Abstract
Human papillomavirus-like particles (HPV VLPs) have shown considerable promise as a parenteral vaccine for the prevention of cervical cancer and its precursor lesions. Parenteral vaccines are expensive to produce and deliver, however, and therefore are not optimal for use in resource-poor settings, where most cervical HPV disease occurs. Transgenic plants expressing recombinant vaccine immunogens offer an attractive and potentially inexpensive alternative to vaccination by injection. For example, edible plants can be grown locally and can be distributed easily without special training or equipment. To assess the feasibility of an HPV VLP-based edible vaccine, in this study we synthesized a plant codon-optimized version of the HPV type 11 (HPV11) L1 major capsid protein coding sequence and introduced it into tobacco and potato. We show that full-length L1 protein is expressed and localized in plant cell nuclei and that expression of L1 in plants is enhanced by removal of the carboxy-terminal nuclear localization signal sequence. We also show that plant-expressed L1 self-assembles into VLPs with immunological properties comparable to those of native HPV virions. Importantly, ingestion of transgenic L1 potato was associated with activation of an anti-VLP immune response in mice that was qualitatively similar to that induced by VLP parenteral administration, and this response was enhanced significantly by subsequent oral boosting with purified insect cell-derived VLPs. Thus, papillomavirus L1 protein can be expressed in transgenic plants to form immunologically functional VLPs, and ingestion of such material can activate potentially protective humoral immune responses.
Infection with specific human papillomavirus (HPV) genotypes is a necessary factor in the development of cervical cancer and its precursor lesions (7, 43). Multiple sexually transmitted “high-risk” genotypes (e.g., types 16, 18, 31, 33, 35, 45, 51, 52, 58, and 59) have been associated with malignant disease, while other sexually transmitted types (notably 6 and 11) primarily cause only benign disease (e.g., anogenital warts) (20). Recombinant virus-like particles (VLPs) are promising immunogens for controlling HPV-associated diseases because they induce high-titer neutralizing antibody responses that have been associated with protection from infectious challenge (27, 36). Results from phase I studies in human subjects indicate that VLPs are safe, well-tolerated, and immunogenic when administered parenterally (12, 16). Encouraging data from a recent phase II study suggest that VLPs may be highly efficacious for HPV prophylaxis (18).
Vaccination for disease prevention is a major success of modern medicine; however, the full potential of this strategy to reduce human suffering has not yet been realized. For example, several so-called “vaccine-preventable” diseases continue to affect the lives of millions of individuals who live mostly in economically disadvantaged regions. With few exceptions, recommended immunizations rely on parenteral administration, and induction of protective immunity often requires a series of injections. Parenteral vaccines are expensive to produce and difficult and costly to administer. Thus, many diseases that are controlled effectively in industrialized countries are controlled poorly at best in less affluent regions.
A promising strategy for controlling infectious diseases involves the expression of recombinant vaccine immunogens in edible plants, which can be grown and distributed easily where they are most needed. It has been shown that VLPs are stable and immunogenic when administered orally (31) and that VLP oral immunogenicity is enhanced significantly by coadministration of a potent mucosal adjuvant [e.g., Escherichia coli LT(R192G) or CpG DNA] (13). Here, we examined whether a VLP-based edible vaccine might be feasible by introducing a codon-optimized version of HPV type 11 (HPV11) L1 into tobacco and potato. We show that L1 protein is expressed in plants and self-assembles into empty capsids that bind conformationally dependent genotype-restricted HPV11 neutralizing antibodies. We also show that transgenic L1 plant material is immunogenic when ingested and activates potentially protective anti-VLP immune responses.
MATERIALS AND METHODS
Synthesis of plant-optimized HPV11 L1.
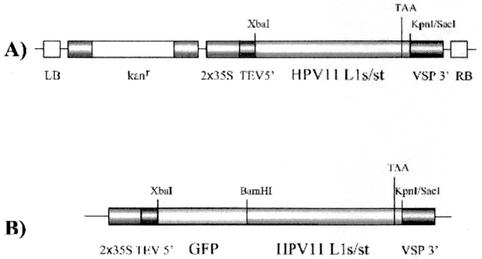
Utilizing published codon usage tables (42), we modified the coding sequence of HPV11 L1 (GenBank accession number M14119) to optimize expression in dicotyledon plants. Briefly, we designed a plant-optimized gene for full-length HPV11 L1 and for a truncated version of L1 lacking the carboxy-terminal nuclear localization signal (NLS) sequence (Fig. 1). Truncation was accomplished by introducing a single-nucleotide substitution by PCR mutagenesis (from A to T at nucleotide position 1441 in the HPV11 L1s [the lowercase “s” represents “synthetic”] sequence) (Fig. 1), which altered the amino acid codon at that location from AAA (Lys) to TAA (stop) (Fig. 1). To further enhance L1 expression in plants, our synthesis strategy also provided for removal of plant polyadenylation signals and RNA-destabilizing elements, both of which occur naturally in HPV11 L1 (data not shown). Synthetic L1 was assembled by the method of Stemmer et al. (38). A total of 75 oligodeoxyribonucleotides that collectively encoded both strands of the plant-optimized L1 coding sequence were synthesized. To facilitate subcloning, restriction enzyme sites (XbaI and KpnI) were introduced at the 5′ and 3′ ends, respectively. The oligonucleotides ranged from 34 to 46 nucleotides in length and had theoretical melting temperatures for overlaps ranging from 58 to 62°C. Gene assembly and amplification was performed as described previously (38). A single amplification product with the expected size of full-length HPV11 L1 (∼1,500 bp) was purified by agarose gel electrophoresis and cloned in pCR2.1 (Invitrogen, Carlsbad, Calif.) to generate pCRL1s. Following DNA sequence verification, the L1s sequence was transferred to a plant expression construct, pPS1, a derivative of pGPTV-Kan (4), which contains the cauliflower mosaic virus (CaMV) 35S promoter, the 5′ untranslated region (UTR) of tobacco etch virus (TEV), and the soybean vspB terminator (15). The resulting construct was named pHPV11L1s (Fig. 2). HPV11 L1s is 80% homologous in nucleotide sequence with native L1 and is 100% identical in amino acid sequence (data not shown). Because the carboxy-terminal arm of papillomavirus L1 contains an NLS sequence that could affect plant viability, and because the L1 NLS is not required for VLP assembly (25), we also constructed a truncated version of L1 (L1st, where “t” represents “truncated”) that lacked this domain. This was accomplished by PCR mutagenesis to introduce a stop codon in L1s at amino acid position 480 to generate p11L1st, which thus encoded an L1 lacking 21 C-terminal amino acids (Fig. 1 and 2). To verify L1s and L1st expression and to examine the intracellular localizations of encoded proteins, we generated green fluorescent protein (GFP)-L1s and -L1st fusion constructs by fusing the L1s or L1st sequence with the C terminus of GFP encoded by pGFP210, which expresses a plant codon-optimized version of GFP under the control of the CaMV 35S promoter (8) (Fig. 2).
