Abstract
Immunity elicited by multicomponent vaccines delivered by replication-competent Ad5hr-simian immunodeficiency virus (SIV) recombinants was systematically investigated. Rhesus macaques were immunized mucosally at weeks 0 and 12 with Ad5hr-SIVsmH4 env/rev, with or without Ad5hr-SIVmac239 gag or Ad5hr-SIVmac239 nef, or with all three recombinants. The total Ad5hr dosage was comparably adjusted among all animals with empty Ad5hr-ΔE3 vector. The macaques were boosted with SIV gp120 in monophosphoryl A-stable emulsion adjuvant at 24 and 36 weeks. Controls received Ad5hr-ΔE3 vector or adjuvant only. By ELISPOT analysis, all four SIV gene products elicited potent cellular immune responses that persisted 42 weeks post-initial immunization. Unexpectedly, modulation of this cellular immune response was observed among macaques receiving one, two, or three Ad5hr-SIV recombinants. Env responses were significantly enhanced throughout the immunization period in macaques immunized with Ad5hr-SIV env/rev plus Ad5hr-SIV gag and tended to be higher in macaques that also received Ad5hr-SIV nef. Macaques primed with all three recombinants displayed significant down-modulation in numbers of gamma interferon (IFN-γ)-secreting cells specific for SIV Nef, and the Env- and Gag-specific responses were also diminished. Modulation of antibody responses was not observed. Down-modulation was seen only during the period of Ad5hr-recombinant priming, not during subunit boosting, although SIV-specific IFN-γ-secreting cells persisted. The effect was not attributable to Ad5hr replication differences among immunization groups. Vaccine delivery via replication-competent live vectors, which can persistently infect new cells and continuously present low-level antigen, may be advantageous in overcoming competition among complex immunogens for immune recognition. Effects of current multicomponent vaccines on individual immune responses should be evaluated with regard to future vaccine design.
While a number of promising human immunodeficiency virus (HIV) vaccines are currently moving to human trials, additional work is yet to be conducted in primate models to achieve immunity that translates into protection from a virulent challenge (18). A number of different vaccine approaches have shown good protection against a pathogenic simian-human immunodeficiency virus (SHIV) 89.6P isolate (1, 2, 6, 50, 57), but development of vaccines that elicit comparable protection against virulent simian immunodeficiency virus (SIV) strains that cause a disease more like AIDS have remained elusive (28). Live attenuated viral vaccines have elicited good protection (20), but in addition to safety issues concerning use of such vaccines (4, 55) more recent studies have indicated that even this strategy has difficulty protecting against a pathogenic heterologous virus (61). Several other approaches have shown promise in eliciting degrees of protection against the more vigorous SIV challenges (7, 12, 19, 23, 25, 43, 64), although sustained, reliable protective efficacy has yet to be attained.
The importance of developing vaccines able to induce cellular immunity is evident, since numerous studies have shown that cellular immune responses are correlated with reduced viremia in HIV infection (8, 33, 51) and that cytotoxic T lymphocytes (CTL) are critical in SIV clearance and control in monkeys (30, 34, 56). However, concerns have arisen about the reliance on vaccines directed solely to limited CTL epitopes, since viral escape from CTL occurs frequently (42) and can lead to AIDS progression (5). It has also been shown that passive immunization with potent, neutralizing human monoclonal antibodies can prevent infection from pathogenic SHIV in primates (3, 22, 27, 38, 39, 44). Therefore, a vaccine that can induce broad, potent cellular and humoral immune responses would not only be highly desirable but also more likely to exhibit improved protective efficacy against a virulent SIV challenge. To this end, we have pursued a prime-boost vaccine regimen to stimulate both arms of the immune system.
As priming immunogen, we have chosen adenovirus (Ad) vectors, which have numerous advantages as vehicles for vaccine development (29, 45). Viral vectors in general can elicit cellular immunity and prime antibody responses to inserted foreign genes. With regard to HIV vaccine design, Ad vectors have a significant advantage over some others in targeting epithelial cells on mucosal surfaces, thereby eliciting mucosal immunity, critical for preventing HIV infection and transmission across mucosal barriers. Our vaccine strategy employs replication-competent Ad recombinants, in which the Ad vector is deleted only in the E3 region. Previous studies have shown the ability of Ad4-, Ad5-, and Ad7-HIV recombinants together with boosts of subunit envelope protein to prime high-titer neutralizing antibodies in dogs (40) and chimpanzees (65). In the chimpanzee model, such antibodies were persistent and able to neutralize HIV primary isolates. This vaccine approach also elicited cellular and mucosal immune responses to HIV env and/or gag gene products in chimpanzees (35, 36, 41). The immunized chimpanzees exhibited long-lasting protection against both low- and high-dose HIV challenges, including a high-dose challenge with a primary, heterologous HIV isolate (36, 49).
Others have exploited replication-defective Ad recombinants (9, 57, 62). Recently, significantly reduced viral burdens and protection from SHIV89.6P-induced CD4+ T-cell loss was shown in the macaque model (57) by immunizing with a replication-defective Ad5-SIV gag recombinant, with or without prior SIV gag DNA priming.
To pursue further studies in a nonhuman primate model in which sufficient numbers of animals can be used to evaluate results statistically, we turned to the SIV/rhesus macaque model, employing an E3 region-deleted Ad5 host-range (Ad5hr) mutant vector shown able to replicate in vitro and express inserted SIVsmH4 env and rev genes in monkey cells (16). After two immunizations with this Ad5hr-SIV env/rev recombinant followed by two sequential SIV gp120 protein boosts, partial protection against an SIVmac251 vaginal challenge was obtained (11, 12). Improved protective efficacy against a rectal challenge was achieved in later studies when priming was carried out with an additional Ad5hr-SIV gag recombinant (64).
To further improve on this strategy, an additional Ad5hr-SIV recombinant was made which expressed a nonmyristoylated SIVmac239 nef, and a study was designed to systematically investigate the contribution of the several SIV gene products (Env, Rev, Gag, and Nef) to vaccine immunogenicity and protective efficacy. Here we summarize the potent immune responses elicited by the multicomponent, replication-competent Ad5hr-SIV recombinant priming-subunit boosting regimen. We report the unexpected finding that cellular immune response levels to individual gene products were modulated depending on the combination of Ad5hr-SIV recombinants administered.
MATERIALS AND METHODS
Animals.
Forty juvenile rhesus macaques, 24 males and 16 females, were selected for this study. All tested negative for prior exposure to SIV, simian retrovirus type D, and simian T-cell leukemia virus. Macaque 21, assigned to group II (see below), succumbed at week 12 to non-study-related renal failure and anemia and was eliminated from the final data set. Ten of the 40 macaques were Mamu-A*01 positive and two were assigned to each immunization group.
Immunogens.
Three replication-competent Ad5hr-SIV recombinants were used in this study. The construction and in vivo replication of Ad5hr-SIVsmH4 env/rev (11, 12, 16, 46, 63) and Ad5hr-SIVmac239 gag encoding rev-independent Gag (37, 63) have been previously described. In addition, an Ad5hr-SIVmac239 nef recombinant encoding a nonmyristoylated Nef protein was constructed as follows. Plasmid p239SpE3′ nef Open was obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). In p239SpE3′ nef Open, the premature stop codon TAA at position 93 in the SIVmac239 nef gene was replaced with GAA, resulting in a full open and functional Nef open reading frame (24, 31, 48). pBRAd5hrΔE3, obtained from Wyeth-Lederle Vaccines (Pearl River, N.Y.), contains the Ad5hr sequence from 59.5 to 100 map units (m.u.), in which the E3 region from 78.8 to 85.7 m.u. is deleted. SIVmac239 nef DNA, with nucleotides 1 to 39 deleted, was amplified from p239SpE3′ nef Open using PCR and then inserted into the XbaI site of pBRAd5hrΔE3 at 78.8 m.u. of the Ad5hr sequence. The resulting pBRAd5hrΔE3-SIVmac239 nefΔ1-13 was confirmed by DNA sequencing. The Ad5hr-SIV nefΔ1-13 recombinant was generated by homologous recombination as described previously (14). Briefly, 4 μg each of EcoR1-cut Ad5hr DNA (0 to 75 m.u.) and pBRAd5hrΔE3SIVmac239 nefΔ1-13 (59.5 to 100 m.u. of Ad5) was cotransfected into 293 cells with Lipofectamine according to the manufacturer's protocol (Life Technologies, Gaithersburg, Md.). After passaging the 293 cells for 1 to 2 weeks to maintain cells in log phase, a typical Ad5 cytopathic effect was observed. Recombinant viruses from this culture were plaque purified once, and Nef expression in 293 cells was verified by an immunofluorescence assay using mouse monoclonal antibody N27 specific for SIV Nef (15). Ad5hr-SIV nefΔ1-13 was then plaque purified three times before final amplification on 293 cells and double cesium chloride gradient purification and concentration. SIV Nef expression following infection of cells with the recombinant virus was confirmed by Western blotting using the anti-SIV Nef 17.2 monoclonal antibody (AIDS Research and Reference Reagent Program), followed by a goat anti-mouse immunoglobulin G-horseradish peroxidase conjugate (Sigma, St. Louis, Mo.) and standard electrochemiluminescent reagents (Amersham Pharmacia Biotech, Piscataway, N.J.) for final detection.
