Abstract
Our understanding of the interaction of carbon (C) metabolism with nitrogen (N) metabolism and growth is based mainly on studies of responses to environmental treatments, and studies of mutants and transformants. Here, we investigate which metabolic parameters vary and which parameters change in a coordinated manner in 24 genetically diverse Arabidopsis (Arabidopsis thaliana) accessions, grown in C-limited conditions. The accessions were grown in short days, moderate light, and high nitrate, and analyzed for rosette biomass, levels of structural components (protein, chlorophyll), total phenols and major metabolic intermediates (sugars, starch, nitrate, amino acids), and the activities of seven representative enzymes from central C and N metabolism. The largest variation was found for plant weight, reducing sugars, starch at the end of the night, and several enzyme activities. High levels of one sugar correlated with high levels of other sugars and starch, and a trend to increased amino acids, slightly lower nitrate, and higher protein. The activities of enzymes at the interface of C and N metabolism correlated with each other, but were unrelated to carbohydrates, amino acid levels, and total protein. Rosette weight was unrelated or showed a weak negative trend to sugar and amino acid contents at the end of the day in most of the accessions, and was negatively correlated with starch at the end of the night. Rosette weight was positively correlated with several enzyme activities. We propose that growth is not related to the absolute levels of starch, sugars, and amino acids; instead, it is related to flux, which is indicated by the enzymatic capacity to use these central resources.
Plant growth is fueled by photosynthetic carbon (C) assimilation and the assimilation of inorganic nutrients, of which nitrogen (N) is quantitatively the most important. The diurnal alternation of light and dark leads to large changes in the C balance of the plant. These are buffered by accumulating part of the photosynthate as starch in the light and remobilizing it in the dark (Geiger and Servaites, 1994; Gibon et al., 2004b). The C supply is modified by further changes in the environment that affect the rate of photosynthesis, and by environmental and developmental events that alter the balance between photosynthetic “source” organs and growing or storage “sink” organs. In conditions where photosynthesis decreases, for example, short days or low light, an increased proportion of the photosynthate is accumulated as starch during the light period. This allows starch mobilization to continue and sugar levels to be maintained until the end of the dark period (Chatterton and Silvius, 1979, 1981; Gibon et al., 2004b).
Even a few hours of C deficiency lead to changes in the expression of 1,000s of genes (Price et al., 2004; Thimm et al., 2004; Thum et al., 2004; Bläsing et al., 2005), major changes in metabolism, and an inhibition of growth, which is not immediately reversed when C becomes available again (Gibon et al., 2004b; Bläsing et al., 2005). This highlights the importance of coordinating the allocation of C and the rate of growth with the C supply. It also explains why the growth of starchless mutants is inhibited in an alternating light/dark cycle (Gibon et al., 2004b; Bläsing et al., 2005). Recent work has implicated trehalose-6-P as one component of the signaling network, which adjusts C allocation and starch synthesis to changes in the C supply (Kolbe et al., 2005; Lunn et al., 2006). Mutant screens based on the response of seedlings and reporter gene expression to exogenous sugar have identified further genes involved in sugar signaling (Gibson, 2005; Rolland and Sheen, 2005), and uncovered an interaction with abscisic acid and ethylene sensing (Gibson, 2005; Rolland and Sheen, 2005; Rook et al., 2006).
C metabolism interacts closely with N metabolism (Stitt and Krapp, 1999; Krapp et al., 2005). The reducing equivalents, ATP and C skeletons that are required to convert nitrate and ammonium into amino acids, nucleotides, proteins, nucleic acids, cofactors, and other N-containing metabolites, are generated by photosynthesis or, in nonphotosynthetic tissues, carbohydrate breakdown. N metabolism is severely impaired when photosynthesis is decreased, for example, in short-day conditions (Matt et al., 1998) or in genotypes with reduced Rubisco expression (Matt et al., 2002). N metabolism is regulated transcriptionally and posttranscriptionally in response to the C supply. Sugar induction was first noted for genes encoding nitrate reductase, nitrite reductase (Cheng et al., 1992; Vincentz et al., 1993), and nitrate transporters (Lejay et al., 1999, 2003). Transcript profiling subsequently revealed that changes in the sugar supply lead to alterations in the expression of hundreds of genes involved in nitrate uptake, reduction, ammonium metabolism, amino acid synthesis, as well as protein synthesis and nucleotide metabolism (Price et al., 2004; Thimm et al., 2004; Bläsing et al., 2005). Sugars stimulate nitrate reductase translation and activity (Kaiser et al., 1999) and decrease NR degradation (Moorhead et al., 1999).
