Abstract
The Sindbis virus (Alphavirus; Togaviridae) strain MRE16 efficiently infects Aedes aegypti mosquitoes that ingest a blood meal containing 8 to 9 log10 PFU of virus/ml. However, a small-plaque variant of this virus, MRE16sp, poorly infects mosquitoes after oral infection with an equivalent titer. To determine the genetic differences between MRE16 and MRE16sp viruses, we have sequenced the MRE16sp structural genes and found a 90-nucleotide deletion in the E2 glycoprotein that spans the 3′ end of the coding region for the putative cell-receptor binding domain (CRBD). We examined the role of this deletion in oral infection of mosquitoes by constructing infectious clones pMRE16icΔE200-Y229 and pMRE16ic, representing MRE16 virus genomes with and without the deletion, respectively. A third infectious clone, pMRE16icΔE200-C220, was also constructed that contained a smaller deletion extending only to the 3′ terminus of the CRBD coding region. Virus derived from pMRE16ic replicated with the same efficiency as parental virus in vertebrate (BHK-21) and mosquito (C6/36) cells and orally infected A. aegypti. Viruses derived from pMRE16icΔE200-Y229 and pMRE16icΔE200-C220 replicated 10- to 100-fold less efficiently in C6/36 and BHK-21 cells than did MRE16ic virus. Each deletion mutant poorly infected A. aegypti and dramatically reduced midgut infectivity and dissemination. However, all viruses generated nearly equal titers (∼6.0 log10 PFU/ml) in mosquitoes 4 days after infection by intrathoracic inoculation. These results suggest that the deleted portion of the E2 CRBD represents an important determinant of MRE16 virus midgut infectivity in A. aegypti.
Sindbis (SIN) viruses are cycled principally between Culex species of mosquitoes and avian vertebrate hosts (6, 7, 37). SIN viruses also have been isolated from Aedes species of mosquitoes (7). The general features of arthropod-borne virus infection of mosquitoes have been described previously (13). The virus enters the lumen of the midgut with ingestion of a blood meal and replicates in the midgut epithelial cells. Virus then escapes the midgut, enters the hemolymph, and disseminates to other tissues, including head and salivary glands. Following multiplication in the salivary glands, virus is transmitted through saliva to a susceptible vertebrate host. However, our understanding of the molecular determinants of vector-pathogen interaction is minimal.
SIN viruses have a positive-sense, single-stranded RNA genome (11.7 kb) with a 5′ cap and a poly(A) tail (36). The 5′ two-thirds of the genome translates two, N-coterminal nonstructural polyproteins that are posttranslationally cleaved to form the viral replication machinery. Translation of the 3′ third of the genome requires the transcription of subgenomic 26S RNA from the full-length negative-sense RNA intermediate and generates the structural proteins. The 26S RNA is translated into a polyprotein that is co- and posttranslationally cleaved to form the viral capsid (C) protein and envelope glycoproteins (E1 and E2). The glycoproteins of alphaviruses are inserted into a host-derived lipid envelope and are present on the surface of the virion as spike proteins, each composed of three E1-E2 heterodimers. The E2 glycoprotein contains epitopes important for host tropism, receptor recognition, virus neutralization, and virulence (8, 11, 20, 21, 36), while the E1 glycoprotein is important in fusion of the virus envelope with host intracellular membranes (36). Amino acid residues 170 to 220 (SIN virus AR339 numbering) of E2 are postulated to constitute a cell-receptor binding domain (CRBD) in the glycoprotein. Physical properties of the domain and the results of several studies predict that it is exposed on the surface of the viral particle (5, 32-36, 42). Anti-idiotypic antibody and virus mutant studies have established the importance of the region to cell binding (23, 35, 39, 41, 42). Studies have also demonstrated that amino acid changes within this domain affect the ability of an alphavirus to infect different vertebrate cell types (3, 36, 38) and productively infect the arthropod vector (45).
The prototype SIN virus strain, AR339, and viruses derived from this strain have limited infection and transmission potential in Aedes aegypti, the vector of yellow fever and dengue viruses. Approximately 40% of mosquitoes orally infected with AR339 virus develop disseminated infections in head tissues by 14 days postinfection (p.i.) and then are competent to transmit the virus to newborn mice (17, 30). AR339 virus generally produces a limited infection of midguts following oral or parenteral infection of A. aegypti (K. M. Myles, D. J. Pierro, and K. E. Olson, unpublished data). A SIN virus strain based on the mouse neurovirulent TE12 virus (22) also shows restricted midgut infection in A. aegypti, with less than 20% dissemination at 14 days p.i. (31). However, a Malaysian SIN virus strain, MRE16, readily infects midguts of A. aegypti and disseminates in nearly 100% of the mosquitoes by 14 days postfeeding (31). Nucleotide sequence analyses of MRE16 genomic RNA show a close genetic relationship between MRE16 and other SIN viruses within the Oriental/Australian genetic group (29, 31). It has been previously shown that the determinants for this enhanced infection phenotype in A. aegypti resides in the structural genes of MRE16 virus (24, 31), and it has been further shown that determinants for enhanced oral infection reside in the E2 glycoprotein (28).
In the present study we have identified a small-plaque variant of MRE16 virus, termed MRE16sp, which poorly infects A. aegypti by the oral route. Sequence analysis of MRE16sp structural genes shows that the virus genome contains a 90-nucleotide deletion (amino acids E-200 to Y-229) in the E2 glycoprotein gene which includes 63 deleted nucleotides of the CRBD coding region. By using a full-length MRE16 cDNA infectious clone (pMRE16ic) we show that two separate deletions, one encompassing residues E-200 to Y-229 and a second shorter deletion encompassing E-200 to C-220, of E2 have a significant effect on the oral infectivity of MRE16 virus for A. aegypti mosquitoes. These results demonstrate the importance of the putative E2 CRBD for alphavirus midgut infectivity and dissemination in the mosquito.
MATERIALS AND METHODS
Cells and medium.
Baby hamster kidney (BHK-21), Aedes albopictus (C6/36) (16), and African green monkey kidney cells (Vero) were grown in a solution of minimal essential medium (MEM) containing 10% fetal bovine serum (FBS), 1× nonessential amino acids (NEAA) for MEM, 292 μg of l-glutamine/ml, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. BHK-21 and Vero cells were maintained at 37°C. C6/36 cells were maintained at 28°C.
Viruses.
MRE16 virus was isolated in AP61 cells (40) and was passaged six times in AP61 cells and five times in C6/36 cells. MRE16sp virus was plaque purified in Vero cells and then was grown in C6/36 cells. Clone-derived viruses were produced by in vitro transcription of linearized plasmid DNA and electroporation into BHK-21 cells. Virus stocks were titered by plaque assay on Vero cell monolayers. MRE16, MRE16ic, MRE16icΔE200-Y229, and MRE16ic ΔE200-C220 virus working stocks contained 9.6, 9.4, 8.6, and 8.1 log10 PFU/ml, respectively. MRE16sp virus C6/36-1 seed contained 9.0 log10 PFU/ml.