FIG. 1.
Codon-optimized HPV11 L1s and L1st sequences. The single-nucleotide substitution introduced by PCR mutagenesis (from A to T at nucleotide position 1441) is shown in boldface.
FIG. 2.
HPV11 L1 plasmid DNA constructs. Constructs contained either unfused synthetic full-length or truncated (i.e., HPV11 L1s or L1st) coding sequence in binary plant transformation vectors (A) or L1s or L1st sequence fused at the carboxy terminus of the coding sequence for GFP (B). LB, left border of transferred DNA (T-DNA); kanr, nptII gene for kanamycin resistance; 2×35S, CaMV 35S promoter with duplicated enhancer; TEV5′, TEV 5′ UTR translational enhancer; VSP 3′, soybean vspB 3′ UTR and polyadenylation signal; RB, right border of T-DNA.
Transformation of plants and cell suspensions.
To obtain transient expression of GFP-L1 fusions in cell suspension cultures, the biolistic delivery system with a particle gun (PDS1000/He device; Bio-Rad) was employed. Gold particles (1-μm diameter) were coated with expression vectors as described previously (35), and tobacco NT-1 cell suspension cultures (1) were grown in liquid NT-1 medium (Murashige & Skoog [MS][Life Technologies No. 11117-041] salts, 500 mg of 2-[N-morpholino]ethanesulfonic acid [Mes]/liter, 1 mg of thiamine HCl/liter, 100 mg of myo-inositol/liter, 180 mg of K2HPO4, 2.21 mg of 2,4-dichlorophenoxyacetic acid/liter, and 30 g of sucrose [pH 5.7]/liter) in 250-ml flasks on a shaker (27°C; 250 rpm). For bombardment, 1 ml of NT-1 cells grown for 4 days after inoculation was spread on a plate containing solidified NT-1 medium (0.8% agar) covered with a filter disk. Bombardment was carried out as described previously (35), and the cells were kept for 24 h at room temperature in the dark. For visualization of green fluorescence, an Olympus IX 70 system was used. For potato transformation, plasmids pHPV11L1s and pHPV11L1st were mobilized into Agrobacterium tumefaciens LBA4404 via electroporation. Potato internode segments from 6-week-old in vitro-grown plants (Solanum tuberosum cv. Desiree) were immersed for 10 min in a suspension of A. tumefaciens grown to early log phase and then cocultivated on agar plates containing 1 mg of 6-benzylaminopurine/liter and 2 mg of 1-naphthaleneacetic acid/liter in MS salts in 0.8% Phytagar (Life Technologies no. 10675-015). After 48 h, the internode segments were transferred to plates containing 4.3 g of MS salts/liter, 1 mg of thiamine HCl/liter, 0.5 mg of nicotinic acid/liter, 0.5 mg of pyridoxine/liter, 100 mg of myo-inositol/liter, 30 g of sucrose/liter, 0.5 mg of indole-3-acetic acid/liter, 3 mg of zeatin riboside/liter, 100 mg of carbenicillin/liter, and 75 mg of kanamycin/liter. After ∼8 weeks, the regenerated shoots were transferred to rooting medium (4.4 g of MS salts, 100 mg of myo-inositol/liter, 0.4 mg of thiamine HCl/liter, 20 g of sucrose/liter, 100 mg of carbenicillin/liter, and 75 mg of kanamycin/liter). Plantlets rooting on selection medium were clonally propagated and tested for transgene expression. Positive transformants were planted in soil and initially grown in a light chamber for 3 weeks prior to transfer to the greenhouse. The potted plants in the greenhouse were maintained at 20 to 26°C with 16 h of light per day (additional lighting with sodium vapor lamps).
Nucleic acid extraction and analysis.
DNA purification was performed by the cetyltrimethylammonium bromide method described by Rogers et al. (26). Plant DNA (30 μg) was digested with XhoI (100 U) overnight and then separated on a 0.8% agarose gel. Plant total RNA was isolated by the method of Chomczynski and Sacchi (9) (TRIzol RNA extraction reagent; Life Technologies, Rockville, Md.), and 10 μg of purified total RNA per lane was loaded on a 1% agarose denaturing gel. Electrophoretic separation of RNA and DNA, transfer to a membrane, and detection were performed essentially as described previously (34). An L1-specific probe was generated by PCR amplification using a PCR DIG probe synthesis kit (Roche Molecular Biochemicals, Indianapolis, Ind.). Two internal primers were used to generate a 552-bp digoxigenin-labeled dUTP DNA fragment, representing the region between nucleotides 457 and 1009 of the synthetic L1 gene. This probe was used for detection of L1 DNA and RNA in Southern and Northern blots, respectively.
Protein purification.
