Abstract
Durum wheat (Triticum turgidum subsp. durum) is more salt sensitive than bread wheat (Triticum aestivum). A novel source of Na+ exclusion conferring salt tolerance to durum wheat is present in the durum wheat Line 149 derived from Triticum monococcum C68-101, and a quantitative trait locus contributing to low Na+ concentration in leaf blades, Nax1, mapped to chromosome 2AL. In this study, we used the rice (Oryza sativa) genome sequence and data from the wheat expressed sequence tag deletion bin mapping project to identify markers and construct a high-resolution map of the Nax1 region. Genes on wheat chromosome 2AL and rice chromosome 4L had good overall colinearity, but there was an inversion of a chromosomal segment that includes the Nax1 locus. Two putative sodium transporter genes (TmHKT7) related to OsHKT7 were mapped to chromosome 2AL. One TmHKT7 member (TmHKT7-A1) was polymorphic between the salt-tolerant and -sensitive lines, and cosegregated with Nax1 in the high-resolution mapping family. The other TmHKT7 member (TmHKT7-A2) was located within the same bacterial artificial chromosome contig of approximately 145 kb as TmHKT7-A1. TmHKT7-A1 and -A2 showed 83% amino acid identity. TmHKT7-A2, but not TmHKT7-A1, was expressed in roots and leaf sheaths of the salt-tolerant durum wheat Line 149. The expression pattern of TmHKT7-A2 was consistent with the physiological role of Nax1 in reducing Na+ concentration in leaf blades by retaining Na+ in the sheaths. TmHKT7-A2 could control Na+ unloading from xylem in roots and sheaths.
Soil salinity causes a significant reduction in agricultural production (Pitman and Läuchli, 2002). More than 6% of the world's arable land is affected by either salinity or sodicity, and a significant proportion of agricultural land has become saline because of land clearing or irrigation (Munns, 2005). To meet this challenge, it is important to understand mechanisms of salt tolerance for further improving salt tolerance of crops, by either traditional breeding or gene manipulation.
In the Triticeae, sodium exclusion is one of the major mechanisms conferring salt tolerance (Gorham et al., 1990; Munns et al., 2006). Bread wheat (Triticum aestivum, AABBDD) has a low rate of Na+ transport to the shoot and maintains a high ratio of K+ to Na+ in leaves (Gorham et al., 1990). The Kna1 locus, contributing to a higher K+ to Na+ ratio and salt tolerance in bread wheat, was mapped to the distal region of chromosome 4DL (Dubcovsky et al., 1996). Durum wheat (Triticum turgidum L. subsp. durum [Desf.], AABB) is more salt sensitive than bread wheat (Rawson et al., 1988; Gorham et al., 1990) and production suffers when grown on saline soil (Francois et al., 1986; Maas and Grieve, 1990). A new source of Na+ exclusion was found in a durum wheat, Line 149, which had low Na+ concentrations and high K+ to Na+ ratios in the leaf blade similar to bread wheat (Munns et al., 2000). Line 149 was derived from a cross between Triticum monococcum (AA) accession C68-101 and the durum cv Marrocos (The, 1973). T. monococcum C68-101, not Marrocos, is the source of the Nax1 gene in Line 149 (James et al., 2006). Genetic studies indicated that two major loci controlled leaf blade Na+ accumulation in Line 149 (Munns et al., 2003). A gene named Nax1, which accounted for 38% of the phenotypic variation for low Na+ concentration, was mapped to the long arm of chromosome 2A (Lindsay et al., 2004). Physiological studies indicated that net xylem loading and leaf sheath sequestration in Line 149 interacted to control leaf blade Na+ concentration (Davenport et al., 2005). Using near-isogenic lines, it was found that the major role of Nax1 in conferring salt tolerance was through greater removal of Na+ from the xylem in the roots and in the leaf sheath, thereby reducing Na+ concentrations in the leaf blade (James et al., 2006). Some members of the HKT family (high-affinity K+ transporter) function as sodium transporters (Rodríguez-Navarro and Rubio, 2006) and play an important role in regulation of Na+ transport in rice (Oryza sativa) and Arabidopsis (Arabidopsis thaliana; Rus et al., 2001; Ren et al., 2005). HKT transporters appear important in control of Na+ transport in bread wheat (Laurie et al., 2002) and may also transport sodium and contribute to salt tolerance in durum wheat.
The rice genome sequence provides a useful reference for comparative genomics in the cereals (Yu et al., 2002). There are currently more than 620,000 wheat expressed sequence tags (wESTs) in public databases, of which more than 8,200 unique wESTs have been mapped to defined chromosome regions using deletion stocks of wheat (Qi et al., 2004; http://wheat.pw.usda.gov/NSF/progress_mapping.html). Wheat chromosome group 2 contains colinear regions with rice chromosomes 4 and 7, while the deletion mapping of wheat genes provides a tool to examine colinearity with rice at the subchromosome level (Sorrells et al., 2003). The rice genome sequence has been successfully used as entry point for positional cloning of important agronomic traits in wheat (Yan et al., 2003; Griffiths et al., 2006). It is therefore feasible to use the rice genome sequence and data from the wEST deletion bin mapping project to identify additional wheat markers for genetic mapping of Nax1 and, perhaps, identify rice genes with close sequence relatedness to candidate genes for Nax1.
The objective of this study was to use wESTs that were previously positioned in the physical deletion bins of wheat chromosome 2AL in conjunction with the rice genome sequence to define a detailed map position and clone a candidate gene for Nax1. We provide evidence that a putative sodium transporter (closely related to OsHKT7) is a candidate gene for Nax1, which may control Na+ unloading from xylem in roots and sheaths as indicated by its expression pattern and physiological role of Na+ partitioning into leaf sheaths.
