Abstract
Human astrovirus is an important cause of acute gastroenteritis. We have generated, for the first time, a vaccinia virus recombinant expressing the astrovirus 87-kDa structural polyprotein. The results demonstrate that this expression results in the formation of virus-like particles in the absence of other astrovirus proteins and genomic RNA. The purified trypsin-activated virus-like particles strongly resemble the complete astrovirus particles.
Human astroviruses (HAstV), which comprise the Astroviridae family (17), are recognized worldwide as an important cause of gastroenteritis among hospitalized infants (4, 5, 18). To date, eight serotypes of HAstV have been described, with serotypes 1 and 2 being the most prevalent around the world (4, 13, 22). HAstV have a plus-sense, single-stranded RNA genome that is approximately 6,800 nucleotides (nt) in length and which comprises three open reading frames (ORFs), namely, ORF1a, ORF1b, and ORF2 (10). ORF1a and 1b encode the nonstructural proteins (12), while ORF2 encodes the structural proteins, which are likely expressed from a 2.4-kb subgenomic RNA that is coterminal with the 3′ end of the genome (14, 17). The expression of ORF2 yields an approximately 87-kDa polyprotein, which is the precursor to the smaller, 20- to 40-kDa structural proteins that have been identified in human and animal astroviruses (3, 9, 15, 21).
Despite the HAstV ORF2 having been expressed in several systems (14, 24), to date there have been no reports, to our knowledge, on whether it would lead to the formation of virus-like particles (VLPs). Here we describe, for the first time, the expression of the 87-kDa HAstV structural polyprotein in two different mammalian cell types by using a vaccinia virus (VV)-inducible expression system. We have also analyzed the feasibility of the production of VLPs by the 87-kDa polyprotein.
HAstV serotype 2 (HAstV-2) was the starting genetic material for this study after being propagated and purified as previously described (21). Genomic HAstV RNA was isolated from purified virus by the guanidine thiocyanate method using the RNaid w/Spin kit (BIO 101; Anachem Bioscience, Bedfordshire, United Kingdom). A cDNA corresponding to ORF2 was generated by reverse transcription-PCR (RT-PCR) with the genomic RNA as a template and the primers 5′-CGCGAAGCTTCATATGGCTAGCAAGTCTGACAAGC-3′ (containing HindIII and NdeI sites) and 5′-GCGCGGATCCTCGATCCTACTCGGCGTGGCCGCGG-3′ (containing a BamHI site) in accordance with the manufacturer's instructions (Access RT-PCR System; Promega, Madison, Wis.). The RT-PCRs were carried out by incubating the mixture for 45 min at 48°C and cycling 40 times with denaturation for 1 min at 94°C, annealing for 2 min at 54°C, and extension for 4 min at 68°C. A final extension was performed for 7 min at 68°C. The nucleotide sequence of the cloned fragment was determined with the Dye Terminator cycle sequencing kit and ABI Prism 3700 DNA sequencer and shown to be correct. The amplified cDNA was subjected to digestion with HindIII and BamHI and then ligated to pFastBac, previously cleaved with the same enzymes, generating the plasmid pFastBac/POLY. The same fragment was then subcloned from this plasmid into pVOTE2 (23), using the cleavage sites NdeI and BamHI present in both plasmid and DNA fragment. The resulting construction (pVOTE2/POLY) was purified by anion-exchange chromatography on QIAGEN columns (Qiagen, Dusseldorf, Germany) and used to generate the recombinant virus VT7/POLY. Generation and amplification of VT7/POLY were carried out as previously described (7). The plasmid vectors pFastBac and pVOTE2 and the recombinant VV VT7LacOI were kindly provided by J. Francisco Rodriguez (CNB, Madrid, Spain).