Study design.
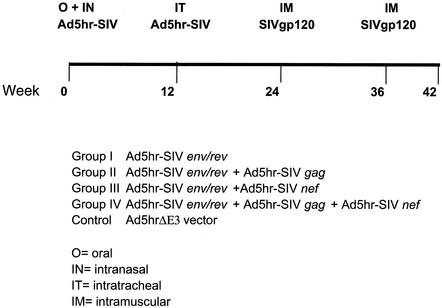
Four groups of eight macaques each (seven in group II) were immunized twice with different combinations of replication-competent Ad5hr-SIV recombinants and then boosted twice with native SIVmac251 gp120 as shown in Fig. 1. All groups received Ad5hr-SIVsmH4 env/rev with or without Ad5hr-SIVmac239 gag and/or Ad5hr-SIVmac239 nefΔ1-13. Eight additional control macaques received the empty vector, Ad5hr-ΔE3. The first Ad immunization was simultaneously administered both orally and nasally at a dose of 5 × 108 PFU/route/recombinant in phosphate-buffered saline at week 0, followed by a second, identical immunization intratracheally at week 12 (12). The intranasal immunizations were administered to the nostrils dropwise while the macaques were sedated. When given orally, the virus was delivered by stomach tube, preceded by 2 ml of bicarbonate solution. To ensure all immunization groups received the same number of infectious Ad particles, macaques immunized with only one or two Ad5hr-SIV recombinants were complemented with enough Ad5hr-ΔE3 vector to maintain a total Ad5hr dose of 1.5 × 109 PFU. Twelve weeks after the second Ad immunization, the macaques were boosted with 100 μg of native SIVmac251 gp120 in MPL-SE (monophosphoryl A-stable emulsion) adjuvant (provided by Wyeth-Lederle Vaccines) by the intramuscular route, rested for 12 weeks, and then boosted again.
FIG. 1.
Immunization schedule and outline of immunization groups. The immunogens, dosages, and adjuvant used are described in Materials and Methods.
Sample collection.
Peripheral blood mononuclear cells (PBMC) were collected throughout the study using lymphocyte separation medium (ICN Pharmaceuticals, Inc.). Either freshly obtained cells or cells viably frozen in fetal bovine serum containing 7% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen were used to evaluate cellular immune responses as indicated below. Serum was obtained and stored at −20°C until use. Stool and nasal samples were collected following Ad immunizations as previously described (11) and stored at −70°C.
Measurement of Ad replication and immunity.
Ad5hr replication was assessed by detection of viral DNA in macaque stool and nasal secretions using a nested PCR assay specific for the conserved Ad5 fiber gene as previously described (12). The method was modified by the addition of 2.5 mM MgCl2 to the reaction mixtures. Five microliters of sample, previously heated to 95°C for 5 min to lyse viral capsids, was used in the first-round PCR, and 1.5 μl was used in the second nested PCR. Reaction products were run on 2% agarose gels to visualize bands and Southern blotted to enhance sensitivity as described elsewhere (12).
Serum neutralization titers to Ad5hr were assayed in a 96-well plate format with A549 cells as detailed previously (17). Macaque sera were tested at serial twofold dilutions, with endpoint titers defined as the reciprocal of the last serum dilution in which no Ad cytopathic effect was seen.
SIV-specific cellular immunity-ELISPOT assay.
Cellular immune responses to SIV EnvsmH4, RevsmH4, Gagmac239, and Nefmac251 proteins were assessed using the ELISPOT assay to detect gamma interferon (IFN-γ)-secreting lymphocytes poststimulation with specific SIV peptide pools. Env, Rev, and Nef peptides, consisting of 15-mers overlapping by 11 amino acids, were provided by Advanced BioScience Laboratories, Inc. (Kensington, Md.). Peptides were dissolved in DMSO at a high concentration, pooled together at a working concentration of 100 μg of each peptide/ml, and then diluted to a 1-μg/ml final concentration of each peptide in the assay plate. For envelope peptides representing the SIVsmH4 gp160 protein minus the signal peptide, three pools totaling 214 peptides were tested separately: pool A included peptides 1 to 71; pool B included peptides 72 to 142; and pool C included peptides 143 to 214. SIVsmH4 Rev (peptides 1 to 22) and SIVmac251 Nef (peptides 1 to 59) were tested in single pools. SIVmac239 Gag peptides, obtained from the AIDS Research and Reference Reagent Program, NIAID, consisted of 20-mers overlapping by 10 amino acids. Two separate pools of 25 peptides each were tested. The Gag peptides were either dissolved in water or DMSO, depending on instructions provided, resulting in negligible final DMSO concentrations. Consequently, two separate negative controls were included in each assay: one of R5 medium only (RPMI 1640 containing 5% human AB serum and 2 mM l-glutamine) for measurements with Gag peptides, and the other of R5 medium containing 1.4% DMSO, for measurements with Env, Rev, or Nef peptides.
PBMC were isolated from whole blood and suspended in R5 medium. Serial, twofold dilutions of cells ranging from 1 × 105 to 1.25 × 104 cells/well were plated in triplicate in a 96-well plate that had been previously coated with anti-IFN-γ monoclonal antibody MD-1, washed, and blocked with 2% bovine serum albumin according to the U-Cytech ELISPOT kit instructions (U-Cytech, Utrecht, The Netherlands). Peptides were added at 1 μg/ml, and the plate was incubated overnight (14 h) at 37°C, 5% CO2. Concanavalin A (Sigma) at 5 μg/ml was included as a positive control for each macaque. The next day the plates were washed 20 times and incubated with biotinylated rabbit anti-IFN-γ antibody for 1 h at 37°C. After 10 washes, gold-labeled anti-biotin immunoglobulin G was added for 1 h at 37°C, the plates were again washed 10 times, and developer was added until spots were observed. The plates were rinsed with water to inactivate the developer and dried, and the resulting spots were counted with an inverted microscope. Background spots obtained with medium alone or with medium plus 1.4% DMSO were subtracted from each experimental value. All figures are a representation of the mean number of spot-forming cells (SFC) per 106 PBMC. A positive response was defined as one exhibiting both ≥120 SFC/106 PBMC and four times as many spots as medium-only wells. The high stringency requirement for determining positivity was dictated by high values seen for two control macaques in response to Env peptides at week 26. A responder macaque for each antigen was defined as one exhibiting positive IFN-γ secretion at any time point over the immunization course.
SIV-specific cellular immunity-T-cell proliferation assay.
Frozen cells were used for assays at all time points. PBMC were counted and adjusted to 3 × 105 cells/well in triplicate in 100 μl of RPMI supplemented with 10% fetal bovine serum (R10). Proteins, including native SIVmac251 p27 and SIVmac251 Nef (Advanced Bioscience Laboratories, Inc.), and aldrithiol-2-treated SIVmac239 particles (52) (AIDS Vaccine Program, SAIC NCI-Frederick) or similarly treated negative control SupT1 microvesicles were diluted to 40 μg/ml and added to cells at a final concentration of 4 μg/well. Concanavalin A (4 μg/well) or R10 alone served as positive and background controls, respectively. After a 5-day incubation, [3H]thymidine was added at 1 μCi/well overnight, and the next day plates were harvested using a Mach IIM (Tomtech, Inc.) cell harvester and counted using a Beckman beta plate counter. Average counts for each condition were divided by the mean for R10 alone or SupT1 microvesicles as appropriate to generate the stimulation index (SI). A positive response was defined as one with an SI of >2.
SIV-specific humoral immune responses.