RESULTS
Exploiting Wheat-Rice Synteny to Identify Markers and Candidate Genes
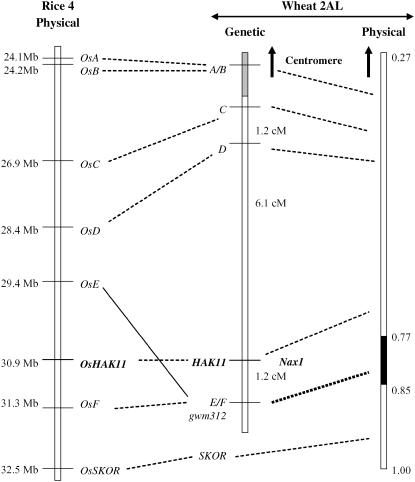
Nax1, a major gene for low Na+ concentration in leaf blades of durum wheat, was mapped as a quantitative trait locus and linked to the microsatellite marker gwm312 on chromosome 2AL (Lindsay et al., 2004). Using a backcross-derived mapping family of 41 BC5F2 lines, Nax1 was determined as a single genetic locus (Fig. 1). In this study, we used wESTs previously mapped to physical deletion bins in ‘Chinese Spring’ wheat as a source of potential markers. The microsatellite gwm312 marker was mapped to the deletion bin flow length (FL) 0.77 to 0.85 (Fig. 2). The position of gwm312 defines the physical interval for Nax1, indicating that the gene was located somewhere between the centromere and distal breakpoint FL 0.85 on chromosome 2AL. Seventy-four wESTs had been mapped into the same physical region that had significant DNA sequence homology to rice genes in the syntenic region of 17.8 to 34.4 Mb on rice chromosome 4L (http://wheat.pwusda.gov/NSF/progress_mapping.html). The putative order for those 74 wESTs was inferred from the corresponding rice gene order and used as basis for developing RFLP markers in durum wheat (Table I). Initially, we selected three wEST markers (A, B, and C) for mapping because the related rice genes spanned the central region of rice chromosome 4L (24.1, 24.2, and 26.9 Mb; see Table I). Both A and B detected polymorphic markers between the donor Line 149 and the recurrent parent Tamaroi. However, only the Tamaroi alleles were present in the BC4 parental line and in the BC5F2 family, suggesting that this chromosomal region was replaced by Tamaroi during the process of backcrossing (Fig. 2). Marker C with sequence relatedness to a rice gene located at 26.9 Mb segregated in the BC5F2 family and mapped 7.3 cM from Nax1 (Fig. 2). To identify additional markers, we therefore focused on wESTs that were closely related to rice genes located distal to 26.9 Mb on chromosome 4L.
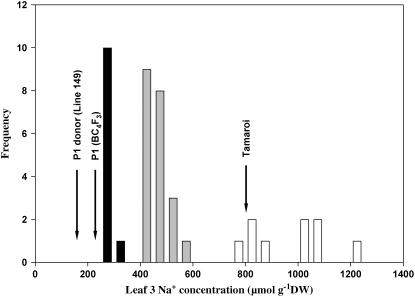
Figure 1.
Frequency distributions for leaf Na+ concentrations of BC5F2 family, grown at 150 mm NaCl for 10 d. The black and white bars represent homozygous lines with low and high Na+ concentration in leaf. The gray bars represent heterozygous lines with medium Na+ concentration in leaf. Arrows indicate parental leaf Na+ concentration means (μmol g−1 DW, n = 6). Line 149: 141 ± 14; P1 (BC4F3): 233 ± 39; Tamaroi: 811 ± 31. (From figure 1A of James et al. [2006].)
Figure 2.
Comparative map of wheat chromosome 2AL and rice chromosome 4 using the low-resolution mapping family. Left, Physical map of rice chromosome 4 constructed from the sequence annotations of rice genes as shown on Gramene (http://www.gramene.org). The solid line connects noncolinear markers. Middle, Genetic map of wheat chromosome 2AL in the low-resolution mapping family. The top region (gray highlight) represents Tamaroi chromatin. Right, Physical mapping of markers into deletion bins on wheat chromosome 2AL.
Table I.
BLASTN search results of selected wESTs located in wheat deletion bin 2AL (0.0–0.85) with rice chromosome 4
| Marker | wEST | BLASTN | Locus | Distance | BLASTX |
|---|---|---|---|---|---|
| Mb | |||||
| A | BE498441 | 5E-15 | LOC_Os04g41040 | 24.14 | oj991113_30.8 (rice [japonica cultivar group]) |
| B | BM137419 | 5E-27 | LOC_Os04g41200 | 24.21 | oj991113_30.22 (rice [japonica cultivar group]) |
| C | BF474590 | 7E-16 | LOC_Os04g45800 | 26.89 | Putative sphingosine kinase (rice) |
| D | BE403863 | 9E-44 | LOC_Os04g48130 | 28.42 | Membrane protein; protein id: At5g07250.1, supported by cDNA: gi_16648761 (Arabidopsis) |
| E | BG262791 | 2E-57 | LOC_Os04g49570 | 29.35 | Ligand-gated ion channel, putative; protein id: At1g42540.1 (Arabidopsis) |
| HAK11 | BE423738a (CK205077) | 5E-58 | LOC_Os04g52390 | 30.91 | Putative potassium transporter (rice [japonica cultivar group]) |
| F | BE403217 | 3E-61 | LOC_Os04g52900 | 31.29 | MRP-like ABC transporter (rice [japonica cultivar group]) |
Five additional markers (D, E, HAK11, F, and SKOR) were developed that corresponded to rice genes located in the distal region of chromosome 4 (28.4–32.5 Mb; Fig. 2). Consistent with the physical location of corresponding rice genes, marker D was mapped as an RFLP proximal to Nax1 (6.1 cM), while marker HAK11 cosegregated with Nax1 (Fig. 2). Marker F corresponding to a rice gene near the distal end of chromosome 4L was also located on the distal side of Nax1 cosegregating with gwm312 (Fig. 2). A break in colinearity was observed with marker E; this marker cosegregated with gwm312, although its predicted map location was on the proximal side of Nax1. The genetic order of wEST markers was confirmed by their physical location within one of three deletion bins on chromosome 2AL, including marker SKOR, which was placed into the distal deletion bin (FL 0.85–1.00) consistent with the location of the corresponding rice gene (Fig. 2). These results suggest that an interstitial segment was rearranged between wheat chromosome 2AL and rice chromosome 4L.
