Abstract
Lipid rafts are proposed to function as platforms for both receptor signaling and trafficking. Following interaction with antigenic peptides, the T-cell receptor (TCR) rapidly translocates to lipid rafts, where it transmits signals and subsequently undergoes endocytosis. The Tip protein of herpesvirus saimiri (HVS), which is a T-lymphotropic tumor virus, interacts with cellular Lck tyrosine kinase and p80, a WD domain-containing endosomal protein. Interaction of Tip with p80 induces enlarged vesicles and recruits Lck and TCR complex into these vesicles for trafficking. We report here that Tip is constitutively present in lipid rafts and that Tip interaction with p80 but not with Lck is necessary for its efficient localization in lipid rafts. The Tip-Lck interaction was required for recruitment of the TCR complex to lipid rafts, and the Tip-p80 interaction was critical for the aggregation and internalization of lipid rafts. These results suggest the potential mechanism for Tip-mediated TCR downregulation: Tip interacts with Lck to recruit TCR complex to lipid rafts, and it subsequently interacts with p80 to initiate the aggregation and internalization of the lipid raft domain and thereby downregulate the TCR complex. Thus, the signaling and targeting functions of HVS Tip rely on two functionally and genetically separable mechanisms that independently target cellular Lck tyrosine kinase and p80 endosomal protein.
Lipid rafts are cholesterol- and sphingolipid-rich plasma membrane microdomains (1, 4, 36) that may take part in coordinating the signaling functions of the T-cell receptor (TCR). Following interaction with major histocompatibility complex class II molecules containing antigenic peptide, the TCR has been shown to rapidly translocate into Lck-enriched, CD45-deficient lipid rafts, where both TCR and Lck become phosphorylated (40). Upon translocation to the lipid rafts, the TCR transmits signals through several signaling pathways that result in the expression of a variety of genes associated with T-cell activation. Subsequently, the TCR complexes are subjected to endocytosis, which delivers the TCR complex to lysosomes for degradation (9). This targeting process appears to be initiated from the lipid rafts, as a portion of the raft constituent sphingolipid GM1 is internalized along with the TCR and trafficked to the lysosomal compartments (20). Thus, current evidence indicates that lipid rafts function as platforms for both the signaling and endocytic targeting functions of the TCR.
Herpesvirus persists in its host through an ability to establish a latent infection and periodically reactivates to produce infectious virus. Herpesvirus saimiri (HVS), an oncogenic γ2 herpesvirus, persists in the T lymphocytes of the natural host (squirrel monkey) without any apparent disease, but infection of other species of New World and Old World primates results in fulminant T-cell lymphomas (12, 22). In addition, when HVS infects primary T lymphocytes of humans, Old World primates, New World primates, and rabbits, the cells become immortalized and cytokine independent (2, 8).
An HVS protein called Tip (tyrosine kinase-interacting protein) is encoded by an open reading frame at the left end of the viral genome. Tip is not required for viral replication, but it is required for T-cell transformation in culture and for lymphoma induction in primates (10). Tip interacts with the src homology 3 (SH3) domain of Lck tyrosine kinase, and this interaction interferes with early events in the TCR signal transduction pathway (3, 23, 27). The molecular interaction between Lck and Tip has been mapped to 37 amino acid residues of Tip, termed the Lck binding domain. The Lck binding domain consists of two motifs, a carboxyl-terminal Src family kinase homology motif and an SH3 binding (SH3B) motif (3, 23, 27).
In an effort to delineate the role of Tip in the modulation of the TCR signal transduction pathway, we recently demonstrated that Tip interacts with a novel cellular lysosomal protein, p80, that contains an amino-terminal WD repeat domain and a carboxyl-terminal coiled-coil domain (34). Interaction of Tip with p80 facilitates lysosomal vesicle formation and subsequent recruitment of Lck into the lysosomes for degradation. Consequently, the interaction of Tip with Lck and p80 results in downregulation of both TCR and CD4 surface expression. Remarkably, these actions of Tip are functionally and genetically separable: the interaction of its amino-terminal region with p80 is responsible for TCR downregulation, and the interaction of its carboxyl region with Lck is responsible for CD4 downregulation (34). This indicates that lymphotropic HVS has evolved an elaborate mechanism to deregulate lymphocyte receptor expression to disarm host immune control. Thus, it is likely that this mechanism plays an important role in viral latency.
Analogous to HVS Tip is Epstein-Barr virus latent membrane protein 2A (LMP2A), which has amino-terminal src homology 2 (SH2) binding motifs. LMP2A is a 12-membrane-spanning-domain integral membrane protein with cytoplasmic amino- and carboxy-terminal domains (25, 26). The amino-terminal domain of LMP2A contains a variety of motifs involved in protein-protein interactions, including eight tyrosine residues, two of which form an immunoreceptor tyrosine-based activation motif (25, 30). LMP2A binds to Lyn and Syk tyrosine kinase, and the phosphorylation of LMP2A correlates with its association with Lyn and Syk (26). The association of LMP2A with Lyn and Syk has been shown to prevent these essential tyrosine kinases from their participation in B-cell receptor (BCR) signal transduction (29). Finally, LMP2A has been shown to be constitutively present in lipid rafts of Epstein-Barr virus-immortalized human B-cell lines (11). Interestingly, LMP2A functions in the rafts to block the translocation of the BCR into lipid rafts, which leads to inhibition of the subsequent signaling and accelerated internalization of the BCR upon BCR cross-linking (11). These results indicate that the signaling and targeting functions of the BCR rely on distinct molecular mechanisms and that LMP2A has adopted two separate strategies to block these two functions of the BCR.
We report here that Tip is constitutively present in lipid rafts and that the interaction between Tip and p80 is mutually necessary for its efficient localization in lipid rafts. Furthermore, detailed confocal microscopy and cell fractionation analyses showed that Tip-Lck interaction is necessary for recruitment of the TCR complex to lipid rafts and that the Tip-p80 interaction is critical for the internalization of lipid rafts. Taken together, these results provide evidence that the interaction of Tip with Lck and p80 is involved in Tip-mediated signaling and trafficking.
MATERIALS AND METHODS
Cell culture and transfection.
