Abstract
During Proterozoic time, Earth experienced two intervals with one or more episodes of low-latitude glaciation, which are probable “Snowball Earth” events. Although the severity of the historical glaciations is debated, theoretical “hard Snowball” conditions are associated with the nearly complete shutdown of the hydrological cycle. We show here that, during such long and severe glacial intervals, a weak hydrological cycle coupled with photochemical reactions involving water vapor would give rise to the sustained production of hydrogen peroxide. The photochemical production of hydrogen peroxide has been proposed previously as the primary mechanism for oxidizing the surface of Mars. During a Snowball, hydrogen peroxide could be stored in the ice; it would then be released directly into the ocean and the atmosphere upon melting and could mediate global oxidation events in the aftermath of the Snowball, such as that recorded in the Fe and Mn oxides of the Kalahari Manganese Field, deposited after the Paleoproterozoic low-latitude Makganyene glaciation. Low levels of peroxides and molecular oxygen generated during Archean and earliest Proterozoic non-Snowball glacial intervals could have driven the evolution of oxygen-mediating and -using enzymes and thereby paved the way for the eventual appearance of oxygenic photosynthesis.
Keywords: atmospheric processes, Paleoproterozoic, photochemistry
Hydrogen peroxide provides a powerful oxidant in anoxic environments. The recent discovery of H2O2 in the atmosphere of Mars (1, 2) supports the hypothesis that oxidation of iron by photochemically generated H2O2 over the past 4 billion years may have yielded the present oxidized Martian surface (3). The cold, dry, and low-oxygen (≈7 μbar) Martian atmosphere concentrates H2O2 production near the surface (4, 5). Hydrogen peroxide can be preserved readily in ice, because H2O2 has a slightly lower freezing point (−1°C) than H2O; a concentration of H2O2 as high as 0.13% is observed on the surface of the Galilean satellite Europa (6). The closest analog in Earth history for the cold, dry, low-oxygen conditions of Mars and Europa may have occurred during the proposed Paleoproterozoic Snowball Earth event (7, 8) and perhaps during one or both of the proposed Neoproterozoic Snowball Earth events (9, 10).
Although there have been many glacial events recorded in the history of the Earth (9–11), two major periods of low-latitude glaciation in the Proterozoic appear correlated with significant changes in the evolution of life (8, 12–14) and atmospheric oxygen level (15–19). The Paleoproterozoic Makganyene glaciation occurred approximately between 2.3 and 2.2 Ga, and at least two other low-latitude glaciations occurred during the Cryogenian period, between ≈740 and 630 Ma (9, 10, 20). The severity of these “Snowball Earth” events is debated, but the low latitude of the glaciations indicates that, at least on the continents, ice extended to the equator, average global temperatures were likely well below freezing, and the hydrological cycle was much diminished (21, 22). The rock record indicates that the atmosphere and ocean were oxygen-poor until shortly before the onset of the Paleoproterozoic Snowball at ≈2.3 Ga (15–19), and the weakening of the biosphere and hydrological cycle would likely have decreased atmospheric oxygen levels during the event. Here we adopt a photochemical model similar to the one that Nair et al. (4) successfully applied to the Martian atmosphere to investigate the production and deposition of H2O2 during low-oxygen Snowball Earth conditions.
We examined atmospheric photochemistry during the Paleoproterozoic Snowball Earth event, as well as the impact of earlier glaciations on the evolution of cellular life. Assuming the Paleoproterozoic Makganyene glaciation was a “hard Snowball,” it led to an environment similar to that on Mars and icy satellites, where H2O2 could be produced and preserved in ice. During the deglaciation, the deposited H2O2 would have been released into the oceans and atmosphere, as occurs during the spring and summer in Greenland and at the South Pole (23, 24). On a low-oxygen planet, the H2O2 could have provided an important source of oxidants for driving the evolution of oxygen-mediating and -using enzymes.
