Abstract
The F13L protein of vaccinia virus, an essential and abundant palmitoylated peripheral membrane component of intra- and extracellular enveloped virions, associates with Golgi, endosomal, and plasma membranes in the presence or absence of other viral proteins. In the present study, the trafficking of a fully functional F13L-green fluorescent protein (GFP) chimera in transfected and productively infected cells was analyzed using specific markers and inhibitors. We found that Sar1H79G, a trans-dominant-negative protein inhibitor of cargo transport from the endoplasmic reticulum, had no apparent effect on the intracellular distribution of F13L-GFP, suggesting that the initial membrane localization occurs at a downstream compartment of the secretory pathway. Recycling of F13L-GFP from the plasma membrane was demonstrated by partial colocalization with FM4-64, a fluorescent membrane marker of endocytosis. Punctate F13L-GFP fluorescence overlapped with clathrin and Texas red-conjugated transferrin, suggesting that endocytosis occurred via clathrin-coated pits. The inhibitory effects of chlorpromazine and trans-dominant-negative forms of dynamin and Eps15 protein on the recycling of F13L-GFP provided further evidence for clathrin-mediated endocytosis. In addition, the F13L protein was specifically coimmunoprecipitated with α-adaptin, a component of the AP-2 complex that interacts with Eps15. Nocodazole and wortmannin perturbed the intracellular trafficking of F13L-GFP, consistent with its entry into late and early endosomes through the secretory and endocytic pathways, respectively. The recycling pathway described here provides a mechanism for the reutilization of the F13L protein following its deposition in the plasma membrane during the exocytosis of enveloped virions.
Poxviruses are large, enveloped DNA viruses that replicate in the cytoplasm of host cells (43). The assembly and extracellular release of vaccinia virus, the model poxvirus, can be broadly divided into three phases: formation of membrane crescents and their maturation into dense, brick-shaped, infectious intracellular mature virions (IMV); wrapping of IMV with cellular membranes derived from trans-Golgi or early endosomal cisternae to form intracellular enveloped virions (IEV); and transport of IEV along microtubules to the periphery, where their outer membranes fuse with the plasma membrane to form cell-associated enveloped virions (CEV), some of which acquire motile actin tails and released extracellular enveloped virions (EEV) (reviewed in references 44, 58, and 59).
Seven proteins have been identified in IEV- or EEV-specific membranes. Of these, the two encoded by the F13L and B5R open reading frames (ORFs) are required for the wrapping of IMV to form IEV (11, 19, 70). The B5R protein is a glycosylated type I integral membrane component of EEV (18, 31). The F13L protein, the subject of the present study, is the most abundant component of EEV membranes (27, 45). This nonglycosylated protein is palmitoylated at cysteines 185 and 186, a modification that is required for the association of the F13L protein with membranes, proper intracellular targeting, and IMV wrapping (13, 23, 24, 29, 56). Another feature of the F13L protein is the presence of a variant of the HKD motif (which is conserved in members of the phospholipase superfamily [34, 63]) that is required for the formation of the IEV membrane (48). The F13L protein localizes in the Golgi network as well as in endosomes and influences the intracellular location of other viral envelope proteins (26, 28, 29, 55). Topological studies have indicated that the N and C termini of the F13L protein face the cytoplasmic side of the outer IEV membrane and the inner side of the inner IEV membrane, which is destined to become the CEV or EEV envelope (30, 47, 56).
Studies with fluid-phase tracers have indicated that recycling between the endocytic and exocytic compartments is greatly increased following vaccinia virus infection, making it difficult to determine whether the membranes that wrap IMV are derived from endosomal or trans-Golgi cisternae (55, 65). Enhanced recycling could benefit virus replication by allowing recovery of viral proteins that are deposited in the plasma membrane either through the secretory pathway or by fusion with the outer IEV membrane. Plasma membrane retrieval of the B5R protein was shown by the internalization of a Fab fragment that specifically bound to its extracellular domain; the retrieval signals were identified in the cytoplasmic segment (69). The topology of the F13L protein, specifically the absence of an extracellular domain, precluded the direct approach used to demonstrate recycling of the B5R protein. In the present study, we employed specific markers and inhibitors of intracellular trafficking to perturb the steady-state distribution of the F13L protein in order to demonstrate recycling.
MATERIALS AND METHODS
Cells, viruses, and plasmids.
HeLa and BS-C-1 cells were grown and subcultured in Dulbecco's modified Eagle medium (DMEM) and Earle's modified Eagle medium (EMEM), respectively, supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin, at 37°C under 5% CO2. Recombinant vaccinia virus vF13L-GFP and plasmid pF13L-GFP, which contain an enhanced green fluorescent protein (GFP) coding sequence appended to the C terminus of the F13L ORF, and recombinant vaccinia virus vF13LHAC and plasmid pF13L-HA, which contain the F13L ORF with a C-terminal hemagglutinin (HA) epitope tag, have been described previously (29, 30). pVSVG-GFP and pSAR1H79G with an N-terminal HA tag were provided by Jennifer Lippincott-Schwartz. To replace the HA tag with GFP, the SAR1H79G sequence was amplified by PCR and the product was inserted into plasmid pEGFP-C1 (Clontech). Marc McNiven (14) generously provided wild-type Dyn2 (ab) and dominant-negative Dyn2 (ab) K44A mutant cDNAs in plasmid pEGFP-N1 (Clontech). DNAs encoding dominant-negative EH21 and the control D3Δ2 in plasmid pEGFP-C2 (Clontech) were kind gifts from Alexandre Benmerah (5, 7).
Antibodies and chemicals.
Mouse monoclonal antibody (MAb) HA.11 and rabbit polyclonal antibody HA.11, which recognize the influenza virus HA epitope, were purchased from Covance. A mouse anti-clathrin MAb and a goat anti-clathrin polyclonal antibody were purchased from ICN Biomedicals. Mouse anti-α-adaptin and anti-γ-adaptin MAbs were purchased from Affinity Bioreagents and Sigma, respectively. An anti-LAMP2 mouse MAb was obtained from Thomas August. Anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase was purchased from Amersham. Rhodamine red-conjugated anti-mouse IgG and tetramethyl rhodamine isothiocyanate-conjugated anti-goat IgG were purchased from Jackson ImmunoResearch Laboratories. Texas red-conjugated transferrin (TR-Tfn) and the membrane probe FM4-64 were procured from Molecular Probes. Chlorpromazine, nocodazole, and wortmannin were from Calbiochem.