For biochemical and immunological analyses, wild-type and transgenic L1 tubers were peeled, sliced, diced, and ground under liquid nitrogen. The resulting powder was suspended in 5 volumes of extraction buffer (phosphate-buffered saline [PBS], pH 7.2, 0.5 M NaCl, 50 mM Na-ascorbate, protease inhibitor mix [Roche]) and kept on ice. For purification of L1 complexes, ground tuber tissue was resuspended in extraction buffer (10 sample volumes) and clarified by sequential low-speed centrifugation (30 min at 1,500 × g, followed by 30 min at 10,000 × g). The clarified supernatants were then centrifuged at high speed (3.5 h at 100,000 × g), and the final pellets were resuspended in 1 ml of extraction buffer. Quantitation of plant-expressed L1 was accomplished by polyacrylamide gel electrophoresis and Western blot analysis of freeze-dried specimens (see below), using purified insect cell-produced HPV11 L1 VLPs as a standard.
Preparation of freeze-dried specimens.
Tubers were harvested, washed in a 1% bleach solution, rinsed well with water, and air dried at 23°C. The tubers were then cut into ∼1-cm3 pieces with a knife, placed in a 1% sodium ascorbate solution to avoid oxidation, placed in stainless steel trays, frozen at −40°C, and freeze-dried in a commercial food freeze-drier (model 100-SRC sublimator; Virtis, Inc., Gardiner, N.Y.) for 4 days at a maximum shelf temperature of 20°C. The dried tuber material was ground to powder, sealed in air-tight plastic bags, and stored at 23°C.
Immunological analyses.
Freeze-dried specimens prepared from control and 11L1st line 22 (ST22) potato tubers were evaluated by Western blot immunoassay essentially as described previously (30, 39). Briefly, extracts were loaded on 10% denaturing polyacrylamide gels, electrophoresed and blotted, and probed with a previously characterized rabbit polyclonal antiserum reactive with denatured papillomavirus L1 (39). To obtain evidence of the adoption of higher-order L1 structure, tuber extracts were evaluated by enzyme-linked immunosorbent assay (ELISA) using previously characterized conformationally dependent type-restricted HPV11 or HPV16 virion-neutralizing polyclonal antisera, as previously described (14, 28, 32, 44). Briefly, lysates were aliquoted (100 μl) into wells, and the plates were incubated overnight at 4°C and then washed three times. Following this, HPV11 or HPV16 virion-neutralizing polyclonal antibodies (N-PAb) were diluted, added to the plates, and incubated for 90 min at room temperature. The plates were then developed with secondary antibody (anti-rabbit immunoglobulin G [IgG] polyclonal antibody-enzyme conjugate) and colorimetric substrate as previously described (14). Purified insect cell-derived VLPs (28, 29, 32) were used as reference standards to quantify L1 protein in the plant samples by densitometry, using Digital Science 1D image analysis software, version 3.0 (Eastman Kodak Company Scientific Imaging Systems, Rochester, N.Y.).
Antigen denaturation.
Conformational dependence and genotype specificity are two properties of VLP antibody responses that are associated closely with, and thus are good surrogate markers for, virus-neutralizing activity (37). To assess the conformational dependence of HPV11 L1 immunoreactivity detected in transgenic L1 tubers, homogenates prepared from parental (control) and line ST22 tubers were diluted in carbonate buffer (pH 9.5; 0.01 mg/ml final concentration) and incubated in a boiling-water bath for 10 min prior to evaluation by ELISA, as previously described (10). As a control, an equivalent amount of each preparation was diluted in PBS (pH 7.1) and kept on ice prior to evaluation.
Electron microscopy.
For electron microscopy, pelleted material recovered by high-speed centrifugation (see above) was further purified by sucrose sedimentation (40% [wt/vol]; 100,000 × g; 2 h). The final pellets were resuspended in PBS (1 ml). Small amounts (5 μl) of these preparations were placed on Formvar grids for ∼1 min. Excess liquid was drained by capillary action, and the grids were stained with 2% phosphotungstic acid for 1 min, as previously described (5, 6, 29). The grids were imaged on a Hitachi 7100 transmission electron microscope.
Immunogenicity testing in mice.
Female BALB/c mice (n = 10/group) were fed meals consisting of 5 g of either wild-type or transgenic L1 tuber at weekly intervals as follows: (i) parental (nontransgenic) tuber, (ii) transgenic HPV11L1st-22 tuber, or (iii) transgenic HPV11L1st-22 tuber in combination with LT(R192G) (5 μg). Sera were collected 4 weeks after primary immunizations (postimmunization [p.i.]) and evaluated in an ELISA. Following this, each group was divided into subgroups A and B (four or five animals per subgroup). At 6 and 9 weeks p.i., subgroups A were fed as before, whereas subgroups B received VLP oral booster immunization (by gavage) as previously described (13, 31) with a subimmunogenic oral dose of insect cell-produced HPV11 L1 VLPs (0.5 μg) in combination with adjuvant [LT(R192G); 5 μg]. Postboost sera were collected 8 and 11 weeks p.i. and evaluated by ELISA as described previously (14).
Nucleotide sequence accession numbers.
The HPV11L1s and HPV11L1st sequences have been submitted to GenBank under accession numbers AY191838 and AY191839, respectively.
RESULTS
Expression of HPV11 GFP-L1s and GFP-L1st fusion proteins in tobacco.