Atmospheric Chemical Models
We performed a one-dimensional diurnally averaged simulation of the chemical processes in the atmosphere of a Snowball Earth. Our model calculates the profiles of O, O(1D), O2, O3, H, H2, OH, HO2, H2O2, CO, CO2, HCO, and H2CO by solving the mass continuity equation (∂ni/∂t) + (∂ϕi/∂z) = Pi − Li, where ni is the number density for species i, ϕi is the vertical flux, Pi is the chemical production rate, and Li is the chemical loss rate, all evaluated at time t and altitude z. Pi and Li are calculated based on the chemical schemes published in the literature (4, 25–27). The vertical flux is given by
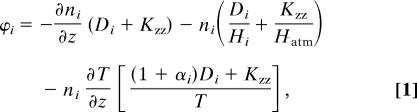 |
where Di is the species' molecular diffusion, Hi is the species' scale height, Hatm is the atmospheric scale height, αi is the thermal diffusion parameter, and T is the temperature. The vertical eddy mixing coefficient Kzz in this work is set to be 2 × 105 cm2·s−1, which is close to the present-day value near the surface (25). With this Kzz, the vertical mixing time is on the order of 106 s, a value much longer than the lifetime of H2O2 of ≈104 s in the atmosphere. We therefore expect that Kzz plays a minor role in the vertical profile of H2O2.
Because of model limitations, we assume the surface pressure is 1 bar over the course of model time. We fix the H2O profile at that determined by its saturation pressure in the atmosphere; we vary the profile by modifying the surface temperature. For the temperature profile, the surface temperature is taken to be 240 K, with a vertical temperature gradient of −10 K·km−1 (dry-adiabatic lapse rate) and a constant temperature of 150 K in regions where the extrapolation of the temperature with the assumed gradient yields values <150 K; this is quoted as the reference model. We also assume a present-day solar UV spectrum. Initially, the atmosphere contains N2 and H2O only. The model starts with an upward H2 and CO2 flux of 1010 molecules cm−2·s−1, which are close to the current volcanic outgassing rates (13). Species other than H2, H2O, and H2O2 are impermeable at the boundary. We allow H2 to escape to space hydrodynamically when the conditions of Tian et al. (28) are met, although the strength of hydrodynamic escape in this paleoatmosphere remains inconclusive (29). The model results with both higher and lower levels of hydrogen escape are also presented. With the present-day solar spectrum, hydrodynamic escape happens when the homopause (≈65 km) mixing ratio of H2 is >0.05. To a first-order approximation, we assume that H2 escape with a flux of 1010 molecules cm−2·s−1 is initiated when the H2 mixing ratio is >0.1 and that it remains at this level as long as the ratio is >0.05. We let the model run for the entire lifetime of the Snowball (see below). Vertical profiles of species important to this study are summarized in Fig. 1; some other profiles are presented in Fig. 2. Note that formaldehyde, which can be preserved on the surface by precipitation (27), is not produced in large amounts, so the deposition of H2 by burying H2CO is insignificant.
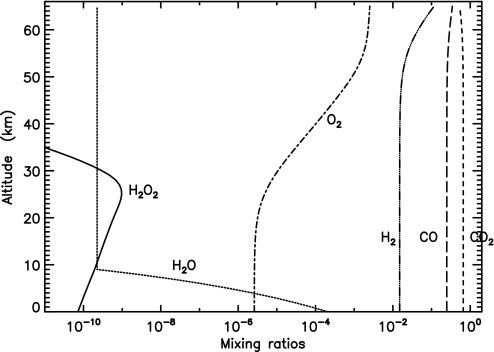
Fig. 1.
Profiles of H2O (dotted line), H2O2 (solid line), O2 (dash-dotted line), H2 (triple dot-dashed line), CO (long-dashed line), and CO2 (dashed line) calculated with the reference model, in which surface temperature is 240 K, temperature gradient is −10 K km−1, and constant temperature is 150 K above the tropopause (≈10 km). The values are shown for the model calculation at one instance at the end of the Snowball Earth. The downward and escape fluxes of H2O2 and H2 are ≈5 × 108 and 1010 molecules cm−2·s−1, respectively. The mixing ratio of H2O above the tropopause is set equal to that at the tropopause.
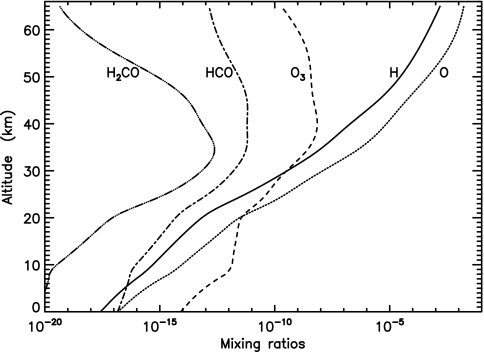
Fig. 2.
