Abstract
Objective
To compare the 24-h cortisol secretion profiles of normal control subjects and patients with bipolar disorder who were in the depressive, manic and euthymic phases of the disorder.
Participants
Eighteen patients, 25–62 years of age, in depressed (n = 5), manic (n = 5) or euthymic (n = 8) phase of bipolar disorder recruited through a psychiatric outpatient clinic, and 5 control subjects, 24–41 years of age, recruited through advertisement or word of mouth.
Outcome measures
Subjects were interviewed and symptom ratings were obtained using the Hamilton Depression Rating Scale, Beck Depression Inventory and Young Mania Scale. Blood collection began at 0800 and continued at hourly intervals for 24 h. Serum cortisol levels were assayed using a validated commercial radioimmunoassay kit.
Results
An analysis of variance of the area under the cortisol 24-h time-concentration curve (AUC) revealed a significant difference between the control group and patient groups (F = 3.69, p = 0.03). The mean AUCs of the patients in the depressed (263.4 μg/dL) and hypomanic (262.2 μg/dL) phases were beyond the 95% confidence interval for the controls (120.9–253.3 μg/dL). There were no significant group differences in cosinor acrophase and no significant effects of sex, education, age of illness onset, duration of illness or duration of mood state at time of testing on the cortisol measures. Pearson correlations between symptom rating scores and cortisol secretion variables were not significant.
Conclusion
The increases in cortisol secretion in patients in both the depressed and manic phases of bipolar disorder suggest that cortisol level is probably not a state marker in bipolar disorder.
Medical subject headings: affective symptoms, bipolar disorder, circadian rhythms, depression, hydrocortisone, laboratory techniques and procedures, psychiatric status rating scales
Abstract
Objectif
Comparer les profils de sécrétion du cortisol sur 24 heures de sujets témoins normaux à ceux de patients atteints d'un trouble bipolaire qui étaient en phase dépressive, maniaque et euthymique du trouble.
Participants
Dix-huit patients âgés de 25 à 62 ans en phase déprimée (n = 5), maniaque (n = 5) ou euthymique (n = 8) du trouble bipolaire recrutés par l'entremise d'une clinique externe de psychiatrie, et 5 sujets témoins âgés de 24 à 41 ans, recrutés au moyen d'annonces ou par le bouche à oreille.
Mesures de résultats
On a interviewé des sujets et évalué les symptômes au moyen de l'échelle de dépression de Hamilton, de l'Inventaire de dépression de Beck et de l'échelle de la manie de Young. On a commencé à prélever du sang à 8 h et les prélèvements se sont poursuivis aux heures pendant 24 heures. On a dosé les taux de cortisol sérique au moyen d'une trousse de dosage radio-immunologique commerciale validée.
Résultats
Une analyse des écarts de la superficie délimitée par la courbe de la concentration du cortisol sur 24 heures a révélé une différence importante entre le groupe témoin et le groupe de patients (F = 3,69, p = 0,03). Les superficies moyennes dans le cas des patients en phase déprimée (263,4 μg/dL) et hypomaniaque (262,2 μg/dL) dépassaient l'intervalle de confiance à 95 % des sujets témoins. Il n'y avait pas de différences importantes entre les groupes en ce qui concerne le cosinor de l'acrophase ni d'effets importants liés au sexe, à l'éducation et à l'âge au moment de l'apparition de la maladie, à la durée de la maladie ou à celle de l'état de l'humeur au moment des mesures des taux de cortisol. Les corrélations de Pearson entre les résultats d'évaluation des symptômes et les variables reliées à la sécrétion de cortisol n'étaient pas importantes.
Conclusion
Les augmentations de la sécrétion de cortisol chez les patients en phase déprimée et maniaque du trouble bipolaire indiquent que le taux de cortisol n'est probablement pas un marqueur de l'état dans les cas de trouble bipolaire.
Introduction
Hypersecretion of cortisol is a consistent finding in patients with major depression,1 but few studies have included patients in the manic and euthymic phases of bipolar disorder.2,3,4,5 It has been suggested that the circadian rhythm may be disturbed in mania,4 but, here again, only a few such studies have assessed patients in all phases of bipolar disorder.6,7,8,9 To study whether the abnormal secretion of cortisol found in the depressed phase of bipolar disorders is state dependent, we compared the 24-h secretion profiles of cortisol of control subjects and patients who were in the depressed, hypomanic and euthymic phases of bipolar disorder.
