Abstract
Vesicular stomatitis virus (VSV) is a negative-stranded RNA virus normally sensitive to the antiviral actions of alpha/beta interferon (IFN-α/β). Recently, we reported that VSV replicates to high levels in many transformed cells due, in part, to susceptible cells harboring defects in the IFN system. These observations were exploited to demonstrate that VSV can be used as a viral oncolytic agent to eradicate malignant cells in vivo while leaving normal tissue relatively unaffected. To attempt to improve the specificity and efficacy of this system as a potential tool in gene therapy and against malignant disease, we have genetically engineered VSV that expresses the murine IFN-β gene. The resultant virus (VSV-IFNβ) was successfully propagated in cells not receptive to murine IFN-α/β and expressed high levels of functional heterologous IFN-β. In normal murine embryonic fibroblasts (MEFs), the growth of VSV-IFNβ was greatly reduced and diminished cytopathic effect was observed due to the production of recombinant IFN-β, which by functioning in a manner involving autocrine and paracrine mechanisms induced an antiviral effect, preventing virus growth. However, VSV-IFNβ grew to high levels and induced the rapid apoptosis of transformed cells due to defective IFN pathways being prevalent and thus unable to initiate proficient IFN-mediated host defense. Importantly, VSV expressing the human IFN-β gene (VSV-hIFNβ) behaved comparably and, while nonlytic to normal human cells, readily killed their malignant counterparts. Similar to our in vitro observations, following intravenous and intranasal inoculation in mice, recombinant VSV (rVSV)-IFNβ was also significantly attenuated compared to wild-type VSV or rVSV expressing green fluorescent protein. However, VSV-IFNβ retained propitious oncolytic activity against metastatic lung disease in immunocompetent animals and was able to generate robust antitumor T-cell responses. Our data indicate that rVSV designed to exploit defects in mechanisms of host defense can provide the basis for new generations of effective, specific, and safer viral vectors for the treatment of malignant and other disease.
A number of replicating DNA and RNA viruses, including adenovirus, reovirus, and herpes simplex virus, have demonstrated significant oncolytic activity and as such are being evaluated as agents for the treatment of cancer (3, 9, 12, 42). Although the mechanisms by which oncolytic viruses exert their antitumor efficacy are largely unclarified, it is thought that these viruses exploit defective antiviral host defense mechanisms, potentially widespread in transformed cells. One such virus demonstrating enhanced replication and cytolytic ability in transformed cells is vesicular stomatitis virus (VSV), a member of the genus Vesiculovirus of the family Rhabdoviridae. VSV is an enveloped virus with a single negative strand of genomic RNA comprising 11 kb. Five proteins are encoded by the template, namely, a nucleocapsid protein, a phosphoprotein, a matrix protein, a glycoprotein, and an RNA-dependent RNA polymerase, all of which are synthesized from the genomic template as monocistronic, capped, polyadenylated mRNAs (50). VSV is known to infect selected insects and mammals and can cause nonfatal disease in swine and cattle, although infection in humans is rare, with such infections typically being asymptomatic. Effective immune defense to VSV predominantly involves the antiviral activity of endogenously produced interferon (IFN) and subsequently the generation of neutralizing antibody to the glycoprotein (28, 34, 45, 48). Indeed, the importance of the IFN in host defense against VSV is underscored by demonstrating that mice harboring a defective IFN system are exquisitely sensitive to normally innocuous exposure to VSV (15, 31, 32).
The IFNs were discovered in the late 1950s as antiviral cytokines that were induced and secreted from cells exposed to virus infection (23). It is now known that the IFNs are comprised of two groups referred to as type I (α/β) and type II (γ). IFN-α/β genes include several α genes and a single β gene, are clustered onto the short arm of human chromosome 9, and are expressed from most cell types (39, 41). In contrast, IFN-γ is encoded by a single gene on chromosome 12 and is mainly secreted by Th-1 lymphocytes and natural killer (NK) cells (33, 41). Aside from virus, the expression of the IFN-α/βs can be induced by a number of stimuli, including double-stranded RNA and lipopolysaccharides. It is thought that RNA species arising from invading viruses trigger signaling cascades, involving the NF-κB and IFN regulatory factor 3 (IRF-3) pathways, that lead to the transcriptional activation of IFN-β, which is then secreted (20, 39, 41). IFN-α/βs bind to species-specific cell surface receptors and trigger the activation of the Janus protein kinases (specifically JAK-1 and TYK2) and the signal transducer and activator of transcription (STAT1 and STAT2) pathway (13, 20, 22). In conjunction with p48 (ISGF3/IRF-9), phosphorylated STAT1 and STAT2 heterodimers bind to cognate DNA recognition motifs referred to as IFN-stimulated response elements, which are located in the promoter regions of numerous genes. This event invokes transcriptional activation of target genes such as the double-stranded RNA-dependent protein kinase PKR, the death ligand TRAIL, 2′-5′ oligoadenylate/RNAse L proteins, heat shock proteins, major histocompatibility class antigens, PML, STAT1, and IRF-7, among many others (7, 14, 41). In addition to being able to potently prevent virus replication through intracellular mechanisms, the IFNs are known as important modulators of the immune system and are able to activate NK and T cells and facilitate the maturation of professional antigen-presenting cells such as dendritic cells (DC) (8, 24, 35). IFNs are also capable of inhibiting cell growth and differentiation and have been used in the treatment of a number of types of cancer, including hematological malignancies and certain solid tumors such as melanoma, renal carcinoma, and Kaposi's sarcoma (17). IFN is also used worldwide for the treatment of chronic infection with hepatitis B and C viruses and for the treatment of gammaherpesvirus-associated lymphoma, although the mechanisms of these actions remain unclarified (11, 21, 46).
Our data and those of others have indicated that VSV exhibits increased growth kinetics in transformed cells and to a lesser extent in normal cells unless the IFN pathway or PKR is defective (5, 6, 43, 53). Subsequent analysis has indicated that the antiviral action of IFN is possibly flawed in many transformed cells, which presumably permits VSV to replicate rapidly to high levels, eventually inducing cytolysis with the characteristic hallmarks of apoptosis (4, 5). The genetic malleability of VSV and lack of genetic transforming, integrating, or reassortment properties further afford the opportunity to improve the oncolytic therapeutic potential of VSV. Accordingly, Fernandez et al. have demonstrated in tumor therapy studies that recombinant VSV (rVSV) expressing the herpesvirus thymidine kinase (TK) or cytokine interleukin 4 (IL-4) genes exhibits greater oncolytic activity, including activity against metastatic disease, than wild-type VSV (16). To further potentially improve specificity and safety in the use of VSV in gene therapy, we have generated rVSV that expresses the IFN-β gene. We hypothesized that VSV-IFNβ would be less cytolytic against normal cells and tissue and therefore significantly attenuated in vivo, since these viruses would impede their own replication by activating the IFN antiviral pathway. However, since the IFN pathway is defective in transformed cells, VSV-IFNβ should retain oncolytic activity by replicating efficiently. In addition, VSV-synthesized IFN could plausibly activate the IFN pathway in surrounding normal cells (enforcing protection against superfluous VSV), may exert antiproliferative effects against the tumor itself, and, finally, may enhance the antitumor immune response by stimulating NK and cytotoxic T cells as well as DC activity. Here, we report that VSV-IFNβ indeed retains specific oncolytic activity and is significantly attenuated in vivo.
MATERIALS AND METHODS
Cells.
BHK-21 cells and primary and transformed murine embryonic fibroblasts (MEFs) derived from BALB/c or C57BL/6 mice were generated as described previously (29) and maintained in Dulbecco's modified essential medium supplemented with 10% fetal bovine serum (HyClone Laboratories Inc., Logan, Utah), 100 U of penicillin G/ml, 100 U of streptomycin/ml, and 0.25 μg of amphotericin B. STAT1-lacking (STAT1−/−) cells were a gift from J. Durbin (15). B16(F10) melanoma cells and Renca renal carcinoma and DA-3 cells derived from D1-DMBA3 breast tumor were maintained in the same medium with 1.5 g of sodium bicarbonate per liter and OPI medium supplement (Sigma, St. Louis, Mo.), respectively (40). TS/A mammary adenocarcinoma cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (36). Human tumor cell lines, HeLa cells, MCF-7 mammary adenocarcinoma cells, PC-3 prostatic adenocarcinoma cells, and K562 myelogenous leukemia cells were obtained from the American Type Culture Collection (Manassas, Va.) and were maintained according to the manufacturer's specifications. Human normal cells, human telomerase reverse transcriptase (hTERT)-expressing foreskin fibroblast cells (Clontech, Palo Alto, Calif.), human microvascular endothelial cells (HMVECs), and human mammary epithelial cells (HMECs) (Clonetics Corp., San Diego, Calif.) were grown according to the manufacturers' specifications.