Profiles of H (solid line), O (dotted line), O3 (dashed line), HCO (dash-dotted line), and H2CO (triple dot-dashed line) under the same conditions as Fig. 1.
Current thinking suggests that deglaciation would proceed from build-up of greenhouse gases such as CO2 due to persistent volcanic activity uncompensated by silicate weathering. As ice cover and low temperatures would have greatly hindered silicate weathering, atmospheric accumulation of greenhouse gases, particularly CO2, would have eventually allowed temperatures to rise above freezing (30). In Neoproterozoic time, over the likely 12-million-year lifetime of the Snowball event (20), atmospheric CO2 could have reached a level of 0.2 bar at the present-day outgassing rate of ≈1010 molecules cm−2·s−1. Although it has recently been noted that greenhouse warming caused directly by CO2 at a level as high as 0.2 bar may not have been sufficient to initiate the deglaciation of Neoproterozoic Snowballs (30, 31), in our model we still follow typical suggestions to estimate the duration of the Paleoproterozoic Snowball event. Assuming 0.2 bar CO2 was actually required to start the Neoproterozoic deglaciations, the lower solar constant in the Paleoproterozoic (85% of modern insolation, rather than 96%) implies that triggering the end of a Paleoproterozoic Snowball would have required ≈0.6 bar CO2. At present degassing rates, such an amount would have taken ≈35 million years to build up (32).
Hydrogen Peroxide Deposition
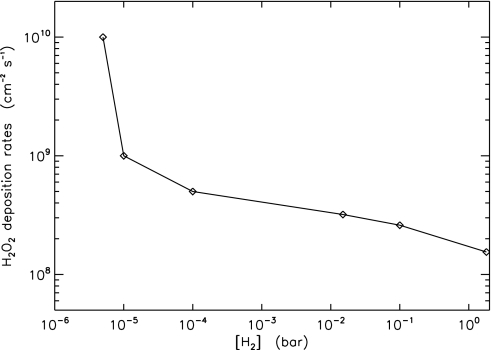
Hydrogen peroxide can be deposited on ice by diffusional contact. This deposition rate is limited by the lifetime of H2O2 against photolysis in the atmosphere (≈6 h), equivalent to a traveling distance of <1 km for a diffusion coefficient of 2 × 105 cm−2·s−1. The diffusional deposition of H2O2 is also sensitive to the H2/O2 ratio (Fig. 3). The abundance of H2 reflects the strength of hydrogen escape to space. With a ratio of ≈2 the deposition rate is as high as 1010 molecules cm−2·s−1. For ratios >5, the rates are suppressed by a factor of >10. The accumulation of H2 in the atmosphere would soon stop the diffusional deposition of H2O2 if the replenishment of O2 were insufficient to support an O2 abundance as high as that in Fig. 1, because of the reaction 2H2 + O2 → 2H2O. We find that the continuous accumulation and photolysis of CO2 in the atmosphere can maintain such a level of O2 (2CO2 → 2CO + O2) via well known HOx chemistry (26). Consequently, CO is in high abundance (Fig. 1). Because the photolytic processes of CO2 and H2O are in similar wavelength ranges, by the end of the Snowball CO2 becomes a major UV absorber and H2O photolysis is less significant. In this standard model, the deposition rate of H2O2 by diffusion is 3 × 108 molecules cm−2·s−1.
Fig. 3.
Sensitivity of surface H2 abundance, obtained by varying the strength of hydrogen hydrodynamic escape, on the deposition rate of H2O2 by diffusion. The abundance of O2 as high as that in Fig. 1 is maintained by CO2 photolysis (see text). The rightmost point is calculated by assuming that H2 escape vanishes. For the diffusion-limit hydrogen escape case, the surface H2 mixing ratio is ≈5 × 10−4 and the H2O2 deposition rate is ≈4 × 108 molecules cm−2·s−1.
