Abstract
UV irradiation of a mixture of an isolated tobacco mosaic virus (TMV; tomato strain L [TMV-L]) RNA-dependent RNA polymerase complex and the TMV-L RNA 3′-terminal region (3′-TR) resulted in cross-linking of the TMV-L 126-kDa replication protein to the TMV-L 3′-TR. Using both Escherichia coli-expressed proteins corresponding to parts of the 126-kDa protein and mutants of the 3′-TR, the interacting sites were located to a 110-amino-acid region just downstream of the core methyltransferase domain in the protein and a region comprising the central core C and domain D2 in the 3′-TR. Mutation to alanine of a tyrosine residue at position 409 or a tyrosine residue at position 416 in the protein binding region abolished cross-linking to the 3′-TR, and corresponding mutations introduced into TMV-L RNA abolished its ability to replicate in tomato protoplasts, with no detectable production of either plus- or minus-strand RNA. The results are compatible with a model for initiation of TMV-L minus-strand RNA synthesis in which an internal region of the TMV-L 126-kDa protein first binds to the central core C and domain D2 region of the TMV-L 3′-TR and is then followed by binding of the 183-kDa protein to this complex and positioning of the catalytically active site of the polymerase domain close to the 3′-terminal CCCA initiation site.
Tobacco mosaic virus (TMV) has a 6.3-kb positive-stranded RNA genome which encodes four proteins (reviewed in references 1, 2, and 6). The 126- and 183-kDa proteins have essential replication functions and are translated from the virus genomic RNA. The 126-kDa protein has an N-terminal methyltransferase and guanylyltransferase or capping domain (14, 16) and a C-terminal helicase domain (10, 14). The 183-kDa protein, which is translated as a result of ribosomal read-through of a termination codon at the end of the open reading frame for the 126-kDa protein, additionally has a domain typical of RNA-dependent RNA polymerases (RdRps) (14). The 35-kDa cell-to-cell movement protein and the 17.5-kDa coat protein are translated from 3′-coterminal subgenomic RNAs.
The 3′-terminal region (3′-TR) of TMV RNA can be folded into a tRNA-like structure and adjacent upstream pseudoknots (7, 31) which have been shown to be important for TMV RNA replication in vivo (3, 28) and negative-strand RNA synthesis in vitro (22, 33). Regions which were identified as being important for negative-strand synthesis in vitro included the 3′-terminal CA sequence, domains D1 (equivalent to a tRNA acceptor arm), D2 (similar to a tRNA anticodon arm), and D3 (an upstream pseudoknotted region), and a central core region C which connects domains D1, D2, and D3. Domain D2 and the central core region C were shown to be the most important elements for binding of the TMV RdRp complex to the viral 3′-TR (22).
The TMV RdRp complex, isolated from infected plants, consists of the 126-kDa protein, the 183-kDa protein, and a number of plant proteins (21, 29, 33). Until now, the protein component of the RdRp complex responsible for the specific binding to the TMV RNA 3′-TR was unknown. Here we have used UV cross-linking to show that a region of the 126- and 183-kDa proteins downstream of the core methyltransferase domain is responsible for binding of the RdRp to the RNA 3′-TR in vitro. We have also identified two aromatic amino acids in this region of the 126- and 183-kDa proteins that are essential for cross-linking to the RNA 3′-TR in vitro and for replication of TMV RNA in tomato protoplasts.
MATERIALS AND METHODS
Preparation of TMV-L RdRp.
Membrane-bound viral RdRp was isolated from tomato plants infected with TMV-L by differential and density gradient centrifugation and then solubilized and purified as described by Osman and Buck (20, 21).
Preparation of cDNA templates and in vitro transcription.
RNA t2 and the mutants described in Table 1 were synthesized by in vitro transcription using T7 RNA polymerase and a DNA template generated by PCR from cDNA clones of TMV-L RNA as described previously (22). RNAs corresponding to the 3′-terminal 273 nucleotides (nt) of red clover necrotic mosaic virus RNA2 and nt 2466 to 2738 of the vector LITMUS28 were synthesized by in vitro transcription using T7 RNA polymerase and templates described previously (22). RNA transcripts were purified as described in the Ambion manual and assayed spectrophotometrically.
TABLE 1.
Effect of mutations in the TMV-L 3′-TR on its ability to cross-link to protein P1
| Transcript | Mutation in TMV-L 3′-TR (transcript t2) | IC50 (nM)a | Ability to cross-link to P1b |
|---|---|---|---|
| t2 | None | 12.0 | +++ |
| t15a | Deletion of 3′-terminal CCCA | 10.5 | +++ |
| t31 | Deletion of domains D2 and D3 and of core C | >100 | − |
| t37 | Disruption of stem S4 in core C | 94 | − |
| t39 | Deletion of domains D1 and D3 except for 3′-terminal CCCA | 8.2 | +++ |
| t44 | Deletion of core C and domains D1 and D2 except for 3′-terminal CCCA | 78 | − |
| t45 | 5′ nt 1-73 only | >100 | − |
| t46 | 5′ nt 1-73 plus 3′-terminal CCCA | ND | − |
| RCNMVc | 3′-terminal 273 nt of RCNMV RNA2 | >100 | − |
| L1 | nt 2466-2738 of vector LITMUS28 | >100 | − |
Data from Osman et al. (20). IC50, 50% inhibitory concentration, i.e., the concentration of each competitor RNA necessary to reduce minus-strand synthesis from template t1 (which contains the 3′ 277 nt of TMV-L RNA) by 50%. ND, not determined.
