Abstract
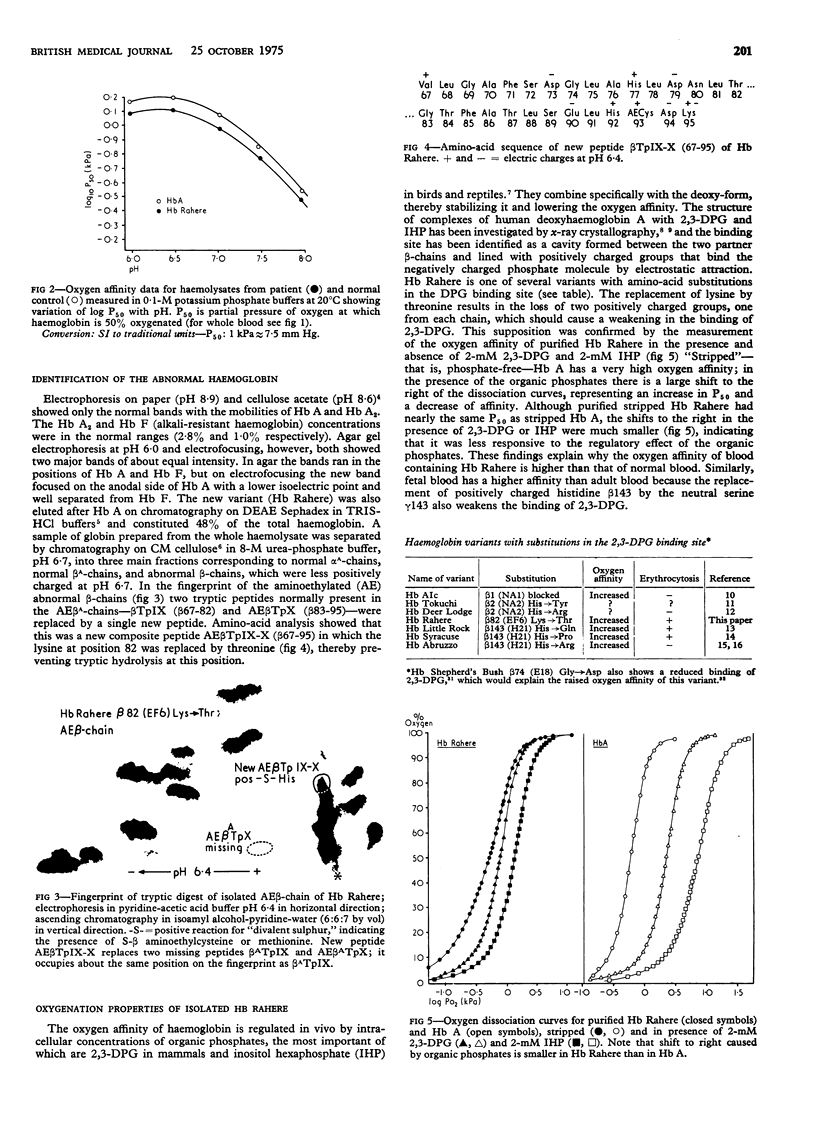
A new haemoglobin with increased oxygen affinity, beta82 (EF6) lysine leads to threonine (Hb Rahere), was found during the investigation of a patient who was found to have a raised haemoglobin concentration after a routine blood count. The substitution affects one of the 2, 3-diphosphoglycerate binding sites, resulting in an increased affinity for oxygen, but both the haem-haem interaction and the alkaline Bohr effect are normal in the haemolysate. This variant had the same mobility as haemoglobin A on electrophoresis at alkaline pH but was detected by measuring the whole blood oxygen affinity; it could be separated from haemoglobin A, however, by electrophoresis in agar at acid pH. The raised haemoglobin concentration was mainly due to a reduction in plasma volume (a relative polycythaemia) and was associated with a persistently raised white blood count. This case emphasises the need to measure the oxygen affinity of haemoglobin in all patients with absolute or relative polycythaemia when some obvious cause is not evident.
