotransmitter systems will help to delineate the neurochemical mechanisms leading to alcoholism and may lead to the development of novel treatments.
Medical subject headings: alcoholism; dopamine; endorphins; enkephalins; ethanol; dynorphins; genetic predisposition to disease; genetics; opioid peptides; receptors, opioid; reward
Abstract
Des données probantes de plus en plus nombreuses appuient l'existence d'un lien entre le système opioïde endogène et la surconsommation d'alcool. La consommation aiguë ou légère d'alcool stimule la libération, dans des régions du cerveau qui sont associées à la récompense et au renforcement, de peptides opioïdes qui déclenchent, du moins en partie, les effets de renforcement de l'éthanol. Une consommation importante et chronique d'alcool provoque toutefois une déficience centrale des opioïdes qui peut être perçue comme un sevrage des opioïdes et peut favoriser la consommation d'alcool par les mécanismes du renforcement négatif. Le rôle des facteurs génétiques dans la dépendance de l'alcool est bien connu et des données probantes indiquent que l'activité du système opioïde endogène dans des conditions de base et en réaction à la présence d'éthanol peut jouer un rôle dans la détermination de la prédisposition d'une personne à l'alcoolisme. L'efficacité des antagonistes des récepteurs des opioïdes dans la réduction de la consommation d'alcool chez les personnes qui ont une dépendance à l'égard de l'alcool et chez des modèles animaux appuie encore davantage l'opinion selon laquelle le système opioïde peut régulariser la consommation d'alcool, directement ou par interaction avec d'autres neurotransmetteurs. Une meilleure compréhension des interactions complexes entre l'éthanol, les opioïdes endogènes et d'autres systèmes neurotransmetteurs aidera à définir les mécanismes neurochimiques à l'origine de l'alcoolisme et pourrait déboucher sur la mise au point de traitements nouveaux.
Introduction
Alcoholism is a major public health problem that not only causes enormous damage to health and quality of life, but also undermines the well being of family and society. In an attempt to deal with alcohol-related problems at the beginning of the 20th century, the focus was on the prohibition of alcohol use, and for a time in the United States, there were sanctions against the sale and use of alcohol. More recently, however, the focus has been placed on better understanding the medical and psychosocial problems associated with excessive alcohol consumption, as well as the neurochemical substrates mediating alcohol reinforcement.1 Studies on the genetic epidemiology of alcoholism, such as twin, family and adoption studies, clearly demonstrate that genetic factors play an important role in the development of alcoholism.2 These findings are further supported by the development of inbred3,4,5,6 and outbred7,8,9,10,11,12,13 lines and strains of animals with high or low preferences for ethanol solutions. These animals are used to study the biochemical basis of alcoholism.
Among the neurotransmitter systems proposed to be important in controlling alcohol-seeking behaviour is the endogenous opioid system.14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31 It has been proposed that alcohol may stimulate the release of certain opioid peptides, which in turn, could interact with the centres of the brain associated with reward and positive reinforcement and lead to further alcohol consumption.32,33,34,35,36 It has been suggested that increased activity of brain enkephalin or β -endorphin opioid peptide systems, either under basal conditions or in response to ethanol exposure, may be important for initiating and maintaining high alcohol consumption.37,38,39,40,41 Likewise, increased density of δ or μ (or both) opioid receptors in brain regions known to mediate the positive reinforcing effects of drugs of abuse may also be important in initiating and maintaining high alcohol consumption.42,43,44,45,46,47 Conversely, increased dynorphin activity or increased κ binding site density may inhibit high ethanol consumption.14,36
In this review, we present the experimental evidence that suggests the endogenous opioid system plays a role in controlling alcohol consumption, examine the opioid system's possible contribution to the genetic predisposition to alcohol dependency and summarize the effectiveness of pharmacotherapy based on opioid antagonists.
Endogenous opioid system
The endogenous opioid system is involved in 3 major functions: modulation of the response to painful stimuli and stressors; reward and reinforcement; and homeostatic adaptive functions, such as regulating body temperature and food and water intake.48 The 3 distinct families of endogenous opioid peptides are defined by their precursor molecules.49
· Pro-opiomelanocortin (POMC) gives rise to b-endorphin, which is synthesized in the brain in the arcuate nucleus and a small group of neurons in the nucleus tractus solitarii.50,51 β -Endorphin neurons of the arcuate nucleus project to various brain regions including the ventral tegmental area (VTA), nucleus accumbens, septum, amygdala, hippocampus, frontal cortex and periaqueductal gray.49
· Proenkephalin gives rise to 4 methionine (met)-enkephalin molecules and 1 of each met-enkephalin-Arg6-Phe7, met-enkephalin-Arg6-Gly7-Leu8 and leucine (leu)-enkephalin.52
· Prodynorphin gives rise to dynorphins, α -neoendorphins and leu-enkephalin.53 Neurons synthesizing enkephalins and dynorphins are widely distributed throughout the brain.49
At least 3 major classes of opioid receptors — μ, δ and κ — have been identified and characterized. The opioid peptides present different affinities for each of the opioid receptors. β -endorphin binds with about equal affinity to μ and δ opioid receptors, met- and leu-enkephalins bind with 10- to 25-fold greater affinity to δ than μ opioid receptors, and dynorphins bind selectively to κ opioid receptors54 (Table 1).
Table 1
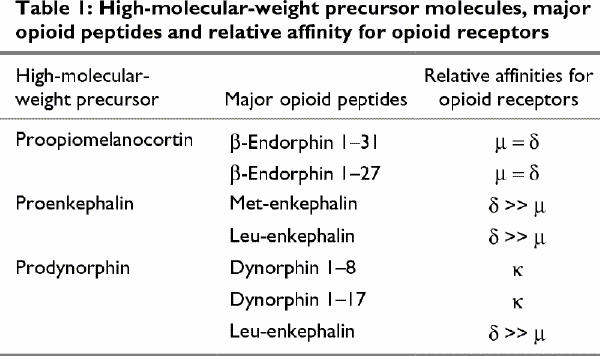
Interactions of endorphins and enkephalins with μ and δ opioid receptors increase dopamine (DA) release in the nucleus accumbens and may initiate processes associated with reward and reinforcement,55 whereas dynorphins binding to κ opioid receptors, which has been shown to produce aversive states and decrease DA release, may prevent reinforcement.55
Brain reward system
The anatomical pathway of the brain reward system consists of a midbrain–forebrain–extrapyramidal circuit centred on the nucleus accumbens. The main regions associated with the reward system are the VTA, the nucleus accumbens, the septal area, the amygdala, the hypothalamus, the hippocampus and the frontal cortex.55,56,57 Significant experimental evidence suggests that the reinforcing effects of many drugs of abuse, including ethanol, are mediated by the mesolimbic DA pathway,55,56,57 consisting mainly by the A10 group of DA neurons. The A10 cell bodies in the VTA project to nuclei of the forebrain, mainly the nucleus accumbens, caudate, olfactory tubercles, frontal cortex, amygdala and septum.
Systemic administration of many drugs of abuse increases DA release in the nucleus accumbens.55,56,57 Although evidence suggests that DA release is sufficient to produce reward, it may not be “required”; nondopaminergic mechanisms may be also involved.55,56,57 Interestingly, data indicate that the acquisition of alcohol drinking behaviour may be dependent on the activation of mesolimbic DA neurons, but the maintenance of the behaviour does not require the functional integrity of mesolimbic DA neurons.57,58,59 Therefore, different neuronal mechanisms may mediate the acquisition and the maintenance of alcohol drinking behaviour.57,58,59,60 Furthermore, ethanol-induced activation of DA neurons may be mediated by the endogenous opioid system at the level of VTA and nucleus accumbens,57,61,62 thus supporting a role of the endogenous opioid system in controlling alcohol consumption (Fig. 1).
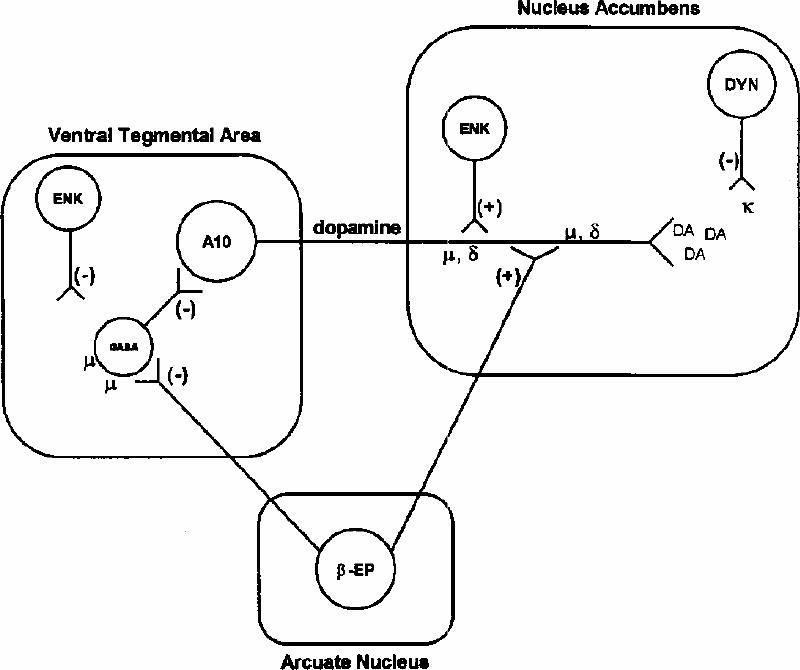
Fig. 1: Diagramatic representation of the possible interactions between the endogenous opioid system and the brain nuclei responsible for mediating the positive reinforcing effects of ethanol. A10 dopaminergic (DA) neurons, whose cell bodies are located in the ventral tegmental area (VTA) and whose axonal terminals are in the nucleus accumbens, are under tonic GABAergic inhibition. This inhibition may be removed when μ opioid receptors (located in the cell body of the GABA interneuron) are stimulated either by β -endorphin (originating in the arcuate nucleus and projecting to the VTA) or by enkephalins. In addition, DA release may be increased by stimulation of δ or μ opioid receptors in the nucleus accumbens. Ethanol stimulates β -endorphin or enkephalin release, and this may lead to increased DA release in the nucleus accumbens. In contrast, stimulation of κ opioid receptors in the nucleus accumbens by dynorphin peptides decreases DA release and may produce aversive states. GABA, γ-aminobutyric acid; β -EP, β -endorphin, ENK, enkephalin; DYN, dynorphin; (–) indicates inhibition; (+) indicates stimulation. Reproduced from Jamensky and Gianoulakis,36 with permission of the publisher.
