Abstract
Objective
To determine the feasibility of using the Medication Event Monitoring System (MEMS) to estimate medication compliance in patients with schizophrenia or schizoaffective disorder.
Subjects and setting
Fourteen of 35 consecutive patients admitted to a psychiatric inpatient hospital with schizophrenia or schizoaffective disorder who met eligibility requirements and gave informed consent.
Intervention
After random assignment to either risperidone or typical antipsychotic treatment, medication upon discharge from hospital was dispensed in a bottle with a MEMS cap which recorded the number of bottle openings and the date and time of each opening. The first 6 patients were asked to return monthly for data downloading. The next 8 were asked to return weekly during the first month and every 2 weeks thereafter; they were also paid $5 for returning each bottle.
Outcome measures
MEMS data collected over a 6-month period and hospital readmission data.
Results
Patient medication compliance data were collected from 10 (71%) of 14 patients during the first month, from 7 (58%) of 12 (2 patients dropped out) during the second and from 5 (45%) of 11 (a third patient dropped out) during months 3–6. Mean compliance rates were 63% for the first month and ranged from 56% to 45% over the next 5. First-month compliance rates were significantly lower for those who were subsequently readmitted to hospital (n = 7) than for those who were not (p < 0.01).
Conclusions
Electronic monitoring devices can be used to estimate compliance with medication regimens in patients with severe schizophrenic disorders, but there are methodological improvements that can be made to increase data recovery and compliance, and these are discussed.
Medical subject headings: antipsychotic agents, drug therapy, patient compliance, psychotic disorders, schizophrenia, self administration
Abstract
Objectif
Déterminer la possibilité d'utiliser le système de surveillance des événements de médication (SSEM) afin d'évaluer le respect de la pharmacothérapie chez les patients atteints de schizophrénie ou de troubles schizo-affectifs.
Sujets et contexte
Quatorze patient sur 35 consécutifs admis à un hôpital psychiatrique interne atteints de schizophrénie ou de troubles schizo-affectifs qui satisfaisaient aux conditions d'admissibilité et ont donné leur consentement éclairé.
Intervention
Après une affectation aléatoire à un traitement à la rispéridone ou aux antipsychotiques typiques, les patients ont reçu, au moment de leur congé de l'hôpital, leurs médicaments dans une bouteille munie d'un bouchon SSEM, qui enregistrait le nombre d'ouvertures de la bouteille, ainsi que la date et l'heure de chaque ouverture. On a demandé aux six premiers patients de revenir une fois par mois pour télécharger les données, et aux huit patients suivants de revenir une fois par semaine pendant le premier mois et aux deux semaines par la suite. Ils ont aussi touché 5 $ pour chaque bouteille rapportée.
Mesures de résultats
Données du SSEM recueillies pendant six mois et données sur les réhospitalisations.
Résultats
On a recueilli des données sur l'observation de la pharmacothérapie par le patient de 10 (71 %) des 14 patients au cours du premier mois, de 7 patients (58 %) sur 12 (2 ont abandonné) pendant le deuxième mois et de 5 patients (45 %) sur 11 (un troisième patient a abandonné) au cours des mois 3 à 6. Les taux moyens d'observation se sont établis à 63 % pendant le premier mois et ont varié de 56 % à 45 % au cours des cinq mois suivants. Les taux d'observation au cours du premier mois étaient beaucoup moins élevés chez les sujets qui ont été réhospitalisés par la suite (n = 7) que chez ceux qui ne l'ont pas été (p < 0,01).
Conclusions
On peut utiliser des dispositifs de surveillance électronique pour estimer l'observation de la pharmacothérapie chez les patients atteints de troubles schizophréniques graves, mais il serait possible d'améliorer les méthodologies afin d'augmenter la récupération des données et l'observation, et ces améliorations font l'objet de discussions.
Introduction
Medication compliance is a key factor in the treatment of mental illnesses such as schizophrenia. Relapse is common in patients with chronic psychosis, and one of the most important causes for relapse is noncompliance with medications.1,2 One of the problems in studying compliance with medications is that it is difficult to monitor. A review of the literature comparing medication compliance in patients with mental disorders and patients with physical disorders revealed different compliance rates for those taking antipsychotics, antidepressants and medications for physical disorders; however, the differences may have been attributable to the method of estimating compliance.3
Although some studies have used quantitative methods such as patient reports, clinician reports and objective measures, questions can be raised about the accuracy of these methods as well.