Based on these results, Nax1 was located within a 7-cM genetic interval and was flanked by markers D and F. This genetic interval corresponded to a physical interval between 28.4 and 31.3 Mb on rice chromosome 4L. To identify rice genes that may be related to candidate genes for Nax1, the 3-Mb interval (28.4–31.3 Mb) was searched for genes encoding putative potassium or sodium transporters (http://www.gramene.org). Besides OsHAK11, three additional rice genes were identified with homology to putative potassium transporter (AL817940, OsHAK15) and sodium transporters (BE604162, OsHKT7; BJ472463, OsHKT4) in wheat and barley (Hordeum vulgare; Table II). A high-resolution mapping family was developed to resolve the position of candidate genes relative to Nax1.
Table II.
List of candidate genes for Nax1 on rice chromosome 4La
| Marker | Wheat or Barley EST | E Value | Locus | Distance | BLASTX |
|---|---|---|---|---|---|
| Mb | |||||
| HKT4 | BJ472463 | 2E-43 | LOC_Os04g51820 | 30.51b | Putative Na+ transporter |
| HKT7 | BE604162 | 3E-67 | LOC_Os04g51830 | 30.52 | Putative Na+ transporter |
| HAK15 | AL817940 | 3E-97 | LOC_Os04g52120 | 30.73 | Putative K+ transporter |
| HAK11 | CK205077 | 1E-79 | LOC_Os04g52390 | 30.91 | Putative K+ transporter |
| SKOR | CA498418 | 2E-99 | LOC_Os04g55080 | 32.53 | Cyclic nucleotide and calmodulin-regulated K+ channel |
The HKT and HAK families in rice were systematically named by Garciadeblás et al. (2003) and Bañuelos et al. (2002), respectively.
Based on search results from Gramene database (http://www.gramene.org), OsHKT4 and OsHKT7 located side by side separated by approximately 3 kb on chromosome 4.
Map Position of Candidates Relative to Nax1
To produce a high-resolution mapping family, tightly linked flanking PCR-based markers were required for screening a large number of F2 lines. To investigate the possibility of markers HAK11 and gwm312 flanking Nax1, we developed a cleavage amplification polymorphism sequence (CAPS) marker from CK205077 (Table I; Supplemental Fig. S1) and screened 100 lines with both markers. Three recombinant F2 individuals were identified and phenotyped for Na+ accumulation. Based on these results, the most likely position for Nax1 was in between HAK11 and gwm312. The markers were subsequently used to screen a larger number of F2 lines to identify 22 F2 lines that incorporated recombination events within the HAK11- gwm312 interval (from a total of 864 F2 lines screened).
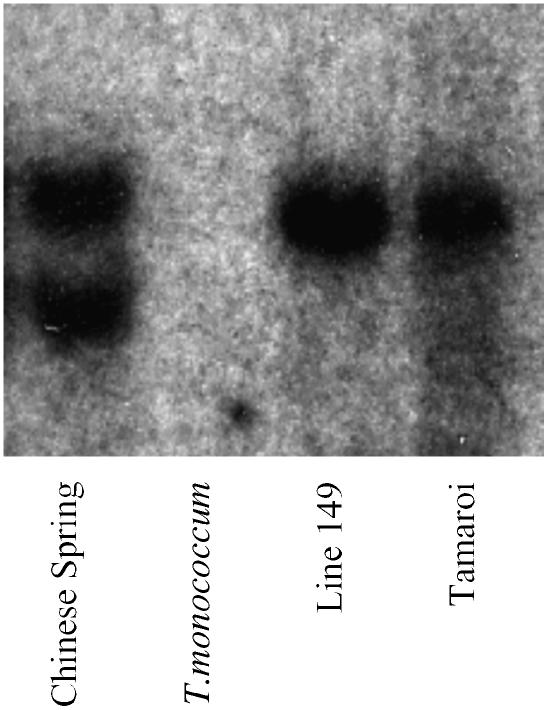
The high-resolution family of 22 F2 lines was used to separate markers (HAK11 and HAK15) derived from putative potassium transporter genes from Nax1 by recombination, ruling them out as candidate genes (Fig. 3). Furthermore, a probe derived from the barley EST BJ472463, closely related to a putative sodium transporter gene (OsHKT4), failed to hybridize to genomic DNA of T. monococcum C68-101 (AA), the donor of Nax1 in Line 149, using five restriction enzymes (EcoRI, EcoRV, HindIII, NcoI, and XbaI; see example in Fig. 4). This result indicated that the A genome of Line 149 had no HKT4-like gene. However, this probe hybridized to at least one and two gene members in tetraploid and hexaploid wheats, indicating that the B and D genomes contained HKT4-like genes (Fig. 4).
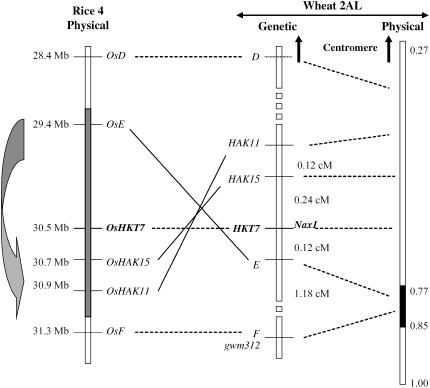
Figure 3.
Comparative map of wheat chromosome 2AL and rice chromosome 4 using the high-resolution mapping family. Left, Physical map of rice chromosome 4 constructed from the sequence annotations on Gramene (http://www.gramene.org). The solid lines highlight the rearrangement between wheat and rice. Middle, Genetic map of Nax1 region using the high-resolution mapping family. Right, Physical mapping of markers into deletion bins of wheat chromosome 2AL. The broad gray arrow on the left indicates the interstitial inversion event.
Figure 4.
DNA gel blot hybridized with barley BJ472463 corresponding to OsHKT4. The genomic DNA was digested by HindIII.