Sequencing of MRE16 virus genome and MRE16sp structural genes.
Primers for reverse transcriptase PCR (RT-PCR) were designed from a consensus sequence generated from six previously published SIN virus or SIN virus-like genome sequences and a previously published MRE16 sequence (31). Genomic RNA was isolated from MRE16 virus seed by using a QIAamp viral RNA mini kit as recommended by the manufacturer (Qiagen, Valencia, Calif.). Primers were used to transcribe and amplify six overlapping cDNA fragments from the nonstructural gene region (nsP1-nsP4) and the 5′ noncoding region (NCR). RNA was transcribed into cDNA by using Roche Biochemicals' (Indianapolis, Ind.) Titan one-tube RT-PCR system. The RT-PCR products were TA cloned into the pCR2.1 vector (Invitrogen, Carlsbad, Calif.). Automated DNA sequencing was performed as recommended by Applied Biosystems (310 Gene Analyzer; Foster City, Calif.) and Perkin-Elmer (Shelton, Conn.). The 5′ terminus of the genome was cloned and sequenced by using Ambion's (Austin, Tex.) First Choice RLM-RACE kit as recommended. The 3′-terminal sequence had been previously reported (31). New sequencing primers efficiently spaced to span the entire genome were designed on the basis of MRE16 sequence and previously published data (31). Genomic RNA was isolated from MRE16sp virus seed stock as described previously. The primers used in the construction of the MRE16 infectious clone were used in RT-PCRs to amplify overlapping cDNA fragments containing the region of the genome carrying the structural genes. The cDNA fragments were sequenced directly without cloning.
Construction of full-length MRE16 infectious cDNA clone.
Primers flanking the restriction sites to be used in the final assembly of the clone were designed from MRE16 sequence. Additional restriction sites were also engineered into the primers to facilitate cloning, including unique HindIII and PacI restriction sites as well as an SP6 promoter sequence upstream of the 5′ genomic terminus. An additional guanosine nucleotide was inserted between the SP6 promoter sequence and the 5′-terminal nucleotide of the genome. Unique restriction sites AscI and SacI were engineered 3′ of the poly(A) tail. The unique AscI site was engineered to permit linearization prior to transcription of RNA. These primers were used to amplify six overlapping cDNA fragments containing genome regions (nucleotides 1 to 1446 [EcoRV], 1446 to 3388 [BclI], 3388 to 5196 [FseI], 5196 to 7489 [AgeI], 7489 to 9757 [AvrII], 9757 to 11693]). The fragment carrying sequences for nsP1-nsP4 and the 5′ NCR were directly sequenced without cloning in both directions with the new sequencing primer set. Each of the amplified fragments was subcloned into the multiple cloning site of the pBRUC vector (19). At least one strand of two independent subclones was sequenced for each amplified region of the full-length MRE16 viral genome. The sequence of each subclone was compared to the viral sequence previously determined from overlapping uncloned RT-PCR amplicon fragments (5′ NCR, nsP1-nsP4) or previously published sequence (26S junction region, structural polyprotein, and 3′ NCR) (31). Subclones containing errors resulting in a difference in the deduced amino acid sequence were not used in the final assembly of the full-length cDNA clone. Silent nucleotide substitutions were retained as molecular markers to distinguish the clone-derived virus from the parental virus and are summarized in Table 1. The cDNA subclone-derived sequences differed from the previously published sequence data at several nucleotide positions, presumably as a result of passage history. Differences were not considered to have arisen through cloning error if they were present in the sequence of at least two independent subclones. Nucleotide differences between the full-length MRE16 cDNA clone and the previously published sequence data are summarized in Table 1. The full-length genome cDNA clone was constructed by sequentially ligating the fragments from the six subclones into the multiple cloning site of a single pBRUC plasmid (Fig. 1).
TABLE 1.
Summary of nucleotide and amino acid sequence differences between the MRE16 full-length infectious cDNA clone and its parental MRE16 virus or previously published sequence data for MRE16 virusa
| Nucleotide position | Nucleotide for:
|
Amino acid for:
|
|||
|---|---|---|---|---|---|
| MRE16ic | Parental virus or published data | MRE16ic | Parental virus or published data | Amino acid position | |
| 1279b | G | A | K | K | nsP1-407 |
| 8422 | G | A | E | E | Capsid-264 |
| 9476 | C | G | Q | E | E2-286 |
| 10011 | C | U | P | L | 6K-41 |
| 10021 | G | A | V | V | 6K-44 |
| 10858 | A | G | A | A | E1-268 |
| 10892 | C | G | L | V | EI-280 |
| 10930b | T | G | D | D | EI-292 |
For previously published sequence data see reference 31.
Error was incorporated during cloning.
FIG. 1.
Strategy for the final assembly of the full-length infectious cDNA clone MRE16ic. Six subclones contained the complete 11,693-nt-long genome, SP6 promoter, 5′ cap, and a poly(A) tail as overlapping amplified cDNA regions. The pBRUC vector was used for all cloning steps.
Site-directed mutagenesis and construction of MRE16ic ΔE200-Y229 and MRE16ic ΔE200-C220.
Site-directed mutagenesis and construction of MRE16ic ΔE200-Y229 and MRE16ic ΔE200-C220 was performed by using phosphorylated forward (5′AAAAGTGACCAGACAAAGTGGGTC3′) and reverse (5′GTACGTGACGTTCTTTCCAGAAGGGGGC3′) primers and the ExSite PCR-Based Site-Directed Mutagenesis kit (Stratagene) as recommended. A deletion mutant was generated lacking 30 contiguous amino acids from E2-200-Glu through E2-229-Tyr in pMRE16ic (Fig. 1). Another deletion mutant was generated lacking 21 contiguous amino acids from E2-200-Glu through E2-220-Cys in pMRE16ic by using phosphorylated forward (5′ACGGCATTGAAACAATGCATCGCC3′) and reverse (5′GTACGTGACGTTCTTTCCAGAAGGGGGC3′) primers. DNA sequences containing the deleted portions of E2 were then cloned into the AgeI and AvrII unique restriction sites of the full-length MRE16ic cDNA.
Mosquitoes.
A. aegypti Rexville D strain mosquitoes originating from Rexville, Puerto Rico (Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, Colo.) were reared and maintained at 28°C and 80% relative humidity with a photoperiod of 16 h of light and 8 h of darkness.
Oral infection of mosquitoes by artificial blood meal.
