Abstract
Hepatitis B immunoglobulin is used for prophylaxis against hepatitis B virus (HBV) and is thought to act by neutralization of virions and hepatitis B virus surface antigen (HBsAg)-containing particles in circulation. Using a panel of hepatocyte-derived cell lines, the present study investigated in vitro whether HBs-specific immunoglobulin G (IgG) is internalized in hepatocytes and whether it interacts with HBsAg in the cells. By immunoelectron microscopy and immunoblotting, human IgG and FcRn receptor for IgG were demonstrated on cellular membranes and in cytoplasmic extracts, irrespective of the HBsAg status of the cells. Furthermore, HBsAg and anti-HBs were shown to be colocalized in the same cellular compartment by two-color confocal microscopy. Endocytosis of HBs-specific IgG caused intracellular accumulation of HBsAg in a dose-dependent manner and inhibited the secretion of HBsAg and HBV virions from the cells. These effects were not observed with F(ab)2 fragments or nonimmune IgG as controls. The specificity of intracellular HBsAg- anti-HBs interaction was further investigated in cells transfected with HBV genomes expressing wild-type HBsAg or immune escape HBsAg (with a G145R mutation). Monoclonal anti-HBs markedly reduced the secretion of wild-type HBsAg, while the secretion of mutant HBsAg was not affected. These results suggest that HBs-specific IgG binds to hepatocytes and interacts with HBsAg within the cells. This may be relevant for the selection of surface antibody escape mutations.
Hepatitis B immunoglobulin (HBIG) is used clinically as passive immunoprophylaxis for accidental exposure to hepatitis B virus (HBV) and long term to prevent HBV recurrence in the graft after liver transplantation (24). It contains high-titer antibodies against HBV surface antigen (HBsAg), which is the major component of the outer envelope of the 42-nm-diameter hepatitis B virion, as well as the 22-nm-diameter subviral particles. The therapeutic effect of HBIG is believed to be due to high-affinity binding with HBs-containing particles and neutralization of HBV in the circulation.
Despite HBIG prophylaxis, HBV infection recurs in 30% of patients who receive transplants for HBsAg-positive cirrhosis (24). The failure of immunoprophylaxis is due either to a high HBV load and inadequate neutralization by HBIG or to the emergence of antibody-induced escape HBV mutants (3, 6, 25). These mutant HBV strains contain amino acid substitutions within the conserved a-determinant (a group-reactive region between amino acids 124 and 149 of HBsAg), which abrogate the binding affinity of anti-HBs (4, 5, 22, 27). The most frequent mutation occurs at codon 145 of the surface open reading frame, leading to a glycine (G)-to-arginine (R) substitution—G145R—which has been shown to emerge both in liver transplant patients receiving HBIG prophylaxis and in HBV vaccine recipients (2, 6, 22). The mechanism for the emergence of HBsAg mutations that escape antibody recognition has not been defined.
Earlier studies have demonstrated the presence of membrane-bound and/or nuclear localization of immunoglobulin G (IgG) in hepatocytes of patients with chronic HBV infection, who express HBV core antigen or hepatitis delta virus antigen in the liver (17, 19, 23). Recently, a novel Fc receptor for IgG (FcRn) which mediates the transcytosis of IgG from serum to bile and protects the internalized IgG from catabolism has been identified on the plasma membranes of adult rat hepatocytes (1, 9). FcRn is a heterodimer of β2-microglobulin light chain and a major histocompatibility complex class I-like heavy chain that binds IgG via Fc residues in a pH-dependent manner. IgG binding to FcRn is followed by endocytosis of the complex in the acidic endosome environment, trafficking through cellular conduits to bypass lysosomal activities and finally releasing IgG in the extracellular fluids (7). Whether hepatitis B immunoglobulin enters HBV-infected hepatocytes and whether an interaction with HBsAg occurs within cells, in addition to the interaction in serum, have not been investigated.
In the present study, we investigated the hypothesis that HBIG is able to bind to hepatocytes and affect the secretion of HBsAg and HBV virions from the cells. For this purpose, we used a panel of human hepatocyte-derived cell lines cultured together with monoclonal and polyclonal HBs-specific antibodies. The results demonstrate that anti-HBs IgG is internalized in the cells irrespective of the presence or absence of HBsAg expression. In HBV-positive cells, HBsAg and anti-HBs were colocalized in the same compartment, and the amount of intracellular HBsAg in cells cultured with human anti-HBs IgG was increased in a dose-dependent manner. The specificity of the antigen-antibody interaction within hepatocytes was further investigated in cells transfected with replication-competent HBV genomes. This revealed that monoclonal HBs-specific IgG markedly reduced the secretion of wild-type HBsAg, whereas the secretion of HBsAg with a G145R mutation, which abrogates the binding of anti-HBs antibodies, was not affected. These findings suggest that antiviral antibodies against HBsAg exert intracellular selection pressure, which may represent a mechanism for the emergence of immune escape HBV mutants.
MATERIALS AND METHODS
Human hepatoma cell lines.
Several human hepatocyte-derived cell lines were used in this study: HuH-7 (HBV negative), PLC/PRF/5 (HBV positive, producing HBsAg only), and HepG2.2.15 (HBV positive, supporting full HBV replication). We also used another HepG2 cell line (HepAD38) supporting full HBV replication which is stably transfected with a wild-type HBV construct under the control of the tetracycline (Tet)-responsive promoter (12). The cells were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium-F12 (GIBCO BRL, Glasgow, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO BRL) in the absence or presence of different concentrations of antibodies against HBsAg.
Immunoglobulins for cell culture experiments.
Two antibodies against epitopes within the a-determinant of HBsAg were used: humanized monoclonal anti-HBs of the IgG1 subclass (BM 80.1003; Boehringer Mannheim, Mannheim, Germany) and human polyclonal anti-HBs IgG (HBIG; BioProducts Laboratory, Elstree, United Kingdom). The protein concentration of HBIG is 100 g/liter, and it contains at least 90% human IgG. Liver transplant patients normally receive 10 vials of HBIG (5 ml each) intravenously, equivalent to 5,000 IU of anti-HBs. Thus, the estimated HBIG concentration in the bloodstream of the recipient is ∼1.0 mg/ml. This concentration was used for guidance in choosing the final concentration of human IgG in our in vitro experiments. The cells were cultured with and without anti-HBs for 3 days, and the experimental design included the following conditions: (i) cells cultured in medium with 10% FCS (control), (ii) cells cultured in medium with 10% FCS plus monoclonal anti-HBs IgG (at concentrations between 0.1 and 2.0 mg/ml), and (iii) cells cultured in medium with 10% FCS plus human polyclonal anti-HBs IgG (concentrations between 0.1 and 2.0 mg/ml).
As specificity controls, we used human IgG with anti-HBc reactivity and nonimmune human IgG. Total IgG was extracted from a serum sample from a patient with chronic HBV infection (HBeAg positive) with a high titer of anti-HBc but no anti-HBs. After overnight dialysis in 10 mM phosphate buffer at 4°C, the serum sample was layered on top of a DE52 column and absorbed over 30 min. By elution with 20 mM phosphate buffer, only a single peak at 280-nm optical density, corresponding to the IgG fraction, was recovered and adjusted to a concentration of 1.0 mg/ml. As a further control, cells were cultured in the presence of 5% human AB serum (ABS; Gemini Bioproducts, Calabasas, Calif.), which contains ∼1.0 mg of nonimmune human IgG/ml. In addition, to test the binding of HBs-specific IgG, we prepared F(ab)2 fragments from HBIG. For this purpose, 20 mg of HBIG was digested with 1 mg of pepsin (Sigma Chemicals, Dorset, United Kingdom) in 500 μl of 100 mM sodium citrate, pH 3.5, for 18 h at 37°C. The reaction was stopped with 50 μl of 3 M Tris buffer, pH 8.8, and the solution was loaded onto a HiTrap protein G affinity column (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) to remove Fc fragments and undigested IgG molecules. The column was washed with 20 mM sodium phosphate, pH 7.0, to collect HBs-specific F(ab)2 fragments and was used at a final concentration of 1.0 mg/ml in cell cultures.