Methods
Eighteen patients in depressed (n = 5), hypomanic (n = 5) or euthymic phase (n = 8) of bipolar disorder, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition,10 were recruited through the psychiatric outpatient clinic of the Montreal General Hospital, Montreal, Que. Two patients (J.B. and G.C.) underwent 24-h blood sampling during all 3 phases of their illness, and their data are presented separately. Only data from J.B., a 53-year-old man who had been ill for 28 years, in the depressed phase and G.C., a 39-year-old women who had been ill for 16 years, in the hypomanic phase were included in their respective groups because they first entered the study during those phases. All depressed patients (n = 5) were receiving antidepressants in combination with lithium (n = 4) or divalproex (Epival) (n = 1). Four of 5 patients in hypomanic phase were receiving lithium; 1 was also taking neuroleptics and 1 was taking neuroleptics alone. Six of 8 patients in euthymic phase were taking medication (5 lithium and 1 antidepressant). Five normal subjects, recruited through advertisement and word of mouth, served as controls. Subjects were excluded from the study if physical examination or clinical laboratory results (including urinary screen for drugs of abuse) were abnormal. After the procedures of the study were explained to the subjects, written consent to participate in the study was obtained.
Symptom assessment and blood sampling procedure
The Hamilton Depression Rating Scale11 (HDRS) and the Young Mania Scale12 (YMS) were used to assess symptom severity. In addition, patients rated their symptoms on the Beck Depression Inventory13 (BDI).
On the day of 24-h sampling, subjects arrived at the Clinical Investigation Unit of the Douglas Hospital Research Centre at approximately 0630. An indwelling intravenous catheter, kept patent with a 0.3% heparin solution, was installed in the subject's anterocubital vein at 0700. Blood collection began at 0800 and continued at hourly intervals for 24 hours. Throughout the course of sampling, illumination was maintained at 300 lux during the “daytime” (0700–2300) and at 50 lux during the “nighttime” (2300–0700). Blood samples (7 mL each) were drawn into a Vacutainer tube and allowed to clot for 30 min at room temperature, and blood sera were isolated using a refrigerated centrifuge and stored at –20°C until assayed. Serum cortisol levels were measured using a validated commercial radioimmunoassay kit (Ortho-Clinical Diagnostics, Amersham, UK).
Meals were eaten at 0730, 1215 and 1715, and a snack was provided at 1930. All subjects remained indoors throughout the procedure. Smoking was permitted in a ventilated room, and regular medication was not withheld during the sampling day.
Data analysis
The cosinor method,14 which derives a best-fit sinusoidal curve from individual time points, was used to obtain information on the timing parameters.15 The acrophase represents the time of the peak value of the fitted curve of the cortisol circadian rhythm. The mesor is the mean 24-h serum level of cortisol, as estimated by the cosinor curve. The amplitude is the distance between the mesor and the highest or lowest values on the cosinor curve.
The circadian secretion of cortisol was calculated from the area under the 24-h time-concentration curve (AUC). Analyses of variance (ANOVA) were used to compare demographic and cortisol variables of interest among the depressed, hypomanic, euthymic and control groups. χ 2 was used to assess significant differences among categorical variables. Repeated measures ANOVAs were used to compare changes in cortisol levels across the 24-h time period among the groups. Two-tailed tests of significance at the 0.05 level were used, and post-hoc tests were carried out with Scheffé's test. Pearson product-moment correlations between AUC, peak cortisol levels and symptoms on the HDRS and YMS were calculated.