For the H2O2 precipitation rate, we follow the method developed for modern glacial environments (23, 24) to estimate a concentration of H2O2 in snowfall of ≈20 μM. Incorporating the estimated H2O2 diffusional flux to surface ice and assuming a hydrological cycle of f mm·yr−1, this standard model yields a volume mixing ratio of H2O2 in the snow/ice as high as ≈3 × 10−6(1/f) if f < 10, and as low as ≈5 × 10−7 if f > 10. Enhancing the hydrological cycle will always enhance the rainout rate of H2O2 as well as the concentration of H2O2 in H2O ice, by analogy with evaporation processes in H2O2-H2O solution, which enhance the concentration of H2O2 in the solution (33). This further concentration of H2O2 in the ice depends on the partitioning of H2O2 and H2O in vapor during evaporation (knowledge of which is not available for low-temperature conditions), the lifetime of ice sheet (34), and the strength of hydrological cycles or precipitation/evaporation rates (31, 34). A one-dimensional model of the dynamics of ice on a Snowball Earth has been published (34).
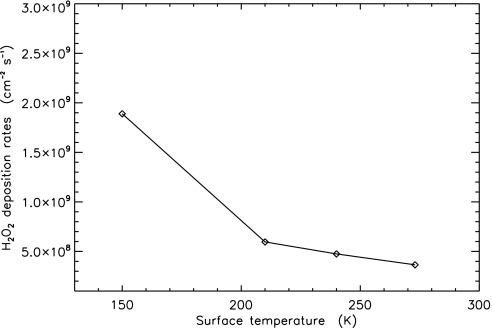
Changing surface temperature will also modify the calculated deposition rate of H2O2 by diffusional contact. Reducing surface temperature will correspondingly reduce the atmospheric H2O vapor abundance and hence move the oxidation line (the region of the atmosphere where H2O2 is mostly produced) closer to the surface. This shift will enhance diffusional H2O2 deposition, because the lifetime of H2O2 is short compared with vertical transport time (104 vs. 106 s; see above). The results of tests of sensitivity to changes of surface temperature are summarized in Fig. 4. In general, reducing surface temperatures will enhance the deposition rate by diffusion but will reduce the rainout rate.
Fig. 4.
Sensitivity of surface temperature on the deposition rate of H2O2 by diffusion. The vertical profiles of major compounds (e.g., H2, O2, CO, and CO2) are similar to those in Figs. 1 and 2.
Direct rainout from H2O2 nucleation in the region between ≈10 and 20 km is not included in the current model; the partial vapor pressure of H2O2 is ≈5 × 10−8 mbar, which is greater than the saturation pressure (33) of ≈10−9 mbar at 150 K. If this amount of H2O2 is removed by rainout, an additional precipitation rate of ≈1010 molecules cm−2·s−1 (equal to the production rate in this region) is imposed, yielding the total volume mixing ratio of H2O2 in the ice as high as 10−4(1/f). Note that the total production rate of H2O2 by gas-phase chemistry is ≈1011 molecules cm−2·s−1, which provides an upper limit to the rate of H2O2 deposition by diffusion and precipitation.
We stress that the calculated concentration of H2O2 in the snow/ice is a lower limit. With the present-day solar spectrum, the maximum photolysis rate of H2O is ≈1012 molecules cm−2·s−1. If UV photons directly irradiated the snow/ice, H2O2 could be produced and preserved readily in the ice. For example, the production rate of H2O2 in Antarctic seawater samples by direct sun light irradiation has been shown to approach this maximum H2O photolysis rate (35). Recently, it has also been shown that H2O2 at the surface is enhanced when stratospheric ozone is depleted (36), as in an anaerobic atmosphere. This mechanism of depositing H2O2 would be insensitive to the abundance of atmospheric H2.
In summary, we find that the volume mixing ratio of H2O2 in the ice falls between 5 × 10−7 and 10−4(1/f). The lower limit represents the regions where water cycles (precipitation/evaporation and ice melting) are active, as might occur at midday, in the summer, and in low-latitude regions. The upper limit can be achieved where water cycles are weak, such as in mid to high latitudes of the winter hemisphere (31). For example, at low latitudes of the summer hemisphere, the water precipitation could be as high as 45 cm·yr−1 (31), resulting in the mixing ratio of H2O2 in the ice ≈5 × 10−7. At winter hemisphere mid to high latitudes, pure H2O2 rainout is possible, because of low water cycles (31). Coupled with sea ice flow (34), a high level of H2O2 ice formed at mid to high latitudes can be transported to a lower latitude. Consequently, high-concentration H2O2 ice is available globally on the Snowball Earth. (A volume mixing ratio of H2O2 of 10−3 stored in 1-km-thick ice could disproportionate to form the equivalent to 0.1 bar of O2.) We note that the above estimation is simply based on snowfalls and dry deposition. The direct freezing of sea water could affect the concentration of H2O2 in the frozen ice; however, this process is sensitive to the strength of dynamical mixing in sea water. High concentrations of H2O2 in the ice may significantly suppress hydrological cycles. Future work is needed to resolve the aforementioned uncertainties.