To better understand how the endogenous opioids may influence alcohol consumption, it is helpful to review the effects ethanol has on the various opioid peptides and receptors.
Ethanol and β -endorphin
Evidence suggests that ethanol alters the activity of the endogenous opioid peptides in the brain and pituitary, and these alterations may modulate, at least in part, many of the behavioural and neuroendocrine effects of ethanol, including that of reinforcement. Although all 3 opioid peptide systems have been implicated in this process, most investigations to date have focused on the effects of ethanol on β -endorphins in the brain and pituitary.
In the short term, in vivo ethanol administration increases the release of β -endorphin by the pituitary gland, the hypothalamus and other distinct regions of the brain.41,63,64 The increased release by the pituitary is mediated by the ethanol-induced increase of hypothalamic corticotropin releasing hormone (CRH) and parallels the activation of the hypothalamic–pituitary–adrenal axis, as indicated by the changes in plasma levels of adrenocorticotropic hormone (ACTH) and glucocorticoids. The ethanol-induced increase of hypothalamic CRH mediates, at least in part, the enhanced release of β -endorphin, not only by the pituitary, but also by the hypothalamus (Fig. 2).
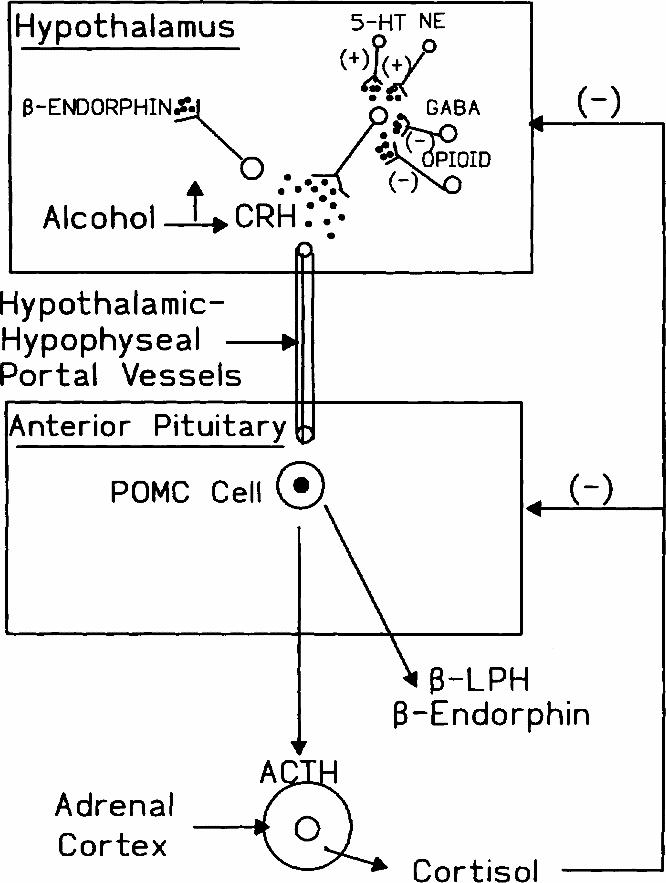
Fig. 2: Major neuroendocrine components of the hypothalamic–pituitary–adrenal axis. Hypothalamic neurons synthesize and release corticotropin releasing hormone (CRH), which is transported to the anterior pituitary via the hypothalamic-hypophyseal portal vessels, interacts with pro-opiomelanocortin (POMC) producing cells and stimulates the synthesis and release adrenalcorticotropin (ACTH), β -lipotropin (β -LPH) and β -endorphin. CRH also interacts with hypothalamic endorphinergic neurons and stimulates the release of β -endorphin in the brain. ACTH stimulates cells of the adrenal cortex to increase the synthesis and release of cortisol, and this increase in plasma cortisol inhibits the release of CRH and ACTH via a negative-feedback mechanism. The release of CRH is also inhibited by opioidergic and GABAergic neurons and stimulated by serotonergic (5-HT) and noradrenergic (NE) neurons. Ethanol stimulates the release of CRH, and this leads to increased release of β -endorphin, both by the pituitary and the hypothalamus.
In vitro exposure of tissue of the pituitary gland or the hypothalamus to ethanol stimulates the release of β -endorphin in a dose-dependent manner;40,65,66,67 low concentrations of ethanol induce a more pronounced increase in β -endorphin release than high concentrations, leading to an inverse U-shaped dose–response curve40,66,68 (see Fig. 3). Furthermore, time-course studies indicate that this enhanced release is not maintained under prolonged exposure of the tissue to the same concentration of alcohol.65,67 Indeed, ethanol induces a fast transient increase of β -endorphin release lasting about 15–20 min, and this is followed by a return to basal levels by both the pituitary and the hypothalamus.65,67 It is noteworthy that in vivo studies also indicate a nonsustained ethanol-stimulated release of pituitary β -endorphin (Guillaume and Gianoulakis, McGill University, unpublished data, 1997).
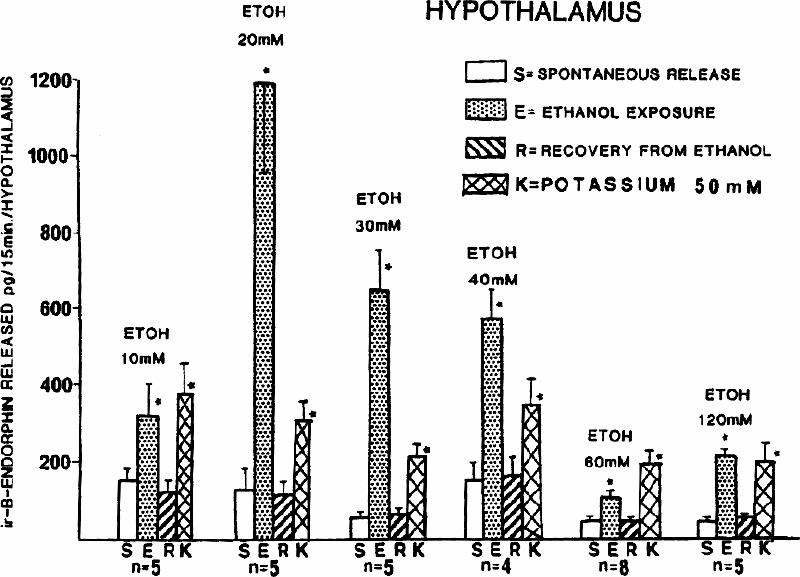
Fig. 3: The effect of various concentrations of ethanol (ETOH) and potassium (K) on the release of immunoreactive β -endorphin by the hypothalamus. Bars represent means and standard errors. n = number of distinct experiments. *Significantly different from spontaneous release (p < 0.05). Reproduced from Gianoulakis,66 with permission of the publisher.
Results of studies investigating the effects of long-term alcohol administration on pituitary and hypothalamic β -endorphin have been inconsistent. Some studies report that long-term alcohol administration leads to a significant increase in POMC mRNA, as well as in the biosynthesis of POMC and its post-translational processing to β -endorphin by the pituitary.69,70 Others report a reduction in the biosynthesis and release of POMC-derived peptides;71,72 another study reported an initial increase of POMC mRNA and a gradual return to control or even below-control levels, suggesting the development of ethanol tolerance by POMC-producing cells of the anterior pituitary.73
The effects of long-term alcohol administration on hypothalamic β -endorphin are also inconsistent, with some studies reporting enhanced activity,74,75,76 some no change77 and others decreased activity.78 The inconsistencies are likely due to the length and method of ethanol administration (i.e., drinking, liquid diet, vapor inhalation), as well as to the quantity of ethanol consumed and species and strain differences. The conflicting results may also be associated with differences in the development of tolerance. The development of tolerance to specific effects of ethanol, such as the hypothermic effect, or the development of physical dependence was investigated by some,70,74,78 but most studies69,71,72,73,75,76,77 did not report tolerance-related effects.