Macaque sera were tested throughout the immunization course for the presence of binding antibody to SIV gp120. Nunc plates were coated with purified SIVmac251 gp120 (50 ng/well) in bicarbonate buffer with 100 ng of bovine serum albumin/well. All sera were initially tested at a 1:50 dilution and then titered to end point as needed. Endpoint titers are reported as the reciprocal of the highest serum dilution giving an absorbance value twice that of serum from an SIV-negative macaque diluted 1:50. SIV-specific neutralization antibodies were measured against a lab-adapted strain of SIVmac251 as previously described (64). Virus infectivity was measured by a p27 antigen capture assay (Coulter Corp., Miami, Fla.). Endpoint titers, defined as the reciprocal of the serum dilution at which viral infectivity was 50% of that in the presence of control serum, are shown.
Statistical analysis.
Two-group comparisons of repeated measurements over intervals were made using Wei-Johnson tests (60). Comparisons at individual times employed the exact Wilcoxon rank sum test. P values for pairs selected from among the four immunized groups and the control group were corrected by the method of Hochberg (26) for the number of tests (10 for responses to Env and Rev, 3 for Gag and Nef). All P values are two-tailed.
RESULTS
Construction and in vitro expression of Ad5hr-SIVmac239 nefΔ1-13.
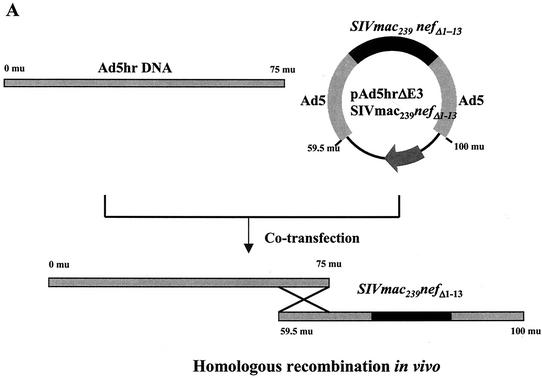
In prior studies an Ad5hr-SIVsmH4 env/rev recombinant effectively primed SIV-specific cellular, humoral, and mucosal immunity in rhesus macaques (11, 12, 46, 64). Additionally, our group has recently shown that priming immunizations with a combination of Ad5hr-SIVsmH4 env/rev and an Ad5hr-SIVmac239 gag recombinant encoding Rev-independent Gag similarly induced good cellular immune responses to the SIV Gag component as well as Rev and Env (63) and elicited improved protective efficacy against a mucosal SIVmac251 challenge (64). In the study reported here, we added an additional SIV component into the vaccine regimen to provide additional targets for cellular immune responses, with the ultimate goal of further enhancing protection against a virulent, pathogenic challenge. Nef was selected, as it is expressed early and abundantly in infection and possesses numerous CTL and B-cell epitopes. The decision to incorporate a mutated Nef recombinant, Ad5hr-SIVmac239 nefΔ1-13, was based on results obtained in a functional study of HIV Nef mutants (47). This study reported that deletion of Nef N-terminal amino acids 1 through 19, which included the myristoylation site, resulted in elimination of Nef-induced down-modulation of CD4 and major histocompatibility complex class I expression at the cell surface. Both of these Nef functions might impair vaccine-elicited immune responses. Therefore, the Ad5hr-SIV nefΔ1-13 recombinant was constructed with the coding sequences for N-terminal amino acids 1 through 13 of SIV Nef deleted. The design and construction of this recombinant is summarized in Materials and Methods and illustrated in Fig. 2A.
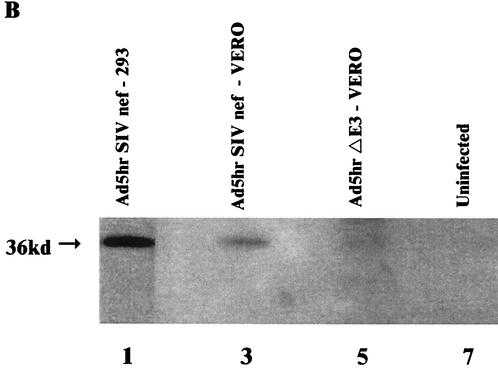
FIG. 2.
Construction and expression of Ad5hr-SIV nefΔ1-13. (A) Schematic showing Ad5hr-SIV nefΔ1-13 recombinant design and construction. (B) Western blot of Ad5hr-SIV nefΔ1-13 expression on human 293 (lane 1) and monkey-derived VERO (lane 3) cell lines. Negative controls include uninfected VERO cells (lane 7) and VERO cells infected with Ad5hrΔE3 vector without the nef insert (lane 5).
Before determining the potential benefit of this recombinant as a vaccine, it was first necessary to demonstrate Nef expression in vitro following infection of cells with Ad5hr-SIVmac239 nefΔ1-13. The Ad5hr-SIVmac239 nefΔ1-13 recombinant grew to high titer in both human 293 cells and monkey Vero cells. In Fig. 2B, Western blots probed with anti-Nef 17.2 monoclonal antibody show that both cell types expressed the mutated Nef protein, which had an apparent molecular mass of 36 kDa.
Cellular immune responses.
To systematically investigate the immunogenicity of the individual components of the multicomponent vaccine regimen, we first examined cellular immune responses to all SIV gene products encoded in the Ad5hr recombinants. The ELISPOT assay was used to detect IFN-γ secretion by macaque PBMC in response to SIV Gag, Env, Rev, or Nef peptides as described in Materials and Methods prior to immunization and at weeks 2 and 10 following each Ad recombinant or gp120 administration, out to 42 weeks post-initial immunization. Results using fresh PBMC are reported in all cases except for IFN-γ secretion in response to Env, Rev, and Nef peptides at weeks 0, 2, and 10 post-first Ad immunization, where the appropriately matched medium-1.4% DMSO control was not included. This control was included in all further assays, and IFN-γ secretion at weeks 0, 2, and 10 in response to Env, Rev, and Nef peptides was reevaluated using viably frozen PBMC.
The Ad5hr-SIV recombinants were highly effective in eliciting cellular immunity to all SIV proteins: Env, Gag, Nef, and Rev (Table 1). Comparison of IFN-γ secretion by immunized and control macaques in response to each immunogen, using nonparametric analysis of medians over multiple time points, showed highly statistically significant differences during the period of Ad5hr-SIV recombinant priming (weeks 2 to 22) that persisted during gp120 boosting (weeks 26 to 42). Analysis of immune responses induced over the entire immunization course (weeks 2 to 42) also showed highly significant differences between immunized and control macaques. Using the criteria in Materials and Methods defined for a positive response and for a responder macaque, 90% of all animals immunized with Ad5hr-SIVsmH4 env/rev exhibited a positive cellular immune response to Env peptide pools, the highest frequency of response for any of the SIV immunogens. Moreover, the response was highly potent, with a mean peak ELISPOT value during priming of 959 SFC/106 PBMC. Importantly, this response persisted during and after subsequent gp120 boosting, resulting in potent SFC counts that were sustained as long as 30 weeks after the second Ad5hr recombinant immunization. The response to Gag in immunized macaques was equally potent, exhibiting a mean peak response of 1,003 SFC/106 PBMC during the Ad5hr-SIVmac251 gag recombinant priming period. Again, Gag-specific IFN-γ-secreting cells persisted over the period of boosting with gp120, maintaining an equivalent level of SFC (Table 1). Overall, two-thirds of the immunized macaques exhibited a positive response to Gag peptides.
TABLE 1.
IFN-γ secretion by PBMC of immunized macaques in response to SIV peptide pools
| Peptide pool | No. of macaques immunized | Mean peak IFN-γ secretion for all responders
|
Overall % respondingc | P valuea (wks 2-42) | |||
|---|---|---|---|---|---|---|---|
| During Ad5hr recombinant priming (wks 2-22) | P valuea (wks 2-22 or 14-22)b | During gp120 boosting (wks 26-42) | P valuea (wks 26-42) | ||||
| Env | 31 | 959 ± 352 | 0.0003 | 984 ± 222 | 0.0026 | 90 | 0.0008 |
| Gag | 15 | 1,003 ± 115 | 0.0094 | 1,494 ± 751 | 0.0026 | 67 | 0.0033 |
| Nef | 16 | 347 ± 164 | 0.0016 | 269 ± 53 | 0.058 | 44 | 0.012 |
| Rev | 31 | 315 ± 59 | 0.0007 | 319 ± 76 | 0.0022 | 39 | 0.0003 |
P values reflect comparison of SFC for all immunized macaques versus controls over the indicated time periods.