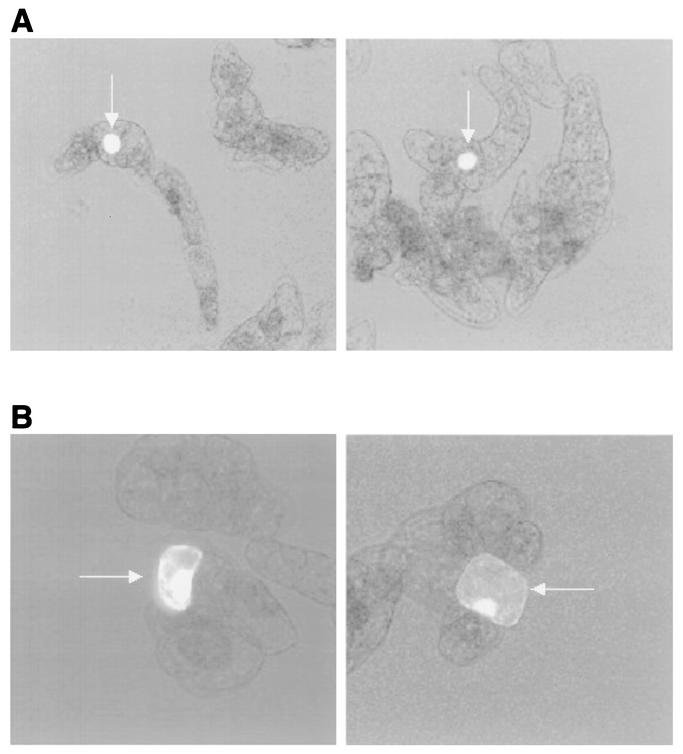
To evaluate papillomavirus L1 expression in a plant-based system, we transiently introduced GFP fusion constructs (i.e., GFP-11L1s and GFP-11L1st) into tobacco cells (NT-1) by microprojectile bombardment and examined the cells by fluorescence microscopy 24 h later. The results indicated that full-length GFP-L1s localized essentially entirely within the nucleus, whereas cells that received the truncated form of L1 (i.e., GFP-11L1st) exhibited a more diffuse pattern of fluorescence throughout the cell (Fig. 3).
FIG. 3.
Expression of GFP-HPV11 L1 fusion proteins in tobacco cells. Plasmid DNA constructs expressing either full-length (L1s) (A) or truncated (L1st) (B) L1 coding sequence fused in frame at the carboxy terminus of GFP were delivered biolistically into 4-day-old tobacco cells grown in suspension. Fluorescence appears as bright areas (arrows).
Expression of HPV11 L1 in potato.
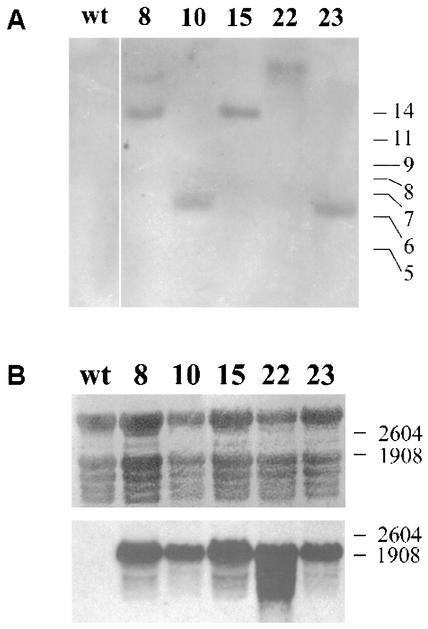
HPV11 L1s and L1st genes were cloned into a plant expression cassette featuring the nptII gene for selection on kanamycin; the 35S promoter for strong, constitutive expression; the TEV 5′ UTR for enhanced translation; and the VSP 3′ UTR and polyadenylation signal. For each construct, 100 potato internode segments were transformed via A. tumefaciens-mediated transformation. With both transformations, only a low number of plants could be regenerated (three for 11L1s and seven for 11L1st), indicating that L1 expression may interfere with plant growth and viability. Since genome integration of DNA via Agrobacterium is a random event and can occur multiple times, we checked the number of transgene copies in the genomes of the viable transformants. The restriction enzyme XhoI has one recognition site inside the expression cassette, upstream of the 11L1 coding sequence. DNA analysis by Southern blotting revealed that only ST8 showed two insertion sites, while all other lines contained only a single copy (Fig. 4A). RNA analysis by Northern blotting demonstrated that all 11L1st lines generated transcripts of the expected size (∼2 kb) (Fig. 4B); however, none of the 11L1s lines contained RNA of the expected size (data not shown). The failure of L1 mRNA to accumulate in 11L1s transgenic lines suggests the possibility that nuclear localization of L1 protein may alter plant viability. The HPV11 L1st lines were transferred to the greenhouse for development of tubers. Of seven lines found to express L1st mRNA, only three yielded tubers (lines ST10, ST22, and ST23). Two other lines were not transferred to the greenhouse due to poor growth of tissue culture plantlets, and line ST8 showed stunted growth in the greenhouse and did not form tubers (data not shown). Line ST15 had a normal phenotype but did not yield tubers.
FIG. 4.
Southern and Northern blot analyses of selected HPV11 L1st potato transformants. (A) Plant genomic DNA was prepared and Southern blotted as described in Materials and Methods. DNAs extracted from several HPV11 L1st lines (i.e., lines 8, 10, 15, 22, and 23) contained bands that varied in molecular weight, consistent with A. tumefaciens-mediated random insertions that were reactive with an HPV11 L1-specific nucleic acid probe. wt, wild type. (B) Total RNAs extracted from the same lines were Northern blotted and probed as for panel A. Top, methylene blue-stained blot to verify loading (rRNA bands); bottom, blot hybridized with HPV11 L1 probe.
Detection of HPV11 capsid antigenicity in transgenic L1 potato lines by ELISA.