Thus, experimental data clearly indicates that short-term ethanol exposure stimulates the release of brain β -endorphin, which may interact with specific μ and δ opioid receptors in regions of the brain that mediate, at least in part, many of the neurobehavioural effects of ethanol. However, because the release of β -endorphin is not sustained under prolonged ethanol exposure, enhanced β -endorphin activity may not be involved in maintaining the alcohol-drinking behaviour. On the contrary, the decrease in β -endorphin activity after prolonged exposure to ethanol may promote and maintain alcohol consumption through the mechanisms of negative rather than positive reinforcement. Indeed, after long-term exposure, many neuronal systems undergo adaptive changes to overcome the effects of alcohol and maintain their functional activity at about normal levels. Thus, when alcohol is no longer present, abnormalities in distinct neuronal systems may cause discomfort or pain and may increase craving and motivation to consume ethanol. This motivation to avoid discomfort is known as negative reinforcement. Decreased β -endorphin release after prolonged alcohol exposure may therefore lead to an increase in consumption through negative reinforcement.
Ethanol and the enkephalins and dynorphins
Although there are significantly less data on interactions of ethanol with the enkephalin and dynorphin opioid peptides, there is some evidence to indicate that both systems are influenced by alcohol; however, the effects seem to be tissue and species, strain or line specific.
Short-term ethanol administration has been reported to increase met-enkephalin levels in the striatum and hypothalamus77 of rats, whereas long-term administration decreased met-enkephalin levels in many, but not all, brain regions assayed.74,77 Other studies report no significant changes in met-enkephalin levels in the striatum and hypothalamus of the rat after short-term ethanol administration.79 Ethanol intake was found to increase proenkephalin mRNA in the nucleus accumbens,80 and prolonged administration increased met-enkephalin-Arg6-Phe7 in the same region; these effects were not observed in all lines of animals tested, however.80,81
Milton and Erickson79 found that long-term ethanol administration decreased dynorphin and α -neoendorphin levels in the hypothalamus and hippocampus, but not in the striatum, midbrain and pituitary gland of male Sprague-Dawley rats. A reported increase in prodynorphin peptides in the nucleus accumbens after prolonged ethanol self-administration was also animal-line specific.81
It is evident that more studies are needed to clarify the effects of ethanol on enkephalin and dynorphin opioid peptides.
Ethanol and opioid receptors
Alcohol intake may alter, not only the activity of the endogenous opioid peptide system, but also the density or affinity of specific opioid receptors in distinct regions of the brain. Such changes would alter the interactions of the opioid receptors with their respective ligands and, as a result, would alter the functional activity of the whole system.
Opioid receptor changes may mediate some of the neurobehavioural effects of ethanol, including ethanol reinforcement. Indeed, experimental evidence indicates that both short- and long-term ethanol administration may affect opioid receptors. Again, study results are inconsistent; these inconsistencies may be due to differences in the type of opioid receptors and brain regions studied, alcohol concentration, duration and mode of alcohol administration, presence or absence of alcohol in the incubation medium during in vitro binding experiments, as well as the species, strains and lines of animals tested.
Early studies reported short-term in vitro exposure of brain membrane preparations to ethanol selectively decreased the binding to δ, but not to μ or κ opioid receptors,82,83,84 whereas others reported an increased binding to μ opioid receptors.84,85,86,87 Most early studies report that prolonged ethanol administration decreases binding to δ opioid receptors,54,84,88,89 but data on the effect of long-term treatment on μ receptors are inconsistent (i.e., increase,84,85 decrease90,91 and no effect92 on binding to μ opioid receptors). However, in many of the early studies, tissue preparations contained many receptor subtypes and the ligands were not very selective and recognized (with different affinities) more than 1 receptor subtype.54
In more recent studies, autoradiographic techniques have allowed the investigation of the effects of ethanol in specific brain regions, and the development of more selective ligands has allowed a better characterization of the effects of ethanol on specific opioid receptor systems. Thus, voluntary ethanol consumption for 30 days by the Sardinian alcohol-preferring rats increased binding to both μ and δ opioid receptors in the caudate putamen, but had no effect in the other regions studied.45 However, ethanol in the drinking water of Wistar rats induced a down-regulation of the μ opioid receptors in the nucleus accumbens and striatum but had no effect on δ1 and δ2 opioid receptors.93 These findings confirm earlier observations that alcohol-induced changes vary with the brain region investigated as well as the species and strain of animals used.
Despite the inconsistent reports on the effects of ethanol on opioid receptors, further evidence supporting a link between the opioids and alcohol consumption can be found in studies reporting the attenuation of ethanol self-administration after the administration of specific and nonspecific opioid receptor antagonists.15,31,95 Such studies indicated that μ and δ opioid receptor antagonists are more effective in decreasing alcohol consumption15,31 than κ opioid receptor antagonists.94,95,96,97,98
In addition, microinjection of an antisense oligodeoxynucleotide targetted to μ opioid receptors in the nucleus accumbens disrupted ongoing alcohol drinking by the ethanol-preferring (HEP) rats,99 and knock-out mice, either lacking β -endorphin or presenting half of the normal expression of β -endorphin, worked harder to obtain ethanol delivered either orally or intravenously.100,101 On the other hand, C57BL/6 mice lacking the expression of the μ opioid receptor showed a decreased preference for ethanol solutions.102
Clinical studies have also shown that opioid receptor antagonists decrease alcohol consumption in those with an alcohol dependency.29,30,31
Genetic influence on alcohol consumption: implications of the endogenous opioid system
Epidemiological studies clearly indicate that genetic factors and family history play a significant role in determining a person's vulnerability for high alcohol consumption and alcoholism. Twin studies, adoption and cross-fostering studies, as well as detailed pedigree analyses all suggest that alcoholism “runs” in families. However, there are many genes that interact with environmental factors in a complex manner to increase or decrease an individual's vulnerability to alcoholism.103,104 In fact, studies indicate that sons of those with an alcohol dependency have a 4- to 9-time greater risk of becoming an alcoholic than sons of nondependent parents.103,104
However, not all the children inherit the genetic factors associated with increased vulnerability to high alcohol consumption. The physiologic, hormonal and psychological responses to alcohol of those with a positive or negative family history of alcoholism have been compared to determine if any biological markers might be used to identify individuals in high-risk families who have inherited the vulnerability for high alcohol consumption. These markers could be behavioural (e.g., impulsive or violent behaviour),103 physiological (e.g., electroencephalographic [EEG] abnormalities105 or body sway)106 or biochemical markers (e.g., enzymes, hormones, neurotransmitters and neuromodulators).37,38,107,108,109,110,111,112 Indeed, results from such studies indicate that a number of physiological responses, including electroencephalographic, heart rate and hormonal changes, might differ between individuals with and without a family history of alcoholism. Furthermore, sociocultural studies point to a number of environmental factors, for example culture and stress,103,104,113 that increase or decrease the risk for alcoholism. Thus, it may be proposed that alcoholism is a multifactorial disorder, with an inherited predisposition interacting with specific environmental factors.103,104
Genetic differences in the activity of the endogenous opioid system may mediate the reinforcing effects of alcohol and play a role in controlling alcohol consumption. For example, in some individuals, genetic factors may cause a more pronounced release of β -endorphin or enkephalin in the VTA and nucleus accumbens in response to alcohol. Genetic factors may also lead to higher densities of μ or δ opioid receptors, creating more opportunities for interactions between opioid peptides and their receptors. In others, as proposed by the opioid deficiency hypothesis, genetic factors may be responsible for low opioid activity under basal conditions.114
Ethanol, by stimulating the release of opioid peptides, increases central opioid activity and stimulates the release of DA, leading to alcohol reinforcement. Indeed, genetic differences were observed between young nonalcoholic individuals with and without a family history of alcoholism. Subjects with a family history of alcoholism presented lower concentrations of plasma β -endorphin in the early morning hours and a more pronounced increase in pituitary β -endorphin release after the ingestion of moderate doses of alcohol.37,38 Thus, low basal morning levels of β -endorphin and an increased release of pituitary β -endorphin in response to ethanol may be a biological marker and could be used together with other markers to distinguish individuals who have inherited a vulnerability for high alcohol consumption. In fact, a recent study of identical (monozygotic) and fraternal (dizygotic) twins to determine the heritability of hormonal responses to alcohol found that the β -endorphin response to alcohol presented significant heritability.39 Moreover, increased levels of peripheral β -endorphin during physical activity or altered states of consciousness are associated with improved mood and a general feeling of well being,115,116,117 suggesting that the pronounced ethanol-induced increase of pituitary β -endorphin observed in those with a family history of alcoholism may have a functional significance.
In addition to the differences in the pituitary β -endorphin system between subjects with and without a family history of alcoholism, differences have also been reported in the activity of the hypothalamic opioid system. The endogenous opioid system exerts inhibitory control over hypothalamic CRH-producing neurons.118 Removing this inhibition by administering opioid antagonists such as naloxone increases the release of CRH, which leads to increased release of pituitary ACTH and adrenal cortisol.118 The stronger the opioid-related inhibitory control on CRH neurons, the higher the dose of naloxone needed for disinhibition. Wand et al118 found that significantly lower concentrations of naloxone were needed to remove the opioid inhibitory control of the CRH neurons in nonalcoholic offspring of alcohol-dependent individuals than in offspring of nondependent parents, perhaps indicating diminished hypothalamic opioid activity.118 This deficiency may be attributed either to decreased levels of one or more opioid peptides or to decreased density of one or more opioid receptors. Presently, it is not known which component of the hypothalamic endogenous opioid system influences the hypoactivity under basal conditions.118 However, this low central opioid activity in individuals with a family history of alcohol dependence may increase their vulnerability to alcoholism by altering the DA release from the nucleus accumbens. For example, it may be hypothesized that under basal conditions, the opioid-stimulated release of DA is low, reflecting the low activity of the central endogenous opioid system. Alcohol stimulates the central endogenous opioid system, either by increasing the release of opioid peptides37,38,39,40,41,63,64,65,66,67,68 or by altering the binding properties of opioid receptors82,83,84,85,86,87,88,89,90,91,92,93 or both. Opioid activity will also stimulate DA release and, thus, enhance ethanol's reinforcing effect, and this may lead to increased alcohol consumption. Future brain imaging studies may provide support for this hypothesis.