Transfection and infection.
Plasmids were prepared using the Qiagen plasmid preparation kit. HeLa cells were grown on glass coverslips until they reached 80 to 90% confluence. Routinely, 2 μg of Lipofectamine 2000 (Invitrogen) and 0.5 to 1 μg of DNA were diluted separately in Opti-MEM I medium (Invitrogen), mixed, incubated at room temperature for 20 min, and added to the cells for 4 to 5 h at 37°C. The Lipofectamine-DNA complex was replaced with DMEM supplemented with 10% FBS, and the incubation was continued for a total of 24 h.
Virus stocks, diluted in DMEM or EMEM supplemented with 2.5% FBS, were added to cell monolayers on coverslips or in wells. After 1 to 2 h of incubation at 37°C, the virus inocula were replaced with fresh medium containing 2.5% FBS, and cells were incubated for a further 18 h.
Coimmunoprecipitation and Western blotting.
BS-C-1 cells infected with vF13LHAC were harvested in cold phosphate-buffered saline (PBS). Cells were lysed in cold nondenaturing lysis buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 5 mM EDTA, 1% Triton X-100) or radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate). The lysis mixtures were vortexed briefly, incubated on ice for 10 min, and centrifuged at 20,000 × g for 10 min at 4°C. Each supernatant was collected and used directly (cell lysate) or mixed with 5 μl of MAb and incubated overnight at 4°C with constant shaking. On the next day, 20 μl of protein G-Sepharose (Pierce) was added, and the mixture was incubated as described above for 2 h. The Sepharose beads were pelleted at 20,000 × g for 30 s at 4°C, washed four times with nondenaturing lysis buffer, and lastly washed with PBS. SDS sample buffer was added to the beads, and the extracted proteins were resolved by SDS-12% polyacrylamide gel electrophoresis (SDS-12% PAGE).
For Western blotting, proteins were transferred to a nitrocellulose membrane and incubated overnight in PBS with 5% milk at 4°C. The membrane was then washed three times with PBS and incubated with the anti-HA polyclonal antibody diluted 1:500 in 5% milk in PBS for 1.5 h at room temperature with constant shaking. After the membrane was washed four times with PBS containing 0.1% Tween 20, the membrane was incubated as described above for 1 h with a horseradish peroxidase-conjugated anti-rabbit secondary antibody diluted 1:2,000. The membrane was washed as described above, and proteins were visualized with the Super Signal chemiluminescence substrate (Pierce).
Confocal microscopy.
Transfected or infected cells on coverslips were fixed with cold 4% paraformaldehyde in PBS and then incubated at room temperature for 20 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. The permeabilized cells were washed three times with PBS and incubated with primary antibodies diluted in PBS containing 10% FBS for 1 h at room temperature. The cells were again washed three times with PBS and then incubated with a secondary antibody diluted in PBS containing 10% FBS for 30 min at room temperature. After further washing with PBS, the coverslips were mounted in 20% glycerol. Fluorescence was examined with a Leica TCS NT inverted confocal microscope, and images were overlaid using Adobe Photoshop, version 5.0.2.
Endocytosis experiments.
Endocytosis of TR-Tfn or membranes labeled with FM4-64 was examined as described previously with little modification (5). At 24 h after transfection or 18 h after infection, HeLa cells were washed three to four times with DMEM and incubated with 200 μg of TR-Tfn/ml or 16 μM FM4-64 diluted in DMEM for 10 to 20 min at 37°C. The cells were rapidly cooled to 4°C, washed twice with cold PBS, and fixed in cold paraformaldehyde as described above. For inhibition studies, cells were pretreated with chlorpromazine (20 μg/ml), nocodazole (30 μM), or wortmannin (100 nM) for 10 min at 37°C. Equivalent amounts of dimethyl sulfoxide, used to dissolve the drugs, were added to the media of untreated cells. After pretreatment, cells were incubated with TR-Tfn as described above in the continuous presence of drugs or dimethyl sulfoxide.
RESULTS
Intracellular localization of F13L-GFP is not dependent on endoplasmic reticulum (ER) cargo transport.
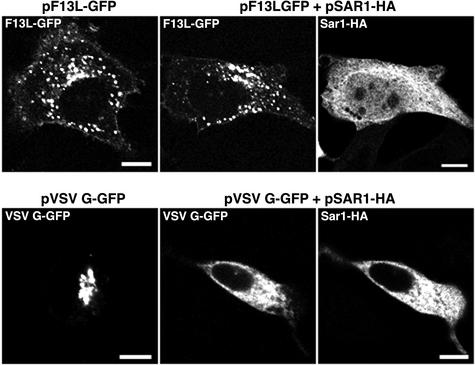
In previous studies, we demonstrated that an F13L-GFP fusion protein was fully functional and could replace the wild-type F13L protein for vaccinia virus replication and spread (29). F13L-GFP was detected by confocal microscopy in Golgi, endosomal, and plasma membranes when expressed alone in transfected cells or by a recombinant vaccinia virus in infected cells (29). This steady-state distribution, however, did not preclude initial association and export from the ER. Export of proteins from the ER is mediated by the COPII coat machinery, which includes the GTPase Sar1 and the Sec23/24 and Sec13/31 complexes (4, 40). We employed Sar1H79G-HA, a trans-dominant mutated form of the Sar1 protein that is retained in the ER (3), to determine whether the Golgi and endosomal membrane localization of the F13L protein is dependent on export from the ER. Both HA- and GFP-tagged versions of Sar1H79G were used, with equivalent results. In the experiments described below, plasmids expressing Sar1H79G, VSVG-GFP, or F13L-GFP were transfected separately or in combinations. Expression of Sar1H79G-HA in transfected cells did not appear to perturb the localization of endogenous p115, LAMP2, and EEA1, markers of cis-Golgi membranes, late endosomes, and early endosomes, respectively, during the time frame of the transfection experiments (data not shown). VSVG-GFP, however, showed an ER-like staining pattern in the presence of Sar1H79G-HA, indicating the failure of VSVG-GFP transport to the juxtanuclear Golgi region (Fig. 1). In contrast, F13L-GFP showed its typical cytoplasmic punctate staining pattern in the presence of Sar1H79G-HA (Fig. 1), indicating that significant amounts of F13L-GFP do not traffic from the ER to the Golgi apparatus and endosomes through the established cargo pathway. In the experiment described above, the Sar1H79G-HA and F13L-GFP expression plasmids were transfected simultaneously. However, similar results were obtained even when the former was transfected 24 h before the latter (data not shown).
