Abstract
Children with human immunodeficiency virus infection often have higher viral loads and progress to AIDS more rapidly than adults. Since the intestinal tract is a major site of early viral replication and CD4+ T-cell depletion in adults, we examined the effects of simian immunodeficiency virus (SIV) on both peripheral and intestinal lymphocytes from 13 neonatal macaques infected with SIVmac239. Normal neonates had more CD4+ T cells and fewer CD8+ T cells in all tissues than adults. Surprisingly, neonates had substantial percentages of CD4+ T cells with an activated, memory phenotype (effector CD4+ T cells) in the lamina propria of the intestine compared to peripheral lymphoid tissues, even when examined on the day of birth. Moreover, profound and selective depletion of jejunum lamina propria CD4+ T cells occurred in neonatal macaques within 21 days of infection, which was preceded by large numbers of SIV-infected cells in this compartment. Furthermore, neonates with less CD4+ T-cell depletion in tissues tended to have higher viral loads. The persistence of intestinal lamina propria CD4+ T cells in some neonates with high viral loads suggests that increased turnover and/or resistance to CD4+ T-cell loss may contribute to the higher viral loads and increased severity of disease in neonatal hosts.
Children infected with human immunodeficiency virus (HIV) often have higher viral loads and a more rapid progression to AIDS than adults (2, 5, 11, 12, 28, 35, 44). Approximately one-third of untreated HIV-infected children develop severe symptoms in the first 2 years of life, and at least 20% die within the first 4 years of infection (20, 25, 35, 44, 45). While early viral loads in children are comparable to those of adults with acute infection (105 to 107 RNA copies/ml of plasma), high viral loads usually persist in the pediatric patient (7, 21, 22). The reasons for the enhanced progression to disease and sustained viral loads are not known but are generally attributed to the immaturity of the immune system. However, many children demonstrate active cellular and humoral immune responses to HIV infection, indicating that the neonatal immune system is fully capable of mounting an immune response (11, 24, 42).
Evidence suggests that an increased level of cellular activation may be associated with the high viral loads and rapid disease progression in the pediatric patient (11). Levels of soluble l-selectin (CD62L) have been shown to be higher in neonates than adults (11). Since l-selectin is present on naive cells yet rapidly shed upon lymphocyte activation, this suggests that neonates have a higher level of basal lymphocyte activation (11). Furthermore, levels of soluble l-selectin were shown to be higher in HIV-infected neonates than in uninfected children (11). More recent studies have demonstrated that functional, cytokine- and/or immunoglobulin-secreting cells develop very early in utero in nonhuman primates and that these cells may be functional at birth (17). Since HIV and simian immunodeficiency virus (SIV) replicate most efficiently in activated CD4+ T cells of adults (32, 33, 46) and children (29), increased levels of CD4+ T-cell activation could, at least partially, explain the higher viral loads in infected neonates.
Activated, memory CD4+ T cells are rapidly and selectively lost within days of SIV infection in adult macaques, which is usually followed by a reduction in viral loads (36, 37, 41). Since the thymus of infants is much more active during fetal and neonatal life than in adults (8), increased production and turnover of activated CD4+ T cells could explain the persistently high viral loads and more rapid progression to AIDS in pediatric patients, particularly if there is a limit to the regenerative capacity of the thymus. Furthermore, marked differences in the status of the immune system exist between neonates and adults. For example, CD4+ T-lymphocyte counts in the blood of human and macaque infants are many-fold higher than in older children and adults (4, 6). Since CD4+ T cells are the major target cells in HIV infection, simple numerical differences in CD4+ T cells could also play a role in the pathogenesis of HIV infection of children.
Serial time course studies are difficult to perform in HIV-infected children. As a result, pediatric HIV studies often resort to grouping data from children of different ages. Furthermore, very little is known regarding the immunologic events that occur in other lymphoid tissues, such as the spleen, lymph nodes, and intestinal tract, in HIV-infected children.
To date, immunologic studies of human neonates, performed on either peripheral blood or umbilical cord blood, suggest that neonatal lymphocytes essentially all have a naïve, resting phenotype. However, very little is known regarding the phenotype of lymphocytes in other neonatal tissues. In this study, we examined the phenotypes of lymphocytes obtained from the blood, lymph nodes, spleen, and intestines of pediatric macaques infected with the molecular clone SIVmac239 and compared them with values from age-matched uninfected controls. Macaques were intravenously infected with SIVmac239 within 24 h of birth and examined at multiple time points, focusing on the first 2 months of infection.
This report demonstrates that, similar to human infants, peripheral CD4+ T cells of neonatal macaques primarily have a naïve, resting phenotype. Surprisingly, however, newborn macaques have large numbers of activated, memory CD4+ T cells in the lamina propria of the jejunum, and these are major early targets for SIV infection, amplification, and rapid CD4+ T-cell depletion in primary infection. Furthermore, these data suggest that viral loads in plasma and tissues directly correlate with the number of CD4+ T cells remaining in lymphoid tissues. Combined, these data suggest that higher absolute numbers and higher rates of turnover and/or survival of CD4+ T cells in the neonatal host may be responsible for the more severe disease course often observed in pediatric patients.
MATERIALS AND METHODS
Animals and virus.
Complete sets of tissues were examined from a total of 21 neonatal macaques. All macaques were derived from their dams by cesarean section and nursery reared. Ten were uninfected controls examined on the day of birth (n = 4) or at 3 (n = 1), 7 (n = 1), or 50 (n = 2) days of age. Experimental macaques were intravenously infected with molecularly cloned SIVmac239 (10 ng of p27; provided by Ronald Desrosiers) 1 day after birth, and two macaques were humanely euthanized at 3, 7, 14, 21, and 50 days after infection. An additional three neonates were similarly inoculated but allowed to progress to AIDS. Blood was collected at days 3, 7, 14, 21, and 50, monthly thereafter, and at necropsy. Each of these animals was euthanized when clinical signs of AIDS developed, which occurred at 81, 142, and 209 days after infection. All animals were housed and cared for in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and the guidelines of the Committee on Animals of Harvard Medical School.
Tissue collection and analysis.
SIV-infected neonates were euthanized in pairs at intervals of 3, 7, 14, 21, or 50 days (n = 2) or allowed to progress to terminal disease (n = 3). Immediately after euthanasia, complete sets of tissues were harvested and preserved in formalin (for histopathology and in situ hybridization). Portions of spleen, axillary and mesenteric lymph nodes, and 6- to 8-cm-long sections of the jejunum, ileum, and colon were also collected fresh in complete RPMI 1640 medium and transported to the laboratory for lymphocyte isolation.