+++, intensity of cross-linked band is not significantly different from that obtained with t2. −, no detectable cross-linked band.
RCNMV, red clover necrotic mosaic virus.
Expression of proteins in Escherichia coli.
Proteins corresponding to regions of the TMV-L 126- and 183-kDa proteins were expressed with N-terminal His tags in E. coli. The coding regions were amplified from pTMV5, a full-length cDNA clone of TMV-L RNA (20), by using Pfu Turbo DNA polymerase and the following pairs of forward and reverse primers: (i) for amino acids 1 to 654, forward primer HisI (CGAGCTCCATGGCATACACACAAACAGCCAC) (contains an NcoI site and TMV-L nt 72 to 94) and reverse primer HisVII (CATGTGTCGACCTATTGTAACTCACCACGGGCCAT) (contains a SalI site and sequence complementary to TMV-L nt 2031 to 2013); (ii) for amino acids 648 to 1116, forward primer HisIII (GGAGTCCCATGGCCCGTGGTGAGTTACAATTGG) (contains an NcoI site and TMV-L nt 2013 to 2040) and reverse primer HisVIII (TAGCTGAAGCTTCTATTGAGTACCTGCATCTAC) (contains a HindIII site and sequence complementary to TMV-L nt 3422 to 3401); (iii) for amino acids 1117 to 1616, forward primer HisV (CATGGTCCATGGCATACCAATTACAGGTCGACTCTGTG) (contains an NcoI site and TMV-L nt 3420 to 3443) and reverse primer HisIX (TCGATAAAGCTTTTAACAACTAGAGCCATCAAG) (contains a HindIII site and sequence complementary to TMV-L nt 4922 to 4901); (iv) for amino acids 1 to 313, forward primer HisI and reverse primer HisXI (CATGTGTCGACCTATATTCTAGAAAATTTACAAAACCAGG) (contains a SalI site and sequence complementary to TMV-L nt 1010 to 985); (v) for amino acids 314 to 654, forward primer HisXII (CGAGCTCCATGGATACTTTCTTATTGTACAAAGG) (contains an NcoI site and TMV-L nt 1011 to 1033) and reverse primer HisVII; (vi) for amino acids 1 to 423, forward primer HisI and reverse primer HisX (CATGTGTCGACCTAGACGAAAGATAACACGTTGG) (contains a SalI site and sequence complementary to TMV-L nt 1340 to 1321); (vii) for amino acids 424 to 654, forward primer HisXIII (CGAGCTCCATGGAATCAATTCGTTCGAGAGTGATC) and reverse primer HisVII; and (viii) for amino acids 314 to 423, forward primer HisXII and reverse primer HisX. The PCR products were gel purified, cleaved with NcoI and SalI (for primer pairs i, iv, v, vi, vii, and viii above) or NcoI and HindIII (for primer pairs ii and iii), and cloned into the corresponding sites of vector pET30b in E. coli BL21 cells. Proteins were expressed (25) and purified on a Ni2+-nitriloacetic acid column (32). Fusion proteins of the TMV-L 126- and 183-kDa proteins with the E. coli maltose-binding protein were produced and purified as described previously (20, 21).
UV cross-linking and Western blotting.
Preparation, inoculation, and processing of protoplasts.
Mesophyll protoplasts were isolated from leaves of tomato plants (Lycopersicon esculentum Craigella GCR 26) as described by Motoyoshi and Oshima (19). Protoplasts were inoculated and processed for analysis of the products of infection as described by Turner and Buck (30). Labeled probes to detect the TMV-L plus and minus strands by Northern blotting were as described previously (20).
RESULTS
UV cross-linking of purified TMV-L RdRp to the 3′-TR of the viral RNA.
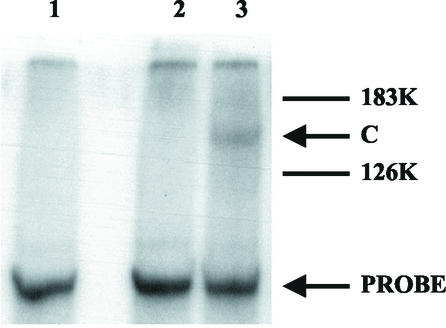
A 32P-labeled RNA transcript, RNA t2, corresponding to the 3′-terminal 195 nt of TMV-L RNA, which contains the whole of the 3′-tRNA-like sequence plus domain D3, was mixed with a purified TMV-L RdRp preparation and UV irradiated. The cross-linked products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and phosphorimaging. As purified RdRp preparations contain very small amounts of protein, the sensitivity of the assay was increased by analysis of the cross-linked protein-RNA complex without the nuclease digestion which is usually used to remove uncross-linked RNA. Hence, the cross-linked protein(s) would be expected to be retarded in SDS-PAGE compared with the uncross-linked protein. A labeled protein band, designated C, was detected on the gel (Fig. 1, lane 3) and migrated more slowly than the TMV-L 126-kDa protein. After digestion with either RNase A or proteinase K, this band could not be detected (data not shown), indicating that it was a cross-linked RNA-protein complex.