Week 2 to 22 time points were evaluated for response to Gag pools, while weeks 14 to 22 were used for Env, Rev, and Nef.
Percentage of macaques exhibiting a positive response, as defined in Materials and Methods, at one or more time points over the entire immunization period.
Significant induction of both Nef- and Rev-specific cellular immune responses was seen in macaques immunized with Ad5hr-SIVmac239 nefΔ1-13 and Ad5hr-SIVsmH4 env/rev in comparison to the control group, and the levels and frequencies of responses were similar (Table 1). However, somewhat less than half of the immunized macaques developed positive responses to Nef and Rev, and the levels of SFC in the responder macaques were approximately one-fifth to one-third of that seen in response to Gag and Env peptides, respectively. Of course Nef and Rev are smaller proteins and possess fewer CD8+ T-cell epitopes, which could account for differences seen in the frequency and magnitude of ELISPOT responses. Nevertheless, the persistence of the immune responses to Nef and Rev, as with Gag and Env, to 42 weeks post-initial immunization was noteworthy (Table 1).
Modulation of peptide-specific IFN-γ production between groups.
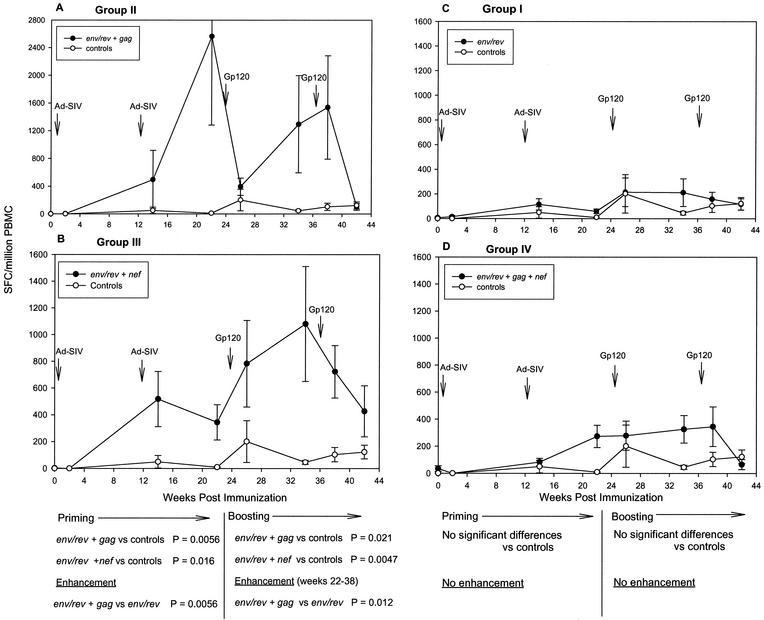
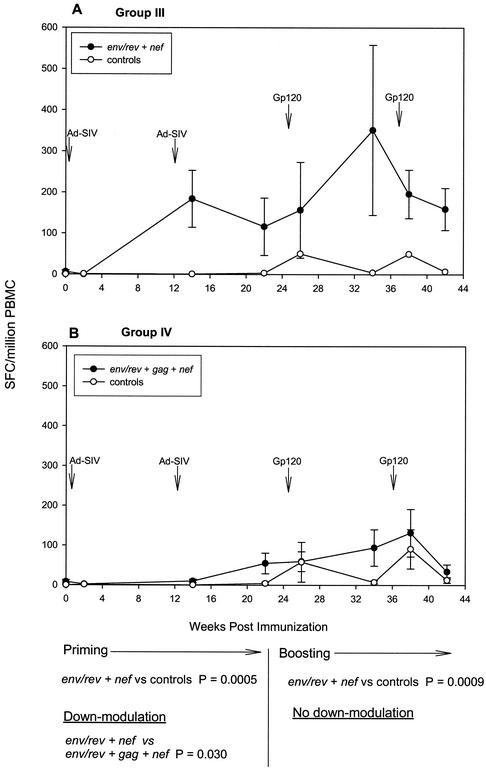
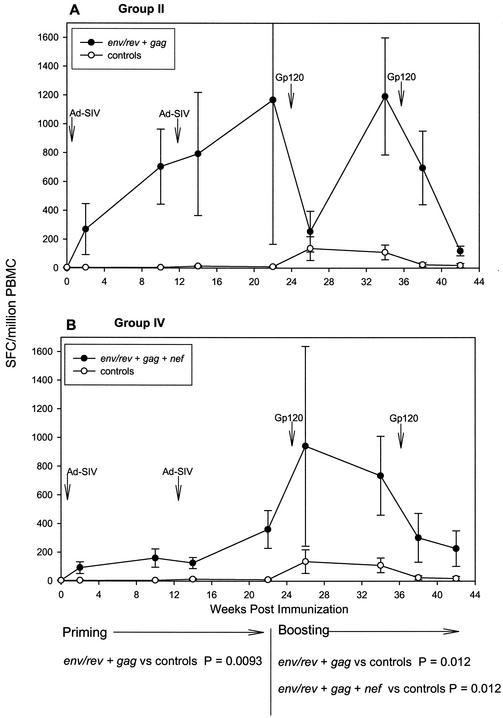
Analysis of peak immune responses showed that all Ad5hr-SIV recombinants elicited potent and persistent cellular immunity. A somewhat surprising finding when responses were evaluated over the entire period of Ad-recombinant priming (weeks 2 to 22) and boosting (weeks 26 to 42), however, was modulation of the cellular immune response to individual SIV antigens depending on the combination of immunogens administered. The design of this study allowed us to compare immune response levels to individual SIV antigens in immunization groups of sufficient size to achieve statistical significance. All the immunized macaques received the Ad5hr-SIVsmH4 env/rev recombinant, so initially we compared IFN-γ secretion in response to Env peptides among the four immunization groups. Figure 3A and B show that only macaques immunized with two recombinants, group II, which received Ad5hr- SIVsmH4 env/rev plus Ad5hr-SIVmac239 gag, and group III, which received Ad5hr- SIVsmH4 env/rev plus Ad5hr-SIVmac239 nefΔ1-13, exhibited significant cellular immune responses to Env compared to controls during priming (P = 0.0056 and 0.016, respectively) and boosting (P = 0.021 and 0.0047). Within-group variability in level of immune response at individual time points is attributed to inclusion of both responder and nonresponder macaques in this analysis as well as different kinetics of immune responses among the animals, with some macaques exhibiting peak responses 2 weeks postimmunization and others 10 weeks postimmunization. In general, the response to Env peptides differed considerably among the four groups in terms of the potency of the cellular immune response. Group II macaques (Fig. 3A) developed the highest response following the second recombinant administration, peaking at a mean of 2,500 SFC/106 PBMC. This was in contrast to the very modest response to Env peptides seen in group I macaques (Fig. 3C) immunized with only Ad5hr-SIVsmH4 env/rev. In fact, the response to Env by group II macaques was significantly enhanced during priming compared to that of group I macaques (P = 0.0056), and this enhancement was maintained out to 38 weeks post-initial immunization (P = 0.012). The level of the Env response was also higher in group III (Fig. 3B) than in group I macaques, but the difference was not statistically significant. Interestingly, when all three recombinants were given to group IV macaques (Ad5hr-SIVsmH4 env/rev, Ad5hr-SIVmac239 gag, and Ad5hr-SIVmac239 nefΔ1-13), the mean Env response was both lower and delayed (Fig. 3D) compared to that in groups II and III, although this down-modulation did not reach statistical significance.
FIG. 3.
Modulation of IFN-γ secretion in response to Env peptide stimuli. Ad5hr-SIV recombinants administered to the various immunization groups are denoted by the inserted SIV genes. Line graphs reflect average SFC per 106 PBMC ± the standard error of the mean at each time point among all macaques in the indicated group. Arrows denote Ad5hr-SIV recombinant immunizations at weeks 0 and 12 and gp120 boosts at weeks 24 and 36.
The rev gene was also expressed in all 39 macaques immunized with the Ad5hr-SIVsmH4 env/rev recombinant, so responses to Rev were evaluated among all four immunization groups. As illustrated in Table 1, responses to Rev were among the lowest observed. This low level of reactivity, together with considerable variability among macaques within immunization groups as discussed above for Env, resulted in significant cellular immune responses when compared to controls only in group III macaques during priming (P = 0.028; data not shown). During the boosting phase, both group II and group III responses were significantly higher than controls (P values of 0.011 and 0.034, respectively). Modulation in Rev responses between immunization groups was not detected.
