Abstract
TT virus (TTV) produces chronic plasma viremia in around 90% of healthy individuals of all ages and has, therefore, been proposed as a commensal human virus. We recently demonstrated that in children hospitalized for acute respiratory diseases high TTV loads were associated with severe forms of disease. Here, we report that in such children TTV loads showed an inverse correlation with the percentage of circulating total T and helper T cells and a direct correlation with the percentage of B cells. Thus, florid TTV replication might contribute to lymphocyte imbalances and, possibly, immunosuppressive effects, thus resembling related animal viruses.
TT virus (TTV) has genome properties similar to those of animal circoviruses, such as chicken anemia virus and porcine circovirus. Because it produces chronic plasma viremia in around 90% of healthy individuals of all ages worldwide and no associated disease has yet been identified, it has been suggested that TTV might be totally apathogenic (for reviews, see references 1 and 11). Recently, we tested for the presence of TTV and assessed viral load by universal untranslated-region real-time PCR in 157 children under 2 years old with acute respiratory diseases (ARD) on the day of hospital admission and obtained convincing data that TTV might replicate in the respiratory tract (5). Also, although we found no evidence that TTV might be the direct cause of ARD, TTV loads in both nasal swabs and plasma samples were substantially higher in subjects with bronchopneumonia (BP) than in the subjects with milder ARD (laryngitis, bronchitis, and bronchiolitis), suggesting among other possibilities that TTV could be locally or systemically immunosuppressive and aggravate disease induced by other agents (5). However, there is no information on this matter except for recent reports showing an inverse relationship between TTV burdens and CD4 cell counts in patients with human immunodeficiency virus type 1 (2, 10, 13).
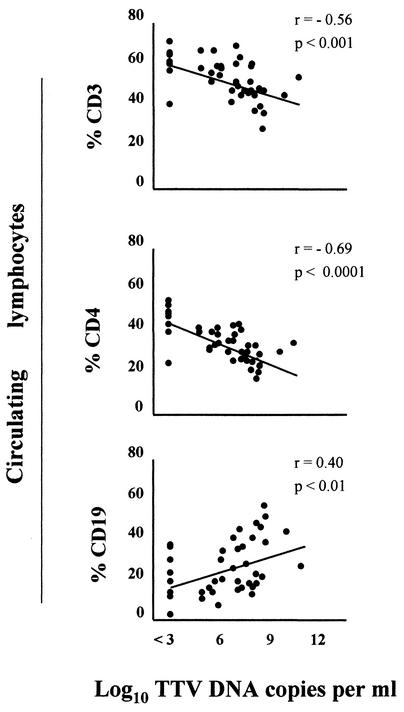
In this study, we examined, with informed parental consent, peripheral leukocyte and lymphocyte subset counts in 40 randomly chosen children with ARD from the above study (5). Of the 40 children, 18 had an X-ray-confirmed diagnosis of BP. The same blood samples used for TTV assays were analyzed. Features similar to those described for the entire sample (5) were confirmed in this subset. In particular, mean TTV loads in plasma were significantly higher in BP patients than in children with milder ARD (7.9 ± 1.2 versus 5.6 ± 2.3 log10 per ml [P < 0.001]). Interestingly, although all absolute cell counts were within the values reported for healthy children of similar age (3) with unknown TTV status (but presumably mostly TTV positive, based on what is currently known of TTV epidemiology [1, 11]), the percentages of CD3 (total T lymphocytes) and CD4 cells (T helper lymphocytes) showed an inverse correlation with the levels of TTV. By contrast, the percentages of CD19 cells (B lymphocytes) showed a positive correlation with TTV levels (Fig. 1). On the other hand, lymphocyte and TTV levels were unrelated to age (data not shown). Thus, high TTV loads, presence of BP, and low percentages of circulating CD4 cells were substantially correlated to one another. However, the correlation between the presence of BP and percentage of CD4 cells was greatly reduced after correcting for TTV loads (from r = −0.53 and P < 0.0001 to r = −0.27 and P = 0.09), whereas the correlation between the TTV level and percentage of CD4 cells persisted after correcting for BP status (from r = −0.69 and P < 0.0001 to r = −0.57 and P < 0.0001). Of note, the nasal swabs of 24 subjects tested positive for common respiratory viruses (CRV) (respiratory syncytial virus [RSV] [n = 19], cytomegalovirus [n = 3], parainfluenza virus [n = 1], and RSV and parainfluenza virus [n = 1]), whereas no CRV were detected in 16 subjects. These two subgroups showed no significant differences in TTV loads, and again, each exhibited most of the above correlations between TTV and lymphocyte subset count (data not shown). Thus, collectively these findings support the idea that vigorous TTV replication per se might contribute to important lymphocyte subpopulation imbalances.
FIG. 1.