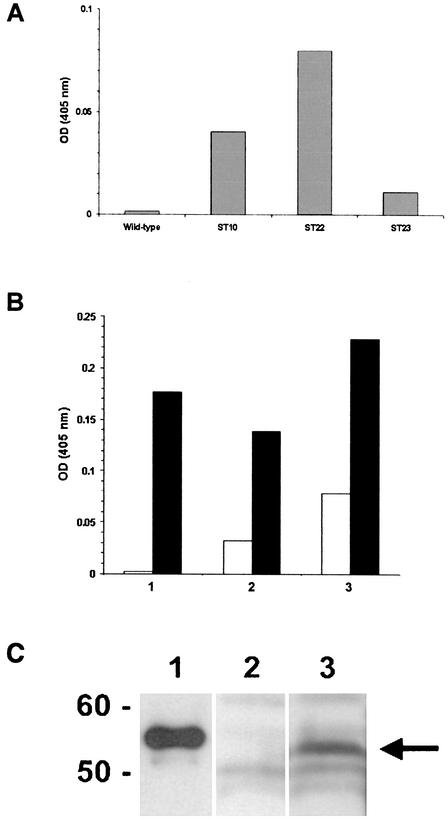
Extracts were prepared from the potato host line and from lines ST10, ST22, and ST23 and evaluated in an ELISA for immunoreactivity with previously characterized conformationally dependent, genotype-restricted HPV11 N-PAb (32). The results (Fig. 5A) indicated that extracts from each of the transgenic L1 lines examined were immunoreactive with this antiserum, suggesting that plant-expressed L1 was capable of correct self-assembly into higher-order L1 structures (i.e., capsomers and/or capsids). The strongest level of immunoreactivity with HPV11 N-PAb was exhibited by ST22 extract (Fig. 5A), and further analysis indicated that this activity could be enriched by centrifugal fractionation (Fig. 5B). Immunoreactivity was also detected in the supernatant fraction following ultracentrifugation, which may be due to the presence of incompletely assembled L1 (e.g., capsomers). As noted previously (33), the antiserum used in this assay (R-399) reacts well with assembled capsids, and also with isolated L1 capsomers.
FIG. 5.
Immunological analyses of transgenic L1 potato. (A) ELISA. Homogenates of wild-type and transgenic L1st lines 10, 22, and 23 were prepared and evaluated by ELISA as described in Materials and Methods. A previously characterized HPV11 virion-neutralizing polyclonal antiserum was used at high dilution (1:10,000) to evaluate these preparations by ELISA. HPV11 N-PAb was most immunoreactive with extract from line ST22 and to a lesser extent with extracts from lines ST10 and ST23 but was not immunoreactive with control extract. OD, optical density. (B) ELISA. HPV genotype-specific virion-neutralizing polyclonal antisera against HPV11 (solid bars) or HPV16 (open bars) were diluted (1:10,000) and tested in an ELISA against fractions prepared by ultracentrifugation of ST22 extract. Bar 1, unfractionated ST22 extract; bar 2, 100,000 × g supernatant; bar 3, 100,000 × g pellet. (C) Western blot. Extracts from untransformed control and transgenic tuber lines were centrifuged at 100,000 × g, and the pellet was resuspended and immunoblotted as described in Materials and Methods. Lane 1, full-length HPV11 L1 (25 ng) produced in insect cells; lane 2, untransformed control extract; lane 3, ST22 extract (transgenic L1 tuber). The immunoblot was probed with polyclonal antiserum raised against PV L1 common epitope (39). (The arrow indicates the position of plant-expressed L1st [∼53 kDa]).
Western blot detection of L1-immunoreactive protein in transgenic L1 potato.
Control and ST22 extracts were evaluated by Western blot immunoassay after partial purification by sedimentation at 100,000 × g to pellet VLPs. The ST22 extract, but not the control extract, contained an L1-immunoreactive band with an apparent mobility consistent with that expected for truncated L1 lacking 21 C-terminal amino acids (i.e., ∼53 kDa; Fig. 5C). Densitometric quantitation of this band, using purified insect cell-produced HPV11 L1 VLPs as a standard, indicated that ST22 potato tubers contained ∼23 ng of L1 VLP per g of fresh tuber (data not shown). L1 immunoreactivity was also detected in the 100,000 × g supernatant, perhaps due to the presence of partially or fully assembled capsomers.
Antigenic specificity of transgenic L1 tuber is conformationally dependent and genotype restricted.
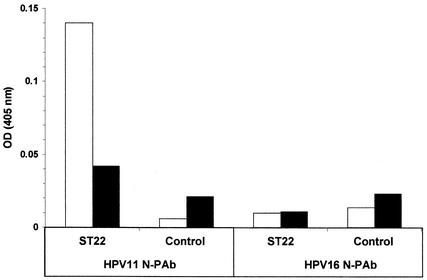
To characterize the antigenic specificity of HPV11 L1 transgenic tuber, we prepared homogenates from control and ST22 tubers and evaluated them in an ELISA in either native or denatured form (see Materials and Methods) using HPV11 and HPV16 N-PAb. As shown in Fig. 6, HPV11 N-PAb reacted well with nondenatured extract prepared from ST22 tubers but was relatively much less immunoreactive with the same extract following heat denaturation and did not react with homogenate prepared from control tubers in either native or denatured form (Fig. 6). With regard to HPV genotype specificity, HPV16 N-PAb was not immunoreactive when tested against control or ST22 homogenates in either native or denatured form (Fig. 6). Thus, antigenic properties correlated previously with neutralization of authentic HPV virions (32, 37, 44) were detected in HPV11 transgenic L1 tubers.
FIG. 6.
Conformational dependence and genotype-specificity of HPV11 L1 expressed in potato. HPV11 L1 transgenic (ST22) and parental (Control) homogenates were prepared as described in Materials and Methods and tested in an ELISA using previously characterized HPV virion-neutralizing polyclonal antisera, as indicated. ST22 and control homogenates were either untreated (open bars) or denatured with heat (solid bars) before being added to ELISA wells. OD, optical density.
Visualization of capsid-like structures in transgenic L1 potato.