Construction of recombinant virus.
Using oligonucleotides 5′-TCCATCCTCGAGCACTATGAACAACAGGTGGATC CTC-3′ (sense) and 5′-AGGTCTGCTAGCCTAGTTTTGGAAGTTTCTGGT-3′ (antisense), mouse IFN-β cDNA was amplified by PCR from plasmid pMK-Mβ, a generous gift from Yoichiro Iwakura, Institute of Medical Science, University of Tokyo. Using oligonucleotides 5′-ACAGGTCTCGAGCAACATGACCAACAAGTGTCTCCTC-3′ (sense) and 5′-GCTTGCGCTAGCTCAGTTTCGGAGGTAACCTGT-3′ (antisense), human IFN-β (hIFNβ) cDNA was amplified by PCR from plasmid pORF-hIFNβ (InvivoGen, San Diego, Calif.).
The amplified fragment was then inserted into the XhoI-NheI site of pVSV-XN2 (27). The procedure for recovering rVSV was similar to that described previously (27, 52). The seed virus was propagated in BHK-21 cells and stored at −80°C until use. VSV-green fluorescent protein (GFP) and rVSV were prepared as previously described (16).
Virus growth in vitro.
The growth of the recombinant viruses in BHK-21 cells were examined as previously described (16). Cells were seeded in 6-well culture plates at 106 cells per well and infected with each virus at a multiplicity of infection (MOI) of 10 PFU per cell. The culture supernatants were harvested at the indicated times and subjected to titer determination with a standard plaque assay using BHK-21 cells.
For the in vitro cell-killing assay, murine and human cells were seeded in 12- or 24-well culture plates at approximately 2 × 105 cells per well, infected with viruses at the indicated MOI for 24 or 48 h, and then trypsinized and counted by trypan blue exclusion analysis.
Immunoblotting for IFN-β production.
Expression of IFN-β on VSV-IFNβ-infected or VSV-hIFNβ-infected cells was examined by immunoblotting. BHK-21 cells (106) were seeded in a 35-mm-diameter culture dish and infected with viruses at an MOI of 5 or 10 PFU per cell for 24 h. A total of 25 μl of the supernatant was then separated by sodium dodecyl sulfate-13.5% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Polyclonal rabbit antibody against mouse IFN-β (Research Diagnostics Inc., Flanders, N.J.) or goat antibody against hIFNβ (Sigma) was used as the primary antibody. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G or anti-goat immunoglobulin G was used as a secondary antibody and detected by enhanced chemiluminescence (Pierce, Rockford, Ill.). Purified recombinant mouse IFN-β (United States Biological, Swampscott, Mass.) or hIFNβ (Sigma) was used as a positive control.
Biological activity assay for IFN-β.
BHK-21 cells were seeded in a 35-mm-diameter culture dish at 106 cells and infected with viruses at an MOI of 10 PFU per cell for 24 h. The supernatants were harvested and treated at 56°C for 30 min to inactivate the viral infectivity. C57BL/6 MEFs or B16(F10) cells were seeded in a 24-well culture plate at 5 × 104 to 1 × 105 cells per well and incubated with the supernatant diluted by 103 to 108 for 24 h or with 13 to 103 U of mouse IFN-α/β (Sigma)/ml for 24 h. Cells were then infected with VSV-GFP at an MOI of 0.1 or 0.01 for 24 h, and cytopathic effect (CPE) was assessed by microscopy. Activity was calculated in units and calibrated against the standard.
Tumor studies.
Female BALB/c mice (6 to 8 weeks old) were obtained from Jackson Laboratory and maintained under specific pathogen-free conditions. Mice were injected intravenously (i.v.) with 5 × 104 TS/A cells and then infected i.v. with 5 × 107 PFU of rVSV 2 days later. The survival of mice was monitored daily after virus infection. For intratumoral studies, mice were injected subcutaneously (s.c.) with 7 × 105 Renca cells. After tumors had reached palpable size (approximately 0.5 cm2), they were injected with 1.5 × 108 PFU of rVSV. Inoculations were repeated twice more at 2-day intervals. The tumor sizes were measured at 2-day intervals, and the volume was calculated according to the formula 0.5 × length × width2. Statistical significance of intergroup differences was evaluated using Student's t test.
ELISPOT assay.
To detect and enumerate IFN-γ-secreting cells, enzyme-linked immunospot (ELISPOT) assays were done as previously described (1). Briefly, 96-well nitrocellulose plates were coated with anti-IFN-γ monoclonal antibody (MAb) (Pharmingen, San Diego, Calif.) (5 μg/ml) overnight at room temperature, followed by blocking of the wells for 2 h with medium containing 5% fetal bovine serum. Spleen cells from heat-inactivated VSV (HI-VSV)-, VSV-GFP-, and VSV-IFNβ-treated mice were obtained at 10 days postinfection and layered over a Histopaque gradient. The mononuclear cells were harvested and incubated overnight with Renca tumor cells (previously treated with 25 μg of mitomycin C/ml for 2 h at 37°C) at a 20:1 ratio at 37°C. After washing, biotinylated anti-mouse IFN-γ monoclonal antibody (0.5 μg/ml) was added for 90 min at room temperature followed by the addition of streptavidin-alkaline phosphatase and 5-bromo-4-chloro-3-indolylphosphate. The plates were washed and analyzed the next day with a dissecting microscope (×25 magnification).
RESULTS
Generation of VSV expressing IFN-β.
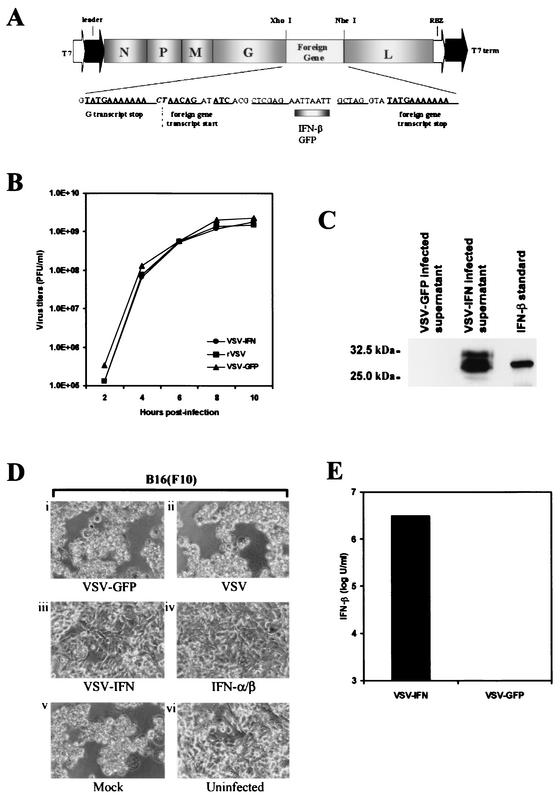
Fernandez et al. have previously demonstrated that the herpes simplex virus-TK or murine IL-4 gene inserted into the VSV genome as an additional transcription unit between the viral glycoprotein and polymerase genes is expressed at high levels and does not affect the growth of the resultant recombinant viruses (16). To further potentially increase the specificity, safety, and efficacy of the use of rVSV in gene therapy regimens targeting malignant disease, we inserted the murine IFN-β gene into the same position of the full-length viral antigenomic cDNA, pVSV-XN2, by using unique restriction enzyme sites as described previously (27) (Fig. 1A). BHK-21 cells were chosen to retrieve rVSV (VSV-IFNβ), since they are of hamster origin and are not receptive to binding murine IFN-β. Thus, any IFN-β generated by VSV-IFNβ would not be expected to inhibit virus replication and subsequent progeny amplification. Following transfection of BHK-21 cells, rVSV-IFNβ was recovered and exhibited plaque sizes similar to those of rVSV, which carries and expresses the GFP gene (VSV-GFP) (data not shown). In BHK-21 cells, the kinetics of VSV-IFNβ growth further indicated the absence of significant growth abnormalities compared to those of rVSV and VSV-GFP, and high virus titers of approximately 109 PFU per ml were obtained from 106 cells at between 8 and 10 h postinoculation (p.i.) (Fig. 1B).
FIG. 1.