FIG. 1.
UV cross-linking of 32P-labeled t2 RNA (3′ 195 nt of TMV-L RNA) to purified TMV-L RdRp. Purified TMV-L RdRp was incubated with 32P-labeled t2 RNA probe and UV irradiated. The product was analyzed by SDS-PAGE (21) and autoradiography. Lane 1, probe (no UV); lane 2, probe (UV irradiated); lane 3, probe plus TMV-L RdRp (UV irradiated). 183K and 126K, the positions of the TMV-L 183- and 126-kDa proteins, respectively; C, the position of the cross-linked product; PROBE, the position of the labeled t2 RNA probe.
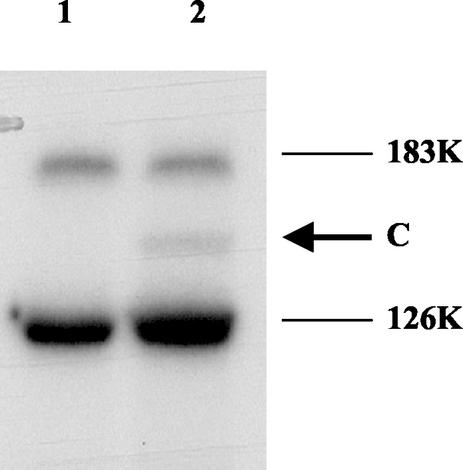
Band C could have been a complex of RNA t2 with either the TMV-L 126-kDa protein or one of the host proteins associated with the purified TMV-L RdRp complex (21, 29, 33). To determine whether band C was derived from the 126-kDa protein, the purified TMV-L RdRp was incubated with unlabeled t2 transcript and UV irradiated. After SDS-PAGE, the products were analyzed by immunoblotting using antibodies to the helicase domain of the 126-kDa protein (20, 21). In addition to bands of free, uncross-linked 126- and 183-kDa proteins, a band which migrated between these proteins with a mobility similar to that of band C, between the 126- and 183-kDa proteins, was detected (Fig. 2, lane 2, arrow). This band was not detected in the absence of UV irradiation (Fig. 2, lane 1). Hence, the results are consistent with band C being the 126-kDa protein cross-linked to RNA t2. The efficiency of cross-linking (about 7%) falls within the typical range of cross-linking efficiencies for nucleic acid-binding proteins (18).
FIG. 2.
UV cross-linking of unlabeled t2 RNA to purified TMV-L RdRp. The product was subjected to SDS-PAGE (21) and then blotted onto a membrane. The membrane was probed with antibodies to the 126-kDa protein N-terminal domain (21). Lane 1, RNA probe plus TMV-L RdRp (no UV); lane 2, RNA probe plus TMV-L RdRp (UV irradiated). 183K and 126K, the positions of the TMV-L 183- and 126-kDa proteins, respectively; C, the position of the cross-linked product.
Identification of the region of the TMV-L 126- and 183-kDa proteins which binds to the TMV-L RNA 3′-TR.
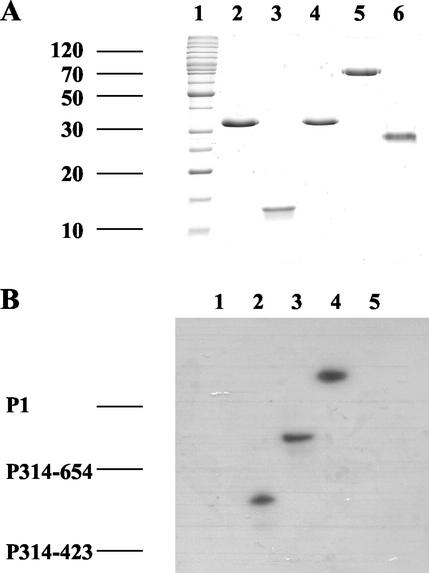
Regions of the TMV-L 126- and 183-kDa proteins corresponding to amino acids 1 to 653 (containing the methyltransferase domain and an intervening region), 648 to 1116 (containing the helicase domain), and 1144 to 1627 (containing the RdRp domain) were expressed as His-tagged proteins in E. coli, and the proteins were designated P1, P2, and P3, respectively (Fig. 3A). Each purified soluble protein (Fig. 3B) was mixed with 32P-labeled RNA transcript t2 and subjected to UV irradiation. Analysis of the products by SDS-PAGE showed a band, designated P1a, arising from the P1-t2 irradiated sample, which migrated more slowly than the P1 protein (Fig. 3C, lane 2). This band was not detected in the absence of UV irradiation (Fig. 3C, lane 1), when RNA t2 was UV irradiated in the absence of protein P1 or when the P2-t2 mixture was UV irradiated and treated with proteinase K prior to SDS-PAGE (data not shown). When the P1-t2 mixture was UV irradiated and then treated with RNase A, band P1a was no longer detected, but a fainter band, designated P1b, with a mobility only slightly less than that of P1, was detected (Fig. 4, lane 2). Taken together, these results indicate that band P1a consists of protein P1 covalently cross-linked to RNA t2 and that band P1b consists of protein P1 cross-linked to a small number of nucleotides which remain after nuclease digestion. No cross-linking to proteins P2 and P3 could be detected (Fig. 3C, lanes 3 to 6). Similar results were obtained in cross-linking experiments using RNA t2 and P1, P2, and P3 proteins expressed as fusions with the E. coli maltose-binding protein (data not shown).