HBV DNA transfection of HuH-7 cells.
For transfection experiments, we used two plasmids containing a pBlueScript KS(+) vector with a 1.5 HBV genomes of subtype adw2. These replication-competent HBV genomes differ only in a single nucleotide and express wild-type HBsAg or naturally occurring mutant HBsAg, which contains a Gly-to-Arg substitution at position 145 within the a-determinant of HBsAg (27). Subconfluent HuH-7 cells were transfected with 5 μg of plasmid DNA per 60-mm-diameter dish using 10 μl of Superfect reagent (Qiagen, Crawley, United Kingdom). The supernatants were replaced 18 h posttransfection with medium supplemented with 10% FCS, and the cells were cultured for 3 days.
Immunocytochemistry for detection of IgG and FcRn.
HuH-7 cells were cultured on coverslips in the presence or absence of 1.0 mg of monoclonal or polyclonal anti-HBs IgG/ml. The coverslips were washed three times with phosphate-buffered saline (PBS). The cells were fixed for 5 min in ice-cold acetone, and the endogenous peroxidase activity was blocked for 15 min in methanol- 1% H2O2. The fixed cells were analyzed for the presence of IgG using rabbit anti-human IgG (diluted 1:100; Dako, Cambridge, United Kingdom) and for FcRn using mouse antiserum against human FcRn (diluted 1:50), generously donated by Richard Blumberg (Brigham and Women's Hospital, Harvard Medical School, Boston, Mass.). After a 30-min incubation, the cells were washed three times in PBS- 0.5% Tween 20, followed by incubation with a secondary antibody—horseradish peroxidase (HRPO)-labeled swine anti-rabbit or rabbit anti-mouse immunoglobulin (Dako, Ely, United Kingdom). After three washes in PBS- 0.5% Tween 20, the cells were stained using 0.03% 3′,3′-diaminobenzidine tetrahydrochloride (Sigma Chemicals) as a substrate for HRPO enzymatic activity and counterstained with hematoxylin (Sigma Chemicals).
Immunoelectron microscopy (IEM) for detection of IgG and FcRn.
HuH-7 cells were cultured for 3 days in the presence or absence of HBs-specific IgG on coverslips precoated with 0.5% gelatin- 0.05% chromium-alum. At the end of the culture period, the cells were washed three times in ice-cold PBS and fixed with 4% paraformaldehyde- 0.08% glutaraldehyde for 10 min at 4°C. As primary antibodies for the detection of human IgG and FcRn, we used rabbit anti-human IgG (diluted 1:500; Dako) and mouse antiserum against human FcRn at a dilution of 1:50 (29). As a second antibody, anti-rabbit IgG-biotin or anti-mouse IgG-biotin (both from Dako) was used. Finally, avidin-biotin-peroxidase complex (diluted 1:250; Vector, Burlingame, Calif.) was used. The cells were then fixed with 2.5% glutaraldehyde in PBS for 10 min and thoroughly washed, and the peroxidase activity was visualized with a solution containing 5 mg of 3′,3′-diaminobenzidine tetrahydrochloride and 60 μl of 1% hydrogen peroxide in 25 ml of 0.05 M Tris-HCl, pH 7.6. After treatment with 0.1% OsO4, the cells were embedded in Durcopan and ultrathin sections were examined noncontrasted with an EM300 microscope (Philips Medical Systems, Surrey, United Kingdom). As negative controls, the cells were incubated with PBS instead of the primary antibodies.
IFA.
HepAD38 cells grown on glass coverslips were maintained in culture medium in the presence (Tet-on) or absence (Tet-off) of Tet; HBV expression was detected in the cells grown under Tet-off conditions. From day 3 of culture, the cells were mock treated or treated daily with 0.5 mg of HBIG/ml under Tet-on or Tet-off conditions. On day 12, the cells were fixed with absolute methanol for 5 min at room temperature (RT). The fixed cells were processed for single-labeled or dual-labeled immunofluorescence assay (IFA) as described previously (13). For dual-labeled IFA, the cells were incubated for 30 min at 37°C with or without mouse monoclonal antibody to HBV pre-S2 antigen (Abcam Ltd., Cambridge, United Kingdom) diluted 1/100 in PBS. After several washes in PBS, the cells were reacted for 30 min at 37°C with a combination of a 1/200 dilution of goat Texas Red-X-conjugated anti-mouse immunoglobulin (Molecular Probes, Eugene, Oreg.) and a 1/40 dilution of rabbit fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin (Dako Corp., Carpenteria, Calif.). For single-labeled IFA, fixed cells were reacted with rabbit polyclonal antibody to HBV core protein (Dako Corp.) or anti-HBV pre-S2 antigen followed by reactivity to the appropriate fluoroprobe-conjugated secondary antibodies. Coverslip preparations were mounted in fluorescent mounting medium (Dako Corp.) and viewed with a Bio-Rad (Hemel Hempstead, United Kingdom) MRC 1024 laser confocal system attached to a Zeiss microscope. Image collection parameters were adjusted to minimize cross-channel leak over and tested using appropriate single- and dual-labeled preparations (13).
Immunoblot analysis for detection of IgG, HBsAg, and FcRn.
HuH-7 or PLC/PRF/5 cells were cultured in the absence or presence of monoclonal or polyclonal anti-HBs IgG, as outlined above. After 3 days, the cells were harvested using ice-cold PBS and 0.1% EDTA, washed three times in PBS, and resuspended in 100 μl of lysis buffer (1% NP-40, 80 mM Tris [pH 8.0], 150 mM NaCl, 10 mM EDTA). The cell nuclei were removed from the lysates by centrifugation at 1,200 × g for 5 min. The remaining cytoplasmic extracts were precipitated with 10× [vol/vol] acetone for 2 h at −20°C and centrifuged at 15,000 × g for 10 min. The air-dried precipitates were resuspended in 2× sodium dodecyl sulfate (SDS) buffer (160 mM Tris-HCl [pH 6.8], 4% SDS, 200 mM dithiothreitol), denatured for 5 min at 95°C, chilled on ice, and separated through 17.5% SDS-polyacrylamide gel electrophoresis. For immunoblot analysis, the proteins were transferred for 1 h onto a nitrocellulose membrane (ECL Hybond; Amersham) using semidry Transblot SD (Bio-Rad). The membrane was blocked overnight at 4°C in PBS plus 5% fat-free milk powder (MP) and incubated for 1 h at RT with rabbit anti-human IgG (Dako) diluted 1:5,000 in PBS plus 1% MP. The detection of human Fc receptor FcRn was performed for 1 h at RT using mouse antiserum against human FcRn diluted 1:100 in PBS plus 1% MP. For the detection of HBsAg, the membrane was incubated for 1 h at RT with the mouse anti-HBs antibody P2D3 diluted 1:250 in PBS plus 1% MP. The P2D3 antibody is known to react with both wild-type and mutant G145R HBsAg (8). After three washes in PBS- 1% Tween 20, the blot was incubated for 30 min at RT with HRPO-labeled rabbit anti-mouse IgG or swine anti-rabbit IgG (Dako) diluted 1:10,000 in PBS plus 1% MP. The immunoblot was washed three times in PBS- 1% Tween 20, and chemiluminiscence detection was performed using SuperSignal Ultra (Pierce, Rockford, Ill.) as a substrate for the HRPO enzymatic activity. The blots were exposed to an X-ray film or analyzed with the Fluor-S MultiImager (Bio-Rad). Using the imager, the chemiluminescence signal from IgG in the cytoplasmic extracts was quantitated in arbitrary units and compared to the signal obtained from standard amounts of anti-HBs IgG in the blot. This allowed an assessment of the amounts of IgG present in the cytoplasmic fractions of cells cultured under different conditions.