Patient self-reports might be influenced by memory deficits, level of psychosis, use of illegal substances or denial of illness. Patient reports, as recorded by an interviewer, may be influenced by who asks the questions.
Compliance information obtained through clinician reports could be biased by the investment the clinician may have in the patient's treatment. Additionally, clinicians' reports of compliance may not be independent from important predictor variables.4
Objective measures include pill counts, blood tests, medication markers, direct observation and technical monitoring. Pill counts may not detect alternating under- and overcompliance or discarding of pills.5 Traces of neuroleptic medications can be be detected in the blood long after the medications have been stopped, and plasma levels can vary widely among patients taking the same dose.6 Medication markers such as riboflavin are also nonquantitative,7 and direct observation of pill-taking is costly and intrusive. Technical monitors, however, give a quantitative estimate that is objective, independent from predictor variables and possibly less intrusive than direct observation. Moreover, technical devices may have some advantage over pill counting in detecting the discarding of pills because, if patients wish to deceive the method, they must open the pill bottle on the same schedule they would if they were actually taking the pills.
The Medication Event Monitoring System, ([MEMS], Aprex Corp., Fremont, Calif.) is a medication bottle cap with a microprocessor that records the occurrence and time of each bottle opening. The MEMS has been used in a variety of populations with medical disorders.5 The only study we know of to date using an electronic medication monitor for patients with chronic mental illnesses (i.e., schizophrenia, schizoaffective disorder and severe mood disorders) was conducted in a population of veterans patients using a day hospital and intensive support.8
In this pilot study, we aimed to determine the feasibility of using a technical monitor such as the MEMS to estimate medication compliance in socially disadvantaged patients with chronic mental illness.
Methods
Patients with schizophrenia or schizoaffective disorder who were readmitted to a psychiatric inpatient unit were considered for the study. Upon admission and consent to the study, patients were assessed with the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV, SCID patient version),9 and the Positive and Negative Symptom Scale (PANSS)10 and a demographic and clinical history was recorded. To be included in the study, patients had to be dispensing oral medications to themselves as outpatients. Those who were believed to have been fully compliant with their medication before readmission were excluded from the study.
For 6 months, we conducted a pilot project to determine the feasibility of randomly assigning patients with schizophrenia or schizoaffective disorder to risperidone or typical antipsychotic treatment and of collecting MEMS data as an outcome measure after discharge from a psychiatric inpatient unit.
Patients meeting eligibility criteria and giving informed consent were randomly assigned to receive risperidone or typical antipsychotic treatment. Upon discharge, all patients were given their antipsychotic medication in a MEMS bottle and were told that the bottle cap recorded the number of openings, with date and time, and that the purpose of the study was to use these electronic monitors to estimate compliance with their medication regimen. They were also told that medication compliance was going to be used in conjunction with answers about demographics, clinical state and symptoms to learn more about the patient characteristics that influence whether a medication regimen is adhered to. It was explained that this information would be kept confidential.
The first 6 patients were given monthly appointments (method 1) to download compliance data and provide information on follow-up measures. The next 8 patients were given appointments to download compliance data on a weekly basis during the first month and every 2 weeks thereafter (method 2). Follow-up on the other measures was still done monthly. To maximize data collection, these patients were paid $5 when they returned the bottle. We continued compliance data collection for 6 months and tracked readmission to hospital for up to a year.
A summary statistic, dose compliance, was defined as the mean of the number of bottle openings not exceeding the number of doses prescribed for the day divided by the number of prescribed daily doses, for all available data for the month. We excluded bottle openings that exceeded the prescribed number because we did not want to permit a spurious appearance of full compliance that could occur if overcompliance on one day was averaged with undercompliance on another. Fisher's exact test was used for dichotomous variables across groups, and Student's and paired t-tests were used for continuous variables across and within groups, respectively.