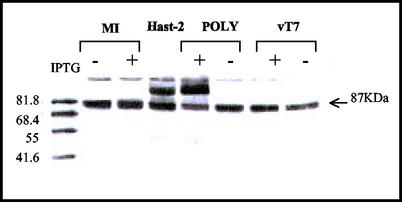
The expression of the structural polyprotein was analyzed in two mammalian cell types, namely, BSC-40 (African green monkey kidney epithelial cells, derived from the original BSC-1 cell line) and LLCMK2 (rhesus monkey kidney cells), from the American Type Culture Collection. Preconfluent monolayers were infected with either VT7LacOI or VT7/POLY at 5 PFU/cell and maintained in either the presence or the absence of the inducer isopropyl β-d-thiogalactosidase (IPTG) (1 mM final concentration). Alternatively, cells were trypsin-free infected with HAstV-2 at a multiplicity of infection (MOI) of 0.1 PFU/cell. At 24 or 48 h postinfection (p.i.), cells were washed with phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer (50 mM Tris [pH 8], 150 mM NaCl, and 1% NP-40) containing a complete protease inhibitor cocktail (Boehringer-Mannheim, Mannheim, Germany). The cell extracts were mixed with 1× polyacrylamide gel electrophoresis sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 0.25% bromophenol blue, 5% glycerol, 5% β-mercaptoethanol) and analyzed by Western blotting (WB) using a polyclonal antiserum, derived from the Oxford HAstV-2 strain (19), in combination with a goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase. The same 87-kDa protein was observed in 24-h HAstV- and IPTG-induced VT7/POLY-infected cell extracts of both types of cells (Fig. 1). In contrast with what was described by other authors (2, 15), no other proteins indicating processing of the polyprotein could be detected in any case, even when infections were maintained for 48 h (data not shown). This 87-kDa protein was absent in the negative controls (IPTG-induced cells infected with the parental virus VT7LacOI and uninfected cells).
FIG. 1.
Characterization of proteins expressed in cells infected with the recombinant VV VT7/POLY. LLCMK2 cells were mock infected (MI) or trypsin-free infected with HAstV-2 (Hast-2), VT7/POLY (POLY), or VT7LacOI (vT7), either uninduced (−) or induced (+) with IPTG. Cells were lysed at 24 h p.i. and analyzed by WB by using an HAstV-specific antiserum. The position of the 87-kDa polyprotein is indicated at the right margin of the panel. Molecular weight standards are shown in the left margin.
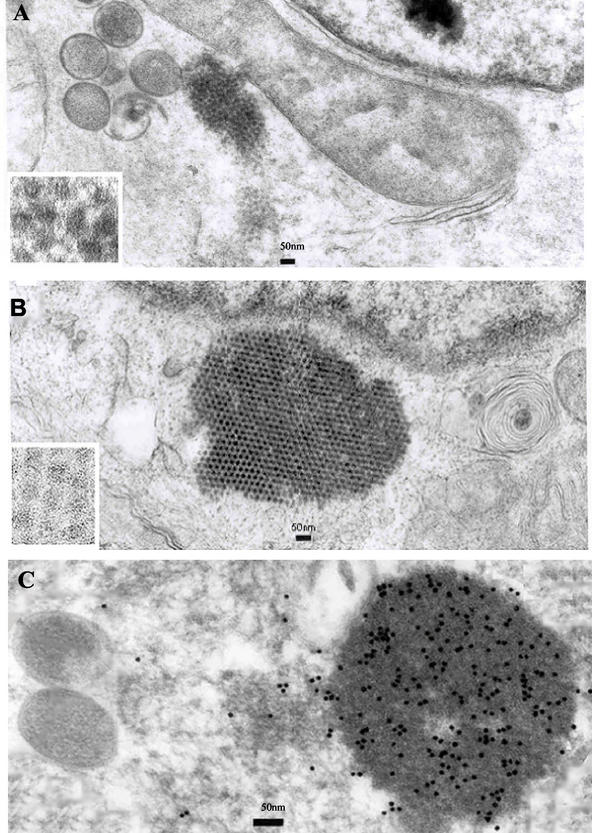
In order to determine whether the 87-kDa polyprotein expressed by VT7/POLY would assemble into VLPs, LLCMK2 cells were infected with VT7/POLY at an MOI of 5 in the absence or presence of 10 μg of trypsin/ml and maintained in the presence of IPTG. As a control, cells were alternatively infected with HAstV-2 at an MOI of 0.1. When trypsin-free infections were performed, 5% fetal bovine serum and/or 10 μg of trypsin inhibitor/ml was added to inactivate any trypsin remnant from the inocula. At 24 h p.i., culture supernatants were collected and cells were fixed in situ for embedding in epoxy resin or in Lowicryl K4 M. Thin sections of the cells were examined by electron microscopy and immunoelectron microscopy, as previously described (1, 11, 19). As shown in Fig. 2A, when infections were assayed in the absence of trypsin, in addition to the presence of large VV particles, the cytoplasm of the recombinant infected cells presented tightly packed arrays of smaller isometric particles. This result is in concordance with that obtained by immunofluorescence analysis of VT7/POLY-infected cells using monoclonal antibody (MAb) PL-2, which showed a cytoplasmatic distribution of the fluorescence signal (data not shown). The morphology and size of the VLPs strongly resemble the appearance of particles found within the cytoplasm of HAstV-2-infected cells (Fig. 2B) and particles previously described by other authors (8, 16, 19). These results are in concordance with what was described by Geigenmüller et al. (8) for HAstV-1 regarding the fact that unprocessed capsid protein can assemble into viral particles. On the other hand, these results are in contrast with those of Bass and Qiu (2) and Méndez et al. (15), who described intracellular processing of the capsid protein as being a prerequisite for virus assembly. In our system, all attempts to find particles composed of a 70- to 79-kDa protein were fruitless, even when infections were developed by using their protocol (120 h p.i.). Although there is no obvious explanation for this discrepancy, this could be due to the susceptibility of the HAstV strains analyzed, as previously mentioned (15). The intracytoplasmatic VLP accumulations are specifically recognized by the polyclonal antiserum mentioned above, confirming their identity (Fig. 2C), but not by the MAb PL-2 (21).