(A) Construction of VSV-IFNβ cDNA. Mouse IFN-β cDNA was inserted between the glycoprotein (G) and polymerase protein (L) genes of pVSV-XN2, a full-length cDNA clone of the Indiana serotype, as described in Materials and Methods. N, nucleocapsid protein gene; P, phosphoprotein gene; M, matrix protein gene. (B) One-step growth curves of recombinant viruses. BHK-21 cells were infected at an MOI of 10 PFU per cell with VSV-IFNβ, VSV-GFP, or rVSV not containing a foreign gene. The supernatants of infected cells were harvested at the indicated times, and viral titers were determined by a standard plaque assay. (C) Expression of IFN-β in VSV-IFNβ-infected cells. BHK-21 cells were infected with VSV-IFNβ or VSV-GFP at an MOI of 10 PFU per cell for 24 h. Supernatants (25 μl from 1 ml of 106 infected cells) were analyzed for IFN-β expression by immunoblotting using an anti-mouse IFN-β polyclonal antibody. Purified recombinant mouse IFN-β (UnitedStates Biological) (120 ng) was used as a positive control. (D) Biological assay of IFN-β expressed by VSV-IFN. BHK-21 cells were mock infected or infected with VSV-GFP, VSV-IFNβ, or rVSV at an MOI of 10 PFU per cell for 24 h. Supernatants (500 μl) were HI to remove residual virus and used to incubate B16(F10) cells for 24 h. Treated cells were then infected with wild-type VSV (Indiana strain) at an MOI of 0.1 for 24 h, and CPE was assessed under microscopy. (Di) B16(F10) cells treated with supernatants from VSV-GFP-infected BHK-21 cells; (Dii) B16(F10) cells treated with supernatants from rVSV-infected BHK-21 cells; (Diii) B16(F10) cells treated with supernatants from VSV-IFNβ-infected BHK-21 cells; (Div) B16(F10) cells treated with purified recombinant murine IFN-α/β (1,000 U/24 h); (Dv) B16(F10) cells treated with medium from uninfected BHK-21 cells; (Dvi) uninfected B16(F10) cells. (E) Quantitation of IFN-β expressed by VSV-IFNβ. The supernatant of BHK-21 cells infected with VSV-IFNβ or VSV-GFP at an MOI of 10 PFU per cell for 24 h was serially diluted and used to incubate C57BL/6 primary cells for 24 h. The cells were infected with wild-type VSV (Indiana strain) at an MOI of 0.01 PFU per cell for 24 h, and CPE was assessed by microscopy. Biological activity data are presented as units per milliliter and represent the means from two experiments.
To determine whether the recovered VSV-IFNβ expressed recombinant IFN-β, BHK-21 cells were infected with VSV-GFP or VSV-IFNβ at an MOI of 10. After 24 h, the supernatants of infected cells were retrieved and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. As a positive control, purified commercially available murine IFN-β (synthesized in Escherichia coli) was also examined. Membranes were subsequently incubated using anti-murine IFN-β polyclonal antibody. Our results indicated that only supernatants from VSV-IFNβ-infected cells and not those from VSV-GFP-infected cells contained detectable IFN-β of the expected size of approximately 30 to 32 kDa, the size fluctuations perhaps representing differing carbohydrate moieties (39) (Fig. 1C). To assess the biological activity of the VSV-expressed IFN-β, we developed an assay using B16(F10) melanoma cells, which are partially receptive to the protective antiviral effects of IFN (16). Essentially, the supernatant of rVSV-IFNβ- or VSV-GFP-infected BHK-21 cells containing secreted, recombinant IFN-β was treated at 56°C to inactivate residual parental VSV-IFNβ. Supernatants were then analyzed by plaque assay to confirm that virus had been HI (data not shown). As shown in Fig. 1D, B16(F10) cells treated for 24 h with supernatants of VSV-IFNβ-infected but not VSV-GFP-infected BHK-21 cells exhibited significant evidence of protection against challenge by VSV-GFP infection. The protective effects manifested using IFN-β generated by VSV-IFNβ were similar to those of commercially available purified recombinant murine IFN-α/β (Fig. 1D). The amount of VSV-IFNβ-synthesized IFN-β was quantitated in a biological protection assay using purified recombinant IFN-α/β as a standard and was calculated at between 106 and 107 U of IFN per ml of 106 cells within 24 h postinfection (Fig. 1E). Collectively, these data strongly indicate that VSV-IFNβ expresses a high level of biologically active IFN-β.
VSV-expressing IFN-β retains oncolytic activity.
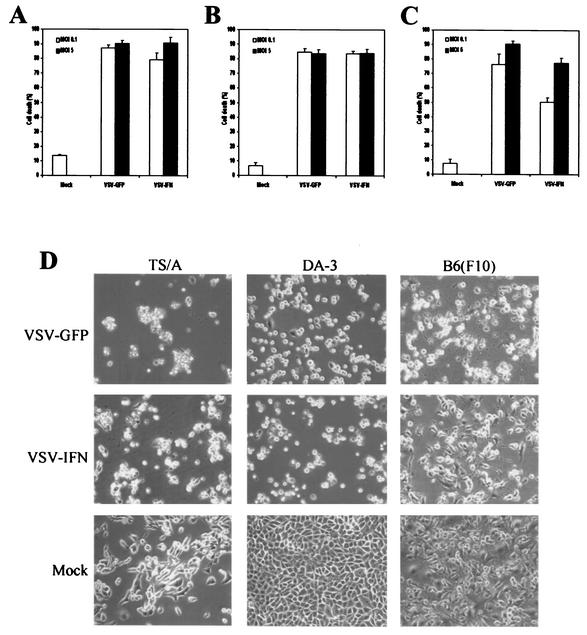
To assess whether VSV-IFNβ retains oncolytic activity, murine tumor cell lines [mammary adenocarcinoma TS/A and DA-3 cells as well as melanoma B16(F10) cells] were infected with VSV-IFNβ or VSV-GFP at an MOI of 0.1 or 5 PFU per cell for 24 h. The percentages of dead cells, as measured by trypan blue exclusion assay, indicated that TS/A, DA-3, and B16(F10) cells were readily killed within 24 h following infection with either VSV-IFNβ or VSV-GFP at an MOI of 0.1 (Fig. 2A to C). Although little additional effect was observed after increasing the amount of virus used to infect TS/A or DA-3 cells, the amount of cell death observed following treatment of B16(F10) with VSV-IFNβ at an MOI of 5 increased to approximately 80% within 24 h. It is interesting that the previous data of Fernandez et al. indicate that pretreatment with IFN afforded some protection to B16(F10) cells exposed to VSV (cell death appeared delayed, although viral replication was less affected) (16). Thus, the slight decrease in cell killing observed when using VSV-IFNβ to infect B16(F10) cells may have been due to heterologous IFN-β produced by VSV-IFNβ, which may confer some initial protective effect. Nevertheless, the data of Fernandez et al. confirm that even tumors with a partially intact IFN pathway from such cell lines as B16(F10) readily acquiesce to VSV-mediated oncolysis (Fig. 2C and D) (16). Confirmation that VSV-IFNβ replicates effectively in tumor cells was carried out using plaque assay analysis, which indicated that VSV-IFNβ and VSV-GFP replicated at high levels (107 to 108 PFU per ml within 24 h p.i.) in TS/A cells following infection at an MOI of 0.01 or 0.1 PFU per cell (Table 1). Similar results were obtained from infected DA-3 and B16(F10) cells (data not shown) (16). These results confirm that VSV-IFNβ replicates efficiently and retains in vitro oncolytic activity similar to that of VSV-GFP in malignant cells.
FIG. 2.
In vitro oncolytic activity of VSV-IFNβ. TS/A (A), DA-3 (B), or B16(F10) (C) cells were mock infected or infected with VSV-IFNβ or VSV-GFP at an MOI of 0.1 (open bars) or 5 (solid bars) PFU per cell for 24 h. After this period, cell viability was assessed by trypan blue exclusion assay (A, B, and C) or visually by microscopy (magnification, ×20) (D).
TABLE 1.
Virus titers in TS/A cells
| Virus | Virus titers (PFU/106 cells)a at an MOI of:
|
|
|---|---|---|
| 0.1 PFU/cell | 0.01 PFU/cell | |
| VSV-GFP | 4.3 × 108 | 1.4 × 108 |
| VSV-IFN | 3.5 × 108 | 1.4 × 107 |
Data show the means of two independent experiments.
The replication of VSV expressing IFN-β is suppressed in normal cells.