Results
Of 35 consecutive patients meeting eligibility criteria, 14 (40%) consented to random assignment to risperidone (n = 7) or typical antipsychotic (n = 7) treatment and were enrolled in the study (mean age 31.5 years, range 21–46 years). Most of the patients had been admitted to hospital on numerous occasions and were homeless; all were living below the poverty level at the time of admission. The mean score for PANSS positive was 22.5, negative 20.0, and total 86. Three patients withdrew consent for the study; 2 within the first 2 weeks and 1 after the second month. All were paranoid about the cap and the research staff.
Data recovery, lost caps and dose compliance
During the first month of the study, we were able to recover compliance data from 10 (71%) of the 14 patients (Table 1). During the second month, we were able to recover data from 7 (58%) of the 12 patients remaining in the study and, in each of months 3, 4, 5 and 6, from 5 (45%) of 11 patients.
Table 1
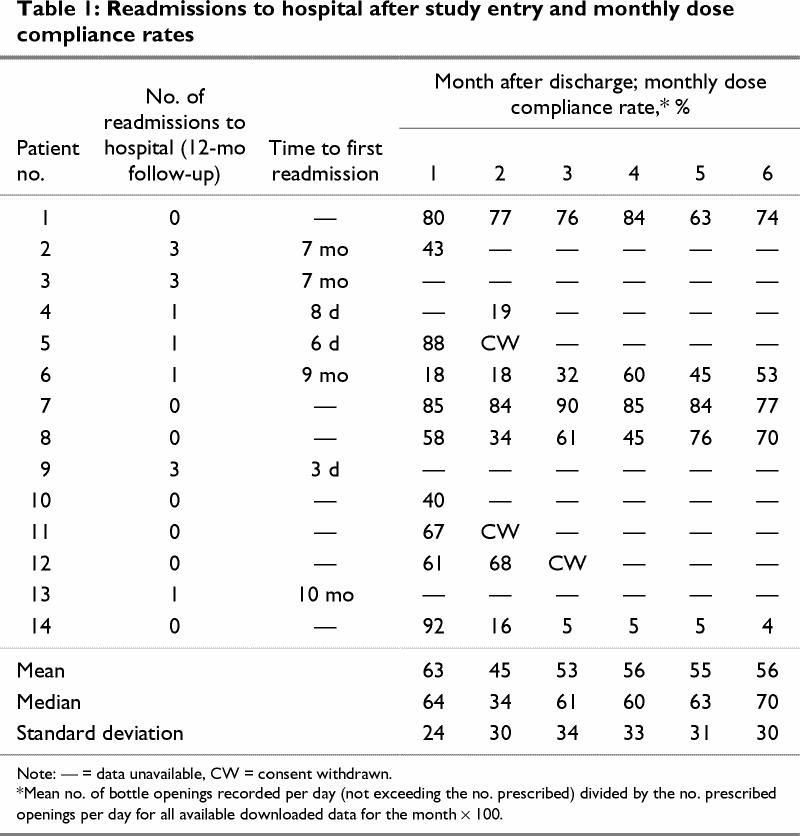
The mean dose-compliance rate for the first month of the study was 63% (range 18%–92%), and over the next 5 months, it ranged from 56% to 45%.
A somewhat higher proportion of compliance data was recovered with method 2 than with method 1, but the differences were not statistically significant. Also, the recovery of data was better in the earlier months of the study with both methods.
Seven patients lost 1 or more caps. Each lost cap represented a cost of $126. Additional study costs included the rental of the caps and hardware and software necessary for data retrieval. The average cost per patient, excluding staff time, bed days and subject fees, was $274 for the 6 months.
First-month compliance rates and readmissions to hospital
Six of the 7 patients whose first-month dose compliance was either uncollectable or below 50% were subsequently readmitted to hospital, but only 1 of the 7 patients whose first-month dose compliance was above 50% was readmitted. This difference was significant (Fisher's exact test, 2-tailed, p < 0.03).
There was also a significant difference between mean first-month compliance for the 7 patients who were readmitted to hospital after index discharge (mean compliance rate 21%, standard deviation [SD] 34%), assuming the patients with no data had zero compliance, and the 7 who were not readmitted (mean 69% SD 18%) (2-tailed t [unequal variances] = 3.3, p < 0.01).