Cultures of 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Jurkat T cells and HVS-transformed common marmoset T cells were grown in RPMI 1640 supplemented with 10% and 20% fetal calf serum, respectively. Fugene 6 (Roche, Indianapolis, Ind.) or calcium phosphate (Clontech, Palo Alto, Calif.) was used for transient expression of Tip in 293T cells. Electroporation at 260 V and 975 μF was used for transient expression of Tip in Jurkat T cells. A stable Jurkat T-cell line expressing AU1 epitope-tagged Tip was selected and maintained by the presence of puromycin (5 μg/ml).
Isolation of lipid rafts.
Lipid rafts were isolated by floatation on discontinuous sucrose gradient (6, 7). Briefly, Jurkat T (108 cells) or 293T (five 100-cm dishes) cells were washed with ice-cold phosphate-buffered saline and lysed for 30 min on ice in 1% Triton X-100 in TNEV (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA) containing phosphatase inhibitors and protease inhibitor cocktail (Roche, Mannheim, Germany). The lysis solution was further homogenized with 10 strokes in a Wheaton loose-fitting Dounce homogenizer. Nuclei and cellular debris were pelleted by centrifugation at 900 × g for 10 min. For the discontinuous sucrose gradient, 1 ml of cleared cell lysates was mixed with 1 ml of 85% sucrose in TNEV and transferred to the bottom of a Beckman centrifuge tube (14 by 89 mm). The diluted lysate was overlaid with 6 ml of 35% sucrose in TNEV and finally with 3.5 ml of 5% sucrose in TNEV. The samples were then centrifuged in an SW41 rotor at 200,000 × g for 20 h at 4°C, and 1-ml fractions were collected from the top of the gradient.
Flow cytometry.
Cells (5 × 105) were washed with RPMI medium containing 10% fetal calf serum and incubated with fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated monoclonal antibodies for 30 min at 4°C. After washing, each sample was fixed with 4% paraformaldehyde solution, and flow cytometry analysis was performed with a FACScan (Becton Dickinson Co., Mountain View, Calif.). Antibodies for CD3 (SK7), CD4 (Leu-3a), and CD45 (HI30) were purchased from Becton Dickinson, and antibody for TCRαβ (BW242/412) was purchased from T Cell Diagnostics (Cambridge, Mass.).
Immunofluorescence.
Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with cold acetone for 15 min, blocked with 10% goat serum in phosphate-buffered saline for 30 min, and reacted with 1:100 to 1:2,000 dilutions of primary antibody in phosphate-buffered saline for 30 min at room temperature. After incubation, cells were washed extensively with phosphate-buffered saline, incubated with 1:100-diluted Alexa 488- or Alexa 568-conjugated rabbit or mouse antibody (Molecular Probes, Eugene, Oreg.) in phosphate-buffered saline for 30 min at room temperature, and washed three times with phosphate-buffered saline. Confocal microscopy was performed with a Leica TCS SP laser-scanning microscope (Leica Microsystems, Exton, Pa.) fitted with a 40× Leica objective (PL APO, 1.4NA) and with Leica imaging software. Images were collected at a resolution of 512 by 512 pixels. The stained cells were optically sectioned in the z axis, and the images in the different channels (photo multiplier tubes) were collected simultaneously. The step size in the z axis varied from 0.2 to 0.5 μm to obtain 30 to 50 slices per imaged file. The images were transferred to a Macintosh G4 computer (Apple Computer, Cupertino, Calif.), and NIH Image version 1.61 software was used to render the images.
Immunoblot, in vitro kinase assay, and antibodies.
Cells were harvested and lysed with lysis buffer (0.15 M NaCl, 1% Nonidet P-40, 50 mM Tris [pH 7.5]) containing 0.1 mM Na2VO3, 1 mM NaF, and protease inhibitors. For immunoblots, polypeptides from whole-cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane filter. Immunoblot detection was performed with a 1:2,000 dilution of primary antibodies. For in vitro protein kinase assays, immune complexes prepared as described above were washed once with kinase buffer (10 mM MgCl2, 1 mM dithiothreitol, 20 mM Tris, pH 7.0), resuspended with 20 μl of kinase buffer containing 5 μCi of γ-[32P]ATP (6,000 Ci/mmol; New England Nuclear) for 20 min at room temperature, and separated by SDS-PAGE. Kinase activity was measured with a Fuji PhosphorImager. Mouse anti-AU1 antibody was purchased from Babco (Richmond, Calif.). Cholera toxin B (CT-B) and antibodies to CD3, CD4, and CD45 were purchased from Santa Cruz Biotech (Santa Cruz, Calif.). Lck antibody was purchased from the Upstate Biotech Institute (Lake Placid, N.Y.). Proteins were visualized with a chemiluminescent detection reagent (Pierce, Rockford, Ill.) and a Fuji PhosphorImager.
RESULTS
Tip-Lck interaction is involved in TCR and CD4 downregulation.
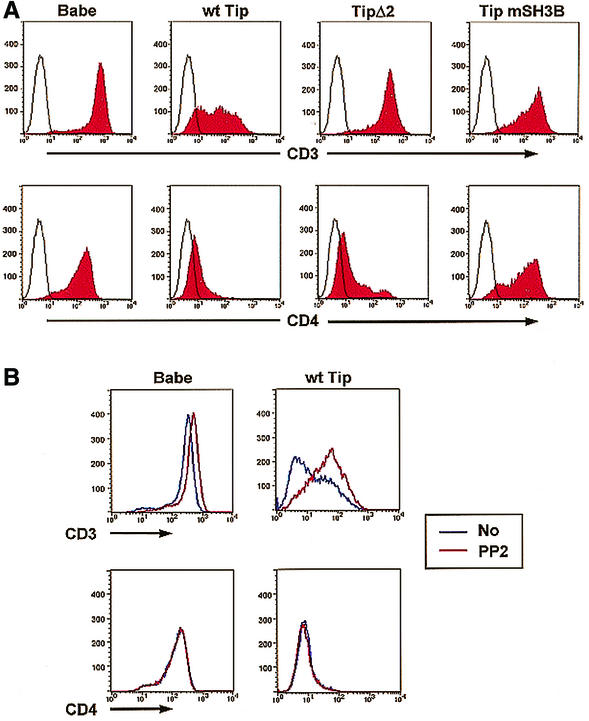
The surface expression of the CD4 molecule is regulated by physical interaction with Lck kinase (38), whereas surface expression of the TCR complex is regulated by the enzymatic activity of Lck kinase (9). To further delineate the role of Lck in the Tip-mediated downregulation of lymphocyte surface antigen expression, Jurkat T cells expressing Tip or its mutants were examined for their levels of CD3 and CD4 surface expression. The Tip mSH3B mutant, which interacts with p80 but not with Lck, and Tip Δ2, which interacts with Lck but not with p80, were expressed in Jurkat T cells with the pBabe-puro retrovirus vector (15, 34). Puromycin-resistant Jurkat T cells were fixed, reacted with anti-CD3 and anti-CD4 antibodies, and assessed by flow cytometry. As previously shown (15, 34), Tip expression strongly downregulated the surface expression of CD3 and CD4, whereas Tip Δ2 expression downregulated the surface expression of CD4 but not CD3 (Fig. 1). In contrast, Tip mSH3B expression did not downregulate the surface expression of either the CD3 or CD4 molecule (Fig. 1). The surface expression of TCRαβ was regulated by Tip similarly to that of CD3 (data not shown). These results indicate that the Tip-Lck interaction is necessary for Tip-mediated downregulation of CD3, CD4, and TCR surface antigens.