FIG. 2.
Visualization of assembled HAstV VLPs. (A and B) Shown is ultrathin section electron microscopy analysis of LLCMK2 cells infected with either HAstV-2 (A) or VT7/POLY (B). (C) The sections were incubated with specific HAstV antiserum followed by incubation with 10-nm colloidal gold-conjugated goat anti-rabbit immunoglobulin G. Immunogold specifically binds to the group of VLPs. Bars, 50 nm.
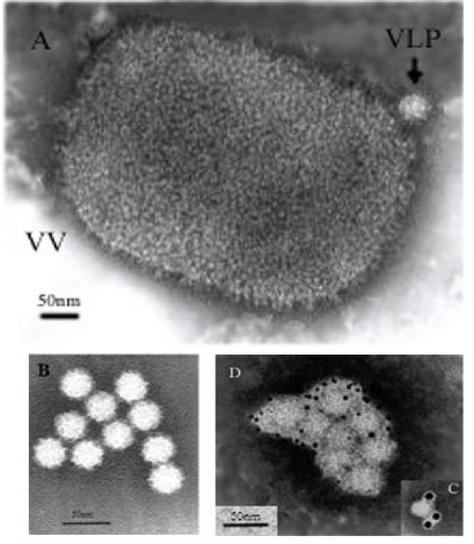
Very few VLPs were detected by negative staining within the extracellular medium of trypsin-free VT7/POLY-infected cells. It was also very difficult to observe viral particles in the culture supernatants of cells infected with HAstV-2 in the absence of trypsin, even when infections were developed as previously mentioned (120 h p.i.). However, when recombinant infections were carried out in the presence of trypsin, HAstV VLPs could be easily observed in the supernatant medium by negative staining (Fig. 3A) and presented a morphology and size similar to those described for native HAstV-2 particles (19). These VLPs remain stable throughout the purification procedure described as follows (Fig. 3B). Culture supernatants were first concentrated by ultracentrifugation at 50,000 × g for 5 h at 4°C. The resulting pellet was resuspended in TNE buffer (1 M Tris-HCl [pH 7], 0.1 M NaCl, and 100 mM EDTA), treated with 1% octyl-glucoside and loaded on top of a cushion of 30% (wt/wt) sucrose in TNE buffer for spinning at 200,000 × g for 2 h at 4°C. The resulting pellet was resuspended in TNE buffer. Alternatively, this pellet was loaded on top of a 5-ml continuous 20 to 60% sucrose gradient and spun at 200,000 × g for 2 h at 4°C. Gradients were fractionated, and the fractions were analyzed by WB. Appropriate fractions were concentrated by centrifugation at 200,000 × g for 2 h at 4°C. The pellets were resuspended in TNE buffer and visualized by electron microscopy and immunoelectron microscopy.
FIG. 3.
Electron microscopy of purified HAstV VLPs isolated from LLCMK2 cells infected with VT7/POLY and maintained in the presence of IPTG. (A) Negative stain of the sediment obtained after centrifugation of culture supernatants through a 30% sucrose cushion. Two types of particles were observed: HAstV-like particles (VLP) and large brick-shaped VV particles (VV). (B) Purified VLPs obtained after centrifugation through a sucrose gradient. (C and D) Purified VLPs were attached to coated electron microscopy grids and incubated with anti-HAstV antiserum (C) or MAb PL-2 (D). Bound antibody was detected using colloidal gold conjugates of 10 and 5 nm, respectively. Bars, 50 nm.