Isolation and characterization of lymphocytes by flow cytometry.
Lymphocytes from the intestine, lymph nodes (axillary and mesenteric), spleen, and blood were isolated and stained for flow cytometry as previously described (36, 40, 41). Briefly, intestinal epithelial lymphocytes and lamina propria lymphocytes (LPL) were isolated and examined separately using serial incubations in EDTA followed by collagenase digestion (40). To prove that intestinal lymphocytes were not being affected by the isolation procedure, blood, spleen, and lymph node from an uninfected macaque were similarly treated with serial incubations in EDTA and collagenase and compared by flow cytometry. Lymph nodes and spleen were minced, passed through nylon mesh screens, and washed twice in RPMI containing 5% fetal calf serum before staining. Red blood cells were lysed in cell preparations from the spleen using a standard ammonium chloride lysing solution. Blood was examined by a whole blood lysis and staining technique (41). Purified lymphocytes were stained for flow cytometry using the panel of monoclonal antibodies shown in Table 1. For staining, cells were adjusted to 107 cells/ml and 100-μl aliquots (106 cells) were stained using appropriately diluted concentrations of antibody for 30 min at 4°C. Cells were then washed and fixed overnight in 2% paraformaldehyde. Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson).
TABLE 1.
Antibody panel used to examine neonatal macaque lymphocytes
| FL-1 | FL-2 | FL-3a | FL-4 |
|---|---|---|---|
| CD45RAb | CD62Lb | CD3c | CD4d |
| CD45RAb | CD62Lb | CD3c | CD8d |
| γδ TCRe | CD8b | CD3c | CD4d |
| CD20f | CD2b | CD3c | CD4d |
| CD25b | CD8b | CD3c | CD4d |
| CD38g | HLA-DRb | CD3c | CD8d |
| CD38g | HLA-DRb | CD3c | CD4d |
| CD8b | CD28b | CD3c | CD4d |
| CD38d | CD56b | CD3c | CD8d |
| CD4g | HLA-DRb | CD34h | CD8d |
FL-3 reagents were biotinylated, and a two-step staining procedure was performed using streptavidin red 613 (SA-613; Gibco, Grande Isle, N.Y.) as the secondary reagent.
Becton Dickinson, San Jose, Calif.
Clone 6G12 provided by J. Wong, Massachusetts General Hospital.
Allophycocyanin-conjugated monoclonal antibodies to CD4 (clone OKT4) and CD8 (clone Leu-2a) were grown and prepared in the laboratory of R. Paul Johnson, New England Regional Primate Research Center.
Pan-TCR gamma delta; Endogen, Inc., Woburn, Mass.
Caltag Inc., Burlingame, Calif.
Ortho Diagnostics Inc., Raritan, N.J.
Clone Q-bend 10; Immunotech, Westbrook, Maine.
Assessment of viral loads.
Virion-associated RNA in plasma was quantified by real-time reverse transcription-PCR on a Prism 7700 sequence detection system (Applied Biosystems) as previously described (34).
Localization of virus in tissues by in situ hybridization.
In situ hybridization for SIV was performed as previously described (13, 43). To semiquantitate infected cells in tissues, sections were examined by light microscopy and positive cells were counted at ×100 magnification. At least 10 fields (0.5 mm2 each) were counted for the jejunum, ileum, and colon, and at least 5 fields were examined from the spleen and lymph nodes. Since viral loads in the intestine differed remarkably between effector lymphoid tissues (lamina propria) and inductive sites (Peyer's patches and lymphoid follicles), these regions were counted separately (see Table 2).
TABLE 2.
Localization of SIV-infected cells in neonatal macaques by in situ hybridizationa
| Animal | Days p.i. | Spleen | Axillary LN | Mesenteric LN | Jejunumb | Ileumb | GALTc |
|---|---|---|---|---|---|---|---|
| 68-97 | 3 | + | − | − | − | − | − |
| 69-97 | 3 | + | − | − | − | − | − |
| 102-97 | 7 | ++++ | +++ | ++++ | + | + | ++++ |
| 103-97 | 7 | ++++ | +++ | ++++ | +++ | +++ | ++++ |
| 136-97 | 14 | +++ | ++++ | ++++ | ++ | ++ | +++ |
| 137-97 | 14 | ++ | ++ | ++ | + | + | +++ |
| 190-97 | 21 | ++ | +++ | +++ | + | + | +++ |
| 191-97 | 21 | ++ | +++ | ++ | + | + | ++ |
| 296-97 | 50 | +++ | ++++ | ND | + | + | ++ |
| 297-97 | 50 | +++ | ++++ | ++ | + | + | ++ |
| 415-97 | 81 | +++ | ++++ | ++++ | + | + | + |
| 484-97 | 142 | ++ | ++ | ++ | + | + | ND |
| 412-97 | 209 | ++ | + | + | + | + | ND |
Plus signs indicate the relative number of SIV+ cells per 0.5-mm2 field (magnification, × 100), as follows: +, <5; ++, 5-10; +++, 11-15; ++++, >15; −, no infected cells were detected. ND, not determined; LN, lymph node.
Lamina propria only, excluding organized lymphoid tissues.
GALT is defined here as organized lymphoid tissues (Peyer's patches and lymphoid aggregates).
RESULTS
Viral loads in blood.
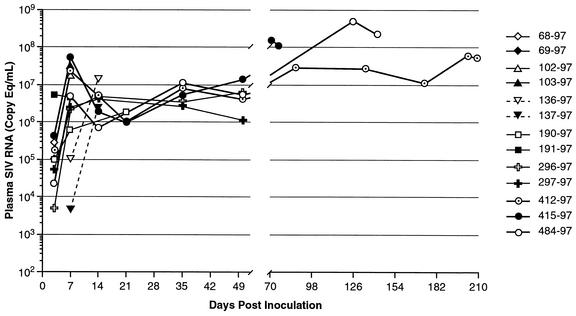
Mean viral loads in these neonates have previously been published elsewhere (43). Individual data are shown here for comparison with changes in T cells in tissues. In general, early peak viral loads were attained between 7 and 14 days of infection, with most neonates peaking by day 7 (Fig. 1). Although early (<21 days postinfection [p.i.]) viral loads were similar to those previously reported for adult macaques (data not shown), viral loads continued to increase in neonates that were allowed to progress to AIDS (Fig. 1). Of note, viral loads at day 7 were highest in macaque 415-97, and this animal continued to maintain high viral loads through day 79, when he was euthanized for diarrhea and neurologic signs. Of the three macaques that progressed to AIDS, the highest viral loads overall were observed in macaque 484-97 at 4 to 5 months of infection (Fig. 1).