Confluent monolayers of C6/36 cells were infected with MRE16ic, MRE16icΔE200-Y229, or MRE16icΔE200-C220 electroporation supernatant at a multiplicity of infection of 0.01. At approximately 60 h p.i., infected cells were harvested and centrifuged (1,000 × g) for 3 min. For oral infection of mosquitoes with MRE16sp virus the C6/36-1 seed was used. Aliquots of the supernatant were stored at −70°C. The blood meal was formed by mixing 1.0 ml of sheep blood (Colorado Serum Co., Boulder, Colo.) with 1.0 ml of virus. The blood meal-virus mixture was warmed to 37°C and was pipetted into the chamber of a water-jacketed (37°C) glass membrane feeder (30). Mosquitoes (5 to 7 days posteclosion) were fed for approximately 1 h through a hog gut membrane. Blood meal samples were collected postfeeding for virus titration. Blood-fed mosquitoes were incubated by using conditions described previously (14). In addition, control A. aegypti mosquitoes were intrathoracically inoculated with 1.0 μl of virus (positive control) or MEM containing 10% FBS plus NEAA, l-glutamine, and antibiotics without virus (negative control) and were maintained at insectary conditions until analyzed (12).
Growth of viruses in mosquitoes.
Stock viruses were diluted in MEM containing 10% FBS plus NEAA, l-glutamine, and antibiotics to equal titers prior to intrathoracic inoculation into mosquitoes. A. aegypti mosquitoes were intrathoracically inoculated with either MEM containing 10% FBS, NEAA, l-glutamine, and antibiotics (negative control) or 10 to 15 PFU of MRE16ic, MRE16icΔE200-Y229, or MRE16icΔE200-C220 virus. Ten mosquitoes from each group were stored at −70°C at timed intervals of 0, 24, 48, and 96 h p.i. Frozen mosquitoes were later tritrated separately in 1 ml of diluent and were assayed for virus.
IFA analysis of mosquito tissues.
SIN virus E1 antigen was detected by an indirect immunofluorescence assay. Heads from infected or control mosquitoes were fixed on slides by immersion in cold acetone for 15 min. Tissues were incubated with mouse anti-SIN virus E1 monoclonal antibody (MAb) 30.11a (4) (1:200) for 40 min (37°C), washed twice in phosphate-buffered saline (PBS), and incubated with biotinylated sheep anti-mouse antibody (1:200; Amersham Corp., Arlington Heights, Ill.) for 40 min (37°C). Tissues were washed again and were incubated with fluorescein isothiocyanate-conjugated streptavidin (1:200; Amersham) for 20 min (37°C). Virus dissemination was determined following oral infection by detecting SIN virus E1 antigen in head tissues by using immunofluorescent antibody (IFA) analysis.
For the IFA analysis of midgut dissections, infected or control midguts were placed in a microtube containing paraformaldehyde (4%, 1× PBS) for at least 2 h. The paraformaldehyde solution was removed and midguts were rinsed in PBS-Triton X-100 (1× Ashburners PBS, 0.005% Triton X-100). IFA detection of SIN virus E1 antigen in midguts was performed as described previously, with the exception that all incubations and rinses were done in PBS-Triton X-100. After two final rinses midguts were mounted in Mowiol mounting medium (Aldrich Chemical Company Inc., Milwaukee, Wis.) and were polymerized overnight. Fluorescence analysis and imaging were carried out by using a fluorescent microscope (Olympus BH2, with 10×, 20×, and 40× objectives).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been deposited with the GenBank nucleotide sequence database under accession no. AF492770.
RESULTS
Completion of nucleotide and deduced amino acid sequence of MRE16 RNA genome.
The nucleotide sequence of the 26S RNA of MRE16 virus has been previously published (31). The nucleotide and deduced amino acid sequences of the 5′ NCR and nonstructural genes of MRE16 virus were generated in preparation for constructing the cDNA infectious clones used in this study. GenBank accession numbers AF492770 and U90536 report the entire genome sequence of MRE16 SIN virus. The 5′ NCR and nonstructural genes of MRE16 virus, excluding the 5′ cap, were 7,582 nucleotides in length. By using the previously published sequence data, the complete MRE16 virus genome was 11,693 nucleotides, excluding the 5′ cap and 3′ poly(A) tail (31).
Characteristics of MRE16 and MRE16ic viruses in cell culture.
Growth of MRE16 (AP61-6 and C6/36-5) stock virus in C6/36 cells followed by plaque assays in Vero cells revealed a predominantly small-plaque phenotype by 36 to 48 h p.i. (Fig. 2A). However, medium harvested at earlier time points from infected C6/36 cells contained virus that produced a predominantly large-plaque phenotype. The large plaque size was consistent with that observed over the duration of growth in BHK-21 cells (Fig. 2A). Growth curves for MRE16 (AP61-6, C6/36-1) and MRE16ic viruses showed that both viruses replicated with nearly equal efficiency in BHK-21 and C6/36 cells (Fig. 2B and C). In BHK-21 cells, maximum titers of 8.8 to 9.2 log10 PFU/ml were reached at 36 to 48 h p.i. In C6/36 cells, titers of 9.5 to 9.8 log10 PFU/ml were achieved at 48 to 60 h p.i. The large-plaque phenotype was found to predominate for MRE16ic virus at each time point taken from either BHK-21 or C6/36 cells (Fig. 2A).
FIG. 2.
Comparative growth characteristics of MRE16, MRE16ic, MRE16sp, MRE16icΔE200-Y229, and MRE16icΔE200-C220 viruses in BHK-21, C6/36, and Vero cells. (A) Relative plaque sizes in Vero cells. Viruses were grown in 25-cm2 tissue culture flasks of BHK-21 (B) and C6/36 (C) cells. Multiplicity of infection was approximately 0.01 PFU/cell. Cell culture medium was harvested from BHK-21 or C6/36 cells infected with MRE16, MRE16ic, MRE16sp, MRE16icΔE200-Y229, or MRE16icΔE200-C220 virus during growth curve experiments and were plaqued on Vero cell monolayers.
Sequence characteristics of MRE16sp structural genes.
MRE16sp virus seed stock obtained from a single round of plaque purification and passaged once in C6/36 cells reproduced the small-plaque phenotype (data not shown). Sequencing of the MRE16sp structural genes revealed a 90-nucleotide deletion in the E2 coding region encompassing amino acid residues E-200 to Y-229 (Fig. 3) as well as the two point mutations, S-60→R in E2 and S-321→L in E1. The deletion in the E2 region of the virus genome included 21 amino acids of the proposed CRBD.
FIG. 3.
Deletion identified in MRE16sp and deletions engineered into MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses. This figure shows the location of the deletion present in the E2 glycoprotein of MRE16sp and MRE16icΔE200-Y229 (bold solid line) and MRE16icΔE200-C220 (bold dotted line). Deduced amino acid residues from prototype AR339 virus sequence are also shown. A total of four amino acid differences are present between AR339 virus and MRE16 virus within the E2 CRDB (E2-172 R→G, E2-178 S→T, E2-197 I→V, and E2-213 T→A). AA, amino acid.