Correlation between the percentages of CD3, CD4, and CD19 cells in peripheral blood samples and TTV loads in plasma samples from 40 infants with ARD. TTV load was determined with a universal real-time PCR assay directed to a highly conserved segment of the untranslated region of the viral genome (lower limit of detection, 103 DNA copies per ml). This assay has the potential to detect most if not all genetic forms of TTV hitherto described but not the TTV-like mini virus (5, 6). Total CD3, total CD4, and total CD19 lymphocyte counts were performed by fluorescence-activated cell sorting.
TTV is genetically highly heterogeneous and has been subdivided into five greatly divergent genogroups (genogroups 1 to 5) (8, 9) that theoretically could have different pathogenic potentials (1, 5, 9). The 32 TTV-positive plasma specimens in this study were characterized using five distinct PCR assays, each specific for one genogroup, and the PCR assay results are shown in Table 1. In line with previous findings (5, 9), there were numerous TTV-positive children who carried more than one genogroup, and these children had higher average total TTV loads and lower percentages of CD3 and CD4 cells than children with only one genogroup. Also in accord with previous findings (5, 9), the TTVs most frequently encountered belonged to genogroups 3, 1, and 4, with genogroup 3 being the most common. When the children harboring one of the three most common genogroups, as a single or a mixed infection, were compared against all other children whose TTV could be typed, their lymphocyte subpopulation values were significantly different in some cases. Collectively, these findings might indicate that certain TTV genogroups affect lymphocyte subpopulations more than others, but further data are clearly needed.
TABLE 1.
TTV genogroups detected in plasma samples of 32 study children found TTV positive by universal PCR
| Category | No. of children | Log10 TTV DNA copies/ml (mean ± SD) | Circulating lymphocytes (mean ± SD)
|
||
|---|---|---|---|---|---|
| % CD3 cells | % CD4 cells | % CD19 cells | |||
| TTV genogroup present | |||||
| Genogroup 1 | 16 | 8.1 ± 1.4a | 47.1 ± 9.5 | 28.3 ± 7.5 | 25.7 ± 13.7 |
| Genogroup 2 | 2 | 9.5 ± 2.2 | 48.5 ± 5.2 | 28.9 ± 4.7 | 20.2 ± 6.1 |
| Genogroup 3 | 21 | 7.8 ± 1.4 | 47.3 ± 8.3b | 28.2 ± 6.2c | 27.2 ± 12.7 |
| Genogroup 4 | 13 | 7.7 ± 1.9 | 50.9 ± 10.0 | 30.6 ± 6.5 | 26.1 ± 13.7 |
| Genogroup 5 | 5 | 8.2 ± 1.5 | 50.7 ± 6.8 | 30.6 ± 2.9 | 32.1 ± 9.5 |
| Untyped TTVd | 5 | 7.6 ± 1.2 | 50.7 ± 12.3 | 32.4 ± 7.4 | 32.2 ± 19.5 |
| No. of TTV genogroups | |||||
| 1 | 8 | 6.2 ± 0.9e | 56.4 ± 7.1f | 34.5 ± 6.3g | 18.8 ± 9.8 |
| 2 | 12 | 7.5 ± 1.1 | 46.6 ± 7.3 | 27.5 ± 7.2 | 24.7 ± 12.1 |
| 3 | 3 | 7.6 ± 1.3 | 43.4 ± 14.9 | 27.0 ± 8.0 | 33.2 ± 19.6 |
| 4 | 4 | 9.6 ± 1.3 | 49.9 ± 7.1 | 29.7 ± 2.6 | 30.0 ± 11.6 |
Statistically different from the value for the 16 children who had TTV genogroups other than genogroup 1 (mean, 6.9 ± 1.2) (P = 0.012 by Student's t test).
Statistically different from the value for the 11 children who had TTV genogroups other than genogroup 3 (mean, 54.6 ± 9.8) (P = 0.04 by Student's t test).
Statistically different from the value for the 11 children who had TTV genogroups other than genogroup 3 (mean, 34.1 ± 7.0) (P = 0.03 by Student's t test).
Specimens which gave positive results by universal PCR but gave negative results by all five genogroup-specific PCR assays.
Statistically different from the value for the 19 children who tested positive for two or more genogroups (mean, 8.0 ± 1.4) (P = 0.0007 by Student's t test).
Statistically different from the value for the 19 children who tested positive for two or more genogroups (mean, 46.8 ± 8.3) (P = 0.008 by Student's t test).
Statistically different from the value for the 19 children who tested positive for two or more genogroups (mean, 27.9 ± 6.4) (P = 0.025 by Student's t test).
