Abstract
During viral entry, herpes simplex virus (HSV) glycoprotein D (gD) interacts with a specific cellular receptor such as nectin-1 (PRR1/HveC/CD111) or the herpesvirus entry mediator A (HVEM/HveA). Nectin-1 is involved in cell-to-cell adhesion. It is located at adherens junctions, where it bridges cells through homophilic or heterophilic interactions with other nectins. Binding of HSV gD prevents nectin-1-mediated cell aggregation. Since HSV gD affects the natural function of nectin-1, we further investigated the effects of gD expression on nectin-1 during HSV infection or in transfected cells. We also studied the importance of the interaction between nectin-1 and the cytoplasmic protein afadin for HSV entry and spread as well as the effects of infection on this interaction. In these investigations, we used a panel of cells expressing nectin-1 or nectin-1-green fluorescent protein fusions as the only mediators of HSV entry. During HSV infection, nectin-1 localization at adherens junction was dramatically altered in a manner dependent on gD expression. Nectin-1 and gD colocalized at cell contact areas between infected and noninfected cells and at the edges of plaques. This specific accumulation of gD at junctions was driven by expression of nectin-1 in trans on the surface of adjacent cells. Reciprocally, nectin-1 was maintained at junctions by the trans expression of gD in the absence of a cellular natural ligand. Our observations indicate that newly synthesized gD substitutes for nectin-1 of infected cells at junctions with noninfected cells. We propose that gD attracts and maintains the receptor at junctions where it can be used for virus spread.
Binding of a specific cell surface receptor to herpes simplex virus (HSV) envelope glycoprotein D (gD) is an essential step in the process of viral entry into mammalian cells (5, 62). This interaction is required but not sufficient for membrane fusion, an event which also requires gB and the gH/gL complex (48, 70). Cellular receptors for HSV gD include herpesvirus entry mediator A (HVEM/HveA) (47), nectin-1 (PRR1/HveC/CD111) (23), and nectin-2 (PRR2/HveB/CD112) (72), as well as a specific type of heparan sulfate modified by 3-OST-3 (d-glucosaminyl 3-O-sulfotransferase 3) (60). HVEM is a lymphotoxin receptor that belongs to the tumor necrosis factor receptor family (6, 41, 47), whereas nectins form a subgroup of immunoglobulin (Ig)-like molecules (16, 37, 68).
HVEM receptor activity is limited to wild-type HSV-1 and HSV-2, whereas nectin-1 acts as a receptor for many alphaherpesviruses (HSV-1, HSV-2, pseudorabies virus [PRV], and bovine herpesvirus 1) (13, 23, 43, 45, 47, 73). The related nectin-2 can be used only by HSV strains carrying specific mutations in the N-terminal part of gD and by HSV-2 (36, 72). Nectin-3 and nectin-4 are not receptors for herpesviruses, but the related human poliovirus receptor (PVR/CD155) can be used by animal herpesviruses such as PRV and bovine herpesvirus 1 (23, 55, 56, 59).
Nectins are widely expressed in tissues and cells (10, 16, 23, 25, 37, 40, 57), where they are located mainly at adherens junctions (AJ) (67). On the cell surface, nectins form homodimers involved in cell-cell adhesion by interacting in trans with other nectins (68). These calcium-independent trans-interactions can be homophilic or heterophilic within the nectin family (1, 67, 68).
Nectins share structural and sequence homology characterized by the presence of three Ig-like domains (one V-domain followed by two C-domains) in their extracellular portion. Isotypes of nectin-1 and nectin-2 generated by alternative splicing vary in their transmembrane region and cytoplasmic tails (10, 16, 23, 37). At least one variant of nectin-1, nectin-2, and nectin-3 (α-isotype) carries a C-terminal PDZ binding domain that is able to interact with afadin/AF6 (37, 38, 55, 59, 67). Afadin is a large protein produced as two isoforms, l-afadin and s-afadin (38, 68). The long form (l-afadin) contains an F-actin binding domain (38). Afadin also appears to be involved in mediating interactions between nectins and the cadherin-catenin system in AJ and between nectins and junctional adhesion molecules (JAM) in tight junctions (TJ) (19, 20, 66).
The V-domain of nectin-1 is critical in the trans-interaction of nectin-1 with itself or with nectin-3 (18, 33, 58, 65). HSV gD binds to the V-domain in a region involving the predicted β-strands C, C′, and C" (7, 8, 22, 34). In this region, mutations at positions between amino acids 64 and 94 abolish or decrease gD binding and receptor function (39, 42). It was shown that gD binding to the V-domain as well as HSV infection directly impaired the adhesive function of nectin-1 (33, 58, 77).
As a structural glycoprotein, gD is expressed relatively early after HSV infection (11). It is found on the surface of infected or transfected cells predominantly at cell contact areas (50, 65) and is targeted to basolateral membranes of epithelial cells (63).
Early in infection, nectin-1 seems to remain on the surface of cells in suspension; however, its epitope accessibility appears modified (33). In this study we qualitatively assessed the effects of HSV infection and gD expression on the cellular distribution of nectin-1 in cell monolayers. Under these conditions, natural AJs are formed and can be monitored directly. We used models consisting of murine melanoma cell lines transfected to constitutively express nectin-1 or nectin-1-green fluorescent protein (GFP) fusions as the only functional mediators of HSV entry (33, 44). First, cell lines carrying various nectin-1-GFP constructs were characterized for nectin-1 localization and ability to interact with afadin, mediate cell aggregation, and sustain HSV entry and spread. Surprisingly, we found that the nectin-1-afadin interaction was not required for nectin-1 accumulation at cell junctions or for efficient viral spread as proposed in earlier studies (58, 67). We further showed that the location of nectin-1 at AJ was altered during HSV infection and that newly synthesized gD was involved in this relocation. Observations by fluorescence microscopy of nectin-1 and gD in infected and transfected cells indicated that the two proteins colocalized at the interface between infected or transfected cells and naive cells, presumably by trans-interacting with each other. We propose a model in which gD plays a role in viral spread by replacing nectin-1 of infected cells at junctions with noninfected cells. By binding nectin-1 of the noninfected cell, gD would maintain the receptor at junctions in a position suitable for virus spread.
MATERIALS AND METHODS
Viruses, cell lines, and constructs. (i) Viruses.
HSV-1 KOS tk12 (72) was grown and subjected to titer determination on Vero cells. HSV KOSgDβ (14) was grown and subjected to titer determination on the complementing cell line VD60, which expresses gD upon infection (35). Viruses were purified as described previously (26).
(ii) Cells.
B78H1-C10 cells and B78H1-Control-16 cells, abbreviated here as C10 and B78 cells, respectively, were derived from B78H1 murine melanoma cells by transfection with pBG38 expressing human nectin-1 and the empty vector pcDNA3, respectively (33, 44). All B78H1-derived cell lines were cloned by limiting dilution in the presence of G418 (1 mg/ml) and subsequently maintained in Dulbecco's modified Eagle's medium with 5% fetal calf serum and G418 at 500 μg/ml.
(iii) Construction of nectin-1 with GFP fused at the C terminus.
The nectin-1 open reading frame (ORF) from pBG38 (23) was directly flanked by HindIII and BamHI sites during PCR amplification using the forward primer FHC5HIND (GCCCAAGCTTATGGCTCGGATGGGGCTTGCGG) and the reverse primer RHC3BAM (GCGGGATCCCACGTACCACTCCTTCTTGG). The digested fragment was inserted into pCDNA3.1 (Invitrogen) that had been digested with HindIII and BamHI to yield plasmid pCK451. The enhanced GFP ORF from plasmid pEGFP (Clontech Laboratories Inc.) was flanked with BamHI and XbaI sites during PCR amplification with the forward primer 5′EGFP (CGCGGATCCATGGTGAGCAAGGGCGAGGAGCTGTT) and the reverse primer 3′GFP (CGGTCTAGACTACTTGTACAGCTCGTCCAT). The digested fragment was inserted into the corresponding sites of pCK451 to yield pCK454.
(iv) Construction of nectin-1 with GFP inserted into the cytoplasmic tail.
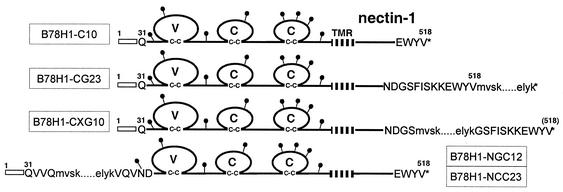
First, a BamHI site was engineered into codons for Gly508 and Ser509 of plasmid pBG38 by using the Quickchange mutagenesis kit (Stratagene). The codons for Gly508 and Ser509 were changed from GGG TCT to GGA TCC without affecting the amino acid sequence. The mutated ORF was flanked by HindIII and BclI sites during PCR amplification using the forward primer FHC5HIND and the reverse primer RHC3BHL (GCGTGATCACACGTACCACTCCTTCTTGG). The digested fragment was introduced into pCDNA3.1 that had been digested with HindIII and BamHI to yield plasmid pCK453. The EGFP ORF was flanked by BamHI sites during PCR amplification using the forward primer 5′EGFP and the reverse primer REGFP3BAM (GCGGGATCCCTTGTACAGCTCGTCCATGCCGAG). The BamHI-digested fragment was inserted into the engineered BamHI site of pCK453, resulting in plasmid pCK455. In this construct, nectin-1 codons Gly508 and Ser509 are duplicated and flank the GFP ORF (Fig. 1).