FIG. 3.
UV cross-linking of 32P-labeled t2 RNA to E. coli-expressed proteins P1 (amino acids 1 to 654), P2 (amino acids 648 to 1116), and P3 (amino acids 1117 to 1616). (A) Map of the TMV-L 126- and 183-kDa proteins showing the positions of the methyltransferase (MT), helicase (HEL), and RdRp (POL) domains and of the E. coli-expressed P1, P2, and P3 proteins. R, the RNA-binding region of the P1 protein (amino acids 314 to 423). (B) SDS-PAGE of soluble, uncross-linked proteins. Lane 1, marker proteins (220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, and 15 kDa); lane 2, P1; lane 3, P2; lane 4, P3. The gel was stained with Coomassie brilliant blue. The sizes (in kilodaltons) of selected protein marker bands are shown on the side of the gel. (C) UV cross-linking. The products were analyzed as described in the legend to Fig. 1. Lane 1, P1 plus t2 RNA (no UV); lane 2, P1 plus t2 RNA plus UV; lane 3, P2 plus t2 RNA (no UV); lane 4, P2 plus t2 RNA plus UV; lane 5, P3 plus t2 RNA (no UV); lane 6, P3 plus t2 RNA plus UV. P1a, the position of protein P1 cross-linked to RNA t2.
FIG. 4.
Digestion of the product of UV cross-linking of 32P-labeled t2 RNA and E. coli-expressed P1 protein with RNase. Products were analyzed as described in the legend to Fig. 1. Lane 1, t2 RNA plus P1 plus UV; lane 2, t2 RNA plus P1 plus UV, followed by RNase A digestion.
Specificity of binding of the P1 protein to the TMV-L 3′-TR.
Previous work has established that the 3′-TR of RNA2 of red clover necrotic mosaic virus, a virus unrelated to TMV, a transcript from the polylinker region of the cloning vector plasmid LITMUS 28 (L1), and the 5′ 73 nt of TMV-L RNA were neither templates for the TMV-L RdRp nor acted as competitors for the binding of the TMV-L 3′-TR to the RdRp (22). When these RNAs were incubated with protein P1, followed by UV irradiation, no cross-linked product could be detected in SDS-PAGE (Table 1). Examination of the effects of mutations in the TMV-L 3′-TR showed that mutations which had previously been shown to abolish the ability of the RNAs to act as competitors for the binding of the 3′-TR to the RdRp also abolished their ability to cross-link to protein P1 (Table 1). Deletion of the 3′-terminal CCCA sequence (t15a) or deletion of domains D1 and D3, except for the 3′-terminal CCCA (t39), had no significant effect on the ability of the RNA to cross-link to protein P1. However, after deletion of domains D2 and D3 and the central core C (t31), deletion of domains D1 and D2 and the central core C (t44), or disruption of stem S4 in the central core C (t37), no cross-linking to protein P1 could be detected. Furthermore, no cross-linking to P1 could be detected when the TMV-L 5′ 73 nt were linked to the 3′-terminal CCCA sequence (t46). Taken together, these results indicate that a region comprising the central core C and domain D2 is important for cross-linking to protein P1, whereas there was no evidence for cross-linking of protein P1 to domain D1 or D3 or the 3′-terminal CCCA sequence.
Mapping the RNA-binding site in protein P1.
Regions of the P1 protein corresponding to amino acids 1 to 313 and 314 to 654 were expressed in E. coli as His-tagged proteins and purified on a Ni2+ column. The purified soluble proteins, P1-313 and P314-654, respectively (Fig. 5A, lanes 2 and 3), were incubated with 32P-labeled TMV-L 3′-TR (transcript t2) and UV irradiated, and the products were analyzed by SDS-PAGE and phosphorimaging. P314-654 gave rise to a band which migrated more slowly than the free P314-654 protein (Fig. 5B, lane 3), but no band was detected with P1-313 (Fig. 5B, lane 1). P1-313 contains the core methyltransferase motifs of the TMV 126- and 183-kDa proteins (14, 16); therefore, the core methyltransferase domain is probably not involved in the binding of P1 to the TMV-L 3′-TR. The binding site lies in a region between the methyltransferase and helicase domains which has been described as the intervening region (10). Further analysis showed that a protein corresponding to amino acids 1 to 423, P1-423, could be efficiently cross-linked to transcript t2 (data not shown), but no cross-linking was detected with a protein corresponding to amino acids 424 to 654, P424-654 (Fig. 5A, lane 6, and 5B, lane 5). This suggested that the RNA-binding site (designated “R” in Fig. 3A) was located between amino acids 314 and 423. This was confirmed by expressing a His-tagged protein corresponding to these amino acids, P314-423, in E. coli. The purified soluble protein (Fig. 5A, lane 3) could be efficiently cross-linked to transcript t2 (Fig. 5B, lane 2).