Immunoassay for detection of secreted HBsAg.
For the detection of HBsAg in cell culture supernatants, we used a radioimmunoassay (RIA). Aliquots of the culture supernatants (100 μl) were added in duplicate to each well of round-bottom plates which had been coated with a polyclonal goat antibody against HBsAg (Murex Biotech, Dartford, United Kingdom). The plates were washed five times with TS buffer (100 mM NaCl, 0.5% Tween 20) and incubated at RT for 4 h with 100 μl of 125I-labeled monoclonal mouse anti-HBs P2D3 (50 nCi diluted in 20 mM Tris buffer containing 2% bovine serum albumin and 20% normal human serum). The epitope recognized by P2D3 was mapped between amino acid positions 121 and 135 of HBsAg, and the antibody was previously shown to react with wild-type and G145R mutant protein (8). The wells were washed twice with TS buffer, and the radioactivity was monitored in a Gammacounter (NE 1600; Nuclear Enterprises, Edinburgh, Scotland). The HBsAg levels were quantitated in nanograms per milliliter based on the testing of 8 to 10 standard amounts of HBsAg between 10 and 2,000 ng, which were included in each run of the assay.
To test whether the presence of HBsAg-anti-HBs complexes interferes with the detection of HBsAg, several concentrations (0.05, 0.1, 0.5, and 1.0 mg/ml) of monoclonal anti-HBs were incubated for 24 h at 37°C with supernatants from PLC/PRF/5 cells. After this period, the HBsAg levels in the supernatants were analyzed by RIA (using anti-HBs P2D3). The results showed that the detection of HBsAg in supernatants containing anti-HBs was not different from that of HBsAg in the supernatant from PLC/PRF/5 cells, which were cultured in parallel without anti-HBs. This control experiment confirmed that the formation of HBsAg- anti-HBs complexes does not hinder the detection of HBsAg by P2D3 antibody.
Southern blot hybridization for detection of HBV replicative intermediates.
HepG2.2.15 cells were cultured in the presence or absence of monoclonal or polyclonal anti-HBs. The cells were harvested and washed three times with ice-cold PBS- 0.1% EDTA. The cell sediments were resuspended in 100 μl of lysis buffer (see above) and incubated at 4°C for 30 min. The nuclei were removed from the cell lysates by centrifugation at 1,200 × g for 5 min. For DNA extraction, the cytoplasmic fractions were incubated with 0.5 mg of proteinase K/ml and 0.5% SDS for 2 h at 56°C, extracted with phenol-chloroforn (1:1), and precipitated by 2× [vol/vol] ethanol with 2 μg of tRNA as a carrier. Nucleic acids were resolved by 1.5% agarose gel electrophoresis and transferred onto nylon membranes (Hybond; Amersham) by overnight capillary transfer. The membrane was UV cross-linked and hybridized for 18 h at 37°C in digoxigenin (DIG) Easy-Hyb (Roche Diagnostics, Lewes, United Kingdom) containing 25 ng of denatured DIG-labeled HBV DNA/ml. A full-length HBV probe was produced by PCR with incorporation of dUTP-DIG (Roche Diagnostics), using primers (5′-CCG GAA AGC TTC TTT TTC ACC TCT GCC TAA TCA-3′, nucleotides 1821 to 1841, and 5′-CCG GAA AGC TTG AGC TCT TCA AAA AGT ATG GTG CTG G-3′, nucleotides 1806 to 1823). For the detection of HBV DNA, we followed the DIG chemiluminescence protocol using alkaline phosphatase-conjugated anti-DIG Fab fragments (Roche Diagnostics) and, as a substrate, CDP* (Roche Diagnostics). The blot was exposed to X-ray film or the signals were quantitatively analyzed in a Fluor-S MultiImager.
Real-time PCR for HBV DNA quantification in cell culture supernatants.
DNA was extracted from 200-μl supernatants of HepG2.2.15 cells cultured in the presence or absence of human anti-HBs IgG. The culture supernatants were incubated with 100 μg of DNase I (Roche Diagnostics) for 2 h at 37°C, and the virion DNA was extracted using the QIAamp-DNA blood minikit (Qiagen). The amplification and detection of HBV DNA were performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Warrington, United Kingdom), as described previously (21). Briefly, the 50-μl reaction mixture contained 2× TaqMan Universal MasterMix (Applied Biosystems), 45 pmol each of forward primer (5′-GGA CCC CTG CTC GTG TTA CA-3′, nucleotides 184 to 203) and reverse primer (5′-GAG AGA AGT CCA CCM CGA GTC TAG A-3′, nucleotides 273 to 249), 15 pmol of the probe (5′-FAM-TGT TGA CAA RAA TCC TCA CCA TAC CRC AGA-TAMRA-3′, nucleotides 218 to 247), and, as a template, 10 μl of DNA extracted from culture supernatants. For quantification, serial dilutions of the HBV plasmid were included in each run.
RESULTS
Detection of human IgG in hepatocyte-derived cell lines.
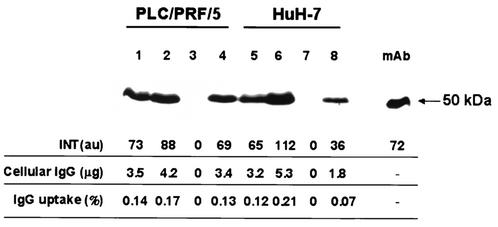
The immunoblot analysis of PLC/PRF/5 and HuH-7 cells, cultured in medium supplemented with 10% FCS plus 1.0 mg of monoclonal antibody against HBsAg/ml, detected the presence of human IgG in cytoplasmic extracts from both cell lines (Fig. 1). A positive signal for IgG was also detected in cells cultured with medium supplemented with human AB serum as a source of nonimmune IgG. Thus, the positive signal for IgG associated with the cells was not dependent on the HBsAg status of the cell lines or on the use of immune or nonimmune IgG.
FIG. 1.
Immunoblot of human IgG in cytoplasmic extracts from PLC/PRF/5 cells (lanes 1 to 4) and HuH-7 cells (lanes 5 to 8). The cells were cultured with medium containing human AB serum (lanes 1 and 5), human AB serum plus 1.0 mg of monoclonal anti-HBs IgG/ml (lanes 2 and 6), medium with FCS alone (lanes 3 and 7), or 1.0 mg of monoclonal anti-HBs IgG/ml (lanes 4 and 8). As a standard, monoclonal anti-HBs IgG (mAb) was included in the blot. INT, intensity of the chemiluminescence signal measured by FluorS MultiImager in arbitrary units (au). Cellular IgG, estimated amount of IgG associated with cells; IgG uptake, relative amount of IgG associated with the cells as a percentage of the IgG in culture supernatants. The amount of the latter was calculated on the basis of the known IgG concentration (1.0 mg/ml) and the known volume of medium (2.5 ml) in each well, i.e., 2,500 μg of IgG.