FIG. 1.
Differential downregulation of CD3 and CD4 surface expression by Tip. (A) Downregulation of CD3 and CD4 by Tip and its mutants. A total of 106 Jurkat-Babe, Jurkat Tip, Jurkat TipY114S, Jurkat Tip Δ2, and Jurkat Tip mSH3B cells were assessed for surface expression of CD3 and CD4 by flow cytometry. Surface expression of CD3 and CD4 on these cells was assessed by staining with isotype control antibody (open area under the black line) or anti-CD3 and anti-CD4 antibodies (red area). (B) Restoration of CD3 but not CD4 surface expression by PP2 treatment. Jurkat-Babe and Jurkat Tip cells (2 × 106) were incubated with PP2 overnight and assessed for surface expression of CD3 and CD4 by flow cytometry. wt, wild type.
To further test whether Lck kinase activity and/or interaction is necessary for Tip-mediated downregulation of surface antigens, Jurkat-Babe and Jurkat Tip cells were treated with the tyrosine kinase inhibitor PP2 for 12 h, followed by flow cytometry analysis for the assessment of CD3 and CD4 surface expression. This showed that PP2 treatment restored CD3 surface expression of Jurkat Tip cells up to 10-fold, whereas only a slight increase in CD3 surface expression was detected on Jurkat-Babe cells (Fig. 1B). Furthermore, the surface expression of TCRαβ was also restored by PP2 treatment in a similar manner to that of CD3 (data not shown). By contrast, PP2 treatment did not restore CD4 surface expression on Jurkat Tip cells (Fig. 1B). These results indicate that Lck interaction is necessary for Tip-mediated downregulation of CD4 surface expression, whereas both Lck interaction and its kinase activity are necessary for Tip-mediated downregulation of TCR and CD3 surface expression.
Tip is constitutively localized to lipid rafts.
The membrane lipid raft microdomain has been described as a functional platform for signaling of various stimuli and trafficking of signal receptor molecules (6, 7, 35, 36). To examine the potential raft localization of Tip, Jurkat-Babe and Jurkat Tip cells were lysed in 1% Triton X-100 and subjected to discontinuous sucrose density gradient centrifugation. Fractions 3 to 5, 6 to 8, and 9 to 11 were previously shown to contain the raft region, the interval region, and the soluble region of the gradients, respectively (7). The position and integrity of the lipid rafts in the sucrose gradient were determined by the presence of the raft-associated GM1 ganglioside, detected with the GM1-specific ligand CT-B (data not shown). Because of the low level of Tip expression in Jurkat Tip, in vitro kinase assays were used to detect Tip protein.
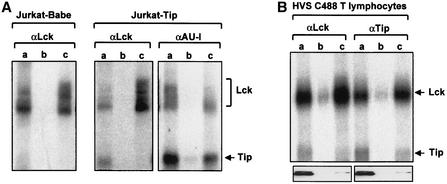
Tip and Lck proteins were immunopurified from each pulled fraction with anti-AU1 and anti-Lck antibodies, respectively, and each immune complex was subjected to in vitro kinase assay (Fig. 2A). This experiment showed that the autophosphorylated Lck protein of 55 to 60 kDa was constitutively present in both the raft and soluble regions of Jurkat-Babe and Jurkat Tip cells, whereas an additional protein of 45 kDa that corresponded to Tip protein was detected primarily from the lipid raft regions of Jurkat Tip cells (Fig. 2A). The in vitro kinase assay of Tip immune complexes further confirmed that Tip was constitutively present in the lipid raft fractions in a complex with Lck (Fig. 2A). Assessment with the PhosphorImager indicated that approximately 70% of Tip protein was located in the lipid raft fraction.
FIG. 2.
Constitutive localization of Tip in lipid rafts. (A) Lipid raft localization of Tip in Jurkat T cells. Jurkat-Babe and Jurkat Tip cells were lysed with Triton X-100 and subjected to discontinuous sucrose density gradient centrifugation. Fractions 3 to 5 (lanes a), 6 to 8 (lanes b), and 9 to 12 (lanes c) were pooled. Tip and Lck proteins were immunopurified from each pulled fraction with anti-AU1 and anti-Lck antibodies. Each immune complex was subjected to the in vitro kinase assay. Arrows indicate Lck and Tip proteins. (B) Lipid raft localization of Tip in HVS-transformed common marmoset T cells. HVS-transformed common marmoset T cells were lysed with Triton X-100 and subjected to discontinuous sucrose density gradient centrifugation. Fractions 3 to 5 (a), 6 to 8 (b), and 9 to 12 (c) were pooled. Tip and Lck proteins were immunopurified from each pooled fraction with anti-Tip and anti-Lck antibodies, respectively, and each immune complex was subjected to the in vitro kinase assay. Arrows indicate Lck and Tip proteins. The bottom panels show the location of GM1.