Effect of alcohol abuse on the opioid system
In contrast to the effects of ethanol challenge and light alcohol consumption, both of which stimulate the brain and pituitary activity of opioid peptides,37,38,39 prolonged alcohol consumption induces a decrease in brain and pituitary β -endorphin activity, as indicated by the low plasma and CSF β -endorphin levels in alcohol-dependent individuals and experimental animals exposed to prolonged alcohol treatment.37,119,120,121,122,123,124,125,126,127,128,129,130 Thus, it may be proposed that individuals with a long history of alcohol dependence will exhibit a central opioid deficiency, which could be associated with decreased hypothalamic and pituitary β -endorphin synthesis and release, as well as with decreased opioid receptor density in distinct brain regions. Furthermore, alcoholic offspring of alcoholic parents, who have been shown to exhibit an opioid deficiency predating heavy alcohol consumption,118 will present a more pronounced central opioid impairment than alcoholic offspring of nonalcoholic parents. This central and peripheral opioid deficiency may be perceived by the individual as a mild opioid withdrawal and may be an additional factor promoting heavy drinking, through the mechanisms of negative reinforcement.
Endogenous opioids in animal models of alcoholism
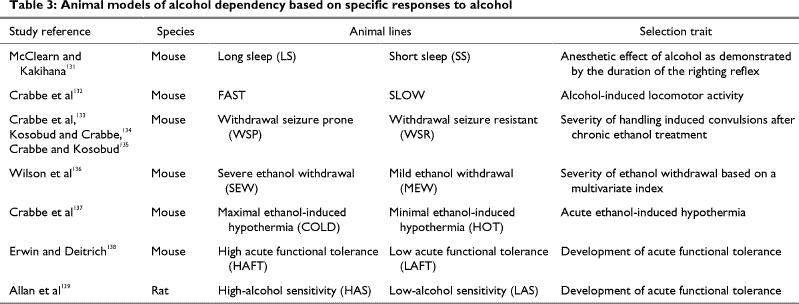
The contribution of genetic factors to the predisposition to excessive alcohol consumption is supported by animal studies of inbred and outbred strains and lines of animals that differ in their preference for drinking alcohol solutions (Table 2)3,4,5,6,7,8,9,10,11,12,13 and in specific responses to short- and long-term alcohol exposure (Table 3).131,132,133,134,135,136,137,138,139 Such selected lines of animals can be used to test various hypotheses of the neurochemical and neurophysiological bases for ethanol-related behaviours (e.g., ethanol-induced activation, anesthesia, hypothermia) and to study the development of tolerance and the severity of withdrawal symptoms. Differences in the sensitivity of selected lines of animals to specific effects of ethanol may also underlie differences in alcohol preference. It has been proposed that the more severe the ethanol withdrawal symptoms an animal experiences, the lower its voluntary ethanol consumption.140 For example, when withdrawal seizure prone (WSP), withdrawal seizure resistant (WSR) and nonselected control mice were withdrawn from ethanol after similar long-term ethanol exposure,140 the WSP mice experienced 10-fold more severe ethanol withdrawal than the WSR mice, and the control mice experienced intermediate withdrawal severity.140 When given the choice between ethanol solutions and water, the WSR mice consumed more ethanol than the WSP, and the WSC mice consumed intermediate amounts.140 A similar relation between severity of ethanol withdrawal and amount of ethanol consumed was observed between DBA/2 and C57BL/6 inbred strains of mice. The ethanol-avoiding DBA/2 mice experienced more severe withdrawal-induced seizures than the alcohol-preferring C57BL/6 mice.141 Thus, different sensitivities to ethanol in selected lines and strains of animals may predict differences in voluntary consumption.140,141
Table 2
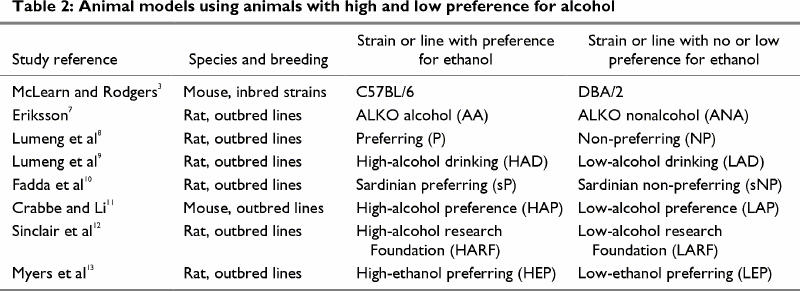
Table 3
Selected lines of animals have been used to investigate the biochemical basis of alcoholism at the level of the central nervous system, where the rewarding and reinforcing effects of drugs of abuse are mediated. Human and animal studies37,38,39,40 suggest a relationship between a highly responsive endogenous opioid system to ethanol and increased vulnerability to high alcohol consumption. If we accept that the endogenous opioids can modulate alcohol consumption, then genetic differences in the activity of one or more distinct components of the endogenous opioid system, either under basal conditions or after exposure to ethanol, may be important in determining the predisposition for excessive alcohol consumption. Indeed, numerous studies have investigated differences in the activity of the endogenous opioid system between ethanol-preferring and ethanol-avoiding animals.36,37,40,42,43,44,45,46,47 For example, using northern blot analysis and in situ hybridization, a higher content of POMC mRNA was observed in the hypothalamus of the AA (alcohol preferring) than ANA (alcohol avoiding) rats47,122 and of the C57BL/6 than DBA/2 mice.40,46,123 Furthermore, C57BL/6 mice presented a higher basal release and a more pronounced and longer lasting hypothalamic β -endorphin response to a single ethanol exposure.40,67 However, the ethanol-preferring AA rats presented a lower spontaneous release of hypothalamic β -endorphin than the ANA rats, although there was no difference in the ethanol-stimulated hypothalamic β -endorphin release between the 2 lines.124
Daily injections of ethanol over 4 days produced a greater increase in POMC mRNA in the anterior and neurointermediate lobes of the pituitary of ethanol-preferring P rats compared with ethanol-avoiding NP rats.125 Studies of long sleep (LS) and short sleep (SS) mice demonstrated no significant differences in the basal levels of pituitary POMC mRNA, and after 4 days of ethanol treatment, there was a significant increase in pituitary POMC mRNA of both LS and SS mice.73 However, after 7 days of ethanol treatment, pituitary POMC mRNA remained elevated in the SS mice, but not in the LS mice which presented a 40% decrease.73 In another study, differences in the basal levels of pituitary β -endorphin among 16 strains of mice were reported, and these differences correlated genetically with the severity of ethanol withdrawal symptoms.142 These studies support the hypothesis that genetically dependent differences in pituitary β -endorphin function may underlie some of the differences in the severity of the ethanol withdrawal symptoms.142
Basal levels of met-enkephalin peptides were reported to be lower in the nucleus accumbens of the AA than ANA rats81 and in the hypothalamus of the C57BL/6 than DBA/2 mice;126 a higher content of proenkephalin mRNA was also observed in the prefrontal cortex of the AA than ANA rats.47 Using northern blot analysis and sensitive radioimmunoassay, it was shown that proenkephalin mRNA and met-enkephalin peptide levels were similar in the hypothalamus, striatum, hippocampus, and medulla pons and lower in the midbrain of the C57BL/6 than in DBA/2 mice.127 The P and NP rats presented similar levels of preproenkephalin-derived peptides;128 however, short-term ethanol treatment increased the preproenkephalin mRNA levels in the nucleus accumbens of the P but not of the NP line of rats. Moreover, after prolonged alcohol treatment, met-enkephalin-Arg6-Phe7 was higher in the nucleus accumbens of the AA than ANA rats.81 AA rats were also found to have lower levels of prodynorphin mRNA in the mediodorsal nucleus of the thalamus47 and lower levels of prodynorphin derived-peptides in the nucleus accumbens and VTA81 than the ANA rats. Prodynorphin mRNA and prodynorphin-derived peptides were also significantly lower in the nucleus accumbens of the C57BL/6 than DBA/2 mice.36
Differences between alcohol-preferring and alcohol-avoiding animals have also been observed in the densities of μ""",""" δ and κ opioid receptors in distinct regions of the brain known to be involved in the processes of drug reward and reinforcement.35,36,42 AA rats were found to have higher density of μ opioid receptors in the shell region of the nucleus accumbens and prefrontal cortex but a lower density of κ opioid receptors in the ventromedial hypothalamus than ANA rats.47 Some studies report higher density of δ opioid receptors in the nucleus accumbens of the AA rats;35 others, using different opioid receptor ligands, report either lower density of δ opioid receptors in the AA rats129 or no difference in δ opioid receptors density between AA and ANA rats.130 The alcohol-preferring C57BL/6 mice were found to have a higher δ opioid receptor density and a lower κ opioid receptor density in the nucleus accumbens than DBA/2 mice,36,42 and the alcohol-preferring P rats presented higher density of μ opioid receptors in some regions of the limbic system than the alcohol-avoiding NP rats.143 However, no differences in μ opioid receptor mRNA were found between the HAD and LAD lines of rats.144
These comparative studies demonstrate that there is no single common component of the endogenous opioid system that is directly responsible for excessive alcohol consumption. Indeed, this observation is in agreement with reports demonstrating that although the μ opioid receptor antagonists are more effective in reducing alcohol consumption by the AA line of rats,25 δ opioid receptor antagonists are more effective in decreasing alcohol consumption by C57BL/6 mice145 as well as by the P and HAD lines of rats.18,23,24 Furthermore, there are indications that the κ opioid receptor dynorphin peptide system also plays a role in controlling alcohol consumption.95,146
Considering the complexity of the endogenous opioid system and the fact that it interacts with a number of other neurotransmitter systems (e.g., GABAergic, serotonergic and DA systems), it is reasonable to conclude that the role of the opioid system in alcohol consumption is a complicated one involving different distinct components of the opioid system for different individuals and selectively bred lines of animals. However, understanding how these components, through their interactions with other neurotransmitter systems, are involved in the control of alcohol consumption may allow the development of effective treatments for alcoholism. Furthermore, future studies investigating genetically determined differences in the activity of the endogenous opioid system or in its interactions with other neurotransmitter systems should include a number of selected lines or strains of animals or a number of recombinant inbred strains. Such studies would allow us to correlate the differences in the activity of distinct components of the endogenous opioid system with differences in the sensitivity to or preferences for ethanol presented by the animals investigated.