FIG. 1.
Schematic representation of nectin-1 and derived fusion proteins. The 518-amino-acid human nectin-1 is represented by residues numbered from methionine 1. The open box indicates the nectin-1 signal peptide, and the transmembrane region (TMR) is hatched. The putative N-linked carbohydrates are represented by black lollipops. The Ig-like domains are labeled V, C, and C by analogy to variable or constant Ig domains. Sequences at GFP and CFP insertion sites are also shown. Capital letters represent the nectin-1 sequence, and lowercase letters represent the GFP and CFP start and end sequences. Asterisks indicate stop codons. The names of representative cell lines derived from B78H1 cells expressing the corresponding constructs are boxed. Nectin-1-GFP cell lines are CG23, CXG10, and NGC12. The NCC23 cell line expresses nectin-1-CFP, whereas C10 cells express wild-type nectin-1.
(v) Construction of nectin-1 with GFP added at the N terminus.
A similar strategy was used to insert the GFP ORF into a newly generated BsrGI restriction site at the N-terminus of nectin-1. A BsrGI site was generated in codons Val32 and Val33 of nectin-1 by using the Quickchange mutagenesis kit on plasmid pBG38. The sequence of these two codons was changed from GTG GTC to GTT GTA and did not affect the amino acid sequence in plasmid pRM467. The EGFP ORF was flanked by BsrGI sites during PCR amplification, and the digested fragment was inserted into the engineered BsrGI site of pBG38, resulting in plasmid pCK495. In this plasmid, the GFP ORF is introduced after codon Gln34, 4 residues downstream of the putative N terminus of the mature nectin-1. Amino acids Val33 and Gln34 are duplicated and flank the GFP ORF (Fig. 1). A similar construct was generated with the cyan fluorescent protein (CFP) ORF (amplified from plasmid pECF-Mem [BD Biosciences]) instead of GFP ORF to give plasmid pCK494.
Plasmids pCK454, pCK455, pCK494 and pCK495 were transfected into B78H1 cells. Based on expression of the relevant fusion protein observed by fluorescence microscopy, several clones were selected for each construct. The resulting cell lines are B78H1-CG23 (nectin-1 with GFP at the C terminus), B78H1-CXG10 (GFP inserted 11 residues prior to the C terminus of nectin-1), B78H1-NGC12 (nectin-1 with GFP at the N terminus), and B78H1-NCC23 (nectin-1 with CFP at the N terminus) (Fig. 1).
Immunofluorescence.
Cells were seeded on glass coverslips and cultured overnight to the desired density. They were fixed with 3% paraformaldehyde for 20 min at room temperature (RT), and then the remaining paraformaldehyde was quenched by incubation in 50 mM NH4Cl for 10 min at RT and the cells were permeabilized with 0.1% Triton X-100 for 5 min at RT (method adapted from that of Sodeik et al. [61]). Fixed cells were incubated in phosphate-buffered saline (PBS) with 10% normal goat serum for 30 min at RT and then labeled with the appropriate antibodies. The following antibodies were used: anti-AF6 (afadin) monoclonal antibody (MAb) (Becton-Dickinson, Transduction Laboratories), anti-gB MAb SS10 (G. H. Cohen and R. J. Eisenberg, unpublished data), and anti-gD polyclonal serum Ig (R7) (28) followed by an adequate Alexa-Fluor594-conjugated goat anti-Ig (Molecular Probes, Eugene, Oreg.). When required, MAb CK41 directly coupled to Oregon Green was subsequently added to detect nonfluorescent nectin-1 as described previously (32, 33). All incubations were performed in PBS plus 10% normal goat serum for 30 min at RT with 1 μg of purified IgG per ml. The coverslips were rinsed three times with PBS and once with H2O and mounted in ProLong Antifade (Molecular Probes). When required, nuclei were stained by the addition of DAPI (4′,6-diamidino-2-phenylindole, 1 μg/ml) to the mounting solution. Preparations were examined with a Nikon Eclipse E600 microscope or with a Nikon TE-300 inverted microscope coupled to a Bio-Rad Radiance 2000-MP confocal imaging system. A two-line argon-krypton laser emitting at 488 and 568 nm was used to excite the fluorescence of Oregon Green and AlexaFluor 594.
Aggregation assay.
The assay monitoring nectin-1-mediated cell aggregation was performed and analyzed as described previously (33). At the beginning (t = 0 min) and at the end (t = 90 min) of the aggregation time, aliquots of cells were fixed by adding 25% glutaraldehyde to a final concentration of 2% before the cells and aggregates were counted for quantitative analysis. Aggregation was reported as A90 = (N90/N0) × 100 where N90 and N0 represent the number of independent entities (clumps and individual cells) at incubation times t = 90 min and t = 0 min, respectively. Accordingly, the more aggregation, the lower the N90 value and therefore the lower the A90 percentage.
Alternatively, cells were fixed by the addition of 2 volumes of a 3% paraformaldehyde solution in PBS for direct observation of GFP by fluorescence microscopy (Nikon Eclipse E600 microscope).
Infections. (i) Entry assay.
Purified HSV-1 KOS tk12 (72) diluted in cell culture medium (100 μl) was added to confluent cell monolayers in 96-well plates at various multiplicities of infection (MOI). The cells were placed directly at 37°C and incubated for 6 h before being lysed with NP-40 (0.5% final concentration). Then, 50 μl of cell lysate was mixed with an equal volume of β-galactosidase substrate (chlorophenol red-β-d-galactopyranoside). The level of entry was monitored by reading the absorbance at 595 nm at intervals of 2 min for 50 min to record the enzymatic activity, expressed as the change in optical density per hour (ΔOD/h).
(ii) Blue-plaque assay.
Cell monolayers were infected for 1 h at 4°C with HSV-1 KOS tk12 diluted in culture medium. The cells were incubated at 37°C for 2 h, and then the culture medium was replaced with an overlay of culture medium containing 0.5% methylcellulose. After 28 h at 37°C, the cells were washed three times with PBS and fixed with cold methanol-acetone (1:1). The enzymatic activity of the virus-encoded β-galactosidase was detected by using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal).
(iii) Immunofluorescence assay.
Cells grown on glass coverslips were infected at high MOI (25 PFU/cell) for 1 h at 4°C with HSV-1 KOS tk12 diluted in culture medium. The virus inoculum was then removed, the cells were washed once with medium, and fresh warm medium was added. Incubation proceeded at 37°C for the indicated time. The cells were then fixed, permeabilized, and stained as described above.
Transfections.
Subconfluent cell monolayers were transfected with the expression plasmid pPEP98 (gB) or pPEP99 (gD) (53) using GenePorter as recommended by the manufacturer (Gene Therapy System). After 24 h, the cells were trypsinized, seeded on glass coverslips, cultured overnight, and fixed and stained as described above. When mixed-cell experiments were performed, B78 cell populations were transfected with gD or gB expression plasmids and cultured for 24 h at 37°C before being mixed with receptor-expressing cells at a 1:1 ratio. Cocultures were grown on glass coverslips for an additional 24 h before being fixed and stained.
RESULTS
Expression of nectin-1-GFP fusion proteins in B78H1 cells.
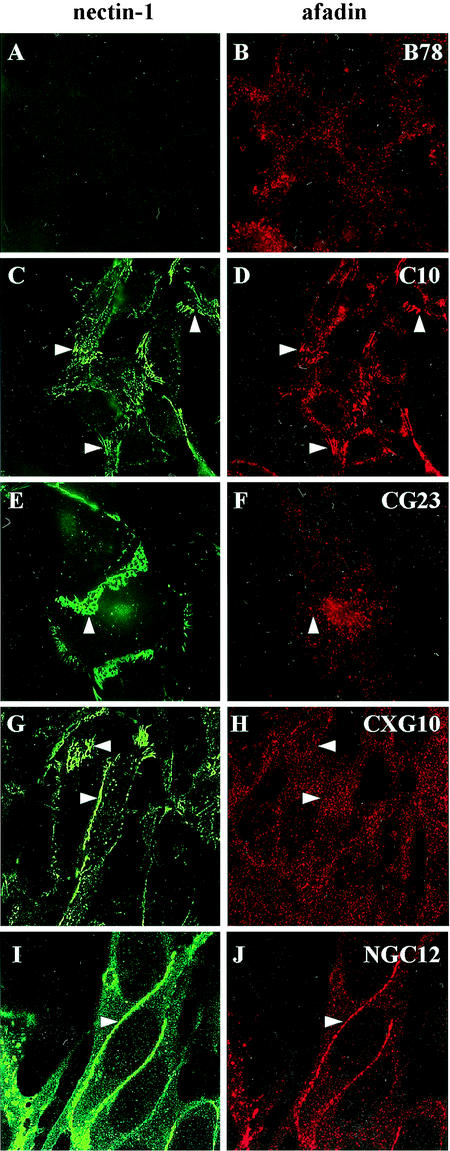
B78H1 mouse melanoma cells are resistant to HSV infection because of the lack of functional gD receptors on their surface (33, 44). Stable expression of full-length human nectin-1 on the surface of transfected B78H1-C10 cells (C10 cells) renders them susceptible to HSV infection. Similarly, we engineered B78H1 lines to express various forms of nectin-1-GFP fusion proteins (Fig. 1). Based on the detection of surface nectin-1-GFP or CFP by immunofluorescence, several lines were selected for each construct. In all cases, nectin-1-GFP accumulated at contact areas between adjacent cells (Fig. 2C, E, G, and I). There was no discernable difference between the patterns of nectin-1-GFP and nectin-1 as detected by immunostaining (C10 cells) (Fig. 2C) (33). The various images in Fig. 2 represent characteristic patterns of expression at AJs. In subconfluent monolayers, a characteristic “leopard skin” type of staining of the contact surface of overlapping cells was observed (Fig. 2E and G), whereas in cells at higher density, the junctions between juxtaposed cells appeared as intense lines (Fig. 2G and I).