Results
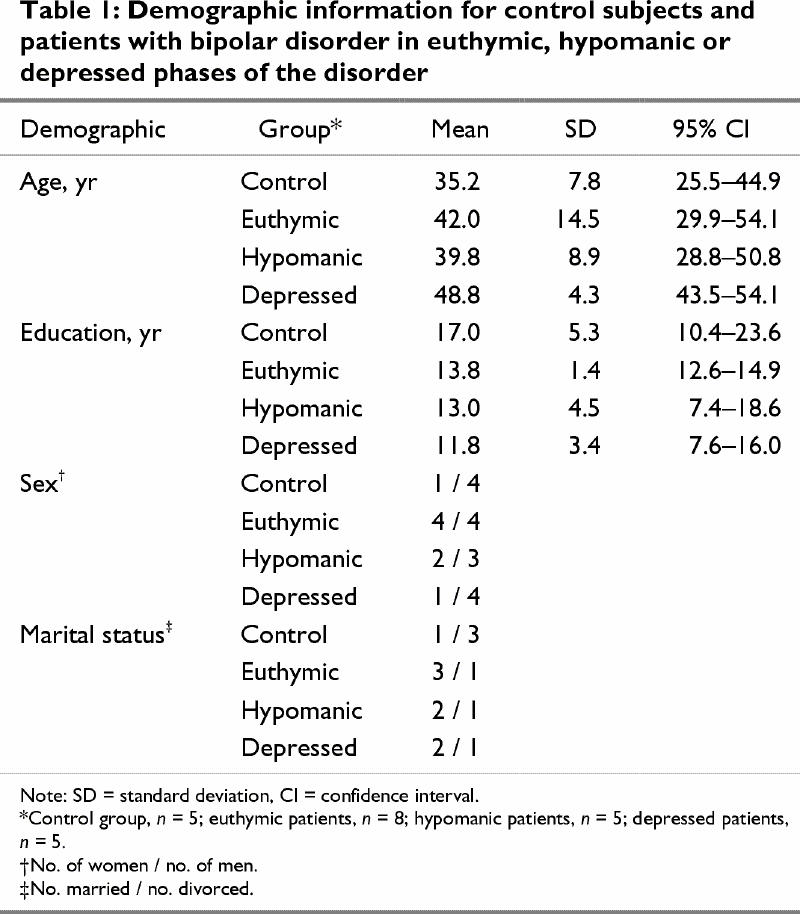
There were no statistically significant differences between control and the patient group overall on any of the demographic variables examined (Table 1). However, it was apparent from the 95% confidence intervals (CIs) that the control subjects were younger than the depressed patients and had more years of education than the depressed and euthymic patients.
Table 1
Ten of the 18 (55%) patients reported a family history of affective disorder. Patients in the depressed phase had a score greater than 17 on the HDRS,16 and those in the hypomanic phase had a score greater than 8 on the YMS17 (Table 2). Both hypomanic and euthymic phases were associated with ratings of 10 or less on the HDRS.18 Phase durations at the time of testing were as follows: all patients in hypomanic phase, 2 weeks or less; 3 patients in depressed phase, 6 months, and 1 patient each, 3 months and 1 month; duration in the euthymic phase was spread evenly between 2 weeks and