FIG. 5.
Mapping of the region of P1 which binds to t2 RNA. Proteins were expressed in E. coli and purified. (A) SDS-PAGE of soluble, uncross-linked proteins. Lane 1, marker proteins (220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, 15, and 10 kDa); lane 2, P1-313; lane 3, P314-423; lane 4, P314-654; lane 5, P1; lane 6, P424-654. The gel was stained with Coomassie brilliant blue. The sizes (in kilodaltons) of selected protein marker bands are shown on the side of the gel. (B) The proteins were incubated with 32P-labeled t2 RNA and UV irradiated. The products were analyzed as described in the legend to Fig. 1. Lane 1, P1-313; lane 2, P314-423; lane 3, P314-654; lane 4, P1; lane 5, P424-654. The approximate positions of free P314-423, P314-654, and P1 are shown on the side of the gel to indicate the degree of retardation resulting from cross-linking to RNA t2.
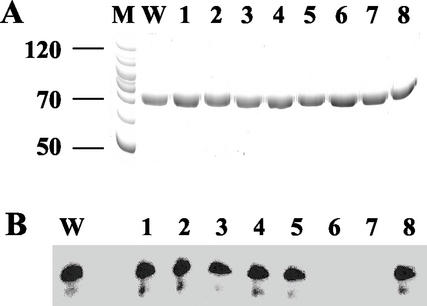
P314-423 contains several aromatic amino acid residues (Fig. 6). Since aromatic amino acids can interact with nucleic acid bases by intercalation, aromatic stacking, or edge-to-face contact (17), it was of interest to determine whether any of these aromatic amino acids was involved in the binding of P1 to the TMV-L 3′-TR. Mutants of the P1 protein were produced in which eight aromatic amino acids (Fig. 6) were changed singly to alanine residues, namely P1-1 (Y333A), P1-2 (W365A), P1-3 (F366A), P1-4 (F378A), P1-5 (F398A), P1-6 (Y409A), P1-7 (Y416A), and P1-8 (F422A). Each mutant was expressed in E. coli as a His-tagged protein, purified, and tested for its ability to be cross-linked to transcript t2 after UV irradiation. Mutant proteins P1-1, P1-2, P1-3, P1-4, P1-5, and P1-8 were all able to form cross-linked adducts with transcript t2 with an efficiency similar to that of the wild-type protein P1 (Fig. 7B, lanes W, 1 to 5, and 8). However, no cross-linking could be detected with mutant proteins P1-6 and P1-7 (Fig. 7B, lanes 6 and 7). Hence, it is likely that tyrosine residues at positions 409 and 416 are important for binding of protein P1 to the TMV-L 3′-TR. These experiments do not eliminate the possibility that the mutations abolished cross-linking without affecting binding, but this seems unlikely. Aromatic amino acids, including tyrosine, are known to be able to form cross-links with nucleic acid bases (8), and these two tyrosine residues may have been involved in forming the cross-links with the TMV-L 3′-TR. If mutation of either of these tyrosine residues to alanine did not affect binding, then it is unlikely that it would have introduced a major conformational change in the protein, and hence cross-linking of the second tyrosine would probably have been unaffected.
FIG. 6.
Aromatic amino acid residues (shown in boldface type) selected for mutation to alanine in the region from amino acid 314 to amino acid 423 of the 126-kDa protein of TMV-L.
FIG. 7.
Effect of mutations of aromatic amino acids to alanine in P1 on ability to cross-link to t2 RNA. (A) SDS-PAGE of soluble, uncross-linked proteins. Lane M, marker proteins (120, 100, 90, 80, 70, 60, and 50 kDa); lane W, wild-type P1; lane 1, Y333A; lane 2, W365A; lane 3, F366A; lane 4, F378A; lane 5, F398A; lane 6, F409A; lane 7, Y416A; lane 8, F422A. The gel was stained with Coomassie brilliant blue. The sizes (in kilodaltons) of selected protein marker bands are shown on the side of the gel. (B) UV cross-linking of 32P-labeled t2 RNA with P1 mutants. After UV cross-linking, the products were analyzed by SDS-PAGE and phosphorimaging. Lane W, wild-type P1; lane 1, Y333A; lane 2, W365A; lane 3, F366A; lane 4, F378A; lane 5, F398A; lane 6, F409A; lane 7, Y416A; lane 8, F422A.
Effect of mutations in the P1 RNA-binding site on the replication of TMV-L RNA in tomato protoplasts.