FIG. 1.
Intracellular distribution of VSVG-GFP and F13L-GFP in cells expressing Sar1H79G-HA. HeLa cells were transfected with plasmids that expressed VSVG-GFP or F13L-GFP in the presence or absence of plasmids that expressed Sar1H79G-HA. After 24 h, the cells were fixed and permeabilized, stained with an anti-HA MAb followed by rhodamine red-conjugated anti-mouse IgG, and examined by confocal microscopy. Bars, 10 μm.
Localization of F13L-GFP and endocytic markers in transfected and infected cells.
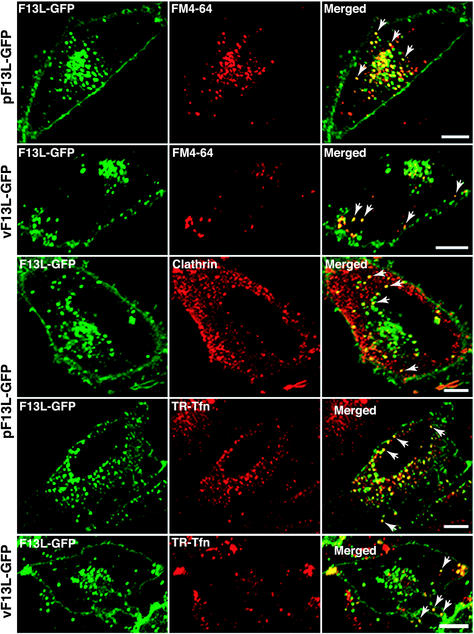
The association of F13L-GFP with early endosomes, demonstrated by staining with an antibody to early endosomal antigen 1 (EEA1), raised the possibility of recycling from the plasma membrane (29). To evaluate this, we used FM4-64, a water-soluble, nontoxic dye that fluoresces after insertion into the outer leaflet of the plasma membrane and becomes an endocytic marker (9). Transfected cells expressing F13L-GFP were first incubated in a medium containing FM4-64 and then fixed and examined by confocal microscopy. Internalized FM4-64 localized in punctate structures, some of which overlapped with F13L-GFP (Fig. 2). Complete colocalization of an endocytic marker with F13L-GFP would not be expected, as the latter is synthesized within the cell and associates with Golgi membranes as well as endosomes. In cells infected with vF13L-GFP, the structures most intensely labeled by FM4-64 were located in the periphery of the cell and contained F13L-GFP (Fig. 2).
FIG. 2.
Localization of F13L-GFP and markers of endocytosis. HeLa cells were transfected with pF13L-GFP or infected with vF13L-GFP. After 24 h of transfection or 18 h of infection, the cells were incubated with FM4-64 or TR-Tfn for 20 min at 37°C. The cells were then fixed and either directly analyzed by confocal microscopy or first permeabilized and stained with an anti-clathrin antibody followed by tetramethyl rhodamine isothiocyanate-conjugated anti-goat IgG. Arrows points to the colocalization of F13L-GFP with FM4-64, clathrin-staining vesicles, or TR-Tfn. Green, GFP; red, rhodamine, Texas red, or FM4-64; yellow, overlap of green and red. Bars, 10 μm.
Because FM4-64 is a membrane marker, it provides no indication of the internalization pathway. Many receptor-ligand complexes accumulate at clathrin-coated pits on the plasma membrane, which bud into the cytoplasm, forming tubules that pinch off and fuse with early endosomes (37, 42). These vesicles may be transported back to the plasma membrane or, via sorting endosomes, to the perinuclear region, where they can fuse with late endosomes. Clathrin-coated vesicles also bud from the trans-Golgi network and fuse with late endosomes. An anti-clathrin antibody stained vesicles of heterogeneous sizes, some of which overlapped with F13L-GFP (Fig. 2).
The internalization of Tfn, which occurs via clathrin-coated pits, provided a more-dynamic marking of the endocytic pathway. After a 20-min incubation in a medium containing TR-Tfn, punctate fluorescence was concentrated in the perinuclear region of transfected (Fig. 2) or untransfected (data not shown) cells. Partial colocalization of F13L-GFP and TR-Tfn was noted (Fig. 2). In cells infected with vF13L-GFP, TR-Tfn staining occurred mostly at the cell periphery, and here too some coincidence of GFP and TR was seen (Fig. 2). Thus, these studies were consistent with the clathrin-mediated recycling of F13L-GFP from the plasma membrane in transfected and infected cells.
Effects of drugs that inhibit clathrin-dependent endocytosis on the localization of F13L-GFP.
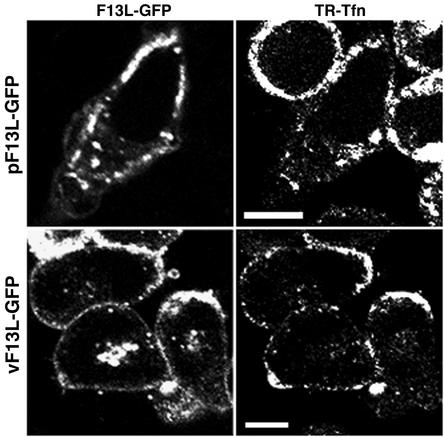
To further investigate the recycling of F13L-GFP, we used drugs that inhibit the clathrin-dependent endocytosis of Tfn in mammalian cells. Chlorpromazine, a cationic amphiphilic compound, induces the redistribution of clathrin-coated components by removing the adaptor protein AP-2 from the plasma membrane (60, 62, 68). Cells transfected with pF13L-GFP were incubated with chlorpromazine and TR-Tfn (Fig. 3). After chlorpromazine treatment, internalization of TR-Tfn was inhibited and a thick region just below the plasma membrane was intensely fluorescent. F13L-GFP also exhibited enhanced fluorescence below the plasma membrane, but with some vesicles in the cytoplasm. Similarly, when cells infected with vF13L-GFP were treated with chlorpromazine, TR-Tfn as well as F13L-GFP accumulated under the cell membrane (Fig. 3). The juxtanuclear fluorescence may represent F13L-GFP associated with Golgi membranes and IEV.