HVS C488-transformed common marmoset T cells were also lysed in 1% Triton X-100 and subjected to discontinuous sucrose density gradient centrifugation. As described above, each fraction was pooled and used for immunoprecipitation with anti-Lck and anti-Tip antibodies, followed by the in vitro kinase reaction. The position and integrity of the lipid rafts in the sucrose gradient were confirmed by the presence of the raft-associated GM1 (Fig. 2B). This also showed that Tip protein was constitutively present in lipid rafts of virally transformed T lymphocytes (Fig. 2B).
Tip recruits p80 to lipid rafts.
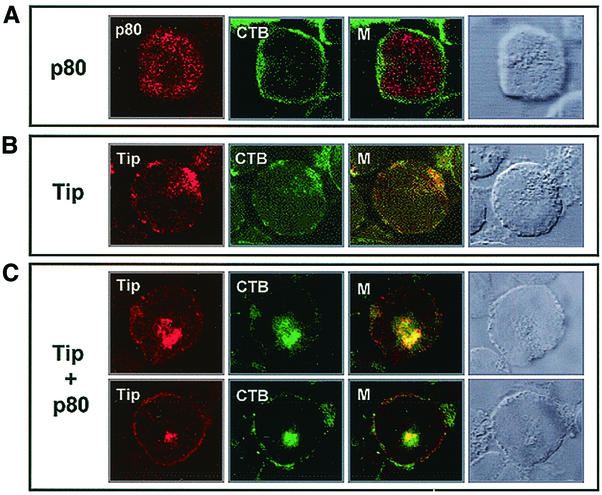
To further investigate the localization of Tip and p80, AU1-tagged Tip and Xpress-tagged p80 were expressed in Jurkat T cells. At 24 h posttransfection, cells were fixed, permeabilized, reacted with anti-Xpress or anti-AU1antibody, and then reacted with fluorescein isothiocyanate (FITC)-conjugated CT-B. A confocal immunofluorescence microscope was used to detect Tip, p80, and lipid rafts. Merged images in multiple optical sections showed that p80 in Jurkat T cells was present in the cytoplasmic region, with little or no colocalization with CT-B staining, whereas Tip was found primarily in patches at the plasma membrane, with considerable colocalization with CT-B staining (Fig. 3). As shown previously (34), coexpression of Tip and p80 greatly facilitated the formation of vesicles where Tip and p80 were recruited (Fig. 3). Remarkably, Tip and p80 coexpression not only led to the colocalization of Tip and p80 with the CT-B-reactive, raft-associated GM1, but also induced the internalization of lipid rafts, indicating that Tip recruits p80 to lipid rafts and that this interaction promotes the internalization of the rafts.
FIG. 3.
Effect of Tip expression on localization of p80 and internalization of lipid rafts. (A) p80 localization. At 24 h posttransfection with Xpress-tagged p80 expression vector, Jurkat T cells were fixed and reacted with mouse monoclonal anti-Xpress antibody (red) and FITC-conjugated CT-B (green). (B) Tip localization. At 24 h posttransfection with the AU1-tagged Tip expression vector, Jurkat T cells were fixed and reacted with mouse monoclonal anti-AU1 antibody (red) and FITC-conjugated CT-B (green). (C) Tip and p80 colocalization. Jurkat T cells were cotransfected with Xpress-tagged p80 and wild-type Tip expression vectors. Cells were fixed and reacted with a rabbit polyclonal anti-Tip antibody (red) and FITC-conjugated CT-B (green). Yellow in the merged panel (M) indicates colocalization of the red and green labels. Cells were visualized with Nomarski optics.
p80 interaction but not Lck interaction of Tip is necessary for the efficient localization of lipid rafts.
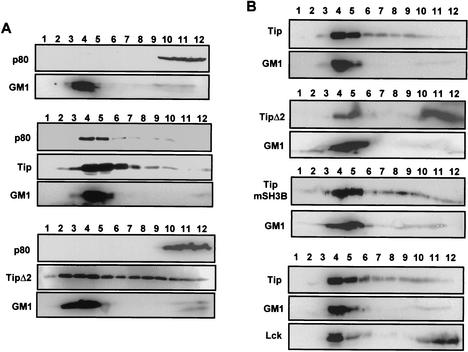
To further investigate the lipid raft localization of Tip and p80, 293T cells were transfected with the p80 expression vector alone or together with wild-type Tip or the Tip Δ2 mutant expression vector. At 48 h posttransfection, cells were lysed with 1% Triton X-100 and subjected to discontinuous centrifugation. An immunoblot assay showed that p80 was primarily present in the soluble regions in the absence of Tip expression, whereas it moved to lipid rafts in the presence of Tip expression (Fig. 4A). In contrast, the Tip Δ2 mutant, which does not interact with p80, was not capable of recruiting p80 to lipid rafts. In parallel, while the majority of Tip Δ2 was present in lipid raft fractions, the level of lipid raft association of Tip Δ2 was significantly lower than that of wild-type Tip (Fig. 4A). These results indicate that Tip recruits p80 to lipid rafts and that an interaction between Tip and p80 is also necessary for the efficient localization of Tip.
FIG. 4.
Requirement of Tip-p80 interaction for localization in lipid rafts. (A) Effect of Tip expression on p80 localization in lipid rafts. 293T cells were transfected with an expression vector containing the Xpress-tagged p80 alone (top two panels) or together with the AU1-tagged wild-type Tip (middle three panels) or the Tip Δ2 mutant (bottom three panels). At 48 h posttransfection, cells were lysed with 1% Triton X-100 and subjected to discontinuous centrifugation. Each fraction was subjected to immunoblot assay with anti-Xpress or anti-AU1 antibody. Horseradish peroxidase-conjugated CT-B (GM1) was used as a control to locate the lipid raft fractions. Fractions 3 to 5 and 9 to 12 contained the raft region and the soluble region of the gradients, respectively. Based on densitometry analysis with the TINA software program, the lipid raft fractions and soluble fractions, respectively, contained 5% and 83% (top panel), 49% and 19% (middle panel), and 4% and 84% (bottom panel) of p80, 65% and 6% (middle panel) and 41% and 20% (bottom panel) of Tip, and 79% and 11% (top panel), 83% and 6% (middle panel), and 78% and 21% (bottom panel) of GM1. (B) Requirement for p80 interaction but not Lck interaction for lipid raft localization of Tip. 293T cells were transfected with an expression vector containing the AU1-tagged wild-type Tip (top two panels), Tip Δ2 (second two panels), or Tip mSH3B (third two panels). Cells were also cotransfected with Tip and Lck expression vectors (bottom three panels). At 48 h posttransfection, cells were lysed with 1% Triton X-100 and subjected to discontinuous centrifugation. Each fraction was subjected to the immunoblot assay with anti-AU1 antibody or anti-Lck antibody. Horseradish peroxidase-conjugated CT-B (GM1) was used as a control to locate the lipid rafts. Based on densitometry analysis with the TINA software program, the lipid raft fractions and soluble fractions, respectively, contained 72% and 5% (top panel), 31% and 52% (second panel), 53% and 9% (third panel), and 71% and 10% (bottom panel) of Tip and 79% and 7% (top panel), 87% and 4% (second panel), 82% and 7% (third panel), and 87% and 4% (bottom panel) of GM1, and 47% and 46% (bottom panel) of Lck.