The same mutations used to produce the eight mutant P1 proteins, P1-1 to P1-8, were introduced into a full-length cDNA clone of TMV-L RNA linked to a T7 promoter in vector pSL1180 (20) to produce TMV-L mutants 1 to 8. Full-length transcripts of each mutant were produced by in vitro transcription and tested for the ability to replicate in tomato protoplasts. Labeled RNA probes were used in Northern blots to detect the TMV-L plus and minus strands separately (Fig. 8A and B). An initial time course showed that replication of wild-type TMV-L RNA was maximal after 24 h (data not shown). Comparison of the replication of transcripts of mutants 1 to 8 in tomato protoplasts after 24 h showed that the production of plus and minus strands by mutants 1, 2, 3, and 4 did not differ significantly from that of transcripts of the wild-type TMV-L cDNA clone. The replication of transcripts of mutants 5 and 8 was about 50% of that of the wild type. However, no production of plus or minus strands could be detected after inoculation of protoplasts with mutants 6 and 7. Hence, the mutations which abolish cross-linking of protein P1 to the TMV-L 3′-TR also abolish replication of TMV-L RNA in tomato protoplasts.
FIG. 8.
Replication of TMV-L mutants in tomato protoplasts. (A and B) Northern blots with the probe to detect TMV-L (plus) RNA (A) and the probe to detect TMV-L (minus) RNA (B). (C) Ethidium bromide-stained gel of rRNA. Lane 1, wild-type TMV-L; lanes 2 to 9, mutants 1 to 8 (aromatic amino acid → alanine), respectively.
DISCUSSION
We have shown that UV irradiation of an isolated TMV-L RdRp complex with the TMV-L 3′-TR results in cross-linking of the TMV-L 126-kDa protein to the TMV-L 3′-TR. Cross-linking of the 183-kDa protein or of host proteins of 50 to 56 kDa found in purified TMV-L RdRp preparations (21) to the 3′-TR was not detected. Using E. coli-expressed proteins P1, P2, and P3 corresponding to the methyltransferase (plus intervening region), helicase, and polymerase domains, respectively, of the 126- and 183-kDa proteins, the binding site was located to a region just downstream of the core methyltransferase domain in the P1 protein. Mutation to alanine of a tyrosine residue at amino acid position 409 or a tyrosine residue at position 416 abolished cross-linking of the P1 protein, and the same mutations introduced into TMV-L RNA abolished its ability to replicate in tomato protoplasts, with no detectable production of either plus- or minus-strand RNA. Since the TMV-L 3′-TR contains all the cis-acting sequences needed for minus-strand synthesis, at least in vitro (22), the mutations introduced into TMV-L RNA probably abolished replication because of the mutations in the 126- and 183-kDa proteins rather than changes in the structure of the RNA. Hence, it is likely that binding of this region of the 126- and 183-kDa proteins to the TMV-L 3′-TR is essential for TML-RNA replication. The aromatic amino acids at positions 409 and 416 may either directly interact with nucleic acid bases of the 3′-TR or be essential for maintaining the structure of the P314-423 region of the 126- or 183-kDa protein that binds to the 3′-TR. Considering these results together with those of previous work (22), it is likely that the P314-423 region binds to the core C and D2 domain of the 3′-TR.
Using a bipartite system in which the 126- and 183-kDa proteins are expressed from separate RNAs, Lewandowski and Dawson (15) showed that the TMV 126-kDa protein appears to function primarily in cis, whereas the 183-kDa protein can function in trans. They suggested that the 126-kDa protein binds to its mRNA and targets it for replication. The 183-kDa protein might then bind to the 126-kDa protein and initiate replication. Interaction of the 126- and 183-kDa proteins has been demonstrated (10), and a solubilized immunoaffinity-purified TMV RdRp preparation containing a 1:1 dimer of the 126- and 183-kDa proteins has been shown to be able to synthesize minus-strand RNA from a plus-strand RNA template in vitro (33). Our finding that a region of the 126-kDa protein binds to the TMV-L 3′-TR, probably via the core C and D2 domain, is consistent with the model of Lewandowski and Dawson (15). Subsequent binding of the 183-kDa protein to the already bound 126-kDa protein may position the catalytically active site of the polymerase domain at the 3′ terminus of the template RNA to enable initiation of minus-strand synthesis. Failure to detect cross-linking of the P3 protein, which contains the polymerase domain, to the 3′-TR suggests that binding of the polymerase domain to the 3′-terminal CCCA sequence may be relatively weak. Hence, the function of the 126-kDa protein may be both to recruit RNA templates for replication and to subsequently bind the 183-kDa protein to position the catalytically active site of the polymerase domain close to the 3′ end of the template RNA.