In conclusion, before TTV is dismissed as clinically irrelevant, it should be thoroughly investigated, as a whole and as individual genogroups, for possible effects on the host's immune system. TTV infection is very dynamic (>3.8 × 1010 virions released into blood daily in chronically infected adults), suggesting that the numbers of cells supporting virus replication (and possibly dying as a result, since the absence of an envelope makes it very unlikely that the virus is released without cytolysis) are quite high at any given time (6). Furthermore, TTV has been shown to replicate in polyclonally stimulated but not resting human peripheral blood mononuclear cells in vitro (4, 7). Should activated lymphoid cells also support TTV replication in vivo, then the consequences for the immune system and its function might be very significant, indeed. For example, TTV infection might favor production of type 2 cytokines, which would explain the observed inverse relationship with T cells and direct correlation with B cells. The fact that the related animal circoviruses mentioned above are markedly immunosuppressive in their respective host species also argues for attentive investigation in this direction (12).
Acknowledgments
This study was supported in part by grants from the Ministero dell'Istruzione, dell'Università e della Ricerca, Rome, Italy.
REFERENCES
- 1.Bendinelli, M., M. Pistello, F. Maggi, C. Fornai, G. Freer, and M. L. Vatteroni. 2001. Molecular properties, biology and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clin. Microbiol. Rev. 14:98-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen, J. K., J. Eugen-Olsen, M. Sorensen, H. Ullum, S. B. Gjedde, B. K. Pedersen, J. O. Nielsen, and K. Krogsgaard. 2000. Prevalence and prognostic significance of infection with TT virus in patients infected with human immunodeficiency virus. J. Infect. Dis. 181:1796-1799. [DOI] [PubMed] [Google Scholar]
- 3.Comas-Bitter, W. M., R. de Groot, R. van den Beemd, H. J. Neijens, W. C. J. Hop, K. Groeneveld, H. Hooijkaas, and J. J. M. van Dongen. 1997. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J. Pediatr. 130:388-393. [DOI] [PubMed] [Google Scholar]
- 4.Maggi, F., C. Fornai, L. Zaccaro, A. Morrica, M. L. Vatteroni, P. Isola, S. Marchi, A. Ricchiuti, M. Pistello, and M. Bendinelli. 2001. TT virus (TTV) loads associated with different peripheral blood cell types and evidence for TTV replication in activated mononuclear cells. J. Med. Virol. 64:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Maggi, F., M. Pifferi, C. Fornai, E. Andreoli, E. Tempestini, M. L. Vatteroni, S. Presciuttini, S. Marchi, A. Pietrobelli, A. Boner, M. Pistello, and M. Bendinelli. 2003. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J. Virol. 77:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggi, F., M. Pistello, M. L. Vatteroni, S. Presciuttini, S. Marchi, P. Isola, C. Fornai, S. Fagnani, E. Andreoli, G. Antonelli, and M. Bendinelli. 2001. Dynamics of persistent TT virus infection, as determined in patients treated with alpha interferon for concomitant hepatitis C virus infection. J. Virol. 75:11999-12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariscal, L. F., J. M. Lopez-Alcorocho, E. Rodriguez-Inigo, N. Ortiz-Movilla, S. de Lucas, J. Bartolome, and V. Carreno. 2002. TT virus replicates in stimulated but not in nonstimulated peripheral blood mononuclear cells. Virology 301:121-129. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto, H., and M. Mayumi. 2001. TT virus: virological and genomic characteristics and disease associations. J. Gastroenterol. 36:519-529. [DOI] [PubMed] [Google Scholar]
- 9.Peng, Y. H., T. Nishizawa, M. Takahashi, T. Ishikawa, A. Yoshikawa, and H. Okamoto. 2002. Analysis of the entire genomes of thirteen TT virus variants classifiable into the fourth and fifth genetic groups, isolated from viremic infants. Arch. Virol. 147:21-41. [DOI] [PubMed] [Google Scholar]
- 10.Shibayama, T., G. Masuda, A. Ajisawa, M. Takahashi, T. Nishizawa, F. Tsuda, and H. Okamoto. 2001. Inverse relationship between the titre of TT virus DNA and the CD4 cell count in patients infected with HIV. AIDS 15:563-570. [DOI] [PubMed] [Google Scholar]
- 11.Simmonds, P. 2002. TT virus infection: a novel virus-host relationship. J. Med. Microbiol. 51:455-458. [DOI] [PubMed] [Google Scholar]
- 12.Todd, D., M. S. McNulty, B. M. Adair, and G. M. Allan. 2001. Animal circoviruses. Adv. Virus Res. 57:1-70. [DOI] [PubMed] [Google Scholar]
- 13.Touinssi, M., P. Gallian, P. Biagini, H. Attoui, B. Vialettes, Y. Berland, C. Tamalet, C. Dhiver, I. Ravaux, P. De Micco, and X. De Lamballerie. 2001. TT virus infection: prevalence of elevated viraemia and arguments for the immune control of viral load. J. Clin. Virol. 21:135-141. [DOI] [PubMed] [Google Scholar]