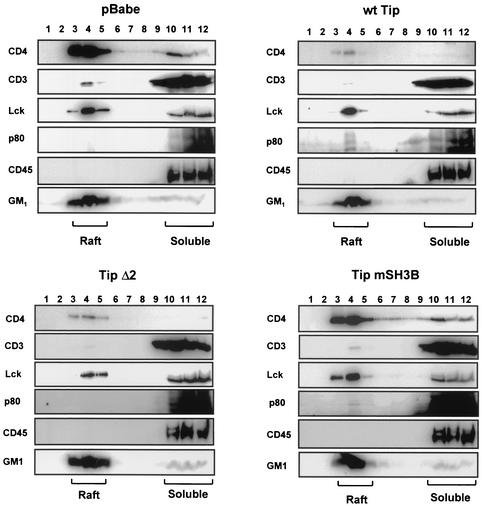
In order to delineate the roles of Lck and p80 in the lipid raft localization of Tip, 293T cells were transfected with an expression vector containing wild-type Tip, Tip Δ2, or Tip mSH3B. At 48 h posttransfection, the cells were lysed with 1% Triton X-100 and subjected to discontinuous centrifugation. The immunoblot assay showed that wild-type Tip and Tip mSH3B were primarily present in the lipid raft regions, whereas Tip Δ2 was present in the soluble regions as abundantly as in the lipid raft regions (Fig. 4B). Furthermore, Lck expression did not affect wild-type Tip localization in lipid rafts to any detectable level (Fig. 4B). These results further indicate that p80 interaction but not Lck interaction is likely necessary for the constitutive localization of Tip in lipid rafts.
Jurkat T cells stably expressing wild-type Tip, Tip Δ2, or Tip mSH3B were also used to investigate the role of the Tip-p80 interaction in the lipid raft localization of p80. Jurkat-Babe, Jurkat-Tip, Jurkat-Tip Δ2, and Jurkat-Tip mSH3 cells were lysed with 1% Triton X-100 and subjected to discontinuous centrifugation. CD45 and GM1 were used as markers for the integrity of raft isolation. Immunoblot assays showed that, as seen in 293T cells (Fig. 4), p80 protein was recruited to lipid rafts at a detectable level in Jurkat-Tip and Jurkat-Tip mSH3B cells, whereas p80 was present exclusively in the soluble fraction in Jurkat-Babe and Jurkat-Tip Δ2 cells (Fig. 5). However, unlike 293T cells (Fig. 4), Jurkat-Tip and Jurkat-Tip mSH3B cells contained only a small amount of p80 in lipid raft fractions (Fig. 5). This result was likely due to a much lower level of Tip expression in Jurkat T cells than in 293T cells.
FIG. 5.
Translocation of p80 to lipid rafts in Jurkat T cells expressing Tip. Jurkat-Babe, Jurkat-Tip (wt), Jurkat-Tip Δ2, and Jurkat-Tip mSH3B cells (108) were lysed in 1% Triton X-100 in cell lysis buffer. Cell lysates were then subjected to discontinuous sucrose gradient centrifugation. Fractions of 1 ml were collected from the top of the gradient and subjected to SDS-10% PAGE, followed by immunoblotting with antibodies specific for CD3, CD4, Lck, p80, and CD45. Horseradish peroxidase-conjugated CT-B (GM1) was used to detect the lipid rafts. Based on densitometry analysis with the TINA software program, the lipid raft fractions and soluble fractions, respectively, of Jurkat-Babe cells contained 79% and 12% of CD4, 3% and 82% of CD3, 57% and 43% of Lck, 0% and 87% of p80, 1% and 95% of CD45, and 88% and 5% of GM1. The lipid raft fractions and soluble fractions, respectively, of Jurkat-Tip cells contained 78% and 13% of CD4, 0% and 72% of CD3, 50% and 48% of Lck, 3% and 74% of p80, 1% and 96% of CD45, and 80% and 6% of GM1. The lipid raft fractions and soluble fractions, respectively, of Jurkat- Tip Δ2 cells contained 80% and 8% of CD4, 1% and 84% of CD3, 41% and 36% of Lck, 1% and 90% of p80, 2% and 96% of CD45, and 86% and 7% of GM1. The lipid raft fractions and soluble fractions, respectively, of Jurkat-Tip mSH3B cells contained 80% and 11% of CD4, 2% and 81% of CD3, 59% and 40% of Lck, 5% and 81% of p80, 2% and 92% CD45, and 89% and 4% of GM1.
The lipid raft localization of Lck was not significantly altered in Jurkat-Tip, Jurkat-Tip Δ2, and Jurkat-Tip mSH3B cells compared to that in Jurkat-Babe cells, while the Lck expression level was detectably lower in Jurkat-Tip and Jurkat-Tip Δ2 cells than in Jurkat-Babe and Jurkat-Tip mSH3B cells (Fig. 5). In concert with its decreased surface expression (Fig. 1), CD4 protein in both raft and nonraft fractions was dramatically reduced in wild-type Tip- and Tip Δ2-expressing Jurkat T cells but not in Tip mSH3-expressing Jurkat T cells (Fig. 5). However, despite its reduced amount, CD4 was tightly associated with lipid rafts in cells expressing Tip or Tip Δ2 (Fig. 5). Surprisingly, unlike its significant reduction in surface expression seen in Fig. 1, CD3 did not display any detectable difference in lipid raft localization in the presence or absence of Tip expression (Fig. 5). Thus, the cell fractionation assay showed that Tip expression led to the relocalization of p80 into lipid rafts, whereas it did not significantly affect the lipid raft localization of Lck, CD3, and CD4.
p80 and Lck interactions have distinct roles in Tip-mediated alteration of lipid rafts and CD3 localization.