FIG. 2.
Expression of nectin-1 and nectin-1-GFP proteins in cell lines derived from B78H1 cells. Nectin-1 in B78H1 and C10 cells was detected using MAb CK41 coupled with Oregon Green (A and C), whereas nectin-1-GFP in CG23, CXG10, and NGC12 cells was observed directly by green fluorescence (E, G, and I). In these permeabilized cells, afadin was detected with anti-AF6 MAb followed by alexa594-coupled secondary antibody (B, D, F, H, and J). Representative pictures of various patterns are shown. These patterns are not cell-type specific and represent cell contact surfaces viewed from different angles (enlarged in panels E and F). Images A to F were collected on a regular fluorescence microscope (Nikon Eclipse E600), and images G to J were collected on a confocal microscope (Nikon TE-300 inverted microscope coupled to a Bio-Rad Radiance 2000-MP confocal imaging system).
Nectin-1 interacts directly with afadin via its C-terminal PDZ binding domain (Glu/Ala-X-Tyr-Val) and the PDZ pocket of afadin (67). In parental B78H1 cells, afadin was detected but did not show preferential association with cell-cell contact areas (Fig. 2B); however, it was recruited to these junctions on expression of human nectin-1 in C10 cells (Fig. 2D) (33). Addition of GFP to the C terminus of nectin-1 should prevent the association with afadin, and, as expected, afadin was not recruited to junctions in CG23 cells (Fig. 2F). Insertion of GFP 11 amino acids prior to the C terminus was predicted to leave the afadin binding site intact (Fig. 1). However, in CXG10 cells, afadin was not detected at cell junctions (Fig. 2H). In contrast, GFP positioned on the N terminus of nectin-1 did not affect its ability to recruit afadin to AJs (Fig. 2J). Thus, for CG23 and CXG10 cells, nectin-1 was located at cell contact areas independently of any interaction with afadin. Therefore, in contrast to observations with transfected L cells (46, 67), afadin did not govern the localization of nectin-1.
Nectin-1-GFP constructs are functional adhesion molecules.
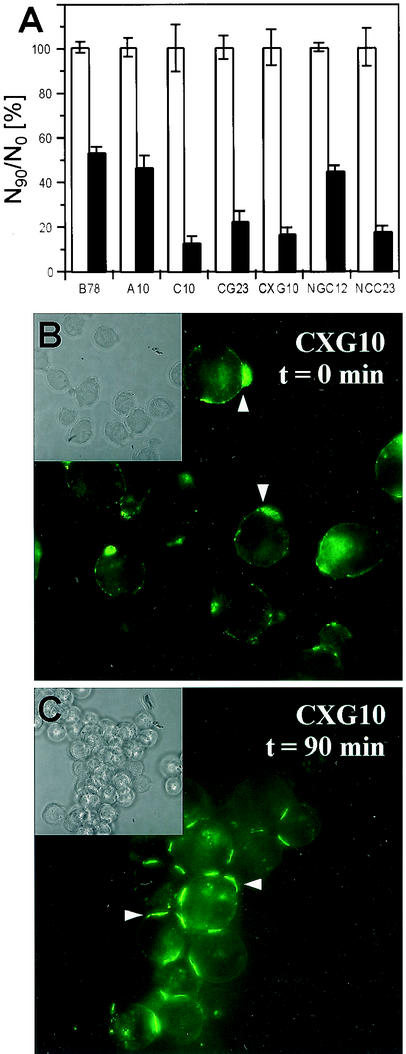
Since nectin-1-GFP was found at cell junctions independently of afadin, we tested whether the various constructs could mediate cell adhesion in a functional aggregation assay by nectin-1-nectin-1 trans-interaction (1, 33, 67). In this assay, cells were trypsinized and resuspended as single cells, which were then allowed to aggregate over time at 37°C in the absence of calcium and magnesium ions (Fig. 3). As shown previously (33), the vector-transfected B78H1-control16 cells (B78) and A10 cells expressing HVEM/HveA showed only a moderate level of aggregation (Fig. 3A). In contrast, expression of nectin-1 induced significant aggregation of C10 cells (Fig. 3A) (33). Addition of GFP within (CXG10) or at the end (CG23) of the cytoplasmic tail of nectin-1 did not affect the ability of nectin-1 to trigger cell aggregation. Addition of CFP at the N terminus did not prevent nectin-1-mediated aggregation of NCC23 cells. However, NGC12 cells that express GFP instead of CFP at the N terminus of nectin-1 did not aggregate significantly above background levels (Fig. 3A). We think this is because NGC12 cells express less nectin-1 than NCC23 cells do (data not shown).
FIG. 3.
Cell aggregation mediated by nectin-1 and nectin-1-GFP. Various cell lines were trypsinized and resuspended in Hanks' balanced salt solution as a single-cell suspension. Trypsin treatment did not affect the detection of the nectin-1 ectodomain by fluorescence-activated cell sorter analysis (data not shown). Cells were then incubated at 37°C for 90 min or fixed immediately with glutaraldehyde (A) or paraformaldehyde (B and C). (A) Quantitative evaluation of nectin-1-mediated cell aggregation. Cells were observed under a microscope and scored as (N90/N0) × 100, where N90 and N0 are the total number of entities (clumps or single cells) observed at t = 90 and 0 min, respectively. Values are the mean and standard deviation of a triplicate representative experiment. A value of 100 indicates no aggregation; the lower the value, the greater the aggregation. White bars represent data at t = 0 min standardized to 100% for each cell line, and black bars represent data after a 90-min incubation. (B and C) CXG10 cells fixed after 0 min (B) or 90 min (C) of incubation at 37°C were observed for nectin-1-GFP localization as detected by green fluorescence emission. Insets show the same field as a phase-contrast image.
Notably, nectin-1-GFP tended to “cap” on freshly trypsinized cells (Fig. 3B). It is not clear if this resulted from the way in which cells gradually detached from each other or was a consequence of the specific proteolytic treatment. In any case, nectin-1 was present and available at the cell surface at time zero. Within the cell aggregates formed during incubation at 37°C, nectin-1-GFP specifically accumulated at contacts between adjacent cells (Fig. 3C). Therefore, GFP present in or at the end of the cytoplasmic tail did not significantly affect nectin-1-mediated cell aggregation. In this assay, cells were isolated in suspension and aggregation was observed soon after the cells came in contact. Since nectin-1 relocated effectively at cell contact areas, we can reasonably assume that these contacts mimic the early stage of formation of AJ similar to AJ seen in cell monolayers. Since nectin-1-GFP in CXG10 and CG23 cells did not associate with afadin in cell monolayers (Fig. 2E to H), this experiment suggests that nectin-1-mediated cell aggregation does not require the interaction of nectin-1 with afadin.
Nectin-1-GFP constructs are functional HSV entry mediators.
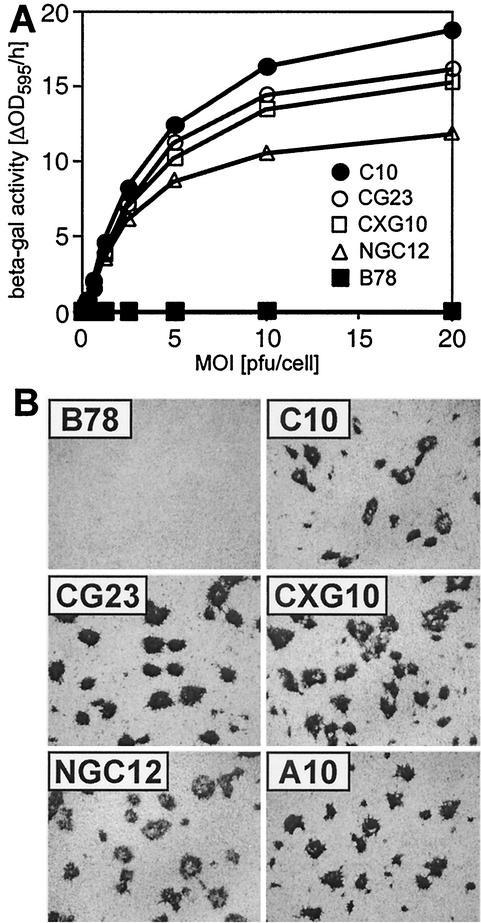
The nectin-1-GFP-bearing cells were tested for their susceptibility to HSV entry and plaque formation. CG23, CXG10, and NGC12 cells were all susceptible to HSV entry compared to the control line, B78 (Fig. 4A). This indicates that all constructs served as receptors for HSV. This assay tests cell lines independently, and the results should not be used to compare the relative efficiency of constructs. Differences in expression levels of nectin-1 on the cell surface probably account for these variations (data not shown).