over 2 years. Of note is that the mean duration of illness for the depressed group (mean 20.4 yr, 95% CI 12.5–28.3 yr) was longer than for the euthymic patients (mean 11.5 yr, 95% CI 6.1–16.9 yr). There were also expected differences on the rating scale scores for the patient subgroups.
Table 2
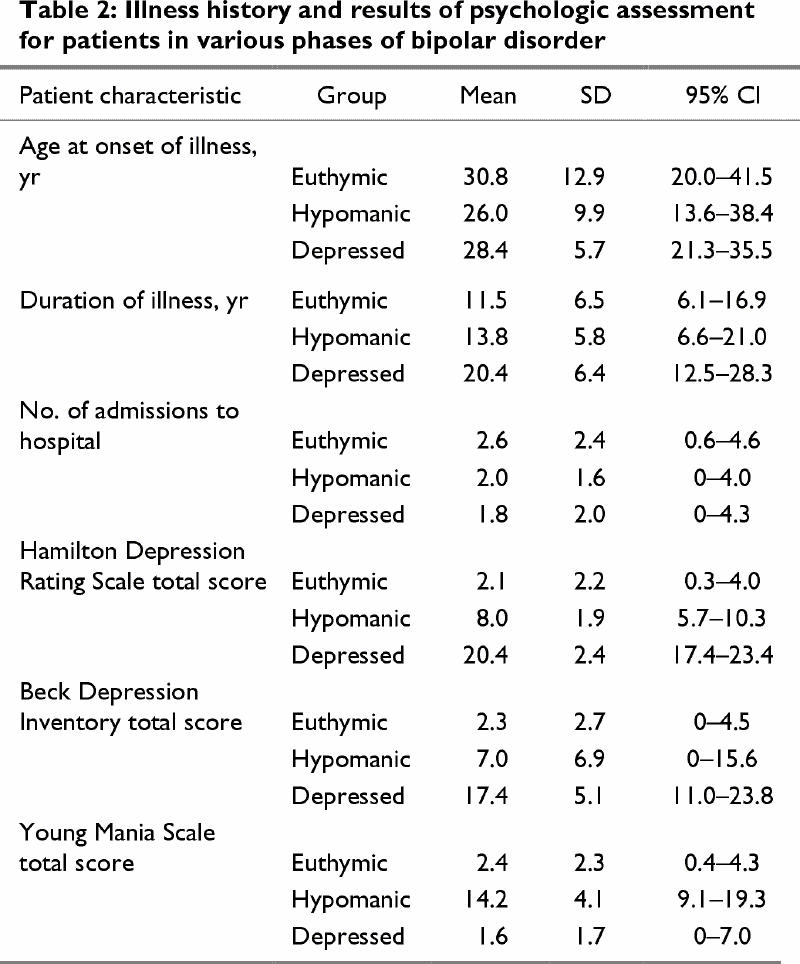
Table 3 presents quantitative and cosinor cortisol secretion information. The intra- and interassay coefficients of variation were 5.7% and 8.9% for mean cortisol concentrations of 2.0 μg/dL and 2.2 μg/dL, respectively, with sensitivity of 0.1 μg/dL.
Table 3
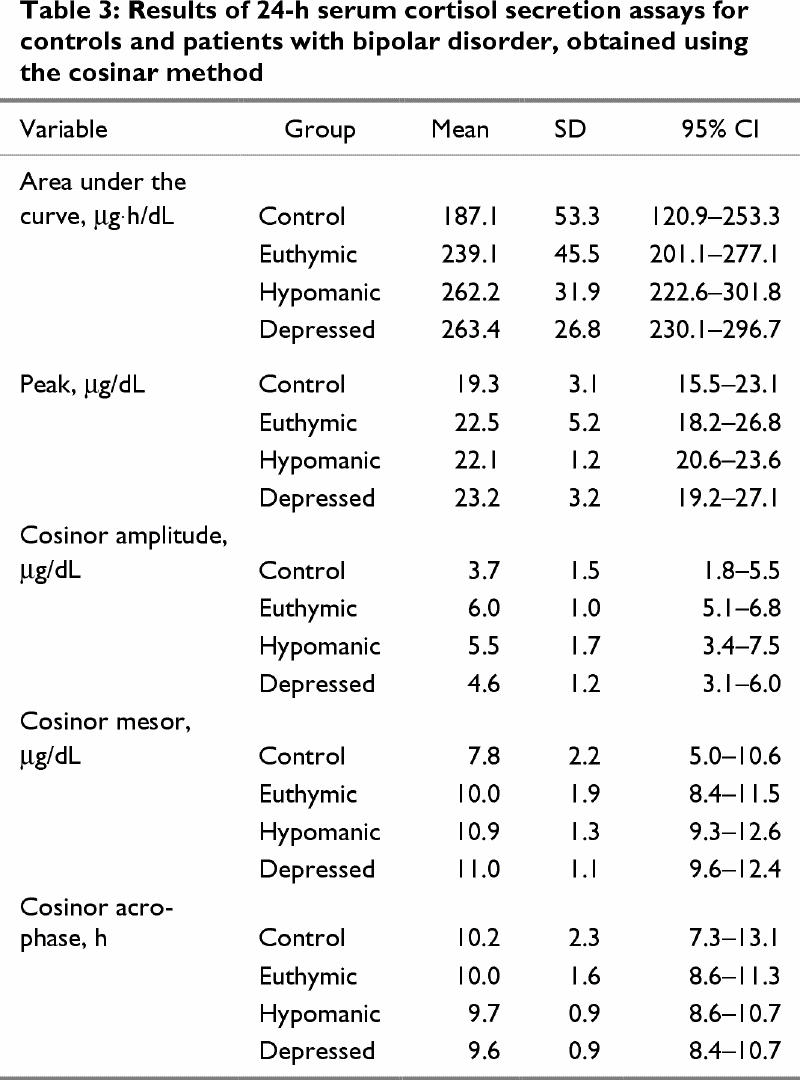
ANOVA of the area under the cortisol 24-h time-concentration curve revealed a significant difference among groups (F = 3.69, p = 0.03). Furthermore, the means for the AUC of the depressed and hypomanic patients were above the upper limit of the 95% CI for the control subjects. There were no significant differences between the depressed, hypomanic and euthymic patients.