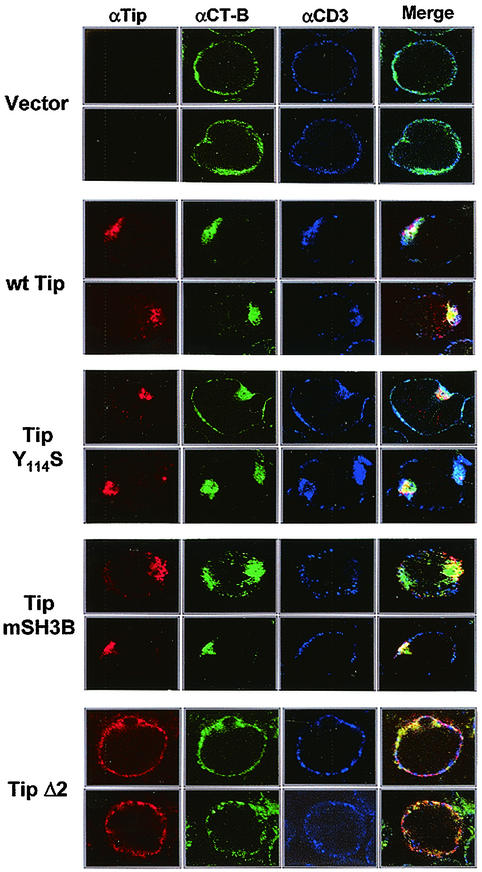
There are several reports indicating that the association of TCR or other proteins with lipid rafts is not well preserved after Triton X-100 extraction (16, 17, 20, 21). Since Tip targets cellular Lck and p80 proteins to generate the pleiotropic effect on T-cell signal transduction, we used immunofluorescence to further examine the properties and localization of lipid rafts and the TCR complex. Jurkat T cells transiently expressing wild-type Tip, Tip Y114S, Tip mSH3B, or Tip Δ2 were used for indirect immunofluorescence confocal microscopy. Jurkat T cells containing a vector alone were included as controls. The Tip Y114S mutant, in which tyrosine 114 has been replaced with serine, has been shown to bind to Lck with approximately fivefold higher affinity than wild-type Tip (15) and to induce a dramatic downregulation of TCRαβ, CD3, and CD4 surface expression (34). FITC-conjugated CT-B was used to detect the distribution of lipid rafts, and anti-Tip and anti-CD3 antibodies were used to detect the Tip and CD3 proteins, respectively.
Merged images in multiple optical sections showed that wild-type Tip, Tip Y114S, and Tip mSH3B in Jurkat T cells were primarily seen in patches at the plasma membrane, whereas Tip Δ2 was evenly dispersed in the plasma membrane (Fig. 6). While lipid rafts were uniformly distributed in the plasma membrane of Jurkat T cells, they were highly concentrated and tightly aggregated at the plasma membrane in Jurkat T cells expressing Tip, Tip Y114S, or Tip mSH3B (Fig. 6). In addition, these patched, CT-B-positive lipid rafts in Jurkat T cells expressing Tip, Tip Y114S, or Tip mSH3B were considerably colocalized with Tip, Tip Y114S, and Tip mSH3B proteins (Fig. 6). In striking contrast, lipid rafts in Jurkat T cells expressing Tip Δ2 cells were evenly dispersed in the plasma membrane, similar to those in control Jurkat T cells (Fig. 6). Finally, CD3 was equally distributed throughout the plasma membrane in control Jurkat T cells and in Jurkat T cells expressing Tip mSH3B or Tip Δ2 (Fig. 6). In contrast, it displayed a patched staining at the plasma membrane in Jurkat T cells expressing Tip or Tip Y114S and was also significantly coloca1ized with Tip and Tip Y114S and with lipid rafts (Fig. 6). Thus, confocal microscopy demonstrates that the expression of Tip, Tip Y114S, and Tip mSH3B, which are capable of interacting with p80, induces the aggregation and internalization of lipid rafts and that the expression of Tip and Tip Y114S, which are capable of interacting with Lck, strongly induces the recruitment of CD3 to lipid rafts.
FIG.6.
Effect of Tip interactions with p80 and Lck on Tip-mediated alteration of lipid raft and CD3 localization. Jurkat T cells were electroporated with an expression vector expressing wild-type (wt) Tip, Tip Y114S, Tip Δ2, or Tip mSH3B. At 48 h postelectroporation, cells were fixed and reacted with rabbit polyclonal anti-Tip antibody (red), mouse monoclonal anti-CD3 antibody (blue), and CT-B (green). Yellow in the merged panel indicates colocalization of the red and green labels. Immunofluorescence was examined with a Leica confocal immunofluorescence microscope, and representative optical sections are presented.
DISCUSSION
A growing body of evidence shows that detergent-resistant rafts comprise anatomically distinct subsets of plasma membrane and that these subsets are enriched for a variety of important signaling proteins (1, 4, 35, 36). Recent studies have also demonstrated that lipid rafts are not static entities but instead dynamic microdomains on the cell surface for which proteins and lipids have various affinities (17, 28, 37). Upon TCR engagement with antigenic peptides complexed to the major histocompatibility complex, lipid raft domains undergo redistribution to induce patched aggregation at the plasma membrane, and TCR-associated signaling molecules and coreceptors then acquire increased affinities for lipid raft domains (31, 32, 40, 41). Thus, the integrity of these domains has been shown to be necessary for efficient signal transduction by the TCR. Here, we report that, as seen in TCR stimulation (31, 32, 40, 41), Tip expression induces the aggregation of lipid rafts and the redistribution of TCR complex into lipid raft domains. This phenotypic resemblance of Tip and TCR suggests that HVS Tip may pirate cellular signaling molecules, Lck and p80, to emulate TCR stimulation, which powerfully activates virus-infected T cells and provides the virus an opportunity for efficient replication, dissemination, and, ultimately, oncogenesis.