Sixteen macaques received the Ad5hr-SIVmac239 nefΔ1-13 recombinant, either with Ad5hr-SIVsmH4 env/rev (group III) (Fig. 4A) or with both Ad5hr-SIVsmH4 env/rev and Ad5hr-SIVmac239 gag (group IV) (Fig. 4B). IFN-γ secretion in response to Nef peptides was significantly elevated above control levels in group III macaques during priming (Fig. 4A) (P = 0.0005) and boosting (P = 0.0009). In contrast, when all three recombinants were administered (group IV) (Fig. 4B), significant down-modulation of the Nef response was seen during priming (P = 0.03). This effect did not extend into the boosting period.
FIG. 4.
Modulation of IFN-γ secretion in response to Nef peptide stimuli. Data are illustrated as described in the legend to Fig. 3.
A similar down-modulation of the Gag response during priming was seen when comparing the seven group II macaques (immunized with Ad5hr-SIVsmH4 env/rev and Ad5hr-SIVmac239 gag) (Fig. 5A) with the eight group IV macaques (immunized with Ad5hr-SIVsmH4 env/rev, Ad5hr-SIVmac239 gag, and Ad5hr-SIVmac239 nefΔ1-13) (Fig. 5B). Statistical significance was not reached, however, due to high within-group variability in responses again seen among the group II macaques. Conversely, Gag-specific responses in group IV macaques were significantly elevated above controls during the boosting period (P = 0.012), with a consequent disappearance of the down-modulation trend. It is notable that for the group II macaques, potent IFN-γ secretion was detected after just one Ad5hr-recombinant immunization, and the response was further boosted with the second priming administration significantly above controls (P = 0.0093). Elevated responses above control values were maintained for much of the boosting period (P = 0.012). In contrast to Env, Rev, and Nef responses for which cryopreserved cells were assayed for the week 0 to 10 time points, all Gag responses were evaluated using fresh PBMC, which provide greater sensitivity in ELISPOT assays in comparison to viably frozen cells (63).
FIG. 5.
Modulation of IFN-γ secretion in response to Gag peptide stimuli. Data are illustrated as described in the legend to Fig. 3.
The diminution of immune responses seen in group IV macaques immunized with all three Ad5hr-SIV recombinants in comparison to either group II or group III macaques, which received two Ad5hr-SIV recombinants in addition to Ad5hr-vector alone, occurred only during the priming period of the immunization regimen (Fig. 3, 4, and 5). No statistically significant down-modulation was seen between response levels of group IV macaques in comparison to either group II or group III animals during boosting. In contrast, as discussed above, the enhanced response to Env peptides seen in group II macaques in comparison to group I was maintained throughout the 38-week immunization period.
T-cell proliferative responses.
In view of the significant modulation of IFN-γ secretion in response to individual SIV antigens depending on the combination of immunogens administered, it was of interest to determine if CD4+ T-cell proliferative responses elicited by the vaccine regimen were similarly modulated. Therefore, viably frozen PBMC from all immunization groups were evaluated for responses to SIV gp120 protein and from immunization groups II and IV for responses to SIV p27. The results are summarized in Table 2. Overall, proliferative responses to both gp120 and p27 stimuli were low, although the use of frozen rather than fresh cells could have resulted in the low responses. Given this, definitive conclusions concerning modulation between groups should await confirmation using fresh cells. Nevertheless, while IFN-γ-secreting cells specific for Env were significantly more numerous among group II immunized macaques compared to group I (Fig. 3A and C), this enhancement was not observed in proliferative assays with gp120 stimuli at the 6-week time point, when cells from both groups of macaques were available for evaluation (Table 2). In addition, while secretion of IFN-γ in response to Gag peptides tended to be down-modulated during the priming period in group IV compared to group II macaques (Fig. 5), no such down-modulation was observed in the proliferative responses to p27 during the same period (Table 2).
TABLE 2.
Proliferative responses of immunized macaques to SIV gp120 and p27
| Response type and macaque groupa | Post-first Ad5hr recombinants (wk 6)
|
Post-second Ad5hr recombinants (wk 18)
|
Post-first gp120 (wk 30)
|
|||
|---|---|---|---|---|---|---|
| No. positive/no. tested (%) | Mean SI ± SEMb | No. positive/no. tested (%) | Mean SI ± SEM | No. positive/no. tested (%) | Mean SI ± SEM | |
| SIV gp120 responses | ||||||
| I, E/R | 0/8 (0) | 0 | NDc | ND | 4/8 (50) | 4.2 ± 1.0 |
| II, E/R+G | 0/7 (0) | 0 | 1/7 (14) | 2.4 | ND | ND |
| III, E/R+N | 1/8 (12) | 2.2 | 5/8 (63) | 4.1 ± 0.7 | 4/8 (50) | 3.4 ± 0.2 |
| IV, E/R+G+N | 2/8 (25) | 2.2 ± 0.2 | 4/8 (50) | 3.6 ± 0.6 | 5/8 (63) | 3.5 ± 0.7 |
| SIV p27 responses | ||||||
| II, E/R+G | 0/7 (0) | 0 | 2/7 (29) | 3.2 ± 0.8 | ND | ND |
| IV, E/R+G+N | 4/8 (50) | 4.0 ± 0.7 | 4/8 (50) | 4.8 ± 1.6 | 5/8 (63) | 3.8 ± 0.9 |
Ad5hr-SIV recombinant inserted genes are abbreviated E/R for env/rev, G for gag, and N for nef.
Mean SI ± the standard error of the mean (SEM) values are reported for positive responders only.
ND, not determined.
Humoral immune responses.
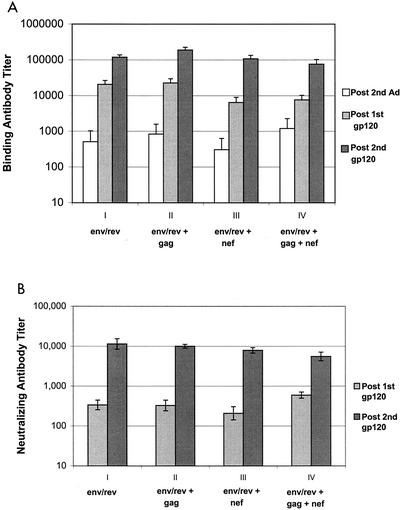
Live viral vectors not only elicit cell-mediated immunity but also prime humoral immune responses. To determine if the modulation of IFN-γ secretion seen between immunization groups in response to individual SIV antigens was reflected in subsequent antibody development, we assessed SIV-specific serum antibodies. Both high-titer anti-SIV gp120 binding and neutralizing antibodies were present in serum after boosting with SIV gp120, denoting effective priming by Ad5hr-SIVsmH4 env/rev recombinant immunization. Binding antibodies with titers of approximately 1,000 were first detected after the second Ad5hr-recombinant immunization. At this time, significantly enhanced IFN-γ secretion in response to Env peptides was observed in group II macaques compared to group I. However, no apparent difference in binding antibody titer between immunization groups was observed (Fig. 6A). With each successive gp120 booster immunization, approximate 10-fold increases in binding titers were seen in all immunized macaques; however, again, no discernible differences in titers were seen between groups. The same pattern of response was repeated for neutralization titers, although neutralizing activity was not detectable until after the first gp120 booster immunization (Fig. 6B). After the second gp120 immunization, serum neutralization titers were boosted 10- to 20-fold, but no significant differences were seen between groups.
FIG. 6.
Lack of modulation of vaccine-induced humoral immune responses. (A) Serum binding titers to SIV gp120. Mean titers ± standard errors of the means are shown for each immunization group, tested 2 weeks after each indicated time point. (B) Neutralizing antibody titers ± standard errors of the means against T-cell line-adapted SIVmac251 are shown for each immunization group at 2 weeks following the gp120 boosts.
Ad replication and immunity.