We assessed the relative amounts of IgG present in the cytoplamsic extracts as percentages of the IgG in the culture supernatants. For this purpose, the amount of human IgG in cytoplasmic extracts was evaluated semiquantitatively using the Fluor-S MultiImager, while the IgG present in the culture supernatants was calculated on the basis of the known IgG concentration (1.0 mg/ml) and the volume of medium (2.5 ml) added to each well of the tissue culture plate, i.e., 2,500 μg. In three separate experiments the amount of anti-HBs IgG associated with the cells, in relation to the amount of IgG present in the culture supernatants, varied between 0.13 and 0.16% for PLC/PRF/5 and between 0.07 and 0.15% for HuH-7 cells. The relative amounts of IgG in the cytoplasmic extracts of cells cultured with the human AB serum were similar—between 0.09 and 0.17% for PLC/PRF/5 and between 0.12 and 0.15% for HuH-7 cells.
Cellular localization of human IgG and FcRn.
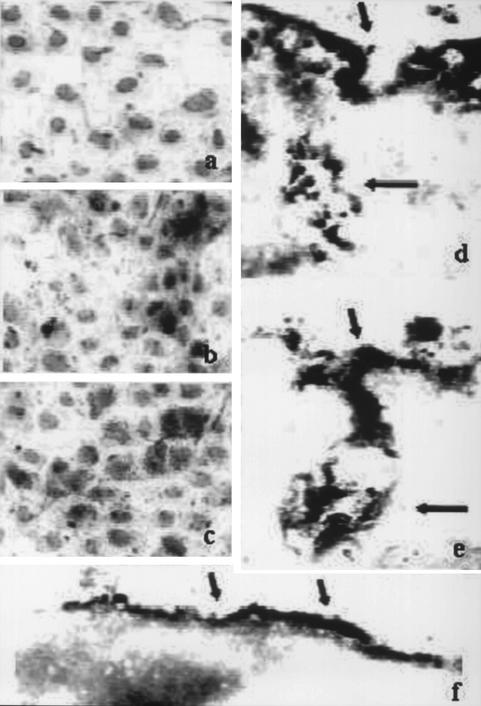
To gain information on the cellular localization of human IgG, HuH-7 cells were cultured in the presence of monoclonal or polyclonal anti-HBs IgG. This was followed by immunostaining with antibodies to IgG and FcRn, and the cells were analyzed by light microscopy or by IEM. The immunostaining detected IgG on the surface and/or in the cytoplasm of cells cultured with either monoclonal or polyclonal anti-HBs but not in the control (Fig. 2a and b). The staining for FcRn revealed cellular localization similar to that for IgG, on the cell surface and in the cytoplasm (Fig. 2c).
FIG. 2.
Detection of human IgG and FcRn receptor by immunocytochemistry and light microscopy (a to c; magnification, ×65,000) or by IEM (d to f; magnification, ×72,000). (a, b, d, and e) Detection of IgG in HuH-7 cells cultured in the absence of human IgG (a), in the presence of 1.0 mg of polyclonal anti-HBs IgG/ml (b and e), or in medium containing human AB serum (d). (c and f) Detection of human FcRn. Hematoxylin staining of nuclei (light grey) and peroxidase staining (black) are shown. The arrows (the short arrows indicate the cellular membrane; the long arrows indicate membranous invaginations) indicate the positive signals for IgG or FcRn.
By IEM, a positive signal for human IgG was detected on the plasma membranes of cells cultured with either nonimmune IgG or HBs-specific IgG (Fig. 2d and e). In addition, a specific signal for IgG was also detected in membranous subsurface invaginations within the cells. In parallel, we analyzed by IEM the presence of the human FcRn receptor in the same cells. Immunoreactivity for FcRn was observed on the plasma membrane (Fig. 2f) and also in some intracellular invaginations, but not in the cytosol.
Colocalization of HBIG and HBV pre-S2 protein.
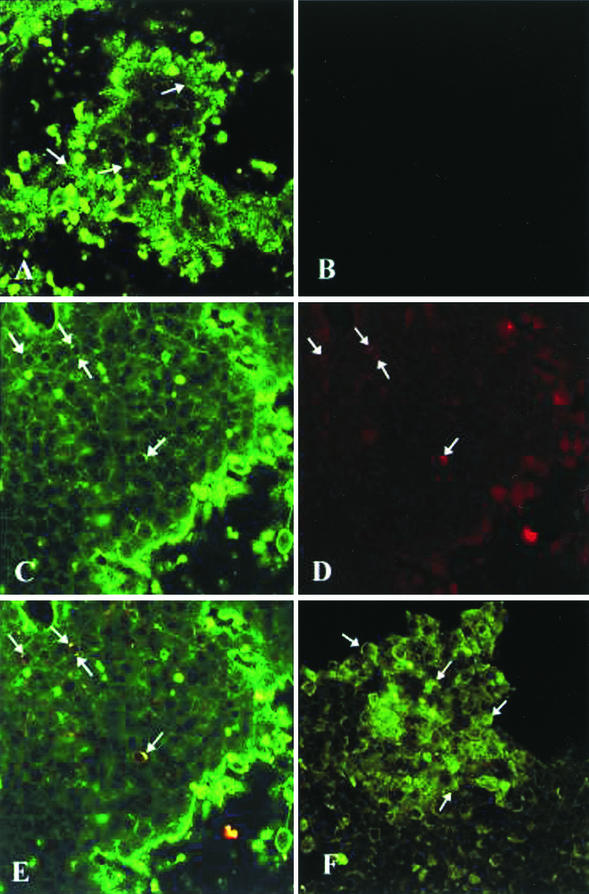
To determine the localization of HBIG in relation to HBsAg in HepAD38 cells under Tet-off conditions, the cells were processed for dual-labeled IFA using monoclonal antibodies to pre-S2 and the respective Texas Red- and FITC-conjugated secondary antibodies. Confocal microscopy analysis of these preparations demonstrated intense cytoplasmic FITC staining, indicative of the presence of HBIG within HBIG-treated HepAD38 cells (Fig. 3A); intense FITC staining was also detected in HBIG-treated cells under Tet-on conditions (results not shown). Under identical image collection parameters, cytoplasmic FITC staining was not observed in parallel cultures that had not been treated with HBIG (Fig. 3B). Some cells were observed to contain both cytoplasmic FITC (Fig. 3C) and Texas Red (Fig. 3D) staining, indicative of the presence of HBIG and HBV pre-S2 protein, respectively. When digital superposition of the colorized HBIG (Fig. 3C) and HBV pre-S2 protein (Fig. 3D) was performed, colocalization of the green and red signals in a single pixel produced yellow (Fig. 3E), while separated signals remained green and red.
FIG.3.
Localization of HBIG and HBV proteins in HBIG-treated HepAD38 cells grown under Tet-off conditions. (A to E) On day 12 of culture, HBIG-treated HepAD38 cells were fixed and processed for IFA. The cells were reacted with monoclonal antibodies to HBV preS-2, followed by reactivity with FITC-conjugated anti-human and Texas Red-X-conjugated anti-mouse immunoglobulins. (A) Uptake of HBIG (arrows) into the cytoplasm of HBIG-treated cells can be detected by fluorescent staining. (B) When identical image collection parameters were applied to view parallel cultures that had not been treated with HBIG, fluorescent staining was not detected. HBIG (arrows) (C) and the HBV pre-S2 protein (arrows) (D) were observed within the cytoplasms of the same cells observed by FITC and Texas Red staining, respectively. (E) Colocalization of HBIG and HBV pre-S2 protein was shown following digital superimposition of the two fluoroprobes (arrows). (F) In addition to the HBV pre-S2 protein, the HBV core protein (arrows) was also detected following reactivity of the cells with rabbit polyclonal antibodies to HBV core. The arrows indicate the positive signals in each panel.
When HepAD38 cells under Tet-off conditions were processed for IFA using rabbit polyclonal antibody to HBV core protein, cytoplasmic FITC staining was observed in the cells (Fig. 3F). The detection of HBV core proteins in HepAD38 cells confirms that these cells are capable of supporting HBV replication (12).