Our understanding of the impact of the C supply on C allocation, N metabolism, and growth derives mainly from studies of experimentally induced transitions, characterization of mutants and transformants with altered activities of enzymes in central metabolism, and investigations of signaling pathway mutants (see above). Natural diversity provides a rich source of change-of-function alleles, which have been selected during evolution. The use of natural diversity has been deepened by advances in quantitative genetics and technologies for gene mapping, which allow rapid cloning of genes involved in a particular process (Lukowitz et al., 2000; Peters et al., 2003). It has been extensively utilized, for example, to identify elements of the flowering signaling pathways or pathogen responses (for review, see Koornneef et al., 1998, 2004). Quantitative trait loci (QTLs) associated with aspects of carbohydrate metabolism (Causse et al., 1995; Eshed and Zamir, 1995; Mitchell-Olds and Pedersen, 1998; Prioul et al., 1999; Chen et al., 2001; Sergeeva et al., 2004), N metabolism (Hirel et al., 2001; Rauh et al., 2002; Loudet et al., 2003a; Harrison et al., 2004), and C-N interactions (Rauh et al., 2002; Loudet et al., 2003a; Krapp et al., 2005) have been identified in Arabidopsis (Arabidopsis thaliana) and a variety of crop plants. An important tomato (Lycopersicon esculentum) QTL affecting sugar levels and the agronomically important BRIX trait has been identified as a change-of-function mutant in a cell wall invertase (Fridman et al., 2004).
Natural variation can be used to identify QTLs and eventually genes that affect a particular metabolic parameter or trait. It can also be used to uncover correlations between parameters across large sets of genotypes, which may provide insights into the structure of physiological, metabolic, or regulatory networks (see e.g. Poorter et al., 2005). This article addresses the second goal. We recently established sensitive microplate-based assays, which allow rapid measurements of metabolites (Gibon et al., 2002) and enzyme activities (Gibon et al., 2004a) in C and N metabolism. This study investigates the natural variation of a small set of metabolic parameters that cover four different levels of function: enzymes, transitory metabolites, semipermanent structural components, and rosette weight as an indicator for biomass production and growth. A set of 24 natural Arabidopsis accessions was used instead of a population generated from a single cross, to sample a wide range of genetic diversity. The plants were grown in conditions in which C was limiting and N in excess, to focus on metabolic responses related to C allocation and the response of N metabolism. The aims were (1) to investigate which metabolic parameters vary in these conditions, and (2) to ask if any patterns emerge that provide a basis for working hypotheses about the relation between the levels of central metabolites, enzyme activities, and rosette weight.
RESULTS
Genotypic Analysis of the Accessions
The Arabidopsis natural accessions Bch-1, Bla-3, Bur, C24, Col-0, Ei, Eil-0, Est, Ler, Lip-0, Mt-0, Nd-0, Ob-0, Pa-1, Pla-0, Rsch-0, Sol-0, Ta, Te, Tsu-0, Wil, Ws, Yo-0, and Ze-0 were selected as representative of different latitudes and altitudes. They derive from a larger set of 406 accessions, which have been genotyped with 115 single-nucleotide polymorphism (SNP) markers (Törjék et al., 2003; Schmid et al., 2006). The genetic diversity of the 24 accessions was compared with (1) the Versailles core collection of 24 accessions that was defined based on DNA sequence data from 10 loci analyzed in 265 accessions (McKhann et al., 2004) and (2) a core collection of 24 accessions defined with the program Mstrat (Gouesnard et al., 2001) using the genotypic data for all of the aforementioned 406 accessions. The markers used for genotyping are comprised of 114 biallelic and one triallelic markers, representing 231 different alleles. All the polymorphisms defined by these markers were present in the set of accessions from this study and the core collection defined with Mstrat, whereas the Versailles core collection included only 196/231 of the polymorphisms. The 115 SNPs were developed for polymorphisms between C24 and Col-0. These two genotypes are included in the present set of accessions but were excluded from this comparison. Diversity scores (Nei's index) calculated with the Mstrat program were 35.1 in the core collections defined from the genotyping of 406 accessions (data not shown), 30.9 in the set used in this study, and 23.6 in the Versailles collection. This indicates that the 24 accessions in our set capture a large amount of the genotypic diversity present in the larger collection of 406 accessions.