FIG. 4.
HSV infection mediated by nectin-1-GFP fusion proteins. (A) HSV entry. Cells were infected at various MOIs with HSV-1 KOS tk12 carrying the lacZ reporter gene. After a 6-h infection, the cells were lysed and β-galactosidase activity (ΔOD595/h) was recorded as a measure of virus entry. (B) Plaque formation. Cell monolayers were infected with HSV-1 KOS tk12 for 28h. The plaques were visualized by X-Gal staining.
When any of the nectin-1-GFP constructs (CG23, CXG10, and NGC12 cell lines) were used to determine the HSV-1 KOS tk12 titer by plaque assay, essentially the same titers were obtained. These titers were 2- to 4-fold higher than that obtained for infection of C10 cells and about 25-fold lower than that determined on Vero cells. We conclude that addition of GFP to the N or C terminus of nectin-1 did not affect its ability to function as an efficient HSV receptor.
Lastly, Sakisaka et al. (58) showed a correlation between viral plaque size and the ability of nectin-1 to interact with afadin in transfected L cells. In their experiments, they infected L cells expressing a mutated nectin-1 that was unable to recruit afadin to junctions. There was inefficient spread, and the resulting plaques were reduced in size. We examined plaque size to assess the ability of the nectin-1-GFP constructs to promote virus spread. All cell lines carrying GFP at the N or C terminus or within the cytoplasmic tail of nectin-1 were equally able to support plaque formation without any noticeable change in plaque size from C10 cells (Fig. 4B). Thus, although afadin was not present at junctions in CXG10 and CG23 cells, in contrast to C10 cells (Fig. 2), virus spread appeared to be similar in all three cell lines.
Effects of HSV infection on nectin-1 localization.
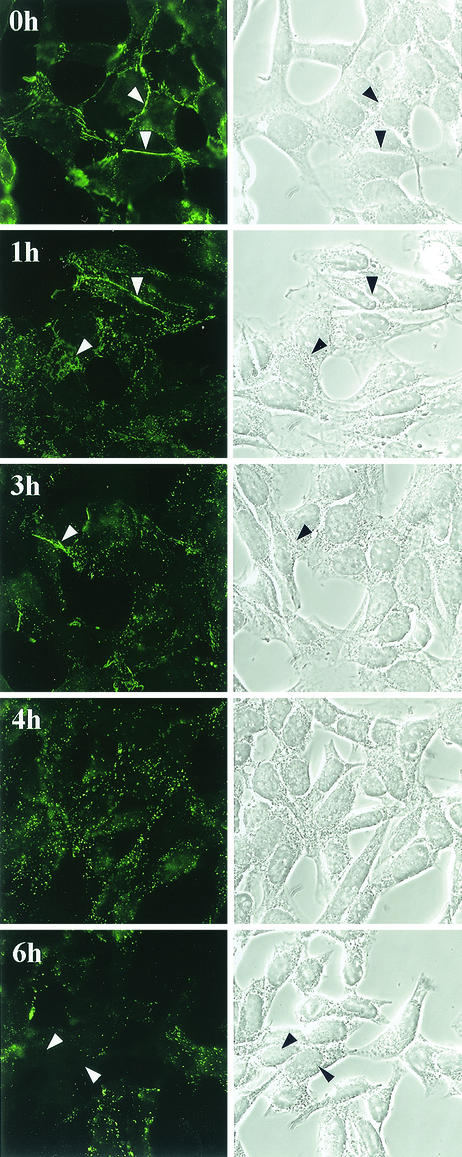
The various nectin-1 cell lines provide good models to study the consequences of HSV infection on nectin-1 distribution. Using C10 cells in suspension, we showed that nectin-1 was not immediately down-regulated from the cell surface during early times of HSV infection but that accessibility to certain epitopes was modified, possibly reflecting a structural change (33). Here we studied the redistribution of nectin-1 over time in infected cell monolayers (Fig. 5). When cells were incubated with virus for 1 h at 4°C (t = 0), nectin-1 was present at cell contact areas and had the same distribution as in mock-infected cells. As early as 1 to 3 h after a temperature shift to 37°C (1 to 3h postinfection [p.i.]), some nectin-1 was still detected at cell contact areas but much of it appeared to be in a distinct dotted pattern. At 4 h p.i. nectin-1 was found mostly in dots and very little was detected at cell contacts. However, the cells still contacted each other, and no obvious cytopathic effect was seen (Fig. 5, right panels). By 6 h p.i., cell morphology was more clearly affected. At this time, many cells appeared negative for nectin-1 staining while some still displayed the dotted pattern. These changes were observed in C10 cells, where nectin-1 was detected by MAb CK41 (Fig. 5). Similarly, nectin-1-GFP relocation was observed on infection of other nectin-1-expressing cells such as CXG10 and NGC12 (see Fig. 7A and 8C). Although we did not time specific events, we noted that in dense monolayers where cell junctions might be stronger, nectin-1 relocation appeared to occur slightly more slowly under our conditions of infection.
FIG. 5.
Detection of nectin-1 in infected cells. Subconfluent C10 cells were infected at high MOI with HSV-1 KOS tk12 as described in Materials and Methods. After a 1-h incubation at 4°C in the presence of virus, the cells were washed and incubated at 37°C for the indicated time before being fixed. Nectin-1 was detected with CK41-Oregon Green antibody (left panels). Phase-contrast images of the same fields are also shown (right panels). Arrowheads indicate the cell junctions where nectin-1 accumulated.
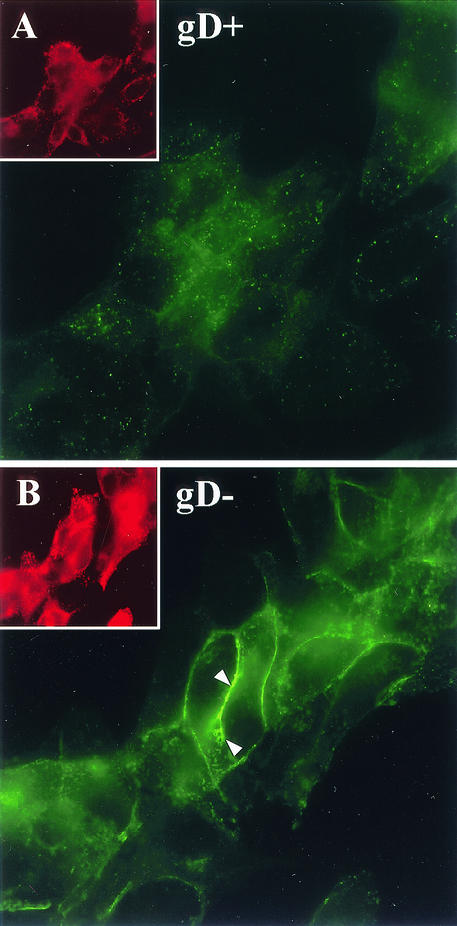
FIG. 7.
Nectin-1 detection after infection with gD-negative HSV-1. NGC12 cells were infected as above with HSV KOS tk12 (wild-type gD positive) (A) or with complemented HSV KOSgDβ (gD negative) (B) for 6 h. As a control for infection, these cells were permeabilized and counterstained with an anti-gB MAb. The main pictures show nectin-1-GFP localization, and the insets show gB expression on the same field. The level of GFP fluorescence of cells infected with KOSgDβ is much higher than that of the corresponding cells infected with KOS tk12. Therefore, image B was exposed for only half of the time used for image A. The insets have the same exposure time. Arrowheads indicate nectin-1 accumulated at junctions between two highly infected cells.
FIG. 8.
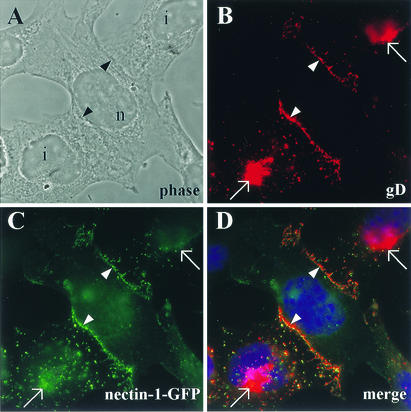
Colocalization of gD and nectin-1 between infected and noninfected cells. (A) Phase-contrast image from the observed field is shown to delineate cell morphology. i, infected cell; n, noninfected cell. (B) Nectin-1-GFP-expressing cells (CXG10) were infected at high MOI with HSV KOS tk12. Cells were fixed at 4 h p.i., permeabilized, and stained for gD expression. (C and D) Expression of nectin-1-GFP (C) and a merge picture where the yellow color indicates colocalization of nectin-1 and gD (D). Nuclei appear blue after DAPI staining (D). Arrows point to gD expressed in the Golgi system, and arrowheads indicate cell junctions.