FIG. 3.
Effects of chlorpromazine on the endocytosis of TR-Tfn and intracellular localization of F13L-GFP. HeLa cells were transfected with pF13L-GFP (upper panels) or infected with vF13L-GFP (lower panels). At 24 h after transfection or 18 h after infection, the cells were pretreated with chlorpromazine for 10 min and then incubated with TR-Tfn for 20 min in the continued presence of chlorpromazine. The cells were then fixed and viewed by confocal microscopy. GFP and Texas red fluorescence is shown in the left and right panels, respectively. Bars, 10 μm.
Nystatin, an inhibitor of caveola-dependent endocytosis (50, 51), did not perturb the intracellular distribution of F13L-GFP in transfected cells (data not shown), suggesting that caveolae are not involved in the recycling of this viral protein.
Effects of dominant-negative protein inhibitors of clathrin-mediated endocytosis on the localization of F13L-GFP.
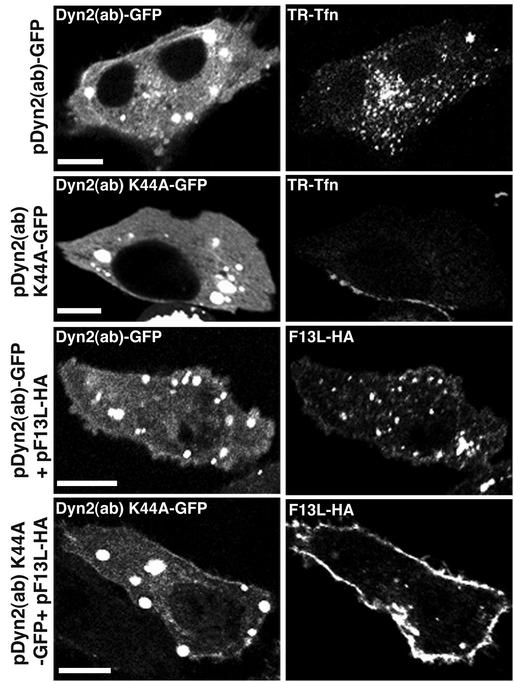
Because drugs can exert multiple effects, we used dominant-negative protein inhibitors of endocytosis to confirm and extend the above findings. Dynamin is a GTPase with an essential role in clathrin-mediated endocytosis (41). A K44A point mutation of Dyn2 (ab), an isoform of dynamin, converts the protein into a dominant-negative inhibitor of clathrin-mediated endocytosis (15, 57). In control experiments, we transfected cells with plasmids expressing either wild-type or mutated Dyn2 (ab) fused to GFP and then incubated the cells with TR-Tfn. TR-Tfn internalization occurred normally in cells expressing wild-type but not mutated dynamin (Fig. 4). In the presence of mutated Dyn2 (ab), TR-Tfn remained associated with the plasma membrane. Similarly, the intracellular distribution of F13L-HA was normal when it was coexpressed with wild-type dynamin, but F13L-HA was mostly associated with the plasma membrane when it was coexpressed with mutated dynamin (Fig. 4).
FIG. 4.
Coexpression of F13L with a trans-dominant-negative dynamin mutant. HeLa cells were transfected or cotransfected with plasmids expressing F13L-HA, functional Dyn 2 (ab)-GFP, or dominant-negative Dyn 2 (ab) K44A-GFP. After 24 h, cells were incubated with TR-Tfn for 20 min and then either fixed and examined by confocal microscopy or first permeabilized and stained with an anti-HA MAb followed by rhodamine red-conjugated anti-mouse IgG. Bars, 10 μm.
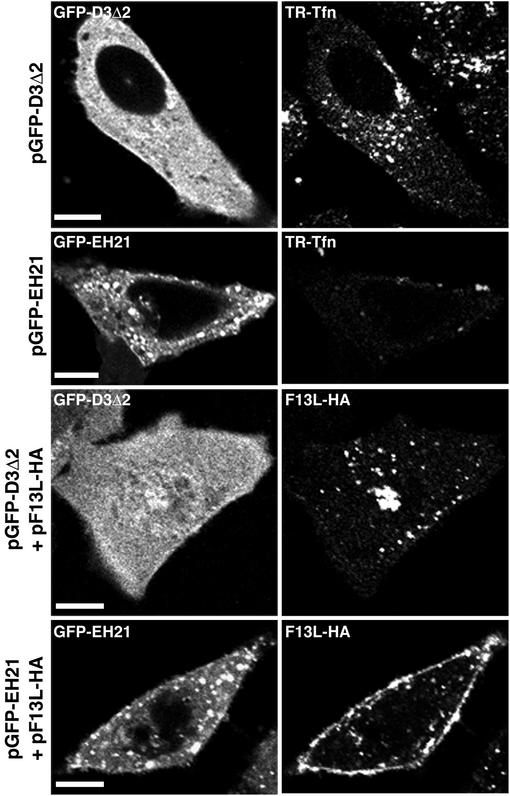
Eps15 is a constituent of clathrin-coated pits that is associated with AP-2 (6, 64). EH21 (EΔ95/295) and D3Δ2 are mutated forms of Eps15; the former acts as a dominant-negative inhibitor of clathrin-mediated endocytosis when overexpressed, whereas the latter has no such effect and is used as a control (5, 64). EH21 causes a severe reduction in the number of coated pits on the plasma membrane and induces a cytoplasmic distribution of clathrin (5). For this reason, we detected very little TR-Tfn associated with cells expressing EH21, whereas the distribution of TR-Tfn was unaffected by the expression of D3Δ2 (Fig. 5). The intracellular distribution of endogenously synthesized F13L-HA was also unaffected by coexpression of D3Δ2, whereas most F13L-HA was associated with the plasma membrane when EH21 was overexpressed (Fig. 5). Taken together, the data obtained with the dominant-negative mutated proteins indicated that the F13L protein recycles from the plasma membrane via clathrin receptors.
FIG. 5.
Coexpression of F13L with a dominant-negative Eps15 mutant. HeLa cells were transfected or cotransfected with plasmids expressing F13L-HA, D3Δ2, or EH21. After 24 h, the cells were incubated with TR-Tfn for 20 min and then either fixed and examined by confocal microscopy or first permeabilized and stained with an anti-HA MAb followed by rhodamine red-conjugated anti-mouse IgG. Bars, 10 μm.