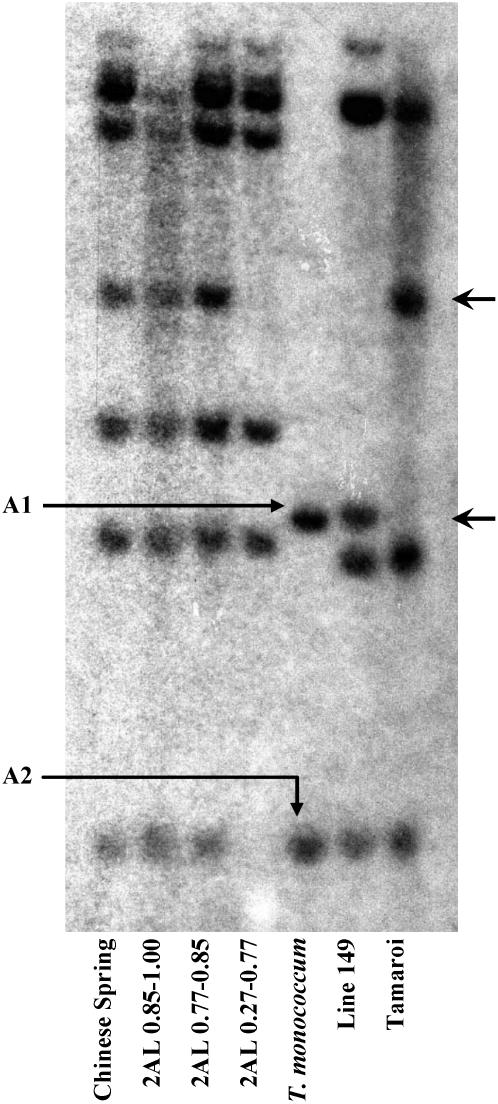
Marker HKT7 cosegregated with Nax1 in the high-resolution mapping family (Fig. 3), suggesting that a HKT7-like gene is a strong candidate for Nax1. The HKT7 probe hybridized to at least two putative gene members in T. monococcum C68-101 (Fig. 5). Line 149 contained both RFLP markers, but only one (HKT7-A1) was polymorphic between Line 149 and Tamaroi and cosegregated with Nax1. The second marker (HKT7-A2) was monomorphic between parents with a range of restriction enzymes but was present in the same deletion bin (FL 0.27–0.77) as HKT7-A1 (Fig. 5). It is possible that the marker HKT7-A2 was part of another candidate gene for Nax1. The HKT7 probe also hybridized to at least four bands in tetraploid (AABB) and six bands in hexaploid (AABBDD) wheat, suggesting that the B and D genomes also carry two copies of HKT7-like genes, respectively (Fig. 5).
Figure 5.
DNA gel blot hybridized with wEST BE604162 corresponding to OsHKT7. The genomic DNA was digested by EcoRV. The arrows on the right side indicate polymorphic allelic bands between Line 149 and Tamaroi which cosegregated with Nax1 in the high-resolution mapping family. The polymorphic and monomorphic alleles in A genome between Line 149 and Tamaroi were named as TmHKT7-A1 and TmHKT7-A2, respectively.
Inversion of Interstitial Region on Chromosome 2AL
The genetic order of HAK11, HAK15, HKT7, E, and F was supported by their physical positions in deletion bins on chromosome 2AL (Fig. 3). The three proximal markers HAK11, HAK15, and HKT7 were also located in the proximal deletion bin FL 0.27 to 0.77, while markers E and F from the distal part of the map were present in the distal deletion bin FL 0.77 to 0.85 (Fig. 3). The physical order of rice genes corresponding to HAK11, HAK15, HKT7, and E was inverted, suggesting that the chromosomal segment between 29.4 and 30.9 Mb was rearranged between wheat and rice. The D and F markers corresponding to rice genes located at 28.4 and 31.3 Mb, respectively, were predicted to flank this interstitial inversion event (Fig. 3).
Isolation of Full-Length HKT7-Like Candidate Genes
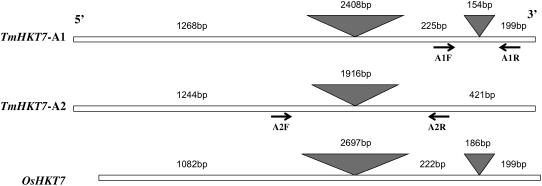
A T. monococcum DV92 bacterial artificial chromosome (BAC) library (Lijavetzky et al., 1999) was screened using wEST BE604162 as probe to isolate full-length sequences corresponding to both TmHKT7-A1 and -A2 gene members. The T. monococcum DV92 had the same low Na+ concentration in leaf blades as T. monococcum C68-101 (data not shown). Nine positive BAC clones were isolated and separated into two groups using a TmHKT7-A1 intron-specific probe (see “Materials and Methods”). Similar fingerprints following digestion with HindIII suggested that BAC clones containing TmHKT7-A1 and TmHKT7-A2 were overlapping (Supplemental Fig. S2). The approximate physical distance between TmHKT7-A1 and TmHKT7-A2 was less than 145 kb based on the estimation of sizes of BAC clone inserts and overlapping fragments (Supplemental Fig. S2). Two open reading frames (ORFs) corresponding to TmHKT7-A1 and TmHKT7-A2 were identified by direct DV92 BAC clone sequencing. The predicted ORF (1,692 bp) of TmHKT7-A1 with two introns shared 88% identity with the predicted ORF of TmHKT7-A2 (1,665 bp), which contained only one intron (Fig. 6). This result was confirmed by the isolation of full-length cDNA of TmHKT7-A2 from T. monococcum C68-101 using reverse transcription (RT)-PCR. Furthermore, the sequences of TmHKT7-A1 and TmHKT7-A2 amplified from T. monococcum C68-101 and Line 149 were 100% identical to those from DV92. At amino acid sequence level, the TmHKT7-A1 and TmHKT7-A2 were 70% and 72% identical to OsHKT7, respectively (Fig. 7). Further amino acid sequence comparisons revealed that TmHKT7-A2 had nine fewer amino acids than TmHKT7-A1, while the OsHKT7 sequence was shorter by 46 amino acids at the N terminus (Fig. 7). A filter Ser residue in P-loop A was present in all three HKT7 transporters (Fig. 7), indicating that they may function as a Na+ transporter (Mäser et al., 2002). Furthermore, TmHKT7-A1 and -A2 shared very similar topological structure except in the N-terminal hydrophilic region (Supplemental Fig. S3). Compared with TmHKT7-A1 and -A2, OsHKT7 lacked the N-terminal hydrophilic tail and had a slight difference in topological structure (Supplemental Fig. S3).