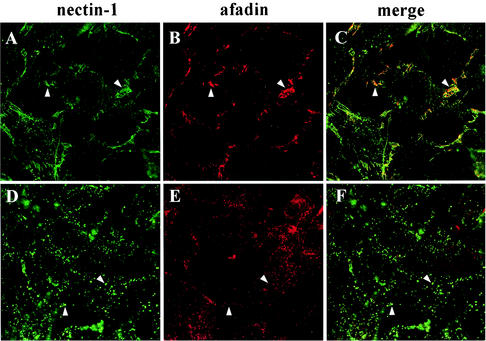
Simultaneously, we looked at the localization of afadin in infected cells. In uninfected C10 cells, nectin-1 and afadin colocalized at cell junctions (Fig. 2C and D and Fig. 6A and B). During infection, the two proteins colocalized as long as nectin-1 was found at AJs (Fig. 6C). Not surprisingly, when nectin-1 ceased to be detected at junctions, afadin no longer accumulated at these areas (Fig. 6E). However, afadin redistribution did not follow the dotted pattern seen for nectin-1 (compare Fig. 6D and E). On the contrary, all afadin staining returned to the cytoplasmic location seen in the parental cell line, B78H1 (Fig. 2B). Subsequently, the level of detection of afadin was not affected by infection whereas detection of nectin-1 appeared to decrease at later time points. This suggests that on HSV infection, afadin and nectin-1 dissociated when nectin-1 left AJs. This conclusion is further supported by the difference between the diffuse, and hence fainter, cytoplasmic stain of afadin compared to the distinctively punctate pattern of nectin-1 (Fig. 6F).
FIG. 6.
Detection of nectin-1 and afadin during HSV infection. C10 cells were infected for 0 h (A to C) or 4 h (D to F) with HSV-1 KOS tk12 at high MOI. The cells were stained with MAb CK41 to detect nectin-1 (A and D) or with MAb anti-AF6 to detect afadin (B and E). Merged images are also shown (C and F). In panels A to C, arrowheads indicate examples of cell junctions where nectin-1 and afadin colocalize. In panels D to F, arrowheads points to dots where nectin-1 was found without colocalization with afadin.
Involvement of newly synthesized gD in nectin-1 relocation.
Since gD is a defined ligand for nectin-1, we investigated its involvement in the repositioning of nectin-1. NGC12 cells were infected in parallel with wild-type HSV-1 KOS tk12 and with the complemented gD-negative virus KOSgDβ (14). The latter virus infects these cells by virtue of envelope gD acquired in complementing cells but is unable to synthesize new gD during infection. After 6 h of infection, cells were stained for gB expression as a marker of infection (Fig. 7). As seen previously for wild-type nectin-1, KOS tk12 also induced the relocation of nectin-1-GFP outside cell contact areas (Fig. 7A). In contrast, when cells were infected with the gD-null KOSgDβ for 6 h, nectin-1 was clearly evident at cell junctions (Fig. 7B). In these cells, no gD was synthesized; however, cytopathic effect and expression of gB were comparable to those in wild-type cells (Fig. 7, insets), indicating that active infection occurred. Similar results were observed when CXG10 (nectin-1-GFP) or C10 (wild-type nectin-1) cells were infected with KOSgDβ (data not shown). These observations suggest that newly synthesized gD plays an active role in altering nectin-1 distribution in the infected cell.
Colocalization of gD with nectin-1 at cell contact areas.
Since gD synthesis appeared to influence nectin-1 localization, it was of interest to investigate if, where, and when gD would interact with nectin-1. Redistribution of nectin-1 in B78H1-derived cell monolayers was observed before 3 h p.i. (Fig. 5). Synthesis of gD was shown to occur as early as 2 h after infection in KB epithelial cells as detected by radiolabeling (11). In this study, newly synthesized gD was not detectable under our immunofluorescence conditions at the early times of nectin-1 relocalization. By 4 h p.i., gD expression was clearly evident, notably by the intense staining at the perinuclear region (Golgi) and of cytoplasmic vesicles (Fig. 8B). Evidence of infection of CXG10 cells was also provided by the punctate pattern of nectin-1-GFP (Fig. 8C). Although some gD-containing vesicles were detected and some colocalization with nectin-1 could be observed, there was no defined overlay since many vesicles contained either gD or GFP only. Therefore, we could not definitively demonstrate colocalization between gD and nectin-1 in vesicles within the infected cell. Similarly, a high level of gD staining in the Golgi, where GFP could also be detected, does not imply specific association. However, gD did accumulate at areas where the infected cell contacted a neighboring noninfected cell (Fig. 8B). Nectin-1 was also very prominent at these locations (Fig. 8C), where it colocalized with gD (Fig. 8D). We did not observe any specific accumulation of gD at junctions between two infected cells (data not shown). Furthermore, gB did not show specific accumulation at junctions in any case (data not shown). Taken together, these data suggest that newly synthesized gD accumulated at cell junctions only when nectin-1 was present on the surface of the opposing cell.
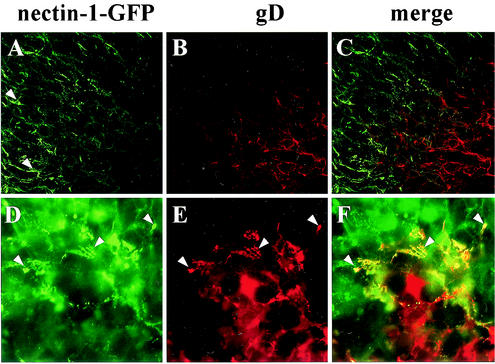
So far we have only investigated cells infected at high MOI. We wanted to confirm these results in situ during an infection progressing in a cell monolayer. By observing HSV-1 plaques formed on CG23 cells expressing nectin-1-GFP, we were able to draw similar conclusions (Fig. 9). First, GFP fluorescence within plaques (counterstained for gD expression [Fig. 9B and C]) appeared less intense than in the surrounding monolayer (Fig. 9A). This correlated with the decreased detection of nectin-1 in infected cells described in Fig. 5. We then magnified the edge of a plaque where infected cells (stained for gD expression [fig. 9E]) contact noninfected CXG10 cells expressing nectin-1-GFP (Fig. 9D). On the periphery of the plaque, gD colocalized with nectin-1, presumably from the noninfected cells, as depicted by the yellow appearance of the typical “leopard skin” pattern of AJs (Fig. 9F).
FIG. 9.
Colocalization of gD and nectin-1 between infected and noninfected cells at the edge of plaques. CG23 cell monolayers were infected with HSV KOS tk12 for 24 h, fixed, and permeabilized. Nectin-1-GFP expression (A and D) and gD staining (B and E) are shown together with merged pictures (C and F). A low magnification of a confocal image of the periphery of a plaque is shown in panels A to C. A higher magnification of a fluorescent microscopy image from the edge of a plaque is shown in panels D to E. Arrowheads in panel A indicate nectin-1-GFP accumulation at cell junctions outside of the viral plaque. Arrowheads in panels D and E point to areas of colocalization of nectin-1 and gD at the periphery of the plaque.
Reciprocal attraction of gD and nectin-1 at cell junctions.
The results obtained for HSV infection suggested that the association or possible targeting of newly synthesized gD to cell junctions might be due to an interaction with nectin-1. We used a transient-transfection assay as a way to dissect the mechanisms involved in this cellular interaction. This approach also allowed us to define whether other viral products were involved in this phenomenon.