Discussion
For many of these severely ill patients, we were able to estimate compliance with antipsychotic medication using a MEMS electronic monitor. Lower than 50% compliance in the first month after discharge was significantly associated with readmission to hospital. These data suggest that medication compliance, as measured with an electronic monitor, is related to outcome. As with other methods used to measure compliance, the MEMS estimate itself is not neutral — it may enhance compliance in some subjects and impact negatively in others. The inclusion of a control group in this study would have been helpful to assess these influences.
Several methodological issues limit the general conclusions that can be drawn from this study about the feasibility of estimating compliance in severe mentally ill patients using an electronic device. Our sample size was limited by the decision to include only patients who gave informed consent for randomization. Another limitation was the group of patients we studied; our population appeared more severely ill, less allied with the treatment system and less cooperative with treatment and study procedures than the “general population” of mentally ill patients. Another possible confound was the change in the frequency of visits midway through the study; better data recovery occurred after we increased the frequency of follow-up from monthly to weekly (i.e., method 1 v. method 2).
There are also some procedural issues that deserve comment. Several patients complained that the bottles were too large to carry in their pockets; supplying a carrying case or “fanny pack” might remedy this problem in the future. Paying the subjects when the caps were returned improved data collection somewhat, but may also have had a reinforcing effect on compliance. Coordinating refills with outpatient pharmacies was a source of difficulty, and clinicians needed to be reminded of the importance of MEMS use so they would continue to encourage the correct use of the cap. Another important consideration is the cost per patient for these devices.
Conclusions
Our data indicate that it is possible to estimate patient compliance with an electronic monitor, even in a population with severe mental illness; however, several difficulties were encountered in this population. Suggestions to increase data recovery rates include more frequent follow-ups in the immediate period after inpatient discharge, paying a fee for cap return and careful coordination of medication refills with clinicians and pharmacies. Our data also confirm the previously reported association between low compliance rates and readmission to hospital.
Acknowledgments
This study was supported in part by a National Association for Research in Schizophrenia and Depression (NARSAD) grant (E.D.), the National Institute of Mental Health MH01912 (E.D.), and by USPHS grant MH54446 (S.W.).
Footnotes
Competing interests: None declared for Dr. Diaz, Ms. Levine, Ms. Sullivan, Dr. Sernyak and Dr. Hawkins. Ms. Cramer received travel assistance from Aprex, a division of Aardex; Dr. Woods received speaker fees and an honorarium from Janssen Pharmaceutica and an educational grant from Eli Lilly and Company.
Correspondence to: Dr. Esperanza Diaz, Associate Professor of Psychiatry, Yale University, Connecticut Mental Health Center, 34 Park St., Office 273A, New Haven CT 06519; fax 203 974-7850; Esperanza.Diaz@yale.edu
Submitted Apr. 18, 2000 Revised Nov. 14, 2000 Accepted Jan. 24, 2001
References
- 1.Sullivan G, Wells KB, Morgenstern H, Leake B. Identifying modifiable risk factors for rehospitalization: a case–control study of seriously mentally ill persons in Mississippi. Am J Psychiatry 1995;152:1749-56. [DOI] [PubMed]
- 2.Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995;21:419-29. [DOI] [PubMed]
- 3.Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 1998; 49:196-210. [DOI] [PubMed]
- 4.Babiker IE. Noncompliance in schizophrenia [review]. Psychiatr Dev 1986;4:329-37. [PubMed]
- 5.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Oulette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA 1989;261:3273-7. [PubMed]
- 6.Dahl SG. Pharmacokinetics of neuroleptic drugs and the utility of plasma level monitoring [review]. Psychopharmacol Ser 1988;5:34-46 [DOI] [PubMed]
- 7.Kapur S, Ganguli R, Ulrich R, Raghu U. Use of random-sequence riboflavin as a marker of medication compliance in chronic schizophrenics. Schizophr Res 1992;6:49-53. [DOI] [PubMed]
- 8.Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. J Nerv Ment Dis 1999; 187:53-5. [DOI] [PubMed]
- 9.Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM III-R (SCID). New York: Biometrics Research, New York State Psychiatric Institute; 1987.
- 10.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardization. Br J Psychiatry 1989;(Suppl 7):59-67. [PubMed]