Effect of anti-HBs IgG on the amount of intracellular HBsAg.
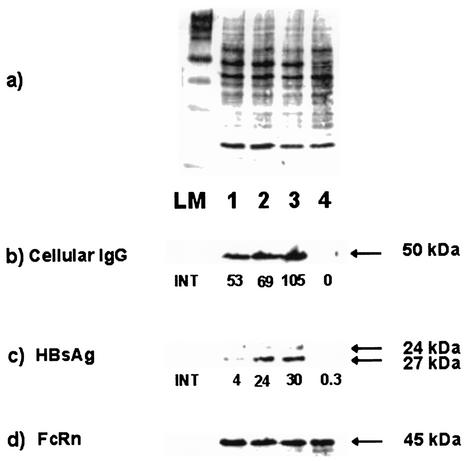
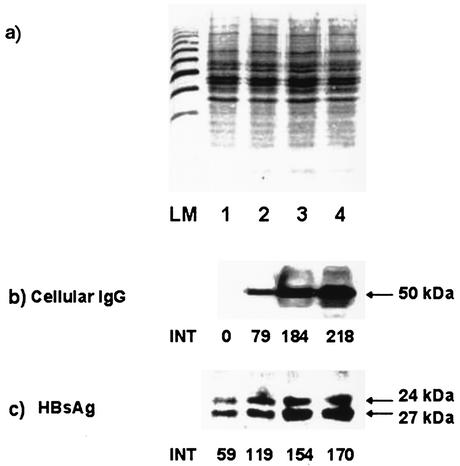
To determine whether the endocytosed HBs-specific IgG interferes with the secretion of HBsAg, PLC/PRF/5 cells were cultured in the presence of different concentrations (0.2, 1.0, and 2.0 mg/ml) of monoclonal HBs-specific IgG. The immunoblot analysis demonstrated that the amount of IgG in cytoplasmic extracts increased progressively in accordance with the concentration of anti-HBs IgG in the culture supernatants (Fig. 4b). The anti-HBs, associated with the cells, resulted in a dose-dependent increase in both forms of surface protein, p24 and gp27, within the cells (Fig. 4c). In addition, a specific signal for a 45-kDa protein, representing the heavy chain of the human major histocompatibility complex class I-related Fc receptor FcRn, was detected consistently in all cytoplasmic extracts (Fig. 4d).
FIG. 4.
Immunoblot analysis of cytoplasmic extracts from PLC/PRF/5 cells cultured in the presence of different concentrations of human monoclonal anti-HBs. The cells were cultured in medium with 0.2 mg of anti-HBs/ml (lanes 1), with 1.0 mg of anti-HBs/ml (lanes 2), with 2.0 mg of anti-HBs/ml (lanes 3), or without human IgG (lanes 4). (a) Amido black staining of proteins. (b) Detection of human IgG. (c) Detection of HBsAg. (d) Detection of FcRn. INT, intensity of the chemiluminescence signal measured by FluorS MultiImager in arbitrary units. The arrows indicate the signals for the γ chain of IgG (50 kDa), for both forms of HBsAg (24 and 27 kDa), and for the heavy chain of FcRn (45 kDa). LM, protein length marker.
Next, we investigated whether polyclonal anti-HBs IgG (HBIG) has a similar effect on the level of intracellular HBsAg. For this purpose, PLC/PRF/5 cells were cultured in the presence of three concentrations (0.2, 1.0, and 2.0 mg/ml) of HBIG. The immunoblot analysis again demonstrated a proportional increase of IgG in the cytoplasmic extracts of cells cultured with HBIG (Fig. 5b). Quantitation of the signal for HBsAg in cells cultured with different concentrations of HBIG revealed increasing intracellular amounts of surface protein in comparison to cells cultured without human IgG. The relative increase of HBsAg in cells cultured with 0.2 mg of HBIG/ml was 101%; in cells with 1.0 mg of HBIG/ml it was 161%, and in cells with 2.0 mg of HBIG/ml it was 188% (Fig. 5c). The amount of surface protein was not significantly affected when cells were cultured with HBs-specific F(ab)2 fragments as a control (data not shown).
FIG. 5.
Immunoblot analysis of cytoplasmic extracts from PLC/PRF/5 cells cultured with different concentrations of polyclonal anti-HBs IgG (HBIG). The cells were cultured in medium without human IgG (lanes 1), with 0.2 mg of HBIG/ml (lanes 2), with 1.0 mg of HBIG/ml (lanes 3), or with 2.0 mg of HBIG/ml (lanes 4). (a) Amido black staining of proteins. (b) Detection of human IgG. (c) Detection of HBsAg. INT, intensity of the chemiluminescence signal measured by FluorS MultiImager in arbitrary units. The arrows indicate the signals for the γ chain of IgG (50 kDa) and for both forms of HBsAg (24 and 27 kDA). LM, protein length marker.
In order to test whether the increased amount of intracellular surface protein was due to extracellular formation and subsequent internalization of HBs-anti-HBs complexes, we cultured HuH-7 cells with preassembled antigen-antibody complexes using HBIG- and HBs-containing culture supernatants from PLC/PRF/5 cells. Immunoblot analysis of cytoplasmic extracts from HuH-7 cells demonstrated the presence of IgG; however, HBsAg was not detectable. Although some HBsAg had been complexed with anti-HBs and might have been associated with the cells, the amount of HBsAg was below the detection limit of the immunoblot assay. Therefore, we have excluded the possibility that formation of HBs-anti-HBs complexes in the culture supernatant may account for the increase of HBsAg within the cells, as demonstrated above.
Internalized anti-HBs IgG inhibits the secretion HBsAg.
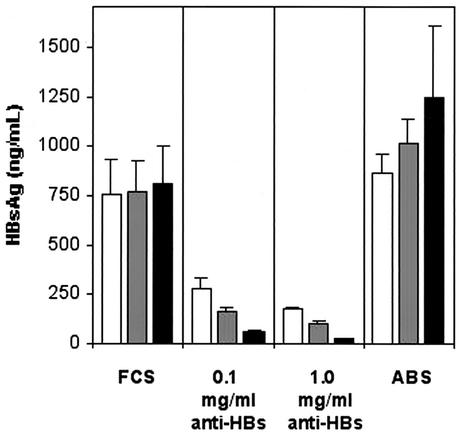
To analyze the effect of anti-HBs on the secretion of HBsAg, PLC/PRF/5 cells were cultured with two concentrations (0.1 and 1.0 mg/ml) of monoclonal anti-HBs IgG. As a control, the cells were cultured in the presence of human AB serum containing nonimmune IgG. After 2 days of culture, the supernatants were collected and the same cells were maintained in culture for a further two intervals of 2 days each in medium with FCS without human IgG. The amounts of HBsAg in the culture supernatants were monitored by RIA. During the first period of the experiment, the amount of HBsAg secreted in supernatants of PLC/PRF/5 cells cultured with 0.1 or 1.0 mg of anti-HBs/ml was reduced by 63 or 77% compared to cells cultured with medium plus FCS only (Fig. 6). In contrast, the secretion of HBsAg from cells cultured with nonimmune human IgG was not different from that from the cells cultured with FCS.
FIG. 6.
RIA quantitation of HBsAg levels in the supernatants of PLC/PRF/5 cells cultured with human monoclonal anti-HBs IgG. During the first period (open bars), the cells were cultured in the presence of medium with FCS and two concentrations of anti-HBs. In parallel, cells were cultured with medium plus FCS only, i.e., without human IgG (FCS) or with nonimmune human IgG (ABS), as controls. After 2 days of culture, the supernatants were collected, and the same cells were maintained in culture for a further two intervals of 2 days each (shaded and solid bars, respectively) in medium with FCS without human IgG. The HBsAg levels in the supernatants were tested at the end of each period, i.e., at three time points. The bars represent the means and standard deviations of two separate experiments, with each experimental condition run in duplicate.