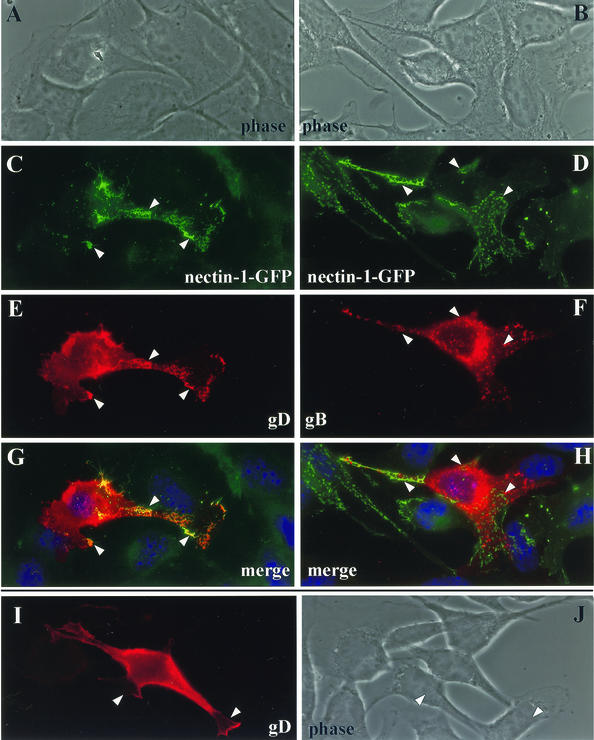
Here we transiently transfected B78H1-CXG10 cells (nectin-1-GFP positive) with an expression plasmid for gD or gB (Fig. 10). Despite overexpression, gD accumulated preferentially at cell junctions, as evidenced by the leopard skin staining pattern of AJ surfaces (Fig. 10E). An identical pattern was visible for nectin-1-GFP (Fig. 10C), and the two overlapped on a merged picture (Fig. 10G). In contrast, when CXG10 cells were transfected with a gB expression plasmid, we observed a different pattern of expression of gB (Fig. 10F) and no colocalization with nectin-1 (Fig. 10H). These results indicate that gD specifically (at least compared to gB) accumulated at cell junctions without the requirement for other viral proteins. When B78H1 cells (nectin-1 negative) were transfected (Fig. 10I), gD was expressed on the cell surface; however, we did not observe specific accumulation of gD at cell contact areas as was seen in transfected CXG10, cells where gD displayed the typical pattern of cell contact surfaces. Therefore, nectin-1 governs the localization of gD, possibly by inducing its accumulation at cell junctions. Since high constitutive expression of gD occurred for a long time in transfected cells, we could not assess the effect of gD expression alone on nectin-1 within the transfected cell itself as we did after infection.
FIG. 10.
Colocalization of gD and nectin-1 in transfected cells expressing nectin-1. Cells expressing nectin-1-GFP (CXG10 cells) were transfected with an expression plasmid for gD (A, C, E, and G) or gB (B, D, F, and H). At 48 h after transfection, gD and gB were detected by immunostaining (E and F, respectively) whereas GFP was directly observed (C and D). Also shown are merged images (G and H). The yellow color indicates colocalization between gD and nectin-1-GFP (G), and nuclei appear blue after DAPI staining (G and H). As controls, nectin-1-negative B78 cells were transfected with the expression plasmid for gD (I and J). B78 cells are nectin-1 and GFP negative; therefore, the green channel and merged pictures have been omitted.
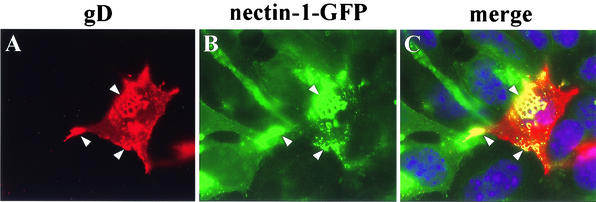
The next experiment was designed to distinguish whether it was nectin-1 within the gD-expressing cell (cis) or nectin-1 on the surface of an adjacent cell (trans) that was responsible for the accumulation of gD at cell junctions. To do this, B78 cells (nectin-1 negative) were transfected with the gD expression plasmid and then mixed with CXG10 cells expressing nectin-1-GFP. After overnight cocultivation, gD on one cell displayed the typical leopard skin appearance when this cell contacted a CXG10 cell (Fig. 11A and B). This experiment showed that gD accumulation at cell contact areas is independent of nectin-1 expression in the same cell. In contrast, it is the presence of nectin-1 on the opposite cell surface (in trans) that is responsible for the attraction and maintenance of gD at the contact areas. Moreover, nectin-1-GFP accumulated at contact areas with cells that express gD but do not express any known cellular ligand for nectin-1. Therefore, we postulate that each partner (nectin-1 and gD) recruits and maintains the other at cell junctions by a molecular trans-interaction between the two.
FIG. 11.
Colocalization of nectin-1 and gD expressed in adjacent cells. Nectin-1-deficient B78 cells were transfected with an expression vector for gD. At 24 h post-transfection, the cells were mixed with nectin-1-GFP-expressing cells (CXG10) and cocultivated for a further 24 h. (A) Fixed and permeabilized cells were stained with anti-gD antibody. (B) Nectin-1-GFP fluorescence was observed directly. The images in panels A and B were merged artificially to show colocalization (yellow) and cell nuclei (blue) (C).
DISCUSSION
Our experiments show that HSV binding and infection affect the natural adhesive function of nectin-1 (33). Numerous types of viruses use cell adhesion molecules as receptors (3, 4, 24, 64, 75), and many modulate the function of these receptors (71, 74). Among them, adenovirus uses its receptor binding fiber protein to enter cells and then newly synthesized fibers disrupt tight junctions by binding to their receptor (CAR), thus allowing new virions to escape (71). To study the effects of gD on nectin-1 during HSV infection, we used a number of cell lines expressing fusion proteins between nectin-1 and GFP in the B78H1 cell background. These cell lines, with their common and different characteristics, allowed us to dissect the features of nectin-1 function and the response to HSV infection and gD expression. We focused on the cellular localization of nectin-1 and newly synthesized gD and found that each molecule can influence the accumulation of the other at cell junctions.
Ability of nectin-1-GFP to function as cell adhesion molecules and HSV receptors.
Nectin-1 is found predominantly at adherens junctions but also participates in tight junctions between epithelial cells (20, 67). At AJs, nectin-1 is part of a complex made of cytoplasmic proteins such as afadin or PAR-3 (20, 67). The nectin-1-afadin complex is connected to the well-characterized cadherin-catenin complex at AJs (66). These junctions are highly organized, and nectins are thought to play a key role in their establishment as well as their maintenance (68).
We found earlier that in B78H1 C10 cells, which express high levels of nectin-1, afadin accumulated at AJs, whereas in parental B78H1 cells, afadin had a cytoplasmic distribution (33). The nectin-1-GFP constructs confirmed that an intact afadin binding site at the C terminus of nectin-1 is required for the recruitment of afadin to AJs. In any case, nectin-1 or nectin-1-GFP constructs were found to accumulate at junctions only when a ligand (nectin-1 or nectin-1-GFP) was available for trans-interaction on the opposite cell. Taken together, our data suggest that nectin-1 is the driving force that recruits afadin to AJs in these cells. This is in partial agreement with published observations showing that nectin-1 or nectin-2 required both the trans-interaction with a ligand and the interaction with afadin in order to be clustered at cell-cell contact sites of L cells (46, 67). The importance of the role of afadin might be cell dependent and might involve other component of AJs. In addition, we found that even when afadin was not recruited to the junctions of CG23 or CXG10 cell monolayers, nectin-1-GFP was still able to promote efficient aggregation of these cells in suspension.
In a similar fashion, the addition of GFP to the PDZ binding domain of JAM-2 did not affect its propensity to accumulate at tight TJs (2). Furthermore, the accumulation of JAM-2 at TJs appears to require homo-trans-interaction of JAM-2 between adjacent cells (2).
The interaction between afadin and nectin-1 in transfected L cells was also reported to influence HSV spread in a plaque formation assay (58). Here we showed that nectin-1 that was fused to GFP at its N terminus or at the C terminus mediated efficient HSV entry. In addition, virus titers obtained on cells expressing the GFP constructs were similar to if not slightly higher than those on cells expressing regular full-length nectin-1 (C10 cells). No difference in plaque size was observed regardless of whether afadin colocalized with nectin-1 at cell junctions. Our results differ from the L-cell situation, where the expression of a nectin-1 mutant unable to interact with afadin resulted in a small-plaque phenotype for HSV infection (58). Again, this might be a cell type-dependent observation. The same site on nectin-1 was recently shown to also interact with PAR3 (69). Therefore, it is possible that other molecules might substitute for afadin function in B78H1-derived cells but not in L cells used by Sakisaka et al. (58). Our data suggest that the role of afadin might not be as essential as previously thought, although we cannot rule out an indirect role for this protein since afadin-deficient cells were not tested. It is possible that other molecules present in large cytoplasmic complexes at AJs, such as PAR3, are involved.
Effects of HSV infection on nectin-1 localization.
Viruses use different strategies to down regulate the surface expression of their receptors (17, 21). We have shown that shortly after infection of cells in suspension, the accessibility of epitopes on nectin-1 was altered (33). These experiments suggested that nectin-1 was not down regulated from the cell surface since at least one epitope was still detected (33). Cell monolayers where junctions are present provide a much better model to observe the effects of infection on nectin-1 distribution. Clearly, the distribution of nectin-1 was drastically affected by HSV infection. In infected cells, nectin-1 no longer accumulated at cell junctions but, rather, was detected in a punctate pattern over the cell. Concomitantly, colocalization with afadin was lost. At later times, nectin-1 disappeared, as detected by MAb CK41 in C10 cells or by GFP fluorescence in cells expressing nectin-1-GFP. This relocation of nectin-1 depended on gD synthesis, since it did not occur after infection with a complemented gD-negative virus.