Parameters Investigated
Suc, reducing sugars, starch, and Glc-6-P (Glc6P; a metabolic intermediate shared between glycolysis and Suc and starch metabolism) were assayed to provide information about C availability. Nitrate, total amino acids, Glu, and Asp were measured to provide information about the availability of N and the levels of reduced N intermediates in the plant. Protein and chlorophyll a were measured as representatives of structural components, and total phenolics as representatives of defense compounds. Ratios were calculated for a small number of parameters; the Suc to reducing sugar and sugar to starch ratios provide information about differences in C partitioning, and the amino acids to nitrate ratio provides an indirect measure of the rate of inorganic N assimilation. Seven enzyme activities were measured to provide information about the capacity for different metabolic processes. The enzymes were chosen because robust and simple assays were available at the start of the project. All assays were optimized to measure maximum catalytic activity (Gibon et al., 2004a). The cytosolic isoform of phosphoglucose isomerase (PGIcy) is involved in Suc synthesis and glycolysis in the cytosol, and the plastid isoform of phosphoglucose isomerase (PGIch) is involved in photosynthetic starch synthesis and glycolysis in the plastid. Fumarase was chosen as an enzyme primarily involved in mitochondrial respiration; phosphoenolpyruvate carboxylase (PEPC) as an anapleurotic enzyme that supplies C for use in organic acid metabolism, amino acid synthesis, and other biosynthetic pathways; Ala aminotransferase (AlaAT) and Asp aminotransferase (AspAT) as representatives for central N metabolism; and Glu dehydrogenase (GluDH) as involved in amino acid catabolism.
Plants were harvested 5 weeks after germination. For the last 3 weeks, they were grown in short-day conditions (8 h light/16 h dark) in well-fertilized soil. The main experiment, in which samples were harvested during the last hour of the day, was repeated three times at intervals of 3 to 4 months (termed end-of-day experiments 1, 2, and 3). Nitrate, total amino acids, protein, chlorophyll, and the enzyme activities do not show large diurnal changes in Arabidopsis in these growth conditions (Gibon et al., 2004a; data not shown). Soluble sugars and starch levels undergo marked diurnal changes (Gibon et al., 2004b). They were analyzed at the end of the night in two additional experiments (termed end-of-night experiments 1 and 2).
Rosette fresh weight (FW) was analyzed as an indirect estimate of plant growth. Rosette dry weight (DW) was analyzed in two experiments (termed DW experiments 1 and 2). Comparison of rosette FW and DW revealed an extremely good agreement with R values of 0.99 and 0.98 (see Supplemental Table S1), showing that rosette FW is a good measure of overground biomass. In a separate experiment with 17 of the 24 accessions, the relative growth rate (RGR) was estimated from images of the leaf area of 4- and 5-week-old plants (see Supplemental Table S2). Leaf area correlated well with rosette FW (R = 0.87), showing it provides a reasonable indirect estimate of plant size (see Supplemental Table S2). The RGR of the accessions varied between 122 and 207 mg g−1 per day. This is in the same range as earlier studies (Purves and Law, 2002; Tholen et al., 2004). RGR correlated with leaf area (R = 0.72) and rosette FW at the time of harvest (R = 0.61). In the following experiments, rosette FW was measured on a routine basis because it could be determined more accurately than DW or RGR in these small samples. Further, this allowed the sample to be completely used for analyses of enzymes and metabolites. Underground plant biomass was not analyzed.
Statistical Analysis of Total and Genetically Determined Variation
A two-way ANOVA with accessions and environment as main effects and an accession × experiment interaction effect was performed to determine the proportion of the variance explained by the accessions (genetic variance). Table I presents the F statistics, the proportion of the variance explained by the accessions and by the interaction between accessions and experiments. In addition, it lists the minimum, maximum, and coefficient of variation of least-square means for accessions calculated across experiments. For all traits, the genetic variance was significant with the exception of chlorophyll a, phenolics, and protein (Table I). For FW, a genetic variance of 50% was recorded. The genetic variance of carbohydrates varied between 17% for starch at the end of the day and 61% for starch at the end of the night, with high levels of explained variance also for total sugars at the end of the night (44%), and for reducing sugars (40%) and total sugars at the end of the day (34%). N metabolites exhibited values of 30% for Asp, 14% for total amino acids, 13% for nitrate, and only 7% for protein. Genetic variances of enzyme activities ranged between 43% for PGIch and 19% for AlaAT. All parameters, with the exception of chlorophyll a, phenolics, and total sugars at the end of the night, showed highly significant genotype × experiment interaction effects. These results may be because, despite randomization of the plants, heterogeneities in the growth chambers have a large effect on the recorded trait variation. In addition, some difference in calibration of analytic methods between experiments that were carried out and analyzed at intervals of 3 to 4 months may have contributed to experimental variation. The original data for all the parameters and individual experiments, information about the distribution of points around median and potential outliers, and the full ANOVA analysis are provided in the supplemental material (Supplemental Tables S1, S3, and S4).