Opioid antagonists to treat alcoholism
The administration of nonspecific opioid receptor antagonists, such as naloxone and naltrexone, as well as of μ and δ selective opioid receptor antagonists has been shown to decrease alcohol consumption in a dose-dependent manner by a number of animal species and in a number of experimental paradigms.14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 Furthermore, studies indicate that κ opioid receptor agonists may also attenuate alcohol consumption.94,95,96,97 Naltrexone has been reported to be an effective treatment for individuals with alcohol dependence,29,30,31 and in reducing relapse rates, as well as craving and alcohol intake, when it was combined with behavioural therapy.31
Currently, naltrexone (ReVia) is approved in Canada and the United States for the treatment of alcoholism. It is the first drug in 50 years to be approved in the US specifically for alcoholism. Naltrexone is, in general, well tolerated at low doses, but hepatotoxicity may occur at doses above 50 mg/day.31,147,148,149 Other opioid receptor antagonists such as nalmefene have also been found to be effective in decreasing alcohol consumption in humans.150 In addition, nalmefene is not associated with hepatotoxicity.150
After the initial studies on the effectiveness of naltrexone to reduce drinking in humans, a number of studies were performed in various treatment centres and laboratories to provide a better understanding of how naltrexone decreases alcohol consumption. Although the major mechanism is considered to be related to the inhibition of ethanol's positive reinforcement,151 animal studies have shown that long-term opioid antagonist administration, such as naltrexone or naloxone, causes increased release of opioid peptides152 and density of opioid receptors,153,154 suggesting an upregulation of the endogenous opioid system; however, this would render naltrexone less effective in preventing alcohol relapse, unless additional nonopioid mechanisms are involved in achieving its long-term therapeutic efficacy. Indeed, subjects who had been taking naltrexone, when given alcohol to drink, reported an increase in the subjective ratings of sedative and unpleasant effects and a decrease in the subjective ratings of liking, best effects and desire to drink, but there was no alteration in the subjective or objective indicators of drunkenness.155 Naltrexone has also been shown to dampen the cardiovascular responses to alcohol, which may play an important role in its therapeutic efficacy (JB Peterson, P Conrod, J Vassileva, C Gianoulakis, RO Pihl, McGill University, unpublished data, 2001). Therefore, naltrexone may act by both opioid and nonopioid mechanisms to reduce alcohol consumption. Although naltrexone may be used to treat those who are alcohol-dependent, its negative side effects decrease compliance and, as a result, decrease its effectiveness.156
In future studies, the effectiveness of opioid receptor antagonists selective for specific opioid receptor subclasses, as well as selective κ opioid receptor agonists, in decreasing alcohol consumption should be investigated. Considering the multifactorial nature of alcohol dependence, involving various subtypes of opioid receptors and many neurotransmitter systems, medications directed to more than one neurotransmitter system should be considered.157 Such medications might be effective at lower doses, induce fewer side effects and thus increase compliance and treatment effectiveness.
Summary
There is increasing evidence from human and animal studies supporting a link between the endogenous opioid system and the development of alcoholism. Overactivity or hypoactivity of distinct components of the opioid system in distinct brain regions associated with reward and reinforcement may be associated with an increased vulnerability to excessive alcohol consumption exhibited by some people, as well as by animals selectively bred for alcohol preferences. The effects of endogenous opioids in controlling alcohol consumption may be either direct or through interactions with other neurotransmitter systems that have been shown be important in the control of alcohol consumption. Because of this multifactorial nature of alcohol dependence, efforts must be made to personalize treatment for each individual by trying to identify the appropriate biological, psychosocial and environmental determinant(s) contributing to the development of alcoholism. Depending on the individual patient, such treatment might include both pharmacotherapy and behavioural psychosocial therapy.158,159,160
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada, The Medical Research Council of Canada and the Alcoholic Beverage Medical Research Foundation.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Christina Gianoulakis, Douglas Hospital Research Centre, 6875 LaSalle Blvd., Verdun QC H4H 1R3; fax 514 762-3034; christina.gianoulakis@mcgill.ca
Submitted Oct. 4, 2000 Revised Mar. 19, 2001 Accepted Mar. 29, 2001
References
- 1.Mann K, Hermann D, Heinz A. One hundred years of alcoholism: the Twentieth Century. Alcohol Alcohol 2000;35:10-5. [DOI] [PubMed]
- 2.Hesselbrock VM. The genetic epidemiology of alcoholism. In: Begleiter H, Kissin B, editors. Alcohol and alcoholism. Vol. 1: the genetics of alcoholism. New York: Oxford University Press; 1995. p. 17-39.
- 3.McLearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol 1959;20:691-5.
- 4.Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol 1983;5:171-8. [PubMed]
- 5.Rezvani AH, Overstreet DH, Janowsky DS. Genetic serotonin deficiency and alcohol preference in the Fawn-Hooded rat. Alcohol Alcohol 1990;25:573-5. [PubMed]
- 6.Beauge FJ, Aufrere G, Colombo G, Rezvani AH, Overstreet DH. Alcohol dependence-induced facilitation of drinking in two fawn-hooded strains of rats [abstract]. Society for Neuroscience Abstracts of the 28th Annual Meeting; 1998 Nov 7–12; Los Angeles. Washington (DC): Society for Neuroscience. 1998;24:1240.
- 7.Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science 1968;159:739-41. [DOI] [PubMed]
- 8.Lumeng L, Hawkins TD, Li T-K. New strains of rats with alcohol preference and non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and aldehyde metabolizing systems. Vol. 3. New York: Academic Press; 1977. p. 537-44.
- 9.Lumeng L, Doolittle DP, Li T-K. New duplicate lines of rats that differ in voluntary alcohol consumption. Alcohol Alcohol 1986;21:A37.
- 10.Fadda F, Mosca E, Colombo G, Gessa GL. Alcohol-preferring rats: genetic sensitivity to alcohol-induced stimulation of dopamine metabolism. Physiol Behav 1990;47:727-9. [DOI] [PubMed]
- 11.Crabbe JC Jr, Li T-K. Genetic strategies in preclinical substance abuse research. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. 4th ed. New York: Raven Press; 1995. p. 799-811.