It is not clear yet by which mechanism gD affects nectin-1, but it is reasonable to postulate a direct interaction. gD might be involved in the active removal of nectin-1 from junctions, leading to the retargeting of nectin-1 to a different compartment. Indeed, since gD accumulated at cell junctions, this might be the result of active displacement of nectin-1. Alternatively, gD might interfere with targeting of nectin-1 to AJs, thus depleting nectin-1 from AJs by regular protein turnover. A high level of gD was detected in the Golgi apparatus during infection, where some level of colocalization with nectin-1 could be found, but a specific interaction was not clearly demonstrated. AJs can be remodeled, and it is not known how stable nectin-1 is once it is involved in cell adhesion; therefore both hypotheses are valid and remain to be tested. Once gD acted on nectin-1, the relocation of the receptor appeared quite specific; however the pathway that is involved needs to be clarified. In preliminary experiments, we were unable to associate the punctate pattern of nectin-1 in infected cells with early endosomes, late endosomes, or lysosomes as detected by the specific markers LAMP-1 and EEA-1 (data not shown). Regardless of which pathway and compartments are involved, it remains clear that HSV infection strongly affects nectin-1 expression via a mechanism involving gD.
Reciprocal influence of gD and nectin-1 on the accumulation of these proteins to cell junctions.
Removal of nectin-1 from AJs appears to be a consequence of gD expression in infected cells. The newly expressed gD accumulated at cell contact areas with adjacent noninfected cells expressing nectin-1. Glycoprotein D targeting to vinculin-containing junction areas (AJs) was observed in HSV-infected Vero cells and was proposed to affect the integrity of these junctions (50). This early observation can now be explained by the presence of nectin-1 on Vero cells (32, 45). The accumulation of gD at cell contact areas driven by nectin-1 expressed in trans may result from an interaction between gD and nectin-1 which remains to be demonstrated directly. It suggests that each partner reciprocally recruits and maintains the other at cell contact areas. Indeed, nectin-1 does not accumulate at AJs unless it is involved in trans-interactions with a ligand (normally nectin-1 or nectin-3) (59). Here we showed that gD could fulfill the role of a ligand to maintain nectin-1 in a junction. We also hypothesized that the type of binding between nectin-1 and another nectin in trans is similar to the trans interaction with gD. It is known that gD binding interferes with nectin-1 homotypic trans interaction (33, 58); therefore, it is not unreasonable to think that gD binding mimics the binding of a natural ligand.
Biological significance of gD-nectin-1 interactions during HSV infection.
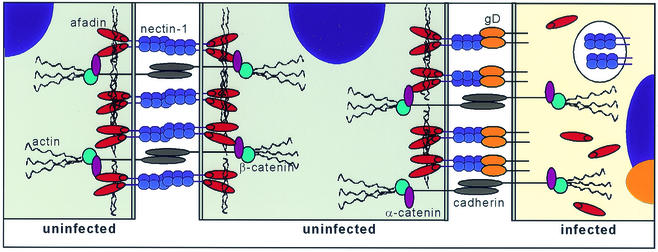
The above observations lead us to propose a model consistent with our knowledge of gD-nectin-1 interactions during infection (Fig. 12). This simplified representation shows how the expression of gD in infected cells might lead to an alteration of nectin-1 cellular localization and to its replacement at junctions by gD. In turn, gD is probably acting as a ligand in trans for nectin-1 on the surface of a neighboring noninfected cell, although direct binding remains to be demonstrated under these circumstances. As a result, nectin-1 in the noninfected cell is maintained at junctions with the infected cells despite the down regulation of the cellular ligand.
FIG. 12.
Schematic representation of components of AJ, between two normal cells (left) and hypothetical contacts between a regular cell and an infected cell (right).
From the point of view of the virus, it might be important to retain a receptor on the next target cell in a situation favorable for virus to spread. Thus, this hypothesis explains the relatively early expression of gD as compared to other HSV envelope glycoproteins (11). We also postulate that gD mimics natural nectin-1 activity as a ligand in trans interactions. Consequently, gD binding might also prevent a change in signaling in the target cell that could trigger some adverse cellular response. Signals transduced by nectins are mostly unknown, although it is clear that the cytoplasmic tail of nectin-1 can interact directly or indirectly with a variety of cytoplasmic proteins involved in signaling or cytoskeleton binding (such as afadin, PAR3, and α-catenin) (52, 66, 67, 69). The consequences of gD binding to nectin-1 in the context of virus spread as well as during HSV entry remain to be addressed from the signaling point of view.
The accumulation of gD at cell contact areas at late times of infection might also play a more direct role in virus spread. It is possible that it might act as a tag to target outgoing capsids to regions on a nearby target cell. In this case, it might be a redundant mechanism since there is strong evidence that the gE-gI complex is important for sorting nascent virions to the junctions of epithelial cells (31; for a review, see reference 30). The gE-gI complex first accumulates at the trans-Golgi network, where nucleocapsids are enveloped, and then it is involved in sorting virus to the lateral cell surface of polarized cells. The trans-Golgi network accumulation of gE-gI requires an interaction between the cytoplasmic domain of gE and the cellular sorting machinery. At later times during infection, gE-gI is relocated to cell junctions and is postulated to drive the nascent virion AJ. Although accumulation of gE-gI at cell contact areas is mediated by the extracellular domain of gE, this region is not sufficient to promote cell-to-cell spread of HSV (76). A ligand for the gE-gI complex at AJ was postulated; however, it has not yet been identified (12, 15).
gD is required for HSV to spread from cell to cell (9, 27, 29, 35, 49), either because it acts as proposed above or, more basically, because its role as receptor binding protein is the same as during the entry of free particles (9). In PRV, gD is dispensable for virus spread (51, 54) even though PRV uses nectin-1 as a receptor for entry (23). However, PRV gD interacts with nectin-1 in a way similar but not identical to that used by HSV gD (13, 22, 45). This suggests that HSV gD might have two separate functions, but at present there are no gD mutants that are impaired in spread but not entry. This is reminiscent of the adenovirus fiber protein which binds directly to its receptor (CAR) during entry as part of virions and also during the virus release phase as a nonstructural protein (71).
In summary, the direct observations of nectin-1, afadin, and HSV glycoproteins, principally gD, during the course of infection suggest that reciprocal influences of gD and nectin-1 occur during different phases of infection. At early times p.i., nectin-1 is relocated from AJs within the infected cell and possibly targeted to degradation in a process that involves newly synthesized gD. At the periphery of the infection, specific accumulation of gD at cell junctions is driven by the presence of nectin-1 in the adjacent cell. This same naive cell is a putative target for infection, and it is our hypothesis that its nectin-1 is retained in junctions with the infected cell via an interaction with gD, thereby facilitating spread of the virus. This hypothesis on viral spread needs to be challenged and confirmed.
Acknowledgments
We thank Pat Spear for the KOStk12 and KOSgDβ viruses, the pBG38 plasmid, and the C10 and A10 cells. We thank David Johnson for the VD60 cells and Peter Pertel for plasmids pPEP98 and pPEP99. We thank Bruce Shenker and Kelly Jordan-Sciutto for their assistance with the confocal microscope. We are grateful to Richard Milne for plasmid pRM467 and for critical reading of the manuscript and to all the members of the Cohen and Eisenberg laboratory for helpful discussions.
This investigation was supported by Public Health Service grants NS-30606 and NS-36731 from the National Institute of Neurological Disorders and Stroke (to R.J.E. and G.H.C.) and AI-18289 from the National Institute of Allergy and Infectious Diseases (to R.J.E. and G.H.C.).