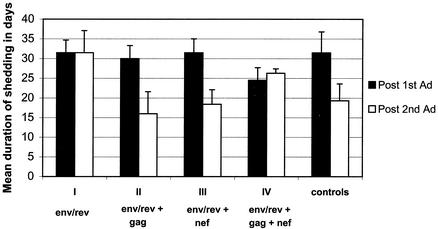
Modulation of immune responses among immunization groups was evident during the period of Ad5hr-SIV recombinant immunization and was restricted to effects on SIV-specific IFN-γ-secreting cells rather than on proliferative responses or antibody development. Therefore, we investigated whether the effect arose from differences in replication of the Ad5hr viruses in vivo following administration to the four immunization groups. In order to maintain an equivalent dose of Ad5hr antigen among all macaque groups, we included Ad5hr-E3-deleted vector where needed to keep the total Ad5hr dose at 1.5 × 109 PFU. For example, group I macaques received 5 × 108 PFU of the Ad5hr-SIVsmH4 env/rev recombinant and 109 PFU of the Ad5hr-E3-deleted vector, whereas group IV macaques received 5 × 108 PFU of each of three Ad5hr-SIV recombinants. Replication within each immunization group was evaluated by duration of shedding of Ad5hr virus in nasal secretions and stool as described in Materials and Methods. As illustrated in Fig. 7, differences in Ad5hr virus replication in nasal secretions among immunization groups were not observed and could not account for the down-modulation observed in comparing group II and group III macaques with group IV macaques. The shedding durations observed for all macaques following the first Ad5hr-recombinant administrations were all very similar. While greater variability in replication was observed following the second administrations, no significant differences were observed. Moreover, the trends seen could not be resolved with the observed differences in immune responses. Notably, following the second Ad5hr-SIV recombinant immunizations, group IV macaques exhibited somewhat higher vaccine virus replication than did groups II and III, yet immune responses were down-modulated in group IV macaques. The Ad5hr viruses did not replicate in the gut to the same degree as in the respiratory tract. Low-level shedding in stool, ranging from 4 to 7 days, was detected for all groups, with no significant differences between groups in terms of duration of replication (data not shown). Therefore, modulation of the immune response between groups was not due to differences in replication of Ad5hr recombinants in the intestinal tract.
FIG. 7.
Ad replication within immunization groups. Ad replication was assessed by determining the duration of viral shedding into nasal secretions, as described in Materials and Methods. The mean duration ± the standard error of the mean is shown.
DISCUSSION
The primary goal of this study was to evaluate the immunogenicity of a multicomponent SIV vaccine approach by using replication-competent Ad5hr-SIV recombinants for priming together with SIV gp120 protein boosts. An advantage of the experimental design was the relatively large number of macaques per immunization group (seven or eight), providing power for statistical analysis. In particular, all 31 immunized macaques received the Ad5hr-SIVsmH4 env/rev recombinant, and 15 or 16 received Ad5hr-SIVmac239 gag and/or Ad5hr-SIVmac239 nefΔ1-13, resulting in meaningful determinations of response levels and frequencies. Overall, the Ad5hr recombinant priming regimen elicited potent cellular immune responses to each SIV immunogen as assessed by ELISPOT assay for IFN-γ-secreting cells. The highest response levels were observed using Env and Gag peptide stimuli, but strong responses were also exhibited in response to Rev and Nef peptides, notable in view of the small size of these proteins and hence fewer CTL epitopes. The small number of Mamu-A*01-positive macaques in this study (two/group) precluded between-group analyses of tetramer binding responses by their CD8+ T cells. However, we observed elicitation of potent, persistent tetramer binding and ELISPOT responses to the dominant p11c Gag and subdominant p15m and p54m Env epitopes as summarized elsewhere (37).
Interestingly, among immunized groups most cellular immune responses did not peak until 10 weeks following each Ad immunization. Similar delayed kinetics have been observed in ELISPOT responses of the Mamu-A*01 macaques to specific Gag p11C and Env p15m and p54m epitopes (37). A slower or persistent release of antigen over a longer time period resulting from use of replicating Ad recombinants may account for this gradual increase in IFN-γ-secreting cells.
This is the first study in which the Ad5hr-SIVmac239 nefΔ1-13 recombinant was used as a vaccine component, and it proved to be highly immunogenic. A high frequency of macaques responded to all antigens, but more responded to Env and Gag than to Nef and Rev. Sixty-seven percent responded to the Rev-independent Gag immunogen, and 90% responded to Rev-dependent Env. The latter result suggests that the native Ad promoter together with the replication competence of the recombinant vaccine leads to significant expression of inserted foreign genes. The potent cellular immunity elicited by the vaccine regimen was also persistent, with IFN-γ secretion observed for 42 weeks over the duration of immunization period.
The cellular immune responses induced by the replication-competent Ad5hr-SIV recombinant priming-subunit boosting regimen utilized here compare favorably with those achieved by other approaches. This is most easily seen in comparing IFN-γ secretion induced by the Ad5hr-SIV recombinant expressing Rev-independent gag with other vaccine regimens that have used SIV gag optimized for expression in human cells. The cellular immune response to Gag peptide pools was equivalent to or higher than that reported in a recent study in which DNA priming was followed by replication-incompetent Ad5-SIV gag boosting in rhesus macaques (reference 57 and supplemental information). Three SIV gag DNA priming immunizations followed by one Ad5-SIV gag boost resulted in mean ELISPOT values of 855 SFC/106 PBMC. Here, in comparison, after one immunization with replication-competent Ad5hr-SIV gag plus Ad5hr-SIV env/rev, Gag-specific ELISPOT responses averaged 700 SFC/106 PBMC and rose after the second Ad5hr-recombinant immunization to 1,200 SFC/106 PBMC (Fig. 5A). This latter value is also similar to reported values ranging from approximately 250 to 1,400 SFC/106 PBMC observed following three DNA-adjuvant priming immunizations and a low-dose (107 viral particles) nonreplicating Ad5 gag boost (13), but it is 2.5-fold lower than the highest values reported in the same study following a high-dose (1011 viral particles) Ad5 gag boost. Peak responses after two SIV gag/pol/env high-dose DNA priming immunizations and one recombinant modified vaccinia virus Ankara (rMVA) boost were twofold higher than our peak responses (2). Subsequently, higher Gag responses, fourfold greater than those reported here, were obtained for gag/pol DNA-primed, gag/pol rMVA-boosted macaques (1). It would be of interest to know if the multigene regimen (gag/pol/env) also modulated immune responses in the DNA/MVA system.
Although reported elsewhere (37), it is informative to summarize comparative results for the Mamu-A*01 macaques in this study. Their cellular responses also compared favorably with other vaccine approaches. The peak percentage of unstimulated CD8+ T cells able to bind p11C tetramer post-Ad5hr-SIV gag immunization ranged from 0.5 to 1.6%. This is comparable to some DNA-cytokine approaches (6) and greater than some alternate vector approaches (21, 53), but less than that achieved by two high-dose replication-defective Ad5-SIV gag immunizations (57) and by a DNA/MVA approach (2). In contrast, IFN-γ secretion in response to the p11C peptide was higher in our study following the two replicating Ad5hr-SIV gag immunizations (mean of 1,600 SFC/106 PBMC) than responses following two or three replication-defective Ad5-SIV gag immunizations (reference 57 and supplemental information).
That this SIV-specific immunity was achieved with sequential immunizations using an Ad recombinant of the same serotype suggests that initial Ad immunization does not preclude a further boost of immune responses by a second administration. Ad-neutralizing antibodies were detected in macaque sera after both immunizations; however, the mean titers (reported as reciprocal serum dilutions) in the various immunization groups were low, ranging from 5 to 30 (data not shown). Importantly, the immunization schedule provided for a 12-week delay before the second immunization, allowing time for the Ad antibody titers to decline. A significant time delay in sequential immunizations has also been shown to be beneficial in inducing memory cells (10).
Comparison of IFN-γ secretion in response to Env and Gag peptides by group II macaques in this study with that in macaques that received the same immunogens in a previous study (63) shows that a higher cellular response was elicited here. One technical explanation for this difference is that here fresh PBMC were evaluated, whereas cryogenically preserved cells were used in the earlier work. Previously frozen monkey PBMC have been shown to exhibit lower levels of cellular immune responses compared to fresh cells (63), perhaps due to poor preservation of some memory cells or antigen-presenting cells. More importantly, however, slightly different immunization routes were utilized for the two studies. In the previous study, the two sequential immunizations were given orally by stomach tube in the presence of sodium bicarbonate buffer and intranasally at the same time. Here, following a similar oral plus intranasal immunization, the second administration of Ad5hr-SIV recombinants was intratracheal, possibly targeting additional cells in the upper respiratory tract, leading to an enhanced immune response.