Growth of MRE16sp, MRE16icΔE200-Y229, and MRE16icΔE200-C220 viruses in vertebrate and mosquito cells.
MRE16icΔE200-Y229 virus plaque sizes in Vero cells (Fig. 2A) were similar to the plaque sizes observed for MRE16icΔE200-C220 and MRE16sp virus (data not shown). Growth curves for MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses indicated that both replicated with nearly equal efficiency in BHK-21 and C6/36 cells (Fig. 2B and C). In BHK-21 cells, maximum titers of 6.7 to 7.0 log10 PFU/ml were reached at 48 h p.i. In C6/36 cells, titers of 7.8 to 8.1 log10 PFU/ml were achieved at 60 to 72 h p.i. The growth curve assays also indicated that the replication kinetics of MRE16ic virus was altered nearly equally in both cell types by the presence of each of the two deletions. The deletions had a greater effect on replication in vertebrate cells (BHK-21), reducing titers by approximately 100-fold at each time point, than in mosquito cells (C6/36) in which titers typically underwent only a 10-fold reduction at each time point. Growth curves for MRE16sp virus showed that the replication of this virus also differed depending on the cell type which it was grown in, BHK-21 or C6/36 cells (Fig. 2B and C). The replication kinetics of MRE16sp virus was similar to those of both MRE16icΔE200-Y229 and MRE16icΔE200-C220 virus in BHK-21 cells. MRE16sp achieved a maximum titer of 7.6 log10 PFU/ml at 48 h p.i. in BHK-21 cells (Fig. 2B). However, the replication kinetics of MRE16sp virus was more similar to those of the MRE16 and MRE16ic viruses in C6/36 cells than either MRE16icΔE200-Y229 or MRE16icΔE200-C220 virus, achieving a maximum titer of 9.4 log10 PFU/ml at 48 h p.i. in this cell type (Fig. 2C). It is likely that one or more mutations in the MRE16sp virus genome that were not in MRE16icΔE200-Y229 and MRE16icΔE200-C220 virus genomes were necessary to achieve high titers in C6/36 cells. These mutations could be one or both of the two point mutations (E2 S-60→R and E1 S-321→L) identified in the MRE16sp structural genes. Alternatively, mutations may also be present in the nonstructural genes of the virus. Such mutations either acting independently or in combination with other mutations might also be responsible for the phenotypic differences.
Immunofluorescent analysis of mosquito head tissues.
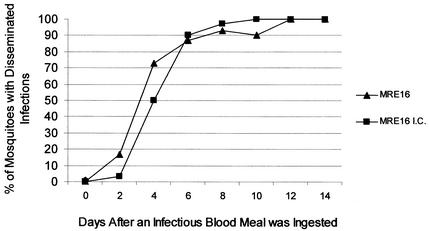
The dissemination of MRE16 and MRE16ic viruses from the midgut was compared following oral infection. MRE16 and MRE16ic virus E1 antigen was detected as early as 2 days p.i. in head tissue. For each virus, SIN virus E1 antigen was detected in greater than 90% of the heads of infected mosquitoes by 8 days p.i., and 100% of mosquitoes were positive for a disseminated infection by 10 to 12 days p.i. (Fig. 4).
FIG. 4.
Comparative disseminated infection rates of MRE16 and MRE16ic viruses in A. aegypti mosquitoes. Shown are the percentages of mosquitoes orally exposed to either MRE16 or MRE16ic virus with disseminated infections (e.g., positive for SIN virus-specific antigen by IFA of head tissues) at timed intervals (n ≥ 30).
MRE16sp virus was assayed for oral infectivity in A. aegypti. Analysis of mosquito head tissues revealed that only 17% (8 of 47) of mosquitoes ingesting a blood meal containing 9.0 log10 PFU/ml of MRE16sp virus had developed disseminated infections by 14 days p.i. (Table 2). MRE16icΔE200-Y229 and MRE16icΔE200-C220 virus dissemination was assayed for SIN virus antigen in head tissues at 9 and 14 days p.i. Only 3.3% (1 of 30) of the mosquitoes ingesting MRE16icΔE200-Y229 virus and none (0 of 30) of those ingesting MRE16icΔE200-C220 virus exhibited a disseminated infection after 9 days. The number of mosquitoes that had disseminated infections at 9 days was not significantly different (P = 1.000, Fisher exact test). By 14 days, 20% (10 of 50) of mosquitoes ingesting MRE16icΔE200-Y229 virus were positive for a disseminated infection. However, only 2% (1 of 50) of those ingesting MRE16icΔE200-C220 virus were positive at this time point, a significant difference (P = 0.008, Fisher exact test). The number of disseminated MRE16icΔE200-Y229 virus infections after 14 days was not significantly greater than the number observed in mosquitoes ingesting MRE16sp after 14 days (17%, or 8 of 47) (P = 0.797, Fisher exact test). The number of mosquitoes positive for dissemination of MRE16icΔE200-Y229 virus (10 of 50) contrasted with that of MRE16ic virus (60 of 60) at the same time point (P < 0.001, Fisher exact test). One possible explanation for the observed reduction in the dissemination rate of MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses was that the blood meal titers of these viruses were not as high as the titer of MRE16 and MRE16ic viruses. This possibility was addressed by determining the number of A. aegypti mosquitoes positive for a disseminated infection 14 days after ingestion of serially diluted MRE16ic virus (Table 2). Mosquitoes that ingested a blood meal containing 7.2 log10 PFU of MRE16ic virus/ml still showed significantly higher virus dissemination than did the MRE16ic ΔE200-Y229 deletion mutant (8.6 log10 PFU/ml in blood meal) (P < 0.001, Fisher exact test). These results clearly rule out a virus titer-specific effect.
TABLE 2.
A. aegypti mosquitoes displaying virus dissemination 14 days after ingestion of an infectious blood meal
| SIN virus | Blood meal titer (log10 PFU/ml) | Immunofluorescence results for head tissue
|
||
|---|---|---|---|---|
| No. positive | Total | % Positive | ||
| MRE16 | 9.6 | 60 | 60 | 100 |
| MRE16ic | 9.4 | 60 | 60 | 100 |
| MRE16sp | 9.0 | 8 | 47 | 17 |
| MRE16ic | 8.6 | 10 | 50 | 20 |
| ΔE200-Y229 | ||||
| MRE16ic | 8.1 | 1 | 50 | 2 |
| ΔE200-C220 | ||||
| MRE16ic (10−1) | 8.4 | 56 | 60 | 93 |
| MRE16ic (10−2) | 7.2 | 44 | 60 | 73 |
Immunofluorescent analysis of mosquito midgut tissues.