FIG. 1.
Viral loads in plasma of neonatal macaques infected with SIVmac239 as determined by real-time reverse transcription-PCR. Note that viral loads stay relatively high in pediatric macaques throughout the course of infection.
Localization of virus in tissues.
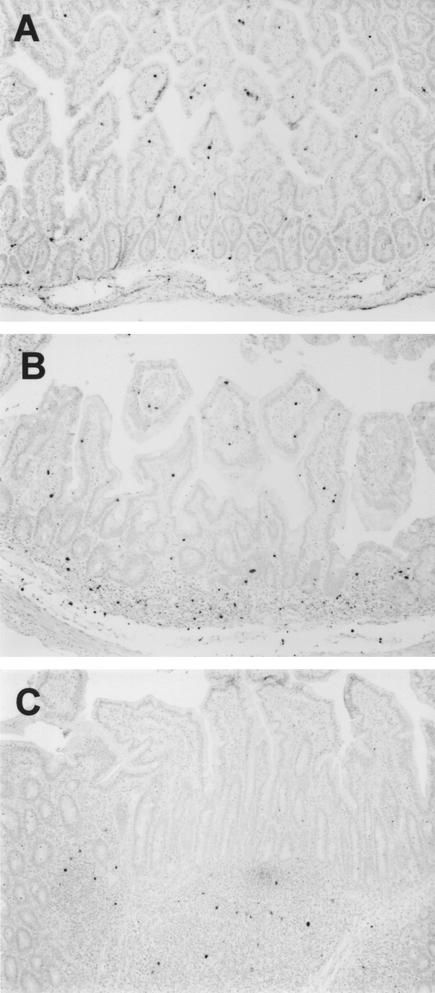
The first tissue in which virus could be detected by in situ hybridization was the spleen (Table 2). Small numbers of virus-infected cells (fewer than 5 per 0.5-mm2 field) were detected in the spleens of both macaques euthanized 3 days after infection. By 7 days, the infection was widely disseminated, with large numbers of SIV-infected cells in the spleen, lymph nodes, and the intestinal inductive lymphoid tissues (organized gut-associated lymphoid tissues [GALT]) of both macaques (Table 2). One of these macaques (mm103-97) also had large numbers of SIV-infected cells in the intestinal effector lymphoid tissues (diffuse lamina propria) of the jejunum (Fig. 2A). The other (mm102-97) had detectable, but fewer infected cells in the intestinal lamina propria. By 14 days of infection, one macaque (mm136-97) had large numbers of SIV-infected cells in the axillary and mesenteric lymph nodes, spleen, and GALT and moderate numbers of infected cells in the intestinal lamina propria (Fig. 2B). Macaque 137-97 also had large numbers of SIV-infected cells in the GALT but few to moderate numbers of infected cells in other tissues (Table 2). After 14 days of infection, large numbers of SIV-infected cells persisted in the axillary lymph nodes and spleen. By day 21, SIV-infected cells were still present in the Peyer's patches, but very few infected cells were detected in the intestinal lamina propria at this time point (Fig. 2C; Table 2).
FIG. 2.
In situ hybridization for SIV in the intestine of neonatal macaques 7 days (mm103-97) (A), 14 days (mm136-97) (B), and 21 days (mm191-97) (C) after intravenous SIVmac239 infection. Note that in early infection, large numbers of SIV-infected cells are detected throughout the lamina propria of the jejunum (A). By 14 days of infection, when intestinal CD4+ T cells are being depleted (B), there are fewer SIV-infected cells in the lamina propria of the ileum but large numbers remain in the inductive lymphoid tissues (Peyer's patches and organized lymphoid tissue). Finally, by 21 days of infection (a period of maximal lamina propria CD4+ T-cell depletion), very few infected cells are observed in the lamina propria of the ileum and most of the SIV-infected cells are observed in the inductive sites (C). Also note that the ileum (B and C) contains both effector lymphoid issues (lamina propria) and inductive lymphoid tissues (organized lymphoid follicles), whereas the jejunum lacks organized lymphoid tissues (A).
Among the three animals examined with AIDS, there were variable numbers of infected cells in tissues. One macaque killed at day 81 (mm415-97) had large numbers of infected cells, whereas the macaque sacrificed at 209 days of infection had markedly fewer infected cells in all lymphoid tissues (Table 2). In general, viral loads in tissues correlated well with viral loads in plasma, particularly in animals that were allowed to progress to AIDS. Note that in both tissues and plasma, viral loads spiked at 7 to 14 days p.i. and again at day 50 or later (Fig. 1; Table 2). In macaque 136-97, viral loads in plasma peaked at day 14, which was also reflected by high viral loads in tissues at this time point (Fig. 1; Table 2). The relative percentage of SIV-infected cells in lymphoid tissues as well as plasma viral load levels also positively correlated with the percentage of CD4+ T cells remaining in the lymphoid tissues (see the description of intestinal CD4 depletion, below).
Neonates have more CD4+ T cells in blood and tissues than adults.
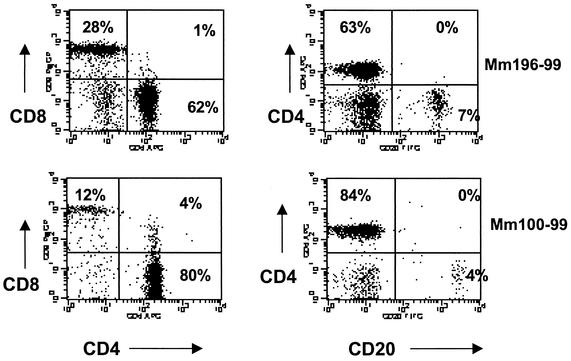
Uninfected normal neonates had significantly higher CD4+ T-cell ratios in their peripheral blood (P < 0.01) and axillary lymph nodes (P < 0.02) than adults, particularly in newborns (Table 3). Although considerable variation was observed between individuals, most newborn macaques had fewer circulating B cells and CD8+ T cells, with CD4+ T cells making up the majority of the lymphocyte population of the blood (Fig. 3). The CD4/CD8 ratios in the axillary lymph nodes and blood of neonates averaged 3.9 and 3.5, whereas those for adults were 1.6 and 1.0, respectively (Table 3). Significantly higher CD4/CD8 ratios were also detected in the colon (P < 0.05). Higher CD4/CD8 ratios were also present in the mesenteric lymph nodes, spleen, jejunum, and ileum of newborn macaques, but these differences were not statistically significant, probably due to the small numbers of animals examined (Table 3). Still, considerably higher percentages of CD8+ T cells were detected in the intestine than in the peripheral lymphoid tissues.