It has been shown that the brome mosaic virus (BMV) 1a protein, which is analogous to the TMV 126-kDa protein, recruits RNA templates for replication (26). The genome of BMV is divided between three RNAs. RNA1 encodes the 1a protein which has an N-terminal methyltransferase and a C-terminal helicase domain, RNA2 encodes the 2a protein which has an N-terminal domain that interacts with the 1a helicase domain and a central RNA polymerase domain, and RNA3 encodes the movement and capsid proteins. The BMV 1a protein recruits RNA2 and RNA3 templates for replication by binding to conserved tRNA-like TψC stem-loops located at the 5′ terminus and intergenic regions, respectively (5, 27). The 2a protein binds to the 1a protein and initiates minus-strand synthesis at the 3′-terminal tRNA-like structure (4, 13, 26). Therefore, BMV and TMV appear to have evolved similar mechanisms for recruitment of RNA templates and initiation of minus-strand RNA synthesis. RNA replication of both viruses takes place on membranes derived from the endoplasmic reticulum. In membrane-bound replication complexes, the ratio of BMV 1a to 2a proteins is about 25:1, and since the 1a protein forms spherules which bud into the endoplasmic reticulum, it has been suggested that the 1a protein plays both structural and functional roles in assembling the membrane-bound replication complexes and sequestering the 2a polymerase and BMV RNA templates within them (24). The TMV 126-kDa protein is present in much larger amounts than the 183-kDa protein in isolated membrane-bound replication complexes (20, 33, 34) and may play a role in assembling TMV replication complexes and recruiting the 183-kDa protein and RNA template that is similar to the role of the BMV 1a protein in assembling BMV replication complexes. Oligomerization of the helicase domain of the TMV 126- and 183-kDa proteins has been demonstrated previously (9). A difference in the mechanisms of sequestering the RNA templates between the two viruses is that, unlike in the case of BMV, there is no easily recognizable tRNA-like TψC stem-loop structure at the 5′ terminus, or internally, in TMV plus-strand RNA. Hence, rather than binding to sequences at the 5′ end of the RNA or internally, as with the BMV 1a protein, the TMV 126-kDa protein binds directly to the 3′-terminal tRNA-like structure.
It is known that TMV mutants able to express the 183-kDa protein, but not the 126-kDa protein, are able to replicate, albeit with reduced efficiency (12, 15). Hence, the 183-kDa protein can bind to TMV RNA in the absence of the 126-kDa protein. Our results suggest that in these mutants the 183-kDa protein binds to the 3′-TR via the binding region we have identified in the 126-kDa protein. This could also allow positioning of the polymerase domain close to the 3′-terminal CCCA sequence, but less efficiently than when the 183-kDa protein binds to the 126-kDa protein which is already bound to the 3′-TR. It is also possible that the 183-kDa protein alone would be less efficient in the assembly and functioning of the membrane-bound replication complexes.
Although we did not detect binding of the isolated TMV-L RdRp domain to the TMV-L 3′-TR in our UV cross-linking assay, binding of the isolated 80-kDa RdRp domain of the bamboo mosaic virus (BaMV) 155-kDa replication protein to the BaMV 3′ untranslated region was detected using a band-shift assay (11). The BaMV 155-kDa replication protein is analogous to the TMV 183-kDa protein and contains methyltransferase and helicase domains as well as the C-terminal RdRp domain. However, the BaMV 155-kDa protein has no stop codon after the helicase domain and no protein corresponding to the TMV 126-kDa protein is produced. Whether sequences upstream of the BaMV RdRp domain in the 155-kDa protein are also involved in binding to the BaMV 3′ untranslated region was not reported.
Acknowledgments
This work was supported by the United Kingdom Biotechnology and Biological Sciences Research Council.
REFERENCES
- 1.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck, K. W. 1999. Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:613-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrika, R., S. Rabindran, D. J. Lewandowski, K. L. Manjunath, and W. O. Dawson. 2000. Full-length tobacco mosaic virus RNAs and defective RNAs have different replication signals. Virology 273:198-209. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., A. Noueiry, and P. Ahlquist. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75:3207-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson, W. O., and K. M. Lehto. 1992. Regulation of tobamovirus gene expression. Adv. Virus Res. 38:307-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felden, B., C. Florentz, R. Giegé, and E. Westhof. 1996. A central pseudoknotted three-way junction imposes tRNA-like mimicry and the orientation of three 5′ upstream pseudoknots in the 3′ terminus of tobacco mosaic virus RNA. RNA 2:201-212. [PMC free article] [PubMed] [Google Scholar]
- 8.Golden, M. C., K. A. Resing, B. D. Collins, M. C. Willis, and T. H. Koch. 1999. Mass spectral characterisation of a protein-nucleic acid photocrosslink. Prot. Sci. 8:2806-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goregaoker, S. P., and J. N. Culver. 2003. Oligomerization and activity of the helicase domain of the tobacco mosaic virus 126- and 183-kilodalton replicase proteins. J. Virol. 77:3549-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goregaoker, S. P., D. J. Lewandowski, and J. N. Culver. 2001. Identification and functional analysis of an interaction between domains of the 126/183-kDa replicase-associated proteins of tobacco mosaic virus. Virology 282:320-328. [DOI] [PubMed] [Google Scholar]