In the second period, when no anti-HBs was present in the medium, the secretion of HBsAg from PLC/PRF/5 cells, initially cultured with 0.1 mg of anti-HBs/ml, remained very low. The HBsAg concentrations at the two time points were 78 and 92% lower than the corresponding HBsAg concentrations in supernatants from control cells which had been cultured with FCS only (Fig. 6). The reduction of HBsAg secretion from PLC/PRF/5 cells (which had been exposed to 1.0 mg of anti-HBs/ml during the first period) during the second period was even greater. The mean HBsAg concentrations in the supernatants from these cells were only 104 ± 5 and 22 ± 3 ng/ml, which were 86 and 97% lower than the corresponding HBsAg levels detected in the supernatants from PLC/PRF/5 cells cultured with medium plus FCS throughout the experiment. The secretion of HBsAg from the PLC/PRF/5 cells cultured with human AB serum was enhanced in the second period, which is in contrast with the marked decrease in HBsAg secretion from the cells that were exposed to anti-HBs during the first period and subsequently maintained in culture with medium containing FCS (Fig. 6).
Internalized anti-HBs IgG inhibits the secretion of virions from cells with HBV replication.
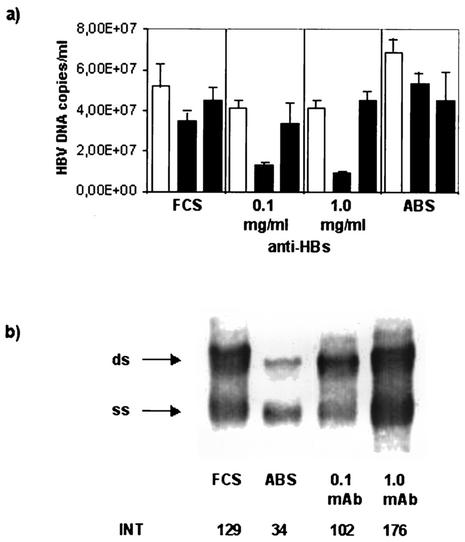
To analyze the effect of anti-HBs on the secretion of HBV virions, HepG2.2.15 cells were cultured with two concentrations (0.1 and 1.0 mg/ml) of monoclonal anti-HBs IgG. As a control, the cells were cultured in the presence of human AB serum. After 2 days, the supernatants were collected and the same cells were maintained in culture for a further two intervals of 2 days each in medium without human IgG. In the first period of the experiment, the HBV DNA levels in the supernatants of cells cultured in the presence of 0.1 or 1.0 mg of anti-HBs/ml were reduced by 20% in comparison to HBV DNA levels in supernatants from cells cultured with medium plus FCS only (Fig. 7a). During the second period, when no anti-HBs was present in the medium, the HBV DNA levels were even lower at the first time point: by 60% in supernatants from HepG2.2.15 cells initially cultured with 0.1 mg of anti-HBs/ml and by 75% in supernatants of cells cultured initially with 1.0 mg of anti-HBs/ml (Fig. 7a). This inhibitory effect on the secretion of HBV virions from the cells was no longer present at the end of the second period, as HBV DNA levels in supernatants from these cells were similar to HBV DNA levels in supernatants from control cells cultured with medium plus FCS only. In the presence of human nonimmune IgG (AB serum), the HBV DNA levels did not decrease during the culture period and were similar to the levels in the supernatants of control cells cultured with FCS only.
FIG. 7.
Detection of HBV DNA in supernatants and cytoplasmic extracts of HepG2.215 cells. (a) Quantitative real-time PCR for the detection of HBV DNA in cell culture supernatants. HepG2.2.15 cells were cultured for an initial period (open bars) without human IgG (FCS), with nonimmune IgG (ABS), or with 0.1 or 1.0 mg of monoclonal HBs-specific IgG/ml (anti-HBs). In the second period (solid bars), the same cells were maintained in culture for a further two time intervals without human IgG. The bars represent the means and the standard deviations of duplicate samples. (b) Southern blot hybridization for the detection of HBV replicative intermediates in the cytoplasm of HepG2.215 cells cultured without human IgG (FCS), with nonimmune human IgG (ABS), or with monoclonal anti-HBs (mAb). The arrows indicate the signals for single-stranded (ss) and double-stranded (ds) HBV DNAs. INT, intensity of the chemiluminescence signal measured by FluorS MultiImager in arbitrary units.
To examine the effect of internalized anti-HBs on HBV DNA in the cells, total DNA was isolated from cytoplasmic fractions of HepG2.2.15 cells cultured with 0.1 and 1.0 mg of monoclonal anti-HBs/ml for 2 days and analyzed by Southern blot hybridization. In the presence of 0.1 mg of anti-HBs/ml, the amounts of single-stranded and double-stranded HBV DNAs were not different from those present in control cells, which were cultured without human IgG (Fig. 7b). In contrast, the amount of HBV replicative intermediates was increased by 36% in cells cultured with 1.0 mg of monoclonal anti-HBs IgG/ml in comparison with the cells cultured with FCS only. The cells cultured with human AB serum showed lower levels of HBV replicative intermediates than cells cultured in the presence of 1.0 mg of anti-HBs IgG/ml.
Specific effect of anti-HBs IgG on the secretion of wild-type HBsAg.
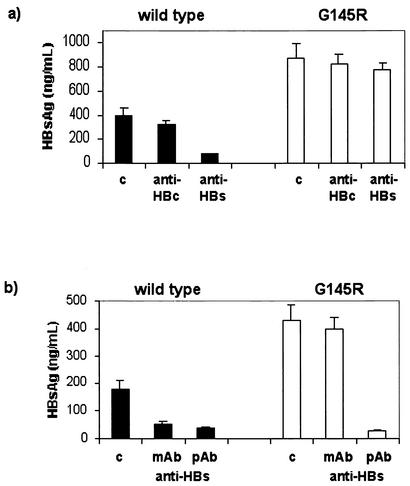
To determine the specificity of the effect of internalized anti-HBs on the secretion of HBsAg, HuH-7 cells were transfected in parallel with a plasmid containing the wild-type HBV genome or with a construct which differs from the wild type only by a single amino acid substitution—G145R in HBsAg. The HBV-transfected cells were cultured in the presence of 1.0 mg of monoclonal or polyclonal HBs-specific IgG/ml. As a control, we cultured the cells with 1.0 mg of IgG/ml containing a high titer of anti-HBc but no anti-HBs. The secretion of HBsAg into the culture supernatants was monitored by RIA using the HBs-specific monoclonal antibody P2D3, which detects both wild-type and G145R mutant HBsAg (8). Cells transfected with G145R mutant HBV produced larger amounts of HBsAg in the supernatants than the wild-type HBV. This is most likely a result of the enhanced replication competence of the G145R HBV mutant, as recently reported (10).
The amount of wild-type HBsAg secreted from cells cultured in the presence of monoclonal anti-HBs was reduced by 81% in comparison with that of transfected cells cultured without human IgG (Fig. 8a). In contrast, the secretion of HBsAg from cells transfected with HBV genome containing the antibody escape G145R mutation was not affected in the presence of monoclonal HBs-specific IgG, as the HBsAg concentration in the supernatant showed only 11% difference from that in cells cultured with FCS. In addition, the secretion of HBsAg was not considerably different in the cells transfected with wild-type HBV or G145R mutant HBV that were cultured with anti-HBc in comparison to the respective controls with FCS only. In another experiment, HuH-7 cells were transfected with either the wild-type or G145R HBV genome, and we compared the effects of monoclonal and polyclonal anti-HBs (Fig. 8b). The amount of HBsAg secreted from G145R was not affected by the monoclonal anti-HBs IgG, whereas polyclonal anti-HBs markedly reduced the secretion of HBsAg from cells transfected with wild-type or G145R—by 81 and 93%, respectively, in comparison to cells cultured without human IgG (Fig. 8b).