TABLE 3.
Comparison of CD4/CD8 ratios in lymphoid tissues of newborn and adult macaquesa
| Tissueb | CD4/CD8 ratio
|
P value | |
|---|---|---|---|
| Newborn | Adult (>4 yrs) | ||
| Axillary LN | 3.9 ± 0.7 | 1.6 ± 0.3 | <0.02 |
| Mesenteric LN | 3.3 ± 0.7 | 2.4 ± 0.5 | <0.7 |
| Spleen | 2.1 ± 0.3 | 1.2 ± 0.4 | <0.2 |
| Jejunum LPL | 1.5 ± 0.4 | 0.9 ± 0.2 | <0.3 |
| Ileum LPL | 1.7 ± 0.4 | 1.3 ± 0.4 | <0.5 |
| Colon LPL | 2.0 ± 0.5 | 1.1 ± 0.3 | <0.05 |
| Blood | 3.5 ± 0.3 | 1.0 ± 0.1 | <0.01 |
Values are means ± standard errors of the means.
LN, lymph node.
FIG. 3.
Lymphocyte phenotypes in the blood of newborn macaques. Flow cytometry dot plots demonstrate lymphocyte subsets in the blood of two different newborn macaques (mm196-99 and mm100-99). Plots were generated by gating through lymphocytes.
CD4+ T cells in the jejunum of newborns lack CD45RA, and many coexpress HLA-DR (an activated, memory phenotype).
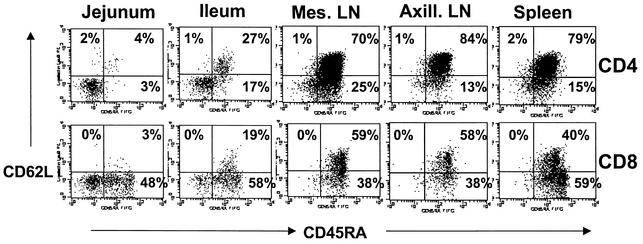
As with human infants (9), peripheral blood CD4+ and CD8+ T cells of neonatal macaques were mostly naïve, as determined by coexpression of both CD45RA and CD62L (data not shown). In contrast, the immunophenotype of CD4+ and CD8+ T cells isolated from the intestines of neonates, particularly in the lamina propria of the jejunum, was markedly different. In Fig. 4, note that most of the CD4+ T cells in the peripheral lymphoid tissues (lymph nodes, spleen) are naive (CD45RA+ CD62L+), whereas CD4+ T cells isolated from the jejunum lack these markers. Of the four newborn macaques examined, most of the intestinal CD4+ T cells were CD45RA negative (Fig. 4). Three of the four newborn macaques examined also had very low (less than 7%) expression of CD62L on CD4 cells from the jejunum, but one neonate had slightly higher levels of CD62L (22% of jejunum CD4+ T cells). With this exception, the data in Fig. 4 are representative of the four newborn macaques examined.
FIG. 4.
Expression patterns of CD45RA and CD62L (L-selectin) on CD4+ and CD8+ T cells from various tissues of a newborn macaque. Plots were generated by gating first on lymphocytes and then through CD4+ (top panels) or CD8+ (bottom panels) T cells. Data are representative of three of four newborn macaques examined (see text).
While the majority of CD4+ T cells from the lymph nodes and spleen of newborns coexpressed CD45RA and CD62L, consistent with a naïve phenotype, both of the 14-day-old, uninfected macaques and one of the 50-day-old uninfected macaques had relatively high expression of CD45RA on intestinal CD4+ T cells, although few of these CD45RA+ cells coexpressed CD62L (data not shown). This could reflect either age-related developmental changes and/or continual replenishment and renewal of naïve CD4+ T cells occurring in tissues in the first few weeks after birth. Alternatively, it could be due to marked variation in individuals and reflect the small number of animals examined in this study.
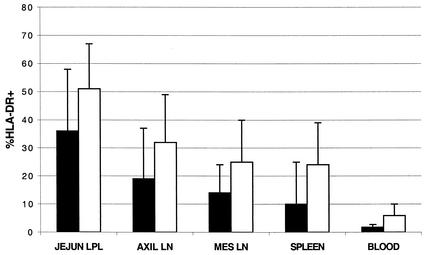
As in adults (36), CD4+ T cells in the jejunum of newborn macaques expressed high levels of HLA-DR, although there was substantial variation between individuals. As shown in Fig. 5, much greater percentages of CD4+ and CD8+ T cells expressing HLA-DR were present in the jejunum compared to blood, spleen, or lymph nodes. Between 13 and 67% of the CD4+ T cells from the jejunum coexpressed HLA-DR, indicating that neonates are born with relatively high expression of this activation molecule on their CD4+ T cells, especially those in the jejunum (Fig. 5). In contrast, only 1.5 to 12% of blood, 6 to 44% of lymph node, and 6 to 43% of spleen CD4+ T cells coexpressed HLA-DR (Fig. 5). CD8+ T cells showed similar (but consistently higher) levels of activation in the jejunum (Fig. 5). Ileum and colon LPL had intermediate HLA-DR expression, with slightly lower HLA-DR coexpression than jejunum LPL but higher than that in lymphocytes from peripheral lymphoid tissues (data not shown). The large error bars in Fig. 5 reflect the small number of newborn macaques examined (n = 4) as well as individual animal variation, but among individuals HLA-DR expression was consistently higher on intestinal lymphocytes than peripheral lymphoid tissues. Treating blood, spleen, and lymph node cells using the same procedures involved in isolating intestinal cells failed to change surface markers (including HLA-DR), proving that the increased activation of intestinal lymphocytes was not an artifact of tissue-processing methods (data not shown). Moreover, since these animals were euthanized and examined within minutes of birth, this suggests that some type of activation of intestinal CD4+ T cells may occur in utero.
FIG. 5.
Coexpression of HLA-DR on CD4+ cells (black bars) and CD8+ cells (white bars) from various tissues of a newborn macaque. Bars represent means of four neonates examined on the day of birth ± the standard deviation. LN, lymph node.
Rapid, profound CD4 depletion occurs in the jejunum of neonatal macaques.