Regarding the means of the individual cortisol peak values over the 24-h sampling period, no statistically significant differences were found among the controls and patient groups, although the mean individual cortisol peak for depressed patients (23.2 μg/dL) was above the upper limit of the 95% CI for the control subjects (i.e, 23.1 μg/dL).
There were no significant differences between groups in cosinor acrophase, and no significant effects of gender, education, age of illness onset, duration of illness or duration of mood state at time of testing on the cortisol measures were found.
Repeated-measures ANOVA on the 24-h cortisol secretions for the 4 groups, with Greenhouse-Geisser correction, revealed no significant group х time interaction, but the expected significant overall time effect was found (F = 25.07, p < 0.001).
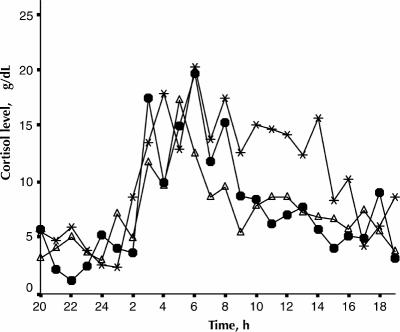
As represented in Fig. 1, the mean cortisol values for the patient groups were above the upper limit of the 95% CI of the control group at the following times: depressed patients: 0900, 1000, 1100, 1200, 1500, 1700, 2100, 0100, 0300 and 0700. Hypomanic patients: 0900, 1000, 1100, 1200, 1300, 1600, 1700 1900, 0300, 0600 and 0700. Euthymic patients: 0900, 1000, 1200, 1700 and 0300.
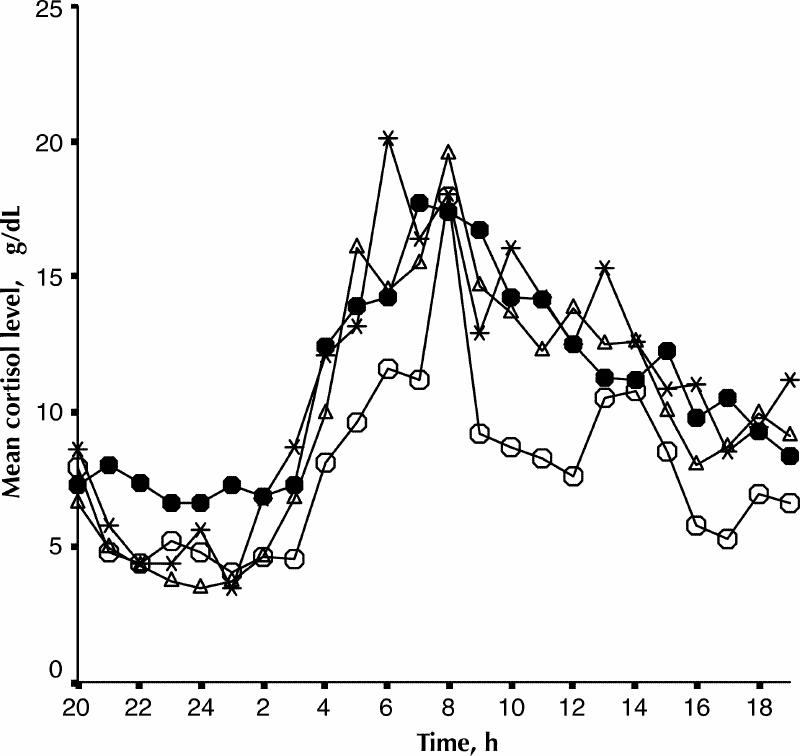
Fig. 1: Mean 24-h cortisol secretion of normal controls (open circles, n = 5) and patients with bipolar disorder in depressed (black circles, n = 5), hypomanic (asterisks, n = 5) and euthymic (open triangles, n = 8) phases of their illness.