Mosquito midguts were assayed for infection with MRE16, MRE16ic, MRE16sp, MRE16icΔE200-Y229, or MRE16icΔE200-C220 virus. A. aegypti ingested a blood meal containing approximately 9.6 log10 PFU/ml (MRE16), 9.4 log10 PFU/ml (MRE16ic), 9.0 log10 PFU/ml (MRE16sp), 8.6 log10 PFU/ml (MRE16icΔE200-Y229), or 8.1 log10 PFU/ml (MRE16icΔE200-C220), respectively. Midguts were dissected and analyzed by IFA at 2 to 3, 4 to 5, or 8 to 9 days p.i. At least 29 midguts from each group were examined for virus infection by IFA (Table 3). Midguts also were dissected from mock-infected mosquitoes and were analyzed by the same IFA protocol. SIN virus-specific antigen was detected in all of the midguts examined from mosquitoes infected with either MRE16 or MRE16ic virus, and widespread distribution was observed in similar proportions at both 2 to 3 and 4 to 5 days p.i. (Fig. 5A and B). SIN virus-specific antigen was detected in the epithelial cell layer in all of the midguts examined but was also detected in the overlying musculature and respiratory tracheoles of many of the A. aegypti infected midguts.
TABLE 3.
A. aegypti mosquitoes displaying midgut infection after ingestion of an infectious blood meal
| SIN virus | Days p.i. | Blood meal titer (log10 PFU/ml) | Immunofluorescence results for midgut
|
||
|---|---|---|---|---|---|
| No. positive | Total | % Positive | |||
| MRE16 | 2-3 | 9.6 | 29 | 29 | 100 |
| MRE16ic | 2-3 | 9.4 | 31 | 31 | 100 |
| MRE16ic | 2-3 | 8.6 | 10 | 32 | 31.3 |
| ΔE200-Y229 | |||||
| MRE16ic | 2-3 | 8.1 | 11 | 31 | 35.5 |
| ΔE200-C220 | |||||
| MRE16 | 4-5 | 9.6 | 30 | 30 | 100 |
| MRE16ic | 4-5 | 9.4 | 29 | 29 | 100 |
| MRE16sp | 8-9 | 9.0 | 18 | 35 | 51.4 |
| MRE16ic | 8-9 | 8.6 | 10 | 30 | 33.3 |
| ΔE200-Y229 | |||||
| MRE16ic | 8-9 | 8.1 | 7 | 30 | 23.3 |
| ΔE200-C220 | |||||
FIG. 5.
Intact A. aegypti midguts assayed for the presence of MRE16, MRE16ic, MRE16icΔE200-Y229, MRE16icΔE200-C220, or MRE16sp virus at various times p.i. (A and B) Composite images of midguts infected with either MRE16 virus (A) or MRE16ic virus (B) at 2 to 3 days p.i. and demonstrating widespread distributions of SIN virus E1 antigen. (C to E) Composite images of midguts infected with MRE16ic ΔE200-Y229 virus at 2 to 3 days p.i. (C), MRE16ic ΔE200-C220 virus at 8 to 9 days p.i. (D), and MRE16sp virus at 8 to 9 days p.i (E). A focus of infection can be seen in the blue square. (SIN virus E1 antigen is shown in green). An Olympus BH2 fluorescent microscope was used. Original magnification, 125×.
Midguts were examined from mosquitoes ingesting MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses to determine if the deletions in E2 had any effect on the ability of the MRE16ic virus to initiate an infection of midgut epithelial cells. SIN virus antigen was detected in only 31.3% (10 of 32) and 35.5% (11 of 31) of midguts from mosquitoes ingesting MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses, respectively, at 2 to 3 days p.i. (P = 0.793, Fisher exact test). In those midguts that did become infected, only 1 or 2 small foci of antigen per midgut were observed (Fig. 5C). At the same time point, 100% (31 of 31) of mosquitoes ingesting MRE16ic virus displayed SIN virus antigen in the midgut indicating infection. SIN virus antigen was not detected in any of the mock-infected mosquitoes (data not shown). Only 33.3% (10 of 30) and 23.3% (7 of 30) of midguts taken 8 to 9 days after ingesting MRE16icΔE200-Y229 virus or MRE16icΔE200-C220 virus were infected, respectively (P = 0.568, Fisher exact test). However, larger foci of antigen were observed, indicating that initial infection had spread to other cells of the midgut (Fig. 5D). SIN virus-specific antigen was detected in only 51.4% (18 of 35) of the midguts examined from mosquitoes that had ingested MRE16sp virus at 8 to 9 days p.i. The infection rate was not significantly greater than that obtained for MRE16icΔE200-Y229 virus (P = 0.069) but was significantly less than that obtained for MRE16ic virus (P < 0.001) at the same time point. Most of the midguts that did become infected with MRE16sp virus had a limited antigen distribution similar to what was observed in MRE16icΔE200-Y229 or MRE16icΔE200-C220 virus-infected midguts at 8 to 9 days p.i. (Fig. 5E).
Virus replication in A. aegypti.
To determine if the observed reduction in infectivity and dissemination by the deletion mutants was specific to midgut epithelial cells, MRE16icΔE200-Y229, MRE16icΔE200-C220, and MRE16ic virus replication was compared following intrathoracic inoculation of mosquitoes. This route of infection circumvents the midgut, injecting virus directly into mosquito hemolymph and allowing infection of non-midgut tissues. Mosquitoes were tritrated at timed intervals, and virus titers were determined by plaque assay in Vero cells (Fig. 6). MRE16ic virus reached a maximum average titer of 7.8 log10 PFU/ml at 48 h p.i. However, MRE16ic virus titer decreased to 6.4 log10 PFU/ml at 96 h p.i. MRE16icΔE200-Y229 virus and MRE16icΔE200-C220 virus attained maximum titers of only 6.1 to 6.5 log10 PFU/ml at 48 h p.i., more than 10-fold lower than MRE16ic titers at the same time point. Significantly, the titers of all three viruses were similar at the 96-h time point.
FIG. 6.
Comparative replication kinetics of MRE16ic, MRE16icΔE200-Y229, and MRE16icΔE200-C220 viruses in A. aegypti. Mosquitoes were inoculated intrathoracically with between 10 to 15 PFU. Ten mosquitoes were taken at each time point for each virus. Individual virus titers obtained from triturated mosquitoes were averaged for each time point. Time zero was included to confirm the initial inoculating dose. The average inoculating dose, determined at the mosquito zero time point, for MRE16ic virus was 7 PFU. Virus was not recovered from mosquitoes inoculated with the deletion mutants at time zero. Virus was recovered from all of the mosquitoes that were triturated at all subsequent time points.