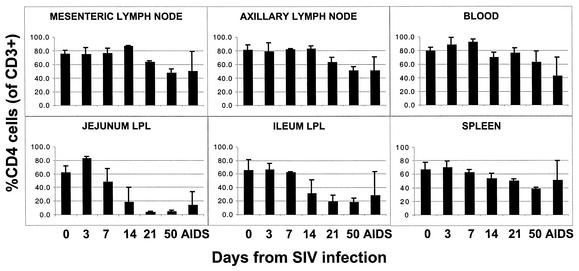
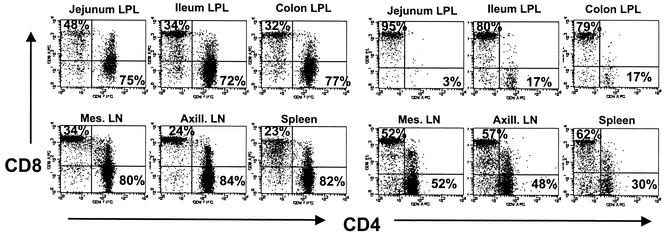
In both acute and chronically infected neonates, the most striking changes in T-cell subsets were observed in the jejunum and, to a slightly lesser extent, the ileum and colon. Profound depletion of CD4+ T cells in the jejunum was observed in SIV-infected neonates by 21 days p.i. (Fig. 6). Although marked and rapid CD4+ T-cell depletion was observed in all three regions of the intestine, it was consistently more pronounced in the jejunum compared to the ileum and colon (Fig. 6 and 7). In uninfected macaques, CD4+ T cells comprised 52 to 75% of the T cells (gating through CD3+) in the jejunum; however, fewer than 5% of the T cells remaining in the jejunum at day 21 p.i. were CD4+ (Fig. 6). Gating through CD3+ T cells confirmed that this was an actual loss of CD4+ T cells, rather than down-regulation or masking of CD4, since no increase in CD4+ CD8+ double-negative cells was detected within the CD3+ T-cell population (Fig. 7). Both neonates examined at 50 days p.i. also had similar marked CD4+ T-cell depletion in the jejunum and moderate depletion in the ileum (Fig. 6 and 7). Data shown in Fig. 7 are representative of all neonates examined at days 21 and 50 and one of two neonates examined at day 14 p.i. Marked CD4 depletion was also observed in the jejunum and ileum of two of three neonates euthanized with AIDS. Remarkably, the other neonate (mm484-97) sacrificed at 141 days of infection with AIDS had large percentages of CD4+ T cells remaining in the LPL of the jejunum (37%), ileum (68%), and colon (59%), which directly correlated with the sustained viral loads in this macaque (Fig. 1). Note that the higher mean CD4+ T-cell percentages depicted in the jejunum and other tissues of neonates with AIDS in Fig. 6 are due to the higher percentages remaining in the intestine of this one neonate (mm484-97), as reflected by the large error bars (Fig. 6).
FIG. 6.
Changes in CD4+ T-cell levels in various tissues of uninfected and SIV-infected neonatal macaques. Each bar reflects the mean of at least two animals ± the standard deviation. Data indicate the percentage of CD3+ T cells coexpressing CD4. Data from uninfected newborn neonates are displayed at day 0.
FIG. 7.
Dot plots comparing T-cell subsets from intestinal (top panels) and peripheral (bottom panels) lymphoid tissues of a normal, age-matched, uninfected neonatal macaque (mm103-98; 50 days old) (left) with those from an SIVmac239-infected neonate (mm296-97; 50 days p.i.) (right). Plots were generated by gating through lymphocytes and then through CD3+ T cells. Thus, the CD4+ T-cell loss is accompanied by an increased percentage of CD8+ T cells remaining in the T-cell gate.
In contrast to the jejunum lamina propria, relatively mild to moderate decreases in CD4+ T cells were observed in other regions of the intestine and the peripheral lymphoid tissues at these early time points (Fig. 6 and 7), similar to what has been observed in adult macaques (36). A steady decrease in CD4+ T cells was observed in the spleen of neonates; however, relatively large numbers of CD4+ T cells (37 to 40%) persisted in this tissue at 50 days p.i. (Fig. 6 and 7). Decreases in CD4+ T cells in the axillary and mesenteric lymph nodes were mild and less pronounced compared to the intestine and spleen at these early time points. In axillary lymph nodes, CD4+ T cells of neonates infected for 50 days represented 48 to 55% of total T (CD3+) cells and 44 to 52% in the mesenteric lymph nodes. For comparison, uninfected neonates at 50 days of age had 69 to 84% CD4+ T cells in the axillary lymph nodes and 73 to 80% CD4+ T cells in the mesenteric lymph nodes (Fig. 6 and 7). Profound CD4+ T-cell depletion was not observed in the lymph nodes and spleen until the development of AIDS, and even then, only one (mm412-97) of the three neonates examined had marked peripheral CD4+ T-cell depletion. This macaque had 17% CD4+ T cells remaining in the lymph nodes and 22% in the spleen. The other two neonates with AIDS had relatively high percentages of CD4+ T cells remaining in the lymph nodes (58 to 71% of total CD3+ T cells) and spleen (52 to 80%) despite the development of AIDS and the presence of opportunistic infections.
A moderate decrease in absolute numbers of CD4+ T cells in the blood was observed in the first few weeks of infection (data not shown). However, most of this early decrease was due to a decrease in total lymphocytes, as the percentages of CD4+ T cells in the blood did not differ significantly from those of uninfected normal macaques (Fig. 6).
Few other significant immunophenotypic changes were detected in SIV-infected neonates. Essentially all CD4+ and CD8+ T cells coexpressed CD38, both in uninfected and SIV-infected macaques (data not shown). In addition, all CD4+ T cells from both infected and uninfected neonates coexpressed CD28 (data not shown). Approximately 50% of CD8+ T cells coexpressed CD28, but there was significant variation between individual neonates (both infected and uninfected); thus, no changes could be directly attributed to SIV infection (data not shown). However, levels of CD28 and CD38 were similar to levels observed on lymphocyte subsets from adult macaques (unpublished observations). Combined, these data and other recent data (17) suggest that neonatal lymphocytes express normal levels of costimulatory and activation molecules and are capable of mounting immune responses at birth.
Neonates also had variable, yet substantial percentages of γδ T cells in the intestinal epithelium (2 to 21% of intestinal epithelial lymphocytes), but no statistically significant changes in this population were observed in SIV-infected macaques (data not shown).