Pearson correlations between symptoms, measured on HDRS total and factor scores, YMS and BDI total scores and cortisol secretion (AUC, peak and cosinor variables) among the patient groups were not significant.
Fig. 2 and Fig. 3 present individual 24-h cortisol secretion curves for 2 patients who underwent repeated testing in 3 phases of their illness. The AUC for the euthymic, depressed, and hypomanic phases of Mr. J.B. were 171, 250 and 322 μġh/dL respectively. The corresponding values for Ms. G.C., were 174, 184 and 251 μġh/dL, respectively.
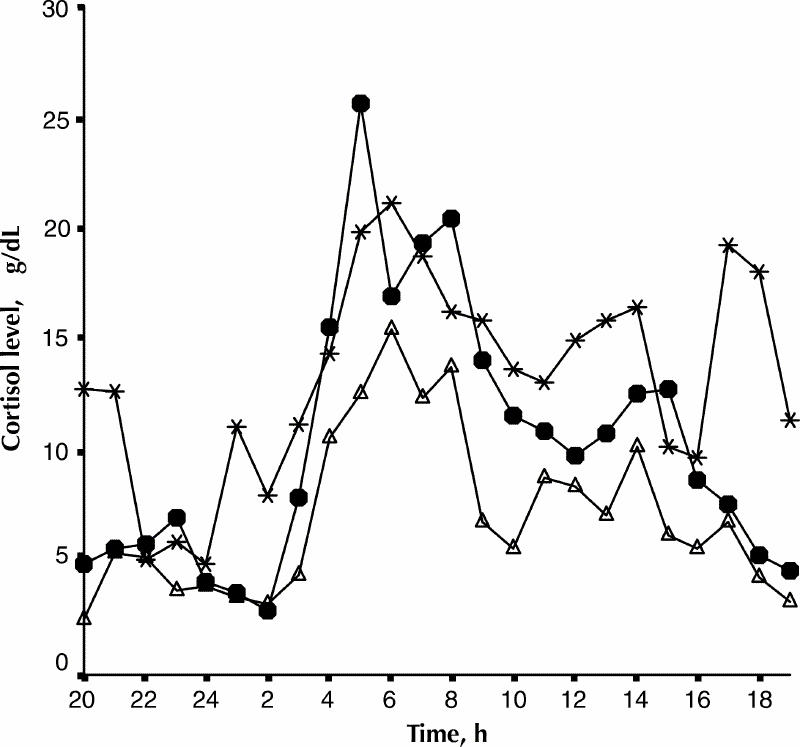
Fig. 2: The 24-h cortisol secretion profile of Mr. J.B. in 3 mood phases of bipolar disorder. Black circles, depressed phase; triangles, euthymic phase; asterisks, hypomanic phase.
Fig. 3: The 24-h cortisol secretion profile of Ms. G.C. in 3 mood phases of bipolar disorder. Black circles, depressed phase; triangles, euthymic phase; asterisks, hypomanic phase.
Discussion
Hypersecretion of cortisol in depression is a commonly reported finding,19 but there has been less consistency in the results of studies on the manic phase of bipolar disorder.4,5 We found no significant differences in cortisol secretion during the 3 mood phases, a finding which may be partially due to the small number of subjects in each mood group. However, compared with controls, significant cortisol hypersecretion occurred during both the depressed and hypomanic phases, and to a less extent in the euthymic phase. It is of interest to note that for 2 patients for whom cortisol measurements were obtained in all 3 mood states, the AUCs were highest in the hypomanic phase.
There does not appear to be any significant circadian phase shift associated with the phase of bipolar illness or any differences from controls. This is in contrast to the reports of Halbreich and colleagues20 and Pepper and Krieger21 of phase advance in the depressed state.
Correlation analyses showed no relationship between psychopathological variables (HDRS, BDI and YMS scores) and cortisol secretion. It may be noted, however, that many patients in the hypomanic phase have insomnia and anxiety symptoms which may contribute to elevated HDRS total scores.
Improvements to this present study include larger samples, sampling the same patients in 3 phases of their illness and matching controls and patients more closely in age.