- 12.Sinclair JD, Li T-K, Gessa GL, Lumeng L, Le AD. High and low drinking rat lines: contributions to current understanding and future development. Biobehav Res 1996;15:10-2. [DOI] [PubMed]
- 13.Myers RD, Robinson DE, West MW, Biggs TAG, McMillen BA. Genetics of alcoholism: rapid development of a new high-ethanol-preferring (HEP) strain of female and male rats. Alcohol 1998;16:343-57. [DOI] [PubMed]
- 14.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology 1997;129:99-111. [DOI] [PubMed]
- 15.Altshuler HL, Philips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci 1980;26:679-88. [DOI] [PubMed]
- 16.Myers RD, Borg S, Mossberg R. Antagonism by naltrexone of voluntary alcohol selection in the chronically drinking macaque monkey. Alcohol 1986;3:383-8. [DOI] [PubMed]
- 17.Froehlich JC, Harts J, Lumeng L, Li T-K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav 1990;35:385-90. [DOI] [PubMed]
- 18.Froehlich JC, Zweifel M, Harts J, Lumeng L, Li T-K. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology 1991;103:467-72. [DOI] [PubMed]
- 19.Weiss F, Mitchiner M, Bloom FE, Koob GF. Free-choice responding for ethanol vs. water in alcohol-preferring (P) and unselected Wistar rats is differentially modified by naloxone, bromocriptin, and methysergide. Psychopharmacology 1990;101:178-86. [DOI] [PubMed]
- 20.Kornet M, Goosen C, Van Ree JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology 1991;104:367-76. [DOI] [PubMed]
- 21.Hubbell CL, Mankes RF, Reid LD. A small dose of morphine leads to drink more alcohol and achieve higher blood alcohol concentrations. Alcohol Clin Exp Res 1993;17:1040-3. [DOI] [PubMed]
- 22.Hyytia P. Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav 1993;45:697-701. [DOI] [PubMed]
- 23.Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li T-K, et al. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology 1995;120:177-85. [DOI] [PubMed]
- 24.Krishnan-Sarin S, Portoghese PS, Li T-K, Froehlich JC. The delta (2)-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav 1995;52:153-9. [DOI] [PubMed]
- 25.Honkanen A, Vilamo L, Wegelius K, Sarviharju M, Hyytia P, Korpi ER. Alcohol drinking is reduced by a mu 1 — but not by a delta-opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol 1996;304:7-13. [DOI] [PubMed]
- 26.Davidson D, Amit Z. Effects of naloxone on limited-access ethanol drinking in rats. Alcohol Clin Exp Res 1996;20:664-9. [DOI] [PubMed]
- 27.Franck J, Lindholm S, Raaschou P. Modulation of volitional ethanol intake in the rat by central δ-opioid receptors. Alcohol Clin Exp Res 1998;22:1185-9. [PubMed]
- 28.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res 1999;23:1468-76. [PubMed]
- 29.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 1992;49:876-80. [DOI] [PubMed]
- 30.Jaffe AJ, Rounsaville B, Chang G, Shottenfeld RS, Meyer RE, O'Malley SS. Naltrexone, relapse prevention and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol 1996;64:1044-53. [DOI] [PubMed]
- 31.O'Malley SS. Opioid antagonists in the treatment of alcohol dependence: clinical efficacy and prevention of relapse. Alcohol Alcohol 1996;31(Suppl 1):77-81. [PubMed]
- 32.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 1998;22:3-9. [PubMed]
- 33.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 1992;80:2046-50. [DOI] [PMC free article] [PubMed]
- 34.Acquas E, Meloni M, Di Chiara G. Blockade of δ-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol 1993;230:139-241. [DOI] [PubMed]
- 35.De Waele JP, Kiianmaa K, Gianoulakis C. Distribution of the μ and δ opioid binding sites in the brain of the alcohol-preferring AA and alcohol-avoiding ANA lines of rats. J Pharmacol Exp Ther 1995;275:518-27. [PubMed]
- 36.Jamensky NT, Gianoulakis C. Content of dynorphins and κ-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice Alcohol Clin Exp Res 1997;21:1455-64. [PubMed]
- 37.Gianoulakis C, Beliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, et al. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci 1989;45:1097-109. [DOI] [PubMed]
- 38.Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary β -endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry 1996;53:250-7. [DOI] [PubMed]
- 39.Froehlich JC, Zink RW, Li T-K, Christian JC. Analysis of heritability of hormonal responses to alcohol in twins: beta-endorphin as a potential biomarker of genetic risk for alcoholism. Alcohol Clin Exp Res 2000;24:265-77. [PubMed]
- 40.de Waele J-P, Papachristou D, Gianoulakis C. The alcohol-preferring C57BL/6 mice present an enhanced sensitivity of the hypothalamic β -endorphin system to ethanol than the alcohol-avoiding DBA/2 mice. J Pharmacol Exp Ther 1992;261:788-94. [PubMed]
- 41.Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res 1998;22:789-801. [PubMed]
- 42.de Waele J-P, Gianoulakis C. Characterization of the μ and δ opioid receptors in the brain of the C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. Alcohol Clin Exp Res 1997;21:754-62. [PubMed]
- 43.Winkler A, Spanagel R. Differences in the kappa opioid receptor mRNA content in distinct brain regions of two inbred mice strains. Neuroreport 1998;9:1459-64. [DOI] [PubMed]
- 44.McBride WJ, Chernet E, McKinzie DL, Lumeng L, Li T-K. Quantitative autoradiography of mu-opioid receptors in the CNS of alcohol-naive alcohol-preferring P and -nonpreferring NP rats. Alcohol 1998;16:317-23. [DOI] [PubMed]
- 45.Fadda F, Tronci S, Colombo G, Fratta W. Diffrences in the opioid system in selected brain regions of alcohol-preferring and alcohol-nonpreferring rats. Alcohol Clin Exp Res 1999;23: 1296-305. [PubMed]
- 46.Jamensky NJ, Gianoulakis C. Comparison of the proopiomelanocortin and proenkephalin opioid peptide systems in brain regions of the alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2 mice. Alcohol 1999;18:177-87. [DOI] [PubMed]
- 47.Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci 2000;66:1915-27. [DOI] [PubMed]
- 48.Olson GA, Olson RD, Kastin AB. Endogenous Opioids [review]. Peptides 1990;11:1277-304. [DOI] [PubMed]
- 49.Khachaturian H, Lewis ME, Schafer MKH, Watson SJ. Anatomy of the CNS opioid systems. Trends Neurosci 1985;10:111-9.
- 50.Crine P, Gianoulakis C, Seidah NG, Gossard F, Pezalla PD, Lis M. Biosynthesis of β -endorphin from β -lipotropin and a larger molecular weight precursor in rat pars intermedia. Proc Natl Acad Sci U S A 1978;75:4719-23. [DOI] [PMC free article] [PubMed]
- 51.Dores RM, Jain M, Akil H. Characterization of the forms of β -endorphin and α -MSH in the caudal medulla of the rat and guinea pig. Brain Res 1986;377:251-60. [DOI] [PubMed]
- 52.Bloom FE. The endorphins: a growing family of pharmacologically pertinent peptides. Annu Rev Pharmacol Toxicol 1983;23: 151-70. [DOI] [PubMed]
- 53.Kakidani H, Furutami Y, Takahashi H, Noda M, Mozimoto Y, Hiroshi T, et al. Cloning and sequence analysis of cDNA for porcine β -neoendorphin/dynorphin precursors. Nature 1982; 298:245-9. [DOI] [PubMed]
- 54.Charnes ME. Ethanol and opioid receptor signalling. Experientia 1989;45:518-28. [DOI] [PubMed]
- 55.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 1992;13:177-93. [DOI] [PubMed]
- 56.Chick J, Erickson C. Conference summary: consensus conference on alcohol dependence and the role of pharmacotherapy in its treatment. Alcohol Clin Exp Res 1996;20:391-402. [DOI] [PubMed]
- 57.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci 1999;22:521-7. [DOI] [PubMed]
- 58.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lessions of the nucleus accumben and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res 1993;623:16-24. [DOI] [PubMed]
- 59.Ikemoto S, McBride WJ, Murphy JM, Lumeng L, Li T-K. 6-OHDA-lesions of the nucleus accumbens disrupt the acquisition but not maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcohol Clin Exp Res 1997;21:1042-6. [PubMed]
- 60.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res 1997;21:1083-91. [DOI] [PMC free article] [PubMed]
- 61.Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopahrmacology 1998;139:79-85. [DOI] [PubMed]
- 62.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 1998;18:10663-71. [DOI] [PMC free article] [PubMed]
- 63.Gianoulakis C, Barcomb A. Effect of acute ethanol in vivo and in vitro on the β -endorphin system in the rat. Life Sci 1987;40:19-28. [DOI] [PubMed]
- 64.Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic–pituitary–adrenal axis; exploration of the mechanism of action. Neuroendocrinology 1989;50:427-32. [DOI] [PubMed]
- 65.Keith LD, Crabbe JC, Robertson LM, Kendall JW. Ethanol stimulated endorphin and corticotropin secretion in vitro. Brain Res 1986;367:222-9. [DOI] [PubMed]
- 66.Gianoulakis C. Characterization of the effect of acute ethanol administration on the release of β -endorphin peptides by the rat hypothalamus. Eur J Pharmacol 1990;180:21-9. [DOI] [PubMed]
- 67.de Waele J-P, Gianoulakis C. Effects of single and repeated exposure to ethanol on hypothalamic β -endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology 1993;57:700-9. [DOI] [PubMed]
- 68.Gianoulakis C, L'Abbee D, Moronval D. Direct evidence of a biphasic effect of ethanol on the pituitary and hypothalamic β -endorphin release. In: Cros J, Meunier J-C, Hamon M, editors. Advances in the biosciences. Vol. 75. Oxford: Pergamon Press; 1989. p. 527-30.