Biological Implications
Even when the concentration of H2O2 in snow and ice is as low as that in modern polar regions, the release of H2O2 into the ocean upon melting could provide an environmental oxidant that would threaten organisms living nearby. Hydrogen peroxide in the presence of ferrous ion produces the hydroxyl radical (and ferryl iron), which is lethal to the cell (37). The Mn-based enzyme catalase, which catalyzes the reaction 2H2O2 → 2H2O + O2 (38, 39), and the superoxide dismutase enzymes, which neutralize O2−, protect the cell against the effects of hydrogen peroxide and the hydroxyl radical. These enzymes likely evolved before the evolution of oxygenic photosynthesis and hence protected the first oxygen-producing phototroph (40), perhaps in response to an environmental peroxide challenge. Blankenship and Hartman (41) further suggested that H2O2 played a crucial role in the origin and evolution of oxygenic photosynthesis because it is capable of being both a powerful oxidant and a reductant and because the oxidation of H2O2 to O2 is fully within the oxidative capabilities of reaction centers from existing anoxygenic photosynthetic bacteria (41). The Huronian glaciations at ≈2.4–2.3 Ga (8), the Pongola glaciations at ≈2.9 Ga (42), and perhaps unrecognized earlier glacial episodes might thus have spurred their development of both oxygen tolerance and oxygenic photosynthesis (40, 41), as well as stimulated the evolution of diverse oxidase and peroxidase enzymes that are now critical for aerobic metabolism.
Kopp et al. (8) proposed that the severity of the Makganyene Snowball Earth event was caused by the evolution of efficient oxygenic photosynthesis during the short interval between the Huronian glaciations and the Makganyene glaciation, and that photosynthetic oxygen production at the onset of the Makganyene glaciation triggered a collapse of the methane greenhouse. The evolution of oxygen tolerance in response to peroxide build-up during the Huronian glaciations could have paved the way for this evolutionary step.
Only a planetary glaciation like a Snowball Earth event, however, would be likely to produce enough H2O2 to leave a global fingerprint. During a Snowball, as ocean waters cycled through hydrothermal vents, the concentration of metals like Fe2+ and Mn2+ would have built up to high levels. After the Snowball, as recorded in the Kalahari Manganese Field (7) and in lesser Neoproterozoic manganese deposits like those of the Urucum district (43), iron and manganese would have been oxidized by oxygen and precipitated out of solution. After the Neoproterozoic Snowballs the main source of oxygen was likely atmospheric, but after the Makganyene Snowball atmospheric oxygen was still fairly low. The oxygen source for the Kalahari Manganese Field was probably a combination of a post-Snowball cyanobacterial bloom (7) and disproportionation of H2O2.
Fennel et al. (44) recognized recently that nitrate limitation is a critical problem in the transition from an anaerobic to an aerobic environment, such as presumably occurred around 2.3 Ga at the “Great Oxygenation Event.” Starting from an anaerobic environment, increasing oxidation removes metal cofactors critical for the function of the nitrogenase enzyme, as well as enabling aerobic denitrification. In turn, nitrogen limitation acts to constrict productivity, keeping the global production of oxygen by the cyanobacteria at levels below those needed to transition into the modern, oxygen-dominated stable environment in which abundant nitrate is biologically available. Fennel et al. (44) offer no clear explanation of how this “biogeochemical bottleneck” barrier between the anaerobic and aerobic stable states of the planetary ecosystem could be crossed. We suggest tentatively that the post-Makganyene snowball “burp” of peroxide (which in some model scenarios could be on the order of 1 bar) might have pushed the global environment over this nitrate limitation barrier by throwing the surface ocean into the oxic, nitrate-rich realm. More sophisticated modeling is necessary to test this idea.