Conclusion
Our results suggests that hypercortisolemia is probably not a state marker in bipolar disorder. No significant circadian phase shifts associated with the phase of illness or any differences from controls were found.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Pablo Cervantes, Director, Affective Disorders Unit, Montreal General Hospital, 1650 Cedar Ave., Montreal QC H3G 1A4; fax 514 934-8284; email: elisabeth.plourde@muhc.mcgill.ca
Submitted Mar. 2, 2000 Revised Mar. 19, 2001, Aug. 9, 2001 Accepted Aug. 16, 2001
References
- 1.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry 1976;33:1051-8. [DOI] [PubMed]
- 2.Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic depressives. Biol Psychiatry 1978; 13:335-51. [PubMed]
- 3.Joyce PR, Donald RA, Elder PA. Individual differences in plasma cortisol changes during mania and depression. J Affect Disord 1987;12:1-5. [DOI] [PubMed]
- 4.Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L'Hermite-Baleriaux M, et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry 1994;51:616-24. [DOI] [PubMed]
- 5.Cassidy F, Ritchie JC, Carroll BJ. Plasma dexamethasone concentration and cortisol response during manic episodes. Biol Psychiatry 1998;43:747-54. [DOI] [PubMed]
- 6.Bunney WE, Hertmann EL, Mason JW. Study of a patient with 48-hour manic depressive cycles. II. Strong positive correlation between endocrine factors and manic defense patterns. Arch Gen Psychiatry 1965;12:619-25. [DOI] [PubMed]
- 7.Kennedy SH, Tighe ST, McVey G, Brown GM. Melatonin and cortisol “switches” during mania, depression, and euthymia in a drug-free bipolar patient. J Nerv Ment Dis 1989;177:300-3. [DOI] [PubMed]
- 8.Gann H, Riemann D, Hohagen F, Strauss LG, Dressing H, Muller WE, et al. 48-hour rapid cycling: results of psychopathometric, polysomnographic, PET imaging and neuro-endocrine longitudinal investigations in a single case. J Affect Disord 1993;28:133-40. [DOI] [PubMed]
- 9.Joyce PR, Fergusson DM, Woolard G, Abbott RM, Horwood, LJ, Upton J. Urinary catecholamines and plasma hormones predict mood state in rapid cycling bipolar affective disorder. J Affect Disord 1995;33:233-43. [DOI] [PubMed]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4th ed. Washington: American Psychiatric Association; 1994.
- 11.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278-96. [DOI] [PubMed]
- 12.Young RC, Biggs JT, Ziegler BE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429-35. [DOI] [PubMed]
- 13.Beck AT, Ward CH, Mendleson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. [DOI] [PubMed]
- 14.Halberg F, Johnson EA, Nelson W, Runge W, Southern R. Autorythmometry procedures for physiologic self-measurements and their analysis. Physiol Teach 1972;1: 1-14.
- 15.Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, et al. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry 1989;25:305-19. [DOI] [PubMed]
- 16.Rubin RT, Poland RE, Lesser IM, Winstox n RA, Blodgett N. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry 1987;44:328-36. [DOI] [PubMed]
- 17.Young RC, Nysenwander RW, Shreiber MT. Mania scale scores, signs and symptoms in forty inpatients. J Clin Psychiatry 1983;44:98-100. [PubMed]
- 18.Greden JF, DeVigne JP, Albala AA, Tarika J, Buttenheim M, Eiser A, et al. Serial dexamethasone suppression tests among rapidly cycling bipolar patients. Biol Psychiatry 1982;17(4):455-62. [PubMed]
- 19.Sachar E, Hellman L, Fukushima DK, Gallagher TF. Cortisol production in depressive illness: a clinical and biochemical clarification. Arch Gen Psychiatry 1970;23:289-98. [DOI] [PubMed]
- 20.Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in endogenous depression. II. Time-related functions. Arch Gen Psychiatry 1985;42(9):909-14. [DOI] [PubMed]
- 21.Pepper GM, Krieger DT. Hypothalamic–pituitary–adrenal abnormalities in depression: their possible relation to central mechanisms regulating ACTH release. In: Post RM, Ballenger JC, editors. Neurobiology of mood disorders. Baltimore (MD): Williams & Wilkins; 1984. p. 245-70.