DISCUSSION
These results demonstrate that, as in adults, acute SIV infection of neonates results in profound, rapid, and selective depletion of intestinal lamina propria CD4+ T cells. Within 14 to 21 days of SIV infection, CD4+ T cells are markedly depleted in the lamina propria of the jejunum and, to a lesser extent, the ileum and colon of neonatal macaques. In considering the GALT, it is important to distinguish effector from inductive lymphoid tissues. As in adult SIV-infected macaques (36) and HIV-infected humans (3, 15), early intestinal depletion of CD4+ T cells is consistently most severe in the lamina propria of the proximal small intestine (jejunum and/or duodenum), because this is a purely effector lymphoid tissue containing predominantly activated, terminally differentiated lymphocytes in the lamina propria. In contrast, relatively large proportions of the lymphocytes in the ileum and large intestine (colon and rectum) reside within inductive lymphoid tissues, namely, Peyer's patches and organized lymphoid follicles. Lymphocytes in inductive lymphoid tissues are mostly naïve, resting cells that may be spared in SIV infection due to differences in both their phenotype and a lower state of cellular activation (3, 14, 26, 37, 41). Since the jejunum lacks organized lymphoid tissues, profound CD4+ T-cell depletion consistently occurs in this region of the intestine. Marked reductions in the number of CD4+ T cells are also observed in the lamina propria of the ileum and colon (Fig. 7). However, cell suspensions prepared from these sites invariably contain cells from organized lymphoid tissues as well, resulting in less overall CD4+ T-cell depletion and larger percentages of naïve, resting T cells remaining in these samples compared to those from the jejunum (Fig. 7).
In contrast to the intestinal lamina propria, CD4+ T cells are relatively spared in peripheral lymphoid tissues (lymph nodes, spleen, and blood) and, in some cases, even in neonates that have progressed to AIDS. One neonate that was allowed to progress to AIDS had substantial numbers (37%) of CD4+ T cells remaining in the jejunum. However, multiparameter flow cytometry showed that the remaining CD4+ T lymphocytes in this neonate were virtually all naïve (CD45RA+ CD62L+) cells, which supports our hypothesis that only terminally differentiated CD4+ T cells are being eliminated in SIV infection, presumably due to the direct effects of viral replication. Although only a small number of animals was examined in this study, we have recently reported a similar phenomenon in an SIV-infected adult macaque with AIDS that had relatively high numbers of naïve, CCR5-negative CD4+ T cells remaining in both mucosal and peripheral lymphoid tissues (39). Combined, these data are consistent with those from SIV-infected adult macaques, in which rapid and profound CD4+ T-cell depletion is limited to effector lymphoid tissues in mucosal sites, and those data indicating that CD4+ T cells in inductive lymphoid tissues (Peyer's patches and peripheral lymphoid tissues) are spared due to the high proportion of naïve, resting cells which may be infected but are apparently less supportive of efficient viral replication (36-39, 41).
In situ hybridization also confirmed that the very early targets for viral infection and amplification in the intestine are located in the effector lymphoid tissues. Relatively large numbers of SIV-infected lymphocytes are detected in the intestinal lamina propria between day 7 and 14 p.i. (Fig. 2A). At this early time point, activated CD4+ T cells are still abundant in the lamina propria. However, once these terminally differentiated CD4+ T cells are depleted (between day 14 and 21 p.i.), most of the remaining SIV-infected cells are found in inductive lymphoid tissues, with considerably fewer infected cells persisting in the lamina propria (Fig. 1C). In other words, once these terminally differentiated, effector CD4+ T cells are eliminated from the lamina propria, they are never fully replenished in animals that progress to disease, and SIV-infected cells at later time points are mostly limited to the inductive lymphoid tissues of the intestine. Thus, we hypothesize that activated, effector CD4+ T cells in the intestinal lamina propria are the major source of early viral amplification and, once eliminated, the inductive lymphoid tissues of the intestine (and peripheral lymphoid tissues) are the major sites of viral persistence in SIV-infected neonates.
Although the results reported in Table 3 suggest that the intestinal lamina propria had fewer infected cells than other tissues, it should be emphasized that the lamina propria of the intestine has a unique morphology and that the lymphocytes are scattered throughout a large bed of connective tissue and interspersed intestinal glands and crypts, resulting in a low lymphocyte-to-area ratio. In contrast, the organized lymphoid tissues (Peyer's patches, lymph nodes, spleen, etc.) consist primarily of densely packed lymphocytes with a very high cell-to-area ratio. Furthermore, the length and mass of the intestinal tract should be considered when comparing the actual numbers of lymphocytes between tissues. Therefore, demonstrating a moderate number of infected cells in one section of intestinal lamina propria reflects a substantial proportion of the infected cells in the entire body when the length and mass of the entire intestinal tract are considered.
It is increasingly evident that the gastrointestinal tract is of major importance in the pathogenesis of HIV infection (10, 38). Using the macaque model of SIV, several studies have confirmed that the intestinal tract is a major target organ for viral replication and CD4+ T-cell depletion in early SIV infection in adult macaques. Within 14 to 21 days of SIV infection, profound depletion of CD4+ T cells occurs specifically within the intestinal tract (10, 30, 36). Since the intestinal tract is the largest lymphoid organ in the body (18), this results in a tremendous loss of CD4+ T cells within the first few weeks of infection. In adults, the majority of CD4+ T cells in the intestinal lamina propria have an activated, memory phenotype and coexpress CCR5 (1, 37, 41). Since HIV and SIV primarily replicate to high levels within activated, memory CD4+ T cells (16, 19, 27, 31, 38), this could explain why the lamina propria is the major target organ in early HIV and SIV infection. Memory CD4+ T cells have also been shown to be the major viral target cell in HIV-infected children (29). However, the majority of lymphocytes in the blood of neonates are naïve, resting cells (4, 9). If SIV and HIV optimally replicate within activated, memory CD4+ T cells, why is it that many children have a more rapid progression to disease? Since neonates were expected to have much lower levels of activated, memory CD4+ T cells, such a profound and rapid depletion of intestinal CD4+ T cells was somewhat unexpected. However, the high percentage of activation markers (HLA-DR) on intestinal CD4+ T cells at birth (Fig. 4) suggests that intestinal lymphocytes may be activated and functional at birth. Furthermore, the paucity of CD45RA and CD62L on intestinal lamina propria CD4+ T cells in newborn macaques indicates that these CD4+ T cells differ from those in peripheral lymphoid tissues (Fig. 3) in that at least some may already be terminally differentiated lymphocytes. Supporting evidence for this has also been demonstrated in fetal macaque tissues (17). Makori et al. recently reported large numbers of functional (cytokine- and/or immunoglobulin-secreting) T cells, B cells, and antigen-presenting cells in the intestine and spleens of second-trimester macaque fetuses (17). The findings reported here demonstrating an abundance of CD4+ T-helper cells in various lymphoid tissues of newborn macaques, many of which express HLA-DR, also support that the newborn immune system is adequately primed to mount an immune response. Taken together, these data suggest that lymphocytes from newborns could be primed in utero to respond to antigens that they may encounter in the environment soon after birth. Furthermore, upon exposure to environmental antigens and/or food, the remaining intestinal CD4+ T cells may be primed for rapid activation and expansion, resulting in an increase in turnover in activated T cells in the intestine and other tissues. Perhaps the key to understanding the pathogenesis of SIV and HIV in the pediatric patient lies in assessing the rate of turnover of CD4+ T cells in tissues during the first few weeks after birth. The rapid disease progression and high viral loads observed in pediatric patients may be associated with a higher rate and magnitude of CD4+ T-cell activation and turnover in the pediatric host.