Table I.
Variation in metabolic parameters relating to central C and N metabolism in 24 Arabidopsis accessions
Three independent experiments were performed harvesting plants at the end of the day and two experiments harvesting plants at the end of the night. Each experiment included five samples for each accession, and each sample contained three pooled rosettes. The entire data set was analyzed using a two-way ANOVA with the accession and experiment as main effect and the accession × experiment as interaction effects. The maximum and minimum values are given as μmol/g FW for all metabolites, μmol g FW−1 min−1 for enzyme activities, and g for rosette FW. Abbreviations not given in text: Redsug, reducing sugars; ΣSug, total sugars at the end of the day; ΣSug_eon, total sugars at the end of the night; Starch, starch at the end of the day; Starch_eon, starch at the end of the night; Nitr, nitrate; AA, amino acids; AA/nitr, amino acids to nitrate ratio; Phen, phenolics; Chl, chlorophyll a; Protein, protein; Fum, fumarase.
| Trait | Effecta | F Statisticb | R2c | Probabilityd | Minimume | Maximume | CVf |
|---|---|---|---|---|---|---|---|
| % | |||||||
| AA | G | 1.92 | 14 | * | 12.4 | 18.9 | 10 |
| G × E | 2.31 | 15 | *** | ||||
| AA/nitr | G | 2.59 | 21 | ** | 0.077 | 0.119 | 13 |
| G × E | 2.69 | 16 | *** | ||||
| AlaAT | G | 1.77 | 19 | * | 1.72 | 3.42 | 16 |
| G × E | 3.28 | 20 | *** | ||||
| Asp | G | 2.64 | 30 | * | 1.72 | 2.98 | 13 |
| G × E | 1.72 | 11 | * | ||||
| AspAT | G | 1.92 | 20 | * | 1.89 | 3.17 | 15 |
| G × E | 2.86 | 20 | *** | ||||
| Chl | G | 1.73 | 4.1 | ns | 0.583 | 0.787 | 7.6 |
| G × E | 1.36 | 4.7 | ns | ||||
| Fru | G | 4.40 | 38 | *** | 0.389 | 1.99 | 48 |
| G × E | 8.37 | 17 | *** | ||||
| Fum | G | 3.58 | 36 | *** | 0.849 | 1.60 | 17 |
| G × E | 3.10 | 19 | *** | ||||
| FW | G | 2.89 | 50 | ** | 0.095 | 0.331 | 31 |
| G × E | 5.22 | 17 | *** | ||||
| Glc | G | 8.83 | 39 | *** | 1.43 | 3.31 | 26 |
| G × E | 1.87 | 8.8 | ** | ||||
| Glc6P | G | 1.74 | 18 | * | 0.102 | 0.175 | 12 |
| G × E | 2.94 | 21 | *** | ||||
| Glu | G | 2.91 | 25 | ** | 2.50 | 3.80 | 11 |
| G × E | 1.96 | 8.4 | ** | ||||
| GluDH | G | 0.89 | 12 | ns | 0.128 | 0.186 | 12 |
| G × E | 2.44 | 26 | *** | ||||
| Nitr | G | 3.54 | 13 | *** | 138 | 197 | 8.4 |
| G × E | 2.79 | 7.4 | *** | ||||
| PEPC | G | 1.85 | 21 | * | 0.345 | 0.626 | 16 |
| G × E | 2.83 | 22 | *** | ||||
| PGIch | G | 5.31 | 43 | *** | 0.539 | 1.98 | 34 |
| G × E | 2.90 | 15 | *** | ||||
| PGIcy | G | 5.63 | 42 | *** | 0.437 | 2.47 | 22 |
| G × E | 3.15 | 14 | *** | ||||
| PGI Total | G | 2.04 | 21 | * | 2.01 | 3.34 | 12 |
| G × E | 2.63 | 20 | *** | ||||
| Phen | G | 1.79 | 16 | ns | 1.79 | 2.45 | 6.2 |
| G × E | 1.12 | 8.9 | ns | ||||
| Protein | G | 1.57 | 7.4 | ns | 10.3 | 13.01 | 5.4 |