Figure 6.
Gene structures of TmHKT7-A1, -A2, and OsHKT7. The gray triangles represent intron region of the gene. The arrows indicate the primers designed for gene expression analysis (Fig. 8; Supplemental Table S2).
Figure 7.
The alignment of amino acid sequences from putative sodium transporters of TmHKT7-A1, -A2, and OsHKT7. The highlighted black boxes indicate the identical amino acids and the highlighted gray boxes indicate the positive amino acids. The P-loop A regions are covered by an open box. The arrow indicates filter Ser in P-loop A.
Expression of TmHKT7-A1 and TmHKT7-A2
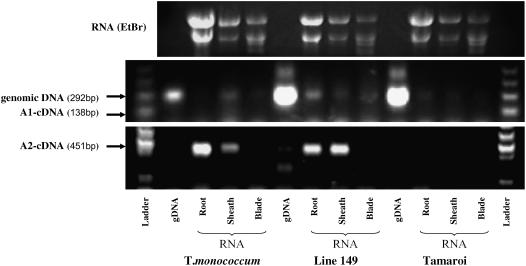
Using gene-specific primers that were flanking introns for RT-PCR analysis, no cDNA product was detected corresponding to TmHKT7-A1 in roots, leaf sheaths, or blades of T. monococcum C68-101, Line 149, or Tamaroi (Fig. 8). This result was confirmed by another pair of specific primers spanning a large intron region (data not shown). However, for TmHKT7-A2 the expected cDNA product was detected in roots and leaf sheaths of T. monococcum C68-101 and Line 149 but not in Tamaroi (Fig. 8). TmHKT7-A2 was not expressed in leaf blades of T. monococcum C68-101 or Line 149, consistent with the physiological role of Nax1 in reducing the Na+ concentration in blades by retaining Na+ in the sheaths (James et al., 2006). Therefore, TmHKT7-A2 is proposed to be the candidate gene for Nax1.
Figure 8.
Expression of TmHKT7-A1 and -A2 in roots, sheaths, and blades of T. monococcum Line 149 and Tamaroi using specific intron-spanning primers (A1F/A1R and A2F/A2R; see Fig. 5; Supplemental Table S2). The expected sizes of genomic DNA (gDNA) and cDNA of TmHKT7-A1 are 292 and 138 bp, respectively. The expected sizes of gDNA and cDNA of TmHKT7-A2 are 2,361 and 451 bp, respectively. There was no amplification of gDNA of TmHKT7-A2 due to spanning a large intron (Fig. 6).
DISCUSSION
We used the rice genome sequence and wESTs mapped in deletion bins to identify markers that assisted in the detailed mapping of Nax1. Comparative mapping results showed that the Nax1 region on wheat chromosome 2AL showed a high level of gene order colinearity with rice chromosome 4L (Fig. 2) and that the rice sequence was useful in identifying candidate gene(s) for Nax1. In another study, good colinearity was found for at least 12 genes in the region containing the vernalization gene Vrn-A1 on chromosome 5AL and the syntenic rice chromosome 3 (Yan et al., 2003). However, colinearity may be interrupted, as observed here by an inversion between wheat 2AL and rice 4L and as reported previously (Brunner et al., 2003; Guyot et al., 2004). Therefore, the success of map-based gene cloning in wheat using a syntenic rice chromosome as reference is dependent on the particular chromosome location of the target gene.
We have developed codominant wEST RFLP markers between Line 149 and Tamaroi for mapping. In all cases, the polymorphic band in Line 149 was the same size as a band in T. monococcum C68-101, while the allelic band in Tamaroi was the same size as the corresponding band in hexaploid ‘Chinese Spring’ (see example in Fig. 5). These results were consistent with our hypothesis that chromosome segment of A genome in Line 149 originates from T. monococcum C68-101 (The, 1973). The A genome in Tamaroi may be more closely related to the A genome in Triticum urartu (AA). Other studies have found A genome-specific markers from tetraploid wheat that were not in T. monococcum but were present in T. urartu (Khlestkina and Salina, 2001). The A genomes of tetraploid (AABB) and hexaploid wheat (AABBDD) may share a common ancestor, and T. urartu is considered to be the closest diploid ancestor surviving today (Dvořák et al., 1988).
The gene copies of HKT members in the wheat genome varied when compared with those in rice (Garciadeblás et al., 2003). The gene corresponding to OsHKT4 was absent in the A genome but is likely to be present in the B and D genomes of wheat (Fig. 4). In rice, OsHKT4 is mainly expressed in shoots (Garciadeblás et al., 2003). There is one copy of OsHKT7 present in the rice genome, but two copies of HKT7-like member in each genome of wheat (Fig. 5). TmHKT7-A2 is unique as it only contains one intron (Fig. 6), while all HKT genes from rice and Arabidopsis have two introns (Uozumi et al., 2000; Garciadeblás et al., 2003). The fact that two HKT7-like genes with different number of introns are located close to each other may indicate that they diversified following duplication to have different functions. TmHKT7-A2 was identified as a candidate gene for Nax1 because it is expressed in root and leaf sheaths but not in leaf blades (Fig. 8). In rice, OsHKT7 was mainly expressed in shoots (Garciadeblás et al., 2003). A barley HKT7-like gene (BQ739876) was also expressed in the leaves of drought-stressed plants (Ozturk et al., 2002). The wEST BE604162 matching OsHKT7 was isolated from a drought-stressed wheat leaf cDNA library, indicating it was also expressed in the leaf tissues (National Center for Biotechnology Information [NCBI] database). BE604162 could be a HKT7 member from the B genome in wheat because the partial nucleotide sequence amplified from Line 149 and Tamaroi were 100% identical to BE604162 (data not shown). It would be interesting to investigate any expression of other HKT7-like genes in roots (Fig. 8).