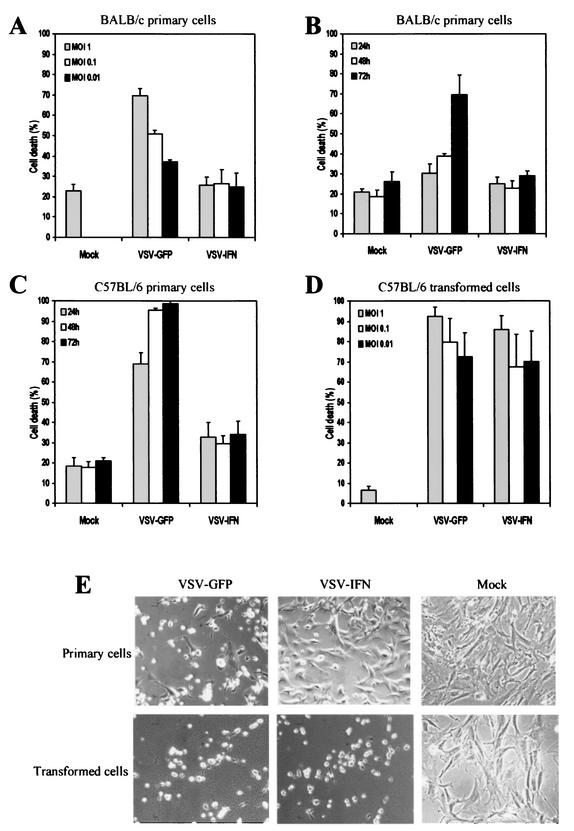
Our data indicate that VSV-IFNβ replicates to high levels in malignant cell lines, inducing cytolysis while producing significant levels of IFN-β. Since our studies indicate that the IFN pathway is defective in many transformed cell lines, the exogenous IFN produced by VSV-IFNβ following infection probably fails to exert significant antiviral activity that would impede virus replication. However, because normal cells retain regular IFN antiviral responses, it is plausible that such cells should be considerably resistant to VSV-IFNβ since the IFN produced by the recombinant virus can function in a manner involving autocrine and paracrine mechanisms to exert a protective effect upon exposed cells. To confirm this hypothesis, we first examined MEFs derived from a 129/Sv, BALB/c, or C57BL/6 background for determination of their susceptibility to VSV infection. As Balachandran et al. have previously shown, MEFs from a 129/Sv lineage are largely resistant to VSV infection unless PKR or the IFN pathway is defective (6) (data not shown). However, MEFs from a BALB/c and C57BL/6 background were found to exhibit some CPE in response to VSV infection after 24 h. MEFs were infected with VSV-IFNβ or VSV-GFP at MOIs of 0.01, 0.1, and 1 PFU per cell. After 24 h, cell viability was measured by trypan blue exclusion assay. In MEFs derived from BALB/c mice, the percentages of dead cells observed following exposure to VSV-GFP increased in a virus dose-dependent manner (Fig. 3A). At an MOI of 1, nearly 70% of cells were trypan blue positive after 24 h, while at an MOI of 0.01, approximately 35% of cells appeared dead. However, we did not observe any CPE when BALB/c cells were infected with VSV-IFNβ at an MOI of 0.01 to 1 PFU per cell (Fig. 3A). Moreover, the viability of BALB/c cells following infection with VSV-IFNβ at an MOI of 0.01 PFU per cell was invariable at up to 72 h p.i., with the background of trypan blue-staining cells remaining at approximately 20% of VSV-GFP-infected cells and nearly 70% of the cells dying after this time (Fig. 3B). Confirmation of these observations was obtained by measuring virus titers in the infected cells. Indeed, infectious VSV-IFNβ was not readily detectable in the supernatant of BALB/c MEFs following infection at an MOI of 0.01 PFU per cell after 24 h (Table 2). In contrast, some progeny VSV-GFP was detected in infected BALB/c cells (1.2 × 105 PFU per 105 cells) unless they were treated with IFN, whereupon virus levels became undetectable (data not shown). For reasons that currently remain unclear, C57BL/6 fibroblasts appear to be considerably more sensitive to VSV infection, with nearly 70% dead cells after 24 h postinfection with VSV-GFP at an MOI of 0.1 (with 1.3 × 107 PFU virus generated per 105 cells) (Fig. 3C and E and Table 2). In contrast, VSV-IFNβ did not cause significant CPE or replicate proficiently in C57BL/6 cells when observed over a 72-h period, in similarity to results obtained using BALB/c cells (Fig. 3C and E and Table 2). In similarity to BALB/c MEFs, C57BL/6 cells were almost completely protected against VSV-GFP infection after exposure to IFN (Table 2). Next, the replication of VSV-IFNβ was compared in transformed C57BL/6 cells that had been developed by serial passaging (S. Balachandran and G. N. Barber, unpublished data). These results indicated that both VSV-GFP and VSV-IFNβ induced rapid cytolysis of transformed cells, routinely invoking 70 to 80% cell death within 12 to 72 h (Fig. 3D and E). Preexposure of transformed C57BL/6 cells to IFN-α/β failed to inhibit VSV-mediated cytolysis, indicating that the transformation process had damaged the IFN antiviral response (Table 2). These data indicate that VSV expressing the murine IFN-β gene exhibits minimal replication and CPE in normal murine cells but retains specific oncolytic properties in transformed cells, conceivably due to defects in IFN function.
FIG. 3.
In vitro replication of VSV-IFNβ in primary or transformed cells. Primary or transformed MEFs derived from BALB/c (A and B) or C57BL/6 (C to E) cells were mock infected or infected with VSV-IFNβ or VSV-GFP at an MOI of 1 (shaded bars), 0.1 (open bars), or 0.01 (solid bars) PFU per cell for 24 h (A and D) or infected at an MOI of 0.01 PFU per cell for 24 (shaded bars in panels B and C and panels in panel E), 48 (open bars), or 72 (solid bars) h (B, C, and E). Cell viability was assessed by trypan blue exclusion assay (A to D) or visually by microscopy (magnification, ×20) (E).
TABLE 2.
Virus titers in primary and transformed mouse fibroblasts
| Virusa | Virus titers (PFU/105 cells)b
|
||||
|---|---|---|---|---|---|
| BALB/c primary cells | C57BL/6 primary cells
|
C57BL/6 transformed cells
|
|||
| IFN− | IFN+ | IFN− | IFN+ | ||
| VSV-GFP | 1.2 × 105 | 1.3 × 107 | NDc | 1.6 × 107 | 1.6 × 106 |
| VSV-IFN | ND | 6.7 × 104 | ND | 2.9 × 105 | 3.7 × 104 |
MOI, 0.01.
Data show the means of two independent experiments.
ND, not detected.
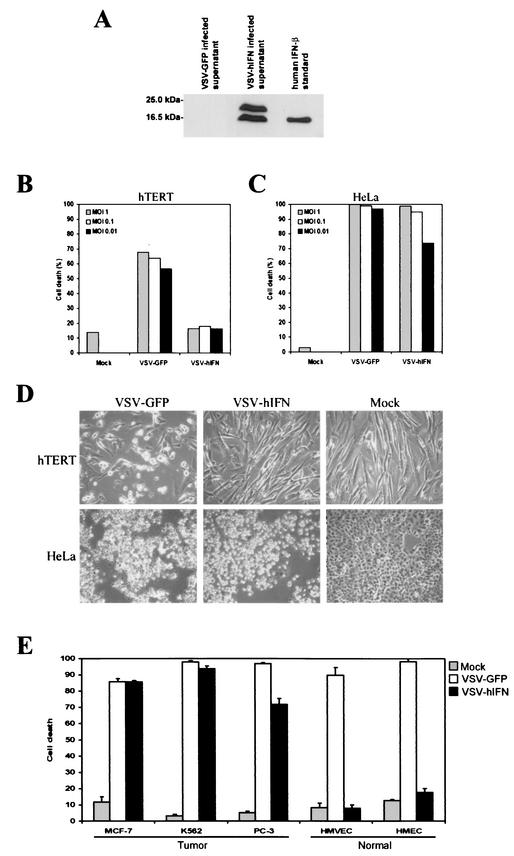
To complement these findings, we additionally cloned the hIFNβ gene into VSV (VSV-hIFN) and confirmed high-level expression and secretion of the heterologous product following infection of BHK-21 cells (Fig. 4A). Presumably, the two bands observed by immunoblot analysis are reflective of different posttranslational processes, as described previously (39). To examine the replication of VSV-hIFN in nontransformed human cells, we infected primary neonatal fibroblasts expressing the catalytic subunit of telomerase (hTERT) at various MOIs and monitored resultant CPE and virus production. As shown in Fig. 4B, VSV-GFP induced approximately 50% cell death within 48 h. However, VSV-hIFN did not provoke any significant cell death under similar conditions. Indeed, no VSV-hIFN replication was detected after 48 h in hTERT fibroblasts infected at an MOI of 0.01 (Table 3). Nevertheless, VSV-hIFN did retain oncolytic activity and readily killed HeLa cells (Fig. 4B and C). To further evaluate the specific oncolytic activity of VSV-hIFN, a panel of transformed cells (MCF-7, breast; K562, leukemia; and PC-3, prostate) were infected at an MOI of 0.1 and monitored over 48 h. As shown in Fig. 4E, all transformed cell lines were susceptible to VSV-hIFN- or VSV-GFP-induced CPE. However, normal HMVECs or HMECs exhibited no CPE in response to VSV-hIFN infection and gave no significant evidence of progeny release (Fig. 4E and data not shown). This result was in contrast to that observed following infection of normal human cells with VSV-GFP, with 20 to 30% cell death observed after 24 h of infection (16) and a significantly higher level observed after 48 h (Fig. 4E).