| G × E | 1.50 | 9.4 | * | ||||
| Redsug | G | 9.50 | 40 | *** | 1.88 | 5.26 | 30 |
| G × E | 2.31 | 8.4 | *** | ||||
| Starch | G | 2.38 | 17 | ** | 38.9 | 54.4 | 8.0 |
| G × E | 2.22 | 14 | *** | ||||
| Starch_eon | G | 8.89 | 61 | *** | 1.98 | 7.18 | 28 |
| G × E | 1.84 | 6.8 | * | ||||
| Suc | G | 2.71 | 25 | ** | 2.92 | 4.75 | 12 |
| G × E | 2.34 | 18 | *** | ||||
| ΣSug | G | 6.26 | 34 | *** | 1.40 | 2.35 | 15 |
| G × E | 2.02 | 11 | *** | ||||
| ΣSug_eon | G | 6.96 | 44 | *** | 4.80 | 9.34 | 18 |
| G × E | 1.42 | 6.4 | ns |
G, main effect of the genotype, the Arabidopsis accession; G × E, interaction effect between the genotype and the experiment.
F values calculated by ANOVA (“Materials and Methods”).
RG2 and R(G×E)2, proportion of the genetic variance, which is explained by the main effect of the genotype or explained by the G × E interaction effect, respectively.
The significance thresholds for F statistics were *, P < 0.05; **, P < 0.01; and ***, P < 0.001. ns, Not significant.
Minimum and maximum of least-square means of trait value for accessions across experiments.
Coefficient of variation (CV) for trait values using least-square means of the accessions.
Variation of Different Parameters
The largest variation is found for reducing sugars at the end of the day, starch at the end of the night, rosette FW, and the enzyme activities (Table I). For most of these parameters, 20% to 50% of the total variation was genetically determined (see above). The least variable parameters are starch at the end of the day, nitrate, protein, and phenolics. About 90% of the starch accumulated in the light is remobilized at night in all accessions (see Supplemental Table S1). This and the high nitrate levels are consistent with the plants being C rather than N limited.
Correlation Analysis of Parameters across the Entire Data Set
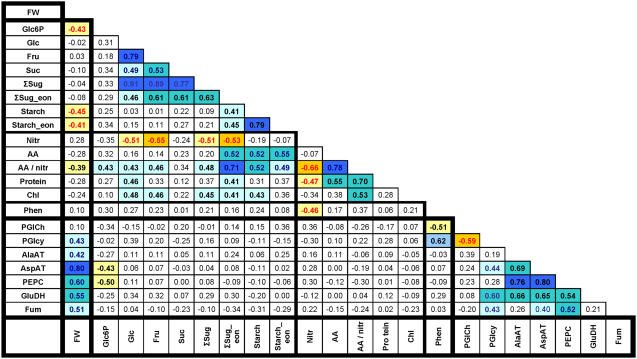
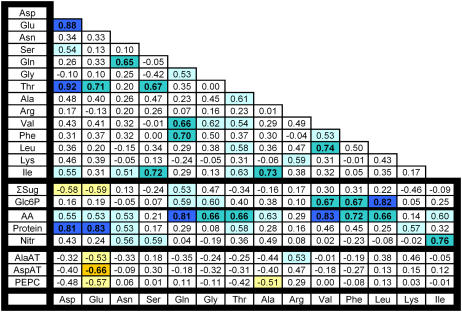
The data were analyzed in two ways to detect trends across the entire set of 24 accessions. In the first, Pearson correlation analysis was performed on the least-square means averages for each parameter pair (Fig. 1). Values are given for the correlation coefficient (R). Parameter pairs that are positively or negatively correlated with a significance greater than P < 0.05, P < 0.01, and P < 0.0001 are highlighted by shading (see legend for details). The values in Figure 1 were derived from parameters expressed on a FW basis. Correlations were also determined for parameters expressed on a DW basis. Both calculations produced virtually identical results (data not shown). As Glu and Asp did not yield any reproducible correlations except for positive relations with total amino acids, these parameters are omitted.
Figure 1.