Other genes belonging to the HKT family have been studied in wheat. TaHKT1 was the first HKT gene cloned from higher plants, showing expression in cortical cells (Schachtman and Schroeder, 1994). The down-regulation (by an antisense construct) of HKT1 in wheat increased shoot fresh weight by 50% to 100% in 200 mm NaCl under conditions of K+ deficiency (Laurie et al., 2002). Following the down-regulation of HKT1, transgenic wheat had smaller Na+-induced depolarizations in root cortical cells than the control and low 22Na+ influx, indicating HKT1 in wheat mediated Na+ influx (Laurie et al., 2002). Further evidence using a root uptake system and a yeast transformation system also supported that TaHKT1 and HvHKT1 (91% identity at amino acid level) functioned as a Na+ uniport (Haro et al., 2005). In Arabidopsis, there is only one sodium transporter, AtHKT1 (Uozumi et al., 2000). AtHKT1 plays a critical role in regulation of Na+ homeostasis (Rus et al., 2001), but its mechanism in conferring salt tolerance is still not clear, as shown by contradictory models for Na+ recirculation (Berthomieu et al., 2003; Sunarpi et al., 2005). In rice, Ren et al. (2005) suggested that OsHKT8 contributed to the maintenance of high shoot K+ and low Na+ accumulation under salt stress in a salt-tolerant cultivar by controlling the unloading of Na+ from the root xylem. TmHKT7-A2, the best candidate for Nax1, could control Na+ unloading from xylem in roots and sheath of durum wheat. This hypothesis is supported by the expression of TmHKT7-A2 in roots and sheath of Line 149 (salt tolerant) but not of Tamaroi (salt sensitive; Fig. 8) and is consistent with the Na+ concentration gradient along the sheath of near-isogenic lines containing Nax1, indicating a retrieval from the sheath xylem, and the greater retrieval of Na+ from the root xylem (James et al., 2006). The removal of Na+ from the xylem resulted in a nearly 4-fold difference in blade Na+ concentration between the low Na+ parental line and Tamaroi (Fig. 1).
In summary, one of two HKT7-like genes (TmHKT7-A2) was identified as a candidate for Nax1. The expression of the TmHKT7-A2 gene in root and leaf sheath tissue of T. monococcum and Line 149 was consistent with the physiological role of Nax1. Functional analysis of TmHKT7-A2 as a sodium transporter using a yeast transformation system is under way. Future work will determine if TmHKT7-A2 is functioning as a sodium transporter in cereals and contributing to salt tolerance by unloading sodium from the xylem in roots and leaf sheaths and by preventing it from accumulating to toxic concentrations in the blade.
MATERIALS AND METHODS
Plant Material and Mapping Families
To generate a low-resolution mapping family, Line 149 (salt tolerant) was crossed with the Australian durum (Triticum turgidum L. subsp. durum) cv Tamaroi (salt sensitive) and backcrossed to produce a homozygous low Na+ BC4F3 line that was used as the parent in an additional backcross (James et al., 2006). The difference in leaf 3 Na+ concentration between low Na+ BC4F3 parent line and Tamaroi was nearly 4-fold (Fig. 1). In this low-resolution mapping family of 41 BC5F2 individuals, the segregation of the sodium exclusion trait fitted the expected ratio for a single major gene (Nax1; expected: 10:21:10; observed: 11:21:9; χ2 = 0.22, P0.05 = 6.00; Fig. 1). Subsequently, a high-resolution mapping family was generated by screening 864 BC5F2 half-seeds without embryos (equivalent to 1,728 gametes) with flanking gwm312 marker and a CAPS marker derived from wEST CK205077 (HAK11; Supplemental Fig. S1). Twenty-two F2 lines that contained recombination events in the marker interval from gwm312 to HAK11 constituted the high-resolution mapping family. At least eight F3 individuals from each F2 plant were phenotyped for Na+ concentration to confirm the phenotypic scores obtained at the F2 generation.
Phenotyping
Plants were grown according to the method of Munns and James (2003). At 8 d after seedling emergence, 25 mm NaCl salt solution was added to the irrigation solution twice daily until a final concentration of 150 mm for low-resolution mapping family and 50 mm for high-resolution mapping family was reached. There was little difference in shoot Na+ concentration between 150 and 50 mm NaCl treatment (Husain et al., 2004). Additional CaCl2 was added to give a final Na+:Ca2+ ratio of 15:1. A lower NaCl concentration was used to phenotype the high-resolution mapping family, to reduce the stress on seedlings that were half-seed derived and less vigorous. Those half-seeds containing embryos with recombination events were treated with the fungicide Thiram (1.4 g/L) and germinated on filter papers with Thiram in sealed sterile dishes at 4°C for 3 d and then at 25°C for 2 d before planting.
Na+ concentration in the blade of the third leaf, 10 d after emergence, was measured according to Munns et al. (2000). Leaves were dried at 70°C for 3 d, extracted in 500 mm HNO3 at 80°C for 1.5 h, and Na+ concentration was measured by an inductively coupled plasma-atomic emission spectrometer (Varian Vista Pro).
DNA Extraction
Plants were transplanted from the salt tanks into soil and allowed to grow for approximately 4 weeks before DNA was extracted as described by Lagudah et al. (1991). For selection of recombinants, a half-seed extraction method according to Mago et al. (2005) was used to isolate DNA from the BC5F2 lines. Four microliters of extracted DNA was used to perform PCR. The embryo sections of recombinants were subsequently germinated for phenotypic analysis.