Once the CD4+ T cells were depleted from the intestinal lamina propria, viral loads in this region remained very low. However, large numbers of SIV-infected cells persisted in the lymph nodes and other organized lymphoid tissues from day 7 through 50 and in two neonates (mm415-97 and 484-97) with AIDS. Interestingly, the animal with the lowest viral loads in tissues (mm412-97) was the only one of the three neonates that demonstrated profound depletion of CD4+ T cells in the lymph nodes, spleen, and blood. Conversely, the macaque with the highest viral loads in both tissues and plasma had the highest percentage of CD4+ T cells remaining in tissues. Although the number of animals examined at each time point was small, the combined data suggest a possible correlation between viral loads and the persistence of viral target cells in the pediatric host. These data are consistent with an important role for target cell limitation in contributing to viral load levels, a hypothesis originally proposed by Phillips (23).
In summary, the spleen and intestinal lamina propria appear to be the major sites of early viral replication and amplification in the neonatal host. Moreover, CD4+ T cells in the intestinal lamina propria of newborns are unique compared to peripheral lymphoid tissues in that they mostly express an activated, memory phenotype similar to that of adults. This suggests that priming of intestinal lymphocytes may occur in utero which renders these cells susceptible to SIV or HIV infection and viral replication. However, additional studies are needed to determine the mechanisms of such priming. These data also suggest that the reason neonates may have higher viral loads and a more rapid progression to disease could be that they have more CD4+ T cells than adults and, more importantly, a higher and/or more persistent turnover of activated, memory CD4+ T cells than adults. Another possibility is that larger numbers of infected CD4+ T cells may persist in neonates, through incomplete activation and/or a greater resistance to apoptosis associated with SIV or HIV infection. Additional studies of CD4+ T-cell activation and turnover in SIV-infected neonates are under way to evaluate the hypothesis that increased target cell turnover in the pediatric patient is responsible for higher viral loads and more rapid disease progression in the neonatal host. If so, therapies directed against specific types of lymphocyte activation and turnover may help to reduce viral loads and disease progression in pediatric patients.
Acknowledgments
We thank Janell LeBlanc, Robin Rodriguez, Irene Tham, and Daniel Shvetz for technical assistance, Michael O'Connell for study coordination, and Elizabeth Curran for her excellent care of the neonatal macaques.
This work was supported by Elizabeth Glaser Pediatric AIDS Foundation grant PG-50861, by National Institutes of Health grants HD36310, NS30769, and DK50550, and by federal funds from the National Cancer Institute, National Institutes of Health, under contract N01 CO12400. A.A.L. is a recipient of an Elizabeth Glaser Scientist award.
REFERENCES
- 1.Anton, P. A., J. Elliott, M. A. Poles, I. M. McGowan, J. Matud, L. E. Hultin, K. Grovit-Ferbas, C. R. Mackay, I. S. Y. Chen, and J. V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14:1761-1765. [DOI] [PubMed] [Google Scholar]
- 2.Butler, K. M., and P. A. Pizzo. 1992. HIV infection in children, p. 285-312. In V. T. DeVita, Jr., S. Hellman, and S. A. Rosenberg (ed.), AIDS: etiology, diagnosis, treatment, and prevention, 3rd ed. J. B. Lippincott Co., Philadelphia, Pa.
- 3.Clayton, F., G. Snow, S. Reka, and D. P. Kotler. 1997. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107:288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeMaria, M. A., M. Casto, M. O'Connell, R. P. Johnson, and M. Rosenzweig. 2000. Characterization of lymphocyte subsets in rhesus macaques during the first year of life. Eur. J. Haematol. 65:245-257. [DOI] [PubMed] [Google Scholar]
- 5.de Martino, M., P.-A. Tovo, M. Balducci, L. Galli, C. Gabiano, G. Rezza, and P. Pezzotti. 2000. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA 284:190-197. [DOI] [PubMed] [Google Scholar]
- 6.Denny, T., R. Yogev, R. Gelman, C. Skuza, J. Oleske, E. Chadwick, S. C. Cheng, and E. Connor. 1992. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA 267:1484-1488. [PubMed] [Google Scholar]
- 7.De Rossi, A., S. Masiero, C. Giaquinto, E. Ruga, M. Comar, M. Giacca, and L. Chieco-Bianchi. 1996. Dynamics of viral replication in infants with vertically acquired human immunodeficiency virus type 1 infection. J. Clin. Investig. 97:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes, B. F., M. E. Martin, H. H. Kay, and J. Kurtzberg. 1988. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J. Exp. Med. 168:1061-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt, P. G. 1995. Postnatal maturation of immune competence during infancy and childhood. Pediatr. Allergy Immunol. 6:59-70. [DOI] [PubMed] [Google Scholar]
- 10.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F. J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 116:1115-1123. [DOI] [PubMed] [Google Scholar]
- 11.Kourtis, A. P., S. R. Nesheim, D. Thea, C. Ibegbu, A. J. Nahmias, and F. K. Lee. 2000. Correlation of virus load and soluble L-selectin, a marker of immune activation, in pediatric HIV-1 infection. AIDS 14:2429-2436. [DOI] [PubMed] [Google Scholar]
- 12.Krogstad, P., C. H. Uittenbogaart, R. Dickover, Y. J. Bryson, S. Plaeger, and A. Garfinkel. 1999. Primary HIV infection of infants: the effects of somatic growth on lymphocyte and virus dynamics. Clin. Immunol. 92:25-33. [DOI] [PubMed] [Google Scholar]
- 13.Lane, J. H., A. F. Tarantal, D. Pauley, M. Marthas, C. J. Miller, and A. A. Lackner. 1996. Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am. J. Pathol. 149:1097-1103. [PMC free article] [PubMed] [Google Scholar]
- 14.Lapenta, C., M. Boirivant, M. Marini, S. M. Santini, M. Logozzi, M. Viora, F. Belardelli, and S. Fais. 1999. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 29:1202-1208. [DOI] [PubMed] [Google Scholar]