F13L-HA coprecipitated with α-adaptin.
We used coimmunoprecipitation to investigate possible interactions between F13L-HA and proteins associated with clathrin. Lysates from vF13LHAC-infected cells were incubated with a MAb to α-adaptin, γ-adaptin, or clathrin. Immune complexes captured on protein A beads were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an anti-HA polyclonal antibody. The antibody reacted with a band corresponding to F13L-HA that immunoprecipitated with the anti-α-adaptin MAb but not with the anti-clathrin or anti-γ-adaptin MAb, indicating the specificity of the interaction (Fig. 6). α-Adaptin, a component of the AP-2 complex, is required for the targeting of AP-2 to the plasma membrane, where it interacts with Eps15 and plays a major role in the organization and function of clathrin-coated pits (46). Attempts to perform the reverse experiment, coimmunoprecipitation of α-adaptin with F13L-HA using the anti-HA polyclonal antibody followed by Western blotting with the anti-α-adaptin MAb, failed. We attribute this failure to the large excess of the abundant F13L-HA viral protein over α-adaptin as well as to the poor ability of the anti-α-adaptin MAb to work in Western blotting.
FIG. 6.
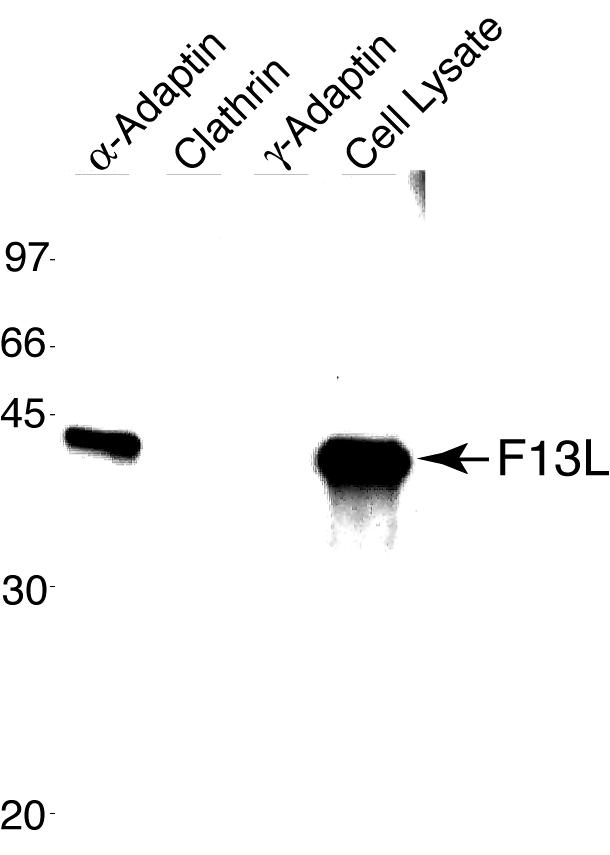
Coimmunoprecipitation of F13L with α-adaptin. BS-C-1 cells infected with vF13LHAC were harvested and lysed. The lysate was incubated with an antibody to α-adaptin, clathrin, or γ-adaptin, and immune complexes were captured on protein A beads, resolved by SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was probed with an anti-HA polyclonal antibody to detect the F13L protein. A sample of the cell lysate was also analyzed directly. The masses of marker proteins (in kilodaltons) are given on the left.
Effects of wortmannin and nocodazole on the intracellular distribution of F13L-GFP.
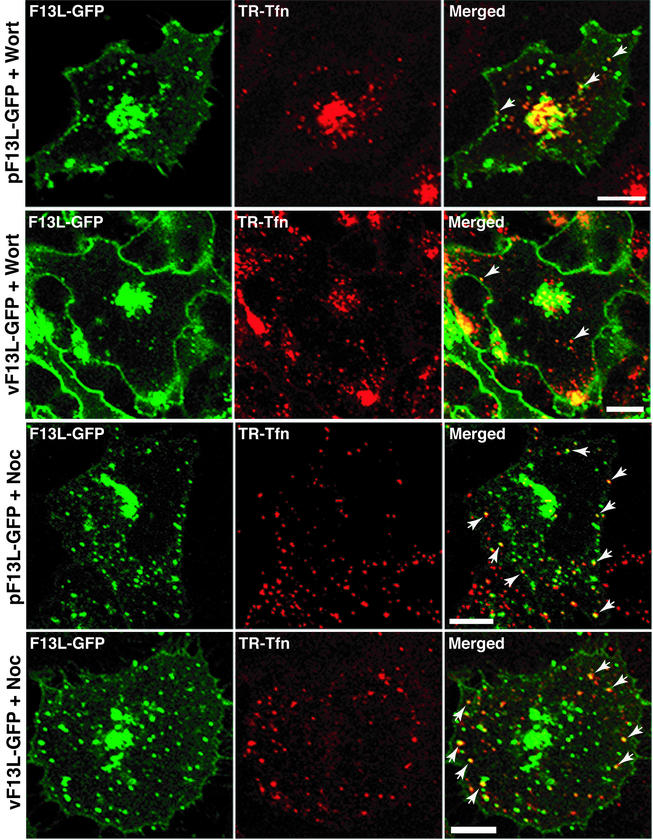
Additional inhibitors were used to perturb the endocytic recycling pathways. Wortmannin, a phosphatidylinositol 3-OH kinase inhibitor, accelerates the rate of internalization of Tfn and slows down endocytic recycling, resulting in the concentration of Tfn in the perinuclear recycling compartment (39). To determine the effect of this drug on the intracellular localization of F13L-GFP, transfected cells were first treated with wortmannin and then incubated with TR-Tfn. As shown in Fig. 7, TR-Tfn was concentrated in the central region of the cell, with relatively little at the periphery, and a similar distribution was found for F13L-GFP. Comparable results were obtained with infected cells except for the masses of F13L-GFP at the periphery, which likely represent IEV (Fig. 7).
FIG. 7.
Effects of wortmannin and nocadazole on the internalization of TR-Tfn and intracellular localization of F13L-GFP. HeLa cells were transfected with pF13L-GFP or infected with vF13L-GFP. At 24 h after transfection or 18 h after infection, the cells were pretreated with wortmannin (Wort) or nocadazole (Noc) for 10 min and then incubated in the continued presence of the drug with TR-Tfn for 20 min. Cells were then fixed and analyzed by confocal microscopy. Arrows point to the overlap of F13L-GFP with TR-Tfn. Green, GFP; red, Texas red; yellow, overlap of green and red. Bars, 10 μm.