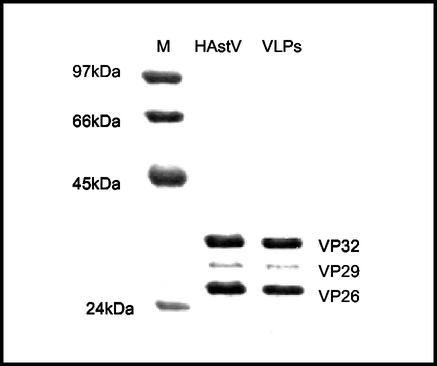
Protein composition of these purified VLPs (structural proteins VP32, VP29, and VP26) revealed no substantial differences from that of authentic virus particles when tested by WB with the mentioned polyclonal antiserum (Fig. 4). For this reason, we state, as other authors (8, 15, 16, 21) previously have, that trypsin cleavage should be necessary to proteolytically process the HAstV polyprotein into the structural proteins, as the 87-kDa IPTG-induced polypeptide also needs the trypsin to reach the mature capsid proteins. The trypsin-activated VLPs purified from the extracellular medium were strongly recognized by immunoelectron microscopy using either the mentioned polyclonal antiserum (Fig. 3C) or PL-2 MAb (Fig. 3D), thus further confirming their similarity with the native virus. This indicates that a conformational change could occur within the maturation process of the structural polyprotein, as Bass and Qiu concluded in a previous report (2). This change is probably unmasking important epitopes of the VP26 or VP29 protein, recognized by PL-2 antibody (21).
FIG. 4.
WB analysis of the protein composition of purified VLPs. Purified HAstV particles (HAstV) and HAstV VLPs (VLPs) were analyzed by electrophoresis on 12% polyacrylamide gels followed by WB using an anti-HAstV polyclonal serum. Molecular mass standards are shown in the left margin (M), and proteins VP32, VP29, and VP26 are shown in the right margin.
All attempts to purify intracytoplasmatic nonactivated VLPs were fruitless; thus, it was impossible to remove the cellular debris from the viral preparations with the purification protocol described. This could suggest that they could be strongly associated with cellular membranes or that they are structurally unstable.
The expression system described in this report might shed some light on the molecular characterization of HAstV proteins. It could contribute to determination of the targets of humoral and cell-mediated immunity without the need of purifying the viral proteins in a native state (6). In addition, it could enhance the obtaining of new specific MAbs (25) to develop specific and sensitive HAstV detection methods and strategies to gain a better understanding of HAstV antigenicity.
We hope that the availability of VLPs will be useful to investigate the types of immune response needed for protection against HAstV infection. In this way, in the future, they might facilitate the generation of an effective vaccine against this virus, as VLPs might induce an immune response similar to that elicited by inactivated virus (20). However, further experimentation is required to evaluate the true significance of our finding on the biology of the HAstV.
Acknowledgments
This work was partly supported by grant no. 200/2000 from the FIS. R. M. Dalton was supported by a grant from ISCIII (ref. 99/4031).
We are indebted to Francisco Rodriguez (CNB, Madrid, Spain) for outstanding assistance and excellent advice with the recombinant vaccinia virus development. We also thank him for the VOTE expression system. We are grateful to A. del Pozo for the photography work and also to G. Jiménez, P. Martínez, and S. Fauquier for help with the artwork. We also thank E. Cubero and V. Montero for technical assistance. Finally, we thank E. Mendez and S. Monroe for fruitful discussions and R. I. Glass for his unending support.
REFERENCES
- 1.Armbruster, B. L., et al. 1982. Specimen preparation for electron microscopy using low temperature embedding resins. J. Microsc. 126:77-85. [DOI] [PubMed] [Google Scholar]
- 2.Bass, D. M., and Shiqiang Qiu. 2000. Proteolytic processing of the astrovirus capsid. J. Virol. 74:1810-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belliot, G., H. Laveran, and S. S. Monroe. 1997. Capsid protein composition of reference strains and wild isolates of human astrovirus. Virus Res. 49:49-57. [DOI] [PubMed] [Google Scholar]
- 4.Dalton, R. M., E. Roman, A. Negredo, I. Wilhelmi, R. I. Glass, and A. Sánchez-Fauquier. 2002. Astrovirus acute gastroenteritis among children in Madrid, Spain. Pediatr. Infect. Dis. J. 21:1-2. [DOI] [PubMed] [Google Scholar]
- 5.Dennehy, P. H., S. M. Nelson, S. Spangenberger, J. S. Noel, S. S. Monroe, and R. I. Glass. 2001. A prospective case-control study of the role of astrovirus in acute diarrhea among hospitalised young children. J. Infect. Dis. 184:10-15. [DOI] [PubMed] [Google Scholar]
- 6.Earl, P. L., M. Robert-Guroff, T. J. Matthews, K. Krohn, W. T. London, and B. Moss. 1989. Isolate- and group-specific immune responses to the envelope protein of human immunodeficiency virus induced by a live recombinant vaccinia virus in macaques. AIDS Res. Hum. Retrovir. 5:23-32. [DOI] [PubMed] [Google Scholar]
- 7.Earl, P. L., and B. Moss. 1993. Generation of recombinant vaccinia viruses. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. More, J. G. Seidman, J. A. Smith and K. Struhl (ed.), Current protocols in molecular biology. John Wiley, New York, N.Y.