Flanking Microsatellite and PCR Markers
Primer sequences of flanking microsatellite marker gwm312 were described by Röder et al. (1998). Amplifications were performed in 20-μL aliquots containing 1.5 mm MgCl2, 2 μm each primer, 200 μm dNTPs, 200 μm 1× PCR buffer, 2 units of Taq DNA polymerase, and 100 ng of genomic DNA. The PCR program and gel running conditions were as described by Lindsay et al. (2004). Primer sequences for wEST CK205077 were as follows: forward primer, 5′ACGTTTCCAGGAACCTGATTTGT; and reverse primer, 5′GTTAGAAGAATTTCCCCGCCTTC. PCR products amplified from both parents contained different RsaI restriction sites. This polymorphism was used to develop a CAPS marker (Supplemental Fig. S1).
RFLP Markers
DNA from ‘Chinese Spring,’ 2AL deletion lines (Endo and Gill, 1996), Triticum monococcum accession C68-101, parental lines, and F2 lines was digested with six restriction enzymes (DraI, EcoRI, EcoRV, HindIII, NcoI, and XbaI). DNA hybridization analysis was conducted according to Seah et al. (1998).
Comparative Analysis of Wheat and Rice Sequences
The region containing the Nax1 locus on wheat chromosome 2AL is syntenic with chromosome 4 of rice (Oryza sativa; Conley et al., 2004). A total of 347 wESTs previously mapped to the deletion bin 2AL 0.0 to 0.85 were downloaded from the Graingenes Web site (http://wheat.pwusda.gov/NSF/progress_mapping.html). BLASTN (E value ≤ 10−15 and the length of identity greater than 100 bp) was used to search the NCBI and Gramene databases (http://www.ncbi.nlm.nih.gov/; http://www.gramene.org). Seventy-four of the 347 wESTs detected closely related genes between 17.8 Mb and 34.4 Mb of rice chromosome 4L. Table I lists wESTs with close matches to rice genes, which were used to develop RFLP or PCR-based markers.
Cloning and Sequencing of wESTs
Primers (Supplemental Table S1) were designed on the basis of the published wESTs listed in Tables I and II. The amplified products were cloned using the pGEM-T Easy vector system (Promega) and confirmed by sequencing. DNA probes were amplified by PCR and labeled with 32P using the Megaprime DNA labeling system (Amersham Biosciences). Because there was no matching wEST for OsHKT4 (a putative sodium transporter) in the database, a closely related barley (Hordeum vulgare) EST (BJ472462) was isolated and used as DNA probe.
BAC Clone Screening and Sequencing
High-density filters for the BAC library from T. monococcum accession DV92 (Lijavetzky et al., 1999) were screened with the probe matching wEST BE604162 as shown in Figure 5. Initially, the partial sequences (close to 3′ end) of TmHKT7-A1 and -A2 were amplified using primers designed from BE604162. A 153-bp intron was present in TmHKT7-A1 but no intron in this region was present in TmHKT7-A2. Clones containing TmHKT7-A1 were separated by a probe matching the TmHKT7-A1 intron. Contigs were assembled by BAC clone fingerprints after digestion with HindIII (Supplemental Fig. S2). Direct BAC clone sequencing was used to determine the full-length sequence of TmHKT7-A1 and -A2. BAC DNA template was purified with the BACMAX DNA purification kit (Epicenter).
RNA Extraction and RT-PCR Assay
Plants were grown as described in the phenotyping section. RNA from roots, leaf sheaths, and leaf blades of 8-d-old plants treated with 50 mm NaCl for 48 h was extracted using the Trizol method (Invitrogen). RT-PCR procedures were performed using the OneStep RT-PCR kit (Qiagen) under the following conditions: 50°C for 30 min; 95°C for 15 min; 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 50 s; and then 72°C for 5 min, 25°C for 1 min. The specific spanning intron primers to TmHKT7-A1 and -A2 for RT-PCR analysis are listed in Supplemental Table S2.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EF062819 (TmHKT7-A2) and EF062820 (TmHKT7-A1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The CAPS marker of CK205077 (HAK11).
Supplemental Figure S2. Fingerprints of BAC clones containing TmHKT7-A1 and -A2 after digestion by HindIII.
Supplemental Figure S3. Topological structures of TmHKT7-A1, -A2, and OsHKT7.
Supplemental Table S1. Primer pairs of markers selected for mapping Nax1 gene in chromosome 2AL of durum wheat.
Supplemental Table S2. Primer pairs spanning intron region for expression analysis of TmHKT7-A1 and -A2 using RT-PCR.
Acknowledgments
We thank Karen Glover, Carol Blake, and Lorraine Mason from CSIRO Plant Industry for technical assistance; Dr. Jorge Dubcovsky from University of California, Davis, Dr. Beat Keller from the University of Zurich, and Dr. Rod Wing from Clemson University for providing high-density T. monococcum DV92 BAC library filters and clones; and Dr. Ray Hare from NSW Department of Primary Industries, Tamworth, for providing Line 149 and T. monococcum C68-101.