We have demonstrated here that the generation of H2O2 through photolytic processes involving H2O is possible in the oxygen-poor early atmosphere of the Archean and early Paleoproterozoic (45, 46). Large amounts of hydrogen peroxide can be preserved only under special conditions, such as during an intense glaciation. Because of the short atmospheric lifetime of H2O2 against UV photolysis, build-up of large amounts requires H2O2 production close to the ground (hence, under low-O2 conditions), as well as a mechanism to trap it in the ice to store and concentrate it. During an intense glaciation, particularly a Snowball event, the H2O2 deposition rate may have been enhanced greatly, as we discuss above, although our model is extremely simple and excludes many important considerations. A coupled chemistry (gas-phase and solid-state) and dynamics (climate and ice-sheet) model is needed to quantify our calculation in detail and to provide a more accurate and quantitative estimation of the deposition of hydrogen peroxide during glaciations in anaerobic atmospheres as well as Snowball Earth events.
Acknowledgments
We thank C. Boxe, J. R. Leadbetter, and A. L. Sessions for helpful discussions and R. Pierrehumbert and an anonymous referee for helping improve this work. M.-C.L. and Y.L.Y. were supported by National Aeronautics and Space Administration Grant NNG06GF33G and astrobiology institutional support under Cooperative Agreement CAN-00-OSS-01. H.H. was supported by National Science Foundation Grant 00205512. J.L.K. and R.E.K. were supported by the Agouron Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Clancy RT, Sandor BJ, Moriarty-Schieven GH. Icarus. 2004;168:116–121. [Google Scholar]
- 2.Encrenaz T, Bezard B, Greathouse TK, Richter MJ, Lacy JH, Atreya SK, Wong AS, Lebonnois S, Lefevre F, Forget F. Icarus. 2004;170:424–429. [Google Scholar]
- 3.Hunten DM. J Mol Evol. 1979;14:71–78. doi: 10.1007/BF01732369. [DOI] [PubMed] [Google Scholar]
- 4.Nair H, Allen M, Anbar AD, Yung YL, Clancy RT. Icarus. 1994;111:124–150. doi: 10.1006/icar.1994.1137. [DOI] [PubMed] [Google Scholar]
- 5.Yung YL, DeMore WB. Photochemistry of Planetary Atmospheres. New York: Oxford Univ Press; 1999. [Google Scholar]
- 6.Carlson RW, Anderson MS, Johnson RE, Smythe WD, Hendrix AR, Barth CA, Soderblom LA, Hansen GB, McCord TB, Dalton JB, et al. Science. 1999;283:2062–2064. doi: 10.1126/science.283.5410.2062. [DOI] [PubMed] [Google Scholar]
- 7.Kirschvink JL, Gaidos EJ, Bertani LE, Beukes NJ, Gutzmer J, Maepa LN, Steinberger RE. Proc Natl Acad Sci USA. 2000;97:1400–1405. doi: 10.1073/pnas.97.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopp RE, Kirschvink JL, Hilburn IA, Nash CZ. Proc Natl Acad Sci USA. 2005;102:11131–11136. doi: 10.1073/pnas.0504878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chumakov NM, Elston DP. Episodes. 1989;12:115–119. [Google Scholar]
- 10.Hambrey MJ, Harland WB. Earth's Pre-Pleistocene Glacial Record. Cambridge, UK: Cambridge Univ Press; 1981. [Google Scholar]
- 11.Young GM, von Brunn V, Gold DJC, Minter WEL. J Geol. 1998;106:523–538. [Google Scholar]
- 12.Chen JY, Bottjer DJ, Oliveri P, Dornbos SQ, Gao F, Ruffins S, Chi HM, Li CW, Davidson EH. Science. 2004;305:218–222. doi: 10.1126/science.1099213. [DOI] [PubMed] [Google Scholar]
- 13.Holland HD. Geochim Cosmochim Acta. 2002;66:3811–3826. [Google Scholar]
- 14.Xiao SH, Zhang Y, Knoll AH. Nature. 1998;391:553–558. [Google Scholar]