FIG.4.
In vitro replication of rVSV expressing the hIFNβ gene in human normal and tumor cell lines. (A) Expression of hIFNβ in VSV-hIFNβ-infected cells. BHK-21 cells were infected with VSV-hIFNβ or VSV-GFP at an MOI of 5 PFU per cell for 24 h. Supernatants (25 μl of 1 ml from 106 infected cells) were analyzed for hIFNβ expression by immunoblotting using an anti-hIFNβ polyclonal antibody. Purified recombinant hIFNβ (5,000 U) was used as a positive control. (B) In vitro replication of VSV-hIFNβ in human normal cells. Human foreskin fibroblast cells (hTERT) were mock infected or infected with VSV-hIFNβ or VSV-GFP at an MOI of 1 (shaded bars), 0.1 (open bars), or 0.01 (solid bars) PFU per cell for 48 h. Cell viability was assessed by trypan blue exclusion assay. (C) In vitro replication of VSV-hIFNβ in human tumor cells. HeLa cells were mock infected or infected with VSV-hIFNβ or VSV-GFP at an MOI of 1 (shaded bars), 0.1 (open bars), or 0.01 (solid bars) PFU per cell for 48 h. Cell viability was assessed by trypan blue exclusion assay. (D) CPE of VSV-hIFNβ-infected human cells. hTERT or HeLa cells were mock infected or infected with VSV-hIFNβ or VSV-GFP at an MOI of 0.01 PFU per cell for 48 h. After this period, cell viability was assessed visually by microscopy (magnification, ×20). (E) In vitro oncolytic activity of VSV-hIFNβ. Human tumor cell lines (MCF-7, K562, and PC-3) or normal cell lines (HMVECs and HMECs) were mock infected or infected with VSV-hIFNβ or VSV-GFP at an MOI of 0.1 PFU per cell for 48 h. Cell viability was assessed by trypan blue exclusion assay.
TABLE 3.
Virus titers in human normal and tumor cells
MOI, 0.01.
Data show the means of two independent experiments.
ND, not detected.
Collectively, these data would suggest that VSV modified to express murine or hIFNβ inhibits its own replication in normal cells through autocrine as well as paracrine activation of the IFN antiviral pathway, given that MOIs of 1 were insufficient to induce global cell death (Fig. 3). Since transformed cells exhibit defective IFN antiviral responses, however, they remain permissive to VSV-IFNβ.
The attenuation of VSV-IFNβ is due to expression of IFN-β.
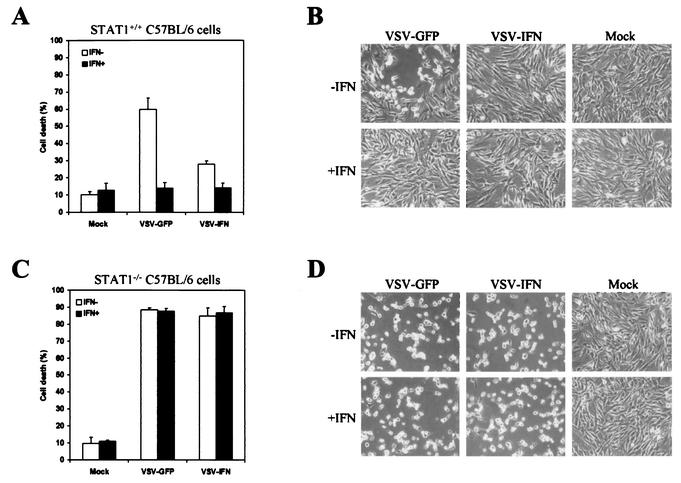
Figure 1B shows that VSV-GFP and VSV-IFNβ exhibited similar growth properties in nonmurine BHK cells, since any murine IFN-β produced by VSV-IFNβ is species specific and does not bind to the cellular IFN receptors to exert antiviral activity. Thus, the lack of replication in normal murine cells exhibited by VSV-IFNβ is presumably due to exogenous IFN exerting potent antiviral effects. To confirm this, we infected immortalized STAT1+/+ and STAT1−/− C57BL/6 cells with VSV-GFP or VSV-IFNβ (15) (MOI, 0.01). As shown in Fig. 5, immortalized STAT1-containing cells exhibited less CPE in response to VSV-IFNβ infection (>20%) than that seen with VSV-GFP infection (>60%). Treatment with murine IFN largely protected the STAT1-containing cells from both VSV-GFP- and VSV-IFNβ-induced CPE (Fig. 5A and B and Table 4). However, STAT−/− cells were equally sensitive to both VSV-GFP and VSV-IFNβ and high levels of CPE were evident within 24 h postinfection. In addition, pretreatment with recombinant IFN failed to protect the STAT1−/− cells, unlike the effect seen with their STAT+/+ counterparts (Fig. 5C and D and Table 4). Thus, the lack of CPE manifested by the presence of VSV-IFNβ is due to exogenous IFN-β produced from the recombinant virus that impedes its own replication via induction of STAT1-dependent host antiviral activity.
FIG. 5.
In vitro replication of VSV-IFNβ in Stat1+/+ or Stat1−/− cells. Immortalized embryonic fibroblasts derived from Stat1+/+ (A and B) or Stat1−/− (C and D) C57BL/6 mice were treated with (solid bars) or without (open bars) murine IFN-α/β (1,000 U/ml) for 24 h and were mock infected or infected with VSV-IFNβ or VSV-GFP at an MOI of 0.01 PFU per cell for 24 h. Cell viability was assessed by trypan blue exclusion assay (A and C) or visually by microscopy (magnification, ×20) (B and D).
TABLE 4.
Virus titers in STAT1+/+ and STAT1−/− C57BL/6 mouse fibroblasts
| Virusa | Virus titers (PFU/105 cells)b
|
|||
|---|---|---|---|---|
| STAT1+/+
|
STAT1−/−
|
|||
| IFN− | IFN+ | IFN− | IFN+ | |
| VSV-GFP | 3.6 × 107 | NDc | 5.2 × 107 | 4.2 × 107 |
| VSV-IFN | 9.7 × 102 | ND | 5.0 × 107 | 3.9 × 107 |
MOI, 0.01.
Data show the means of two independent experiments.
ND, not detected.
VSV expressing IFN-β exhibits increased attenuation.