Nocodazole, a microtubule-depolymerizing drug, inhibits the maturation of early endosomes to sorting endosomes (33, 36) without affecting the internalization and recycling of proteins to the plasma membrane (32, 53). When cells expressing F13L-GFP were first treated with nocodazole and then incubated with TR-Tfn in the presence of the drug, the punctate fluorescence of TR-Tfn appeared mainly at the periphery of the cell, as would be expected if maturation of early endosomes into sorting endosomes was blocked (Fig. 7). This pattern contrasted with the perinuclear pattern that occurred with wortmannin (Fig. 7). In cells treated with nocodazole, punctate F13L-GFP fluorescence was scattered in the cytoplasm, with some in the juxtanuclear area, possibly representing fragmentation and reorganization of Golgi vesicles caused by the drug, as well as endosomes (Fig. 7). Some of the punctate F13L-GFP fluorescence at the periphery of the cell, however, overlapped with TR-Tfn-stained early endosome vesicles, consistent with recycling from the plasma membrane (Fig. 7). Overlap of F13L-GFP fluorescence with TR-Tfn also occurred in virus-infected cells (Fig. 7).
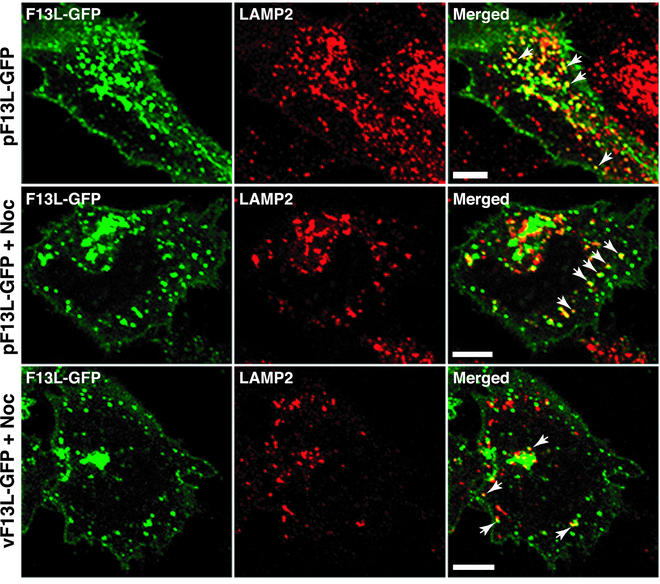
Previously, we showed that F13L-GFP partially colocalized with the LAMP2 protein, a marker for late endosomes and lysosomes (29). At that time, we had mainly considered trafficking from the trans-Golgi network to late endosomes. However, proteins can also associate with late endosomes via the endocytic route (16, 61). Both routes may be used by F13L-GFP, as evidenced by the fact that some punctate F13L-GFP fluorescence overlapped with the LAMP2 late-endosomal/lysosomal marker in transfected and infected cells even in the presence of nocodazole, which blocks the endocytic route (Fig. 8).
FIG. 8.
Effect of nocodazole treatment on the colocalization of F13L-GFP with LAMP2. HeLa cells were transfected with pF13L-GFP or infected with vF13L-GFP. At 24 h after transfection or 18 h after infection, cells were either left untreated or treated with nocodazole (Noc) for 30 min. The cells were then fixed, permeabilized, and stained with an anti-LAMP2 MAb followed by rhodamine red-conjugated anti-mouse IgG. Arrows point to the overlap of F13L-GFP with LAMP2. Green, GFP; red, rhodamine red; yellow, overlap of green and red. Bars, 10 μm.
DISCUSSION
The intracellular trafficking of the F13L protein is of special interest, as the protein is highly conserved among poxviruses and is essential for the wrapping of IMV with trans-Golgi or endosomal membranes to form the IEV (11). The conserved palmitoylation (23, 24, 29) and phospholipase motifs (29, 48) are required for the proper intracellular trafficking of the F13L protein. Previous data have suggested that the F13L protein induces the formation of vesicles that contain other viral envelope proteins and are precursors of the wrapping membranes (28, 29). The F13L protein can also serve as a model for the trafficking of a cytoplasmic, lipid-modified peripheral membrane protein. For example, the F13L protein has palmitoylated residues located in its central domain (24), similar to those of the 25-kDa synaptosome-associated protein (SNAP-25), a t-SNARE that functions in the fusion and exocytosis of secretory vesicles (25). Like the F13L protein, SNAP-25 accumulates in the trans-Golgi network and is transported by vesicles to the plasma membrane (21, 22). The cellular mechanisms involved in palmitoylation are poorly understood (10), and the site at which this occurs may be different for different proteins, since palmitoyltransferases are associated with the plasma membrane as well as with membranes derived from the ER, intermediate compartment, Golgi network, and mitochondria (12, 17, 35, 49, 67). Proteins known to be palmitoylated in the early secretory pathway are integral transmembrane proteins.
To assess a possible requirement that the F13L protein traffic through the ER, we coexpressed a trans-dominant mutated form of the Sar1 protein that is retained in the ER (3) together with F13L or VSVG. Sar1H79G prevented VSVG from exiting the ER but had no effect on the intracellular distribution of the F13L protein, implying that palmitoylation of F13L occurs in a post-ER compartment. Palmitoylation could occur in the Golgi network, as has been suggested for SNAP-25 (22), but this remains to be demonstrated.
Although the importance of endocytosis in virus entry is well known, recent studies suggest additional roles for this process in the virus life cycle (38). Endocytosis may allow the retrieval and reuse of viral membrane proteins; at the same time, it lowers their cell surface expression, which otherwise could enhance the visibility of infected cells to the immune system. Human immunodeficiency virus, simian immunodeficiency virus, herpes simplex virus, varicella-zoster virus, and human cytomegalovirus encode glycoproteins with endocytosis signals which reduce their concentrations on the cell surface (1, 2, 8, 20, 52, 54, 66). The vaccinia virus B5R glycoprotein has endocytosis signals that are not required for virus envelopment but that allow recycling of the protein from the plasma membrane (69). The above proteins all have transmembrane and extracellular domains. Relatively few studies of endocytosis have been carried out with viral or cellular proteins that associate peripherally with the cytoplasmic side of the plasma membranes via a lipid anchor, such as the F13L protein. In this report, we provide the first evidence for the recycling of the F13L protein from the plasma membrane.