Genetic correlations (Pearson coefficients) between different metabolic parameters in 24 accessions. For calculating correlations, the least-square means of the metabolites of each accession averaged across three experiments were used. The results are based on three independent experiments for end-of-day measurements and two experiments for end-of-night and DW studies. Relations that are significant at P < 0.0001, P < 0.01, and P < 0.05 are indicated by dark, medium, and light shading, with positive and negative correlations being distinguished by blue and orange. The original data are given in Supplemental Tables S1 and S3.
Glc, Fru, and Suc are strongly and positively correlated with each other (Glc versus Fru, R = 0.79; Glc versus Suc, R = 0.49; Fru versus Suc, R = 0.53) and the overall sugar level. Starch levels at the end of the day showed relatively little variation and were unrelated to sugar levels. Starch showed more variation at the end of the night and was positively correlated with sugars at this time (R = 0.45). Nitrate showed little variation in these growth conditions. It was weakly negatively correlated with Glc (R = −0.51) and Fru (R = −0.55). Total amino acids showed a positive correlation with sugars (R = 0.20) and starch at the end of the day (R = 0.52). The amino acids to nitrate ratio was positively related to starch at the end of the day (R = 0.52). These results indicate a link between the carbohydrate levels and the conversion of nitrate to amino acids (see “Discussion”). Leaf protein showed a weak positive trend to sugars at the end of the day (R = 0.37) and total amino acids (0.55), a negative trend with nitrate, and positive correlation with the amino acids to nitrate ratio (R = 0.70). Phenolics were unrelated to sugars and amino acids, and displayed a negative trend against nitrate.
There was a striking positive correlation between the activities of PEPC and AspAT (R = 0.80) and of these two enzymes with GluDH (R = 0.54 and 0.65; Fig. 1). These three enzyme activities also correlated, although less strongly, with PGIcy and fumarase (R = 0.21–0.65). They were unrelated to PGIch. Similar results were obtained, irrespective of whether the results are expressed on a FW (Fig. 1), DW, or protein basis (data not shown). The enzyme activities varied independently of sugar, starch, nitrate, and amino acid levels. There was a weak but significant negative correlation of AspAT and PEPC to the glycolytic intermediate Glc6P (R = −0.43 and −0.50).
Rosette FW varied considerably, with about half of the total variation being genetically determined (see above). Rosette FW was unrelated to sugar levels and negatively related to starch at the end of the day and night. Rosette FW showed a weak positive trend compared to nitrate and a weak negative trend compared to total free amino acids (R = −0.28), the amino acid to nitrate ratio (R = −0.39), and protein (R = −0.28), but none was significant except for the amino acids to nitrate ratio. Plant weight was unrelated to phenolics. Organic acids represent a major C pool in leaves (Chia et al., 2000). Malate and fumarate were analyzed in a separate experiment, using 17 of the 24 accessions. Their summed level lay between 4 and 14 μmol/g FW, which is in the same range as total soluble sugars, but lower than total amino acids and considerably lower than starch (data not shown). Malate (R = −0.16) and fumarate (R = 0.07) levels were unrelated to FW.
Rosette FW correlated positively with several enzyme activities, including AspAT (R = 0.80), PEPC (R = 0.60), GluDH (R = 0.55), fumarase (R = 0.51), AlaAT (R = 0.42), and PGIcy (R = 0.43). The changes of enzyme activity are not due to changes of total leaf protein; this is documented by the absence of any correlation between enzyme activities and protein content (see Fig. 1). High enzyme activities were not accompanied by higher levels of carbohydrates or total amino acids. There was a weak but significant negative correlation of AspAT and AlaAT to the glycolytic intermediate Glc6P (R2 = −0.27 and −0.43). In other cases, metabolites were unrelated to enzyme activities.