- 15.Bekker A, Holland HD, Wang PL, Rumble D, III, Stein HJ, Hannah JL, Coetzee LL, Beukes NJ. Nature. 2004;427:117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 16.Farquhar J, Bao H, Thiemens M. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. [DOI] [PubMed] [Google Scholar]
- 17.Karhu JA, Holland HD. Geology. 1996;24:867–870. [Google Scholar]
- 18.Catling DC, Claire MW. Earth Planet Sci Lett. 2005;237:1–20. [Google Scholar]
- 19.Canfield DE. Annu Rev Earth Planet Sci. 2005;33:1–36. [Google Scholar]
- 20.Bodiselitsch B, Koeberl C, Master S, Reimold WU. Science. 2005;308:239–242. doi: 10.1126/science.1104657. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman PF, Schrag DP. Terra Nova. 2002;14:129–155. [Google Scholar]
- 22.Kirschvink JL. The Proterozoic Biosphere: A Multidisciplinary Study. Cambridge, UK: Cambridge Univ Press; 1992. [Google Scholar]
- 23.Hutterli MA, McConnell JR, Stewart RW, Jacobi HW, Bales RC. J Geophys Res Atmos. 2001;106:15395–15404. [Google Scholar]
- 24.Hutterli MA, McConnell JR, Chen G, Bales RC, Davis DD, Lenschow DH. Atmos Environ. 2004;38:5439–5450. [Google Scholar]
- 25.Allen M, Yung YL, Waters JW. J Geophys Res Space Phys. 1981;86:3617–3627. [Google Scholar]
- 26.Liang MC, Lane BF, Pappalardo RT, Allen M, Yung YL. J Geophys Res Planets. 2005;110 doi: 10.1029/2004JE002322. [DOI] [Google Scholar]
- 27.Pinto JP, Gladstone GR, Yung YL. Science. 1980;210:183–184. doi: 10.1126/science.210.4466.183. [DOI] [PubMed] [Google Scholar]
- 28.Tian F, Toon OB, Pavlov AA, Sterck HD. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. [DOI] [PubMed] [Google Scholar]
- 29.Catling DC. Science. 2006;311:38. doi: 10.1126/science.1118412. [DOI] [PubMed] [Google Scholar]
- 30.Pierrehumbert RT. Nature. 2004;429:646–649. doi: 10.1038/nature02640. [DOI] [PubMed] [Google Scholar]
- 31.Pierrehumbert RT. J Geophys Res Atmos. 2005;110 doi: 10.1029/2004JD005162. [DOI] [Google Scholar]
- 32.Tajika E. Earth Planet Sci Lett. 2003;214:443–453. [Google Scholar]
- 33.Manatt SL, Manatt MRR. Chem Eur J. 2004;10:6540–6557. doi: 10.1002/chem.200400104. [DOI] [PubMed] [Google Scholar]
- 34.Goodman JC, Pierrehumbert RT. J Geophys Res Oceans. 2003;108 doi: 10.1029/2002JC001471. [DOI] [Google Scholar]
- 35.Abele D, Ferreyra GA, Schloss I. Antarctic Sci. 1999;11:131–139. [Google Scholar]
- 36.Frey MM, Stewart RW, McConnell JR, Bales RC. J Geophys Res Atmos. 2005;110 doi: 10.1029/2005JD006110. [DOI] [Google Scholar]
- 37.Touati D. Arch Biochem Biophys. 2000;373:1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 38.Penner-Hahn JE. Manganese Redox Enzymes. New York: VCH; 1992. [Google Scholar]
- 39.Stumm W, Morgan JJ. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York: Wiley; 1996. [Google Scholar]
- 40.Schopf JW. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- 41.Blankenship RE, Hartman H. Trends Biochem Sci. 1998;23:94–97. doi: 10.1016/s0968-0004(98)01186-4. [DOI] [PubMed] [Google Scholar]
- 42.Nhelko N. Geology. Johannesburg: Rand Afrikaans Univ; 2004. p. 285. [Google Scholar]
- 43.Klein C, Ladeira EA. Econ Geol. 2004;99:1233–1244. [Google Scholar]
- 44.Fennel K, Follows M, Falkowski PG. Am J Sci. 2005;305:526–545. [Google Scholar]
- 45.McKay CP, Hartman H. Origins Life Evol Biosphere. 1991;21:157–163. doi: 10.1007/BF01809444. [DOI] [PubMed] [Google Scholar]
- 46.Kasting JF, Holland HD, Pinto JP. J Geophys Res Atmos. 1985;90:497–510. doi: 10.1029/jd090id06p10497. [DOI] [PubMed] [Google Scholar]