Ad's can also replicate in the gut, which could provide additional new target cells for Ad replication, and might be effective at enhancing mucosal immune responses. Ad5hr-SIV recombinant replication in rhesus macaques, however, as determined here and in previous studies by length of shedding in stool and nasal samples, has been poor in the intestine compared to the upper respiratory tract (63). It has not yet been determined whether the method of oral immunization used here allows the Ad5hr recombinants to survive the acid environment of the stomach and reach the intestine, or whether intestinal cells of rhesus macaques do not support high-level Ad5hr replication. A future goal will be to examine delivery of Ad5hr-SIV recombinants to the intestine, evaluate intestinal replication, and explore alternative methods for improved oral administration, perhaps by use of enteric-coated capsules, which have been proven effective in delivery of wild-type Ad4 and Ad7 vaccines to people (58, 59).
An unexpected finding of these studies was a modulation of the cellular immune response to individual SIV antigens depending on whether macaques were immunized with one, two, or three Ad5hr-SIV recombinant vaccines. This effect was limited to IFN-γ-secreting cells; there was no effect on CD4+ T-helper cell responses evaluated using cryopreserved cells or on antibody development. During Ad5hr-recombinant priming immunizations, enhancement of Env-specific IFN-γ secretion resulting from immunization with Ad5hr-SIVsmH4 env/rev in addition to either Ad5hr-SIVmac239 gag or SIVmac239 nefΔ1-13 was observed. The enhancement was statistically significant for macaques that received Ad5hr-SIVsmH4 env/rev and Ad5hr-SIVmac239 gag. This phenomenon might be explained by a higher level of activation due to increased cytokine production and proliferation in the doubly immunized macaques. SIV gp120, but not the transmembrane protein, is relatively deficient in T-helper epitopes. Multiple epitopes are also present in SIV Gag (54), which may have provided additional help.
More interesting was the significant down-modulation of IFN-γ secretion in response to Nef peptides in group IV macaques immunized with all three recombinants, compared to responses in group III macaques immunized with only two recombinants, Ad5hr-SIVsmH4 env/rev together with Ad5hr-SIV nefΔ1-13. Even though statistical significance was not reached, a diminished response to Env and Gag peptides was also observed in the group IV macaques immunized with all three recombinants, in comparison to the Env response of group II and group III macaques and the Gag response of group II macaques. Therefore, the down-modulation of responses was not attributable to specific expression of any single gene product: gag, nef, or env. Whether the down-modulation resulted from blocking of enhancement by the third recombinant immunization, rather than from a suppressive mechanism, must be determined with a different study design that includes macaques immunized with either the Ad5hr-SIV gag or Ad5hr-SIV nefΔ1-13 recombinant alone.
Due to the fact that the modulation effect was observed only during the period of Ad-recombinant immunization, we considered that differences in replication of the Ad5hr viruses might be responsible. Assessment of Ad5hr-recombinant replication in the various macaques by determining the duration of Ad shedding in nasal samples did not reveal significant differences among the immunization groups, including the control macaques. It is possible that competition among processed antigens for presentation and/or subsequent T-cell stimulation might account for the phenomenon. However, importantly, the down-modulation was no longer apparent following the protein subunit booster immunizations. This suggests a replicating, persistent vector, by presenting antigen over a prolonged time period and providing a mechanism to target new cells following a single immunization, may confer an advantage in alleviating effects of competition among multicomponent vaccines for immune system components. The vaccine regimen employed here, by including booster immunizations 12 and 24 weeks following the last Ad5hr-recombinant immunization, effectively provided time for maximum presentation of all vaccine priming components to the immune system. The lengthy vaccine regimen has the further advantage, as mentioned above, of better inducing memory cells (10).
Kjerrstrom et al. (32) have reported that expression of the Nef protein was inhibited and T-cell proliferative responses to Rev and Nef were down-modulated when mice were immunized with plasmid DNA vaccines encoding Tat, Rev, and Nef, in comparison to mice immunized with single DNA plasmids. The authors suggested that modulation of protein expression was attributable to competition for transcription factors or effects of unknown enhancer elements on the individual genes. Modulation of the immune responses was discussed in terms of functional effects of the three regulatory proteins. In our study, down-modulation of the cellular immune response could not be attributed to any single inserted gene. With regard to Nef, its functions that might impair immune responses (down-regulation of CD4 and major histocompatibility complex class I) are abolished in a mutant such as Ad5hr-SIVmac239 nefΔ1-13, with the N-terminal region deleted and lacking the myristoylation site (47), and so another mechanism is likely involved.
Here we have shown that sequential immunization with replication-competent Ad5hr-SIV recombinants elicits potent, persistent cellular immune responses. At the same time, we observed significant modulation of immune responses depending on the complexity of the vaccine administered. Significant enhancement of SIV Env cellular responses was observed when both Ad5hr-SIV env/rev and Ad5hr-SIV gag were administered together as opposed to Ad5hr-SIV env/rev alone, arguing for the use of at least two recombinants to induce optimal immunity. On the other hand, that down-modulation occurred following administration of multicomponent (three recombinant) vaccines is highly relevant, especially in view of the current use of similar approaches involving multiple antigens that are believed necessary for mounting effective protective efficacy. The potential for immune modulation should be considered and evaluated in designing and testing future multicomponent vaccines. In our vaccine regimen, however, down-modulation occurred only during the initial period of priming with replication-competent Ad5hr recombinants and did not persist through the subsequent boosting period. We suggest that live, replicating vectors, by presenting antigen over prolonged time periods and targeting new cells, may alleviate competition within the immune system for vaccine immunogens and be advantageous in addressing this issue.
Acknowledgments
We acknowledge the technical assistance of Sonia Grebogi and of Marsha Sowers and Steve Harbaugh for carrying out all animal procedures. We thank Wyeth-Lederle Vaccines for the MPL-SE adjuvant; Larry Arthur and Julian Bess for supplying the aldrithiol-2-inactivated SIV; James Hoxie for kindly providing the anti-SIV Nef monoclonal antibody N27; and Narayan Bhat and Vijaya Gowda for expansion and purification of the Ad5hr-SIV recombinants. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Gag peptides (complete set); p239SpE3′ nef Open from Ronald Desrosiers, Jim Gibbs, and Dean Regier; and SIVmac251 Nef monoclonal (17.2) from Kai Krohn and Vladimir Ovod.