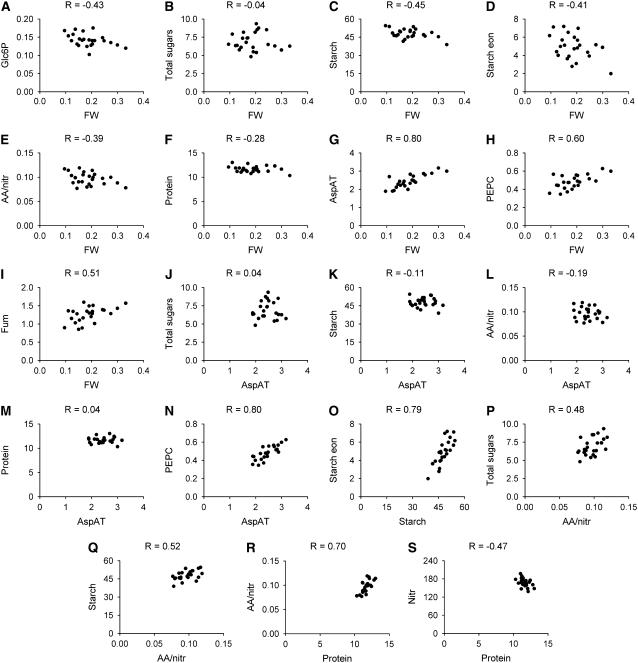
The relationship between the least-square means of selected pairs of parameters is shown in Figure 2. Each accession is shown as a discrete data point. Rosette FW is weakly negatively correlated with Glc6P (Fig. 2A), unrelated to total sugars (Fig. 2B), weakly negatively related to starch at the end of the day (Fig. 2C) and night (Fig. 2D), unrelated or slightly negatively related to the amino acid to nitrate ratio (Fig. 2E) and protein (Fig. 2F), and positively related to several enzyme activities, including AspAT (Fig. 2G), AlaAT (not shown), PEPC (Fig. 2H), and, more weakly, fumarase (Fig. 2I). AspAT is shown as an example of the enzyme activities. It is unrelated to total sugars (Fig. 2J) and starch (Fig. 2K), amino acids (not shown), the amino acids to nitrate ratio (Fig. 2L), and the protein content (Fig. 2M), but correlates with PEPC activity (Fig. 2N) and FW (Fig. 2F). There is a strong correlation between the starch level at the end of the day and the end of the night (Fig. 2O). This section also shows that all of the accessions turn over most of their starch. However, accessions with larger rosettes tend to have marginally lower starch at the end of the day and much lower starch at the end of the night (Fig. 1). The amino acids to nitrate ratio shows a weak positive relation with total sugars (Fig. 2P) and starch (Fig. 2Q). The protein content shows a weak positive relation to the amino acids to nitrate ratio and weak negative relation to nitrate (Fig. 2S).
Figure 2.
Scatter plots of the least-square mean values for selected parameters across 24 accessions. For each accession and parameter, the least-square mean average was calculated across the entire data set (see Fig. 3; Supplemental Table S5). The sections show scatter plots between the averages for selected parameters. The units are μmol/g FW for all metabolites, μmol g FW−1 min−1 for enzyme activities, and g for rosette FW. A to I, Rosette FW (x axis) versus Glc6P (A), total sugars (B), starch at the end of the day (C), starch at the end of the night (D), the amino acids to nitrate ratio (E), protein content (F), AspAT activity (G), PEPC activity (H), and fumarase activity (I). J to N, AspAT activity (x axis) versus total sugars (J), starch at the end of the day (K), the amino acids to nitrate ratio (L), protein content (M), and PEPC activity (N). O, Starch at the end of the day (x axis) versus starch at the end of the night. P and Q, The amino acids to nitrate ratio (x axis) versus total sugars (P) and starch at the end of the day (Q). R and S, Protein content (x axis) versus the amino acids to nitrate ratio (R) and nitrate content (S). The R values for each correlation are given in the sections.
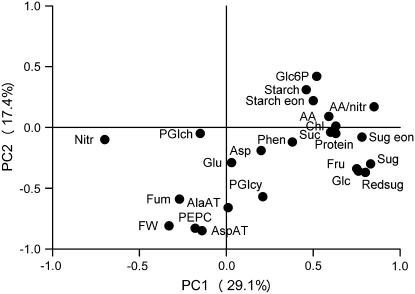
Principal Components Analysis
Principal components analysis was carried out on the least-square means average, using the genotypes as variables. The distribution of metabolites, enzyme activities, and rosette FW in the first and second components is shown in Figure 3. These two components contributed 47.4% of the total variation. All the carbohydrates group together with amino acids, chlorophyll, and protein. Nitrate is strongly separated from the other metabolites. Rosette FW was clearly separated from carbohydrates, amino acids, nitrate, protein, and chlorophyll, but grouped closely with several enzyme activities, especially AspAT and PEPC. This analysis confirms and summarizes the general trends identified in Figures 1 and 2.
Figure 3.