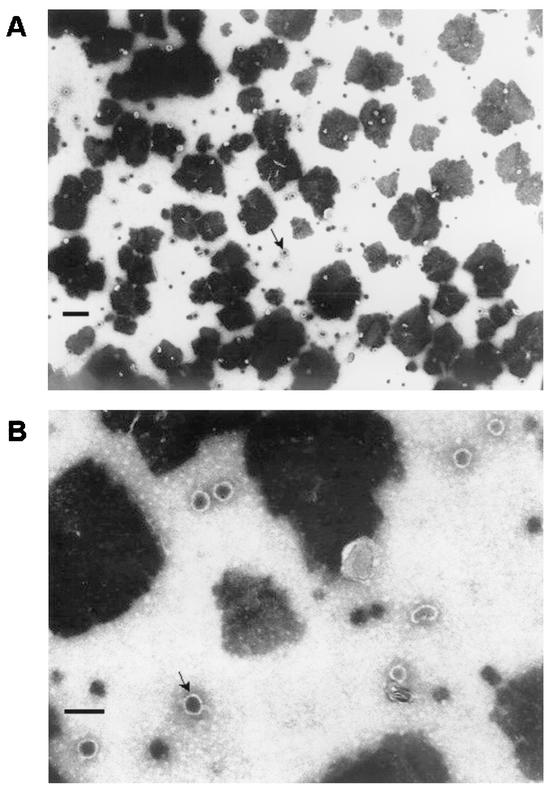
Wild-type and ST22 tuber extracts were prepared for electron microscopy as described in Materials and Methods. Electron microscopic analyses of specimens prepared from line ST22, but not parental tuber, revealed the presence of capsid-like structures with size and morphology consistent with those of native HPV11 virions (i.e., 55-nm-diameter spherical particles) (Fig. 7 and data not shown).
FIG. 7.
Electron microscopy of HPV11 transgenic L1 potato extract. Specimens were prepared as described in Materials and Methods and were examined by electron microscopy. (A) Abundant 55-nm-diameter spherical capsids are present in extract prepared from ST22 transgenic L1 potato tuber (magnification, ×35,000; bar = 0.25 μM). (B) Same field as in panel A at higher magnification (×105,000; bar = 0.1 μM). The arrows indicate representative VLPs.
Oral immunogenicity of transgenic L1 potato.
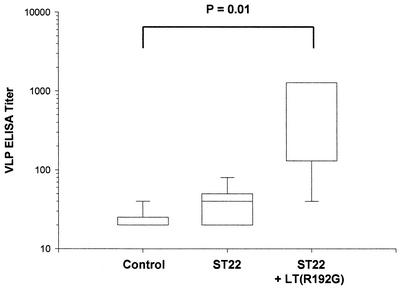
Sera collected 0, 2, and 4 weeks p.i. were evaluated in a VLP ELISA, with little evidence of immunogenicity after oral ingestion of transgenic plant material with or without adjuvant (data not shown). Postimmune sera collected after VLP oral boosting (11 weeks p.i.) were also tested by ELISA, as described previously (13), and the results indicated that ingestion of transgenic L1 potato in combination with LT(R192G) was associated with a significant enhancement of VLP serum antibody responses following oral boosting (Fig. 8; P = 0.01). Mice that did not receive VLP oral boosting but instead received additional feedings of transgenic or nontransgenic materials demonstrated no response at any time in the VLP ELISA (data not shown).
FIG. 8.
Activation of VLP immune responses by ingestion of transgenic L1 potato. Female BALB/c mice were immunized as described in Materials and Methods. Sera were collected 11 weeks p.i. and evaluated in an ELISA against insect cell-derived HPV11 L1 VLPs. The mice received weekly meals consisting of 5 g of nontransgenic tuber (Control), 5 g of transgenic L1 (ST22) tuber, or 5 g of transgenic L1 tuber with LT(R192G) (5 μg), as indicated. Six and 9 weeks p.i., all mice were boosted by oral gavage with HPV11 VLPs (0.5 μg) in combination with LT(R192G) (5 μg). Ingestion of transgenic L1 tuber with LT(R192G) was associated with significant enhancement of serum antibody titers following VLP oral boosting (P = 0.01). Each box indicates the mid-50% of values. The horizontal bar within represents the median endpoint titer. The short horizontal lines at the ends of the vertical lines extending below and above the box are the inner fences, which represent the 5th and 95th percentiles, respectively.
DISCUSSION
An effective orally delivered HPV prophylactic vaccine could facilitate efforts to control cervical HPV disease, particularly in low-resource settings, where the disease is most prevalent. The results of our study demonstrate that HPV11 L1 capsid protein can be expressed in an edible plant (i.e., potato tuber) to form empty capsids that are appropriately antigenic, as judged by the ability to bind antibodies that react specifically with, and efficiently neutralize, native HPV11 virions (28, 32, 33). Importantly, our results demonstrate that ingestion of transgenic L1 tubers activates anti-VLP immune responses that can be boosted by subsequent administration of purified VLPs. From this, we conclude that HPV transgenic L1 plants offer a feasible and potentially useful alternative strategy for immunization against anogenital HPV disease.
The requirement for coadministered adjuvant for induction of observed responses to potato-derived antigen suggests that the effective dose of HPV11 L1 VLPs in these experiments was relatively low. Previously, the induction of anti-VLP responses in mice following oral administration of as little as 1 μg of purified insect cell-derived VLPs was reported (13). In the present study, responses induced by ingestion of transgenic L1 potato were dependent on the coadministration of adjuvant. Consistent with this observation, our quantitative analysis of L1 expression in transgenic tuber indicated that the concentration of L1 VLPs was ∼20 ng per g of fresh tuber. Thus, the effective oral dose level of VLPs in these experiments was ∼100 ng per 5-g feeding, or roughly 1/10 the amount of immunogen previously determined to represent the minimum oral dose level of purified insect cell-derived VLPs without adjuvant (13). Such a low level of expression in plants precludes the direct use of the material from the present study in human subjects; nevertheless, the present results constitute an important proof of principle for this immunization strategy.
The ability of an immunogen to establish a memory response is a key element in the design of an efficacious vaccine. Responses to oral boosting observed in mice that ingested transgenic L1 potato with adjuvant indicated the generation of antigen-specific memory cells. The absence of response to oral boosting in mice fed only nontransgenic potato supports this conclusion. The ability of plant-expressed L1 to establish VLP-specific immune memory, even though VLPs were expressed at a relatively low level in potato, encourages further study of this vaccine material.