- 69.Seizinger BR, Bovermann K, Hollt V, Herz A. Enhanced activity of the endorphinergic system in the anterior and neurointermediate lobe of the pituitary gland after chronic treatment with ethanol liquid diet. J Pharmacol Exp Ther 1984;230:455-61. [PubMed]
- 70.Gianoulakis C, Hutchison WD, Kalant H. Effects of ethanol treatment and withdrawal on the biosynthesis and processing of pro-opiomelanocortin by the rat neurointermediate lobe. Endocrinology 1988;122:817-25. [DOI] [PubMed]
- 71.Seizinger BR, Hollt V, Herz A. Effect of chronic ethanol treatment on the in vitro biosynthesis of pro-opiomelanocortin and its post-translational processing to β -endorphin in the intermediate lobe of the rat pituitary. J Neurochem 1984;43:607-13. [DOI] [PubMed]
- 72.Dave JR, Eiden LE, Karanian JW, Eskay RL. Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis and plasma β -endorphin levels in the rat. Endocrinology 1986; 118:280-6. [DOI] [PubMed]
- 73.Wand CS. Differential regulation of anterior pituitary corticotrope function is observed in vivo but not in vitro in two lines of ethanol sensitive mice. Alcohol Clin Exp Res 1990;14:100-6. [DOI] [PubMed]
- 74.Schulz R, Wuster M, Duka T, Herz A. Acute and chronic ethanol treatment changes endorphin levels in brain and pituitary. Psychopharmacology 1980;68:221-7. [DOI] [PubMed]
- 75.Adams ML, Cicero TJ. Effects of alcohol on β -endorphin and reproductive hormones in the male rat. Alcohol Clin Exp Res 1991; 15:685-92. [DOI] [PubMed]
- 76.Gianoulakis C, Angelogianni P. Chronic ethanol increases proopiomelanocortin gene expression in the rat hypothalamus. Neuroendocrinology 1993;57:106-14. [DOI] [PubMed]
- 77.Seizinger BR, Boverman K, Maysinger D, Hollt V, Herz A. Differential effects of acute and chronic ethanol treatment on particular opioid peptide systems in discrete regions of rat brain and pituitary. Pharmacol Biochem Behav 1983;18:361-9. [DOI] [PubMed]
- 78.Scanlon MN, Lazar-Wesley E, Grant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol Clin Exp Res 1992;16:1147-51. [DOI] [PubMed]
- 79.Milton GV, Erickson CK. Effect of ethanol and discomfort on brain met-enkephalin levels in male Sprague-Dawley rats. Alcohol Clin Exp Res 1991;15:327.
- 80.Li X-W, Li T-K, Froehlich JC. Enhanced activity of the nucleus accumbens enkephalin system to alcohol in rats selectively bred for alcohol preference. Brain Res 1998;794:35-47. [DOI] [PubMed]
- 81.Nylander I, Hyytia P, Forsander O, Terenius L. Differences between alcohol-preferring (AA) and alcohol-avoiding (ANA) rats in the prodynorphin and proenkephalin systems. Alcohol Clin Exp Res 1994;18:1272-9. [DOI] [PubMed]
- 82.Hiller JM, Angel LM, Simon EJ. Multiple opiate receptors: alcohol selectively inhibits binding to delta receptor. Science 1981;214:468-9. [DOI] [PubMed]
- 83.Hiller JM, Angel LM, Simon EJ. Characterization of the selective inhibition of the delta subclass of opioid binding sites by alcohols. Mol Pharmacol 1984;25:249-55. [PubMed]
- 84.Gianoulakis C. Long term ethanol alters the binding of 3H-opiates to brain membranes. Life Sci 1983;33:725-33. [DOI] [PubMed]
- 85.Tabakoff B, Hoffman PL. Alcohol interactions with brain opiate receptors. Life Sci 1983;32:197-204. [DOI] [PubMed]
- 86.Levine AS, Hess SH, Morley JE. Alcohol and the opiate receptor. Alcohol Clin Exp Res 1983;7:83-4. [DOI] [PubMed]
- 87.Hoffman PL, Chung CT, Tabakoff B. Effects of ethanol, temperature, and endogenous regulatory factors on the characteristics of striatal opiate receptors. J Neurochem 1984;43:1003-10. [DOI] [PubMed]
- 88.Hynes MD, Lochner MA, Bemis KG, Hymson DL. Chronic ethanol alters the receptor binding characteristics of the δ-opioid receptor ligand, D-Ala2-D-Leu5 enkephalin in mouse brain. Life Sci 1983;33:2331-7. [DOI] [PubMed]
- 89.Charness ME, Gordon AS, Diamond I. Ethanol modulation of opiate receptors in cultured neural cells. Science 1983;222:1246-8. [DOI] [PubMed]
- 90.Tabakoff B, Urwyler S, Hoffman PL. Ethanol alters kinetic characteristics and function of striatal morphine receptors. J Neurochem 1981;37:518-21. [DOI] [PubMed]
- 91.Hoffman PL, Urwyler S, Tabakoff B. Alterations in opiate receptor function after chronic ethanol exposure. J Pharmacol Exp Ther 1982;222:182-9. [PubMed]
- 92.Khatami S, Hoffman PL, Shibuya T, Salefsky B. Selective effect of ethanol on opiate receptor subtypes in brain. Neuropharmacology 1987;26:1503-7. [DOI] [PubMed]
- 93.Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience 1999;91:971-7. [DOI] [PubMed]
- 94.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in nucleus accumbens and the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 1988;244:1067-80. [PubMed]
- 95.Sandi C, Borell J, Guaza C. Involvement of kappa type opioids on ethanol drinking. Life Sci 1988;42:1067-75. [DOI] [PubMed]
- 96.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: An in vivo microdialysis study. J Neurochem 1990;55:1734-40. [DOI] [PubMed]
- 97.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 1992;89:2046-50. [DOI] [PMC free article] [PubMed]
- 98.Spanagel R. The influence of opioid antagonists on the discriminative stimulus of ethanol. Pharmacol Biochem Behav 1996; 54:645-9. [DOI] [PubMed]
- 99.Myers RD, Robinson DE. Mu and D2 receptor antisense oligonucleotides injected in nucleus accumbens suppress high alcohol intake in genetic drinking HEP rats. Alcohol 1999;18:225-33. [DOI] [PubMed]
- 100.Grisel JE, Mogil JS, Grahame NJ Rubinstein M, Bellknap JK, Crabbe JC, et al. Ethanol oral self-administration is increased in mutant mice with decreased beta-endorphin expression Brain Res 1999;835:6267. [DOI] [PubMed]
- 101.Graham NJ, Low MJ, Cunningham CL. Intravenous self-administration of ethanol in β -endorphin-deficient mice. Alcohol Clin Exp Res 1998;22:1093-8. [PubMed]
- 102.Hall FS, Sora L, Li X-F, Karmacharya N, Goodman N, Uhl GR. Decreased responses to ethanol in μ opiate receptor knockout mice [abstract 535.17]. Society for Neuroscience abstracts of the 29th annual meeting; 1999 Oct 23–28; Miami Beach (FL). Washington (DC): Society for Neuroscience. 1999;25:1327.
- 103.Cloninger CR, Bohman M, Sigvardsson S, Von Knorring AL. Psychopathology in adopted-out children of alcoholics: The Stockholm adoption study. In: Galanger M, editor. Recent developments in alcoholism. New York: Plenum Press; 1985. p. 37-51. [DOI] [PubMed]
- 104.Goldman D, Linnoila M. Genetic approaches to alcoholism. Prog Neuropsychopharmacol Biol Psychiatry 1986;10:237-42. [DOI] [PubMed]
- 105.Bergleiter H, Porjesz B, Bihari B, Kissin B. Event related brain potentials in boys at risk for alcoholism. Science 1984;225:1493-6. [DOI] [PubMed]
- 106.Schuckit MA. Reactions to alcohol in sons of alcoholics and controls. Alcohol Clin Exp Res 1988;12:465-71. [DOI] [PubMed]
- 107.Diamond I, Wrubel B, Estrin W, Gordon A. Basal adenosine receptors-stimulated levels of cyclic AMP are reduced in lymphocytes from alcoholic patients. Proc Natl Acad Sci U S A 1987; 84: 1413-6. [DOI] [PMC free article] [PubMed]
- 108.Tabakoff B, Hoffman PL, Lee JM, Saito T, Willard B, De Leon-Jones F. Differences in platelet enzyme activity between alcoholics and non alcoholics. New Engl J Med 1988;318:134-9. [DOI] [PubMed]
- 109.Blum K, Noble E, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990;263:2055-60. [PubMed]
- 110.Comings DE, Comings BG, Mueleman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptors locus as a modifying gene in neuropsychiatric disorders. JAMA 1991;266: 1793-800. [PubMed]
- 111.Gelenter J, O'Maley S, Risch N, Kranzier HR, Krystal J, Merikangas K. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism. JAMA 1991; 266: 1801-7. [PubMed]
- 112.Froehlich JC. Interactions between alcohol and the endogenous opioid system. In: Zakhari SS, editor. Alcohol and the endocrine system. No. 23 of National Institute of Alcohol Abuse and Alcoholism series. Bethesda (MD): US Government Printing Office; 1993. p. 21-35. Report no.: NIH-93-3533.