REFERENCES
- 1.Aoki, J., S. Koike, H. Asou, I. Ise, H. Suwa, T. Tanaka, M. Miyasaka, and A. Nomoto. 1997. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp. Cell Res. 235:374-384. [DOI] [PubMed] [Google Scholar]
- 2.Aurrand-Lions, M., L. Duncan, C. Ballestrem, and B. A. Imhof. 2001. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276:2733-2741. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 6.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi, F., M. Lopez, P. Dubreuil, G. Campadelli Fiume, and L. Menotti. 2001. Chimeric nectin1-poliovirus receptor molecules identify a nectin1 region functional in herpes simplex virus entry. J. Virol. 75:7987-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi, F., M. Lopez, L. Menotti, M. Aoubala, P. Dubreuil, and G. Campadelli-Fiume. 1998. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc. Natl. Acad. Sci. USA 95:15700-15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi, F., L. Menotti, P. Dubreuil, M. Lopez, and G. Campadelli-Fiume. 2000. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB). J. Virol. 74:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attribute of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, G. H., D. Long, and R. J. Eisenberg. 1980. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J. Virol. 36:429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, W. J., and D. C. Johnson. 2003. Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread. J. Virol. 77:2686-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly, S. A., J. C. Whitbeck, A. H. Rux, C. Krummenacher, S. van Drunen Littel-van den Hurk, G. H. Cohen, and R. J. Eisenberg. 2001. Glycoprotein D homologues in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC (nectin-1) with different affinities. Virology 280:7-18. [DOI] [PubMed] [Google Scholar]
- 14.Dean, H. J., S. S. Terhune, M. Shieh, N. Susmarski, and P. G. Spear. 1994. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology 199:67-80. [DOI] [PubMed] [Google Scholar]
- 15.Dingwell, K. S., and D. C. Johnson. 1998. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberle, F., P. Dubreuil, M. G. Mattei, E. Devilard, and M. Lopez. 1995. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159:267-272. [DOI] [PubMed] [Google Scholar]
- 17.Erlenhoefer, C., W. J. Wurzer, S. Loffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabre, S., N. Reymond, F. Cocchi, L. Menotti, P. Dubreuil, G. Campadelli-Fiume, and M. Lopez. 2002. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin 3 and nectin 4 bind to the predicted C-C′-C"-D beta-strands of the nectin1 V domain. J. Biol. Chem. 277:27006-27013. [DOI] [PubMed] [Google Scholar]
- 19.Fukuhara, A., K. Irie, H. Nakanishi, K. Takekuni, T. Kawakatsu, W. Ikeda, A. Yamada, T. Katata, T. Honda, T. Sato, K. Shimizu, H. Ozaki, H. Horiuchi, T. Kita, and Y. Takai. 2002. Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene 21:7642-7655. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara, A., K. Irie, A. Yamada, T. Katata, T. Honda, K. Shimizu, H. Nakanishi, and Y. Takai. 2002. Role of nectin in organization of tight junctions in epithelial cells. Genes Cells 7:1059-1072. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 22.Geraghty, R. J., A. Fridberg, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 2001. Use of chimeric nectin-1(HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry. Virology 285:366-375. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty, R. J., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 24.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClellend. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 25.Haarr, L., D. Shukla, E. Rodahl, M. C. Dal Canto, and P. G. Spear. 2001. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology 287:301-309. [DOI] [PubMed] [Google Scholar]
- 26.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 70:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, D. C., R. L. Burke, and T. Gregory. 1990. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J. Virol. 64:2569-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on the herpesvirus entry mediator C by using anti-receptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krummenacher, C., I. Baribaud, J. F. Sanzo, G. H. Cohen, and R. J. Eisenberg. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J. Virol. 76:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krummenacher, C., A. H. Rux, J. C. Whitbeck, M. Ponce de Leon, H. Lou, I. Baribaud, W. Hou, C. Zou, R. J. Geraghty, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1999. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity while the third domain is involved in oligomerization of HveC. J. Virol. 73:8127-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez, M., F. Cocchi, L. Menotti, E. Avitabile, P. Dubreuil, and G. Campadelli-Fiume. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 74:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez, M., F. Eberlé, M.-G. Mattei, J. Gabert, F. Birg, F. Bardin, C. Maroc, and P. Dubreuil. 1995. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155:261-265. [DOI] [PubMed] [Google Scholar]
- 38.Mandai, K., H. Nakanishi, A. Satoh, H. Obaishi, M. Wada, H. Nishioka, M. Itoh, A. Mizoguchi, T. Aoki, T. Fujimoto, Y. Matsuda, S. Tsukita, and Y. Takai. 1997. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez, W. M., and P. G. Spear. 2002. Amino acid substitutions in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus types 1 and 2 but not for pseudorabies virus or bovine herpesvirus 1. J. Virol. 76:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mata, M., M. Zhang, X. Hu, and D. J. Fink. 2001. HveC (nectin-1) is expressed at high levels in sensory neurons, but not in motor neurons, of the rat peripheral nervous system. J. Neurovirol. 7:476-480. [DOI] [PubMed] [Google Scholar]
- 41.Mauri, D. N., R. Ebner, K. D. Kochel, R. I. Montgomery, T. C. Cheung, G.-L. Yu, M. Murphy, R. J. Eisenberg, G. H. Cohen, P. G. Spear, and C. F. Ware. 1998. LIGHT, a new member of the TNF superfamily, and lymphotoxin (LT) α are ligands for herpesvirus entry mediator (HVEM). Immunity 8:21-30. [DOI] [PubMed] [Google Scholar]
- 42.Menotti, L., R. Casadio, C. Bertucci, M. Lopez, and G. Campadelli-Fiume. 2002. Substitution in the murine nectin1 receptor of a single conserved amino acid at a position distal from the herpes simplex virus gD binding site confers high-affinity binding to gD. J. Virol. 76:5463-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menotti, L., M. Lopez, E. Avitabile, A. Stefan, F. Cocchi, J. Adelaide, E. Lecocq, P. Dubreuil, and G. Campadelli-Fiume. 2000. The murine homolog of human nectin 1 delta serves as a species non-specific mediator for entry of human and animal alphaherpesviruses in a pathway independent of detectable binding to gD. Proc. Natl. Acad. Sci. USA 97:4867-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 45.Milne, R. S. B., S. A. Connolly, C. Krummenacher, R. J. Eisenberg, and G. H. Cohen. 2001. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315-328. [DOI] [PubMed] [Google Scholar]
- 46.Miyahara, M., H. Nakanishi, K. Takahashi, K. Satoh-Horikawa, K. Tachibana, and Y. Takai. 2000. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J. Biol. Chem. 275:613-618. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 48.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 49.Nicola, A. V., S. H. Willis, N. N. Naidoo, R. J. Eisenberg, and G. H. Cohen. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J. Virol. 70:3815-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norrild, B., I. Virtanen, V. P. Lehto, and B. Pedersen. 1983. Accumulation of herpes simplex virus type 1 glycoprotein D in adhesion areas of infected cells. J. Gen. Virol. 64:2499-2503. [DOI] [PubMed] [Google Scholar]
- 51.Peeters, B., J. Pol, A. Gielkins, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng, Y. F., K. Mandai, H. Nakanishi, W. Ikeda, M. Asada, Y. Momose, S. Shibamoto, K. Yanagihara, H. Shiozaki, M. Monden, M. Takeichi, and Y. Takai. 2002. Restoration of E-cadherin-based cell-cell adhesion by overexpression of nectin in HSC-39 cells, a human signet ring cell gastric cancer cell line. Oncogene 21:4108-4119. [DOI] [PubMed] [Google Scholar]
- 53.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 54.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reymond, N., J. Borg, E. Lecocq, J. Adelaide, G. Campadelli-Fiume, P. Dubreuil, and M. Lopez. 2000. Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene 255:347-355. [DOI] [PubMed] [Google Scholar]
- 56.Reymond, N., S. Fabre, E. Lecocq, J. Adelaide, P. Dubreuil, and M. Lopez. 2001. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276:43205-43215. [DOI] [PubMed] [Google Scholar]
- 57.Richart, S. M., S. A. Simpson, C. Krummenacher, J. C. Whitbeck, L. I. Pizer, G. H. Cohen, R. J. Eisenberg, and C. L. Wilcox. 2003. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J. Virol. 77:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y. F. Peng, K. Yamanishi, and Y. Takai. 2001. Requirement of interaction of nectin-1α/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J. Virol. 75:4734-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh-Horikawa, K., H. Nakanishi, K. Takahashi, M. Miyahara, M. Nishimura, K. Tachibana, A. Mizoguchi, and Y. Takai. 2000. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 275:10291-10299. [DOI] [PubMed] [Google Scholar]
- 60.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 61.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 63.Srinivas, R. V., N. Balachandran, F. V. Alonso-Caplen, and R. W. Compans. 1986. Expression of herpes simplex virus glycoproteins in polarized epithelial cells. J. Virol. 58:689-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stauton, D. E., V. J. Merluzzi, R. Rothlein, R. Barton, S. D. Marlin, and T. A. Springer. 1989. A cell adhesion molecule, ICAM-1, is the major receptor for rhinoviruses. Cell 56:849-853. [DOI] [PubMed] [Google Scholar]
- 65.Struyf, F., W. M. Martinez, and P. G. Spear. 2002. Mutations in the N-terminal domains of nectin-1 and nectin-2 reveal differences in requirements for entry of various alphaherpesviruses and for nectin-nectin interactions. J. Virol. 76:12940-12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tachibana, K., H. Nakanishi, K. Mandai, K. Ozaki, W. Ikeda, Y. Yamamoto, A. Nagafuchi, S. Tsukita, and Y. Takai. 2000. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takai, Y., and H. Nakanishi. 2003. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116:17-27. [DOI] [PubMed] [Google Scholar]
- 69.Takekuni, K., W. Ikeda, T. Fujito, K. Morimoto, M. Takeuchi, M. Monden, and Y. Takai. 2003. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J. Biol. Chem. 278:5497-5500. [DOI] [PubMed] [Google Scholar]
- 70.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789-799. [DOI] [PubMed] [Google Scholar]
- 72.Warner, M. S., W. Martinez, R. J. Geraghty, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by herpes simplex virus type 2, mutants of herpes simplex virus type 1 and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 73.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the TNFR superfamily and a mediator of HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whiteman, S. C., A. Bianco, R. A. Knight, and M. A. Spiteri. 2003. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J. Biol. Chem. 278:11954-11961. [DOI] [PubMed] [Google Scholar]
- 75.Williams, R. K., G. S. Jiang, and K. V. Holmes. 1991. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc. Natl. Acad. Sci. USA 88:5533-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wisner, T., C. Brunetti, K. Dingwell, and D. C. Johnson. 2000. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 74:2278-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon, M., and P. G. Spear. 2002. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J. Virol. 76:7203-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]