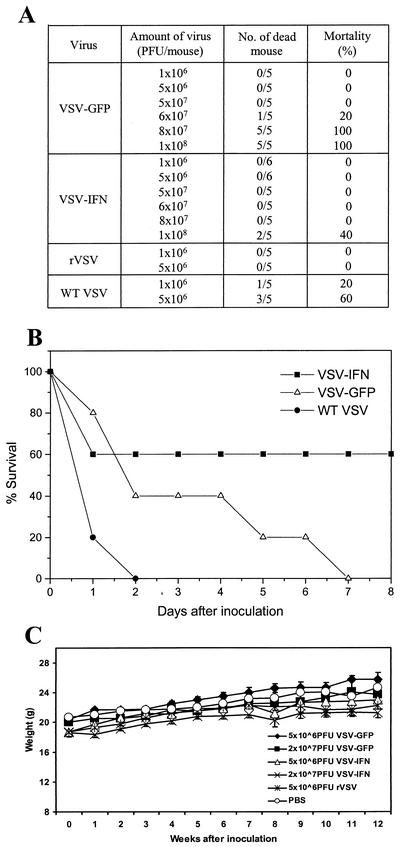
Since our data had demonstrated significant attenuation of VSV-IFNβ with normal cells in vitro, we next examined whether VSV-IFNβ also exhibited increased attenuation in vivo. For these studies, BALB/c animals were inoculated i.v. with different amounts of VSV-IFNβ or VSV-GFP. As has been described previously, approximately 60% of 6- to 8-week-old BALB/c mice receiving 5 × 106 PFU of wild-type VSV (Indiana strain) died within several days of inoculation (Fig. 6A). However, no mice died following inoculation with 5 × 106 PFU of rVSV (either rVSV without a heterologous gene or VSV-GFP or VSV-IFNβ; Fig. 6A). Similar results were obtained using 5 × 107 PFU of all recombinant viruses, confirming that viruses derived from pVSV-XN2 are significantly attenuated in vivo (25, 37). In fact, the maximal tolerable i.v. dose observed for VSV-GFP in BALB/c animals was determined to be 6 × 107 PFU (Fig. 6A). In contrast, mice receiving 6 × 107 PFU of VSV-IFNβ exhibited 100% survival, with no overt anomalies. Indeed, a significantly higher maximal tolerable i.v. dose of 108 PFU was observed for VSV-IFNβ, with 60% of the animals surviving, thus confirming its in vitro behavior and verifying that VSV-IFNβ is also significantly attenuated in vivo (Fig. 6A and B). Similar studies carried out via intranasal inoculation indicated even greater attenuation, with none of the recombinant viruses used (rVSV, VSV-GFP or VSV-IFNβ) causing significant illness or any mortality at doses as high as 2 × 108 PFU (data not shown). Following i.v. inoculation of rVSV, VSV-GFP, or VSV-IFNβ (at from 5 × 106 to 2 × 107 PFU), the monitoring of animals over a 12-week period indicated no significant loss of weight or malaise (Fig. 6C). Further, no virus was detected in a variety of organs (liver, brain, lung, and spleen) from the infected animals when analyzed at 15 weeks postinfection (data not shown). Thus, our data indicate that VSV-IFNβ is significantly attenuated in vitro as well as in vivo and causes no apparent anomalies or persistent infection of exposed animals.
FIG. 6.
VSV-IFNβ is attenuated in vivo. (A) Various amounts of VSV-IFNβ, VSV-GFP, or rVSV without an insert or wild-type (WT) VSV (Indiana strain) were used for i.v. inoculation of BALB/c mice (6 to 8 weeks old). Mortality of the animals was monitored. (B) Survival times of animals receiving i.v. inoculations of 108 PFU of VSV-IFNβ, VSV-GFP, or wild-type VSV (Indiana strain). (C) Weights of BALB/c animals that received i.v. inoculations of phosphate-buffered saline or recombinant viruses at the various doses indicated and that were monitored for 12 weeks postinfection. No residual or persistent virus was detected in a variety of organs analyzed at 20 weeks postinfection.
Oncolytic effect of VSV expressing IFN-β.
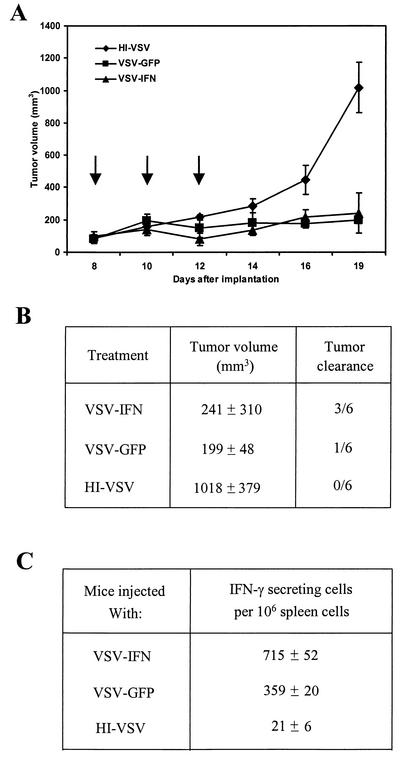
To evaluate the in vivo oncolytic effect of VSV-IFNβ, BALB/c mice were s.c. implanted with 7 × 105 cells derived from syngeneic Renca renal cell carcinoma. After the formation of palpable tumors, 1.5 × 108 PFU of VSV-IFNβ were injected intratumorally three times at intervals of 2 days and tumor size was measured daily. An equivalent amount of HI-VSV or VSV-GFP was used in similar treatments as a control. As shown in Fig. 7A, the tumor volumes of VSV-GFP- or VSV-IFNβ-injected mice were significantly different from those of HI-VSV-injected mice (n = 6, P < 0.01). Indeed, the majority of animals treated with HI-VSV were sacrificed at 19 days postimplantation due to their harboring large tumors (mean tumor volume = 1,018 mm3). However, even though our data indicate that rVSV vectors appear significantly attenuated in vivo, VSV-GFP retained considerable oncolytic activity similar to that of VSV-IFNβ (P = 0.0010 and 0.0028, respectively, as determined using Student's t test). Tumors inoculated with VSV-GFP exhibited slow growth until 3 weeks after the original implantation and 8 days after the last inoculation of virus (Fig. 7A). The mean size of the tumors at this time was 199 mm3. In one out of six mice, no tumor growth was apparent after treatment with VSV-GFP. In the case of VSV-IFNβ-treated animals, three of six mice (50%) exhibited no tumor growth after treatment (Fig. 7B). The remaining three animals exhibited mean tumor sizes of 241 mm3. After this period, some remaining tumors continued to grow slowly and were monitored until day 29 postimplantation, whereupon the animals were sacrificed.
FIG. 7.
rVSV-IFNβ inhibits the growth of syngeneic renal cell carcinoma. (A) BALB/c mice (6 to 8 weeks old) were implanted s.c. with 7 × 105 Renca cells. After palpable tumors had formed, animals were treated intratumorally with 1.5 × 108 PFU of VSV-IFNβ, VSV-GFP, or HI-VSV. Three injections of the virus were given as indicated by the arrows. Tumor volumes were calculated, and values are means ± standard errors of the means (n = 6). (B) Tumor volumes (in cubic millimeters) were measured at day 19 postimplantation, and animals that showed no tumor development at 1 month after treatment were identified. (C) Antitumor IFN-γ-secreting T cells were measured in animals (n = 2) receiving VSV-GFP, VSV-IFNβ, or HI-VSV at 10 days posttreatment as described in Results.
Previous studies have indicated that the antitumor effects of IFN can be mediated by T cells (49). Therefore, to gain insight into the mechanisms of VSV-IFNβ oncolytic activity, spleens from mice (n = 2) inoculated with HI-VSV, VSV-GFP, or VSV-IFNβ were obtained at 10 days posttreatment and analyzed using an ELISPOT assay. Mice treated with VSV-GFP and harboring regressed tumors of a mean size of 266 mm3 were found to have elicited a significant number of IFN-γ-secreting cells in the spleen compared to those with HI-VSV-treated tumors (359 ± 20 versus 21 ± 6 spots, respectively; P = 0.000011, Student's t test) (Fig. 7C). However, spleens from mice inoculated with VSV-IFNβ which subsequently exhibited no tumors after treatment showed a statistically larger number of IFN-γ-secreting cells than those inoculated with VSV-GFP (715 ± 52 IFN-γ-secreting cells per 106 spleen cells; P = 0.000375, Student's t test). These observations strongly suggest that treatment of tumors with VSV-IFNβ activates a robust and specific immune response against the implanted Renca tumor cells and conceivably plays an important role in tumor clearance. Thus, our data indicate that VSV expressing IFN-β exerts potent oncolytic activity against s.c. tumor growth following direct intratumoral inoculation.
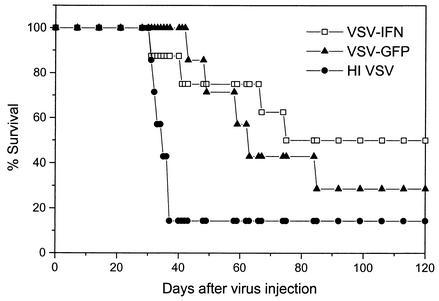
The previous data of Fernandez et al. demonstrated that rVSV expressing the TK suicide cassette and cytokine IL-4 gene dramatically inhibited metastases of a mammary adenocarcinoma (TS/A cells) and could prolong the survival of mice receiving viruses via systemic administration (16). Therefore, we next assessed whether VSV expressing IFN-β exhibited improved survival outcome against mammary-related metastatic disease. BALB/c mice (n = 8) were therefore inoculated with 5 × 104 TS/A cells via the tail vein and then treated with a single i.v. injection of 5 × 107 PFU of VSV-IFNβ 2 days later. As shown in Fig. 8, the majority of mice injected with HI-VSV rapidly died within 30 days postimplantation and seven of eight mice (87.5%) died within 40 days. In contrast, mice treated with VSV-GFP exhibited prolonged life up to day 120 postimplantation, although by this period, six of eight mice (75%) had died (Fig. 8). However, treatment of metastatic disease with a single inoculation of VSV-IFNβ prolonged the survival of the majority of animals (80%) and improved the survival rate of mice compared to that of VSV-GFP-injected mice (50% versus 28.6%, respectively). Log rank test analysis indicated statistically significant survival rates when comparing animals treated with VSV-IFNβ (P = 0.0445) but not VSV-GFP (P = 0.0536) to HI-VSV controls. Collectively, our data indicate that VSV-IFNβ exhibits increased attenuation both in vitro and in vivo and yet exhibits greater oncolytic activity against both s.c. and metastatic malignant disease in immunocompetent animals.