Full text
PDF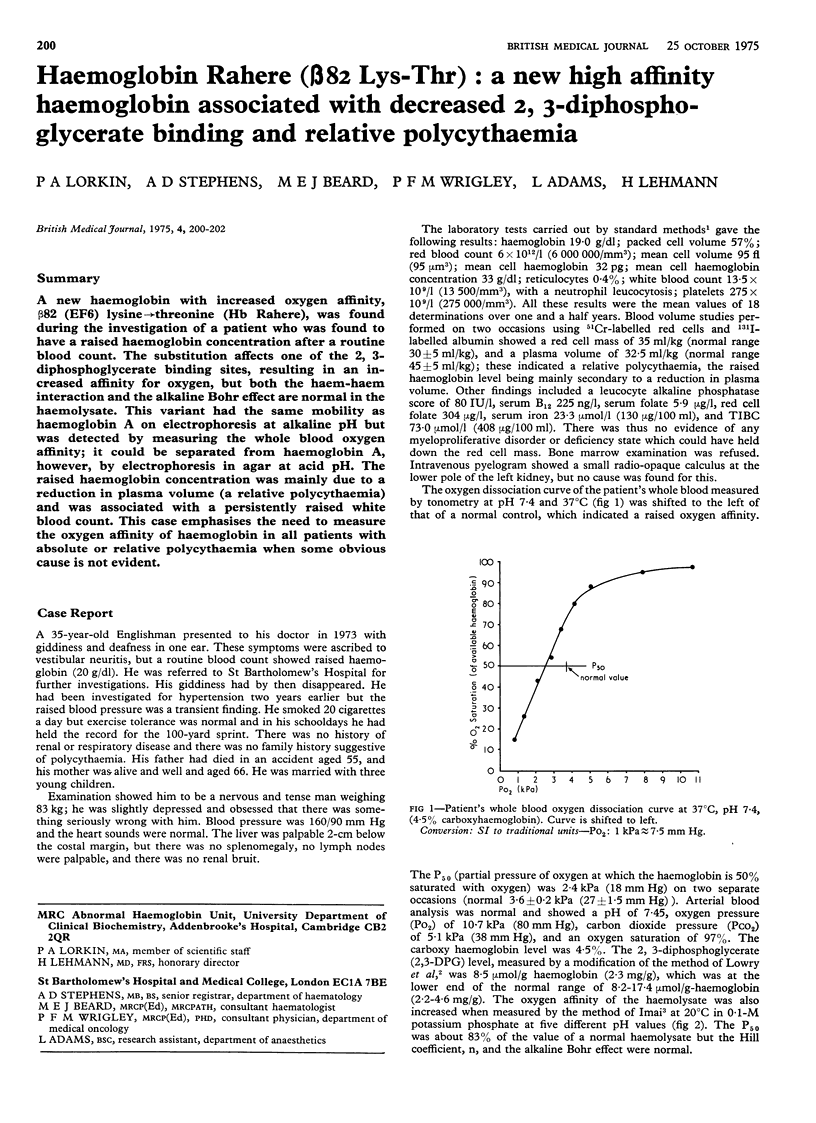

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Botha M. C., Beale D., Isaacs W. A., Lehmann H. Hemoglobin J Cape Town-alpha-2 92 arginine replaced by glutamine beta-2. Nature. 1966 Nov 19;212(5064):792–795. doi: 10.1038/212792a0. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Charache S., Fairbanks V. F., Maldonado J. E., Noyes A., Gayle E. E. Hemoglobin Malmö Beta-97 (FG-4) histidine--glutamine: a cause of polycythemia. J Clin Invest. 1972 Mar;51(3):666–676. doi: 10.1172/JCI106855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg P. A., Alben J. O., Bare G. H., Balcerzak S. P., Jones R. T., Brimhall B., Padilla F. High oxygen affinity variant of haemoglobin Little Rock with unique properties. Nat New Biol. 1973 Jun 6;243(127):177–179. doi: 10.1038/newbio243177a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Brimhall B., Jones R. T. Polycythemia produced by hemoglobin Osler (beta-145 (HC2) Tyr yields Asp). Johns Hopkins Med J. 1975 Mar;136(3):132–136. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Maldonado J. E., Charache S., Boyer S. H., 4th Familial erythrocytosis due to electrophoretically undetectable hemoglobin with impaired oxygen dissociation (hemoglobin Malmö, alpha 2 beta 2 97 gln). Mayo Clin Proc. 1971 Nov;46(11):721–727. [PubMed] [Google Scholar]
- Glynn K. P., Penner J. A., Smith J. R., Rucknagel D. L. Familial erythrocytosis. A description of three families, one with hemoglobin Ypsilanti. Ann Intern Med. 1968 Oct;69(4):769–776. doi: 10.7326/0003-4819-69-4-769. [DOI] [PubMed] [Google Scholar]
- HICKS D. A., HOPE A., TURNBULL A. L., VEREL D. The estimation and prediction of normal blood volume. Clin Sci. 1956 Nov;15(4):557–565. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Hurley P. J. Red cell and plasma volumes in normal adults. J Nucl Med. 1975 Jan;16(1):46–52. [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Jensen M., Oski F. A., Nathan D. G., Bunn H. F. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975 Mar;55(3):469–477. doi: 10.1172/JCI107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Labossiere A., Vella F., Hiebert J., Galbraith P. Hemoglobin Deer Lodge: 2 2 2 His leads to Arg . Clin Biochem. 1972 Mar;5(1):46–50. doi: 10.1016/s0009-9120(72)80007-9. [DOI] [PubMed] [Google Scholar]
- May A., Huehns E. R. The control of oxygen affinity of red cells with Hb-Shepherds Bush. Br J Haematol. 1972 May;22(5):599–607. doi: 10.1111/j.1365-2141.1972.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Morimoto H., Lehmann H., Perutz M. F. Moleuclar pathology of human haemoglobin: stereochemical interpretation of abnormal oxygen affinities. Nature. 1971 Aug 6;232(5310):408–413. doi: 10.1038/232408a0. [DOI] [PubMed] [Google Scholar]
- Tentori L., Carta SORCINI M., Buccella C. Hemoglobin Abruzzo: beta 143 (H 21) His leads to Arg. Clin Chim Acta. 1972 Apr;38(1):258–262. doi: 10.1016/0009-8981(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Yamaoka K. Hemoglobin Hirose: 2 237(C3) tryptophan yielding serine. Blood. 1971 Dec;38(6):730–738. [PubMed] [Google Scholar]