- 113.Ragland DR, Greiner BA, Yen IH, Fisher JM. Occupational stress factors and alcohol-related behavior in urban transit operators. Alcohol Clin Exp Res 2000;24:1011-19. [PubMed]
- 114.Gianoulakis C, de Waele J-P. Genetics of alcoholism: role of the opioid system. Metabolic Brain Dis 1994;9:105-31. [DOI] [PubMed]
- 115.Henry JL. Circulating opioids: possible physiological roles in central nervous function. Neurosci Biobehav Rev 1982;6:229-45. [DOI] [PubMed]
- 116.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain 1984;19:13-25. [DOI] [PubMed]
- 117.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci 1984;7:223-55. [DOI] [PubMed]
- 118.Wand GS, Mangold D, El Deiry S, McCaul ME, Houver D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry 1998;55:1114-9. [DOI] [PubMed]
- 119.Genazzani AR, Nappi G, Facchinetti F. Central deficiency in beta-endorphin in alcohol addicts. J Clin Endocrinol Metab 1982; 55:583-6. [DOI] [PubMed]
- 120.Vescovi PP, Coiro V, Volpi R, Giannini A, Passeri M. Plasma beta-endorphin but not met-enkephalin levels are abnormal in chronic alcoholics. Alcohol Alcohol 1992;27:471-5. [PubMed]
- 121.Aguirre JC, Del Arbol JL, Rico J, Raya J, Miranda MT. Classification of alcoholics on the basis of plasma β -endorphin concentration. Alcohol 1995;12:531-4. [DOI] [PubMed]
- 122.Gianoulakis C, de Waele J-P, Kiianmaa K. Differences in the brain and pituitary β -endorphin system between the alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res 1992;16:453-9. [DOI] [PubMed]
- 123.De Waele J-P, Gianoulakis C. Enhanced activity of the brain β -endorphin system by free-choice ethanol drinking in C57BL/6 but not DBA/2 mice. Eur J Pharmacol 1994;258:119-29. [DOI] [PubMed]
- 124.De Waele J-P, Kiianmaa K, Gianoulakis C. Spontaneous and ethanol stimulated in vitro release of β -endorphin by the hypothalamus of AA and ANA rats. Alcohol Clin Exp Res 1994; 18:1468-73. [DOI] [PubMed]
- 125.Krishnan-Sarin S, Wand GS, Li X-W, Portoghese PS, Froehlich JC. Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav 1998;59:627-35. [DOI] [PubMed]
- 126.Blum K, Briggs AH. Opioid peptides and genotypic responses to ethanol. Biog Amines 1988;5:527-33.
- 127.Ng GYK, O'Dowd BF, George SR. Genotypic differences in mesolimbic enkephalin gene expression in DBA/2J and C57BL/6J inbred mice. Eur J Pharmacol 1996;311:45-52. [DOI] [PubMed]
- 128.Li X-W, Li T-K, Froehlich JC. Enhanced sensitivity of the nucleus accumbens proenkephalin system to alcohol in rats selectively bred for alcohol preference. Brain Res 1998;794:35-47. [DOI] [PubMed]
- 129.Soini SL, Ovaska T, Honkanen A, Hyytia P, Korpi ER. Brain opioid receptor binding of [3H] CTOP and [3H] naltrindole in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol 1998;15:227-32. [DOI] [PubMed]
- 130.Soini SL, Honkanen A, Hyytia P, Korpi ER. [H] ethylketocyclazocine binding to brain opioid receptor subtypes in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol 1999; 18:27-34. [DOI] [PubMed]
- 131.McClearn GE, Kakihana R. Selective breeding for ethanol sensitivty: short-sleep and long-sleep mice. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of animal models as pharmacogenetic tools. Washington (DC): National Institute on Alcohol Abuse and Alcoholism; 1981. Report no. 6. p. 147-59.
- 132.Crabbe JC, Young ER, Deutch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav 1987;27:577-81. [DOI] [PubMed]
- 133.Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Biderectional selection for susceptibility to ethanol withdrawal seizures in mus musculus. Behav Genet 1985;15:521-36. [DOI] [PubMed]
- 134.Kosobud A, Crabbe JC. Ethanol withdrawal in mice bred to be genetically prone (WSP) or resistant (WSR) to ethanol withdrawal seizures. J Pharmacol Exp Ther 1986;238:170-7. [PubMed]
- 135.Crabbe JC, Kosobud A. Sensitivity and tolerance to ethanol in mice bred to be genetically prone (WSP) or resistant (WSR) to ethanol withdrawal seizures. J Pharmacol Exp Ther 1986;239: 327-33. [PubMed]
- 136.Wilson JR, Erwin VG, DeFries JC, Petersen DR, Cole-Harding S. Ethanol dependence in mice: direct and correlated responses to ten generations of selective breeding. Behav Genet 1984;14:235-56. [DOI] [PubMed]
- 137.Crabbe JC, Kosobud A, Tam BR, Young ER, Deutsch CM. Genetic selection of mouse lines sensitive (COLD) and resistant (HOT) to acute ethanol hypothermia. Alcohol Drug Res 1987; 7:163-74. [PubMed]
- 138.Erwin VG, Deitrich RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther 1996;279:1310-7. [PubMed]
- 139.Allan AM, Spuhler KP, Harris RA. Gamma-aminobutyric acid activated chloride channels: relationship to genetic differences in ethanol sensitivity. J Pharmacol Exp Ther 1988;244:866-70. [PubMed]
- 140.Kosobud A, Bodor AS, Crabbe JC. Voluntary consumption of ethanol in WSP, WSC and WSR selectively bred mouse lines. Pharmacol Biochem Behav 1988;29:601-7. [DOI] [PubMed]
- 141.Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic–pituitary–adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res 1992;579: 296-302. [DOI] [PubMed]
- 142.Crabbe JC, Keith LD, Kosobud A, Stack J. Ethanol dependence and the pituitary–adrenal axis in mice. I. Genotypic differences in hormone levels. Life Sci 1983;33:1877-87. [DOI] [PubMed]
- 143.McBride WJ, Chernet E, McKinzie DL, Lumeng L, Li T-K. Quantitative autoradiography of mu-opioid receptors in the CNS of alcohol-naive alcohol-preferring P and non-preferring NP rats. Alcohol 1998;16:317-23. [DOI] [PubMed]
- 144.Gong JH, Li XW, Lai ZN, Froehlich JC, Yu L. Quantitative comparison of mu opioid receptor mRNA in selected CNS regions of alcohol naive rats selectively bred for high and low alcohol drinking. Neurosci Lett 1997;227:9-12. [DOI] [PubMed]
- 145.Le AD, Chow S. Reduction of ethanol intake in C57BL/6 mice by opiate receptor antagonists. Alcohol Alcohol 1992;27(Suppl 1): 49-56.
- 146.Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa- or delta-receptor. Alcohol Clin Exp Res 1998; 22:1634-9. [DOI] [PubMed]
- 147.Litten RZ, Allen J, Ferting J. Pharmacotherapies for alcohol problems: a review of research with focus on developments since 1991. Alcohol Clin Exp Res 1996;20:859-76. [DOI] [PubMed]
- 148.Berg BJ, Pettinati HM, Volpicelli JR. A risk-benefit assessment of naltrexone in the treatment of alcohol dependence. Drug Saf 1996;15:274-82. [DOI] [PubMed]
- 149.Volpicelli JR, Volpicelli LA, O'Brien CP. Medical management of alcohol dependence: clinical use and limitations of naltrexone treatment. Alcohol Alcohol 1995;30:789-98. [PubMed]
- 150.Mason BJ, Ritvo EC, Morgan RO, Salvato FR, Goldberg G, Welch B, et al. A double-blind, placebo-controlled pilot study to evaluate the efficacy and safety of oral nalmefene HCL for alcohol dependence. Alcohol Clin Exp Res 1994;18:1162-7. [DOI] [PubMed]
- 151.Cole JC, Littleton JM, Little HJ. Acamprosate, but not naltrexone, inhibits conditioned abstinence behaviour associated with repeated ethanol administration and exposure to plus-maze. Psychopharmacology 2000;147:403-11. [DOI] [PubMed]
- 152.Gianoulakis C, Gupta A. Neurointermediate lobe transplanted under the kidney capsule modifies the activity of the neurointermediate lobe in situ, but does not respond to opiate treatment. Can J Physiol Pharmacol 1986;64:430-7. [DOI] [PubMed]
- 153.Rothman RB, Long JB, Bykov V, Brady LS, Jacobson AE, Rice KC, et al. Pretreatment of rats with the irreversible μ-receptor antagonist β -FNA, fails to prevent naltrexone-induced upregulation of μ-opioid receptor. Neuropharmacology 1990;29:805-10. [DOI] [PubMed]
- 154.Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RT, Crews FT. Suppression of alcohol intake by chronic naloxone treatment in P rats: tolerance development and elevation of opioid receptor binding. Alcohol Clin Exp Res 1999;23: 1761-71. [PubMed]
- 155.McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 2000;22:480-92. [DOI] [PubMed]
- 156.Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 2000;22:493-503. [DOI] [PubMed]
- 157.Rezvani AH, Overstreet DH, Mason GA, Janowsky DS, Hamedi M, Clark E Jr, et al. Combination pharmacotherapy: a mixture of small dose of naltrexone, fluoxetine, and a thyrotropin-releasing hormone analogue reduces alcohol intake in three strains of alcohol-preferring rats. Alcohol Alcohol 2000;35: 76-83. [DOI] [PubMed]
- 158.Lesch OM, Walter H. Subtypes of alcoholism and their role in therapy. Alcohol Alcohol 1996;31(Suppl 1):63-7. [PubMed]
- 159.Anton RF. Neurobehavioral basis for the pharmacotherapy of alcoholism: current and future directions. Alcohol Alcohol 1996; 31(Suppl 1):43-53. [PubMed]
- 160.Sperling W, Lesch OM. The reduction of alcohol consumption with novel pharmacological intervention. Eur Psychiatry 1996; 11:217-26. [DOI] [PubMed]