FIG. 8.
VSV-IFNβ increases the survival of immunocompetent animals harboring metastatic tumors. BALB/c animals (6 to 8 weeks; n = 8) were injected via tail vein with 5 × 104 TS/A cells, followed 2 days later by a single i.v. injection (5 × 107 PFU) of VSV-GFP, VSV-IFNβ, or HI-VSV. Following injection, the survival rates of the animals were monitored. A total of 50% of animals receiving VSV-IFNβ continued to remain disease free after 120 days.
DISCUSSION
Considerable evidence indicates that the IFN pathway plays a significant antiviral role in host defense against VSV replication. Animals with a defective IFN system (for example, mice lacking the IFN-α/β receptor or STAT1) are extremely sensitive to normally innocuous doses of VSV (15, 31, 32). Further, mice and cells defective in PKR activity have also been demonstrated to be very sensitive to VSV replication and cytolysis (4, 6, 43). Collectively, these data would indicate that PKR acts early in the innate immune response to VSV by preventing translation of viral mRNAs (6). This allows time for sufficient IFN-β to be produced, which, by functioning in a manner involving autocrine and paracrine mechanisms, bolsters the antiviral response by inducing genes, many as yet uncharacterized, that further impede VSV replication. Nevertheless, the PKR and IFN pathways do not appear sufficient to thwart VSV replication in vivo since mice lacking B cells, but not those lacking T cells, rapidly succumb to VSV infection even though the IFN pathway is presumably intact (45). It is likely that in the absence of PKR or IFN, VSV replicates rapidly to produce high levels of progeny virions, effectively outpacing the production and availability of neutralizing antibody that would normally impede lethal infection. These data elegantly demonstrate the synergistic nature of the acquired and innate immune responses required to efficiently combat VSV infection (6).
Considerable data also indicate that VSV is able to replicate very efficiently compared to normal cells in many types of tissue-cultured cell lines, which are generally transformed (4, 5, 44). These observations raise the issue of whether the IFN pathway is defective in transformed cells permissive to VSV. As our studies here emphasize, following exposure to IFN many transformed cells (unlike their normal cell counterparts) do not appear fully protected from VSV replication, indeed implying that the IFN pathway or certain IFN-induced genes may commonly be defective (4, 5, 44). This may explain, in part, the inadequate efficacy of the IFNs in the therapeutic management of many tumor types (17). However, it is also plausible that other defective cellular pathways, in addition to the IFN system, may cooperatively confer permissiveness to VSV replication. These observations allowed our laboratory and others to determine whether VSV could be useful as an oncolytic vector in the treatment of malignant disease. Subsequent studies, including those presented in this report, have confirmed that VSV is indeed able to replicate and exert oncolytic activity in implanted, syngeneic tumors while leaving the host relatively unaffected (5, 16, 44). Balachandran et al. and Fernandez et al. have shown that the mechanisms of oncolytic activity involve virus-induced apoptosis and the generation of cytotoxic T-lymphocyte responses specific to the tumor (5, 16). VSV may thus hold significant promise for evaluation as an oncolytic vector for the following reasons. First, VSV has a simple genome comprising only five genes and replicates in the cytoplasm (50). Secondly, VSV does not undergo genetic reassortment or integration into the host genome and has no transforming properties (50). Third, VSV can be genetically manipulated to contain large foreign gene inserts, which are generally stably incorporated within the virus and expressed to very high levels (16, 27, 38).
We have started to exploit these properties by successfully generating a number of rVSVs that express cytokines (such as IL-4 or suicide cassettes, including that of TK) for oncolytic studies (16). Through these experiments it became apparent that different strains of mice exhibit different degrees of resistance to VSV replication and pathogenesis. For example, cells and animals derived from a 129/Sv background exhibit almost complete resistance to VSV unless PKR is defective (6). In contrast, animals and MEFs from a BALB/c background appear more susceptible to VSV at high doses, while those from C57BL/6 appear to be the most sensitive, as we show in this report (18, 19). Although the reasons behind these observations remain to be clarified, it is plausible that the PKR/IFN pathway is less responsive due to differences in the efficiency of IFN signaling or to acquired mutations being prevalent in critical IFN-induced genes (12). These hypotheses may help explain the characteristic neurotropic hallmarks of VSV when administered at high doses in certain strains of mice (47). Some types of nontransformed human cells such as HMVECs and HMECs also displayed sensitivity to VSV infection in vitro, which was evident after 24 to 48 h posotinfection (16). Despite these differences in responses to VSV infection, however, it is clear that normal human and murine cells collectively exhibit significant delayed resistance to VSV infection compared to malignant cells, which are extremely permissive to VSV and rapidly undergo virus-induced apoptosis within hours (5, 16). Further, IFN pretreatment essentially protects normal cells, including HMVECs, HMECs, and C57BL/6 cells, but not transformed cells from VSV replication (Table 2) (6). Thus, while MEFs from a 129/Sv or BALB/c background seemed relatively resistant to VSV-IFNβ, C57BL/6 cells seemed more sensitive to infection and were relatively unaffected only when exposed to a low MOI, as our data show. Presumably, this was due to VSV-IFNβ-synthesized IFN-β protecting uninfected surrounding C57BL/6 cells through a paracrine mechanism. However, we noted that C57BL/6 cells infected at a high MOI with VSV-IFNβ succumbed to prolific VSV replication prior to the IFN-induced autocrine protection becoming effective.
While some strains of mice appear more sensitive than others to wild-type VSV such as that derived from the Indiana strain, recombinant versions of the virus (derived by reverse genetic techniques using cDNA) have shown greater attenuation in vivo (16, 25, 37). Indeed, our studies reveal that rVSV expressing GFP are 10- to 20-fold more attenuated in BALB/c animals than wild-type VSV for reasons that are not yet clear, although they probably involve subtle, as-yet-uncharacterized nucleotide variations arising in the cloned adaptation (27). Despite this, VSV-GFP retains significant oncolytic activity against s.c. tumors following intratumoral inoculation and can prolong the survival of BALB/c mice exposed to metastatic lung disease. To further potentially improve the attenuation of VSV-based oncolytic vectors, however, we placed the IFN-β gene into VSV and successfully generated a recombinant virus that generated very high levels of biologically active, authentic cytokine following mammalian cell infection. We proposed that VSV-IFNβ and VSV-hIFN should retain some oncolytic activity and be able to replicate in tumor cells, since the IFN pathway appears defective.
Exogenously synthesized IFN from rVSV should function in a manner involving a paracrine mechanism to trigger the antiviral actions of IFN. Thus, normal cells surrounding the tumor mass should be additionally protected against VSV infection since the IFN system is intact and stimulated. Our data as presented in this article confirm that VSV-IFNβ lacks significant cytolytic activity in normal murine cells and is greatly attenuated in vivo. BALB/c and C57BL/6 animals were essentially immune to lethal intranasal VSV-IFNβ infection, and this recombinant virus was approximately 50 times more attenuated following i.v. or intraperitoneal infection in BALB/c mice compared to wild-type VSV. Although VSV-hIFN could not be tested similarly due to the fact that the IFNs are species specific and the hIFNβ protein would not strongly interact with murine IFN-α/β receptors, it is highly plausible that such recombinant viruses would be attenuated in human hosts also. Thus, VSV carrying IFN-β is more attenuated and may provide a safer mechanism for use in oncolytic and even vaccine and gene therapy studies.