- 15.Lim, S. G., A. Condez, C. A. Lee, M. A. Johnson, C. Elia, and L. W. Poulter. 1993. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92:448-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahalingham, M., M. Peakman, E. T. Davies, A. Pozniak, T. J. McManus, and D. Vergani. 1993. T cell activation and disease severity in HIV infection. Clin. Exp. Immunol. 93:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makori, N., A. F. Tarantal, F. X. Lu, T. Rourke, M. L. Marthas, M. B. McChesney, A. G. Hendrickx, and C. J. Miller. 2003. Functional and morphological development of lymphoid tissues and immune regulatory and effector function in rhesus monkeys: cytokine-secreting cells, immunoglobulin-secreting cells, and CD5+ B-1 cells appear in early fetal development. Clin. Diagn. Lab. Immunol. 10:140-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannick, E., and J. N. Udall. 1996. Neonatal gastrointestinal immunology. Clin. Perinatol. 23:287-303. [PubMed] [Google Scholar]
- 19.McDougal, J. S., A. Mawle, S. P. Cort, J. K. A. Nicholson, G. D. Cross, J. A. Schleppler-Campbell, D. Hicks, and J. Sligh. 1985. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J. Immunol. 135:3151-3162. [PubMed] [Google Scholar]
- 20.Ometto, L., R. Bertorelle, M. Mainardi, M. Zanchetta, S. Tognazzo, O. Rampon, E. Ruga, L. Chieco-Bianchi, and A. De Rossi. 2001. Polymorphisms in the CCR5 promoter region influence disease progression in perinatally human immunodeficiency virus type 1-infected children. J. Infect. Dis. 183:814-818. [DOI] [PubMed] [Google Scholar]
- 21.Palumbo, P. E., S. Kwok, S. Waters, Y. Wesley, D. Lewis, N. McKinney, A. Bardeguez, E. M. Connor, and J. M. Oleske. 1995. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J. Pediatr. 126:592-595. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo, P. E., C. Raskino, S. Fiscus, S. Pahwa, M. G. Fowler, S. A. Spector, J. A. Englund, and C. J. Baker. 1998. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA 279:756-761. [DOI] [PubMed] [Google Scholar]
- 23.Phillips, A. N. 1996. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science 271:497-498. [DOI] [PubMed] [Google Scholar]
- 24.Pikora, C. A., J. L. Sullivan, D. Panicali, and K. Luzuriaga. 1997. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J. Exp. Med. 185:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzo, P. A., and C. M. Wilfert. 1995. Markers and determinants of disease progression in children with HIV infection. The Pediatric AIDS Siena Workshop II. J. Acquir. Immune Defic. Syndr. 8:30-44. [PubMed] [Google Scholar]
- 26.Poles, M. A., J. Elliott, P. Taing, P. A. Anton, and I. S. Chen. 2001. A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnittman, S. M., H. C. Lane, J. Greenhouse, J. S. Justement, M. Baseler, and A. S. Fauci. 1990. Preferential infection of CD4+ memory cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. USA 87:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, G. B., C. Hutto, R. W. Makuch, M. T. Mastrucci, T. O'Connor, C. D. Mitchell, E. J. Trapido, and W. P. Parks. 1989. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N. Engl. J. Med. 321:1791-1796. [DOI] [PubMed] [Google Scholar]
- 29.Sleasman, J. W., L. F. Aleixo, A. Morton, S. Skoda-Smith, and M. M. Goodenow. 1996. CD4+ memory T cells are the predominant population of HIV-1 infected lymphocytes in neonates and children. AIDS 10:1477-1484. [DOI] [PubMed] [Google Scholar]
- 30.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel, H. M., E. DeFalcon, G. S. Ogg, M. Larsson, T. J. Beadle, P. Tao, A. J. McMichael, N. Bhardwaj, C. O'Callaghan, W. I. Cox, K. Krasinski, H. Pollack, W. Borkowsky, and D. F. Nixon. 1999. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J. Infect. Dis. 180:359-368. [DOI] [PubMed] [Google Scholar]
- 32.Spina, C. A., H. E. Prince, and D. D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Investig. 99:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 35.Tovo, P. A., M. de Martino, C. Gabiano, N. Cappello, R. D'Elia, A. Loy, A. Plebani, G. V. Zuccotti, P. Dallacasa, and G. Ferraris. 1992. Prognostic factors and survival in children with perinatal HIV-1 infection. The Italian Register for HIV Infections in Children. Lancet 339:1249-1253. [DOI] [PubMed] [Google Scholar]
- 36.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 37.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633. [DOI] [PubMed] [Google Scholar]
- 39.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187:769-776. [DOI] [PubMed] [Google Scholar]
- 40.Veazey, R. S., M. Rosenzweig, D. E. Shvetz, D. R. Pauley, M. DeMaria, L. V. Chalifoux, R. P. Johnson, and A. A. Lackner. 1997. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin. Immunol. Immunopathol. 82:230-242. [DOI] [PubMed] [Google Scholar]
- 41.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiblen, B. J., F. K. Lee, E. R. Cooper, S. H. Landesman, K. McIntosh, J. A. Harris, S. Nesheim, H. Mendez, S. I. Pelton, and A. J. Nahmias. 1990. Early diagnosis of HIV infection in infants by detection of IgA HIV antibodies. Lancet 335:988-990. [DOI] [PubMed] [Google Scholar]
- 43.Westmoreland, S. V., K. C. Williams, M. A. Simon, M. E. Bahn, A. E. Rullkoetter, M. W. Elliott, C. D. deBakker, H. L. Knight, and A. A. Lackner. 1999. Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am. J. Pathol. 155:1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilfert, C. M., and R. E. McKinney, Jr. 1998. When children harbor HIV. Sci. Am. 279:94-95. [DOI] [PubMed] [Google Scholar]
- 45.Wilfert, C. M., C. Wilson, K. Luzuriaga, and L. Epstein. 1994. Pathogenesis of pediatric human immunodeficiency virus type 1 infection. J. Infect. Dis. 170:286-292. [DOI] [PubMed] [Google Scholar]
- 46.Willerford, D. M., M. J. Gale, Jr., R. E. Benveniste, E. A. Clark, and W. M. Gallatin. 1990. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J. Immunol. 144:3779-3783. [PubMed] [Google Scholar]