FIG. 8.
Quantification of HBsAg levels in the culture supernatants of HuH-7 cells transfected with HBV genomes expressing wild-type HBsAg (solid bars) or mutant G145R HBsAg (open bars). (a) HBV-transfected cells were cultured with FCS only as a control (c), with 1.0 mg of human monoclonal anti-HBs IgG/ml, or with 1.0 mg of human anti-HBc IgG/ml. (b) In another experiment, HuH-7 cells were transfected in parallel with the wild-ype HBV or G145R mutant HBV and cultured with 1.0 mg of monoclonal anti-HBs IgG/ml (mAb) or with 1.0 mg of polyclonal anti-HBs IgG/ml (pAb). The bars represent the means and standard deviations of duplicate samples.
DISCUSSION
The present study demonstrates that human IgG, either immune or nonimmune, is endocytosed into a variety of hepatocyte-derived cell lines. The internalized HBs-specific IgG is biologically active, and as a result of a specific antigen-antibody interaction, it inhibits the secretion of HBsAg and HBV virions from the cells. Thus, these results suggest that HBs-specific IgG interacts with HBsAg inside infected cells, in addition to the conventional idea that this antibody acts only by recognition and neutralization of HBV virions in circulation.
The analysis by IEM shows morphologically the presence of HBs-specific IgG on the plasma membrane and membranous invaginations of human hepatocyte-derived cell lines, irrespective of the presence or absence of HBsAg. Furthermore, using two-color confocal microscopy, colocalization of HBV envelope protein (pre-S2) and polyclonal anti-HBs was demonstrated in the same cellular compartment, which provides direct evidence for HBsAg-anti-HBs binding within the cells. Several lines of functional evidence in the present study support the notion that this endocytosed HBs-specific IgG is functionally active within the cells rather than only associated with them. First, human monoclonal and polyclonal HBs-specific IgG caused an accumulation of HBsAg within the cells, which increased in a dose-dependent manner. These effects were not observed in the control experiments with nonimmune IgG. Second, after an initial incubation of HBV-positive cells with anti-HBs in the medium, the inhibitory effect on secretion of HBsAg and HBV DNA continued in the second period, when no human IgG was present in the culture medium. This implies that the sustained effect was due to anti-HBs which had been internalized in the cells during the initial incubation. Third, in the transfection experiments, anti-HBs specifically inhibited the secretion of the wild-type HBsAg but had no effect on the secretion of the antibody escape HBsAg containing the G145R mutation.
Earlier studies identified the presence of membrane-bound IgG and/or nuclear localization of IgG in the hepatocytes of patients with chronic HBV infection, who express HBV core antigen or hepatitis delta virus antigen in the liver (17, 19, 23). An uptake of human IgG into hepatocytes by a process described as macropinocytosis has been demonstrated in a murine system (18). In addition, antibodies to a ribosomal protein have been shown to penetrate live human hepatoma cells and cause profound inhibition of protein synthesis (11). A previous study showed that endocytosis of anti-DNA antibodies into rat hepatoma cells was mediated through the binding of antibodies with myosin on the cell surface (28).
The present study demonstrates that anti-HBs IgG is endocytosed in hepatocyte-derived cell lines irrespective of the presence or absence of HBsAg expression. A possible candidate for a receptor-dependent endocytosis of IgG is the major histocompatibility complex class I-like Fc-receptor, or FcRn. This receptor is expressed on the canalicular and sinusoidal plasma membranes of adult rat hepatocytes and was shown to bind Fc fragments of IgG, thus providing a functional communication between parenchymal immune cells and bile (1). The identification of FcRn-receptor for IgG on the membranes of all cell lines used in the present study indicates the likely mechanism for cellular uptake of human IgG. This is supported by the results of the control experiment with F(ab)2 fragments. The elimination of the Fc fraction of HBIG abrogated IgG binding to the cell lines, as well as the effects on HBsAg secretion from PLC/PRF/5 cells.
The endocytosed anti-HBs IgG appears to inhibit the secretion of HBV virions, and in particular HBsAg, from HBV-infected hepatocytes. Intracellular coexpression of the variable region of monoclonal anti-HBs, together with HBsAg in hepatoma cells, markedly reduced the secretion of HBsAg from these cells (30). This mechanism may be operating in cases of spontaneous resolution of HBV infection, in which significant levels of anti-HBs antibodies are present in the circulation. In the majority of these healthy subjects, HBV DNA remains detectable by PCR, and a recent study showed the presence of ongoing low-level HBV DNA replication in the liver (15). Similarly, in liver transplant patients who receive HBIG prophylaxis and are negative for HBsAg, HBV DNA is frequently detectable in liver tissue by PCR (20). Hepatic uptake of HBs-specific IgG from the serum in these cases may contribute to the containment of HBV within infected hepatocytes. Efficient inhibition of viral replication by intracellular antibodies has been demonstrated by influenza virus-specific IgA within epithelial cells (16). In our experiments using HepG2.2.15 cells, there was no inhibition of viral replication. In the presence of 1.0 mg of anti-HBs/ml, the HBV DNA levels in supernatants decreased, which was associated with a marked increase in HBV DNA replicative intermediates within HepG2.2.15 cells. This is in line with the findings of reduced HBsAg in supernatants (Fig. 6) and the intracellular accumulation of HBsAg within PLC/PRF/5 cells (Fig. 5). A possible interpretation is that anti-HBs, which is internalized in the cells, reduces the excretion of HBV (which leads to the increase of HBV DNA within the cells) or that anti-HBs interferes with the envelopment of viral nucleocapsids containing HBV DNA intermediates.
The emergence of HBV mutant strains under selection pressure from antiviral antibodies, such as the surface antibody escape mutants or the HBe Ag-minus strains with mutations in the precore region, in patients with chronic HBV infection is well recognized (14, 26). These mutations offer a survival advantage for the virus, and in the presence of selection pressure, the mutant HBV partially or completely replaces the wild-type strains in the viral population of a given host. The present study provides new insight into the mechanisms of the emergence of immune escape by demonstrating for the first time that hepatocyte uptake of anti-HBs IgG selectively inhibits the secretion of the wild-type HBV while it has only little effect on HBV with a G145R mutation. These results suggest that the antibody-induced selection of HBV mutants may also take place within the cells during active HBV replication. The emergence of a mutation(s) in the surface gene would offer an advantage to virions with mutated envelopes, which can efficiently bypass the blocking effect of intracellular anti-HBs on HBsAg and virion secretion, while the envelopes of virions containing wild-type HBsAg will be retained within the cells.
The present study reveals that antibodies to HBsAg of the IgG class can bind to the cellular membrane and are endocytosed into live hepatocytes. HBIG is functionally active within the cells and able to contain HBsAg as a result of a specific antigen-antibody interaction. This process may increase the selection pressure and may represent the mechanism for the emergence of surface antibody escape HBV variants.