Previous studies have shown that transgene expression in plants often leads to low expression levels, presumably due to RNA instability or to the use of codons that are unfavorable in plants (24). It has also been shown that resynthesis of complete genes and their adaptation to the plant host can mediate much higher levels of heterologous protein expression (19, 21). Our analysis of the HPV11 L1 coding sequence revealed several internal polyadenylation signals, intron splice recognition sequences, and mRNA-destabilizing motifs that could yield truncated mRNA or decrease mRNA stability in plant cells. The synthetic L1 sequence spares those signals and additionally provides a pattern of codon usage that is highly preferred for expression in dicotyledonous plants (2). With the optimized version of L1, we observed expression of L1 protein only in plants transformed with a truncated form that lacked the C-terminal arm domain, which contains a well-characterized NLS sequence (23). Our observations that (i) full-length HPV11 L1 protein directed fused GFP specifically to the nuclei of plant cells and (ii) full-length L1 protein was not expressed in transgenic potato plants are consistent with the conclusion that expression of full-length L1 protein is not well tolerated in plants. Relatively low expression and poor transformation efficiency with the truncated L1 gene suggest that even with the C-terminal NLS removed, L1 protein could be toxic to plant cells. Use of developmentally regulated or chemically inducible promoters to control expression is likely to solve this problem. Preliminary results for HPV11 L1 expression in tomato show promise for a fruit-specific promoter strategy (data not shown).
The study of plant-based expression and oral delivery of vaccine antigens has expanded greatly in recent years, with a large number of papers showing faithful expression of antigenic proteins (22). Three human clinical trials with orally delivered vaccines produced in plants have been published, all showing stimulation of immune responses against the recombinant antigens expressed in edible plant tissues. The first (40) used potatoes expressing E. coli labile toxin B subunit (LT-B), a strong mucosal immunogen that binds gangliosides displayed on epithelial cell surfaces. Ingestion of raw potato containing up to 750 μg of LT-B on days 0, 7, and 21 resulted in toxin-neutralizing serum IgG antibodies in 10 of 11 subjects as late as day 59 and LT-B-specific IgA in fecal samples of some volunteers. A human study with potato-expressed capsid protein of another enteric pathogen, Norwalk virus, also showed promising results (41), with 95% of subjects showing increases in antibody-secreting cells of the IgA subtype. However, the serum IgG and fecal IgA levels produced by orally delivered antigen in that study were less impressive than in the LT-B study, suggesting that higher doses were needed. The only published human study with a nonenteric vaccine used hepatitis B virus surface antigen expressed in lettuce and stimulated serum IgG at protective levels in two of three volunteers with two doses containing only 1 μg of antigen (17).
Recent evidence of the remarkable protective efficacy of a parenterally administered HPV VLP vaccine (18) bodes well for the possibility of controlling cervical HPV disease through vaccination. Alternative immunization strategies are needed, however, to address the difficulties associated with the distribution of parenteral vaccines in developing regions. As with other promising noninvasive methods of VLP administration (3, 11, 13, 31), the low cost and ease of delivering an edible HPV vaccine could facilitate vaccine distribution in economically disadvantaged regions, which carry a large burden of anogenital HPV disease.
Acknowledgments
This work was funded by NIH R01 CA84105-3 (R.C.R. and H.S.M.) and by a Deutsche Forschungsgemeinschaft (DFG) fellowship (H.W.).
We are indebted to Patricia Keen, Sherry Roof, and Wendy Vonhof (Boyce Thompson Institute) for technical assistance with plant transformation and culture and to Karen L. Bentley (University of Rochester Electron Microscope Research Core) for assistance with electron microscopy.
REFERENCES
- 1.An, G. 1985. High efficiency transformation of cultured tobacco cells. Plant Physiol. 79:568-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology, vol. 3. John Wiley & Sons, Brooklyn, N.Y.
- 3.Balmelli, C., R. Roden, A. Potts, J. Schiller, P. De Grandi, and D. Nardelli-Haefliger. 1998. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol. 72:8220-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, D., E. Kemper, J. Schell, and R. Masterson. 1992. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol. 20:1195-1197. [DOI] [PubMed] [Google Scholar]
- 5.Bonnez, W., C. Da Rin, R. C. Rose, and R. C. Reichman. 1991. Use of human papillomavirus type 11 virions in an ELISA to detect specific antibodies in humans with condylomata acuminata. J. Gen. Virol. 72:1343-1347. [DOI] [PubMed] [Google Scholar]
- 6.Bonnez, W., R. C. Rose, and R. C. Reichman. 1992. Antibody-mediated neutralization of human papillomavirus type 11 (HPV-11) infection in the nude mouse: detection of HPV-11 mRNAs. J. Infect. Dis. 165:376-380. [DOI] [PubMed] [Google Scholar]
- 7.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu, W., Y. Niwa, W. Zeng, T. Hirano, H. Kobayashi, and J. Sheen. 1996. Engineered GFP as a vital reporter in plants. Curr. Biol. 6:325-330. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Dillner, L., P. Heino, J. Moreno-Lopez, and J. Dillner. 1991. Antigenic and immunogenic epitopes shared by human papillomavirus type 16 and bovine, canine, and avian papillomaviruses. J. Virol. 65:6862-6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuy, C., D. Buzoni-Gatel, A. Touze, D. Bout, and P. Coursaget. 1999. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J. Virol. 73:9063-9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, T. G., W. Bonnez, R. C. Rose, S. Koenig, L. Demeter, J. A. Suzich, D. O'Brien, M. Campbell, W. I. White, J. Balsley, and R. C. Reichman. 2001. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J. Infect. Dis. 183:1485-1493. [DOI] [PubMed] [Google Scholar]