DISCUSSION
We have previously described a Malaysian isolate of SIN virus, MRE16, which displays enhanced oral infection of A. aegypti (31). Here we describe a small-plaque mutant, MRE16sp, containing a deletion in the E2 glycoprotein that poorly orally infects A. aegypti. Other groups have demonstrated that small-plaque mutants of alphaviruses, such as Middelburg (26), VEE (10), and SIN virus (27), are nontransmissible by mosquitoes following peroral infection. Well-characterized mutants represent important tools for elucidating the virus-vector interactions associated with midgut infection, dissemination, and transmission. To obtain a more detailed understanding of alphavirus infection of the invertebrate vector, we have applied in vivo, molecular genetic approaches to elucidate viral determinants of A. aegypti infection.
MRE16sp contains a 90-nucleotide deletion that includes a portion at the 3′ terminus of the E2 CRBD coding region. BHK-21 cells infected with MRE16sp virus generated virus titers approximately 50-fold lower than MRE16ic virus titers in the same cell type. However, C6/36 cells infected with MRE16sp generated virus titers in excess of 9.0 log10 PFU/ml, as did MRE16ic virus in the same cell type. An identical deletion (ΔE200-Y229) was introduced into pMRE16ic to reflect the structural gene region of MRE16sp, with the exception of two amino acid substitutions at residues 60 in E2 and 321 in E1. Although C6/36 cells infected with MRE16icΔE200-Y229 virus had lower titers than either MRE16ic or MRE16sp virus, the mutant virus still replicated efficiently, reaching a maximum titer in excess of 8.0 log10 PFU/ml in C6/36 cells at 72 h p.i. The growth of the deletion mutant was even more attenuated in BHK-21 than in C6/36 cells, achieving maximum titers approximately 100-fold lower than those of MRE16ic virus. These results indicate that the deletion (ΔE200-Y229) introduced into pMRE16ic likely requires the presence of a compensatory mutation or mutations to achieve titers in excess of 9.0 log10 PFU/ml in the C6/36 cell type. Once identified it will be interesting to determine what role the mutation or mutations play in the apparent selective advantage of the MRE16sp virus over the MRE16 virus in C6/36 mosquito cells as evidenced by the predominance of the small-plaque phenotype in cultures containing a quasispecies population of virus (Fig. 2A).
The midgut infectivities of MRE16sp and MRE16icΔE200-Y229 viruses were both significantly lower than that of the MRE16ic virus. Thirty three percent of mosquitoes orally infected with MRE16icΔE200-Y229 virus were positive by IFA analysis for midgut infection at 2 to 3 days p.i., and each midgut displayed 1 or 2 foci of infection. In contrast, 2 days after infection of mosquitoes with MRE16ic virus, 100% of midguts were positive for virus antigen, with an average of 20 to 40 times the number of discreet infection sites (K. M. Myles, D. J. Pierro, and K. E. Olson, unpublished data). Although a greater number of the midguts examined from mosquitoes that had ingested MRE16sp virus at 8 to 9 days p.i. were positive for SIN virus-specific antigen (51.4%), the number was still significantly lower than the number of midguts positive for MRE16ic virus infection at 2 to 3 days p.i. Discrepancies between the midgut infection rates of MRE16sp virus and MRE16icΔE200-Y229 virus might be explained by the higher titer of MRE16sp virus used in the oral infections of A. aegypti. Alternatively, the efficiency of initial midgut infection with the MRE16sp and MRE16icΔE200-Y229 viruses might be equivalent, but the presence of compensatory mutations in the MRE16sp genome may allow more efficient replication of MRE16sp virus in the midgut epithelial cells following initial infection. The latter hypothesis is supported by the wider distributions of SIN virus antigen observed in some MRE16sp virus-infected midguts. When the compensatory mutations are identified this hypothesis can be tested. MRE16sp and MRE16icΔE200-Y229 viruses also both displayed poor dissemination in A. aegypti, with ∼20% of mosquitoes positive for MRE16sp or MRE16icΔE200-Y229 virus in head tissues at 14 days p.i. While MRE16icΔE200-Y229 virus initially replicated less efficiently than MRE16ic virus in injected mosquitoes, by 96 h p.i. titers were nearly identical. The ability of MRE16icΔE200-Y229 virus to grow to titers similar to those of MRE16ic virus after intrathoracic inoculation clearly suggests that the deletion's effect on infectivity was specific to the mosquito midgut. Determinants in the E2 glycoprotein not affected by the deletion may be important for the infection of other non-midgut tissues. These remaining determinants may have been sufficient to allow inefficient infection of some midguts following ingestion of MRE16icΔE200-Y229 virus. Alternatively, Omar and Koblet (25) demonstrated that other alphaviruses lacking E2 were infectious, although much less so than wild-type virus, and suggested that virions with E1 alone are capable of inefficiently binding and penetrating cells, probably through direct fusion of the virus envelope with cell membranes.
Residues 170 to 220 (SIN virus AR339 numbering) of E2 are postulated to be particularly important for cell binding and appear to constitute a CRBD in the glycoprotein. The domain has been shown to be important for virus neutralization, and a domain of similar function and location has been identified in the E2 of Venezuelan equine encephalitis (VEE) and Ross River (RR) viruses. This domain may be well conserved among the alphaviruses (32, 36). For SIN virus, two overlapping antigenic sites, A and B, have been defined within this domain (33, 35, 36). Based on changes seen in antibody-resistant variants, it is likely that all epitopes in antigenic sites A and B are contained within this domain (36). The deleted portion of the CRBD included at least portions of both A and B. Mechanisms by which antibodies neutralize virus infectivity can vary, but it has been suggested that SIN virus-specific antibodies that bind to E2 A and E2 B block attachment to cells (41).
Wang et al. (41) demonstrated that anti-idiotypic antibodies recognizing epitopes within this domain (35, 42) blocked the binding of SIN virus to chicken cells. The results suggested that the anti-idiotypic antibody was binding to a cellular receptor for the virus. Ubol and Griffin (39) also reported that an anti-idiotypic antibody recognizing an epitope within the domain was able to block virus binding to murine N18 neuroblastoma cells.
Flynn et al. (9) observed that transitional epitopes are exposed during binding of SIN virus to a host cell. These conformational alterations were mimicked after exposure of virus to heat, and transitional epitopes were neutralized by MAbs (36). Selection for resistance to neutralization by these MAbs gave rise to viruses with amino acid residue changes at E2 position 200 or 202 (anti-E2 MAbs). It is intriguing that the portion of the cell receptor domain deleted in MRE16sp virus lacks the critical transitional epitope residues at E2 positions 200 and 202.
Residue changes within the E2 CRBD have also been shown to affect the infection potential of alphaviruses for different cell types. Amino acid changes within the domain have been found to affect the sensitivity of chicken cells to infection with RR virus (18, 36). The results with RR virus were remarkable in that the parental virus infected only 2% of the cells, and only three amino acid changes in the envelope glycoproteins (one within the E2 CRBD) resulted in a virus capable of infecting all of the cells (36).