As a leukocyte-specific tyrosine kinase, Lck is associated with the cytosolic side of the plasma membrane (19) and interacts specifically with CD4 through noncovalent bonds coordinated by a Zn2+ ion (18, 24). We have demonstrated that a physical interaction with Lck but not Lck kinase activity is necessary for Tip-mediated downregulation of CD4. This is in agreement with the recent analysis of CD4 trafficking showing that Lck binds tightly to CD4 independently of its activation state and inhibits CD4 internalization (13). Thus, Tip interaction with Lck may prevent this kinase from participating in a complex with CD4. Lack of Lck may ultimately facilitate CD4 internalization and thereby degradation. In contrast, treatment with the Src family kinase inhibitor PP2 (42), which inhibits Lck kinase activity, showed that the Lck kinase activity was necessary for Tip-mediated downregulation of the TCR complex but not CD4. Lck has been shown to be required for the initiation of T-cell signaling by phosphorylating the immunoreceptor tyrosine-based activation motif in the T-cell receptor complex cytoplasmic tail. This phosphorylation results in the recruitment and activation of other signaling molecules. Activated TCR complexes are subsequently subjected to endocytosis, which delivers the TCR complex to the lysosomal compartment as a final destination. Thus, Tip targets Lck tyrosine kinase in two distinct ways to downregulate both TCR and its coreceptor CD4.
Tip expression dramatically induced the patched aggregation and internalization of lipid rafts, which is similar to the effect induced by CD3/CD28 costimulation (39). In addition, since the TipY114S and Tip mSH3 mutants but not Tip Δ2 are capable of inducing the aggregation and internalization of lipid rafts, this property of Tip is dependent on p80 interaction but independent of Lck interaction. We recently identified p80 as a novel cellular WD-containing protein that interacts with Tip (34). In the present study, we found that the Tip-p80 interaction induces the generation of large endosomal vesicles that are part of lipid rafts (Fig. 2). Surprisingly, while p80 is present exclusively in soluble fractions in the absence of Tip, it rapidly and efficiently translocates into lipid rafts upon Tip expression. In parallel, p80 interaction is also necessary for the efficient localization of Tip in lipid rafts. Thus, both Tip and p80 are mutually necessary for their localization in lipid rafts, and their interaction induces the aggregation and internalization of lipid rafts.
We have previously shown that the amino-terminal WD repeat domain of p80 interacts with Tip and that the carboxyl coiled-coil region of p80 targets membrane localization (34). WD domain-containing proteins are a family of proteins with diverse cellular functions, including regulation of signal transduction, pre-mRNA processing gene expression, cell cycle progression, development, vesicular traffic, and cytoskeleton assembly (33). The solution of the three-dimensional structure of the WD repeat of Gβ revealed a beta-propeller fold, highly symmetrical structure made up of repeats that each comprised a small four-stranded antiparallel beta sheet (14). Thus, it appears that the amino-terminal WD repeat region of p80 may create a stable stage that can form tight complexes with Tip and that the carboxyl coiled-coil region of p80 may coordinate sequential and/or simultaneous interactions with cellular proteins for aggregation and internalization of lipid rafts. Our recent study found that the carboxyl coiled-coil domain of p80 interacts with cellular cochaperone-like proteins (data not shown). Thus, p80 may act as both a scaffold and chaperone, and the interaction of Tip with p80 may activate p80 function to facilitate membrane fusion of lipid rafts and thereby the generation of aggregated lipid raft microdomains. Then, these aggregated lipid raft domains may function as platforms for recruiting cellular signaling molecules and generating a Tip signalosome.
Our results from confocal microscopy showed an association of the TCR with CT-B-reactive lipid rafts in the presence of Tip expression. This finding contrasts with biochemical studies analyzing lipid raft composition and highlights the limitations of using detergent insolubility as the only criterion to monitor the association of a particular protein with lipid rafts. Although CD3 staining clearly overlapped the CT-B-positive patches in Jurkat Tip cells, this association was completely lost after Triton X-100 extraction. In fact, there are several reports indicating that the association of TCR or other proteins with lipid rafts is not preserved after Triton X-100 extraction (16, 20). It is likely that only proteins that are strongly associated with lipid rafts are Triton X-100 insoluble, whereas weakly associated proteins are extracted. In addition, we found that Tip-induced colocalization of the TCR with CT-B-reactive lipid raft patches required both Lck and p80 interaction. These observations suggest that upon Tip interaction, Lck recruits the receptor to lipid rafts, and that Tip-p80 interactions may then induce coalescence of lipid raft microdomains with which the TCR is already associated. Studies to further define the mechanism of these properties associated with Tip and p80 are currently under way.
Because of its biological significance, the regulation of surface expression of the lymphocyte antigen receptor is a common strategy used by viruses as a way to persist in their host (5). Here, we demonstrate that the signaling and targeting functions of HVS Tip rely on two functionally and genetically separable mechanisms that independently target cellular Lck tyrosine kinase and WD domain-containing p80 protein and that the consequence of these interactions is the downregulation of TCR and CD4 surface expression and thereby their signal transduction pathways. Epstein-Barr virus LMP2A, a homologue of HVS Tip, has also been shown to be constitutively present in lipid rafts of virus-immortalized human B-cell lines (11). However, unlike HVS Tip, LMP2A functions in the rafts to block translocation of the BCR into lipid rafts, which leads to inhibition of the subsequent signaling and accelerated internalization of the BCR upon BCR cross-linking (11). This indicates that the signaling and targeting functions of the HVS Tip and Epstein-Barr virus LMP2A use similar but distinct molecular mechanisms to modulate major cellular signal transduction pathways. Thus, these findings potentially have significant implications for viral persistence and pathogenesis.
Acknowledgments
The first two authors contributed equally to this work.
We thank M. Connole for flow cytometry analysis and J. Macke for manuscript editing.
This work was partly supported by U.S. Public Health Service grants CA31363, AI38131, and RR00168 and ACS grant RPG001102. J. Park is partly supported by the Brain Korea 21 program of the Ministry of Education in Korea, N.-H. Cho is partly supported by the postdoctoral fellowship program of the Korean Science and Engineering Foundation, and J. Jung is a Leukemia & Lymphoma Society Scholar.