Several lines of evidence indicated that endocytosis of the F13L protein from the plasma membrane is clathrin mediated. Thus, the F13L protein showed overlap with vesicles that stained with antibodies to clathrin and to Tfn, which is known to traffic via clathrin-coated pits. Moreover, drugs as well as trans-dominant-negative inhibitors of clathrin-mediated endocytosis prevented recycling of the F13L protein. In addition, the F13L protein coimmunoprecipitated with α-adaptin, a component of the clathrin-associated adaptor complex AP-2. The latter is part of the vesicle coat, which forms at the cytosolic leaflet of the plasma membrane bilayer (46). Since AP-2 is composed of four chains, the immunoprecipitation results do not necessarily indicate that the F13L protein interacts directly with α-adaptin. Inspection of the F13L protein sequence revealed several sites containing dileucine or tyrosine residues that could interact with the adaptor complex. Mutagenesis of these motifs individually, however, did not greatly affect the intracellular distribution of the F13L protein, suggesting redundancy of interaction sites (M. Husain, unpublished data).
Upon internalization from the plasma membrane, most proteins enter sorting endosomes, where they may be delivered to late endosomes and lysosomes or to a separate tubular recycling compartment (42). However, proteins may also enter late endosomes and lysosomes more directly from the trans-Golgi network. The F13L protein may use the latter pathway as well as the recycling pathway, as revealed by the partial colocalization of the F13L protein with LAMP2 even after nocadazole treatment, which is known to inhibit the maturation of early endosomes (33, 36).
Based on previous and present studies of the F13L protein, we propose the following intracellular trafficking scheme. The F13L protein is expressed as a cytoplasmic protein and associates transiently with membranes, probably in the Golgi network, where it becomes palmitoylated by membrane-associated transferases (30). The F13L protein then induces vesicle formation by a mechanism that is dependent on its phospholipase motif (28, 29). The induced vesicles contain additional viral membrane proteins and form cisternae that wrap the IMV. The resulting IEV are transported to the peripheries of the cells, where the outer viral membrane fuses with the plasma membrane. These viral membrane proteins, and additional quantities that may traffic to the plasma membrane by a direct secretory path, would be unavailable for further IEV production unless retrieved. The F13L protein, as well as the cytoplasmic domain of the B5R protein (69), interacts with the clathrin complex, and the associated plasma membrane pinches off to form early endosomal vesicles and tubules. The early endosomes mature into sorting endosomes, which enter the perinuclear recycling compartment, and into vesicles that follow a retrograde path to late endosomes and the trans-Golgi network, a process that appears to be greatly enhanced during vaccinia virus infection (55, 65). Cisternae, containing recycled F13L and other IEV proteins, are used for wrapping IMV to form additional IEV.
Acknowledgments
We thank Owen Schwartz for help and advice with confocal microscopy and Marc McNiven, Alexandre Benmerah, Jennifer Lippincott-Schwartz, and Thomas August for materials.
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aridor, M., S. I. Bannykh, T. Rowe, and W. E. Balch. 1995. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 131:875-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlowe, C., L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M. F. Rexach, M. Ravazzola, M. Amherdt, and R. Schekman. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77:895-907. [DOI] [PubMed] [Google Scholar]
- 5.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 6.Benmerah, A., J. Gagnon, B. Begue, B. Megarbane, A. Dautry-Varsat, and N. Cerf-Bensussan. 1995. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 131:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz, W. J., F. Mao, and C. B. Smith. 1996. Imaging exocytosis and endocytosis. Curr. Opin. Neurobiol. 6:365-371. [DOI] [PubMed] [Google Scholar]
- 10.Bijlmakers, M. J., and M. Marsh. 2003. The on-off story of protein palmitoylation. Trends Cell Biol. 13:32-42. [DOI] [PubMed] [Google Scholar]
- 11.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonatti, S., G. Migliaccio, and K. Simons. 1989. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J. Biol. Chem. 264:12590-12595. [PubMed] [Google Scholar]
- 13.Borrego, B., M. M. Lorenzo, and R. Blasco. 1999. Complementation of P37 (F13L gene) knock-out in vaccinia virus by a cell line expressing the gene constitutively. J. Gen. Virol. 80:425-432. [DOI] [PubMed] [Google Scholar]
- 14.Cao, H., F. Garcia, and M. A. McNiven. 1998. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9:2595-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn, K. W., and F. R. Maxfield. 1992. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J. Cell Biol. 117:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunphy, J. T., W. K. Greentree, C. L. Manahan, and M. E. Linder. 1996. G-protein palmitoyltransferase activity is enriched in plasma membranes. J. Biol. Chem. 271:7154-7159. [DOI] [PubMed] [Google Scholar]
- 18.Engelstad, M., S. T. Howard, and G. L. Smith. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801-810. [DOI] [PubMed] [Google Scholar]
- 19.Engelstad, M., and G. L. Smith. 1993. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology 194:627-637. [DOI] [PubMed] [Google Scholar]
- 20.Fraile-Ramos, A., T. N. Kledal, A. Pelchen-Matthews, K. Bowers, T. W. Schwartz, and M. Marsh. 2001. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 12:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalo, S., W. K. Greentree, and M. E. Linder. 1999. SNAP-25 is targeted to the plasma membrane through a novel membrane-binding domain. J. Biol. Chem. 274:21313-21318. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalo, S., and M. E. Linder. 1998. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol. Biol. Cell 9:585-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosenbach, D. W., and D. E. Hruby. 1998. Analysis of a vaccinia virus mutant expressing a nonpalmitylated form of p37, a mediator of virion envelopment. J. Virol. 72:5108-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosenbach, D. W., D. O. Ulaeto, and D. E. Hruby. 1997. Palmitylation of the vaccinia virus 37-kDa major envelope antigen. Identification of a conserved acceptor motif and biological relevance. J. Biol. Chem. 272:1956-1964. [DOI] [PubMed] [Google Scholar]