- 11.Huang, C.-Y., Y.-L. Huang, M. Meng, Y.-H. Hsu, and C.-H. Tsai. 2001. Sequences at the 3′ untranslated region of bamboo mosaic potexvirus RNA interact with the viral RNA-dependent RNA polymerase. J. Virol. 75:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa, M., T. Meshi, M. Motoyoshi, N. Takamatsu, and Y. Okada. 1986. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 14:8291-8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao, C. C., and P. Ahlquist. 1992. Identification of the domains required for direct interaction of the helicase-like and polymerase-like RNA replication proteins of brome mosaic virus. J. Virol. 66:7293-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koonin, E. V., and V. V. Dolja. 1993. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative sequence analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28:375-430. [DOI] [PubMed] [Google Scholar]
- 15.Lewandowski, D. J., and W. O. Dawson. 2000. Functions of the 126-kDa and 183-kDa proteins of tobacco mosaic virus. Virology 251:427-437. [DOI] [PubMed] [Google Scholar]
- 16.Merits, A., R. Kettunen, K. Mäkinen, A. Lampio, P. Auvinen, L. Kääriänen, and T. Ahola. 1999. Virus-specific capping of tobacco mosaic virus RNA: methylation of GTP prior to formation of covalent complex p126-m7GMP. FEBS Lett. 455:45-48. [DOI] [PubMed] [Google Scholar]
- 17.Misra A., M. Blair, C. Stuart, A. Ozarowski, J. R. Casa-Finet, and A. H. Maki. 2002. Phosphorescence and optically detected magnetic resonance of polynucleotide complexes of tryptophan- and 5-methyltryptophan-containing peptide stereoisomers. J. Phys. Chem. B106:3735-3741. [Google Scholar]
- 18.Molnar, G., N. O'Leary, A. B. Pardee, and D. W. Bradley. 1995. Quantification of DNA-protein interaction by UV crosslinking. Nucleic Acids Res. 23:3318-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motoyoshi, F., and N. Oshima. 1975. Infection with tobacco mosaic virus of leaf mesophyll protoplasts from susceptible and resistant lines of tomato. J. Gen. Virol. 29:81-91. [Google Scholar]
- 20.Osman, T. A. M., and K. W. Buck. 1996. Complete replication in vitro of tobacco mosaic virus RNA by a template-dependent, membrane-bound RNA polymerase. J. Virol. 70:6227-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman, T. A. M., and K. W. Buck. 1997. The tobacco mosaic virus RNA polymerase complex contains a plant protein related to the RNA-binding subunit of yeast eIF-3. J. Virol. 71:6075-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osman, T. A. M., C. L. Hemenway, and K. W. Buck. 2000. Role of the 3′ tRNA-like structure in tobacco mosaic virus minus-strand RNA synthesis by the viral RNA-dependent RNA polymerase in vitro. J. Virol. 74:11671-11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman, T. A. M., P. Thömmes, and K. W. Buck. 1993. Localization of an RNA binding domain in the movement protein of red clover necrotic mosaic virus. J. Gen. Virol. 74:2453-2457. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 25.Studier, F. W., A. H. Rosenburg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan, M., and P. Ahlquist. 1997. cis-acting signals in bromovirus RNA replication and gene expression: networking with viral proteins and host factors. Semin. Virol. 8:221-230. [Google Scholar]
- 27.Sullivan, M., and P. Ahlquist. 1999. A brome mosaic virus intergenic region RNA3 replication signal functions with viral replication protein 1a to dramatically stabilize RNA in vivo. J. Virol. 73:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamatsu, N., Y. Watanabe, T. Meshi, and Y. Okada. 1990. Mutational analysis of the pseudoknot region in the 3′ noncoding region of tobacco mosaic virus RNA. J. Virol. 64:3686-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsujimoto, Y., T. Numaga, K. Ohshima, D. B. Goto, S. Naito, and M. Ishikawa. 2003. Arabidopsis tobamovirus multiplication (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 22:335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner, R. L., and K. W. Buck. 1999. Mutational analysis of cis-acting sequences in the 3′- and 5′-untranslated regions of RNA2 of red clover necrotic mosaic virus. Virology 253:115-124. [DOI] [PubMed] [Google Scholar]
- 31.Van Belkum, A., J. P. Abrahams, C. W. A. Pleij, and L. Bosch. 1985. Five pseudoknots at the 204 nucleotides long 3′ noncoding region of tobacco mosaic virus RNA. Nucleic Acids Res. 13:7673-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walz, A.-C., R. A. Demel, B. de Kruijff, and R. Matzel. 2002. Aerobic sn-glycerol-3-phosphate dehydrogenase from Escherichia coli binds to the cytoplasmic membrane through an amphipathic helix. Biochem. J. 365:471-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe, T., A. Honda, A. Iwata, S. Ueda, T. Hibi, and A. Ishihama. 1999. Isolation from tobacco-mosaic virus-infected tobacco of a solubilized template-specific RNA-dependent RNA polymerase containing a 126K/183K protein heterodimer. J. Virol. 73:2633-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young, N., J. Forney, and M. Zaitlin. 1987. Tobacco mosaic virus replicase and replicative structures. J. Cell Sci. Suppl. 7:277-285. [DOI] [PubMed] [Google Scholar]