REFERENCES
- 1.Blumberg, R. S., T. Koss, C. M. Story, D. Barisani, J. Polischuk, A. Lipin, L. Pablo, R. Green, and N. E. Simister. 1995. A major histocompatibility complex class I-related Fc receptor for IgG on rat hepatocytes. J. Clin. Investig. 95:2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carman, W. F., A. R. Zanetti, P. Karayiannis, J. Waters, G. Manzillo, E. Tanzi, A. J. Zuckerman, and H. C. Thomas. 1990. Vaccine-induced escape mutant of hepatitis B virus. Lancet 336:325-329. [DOI] [PubMed] [Google Scholar]
- 3.Carman, W. F., C. Trautwein, F. J. van Deursen, K. Colman, E. Dornan, G. McIntyre, J. Walters, V. Kliem, R. Muller, H. C. Thomas, and M. P. Manns. 1996. Hepatitis B envelope variation after transplantation with and without hepatitis B immune globulin prophylaxis. Hepatology 24:489-493. [DOI] [PubMed] [Google Scholar]
- 4.Carman, W. F., A. Owsianka, L. A. Wallace, B. C. Dow, and D. J. Mutimer. 1999. Antigenic characterisation of pre- and post-liver transplant hepatitis B surface antigen sequences from patients treated with hepatitis B immune globulin. J. Hepatol. 31:195-201. [DOI] [PubMed] [Google Scholar]
- 5.Cooreman, M. P., M. H. van Roosmalen, R. te Morsche, C. M. Sunnen, E. M. de Ven, J. B. Jansen, G. N. Tytgat, P. L. de Wit, and W. P. Paulij. 1999. Characterization of the reactivity pattern of murine antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring “a” loop escape mutations. Hepatology 30:1287-1292. [DOI] [PubMed] [Google Scholar]
- 6.Ghany, M. G., B. Ayola, F. G. Villamil, R. G. Gish, S. Rojter, J. M. Vierling, and A. S. Lok. 1998. Hepatitis B virus mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology 27:213-222. [DOI] [PubMed] [Google Scholar]
- 7.Ghetie, V., and E. S. Ward. 2000. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 18:739-766. [DOI] [PubMed] [Google Scholar]
- 8.Ijaz, S., F. Torre, R. S. Tedder, R. Williams, and N. V. Naoumov. 2001. Novel immunoassay for the detection of hepatitis B surface ′escape' mutants and its application in liver transplant recipients. J. Med. Virol. 63:210-216. [DOI] [PubMed] [Google Scholar]
- 9.Junghans, R. P., and C. L. Anderson. 1996. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 93:5512-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalinina, T., A. Riu, L. Fischer, H. Will, and M. Sterneck. 2001. A dominant hepatitis B virus population defective in virus secretion because of several S-gene mutations from a patient with fulminant hepatitis. Hepatology 34:385-394. [DOI] [PubMed] [Google Scholar]
- 11.Koscec, M., E. Koren, M. Wolfson-Reichlin, R. D. Fugate, E. Trieu, I. N. Targoff, and M. Reichlin. 1997. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J. Immunol. 159:2033-2041. [PubMed] [Google Scholar]
- 12.Ladner, S. K., M. J. Otto, C. S. Barker, K. Zaifert, G. H. Wang, J. T. Guo, C. Seeger, and R. W. King. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblasma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 41:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, J. Y., J. Culvenor, P. Angus, R. Smallwood, A. Nicoll, and S. A. Locarnini. 2001. Detection of duck hepatitis B virus replicative marker in primary cultures of bile duct epithelial cells. J. Virol. 75:7651-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok, A. V., U. Akarca, and S. Greene. 1994. Mutations in the pre-core region of hepatitis B virus serve to enhance the secondary structure of the pre-genome encapsidation signal. Proc. Natl. Acad. Sci. USA 91:4077-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marusawa, H., S. Uemoto, M. Hijikata, Y. Ueda, K. Tanaka, K. Shimotohno, and T. Chiba. 2000. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 31:488-495. [DOI] [PubMed] [Google Scholar]
- 16.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89:6901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meliconi, R., M. V. Stancari, M. Garagnani, M. Baraldini, G. F. Stefanini, F. Miglio, and G. Gasbarrini. 1983. Hepatocyte membrane-bound IgG and circulating liver-specific autoantibodies in chronic liver disease: relation to hepatitis B virus serum markers and liver histology. Hepatology 3:155-161. [DOI] [PubMed] [Google Scholar]
- 18.Mitrenga, D., W. Arnold, O. Muller, and H. von Mayersbach. 1975. The fate of injected human IgG in the mouse liver. Cell Tissue Res. 156:359-376. [DOI] [PubMed] [Google Scholar]
- 19.Naumov, N. V., M. Mondelli, G. J. Alexander, R. S. Tedder, A. L. Eddleston, and R. Williams. 1984. Relationship between expression of hepatitis B virus antigens in isolated hepatocytes and autologous lymphocyte toxicity in patients with chronic hepatitis B virus infection. Hepatology 4:63-68. [DOI] [PubMed] [Google Scholar]
- 20.Naoumov, N. V., A. R. Lopes, P. Burra, L. Caccamo, R. M. Immolo, R. A. deMan, M. Bassedine, J. G. O'Grady, B. C. Portmann, G. Anschuetz, C. A. Barret, R. Williams, and M. Atkins. 2001. Randomized trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J. Hepatol. 34:888-894. [DOI] [PubMed] [Google Scholar]
- 21.Pas, S. D., E. Fries, R. A. De Man, A. D. Osterhaus, and H. G. Niesters. 2000. Development of a quantitative real-time detection assay for hepatitis B virus DNA and comparison with two commercial assays. J. Clin. Microbiol. 38:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protzer-Knolle, U., U. Naumann, R. Bartenschlager, T. Berg, U. Hopf, K. H. Meyer zum Buschenfelde, P. Neuhaus, and G. Gerken. 1998. Hepatitis B virus with antigenically altered hepatitis B surface antigen is selected by high-dose hepatitis B immune globulin after liver transplantation. Hepatology 27:254-263. [DOI] [PubMed] [Google Scholar]
- 23.Rizzetto, M., M. G. Canese, S. Arico, O. Crivelli, C. Trepo, F. Bonino, and G. Verme. 1977. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 18:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuel, D., R. Muller, G. Alexander, L. Fassati, B. Ducot, J. P. Benhamou, and H. Bismuth. 1993. Liver transplantation in European patients with the hepatitis B surface antigen. N. Engl. J. Med. 329:1842-1847. [DOI] [PubMed] [Google Scholar]
- 25.Terrault, N. A., S. Zhou, C. Combs, J. A. Hahn, J. R. Lake, J. P. Roberts, N. L. Ascher, and T. L. Wright. 1996. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology 24:1327-1333. [DOI] [PubMed] [Google Scholar]
- 26.Thomas, H. C., and W. F. Carman. 1994. Envelope and precore/core variants of hepatitis B virus. Gastroenterol. Clin. N. Am. 23:499-514. [PubMed] [Google Scholar]
- 27.Waters, J. A., M. Kennedy, P. Voet, P. Hauser, J. Petre, W. Carman, and H. C. Thomas. 1992. Loss of the common “A” determinant of hepatitis B surface antigen by a vaccine-induced escape mutant. J. Clin. Investig. 90:2543-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanase, K., R. M. Smith, A. Pucetti, L. Jarett, and M. P. Madaio. 1997. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J. Clin. Investig. 100:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu, X., G. Meng, B. L. Dickinson, X. Li, E. Mizogushi, L. Miao, Y. Wang, C. Robert, B. Wu, P. D. Smith, W. I. Lencer, and R. S. Blumberg. 2001. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 166:3266-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.zu Putlitz, J., A. Skerra, and J. R. Wands. 1999. Intracellular expression of a cloned antibody fragment interferes with hepatitis B virus surface antigen secretion. Biochem. Biophys. Res. Commun. 255:785-791. [DOI] [PubMed] [Google Scholar]