REFERENCES
- 1.Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821-1825. [DOI] [PubMed] [Google Scholar]
- 2.Biesinger, B., I. Muller-Fleckenstein, B. Simmer, G. Lang, S. Wittmann, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biesinger, B., A. Y. Tsygankov, H. Fickenscher, F. Emmrich, B. Fleckenstein, J. B. Bolen, and B. M. Broker. 1995. The product of the Herpesvirus saimiri open reading frame 1 (tip) interacts with T-cell-specific kinase p56lck in transformed cells. J. Biol. Chem. 270:4729-4734. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 5.Callan, M. F., and A. J. McMichael. 1999. T-cell receptor usage in infectious disease. Springer Semin. Immunopathol. 21:37-54. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, P. C., A. Cherukuri, M. Dykstra, S. Malapati, T. Sproul, M. R. Chen, and S. K. Pierce. 2001. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 13:107-114. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, P. C., M. L. Dykstra, R. N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desrosiers, R. C., A. Bakker, J. Kamine, L. A. Falk, R. D. Hunt, and N. W. King. 1985. A region of the herpesvirus saimiri genome required for oncogenicity. Science 228:184-187. [DOI] [PubMed] [Google Scholar]
- 9.D'Oro, U., M. S. Vacchio, A. M. Weissman, and J. D. Ashwell. 1997. Activation of the Lck tyrosine kinase targets cell surface T-cell antigen receptors for lysosomal degradation. Immunity 7:619-628. [DOI] [PubMed] [Google Scholar]
- 10.Duboise, S. M., J. Guo, S. Czajak, R. C. Desrosiers, and J. U. Jung. 1998. STP and Tip are essential for herpesvirus saimiri oncogenicity. J. Virol. 72:1308-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dykstra, M. L., R. Longnecker, and S. K. Pierce. 2001. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14:57-67. [DOI] [PubMed] [Google Scholar]
- 12.Fickenscher, H., and B. Fleckenstein. 2001. Herpesvirus saimiri. Phil. Trans. R. Soc. Lond. B Biol. Sci. 356:545-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foti, M., M. A. Phelouzat, A. Holm, B. J. Rasmusson, and J. L. Carpentier. 2002. p56Lck anchors CD4 to distinct microdomains on microvilli. Proc. Natl. Acad. Sci. USA 99:2008-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Higuera, I., J. Fenoglio, Y. Li, C. Lewis, M. P. Panchenko, O. Reiner, T. F. Smith, and E. J. Neer. 1996. Folding of proteins with WD-repeats: comparison of six members of the WD-repeat superfamily to the G protein beta subunit. Biochemistry 35:13985-13994. [DOI] [PubMed] [Google Scholar]
- 15.Guo, J., M. Duboise, H. Lee, M. Li, J. K. Choi, M. Rosenzweig, and J. U. Jung. 1997. Enhanced downregulation of Lck-mediated signal transduction by a Y114 mutation of herpesvirus saimiri tip. J. Virol. 71:7092-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harder, T., and K. Simons. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534-542. [DOI] [PubMed] [Google Scholar]
- 18.Huse, M., M. J. Eck, and S. C. Harrison. 1998. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J. Biol. Chem. 273:18729-18733. [DOI] [PubMed] [Google Scholar]
- 19.Isakov, N., and B. Biesinger. 2000. Lck protein tyrosine kinase is a key regulator of T-cell activation and a target for signal intervention by Herpesvirus saimiri and other viral gene products. Eur. J. Biochem. 267:3413-3421. [DOI] [PubMed] [Google Scholar]
- 20.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T-cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T-cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 22.Jung, J. U., J. K. Choi, A. Ensser, and B. Biesinger. 1999. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 9:231-239. [DOI] [PubMed] [Google Scholar]
- 23.Jung, J. U., S. M. Lang, U. Friedrich, T. Jun, T. M. Roberts, R. C. Desrosiers, and B. Biesinger. 1995. Identification of Lck-binding elements in tip of herpesvirus saimiri. J. Biol. Chem. 270:20660-20667. [DOI] [PubMed] [Google Scholar]
- 24.Lin, R. S., C. Rodriguez, A. Veillette, and H. F. Lodish. 1998. Zinc is essential for binding of p56lck to CD4 and CD8α. J. Biol. Chem. 273:32878-32882. [DOI] [PubMed] [Google Scholar]
- 25.Longnecker, R. 2000. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv Cancer Res. 79:175-200. [DOI] [PubMed] [Google Scholar]
- 26.Longnecker, R., and C. L. Miller. 1996. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 4:38-42. [DOI] [PubMed] [Google Scholar]
- 27.Lund, T., M. M. Medveczky, and P. G. Medveczky. 1997. Herpesvirus saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J. Virol. 71:378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayor, S., and F. R. Maxfield. 1995. Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol. Biol. Cell 6:929-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. L., J. H. Lee, E. Kieff, A. L. Burkhardt, J. B. Bolen, and R. Longnecker. 1994. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect. Agents Dis. 3:128-136. [PubMed] [Google Scholar]
- 31.Montixi, C., C. Langlet, A. M. Bernard, J. Thimonier, C. Dubois, M. A. Wurbel, J. P. Chauvin, M. Pierres, and H. T. He. 1998. Engagement of T-cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran, M., and M. C. Miceli. 1998. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T-cell activation. Immunity 9:787-796. [DOI] [PubMed] [Google Scholar]
- 33.Neer, E. J., and T. F. Smith. 2000. A groovy new structure. Proc. Natl. Acad. Sci. USA 97:960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park, J., B. S. Lee, J. K. Choi, R. E. Means, J. Choe, and J. U. Jung. 2002. Herpesviral protein targets a cellular WD repeat endosomal protein to downregulate T lymphocyte receptor expression. Immunity 17:221-233. [DOI] [PubMed] [Google Scholar]
- 35.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 36.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 37.Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394:798-801. [DOI] [PubMed] [Google Scholar]
- 38.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T-cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 39.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 40.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T-cell activation. Immunity 8:723-732. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T-cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, X., J. L. Kim, J. R. Newcomb, P. E. Rose, D. R. Stover, L. M. Toledo, H. Zhao, and K. A. Morgenstern. 1999. Structural analysis of the lymphocyte-specific kinase Lck in complex with nonselective and Src family selective kinase inhibitors. Struct. Fold Des. 7:651-661. [DOI] [PubMed] [Google Scholar]