Studies with chimeric SIN and VEE viruses also have described the importance of the alphavirus E2 glycoprotein as a determinant of infection in Aedes species (2, 28). Others have demonstrated that the infectivity and dissemination of VEE in A. aegypti mosquitoes can be altered by a single amino acid change within the CRBD (45).
To further define the genetic basis for the observed effects of the deletion identified in the MRE16sp virus, a truncated deletion encompassing only the 3′ end of the CRBD coding region (E200-C220) was engineered into pMRE16ic. MRE16icΔE200-C220 virus replication in cell culture was not as efficient as MRE16ic virus replication but was very similar to that of MRE16icΔE200-Y229 virus. MRE16icΔE200-Y229 and MRE16icΔE200-C220 viruses infected midguts with similar efficiency. The ΔE200-Y229 and ΔE200-C220 deletions also appear to have an effect on virus escape from the midgut to head tissues, with the ΔE200-C220 deletion having a greater effect. A possible explanation for this observation is that E2 conformation is different at the surface of the MRE16icΔE200-Y229 and MRE16icΔE200-C220 virions, and this affects virus escape. Thus, it is possible that this region of the E2 glycoprotein may play a role in the phenomenon of midgut escape. The nature of midgut escape is presently unknown, although the virus presumably must penetrate the basal lamina separating the midgut from the hemocoel and then pass into the hemolymph (43, 44). Another possibility is that the deletions in the CRBD cause reduced stability as the virions assemble in infected midgut epithelial cells. However, we have noted that other SIN virus strains with full-length E2 glycoproteins show similarly restricted midgut infection patterns as observed with the deletion mutants (28, 31).
The present study suggests that amino acid residues 200 to 220 (SIN virus AR339 numbering) within the CRBD of the MRE16 E2 envelope glycoprotein are important for mosquito infectivity following oral infection. The deletions introduced into the E2 envelope glycoprotein of MRE16ic, generating MRE16icΔE200-Y229 virus and MRE16icΔE200-C220 virus, probably impaired the ability of these viruses to interact with a cellular receptor on the mosquito midgut epithelium. It has been established by using several different experimental approaches that the resistance of Culex pipiens to infection with the alphavirus western equine encephalitis (WEE) is associated with a failure of the virus to bind to the midgut microvillar membrane (1, 15). Furthermore, competitive binding experiments with midgut brush border fragments indicated that the binding of WEE virus was specific in the case of Culex tarsalis, which is susceptible to infection with WEE virus, but was nonspecific in the case of C. pipiens, which is refractory to infection with WEE virus (13). The results of these experiments suggest that the midgut brush border fragments of C. pipiens lack specific receptors for WEE virus. Our results provide additional support for the existence of a specific receptor-ligand interaction by which alphaviruses gain entry into mosquito midgut epithelial cells. Nonetheless, the present study has identified an important molecular viral determinant of mosquito infection. Elucidation of a specific mechanism will require additional studies, but such studies could provide critical insights into the molecular nature of midgut infection barriers and midgut escape barriers and could lead to a better understanding of vector specificity.
Acknowledgments
This work was supported by Public Health Service-NIH grant AI46435.
We thank Richard Kinney and Ann Powers (Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention) for their technical assistance and helpful advice.
REFERENCES
- 1.Arcus, Y. M., E. J. Houk, and J. L. Hardy. 1983. Comparative in vitro binding of an arbovirus to midgut microvillar membranes from susceptible and refractory Culex mosquitoes. Fed. Proc. 42:2141. [Google Scholar]
- 2.Brault, A. C., A. M. Powers, and S. C. Weaver. 2002. Vector infection determinants of Venezuelan equine encephalitis virus reside within the E2 envelope glycoprotein. J. Virol. 76:6387-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burness, A. T., I. Pardoe, S. G. Faragher, S. Vrati, and L. Dalgarno. 1988. Genetic stability of Ross River virus during epidemic spread in nonimmune humans. Virology 167:639-643. [PubMed] [Google Scholar]
- 4.Chanas, A. C., E. A. Gould, J. C. Clegg, and M. G. Varma. 1982. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J. Gen. Virol. 58:37-46. [DOI] [PubMed] [Google Scholar]
- 5.Davis, N. L., D. F. Pence, W. J. Meyer, A. L. Schmaljohn, and R. E. Johnston. 1987. Alternative forms of a strain-specific neutralizing antigenic site on the Sindbis virus E2 glycoprotein. Virology 161:101-108. [DOI] [PubMed] [Google Scholar]
- 6.Doherty, R. L., J. G. Carley, C. Filippich, B. H. Kay, B. M. Gorman, and N. Rajapaksa. 1977. Isolation of Sindbis (alphavirus) and Leanyer viruses from mosquitoes collected in the Northern Territory of Australia, 1974. Aust. J. Exp. Biol. Med. Sci. 55:485-489. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, R. L., J. G. Carley, B. H. Kay, C. Filippich, E. N. Marks, and C. L. Frazier. 1979. Isolation of virus strains from mosquitoes collected in Queensland, 1972-1976. Aust. J. Exp. Biol. Med. Sci. 57:509-520. [DOI] [PubMed] [Google Scholar]
- 8.Dubuisson, J., S. Lustig, N. Ruggli, Y. Akov, and C. M. Rice. 1997. Genetic determinants of Sindbis virus neuroinvasiveness. J. Virol. 71:2636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, D. C., W. J. Meyer, J. M. Mackenzie, Jr., and R. E. Johnston. 1990. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J. Virol. 64:3643-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaidamovich, S. Y., Y. Y. Tsilinsky, A. I. Lvova, and N. V. Khutoretskaya. 1971. Aedes aegypti mosquitoes as an experimental model for studies on the ecology and genetics of Venezuelan equine encephalomyelitis virus. Acta Virol. 15:301-308. [PubMed] [Google Scholar]
- 11.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler, D. J., and L. Rosen. 1976. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am. J. Trop. Med. Hyg. 25:146-150. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, J. L. 1988. Susceptibility and resistance of vector mosquitoes, p. 87-126. In T. P. Monath (ed.), The arboviruses: ecology and epidemiology, vol. 1. CRC Press, Boca Raton, Fla.
- 14.Higgs, S., and B. J. Beaty. 1996. Rearing and containment of mosquito vectors, p. 595-605. In B. J. Beaty and W. C. Marquardt (ed.), The biology of disease vectors. University Press of Colorado, Niwot, Colo.