- 13.Gerber, S., C. Lane, D. Brown, E. Lord, M. DiLorenzo, J. D. Clements, A. L. Williamson, and R. C. Rose. 2001. Human papillomavirus virus-like particles are efficient oral immunogens when co-administered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. J. Virol. 75:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 15.Haq, T. A., H. S. Mason, J. D. Clements, and C. J. Arntzen. 1995. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714-716. [DOI] [PubMed] [Google Scholar]
- 16.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 17.Kapusta, J., A. Modelska, M. Figlerowicz, T. Pniewski, M. Letellier, O. Lisowa, V. Yusibov, H. Koprowski, A. Plucienniczak, and A. B. Legocki. 1999. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 13:1796-1799. [DOI] [PubMed] [Google Scholar]
- 18.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 19.Koziel, M. G., N. B. Carozzi, and N. Desai. 1996. Optimizing expression of transgenes with an emphasis on post-transcriptional events. Plant Mol. Biol. 32:393-405. [DOI] [PubMed] [Google Scholar]
- 20.Lowy, D. R., and P. M. Howley. 2001. Papillomaviruses, p. 2231-2264. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 21.Mason, H. S., T. A. Haq, J. D. Clements, and C. J. Arntzen. 1998. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine 16:1336-1343. [DOI] [PubMed] [Google Scholar]
- 22.Mason, H. S., H. Warzecha, T. Mor, and C. J. Arntzen. 2002. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 8:324-329. [DOI] [PubMed] [Google Scholar]
- 23.Merle, E., R. C. Rose, L. LeRoux, and J. Moroianu. 1999. Nuclear import of HPV11 L1 capsid protein is mediated by karyopherin alpha 2/beta 1 heterodimers. J. Cell. Biochem. 74:628-637. [PubMed] [Google Scholar]
- 24.Meyer, P. 1995. Understanding and controlling transgene expression. Trends Biotechnol. 13:332-337. [Google Scholar]
- 25.Paintsil, J., M. Muller, M. Picken, L. Gissmann, and J. Zhou. 1996. Carboxyl terminus of bovine papillomavirus type-1 L1 protein is not required for capsid formation. Virology 223:238-244. [DOI] [PubMed] [Google Scholar]
- 26.Rogers, J. C., D. Dean, and G. R. Heck. 1985. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc. Natl. Acad. Sci. USA 82:6512-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose, R. C. 2002. Human papillomavirus immunology and vaccine development, p. 165-187. In D. McCance (ed.), Human papillomaviruses, vol. 8. Elsevier, Amsterdam, The Netherlands.
- 28.Rose, R. C., W. Bonnez, C. Da Rin, D. J. McCance, and R. C. Reichman. 1994. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J. Gen. Virol. 75:2445-2449. [DOI] [PubMed] [Google Scholar]
- 29.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose, R. C., W. Bonnez, D. G. Strike, and R. C. Reichman. 1990. Expression of the full-length products of the human papillomavirus type 6b (HPV-6b) and HPV-11 L2 open reading frames by recombinant baculovirus, and antigenic comparisons with HPV-11 whole virus particles. J. Gen. Virol. 71:2725-2729. [DOI] [PubMed] [Google Scholar]
- 31.Rose, R. C., C. Lane, S. Wilson, J. A. Suzich, E. Rybicki, and A. L. Williamson. 1999. Oral vaccination of mice with human papillomavirus virus-like particles induces systemic virus-neutralizing antibodies. Vaccine 17:2129-2135. [DOI] [PubMed] [Google Scholar]
- 32.Rose, R. C., R. C. Reichman, and W. Bonnez. 1994. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J. Gen. Virol. 75:2075-2079. [DOI] [PubMed] [Google Scholar]
- 33.Rose, R. C., W. I. White, M. Li, J. A. Suzich, C. Lane, and R. L. Garcea. 1998. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J. Virol. 72:6151-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanford, J. C., F. D. Smith, and J. A. Russell. 1993. Optimizing the biolistic process for different biological applications. Methods Enzymol. 217:483-509. [DOI] [PubMed] [Google Scholar]
- 36.Schiller, J., and D. Lowy. 2001. Papillomavirus-like particle vaccines. J. Natl. Cancer Inst. Monogr. 28:50-54. [DOI] [PubMed] [Google Scholar]
- 37.Schiller, J. T. 1999. Papillomavirus-like particle vaccines for cervical cancer. Mol. Med. Today 5:209-215. [DOI] [PubMed] [Google Scholar]
- 38.Stemmer, W. P., A. Crameri, K. D. Ha, T. M. Brennan, and H. L. Heyneker. 1995. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164:49-53. [DOI] [PubMed] [Google Scholar]
- 39.Strike, D. G., W. Bonnez, R. C. Rose, and R. C. Reichman. 1989. Expression in Escherichia coli of seven DNA fragments comprising the complete L1 and L2 open reading frames of human papillomavirus type 6b and localization of the ′common antigen' region. J. Gen. Virol. 70:543-555. [DOI] [PubMed] [Google Scholar]
- 40.Tacket, C. O., H. S. Mason, G. Losonsky, J. D. Clements, M. M. Levine, and C. J. Arntzen. 1998. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 4:607-609. [DOI] [PubMed] [Google Scholar]
- 41.Tacket, C. O., H. S. Mason, G. Losonsky, M. K. Estes, M. M. Levine, and C. J. Arntzen. 2000. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 182:302-305. [DOI] [PubMed] [Google Scholar]
- 42.Wada, K., S. Aota, R. Tsuchiya, F. Ishibashi, T. Gojobori, and T. Ikemura. 1990. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 18(Suppl.):2367-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 44.White, W. I., S. D. Wilson, W. Bonnez, R. C. Rose, S. Koenig, and J. A. Suzich. 1998. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J. Virol. 72:959-964. [DOI] [PMC free article] [PubMed] [Google Scholar]