This work was supported by the Commonwealth Scientific and Industrial Research Organization (postdoctoral fellowship to S.H.), the Grains Research and Development Corporation (to R.M.), and the New South Wales Agricultural Genomic Centre (to J.D.P. and E.S.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shaobai Huang (shaobai.huang@csiro.au).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bañuelos MA, Garciadeblás B, Cubero B, Rodríguez-Navarro A (2002) Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol 130: 784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22: 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Keller B, Feuillet C (2003) A large rearrangement involving genes and low copy DNA interrupts the microlinearity between rice and barley at the Rph7 locus. Genetics 164: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley EJ, Nduati V, Gonzlea-Hernandez JL, Mesfin A, Trudeau-Spanjers M, Chao S, Lazo GR, Hummel DD, Anderson OD, Qi LL, et al (2004) A 2600-locus chromosome bin map of wheat homoeologous group 2 reveals interstitial gene-rich islands and colinearity with rice. Genetics 168: 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiol 137: 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Santa Maria G, Epstein E, Luo MC, Dvořák J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor Appl Genet 2: 448–454 [DOI] [PubMed] [Google Scholar]
- Dvořák J, McGuire PE, Cassidy B (1988) Apparent sources of the A genomes of wheats inferred from polymorphism in abundance and restriction fragment length of repeated nucleotide sequences. Genome 30: 680–689 [Google Scholar]
- Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Hered 87: 295–307 [Google Scholar]
- Francois LE, Maas EV, Donovan TJ, Youngs VL (1986) Effect of salinity on grain yield and quality, vegetable growth, and germination of semi-dwarf and durum wheat. Agron J 78: 1053–1058 [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34: 788–801 [DOI] [PubMed] [Google Scholar]
- Gorham J, Wyn Jones RG, Bristol A (1990) Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta 180: 590–597 [DOI] [PubMed] [Google Scholar]
- Griffiths S, Sharp R, Foote TN, Bertin I, Wanous M, Reader S, Colas I, Moore G (2006) Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439: 749–752 [DOI] [PubMed] [Google Scholar]
- Guyot R, Yahiaoui N, Feuillet C, Keller B (2004) In silico comparative analysis reveals a mosaic conservation of genes within a novel collinear region in wheat chromosome 1AS and rice chromosome 5S. Funct Integr Genomics 4: 47–58 [DOI] [PubMed] [Google Scholar]
- Haro R, Banuelos MA, Senn ME, Berrero-Gil J, Rodríguez-Navarro A (2005) HKT1 mediates sodium uniport in roots. Pitfalls in the expression of HKT1 in yeast. Plant Physiol 139: 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S, von Caemmerer S, Munns R (2004) Control of salt transport from roots to shoots of wheat in saline soil. Funct Plant Biol 31: 1115–1126 [DOI] [PubMed] [Google Scholar]
- James RA, Davenport RJ, Munns R (2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol 142: 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlestkina EK, Salina EA (2001) Genome-specific markers of tetraploid wheats and their putative progenitor species. Plant Breed 120: 227–232 [Google Scholar]
- Lagudah ES, Appels R, McNeil D (1991) The Nor-D3 locus of Triticum tauschii: natural variation and genetic linkage to markers in chromosome 5. Genome 34: 387–395 [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32: 139–149 [DOI] [PubMed] [Google Scholar]
- Lijavetzky D, Muzzi G, Wicker T, Keller B, Wing R, Dubcovsky J (1999) Construction and characterization of a bacterial artificial chromosome (BAC) library for the A genome of wheat. Genome 42: 1176–1182 [PubMed] [Google Scholar]
- Lindsay MP, Lagudah ES, Hare RA, Munns R (2004) A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat. Funct Plant Biol 31: 1105–1114 [DOI] [PubMed] [Google Scholar]
- Maas EV, Grieve CM (1990) Spike and leaf development in salt-stressed wheat. Crop Sci 30: 1309–1313 [Google Scholar]
- Mago R, Miah H, Lawrence GJ, Wellings CR, Spielmeyer W, Bariana HS, McIntosh RA, Pryor AJ, Ellis JG (2005) High-resolution mapping and mutation analysis separate the rust resistance genes Sr31, Lr26 and Yr9 on the short arm of rye chromosome 1. Theor Appl Genet 112: 41–50 [DOI] [PubMed] [Google Scholar]
- Mäser P, Hosoo Y, Goshima S, Horie T, Eckelman B, Yamada K, Yoshida K, Bakker EP, Shinmyo A, Oiki S, et al (2002) Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc Natl Acad Sci USA 99: 6428–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167: 645–663 [DOI] [PubMed] [Google Scholar]
- Munns R, Hare RA, James RA, Rebetzke GJ (2000) Genetic variation for improving the salt tolerance of durum wheat. Aust J Agric Res 51: 69–74 [Google Scholar]
- Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57: 1025–1043 [DOI] [PubMed] [Google Scholar]
- Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253: 201–218 [Google Scholar]
- Munns R, Rebetzke GJ, Husain S, James RA, Hare RA (2003) Genetic control of sodium exclusion in durum wheat. Aust J Agric Res 54: 627–635 [Google Scholar]
- Ozturk NZ, Talamé V, Deyholos M, Michalowski CB, David W, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ (2002) Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol Biol 48: 551–573 [DOI] [PubMed] [Google Scholar]
- Pitman MG, Läuchli A (2002) Global impacts of salinity and agricultural ecosystem. In A Läuchli, U Lüttge eds, Salinity: Environment-Plants-Molecules. Kluwer Academic, Dordrecht, The Netherlands, pp 3–20
- Qi LL, Echalier B, Chao S, Lazo GR, Butler E, Anderson OD, Akhunov ED, Dvorák J, Linkiewicz AM, Ratnasiri A, et al (2004) A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168: 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson HM, Richards RA, Munns R (1988) An examination of selection criteria for salt tolerance in wheat, barley and triticale. Aust J Agric Res 39: 759–772 [Google Scholar]
- Ren Z-H, Gao J-P, Li L-G, Cai X-L, Huang W, Chao D-Y, Zhu M-Z, Wang Z-Y, Luan S, Lin H-X (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37: 1141–1146 [DOI] [PubMed] [Google Scholar]
- Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Canal MW (1998) A microsatellite map of wheat. Genetics 149: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A, Rubio F (2006) High-affinity potassium and sodium transport systems in plants. J Exp Bot 57: 1149–1160 [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee B, Matsumoto TK, Koiwa H, Zhu J-K, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98: 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370: 655–658 [DOI] [PubMed] [Google Scholar]
- Seah S, Sivasithamparam K, Karakousis A, Lagudah ES (1998) Cloning and characterization of a family of disease resistance gene analogs from wheat and barley. Theor Appl Genet 97: 937–945 [Google Scholar]
- Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan W-Y, Leung H-Y, Hattori K, et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44: 928–938 [DOI] [PubMed] [Google Scholar]
- Sorrells ME, Rota ML, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Mahmound MA, Ma X, Gustafson PJ, Qi LL, et al (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13: 1818–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The TT (1973) Transference of resistance to stem rust from Triticum monococcum L. to hexaploid wheat. PhD thesis. University of Sydney, Sydney
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JL (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92 [DOI] [PubMed] [Google Scholar]