- 15.Houk, E. J., L. D. Kramer, J. L. Hardy, and S. B. Presser. 1986. An interspecific mosquito model for the mesenteronal infection barrier to western equine encephalomyelitis virus (Culex tarsalis and Culex pipiens). Am. J. Trop. Med. Hyg. 35:632-641. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi, A. 1978. Isolation of a Singh's Aedes albopictus cell clone sensitive to dengue and chikungunya viruses. J. Gen. Virol. 40:531-544. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, A. C., J. C. Bowen, and A. E. Downe. 1993. Experimental infection of Aedes aegypti (Diptera: Culicidae) by the oral route with Sindbis virus. J. Med. Entomol. 30:332-337. [DOI] [PubMed] [Google Scholar]
- 18.Kerr, P. J., R. C. Weir, and L. Dalgarno. 1993. Ross River virus variants selected during passage in chick embryo fibroblasts: serological, genetic, and biological changes. Virology 193:446-449. [DOI] [PubMed] [Google Scholar]
- 19.Kinney, R. M., S. Butrapet, G. J. Chang, K. R. Tsuchiya, J. T. Roehrig, N. Bhamarapravati, and D. J. Gubler. 1997. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 230:300-308. [DOI] [PubMed] [Google Scholar]
- 20.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine, B., H. H. Jiang, L. Kleeman, and G. Yang. 1996. Effect of E2 envelope glycoprotein cytoplasmic domain mutations on Sindbis virus pathogenesis. J. Virol. 70:1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustig, S., A. C. Jackson, C. S. Hahn, D. E. Griffin, E. G. Strauss, and J. H. Strauss. 1988. Molecular basis of Sindbis virus neurovirulence in mice. J. Virol. 62:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza, Q. P., J. Stanley, and D. E. Griffin. 1988. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J. Gen. Virol. 69:3015-3022. [DOI] [PubMed] [Google Scholar]
- 24.Olson, K. E., K. M. Myles, R. C. Seabaugh, S. Higgs, J. O. Carlson, and B. J. Beaty. 2000. Development of a Sindbis virus expression system that efficiently expresses green fluorescent protein in midguts of Aedes aegypti following per os infection. Insect. Mol. Biol. 9:57-65. [DOI] [PubMed] [Google Scholar]
- 25.Omar, A., and H. Koblet. 1988. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology 166:17-23. [DOI] [PubMed] [Google Scholar]
- 26.Pattyn, S. R., and L. De Vleesschauwer. 1968. The multiplication of Middleburg s and l plaque viruses in Aedes aegypti mosquitoes. Acta Virol. 12:347-354. [PubMed] [Google Scholar]
- 27.Peleg, J. 1975. In vivo behavior of a Sindbis virus mutant isolated from persistently infected Aedes aegypti cell cultures. Ann. N.Y. Acad. Sci. 266:204-213. [DOI] [PubMed] [Google Scholar]
- 28.Pierro, D. J., K. M. Myles, B. D. Foy, B. J. Beaty, and K. E. Olson. 2003. Development of an orally infectious Sindbis virus transducing system that efficiently disseminates and expresses green fluorescent protein in Aedes aegypti. Insect Mol. Biol. 12:107-116. [DOI] [PubMed]
- 29.Sammels, L. M., M. D. Lindsay, M. Poidinger, R. J. Coelen, and J. S. Mackenzie. 1999. Geographic distribution and evolution of Sindbis virus in Australia. J. Gen. Virol. 80:739-748. [DOI] [PubMed] [Google Scholar]
- 30.Seabaugh, R. C. 1998. Genetic determinants of Sindbis virus oral infectivity in Aedes aegypti mosquitoes. Ph.D. thesis. Colorado State University, Fort Collins.
- 31.Seabaugh, R. C., K. E. Olson, S. Higgs, J. O. Carlson, and B. J. Beaty. 1998. Development of a chimeric sindbis virus with enhanced per os infection of Aedes aegypti. Virology 243:99-112. [DOI] [PubMed] [Google Scholar]
- 32.Smith, T. J., R. H. Cheng, N. H. Olson, P. Peterson, E. Chase, R. J. Kuhn, and T. S. Baker. 1995. Putative receptor binding sites on alphaviruses as visualized by cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 92:10648-10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stec, D. S., A. Waddell, C. S. Schmaljohn, G. A. Cole, and A. L. Schmaljohn. 1986. Antibody-selected variation and reversion in Sindbis virus neutralization epitopes. J. Virol. 57:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss, E. G., A. L. Schmaljohn, D. S. Stec, and J. H. Strauss. 1990. Mapping of Sindbis virus neutralization epitopes, p. 305-308. In M. A. Brinton and F. X. Heinz (ed.), New aspects of positive-strand RNA viruses. American Society for Microbiology, Washington, D.C.
- 35.Strauss, E. G., D. S. Stec, A. L. Schmaljohn, and J. H. Strauss. 1991. Identification of antigenically important domains in the glycoproteins of Sindbis virus by analysis of antibody escape variants. J. Virol. 65:4654-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, R. M., H. S. Hurlbut, T. H. Work, J. R. Kingsbury, and T. E. Frothingham. 1955. Sindbis virus: a newly recognized arthropod-transmitted virus. Am. J. Trop. Med. Hyg. 4:844-846. [DOI] [PubMed] [Google Scholar]
- 38.Tucker, P. C., and D. E. Griffin. 1991. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 glycoprotein. J. Virol. 65:1551-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ubol, S., and D. E. Griffin. 1991. Identification of a putative alphavirus receptor on mouse neural cells. J. Virol. 65:6913-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varma, M. G., M. Pudney, and C. J. Leake. 1974. Cell lines from larvae of Aedes (Stegomyia) malayensis Colless and Aedes (S) pseudoscutellaris (Theobald) and their infection with some arboviruses. Trans. R Soc. Trop. Med. Hyg. 68:374-382. [DOI] [PubMed] [Google Scholar]
- 41.Wang, K. S., A. L. Schmaljohn, R. J. Kuhn, and J. H. Strauss. 1991. Antiidiotypic antibodies as probes for the Sindbis virus receptor. Virology 181:694-702. [DOI] [PubMed] [Google Scholar]
- 42.Wang, K. S., and J. H. Strauss. 1991. Use of a lambda gt11 expression library to localize a neutralizing antibody-binding site in glycoprotein E2 of Sindbis virus. J. Virol. 65:7037-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver, S. C., T. W. Scott, and L. H. Lorenz. 1990. Patterns of eastern equine encephalomyelitis virus infection in Culiseta melanura (Diptera: Culicidae). J. Med. Entomol. 27:878-891. [DOI] [PubMed] [Google Scholar]
- 44.Whitfield, S. G., F. A. Murphy, and W. D. Sudia. 1973. St. Louis encephalitis virus: an ultrastructural study of infection in a mosquito vector. Virology 56:70-87. [DOI] [PubMed] [Google Scholar]
- 45.Woodward, T. M., B. R. Miller, B. J. Beaty, D. W. Trent, and J. T. Roehrig. 1991. A single amino acid change in the E2 glycoprotein of Venezuelan equine encephalitis virus affects replication and dissemination in Aedes aegypti mosquitoes. J. Gen. Virol. 72:2431-2435. [DOI] [PubMed] [Google Scholar]