- 8.Geigenmüller, U., N. H. Ginzton, and S. M. Matsui. 2002. Studies on intracellular processing of the capsid protein of human astrovirus serotype 1 in infected cells. J. Gen. Virol. 83:1691-1695. [DOI] [PubMed] [Google Scholar]
- 9.Herring, A. J., E. W. Gray, and D. R. Snodgrass. 1981. Purification and characterization of ovine astrovirus. J. Gen. Virol. 53:47-55. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, B., S. S. Monroe, E. V. Koonin, S. E. Stine, and R. I. Glass. 1993. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA 90:10539-10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay, H. 1965. Techniques for electron microscopy, p. 328-355. Blackwell Scientific Publications, Oxford, United Kingdom.
- 12.Kiang, D., and S. M. Matsui. 2002. Proteolitic processing of a human astrovirus nonstructural protein. J. Gen. Virol. 83:25-34. [DOI] [PubMed] [Google Scholar]
- 13.Lee, T. W., and J. B. Kurtz. 1994. Prevalence of human astrovirus serotypes in the Oxford region 1976-92, with evidence for two new serotypes. Epidemiol. Infect. 112:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis, T. L., H. B. Greenberg, J. E. Herrmann, L. S. Smith, and S. M. Matsui. 1994. Analysis of astrovirus serotype 1 RNA, identification of the viral RNA-dependent RNA polymerase motif, and expression of a viral structural protein. J. Virol. 68:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méndez, E., T. Fernández-Luna, S. López, M. Méndez-Toss, and C. F. Arias. 2002. Proteolitic processing of a serotype 8 human astrovirus ORF2 polyprotein. J. Virol. 76:7996-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monroe, S. S., S. E. Stine, L. Gorelkin, J. E. Herrmann, N. R. Blacklow, and R. I. Glass. 1991. Temporal synthesis of proteins and RNAs during human astrovirus infection of cultured cells. J. Virol. 65:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monroe, S. S., B. Jiang, S. E. Stine, M. Koopmans, and R. I. Glass. 1993. Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. J. Virol. 67:3611-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustafa, H., E. Palombo, and R. Bishop. 2000. Epidemiology of astrovirus infection in young children hospitalised with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J. Clin. Microbiol. 38:1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risco, C., J. L. Carrascosa, A. M. Pedregosa, C. D. Humphrey, and A. Sánchez-Fauquier. 1995. Ultrastructure of human astrovirus serotype 2. J. Gen. Virol. 76:2075-2080. [DOI] [PubMed] [Google Scholar]
- 20.Saini, M., and S. Vrati. 2003. A Japanese encephalitis virus peptide present on Johnson Grass Mosaic virus-like particles induces virus-neutralizing antibodies and protects mice against lethal challenge. J. Virol. 77:3487-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Fauquier, A., A. L. Carrascosa, J. L. Carrascosa, et al. 1994. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology 201:312-320. [DOI] [PubMed] [Google Scholar]
- 22.Taylor, M. B., J. Walter, T. Berke, W. D. Cubitt, K. K. Mitchell, and D. O. Matson. 2001. Characterisation of a South African human astrovirus as type 8 by antigenic and genetic analyses. J. Med. Virol. 64:256-261. [DOI] [PubMed] [Google Scholar]
- 23.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 92:6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willcocks, M. M., and M. J. Carter. 1993. Identification and sequence determination of the capsid protein gene of human astrovirus serotype 1. FEMS Microbiol. Lett. 114:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilma, T., S. S. Ristow, B. Moss, and L. Jones. 1987. A novel approach for the production of MAbs using infectious vaccinia virus recombinants. Hybridoma 6:329-335. [DOI] [PubMed] [Google Scholar]