In addition to these specifics, the IFN-β gene has been proposed to exhibit antiproliferative properties and is useful in the treatment of certain instances of leukemia, lymphoma, and Kaposi's sarcoma (17). In this regard, studies of a number of gene delivery systems, such as those based on adenovirus vectors, have indicated the effectiveness of using IFN-α/β-related genes such as IFN-α and IFN-β to enhance viral oncolytic activity (10, 35). Plausibly, IFN-β expressed from oncolytic VSV may directly exert localized antiproliferative activity. However, IFN-α/β is also known to exert powerful immunomodulatory effects, including the activation of NK cells, the maturation of DCs, and the up-regulation of major histocompatibility complex proteins (8, 24, 30). In addition, the induction of apoptotic genes such as the death ligand TRAIL, tumor suppressor genes such as PML, and pleiotropic nitric oxide synthase and antiangiogenic factors may also contribute to the suppression of tumor growth and metastasis (2, 26, 51). Thus, VSV-based oncolytic viruses expressing IFN-β from within targeted tumor cells may invoke enhanced responses involving both the innate and adaptive arms of immunity to facilitate tumor cell clearance.
In this report, we confirm that VSV-IFNβ exerted significant oncolytic activity against s.c. implanted tumors and against metastatic lung disease in immunocompetent animals. In many instances, 50% of animals remained disease free after treatment with VSV-IFNβ, with a log rank test value of P = 0.4974 compared to HI controls. Although the exact mechanisms of VSV-IFNβ remain to be clarified, our data as presented here indicate that the induction of antitumor T-cell responses may have contributed to tumor clearance. Indeed, the induction of cytotoxic T cells against metastatic lung disease was severalfold higher in VSV-IFNβ-treated tumors than in controls and statistically higher than that of VSV-GFP-treated tumors. Thus, our data indicate that rVSV expressing IFN-β may be useful as a therapy against a variety of tumors and could conceivably form the basis of future vectors involving gene therapy, vaccine delivery, and therapeutics.
Acknowledgments
We thank H. Ezelle for technical assistance and J. Durbin for STAT1-deficient fibroblasts.
REFERENCES
- 1.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918-925. [DOI] [PubMed] [Google Scholar]
- 2.Albini, A., C. Marchisone, F. Del Grosso, R. Benelli, L. Masiello, C. Tacchetti, M. Bono, M. Ferrantini, C. Rozera, M. Truini, F. Belardelli, L. Santi, and D. M. Noonan. 2000. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am. J. Pathol. 156:1381-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreansky, S. S., B. He, G. Y. Gillespie, L. Soroceanu, J. Markert, J. Chou, B. Roizman, and R. J. Whitley. 1996. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc. Natl. Acad. Sci. USA 93:11313-11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus therapy of tumors. IUBMB Life 50:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 7.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 8.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. [DOI] [PubMed] [Google Scholar]
- 10.Cao, G., J. Su, W. Lu, F. Zhang, G. Zhao, D. Marteralli, and Z. Dong. 2001. Adenovirus-mediated interferon-beta gene therapy suppresses growth and metastasis of human prostate cancer in nude mice. Cancer Gene Ther. 8:497-505. [DOI] [PubMed] [Google Scholar]
- 11.Chander, G., M. S. Sulkowski, M. W. Jenckes, M. S. Torbenson, H. F. Herlong, E. B. Bass, and K. A. Gebo. 2002. Treatment of chronic hepatitis C: a systematic review. Hepatology 36:S135-S144. [DOI] [PubMed] [Google Scholar]
- 12.Coffey, M. C., J. E. Strong, P. A. Forsyth, and P. W. Lee. 1998. Reovirus therapy of tumors with activated Ras pathway. Science 282:1332-1334. [DOI] [PubMed] [Google Scholar]
- 13.Darnell, J. E., Jr. 1998. Studies of IFN-induced transcriptional activation uncover the Jak-Stat pathway. J. Interferon Cytokine Res. 18:549-554. [DOI] [PubMed] [Google Scholar]
- 14.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin, J. E., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez, M., M. Porosnicu, D. Markovic, and G. N. Barber. 2002. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 76:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrantini, M., and F. Belardelli. 2000. Gene therapy of cancer with interferon: lessons from tumor models and perspectives for clinical applications. Semin. Cancer Biol. 10:145-157. [DOI] [PubMed] [Google Scholar]
- 18.Fultz, P. N., and J. J. Holland. 1985. Differing responses of hamsters to infection by vesicular stomatitis virus Indiana and New Jersey serotypes. Virus Res. 3:129-140. [DOI] [PubMed] [Google Scholar]
- 19.Fultz, P. N., J. A. Shadduck, C.-Y. Kang, and J. W. Streilein. 1982. Mediators of protection against lethal systemic vesicular stomatitis virus infection in hamsters: defective interfering particles, polyinosinate-polycytidylate, and interferon. Infect. Immun. 37:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque, S. J., and B. R. Williams. 1998. Signal transduction in the interferon system. Semin. Oncol. 25:14-22. [PubMed] [Google Scholar]
- 21.Hilleman, M. R. 2001. Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine 19:1837-1848. [DOI] [PubMed] [Google Scholar]
- 22.Ihle, J. N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13:211-217. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs, A., and J. Lindemann. 1957. Virus interference. 1. The interferon. Proc. Roy. Soc. Lond. B Biol. Sci. 147:258-267. [PubMed] [Google Scholar]
- 24.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki, N., N. Yamaguchi, M. Nakayama, H. Eto, K. Okumura, and H. Yagita. 1999. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefrancois, L. 1984. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J. Virol. 51:208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, E., T. H. Bestor, and R. Jaenisch. 1992. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69:915-926. [DOI] [PubMed] [Google Scholar]
- 30.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 31.Meraz, M. A., J. M. White, K. C. Sheehan, E. A. Bach, S. J. Rodig, A. S. Dighe, D. H. Kaplan, J. K. Riley, A. C. Greenlund, D. Campbell, K. Carver-Moore, R. N. DuBois, R. Clark, M. Aguet, and R. D. Schreiber. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 32.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 33.Naylor, S. L., A. Y. Sakaguchi, T. B. Shows, M. L. Law, D. V. Goeddel, and P. W. Gray. 1983. Human immune interferon gene is located on chromosome 12. J. Exp. Med. 157:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 35.Odaka, M., D. H. Sterman, R. Wiewrodt, Y. Zhang, M. Kiefer, K. M. Amin, G. P. Gao, J. M. Wilson, J. Barsoum, L. R. Kaiser, and S. M. Albelda. 2001. Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-beta gene therapy is attributable to induction of systemic immunity. Cancer Res. 61:6201-6212. [PubMed] [Google Scholar]
- 36.Rakhmilevich, A. L., K. Janssen, Z. Hao, P. M. Sondel, and N. S. Yang. 2000. Interleukin-12 gene therapy of a weakly immunogenic mouse mammary carcinoma results in reduction of spontaneous lung metastases via a T-cell-independent mechanism. Cancer Gene Ther. 7:826-838. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 40.Sotomayor, E. M., Y. X. Fu, M. Lopez-Cepero, L. Herbert, J. J. Jimenez, C. Albarracin, and D. M. Lopez. 1991. Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. II. Down-regulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte-macrophage colony-stimulating factor. J. Immunol. 147:2816-2823. [PubMed] [Google Scholar]
- 41.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 42.Steele, T. A. 2000. Recent developments in the virus therapy of cancer. Proc. Soc. Exp. Biol. Med. 223:118-127. [DOI] [PubMed] [Google Scholar]
- 43.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen, A. R., A. Nansen, C. Andersen, J. Johansen, O. Marker, and J. P. Christensen. 1997. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 9:1757-1766. [DOI] [PubMed] [Google Scholar]
- 46.Toomey, N. L., V. V. Deyev, C. Wood, L. H. Boise, D. Scott, L. H. Liu, L. Cabral, E. R. Podack, G. N. Barber, and W. J. Harrington, Jr. 2001. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene 20:7029-7040. [DOI] [PubMed] [Google Scholar]
- 47.van den Pol, A. N., K. P. Dalton, and J. K. Rose. 2002. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 76:1309-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandepol, S. B., L. Lefrancois, and J. J. Holland. 1986. Sequences of the major antibody binding epitopes of the Indiana serotype of vesicular stomatitis virus. Virology 148:312-325. [DOI] [PubMed] [Google Scholar]
- 49.von Hoegen, P. 1995. Synergistic role of type I interferons in the induction of protective cytotoxic T lymphocytes. Immunol. Lett. 47:157-162. [DOI] [PubMed] [Google Scholar]
- 50.Wagner, R. R., and J. K. Rose. 1996. Rhabdoviridae: the viruses and their replication, p. 1121-1135. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lipincott-Raven Publishers, Philadelphia, Pa.
- 51.Wang, Z. G., D. Ruggero, S. Ronchetti, S. Zhong, M. Gaboli, R. Rivi, and P. P. Pandolfi. 1998. PML is essential for multiple apoptotic pathways. Nat. Genet. 20:266-272. [DOI] [PubMed] [Google Scholar]
- 52.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]