REFERENCES
- 1.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T.-C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyeri, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 7.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce, C. B., A. Akrigg, S. A. Sharpe, T. Hanke, G. W. Wilkinson, and M. P. Cranage. 1999. Replication-deficient recombinant adenoviruses expressing the human immunodeficiency virus Env antigen can induce both humoral and CTL immune responses in mice. J. Gen. Virol. 80:2621-2628. [DOI] [PubMed] [Google Scholar]
- 10.Bruna-Romero, O., G. Conzalez-Asequinolaza, J. C. R. Hafalla, M. Tsuji, and R. S. Nussenzweig. 2001. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98:11491-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buge, S. L., L. Murty, K. Arora, V. S. Kalyanaraman, P. D. Markham, E. S. Richardson, K. Aldrich, L. J. Patterson, C. J. Miller, S. Cheng, and M. Robert-Guroff. 1999. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251. J. Virol. 73:7430-7440. (Erratum, 73: 9692.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buge, S. L., E. Richardson, S. Alipanah, P. Markham, S. Cheng, N. Kalyan, C. J. Miller, M. Lubeck, S. Udem, J. Eldridge, and M. Robert-Guroff. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 71:8531-8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casimiro, D. R., L. Chen, T. Fu, R. K. Evans, M. J. Caulfield, M. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Z. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanda, P. K., R. J. Natuk, B. B. Mason, B. M. Bhat, L. Greenberg, S. K. Dheer, K. L. Molnar-Kimber, S. Mizutani, M. D. Lubeck, and A. R. Davis. 1990. High level expression of the envelope glycoproteins of the human immunodeficiency virus type 1 in presence of rev gene using helper-independent adenovirus type 7 recombinants. Virology 175:535-547. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, H., J. Hoxie, and W. P. Parks. 1999. The conserved core of human immunodeficiency virus type 1 Nef is essential for association with LCK and for enhanced viral replication in T-lymphocytes. Virology 264:5-15. [DOI] [PubMed] [Google Scholar]
- 16.Cheng, S. M., S. G. Lee, M. Ronchetti-Blume, K. P. Virk, S. Mizutani, J. W. Eichberg, A. Davis, P. P. Hung, V. M. Hirsch, and R. M. Chanock. 1992. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J. Virol. 66:6721-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chengalvala, M., M. D. Lubeck, A. R. Davis, S. Mizutani, K. Molner-Kimber, J. Morin, and P. P. Hung. 1991. Evaluation of adenovirus type 4 and 7 recombinant hepatitis B vaccines in dogs. Vaccine 9:485-489. [DOI] [PubMed] [Google Scholar]
- 18.Cohen, J. 2002. Monkey puzzles. Science 296:2325-2326. [DOI] [PubMed] [Google Scholar]
- 19.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 21.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology 294:270-281. [DOI] [PubMed] [Google Scholar]
- 22.Ferrantelli, F., and R. M. Ruprecht. 2002. Neutralizing antibodies against HIV B back in the major leagues? Curr. Opin. Immunol. 14:495-502. [DOI] [PubMed] [Google Scholar]
- 23.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs, J. S., D. A. Regier, and R. C. Desrosiers. 1994. Construction and in vitro properties of SIVmac mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retrovir. 10:607-616. [DOI] [PubMed] [Google Scholar]
- 25.Hel, Z., J. Nacsa, E. Tryniszewska, W.-P. Tsai, R. Washington-Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and post-challenge CD4+ and CD8+ T cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg, Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800-802. [Google Scholar]
- 27.Hofmann-Lehmann, R., J. Vlaasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T.-C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz, M. S. 1996. Adenoviruses, p. 2149-2171. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Field's virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 30.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high viral loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 32.Kjerrstrom, A., J. Hinkula, G. Engstrom, V. Ovod, K. Krohn, R. Benthin, and B. Wahren. 2001. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology 284:46-61. [DOI] [PubMed] [Google Scholar]
- 33.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubeck, M. D., R. J. Natuk, M. Chengalvala, P. K. Chanda, K. K. Murthy, S. Murthy, S. Mizutani, S.-G. Lee, M. S. Wade, B. M. Bhat, R. Bhat, S. K. Dheer, J. W. Eichberg, A. R. Davis, and P. P. Hung. 1994. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res. Hum. Retrovir. 10:1443-1449. [DOI] [PubMed] [Google Scholar]
- 36.Lubeck, M. D., R. J. Natuk, M. Myagkikh, N. Kalyan, K. Aldrich, F. Sinangil, L. Alipanah, S. C. S. Murthy, P. K. Chanda, S. Nigida, P. D. Markham, S. Zolla-Pazner, K. Steimer, M. Wade, M. S. Reitz, Jr., L. O. Arthur, S. Mizutani, A. Davis, P. Hung, R. C. Gallo, J. Eichberg, and M. Robert-Guroff. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651-658. [DOI] [PubMed] [Google Scholar]
- 37.Malkevitch, N., L. J. Patterson, K. Aldrich, E. Richardson, W. G. Alvord, and M. Robert-Guroff. 2003. A replication competent Ad5hr-SIV recombinant priming/subunit boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity in Mamu-A*01 rhesus macaques. J. Immunol. 170:4281-4289. [DOI] [PubMed] [Google Scholar]
- 38.Mascola, J. R., G. Stiefler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 39.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natuk, R. J., P. K. Chanda, M. D. Lubeck, A. R. Davis, J. Wilhelm, R. Hjorth, M. S. Wade, B. M. Bhat, L. Mizutani, S. Lee, J. Eichberg, R. C. Gallo, P. P. Hung, and M. Robert-Guroff. 1992. Adenovirus-human immunodeficiency virus (HIV) envelope recombinant vaccines elicit high-titered HIV neutralizing antibodies in the dog model. Proc. Natl. Acad. Sci. USA 89:7777-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natuk, R. J., M. D. Lubeck, P. K. Chanda, M. Chengalvala, M. S. Wade, S. C. S. Murthy, J. Wilhelm, S. K. Vernon, S. K. Dheer, S. Mizutani, S.-G. Lee, K. K. Murthy, J. W. Eichberg, A. R. Davis, and P. P. Hung. 1993. Immunogenicity of recombinant human adenovirus-human immunodeficiency virus vaccines in chimpanzees. AIDS Res. Hum. Retrovir. 9:395-404. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 43.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parren, P. W. H. I., P. A. Marx, A. J. Hessell, J. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson, L. J., B. Peng, X. Nan, and M. Robert-Guroff. Live adenovirus recombinants as vaccine vectors. In M. M. Levine, J. B. Kaper, R. Rappuoli, M. Liu, and M. F. Good (ed.), New generation vaccines, 3rd ed. Marcel Dekker, Inc., New York, N.Y., in press.
- 46.Patterson, L. J., F. Robey, A. Muck, K. VanRemoortere, K. Aldrich, E. Richardson, W. G. Alvord, P. D. Markham, M. Cranage, and M. Robert-Guroff. 2001. A conformational C4 peptide polymer vaccine coupled with live recombinant vector priming is immunogenic but does not protect against rectal SIV challenge. AIDS Res. Hum Retrovir. 17:837-849. [DOI] [PubMed] [Google Scholar]
- 47.Peng, B., and M. Robert-Guroff. 2001. Deletion of N-terminal myristoylation site of HIV nef abrogates both MHC-1 and CD4 down-regulation. Immunol. Lett. 78:195-200. [DOI] [PubMed] [Google Scholar]
- 48.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 49.Robert-Guroff, M., H. Kaur, L. J. Patterson, M. Leno, A. J. Conley, P. M. McKenna, P. D. Markham, E. Richardson, K. Aldrich, K. Arora, L. Murty, L. Carter, S. Zolla-Pazner, and F. Sinangil. 1998. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 72:10275-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. C. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 52.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santra, S., J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, C. I. Lord, R. Pal, G. Franchini, and N. L. Letvin. 2002. Recombinant canarypox vaccine-elicited CTL specific for dominant and subdominant simian immunodeficiency virus epitopes in rhesus monkeys. J. Immunol. 168:1847-1853. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar, S., V. Kalia, M. Murphey-Corb, and R. C. Montelaro. 2002. Detailed analysis of CD4+ Th responses to envelope and Gag proteins of simian immunodeficiency virus reveals an exclusion of broadly reactive Th epitopes from the glycosylated regions of envelope. J. Immunol. 168:4001-4011. [DOI] [PubMed] [Google Scholar]
- 55.Sawai, E. T., M. S. Hamza, M. Ye, K. E. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 57.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. E. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 58.Takafuji, E. T., J. C. Gaydos, R. G. Allen, and F. H. Top, Jr. 1979. Simultaneous administration of live, enteric-coated adenovirus types 4, 7, and 21 vaccines: safety and immunogenicity. J. Infect. Dis. 140:48-53. [DOI] [PubMed] [Google Scholar]
- 59.Top, F. H., Jr., E. L. Buescher, W. H. Bancroft, and P. K. Russell. 1971. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J. Infect. Dis. 124:155-160. [DOI] [PubMed] [Google Scholar]
- 60.Wei, L. J., and W. E. Johnson. 1985. Combining dependent tests with incomplete repeated measurements. Biometrika 72:359-364. [Google Scholar]
- 61.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida, T., K. Okuda, K. Q. Xin, K. Tadokoro, J. Fukushima, S. Toda, E. Hagiwara, K. Hamajima, T. Koshino, and T. Saito. 2001. Activation of HIV-1-specific immune responses to an HIV-1 vaccine constructed from a replication-defective adenovirus vector using various combinations of immunization protocols. Clin. Exp. Immunol. 124:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao, J., Y. Lou, J. Pinczewski, N. Malkevitch, K. Aldrich, V. S. Kalyanaraman, D. Venzon, B. Peng, L. J. Patterson, Y. Edghill-Smith, R. Woodward, G. N. Pavlakis, and M. Robert-Guroff. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIV env/rev and BSIV gag recombinants. Vaccine, in press. [DOI] [PubMed]
- 64.Zhao, J., J. Pinczweski, V. R. Gomez-Roman, D. Venzon, V. S. Kalyanaraman, P. D. Markham, K. Aldrich, M. Moake, D. C. Montefiori, Y. Lou, G. N. Pavlakis, and M. Robert-Guroff. 2003. Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIVmac251 challenge by a replication-competent Ad5hr-SIV env/rev and Ad5hr-SIV gag recombinant priming/gp120 boosting regimen. J. Virol. 77:8354-8365. [DOI] [PMC free article] [PubMed]
- 65.Zolla-Pazner, S., M. Lubeck, S. Xu, S. Burda, R. J. Natuk, F. Sinangil, K. Steimer, R. C. Gallo, J. W. Eichberg, T. Matthews, and M. Robert-Guroff. 1998. Induction of neutralizing antibodies in chimpanzees to T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime/boost vaccine regimen in chimpanzees. J. Virol. 72:1052-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]