- 25.Hess, D. T., T. M. Slater, M. C. Wilson, and J. H. Skene. 1992. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J. Neurosci. 12:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiller, G., and K. Weber. 1985. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 55:651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirt, P., G. Hiller, and R. Wittek. 1986. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J. Virol. 58:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husain, M., and B. Moss. 2002. Similarities in the induction of post-Golgi vesicles by vaccinia virus F13L protein and phospholipase D. J. Virol. 76:7777-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husain, M., and B. Moss. 2001. Vaccinia virus F13L protein with a conserved phospholipase catalytic motif induces colocalization of the B5R envelope glycoprotein in post-Golgi vesicles. J. Virol. 75:7528-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain, M., A. Weisberg, and B. Moss. 2003. Topology of epitope-tagged F13L protein, a major membrane component of extracellular vaccinia virions. Virology 308:233-242. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 66:7217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin, M., and M. D. Snider. 1993. Role of microtubules in transferrin receptor transport from the cell surface to endosomes and the Golgi complex. J. Biol. Chem. 268:18390-18397. [PubMed] [Google Scholar]
- 33.Kok, J. W., K. Hoekstra, S. Eskelinen, and D. Hoekstra. 1992. Recycling pathways of glucosylceramide in BHK cells: distinct involvement of early and late endosomes. J. Cell Sci. 103:1139-1152. [DOI] [PubMed] [Google Scholar]
- 34.Koonin, E. V. 1996. A duplicated catalytic motif in a new superfamily of phosphohydrolases and phospholipid synthases that includes poxvirus envelope proteins. Trends Biochem. Sci. 21:242-243. [PubMed] [Google Scholar]
- 35.Lobo, S., W. K. Greentree, M. E. Linder, and R. J. Deschenes. 2002. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277:41268-41273. [DOI] [PubMed] [Google Scholar]
- 36.Mallet, W. G., and F. R. Maxfield. 1999. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146:345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh, M., and H. T. McMahon. 2000. The structural era of endocytosis. Science 285:215-228. [DOI] [PubMed] [Google Scholar]
- 38.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic 1:525-532. [DOI] [PubMed] [Google Scholar]
- 39.Martys, J. L., C. Wjasow, D. M. Gangi, M. C. Kielian, T. E. McGraw, and J. M. Backer. 1996. Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. Differential effects on endocytosis and lysosomal sorting. J. Biol. Chem. 271:10953-10962. [DOI] [PubMed] [Google Scholar]
- 40.Matsuoka, K., L. Orci, M. Amherdt, S. Y. Bednarek, S. Hamamoto, R. Schekman, and T. Yeung. 1998. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93:263-275. [DOI] [PubMed] [Google Scholar]
- 41.McNiven, M. A. 1998. Dynamin: a molecular motor with pinchase action. Cell 94:151-154. [DOI] [PubMed] [Google Scholar]
- 42.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell. Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 43.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 44.Moss, B., and B. M. Ward. 2001. High-speed mass transit for poxviruses on microtubules. Nat. Cell Biol. 3:E245-E246. [DOI] [PubMed] [Google Scholar]
- 45.Payne, L. 1978. Polypeptide composition of extracellular enveloped vaccinia virus. J. Virol. 27:28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson, M. S. 1994. The role of clathrin, adaptors and dynamin in endocytosis. Curr. Opin. Cell Biol. 6:538-544. [DOI] [PubMed] [Google Scholar]
- 47.Roos, N., M. Cyrklaff, S. Cudmore, R. Blasco, J. Krijnse-Locker, and G. Griffiths. 1996. A novel immunogold cryoelectron microscopic approach to investigate the structure of the intracellular and extracellular forms of vaccinia virus. EMBO J. 15:2343-2355. [PMC free article] [PubMed] [Google Scholar]
- 48.Roper, R. L., and B. Moss. 1999. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J. Virol. 73:1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth, A. F., Y. Feng, L. Chen, and N. G. Davis. 2002. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothberg, K. G., J. E. Heuser, W. C. Donzell, Y. S. Ying, J. R. Glenney, and R. G. Anderson. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68:673-682. [DOI] [PubMed] [Google Scholar]
- 51.Rothberg, K. G., Y. S. Ying, J. F. Kolhouse, B. A. Kamen, and R. G. Anderson. 1990. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J. Cell Biol. 110:637-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 53.Sakai, T., S. Yamashina, and S. Ohnishi. 1991. Microtubule-disrupting drugs blocked delivery of endocytosed transferrin to the cytocenter, but did not affect return of transferrin to plasma membrane. J. Biochem. (Tokyo) 109:528-533. [DOI] [PubMed] [Google Scholar]
- 54.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmutz, C., L. Rindisbacher, M. C. Galmiche, and R. Wittek. 1995. Biochemical analysis of the major vaccinia virus envelope antigen. Virology 213:19-27. [DOI] [PubMed] [Google Scholar]
- 57.Sever, S., H. Damke, and S. L. Schmid. 2000. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 59.Sodeik, B., and J. Krijnse-Locker. 2002. Assembly of vaccinia virus revisited: de novo membrane synthesis or acquisition from the host? Trends Microbiol. 10:15-24. [DOI] [PubMed] [Google Scholar]
- 60.Sofer, A., and A. H. Futerman. 1995. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J. Biol. Chem. 270:12117-12122. [DOI] [PubMed] [Google Scholar]
- 61.Stoorvogel, W., G. J. Strous, H. J. Geuze, V. Oorschot, and A. L. Schwartz. 1991. Late endosomes derive from early endosomes by maturation. Cell 65:417-427. [DOI] [PubMed] [Google Scholar]
- 62.Subtil, A., A. Hemar, and A. Dautry-Varsat. 1994. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J. Cell Sci. 107:3461-3468. [DOI] [PubMed] [Google Scholar]
- 63.Sung, T.-C., R. L. Roper, Y. Zhang, S. A. Rudge, R. Temel, S. M. Hammond, A. J. Morris, B. Moss, J. Engebrecht, and M. A. Frohman. 1997. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 16:4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tebar, F., T. Sorkina, A. Sorkin, M. Ericsson, and T. Kirchhausen. 1996. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271:28727-28730. [DOI] [PubMed] [Google Scholar]
- 65.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 66.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veit, M., and M. F. Schmidt. 1993. Timing of palmitoylation of influenza virus hemagglutinin. FEBS Lett. 336:243-247. [DOI] [PubMed] [Google Scholar]
- 68.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 74:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolffe, E. J., S. N. Isaacs, and B. Moss. 1993. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J. Virol. 67:4732-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]