Principal components analysis for 24 metabolites. Principal components analysis was performed on the correlation matrix of least-square means of accessions averaged across experiments (see Table I; Supplemental Table S5). Numbers in parentheses give the percent variation explained by the first and the second principal component. The figure shows the resulting distribution of metabolites, enzyme activities, and FW.
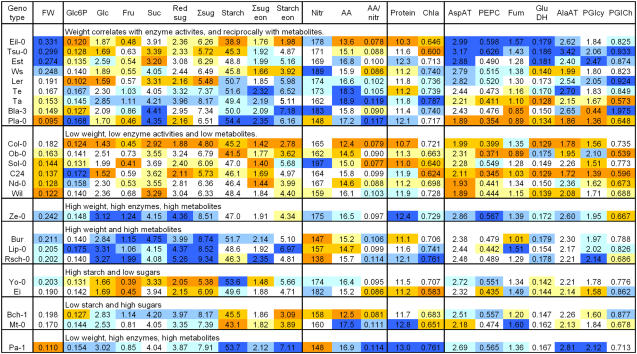
Responses of Individual Accessions
It is evident from Figure 2 that some accessions deviate from the general trends. Figure 4 lists the least-square means averages for each parameter and accession, and color codes them according to their ranking. To aid visual inspection, the accessions are subjectively grouped according to the relationship between the ranking of rosette FW, enzyme activities (especially PEPC and AspAT, which correlate best across the entire set with weight), and metabolites (especially carbohydrates and amino acids). An xls file is provided in the supplemental material (Supplemental Table S5) to allow the data to be sorted in other ways.
Figure 4.
Least-square mean values of the metabolic parameters in individual accessions. For each accession and parameter, the least-square mean average was calculated across the entire data set. This least-square mean is given in the figure. Units are μmol/g FW for all metabolites, μmol g FW−1 min−1 for enzyme activities, and g for rosette FW. Each parameter was color coded, with deep, medium, and light blue indicating first-, second-, and third-, fourth-, fifth-, and sixth-, and seventh-, eighth-, and ninth-ranked genotypes, and light, medium, and deep orange indicating the 16th-, 17th-, and 18th-, 19th-, 20th-, and 21st-, and 22nd-, 23rd-, and 24th-ranked genotypes, respectively. The accessions are ranked according to the rosette FW in this figure. The accessions were then organized into groups that show a qualitatively similar response. The data are provided in xls format as Supplemental Table S5, to allow sorting according to other parameters. For abbreviations, see Figure 1.
Some accessions closely match the general pattern found by analyzing the overall data set. Thus, Eil-0, Tsu-0, Est, Ws, and Yo-0 (which rank first, second, third, fourth, and eighth in rosette weight) have high rosette weight, average or below-average carbohydrates and amino acid levels, and high enzyme activities, and Pla-0, Bla-3, and Ta (which rank 24th, 18th, and 17th in rosette weight) have low rosette weight, average or above-average carbohydrates and amino acids, and low enzyme activities. Other accessions show a deviating response. Some still show the correlation between rosette weight and enzyme activities, but these parameters show a positive rather than negative trend with carbohydrates and amino acids. Examples include Wil, Ob-0, Nd-0, C24, Sol-0, and Ob-0 that show the trend to low rosette weight, low enzyme activities, and low carbohydrate and amino acid levels, Ze-0 that has high rosette weight, enzyme activities, and carbohydrate and amino acid levels, and Yo-0 that has high rosette weight, enzyme activities, and starch and amino acids but low soluble sugars. In four accessions, the relationship between rosette weight and enzyme activity breaks down. In three of these (Bur, Lip-0, and Rsch-0), an above-average rosette weight is accompanied by high levels of sugars, amino acids, and, to a lesser extent, starch, but only mid-ranking AspAT and PEPC activity. In Pa-1, low rosette weight is accompanied by above-average enzyme activities. The high protein content in this accession may be responsible for the high enzyme activities. The overall picture is that the majority of accessions follow the general positive trend between rosette weight and enzyme activities, but there is no overall trend of rosette weight with metabolites because roughly similar numbers of accession show a positive and negative trend of metabolites with rosette weight. This is reflected in Figure 2, B and J, where a group of accessions with high sugars also have relatively large rosettes and high AspAT activity.
Carbohydrates were strongly correlated with each other in most of the 24 accessions. There were five exceptions; Yo-0 and Ei have high starch and low sugar, Bch-1 and Mt-0 have high sugar and low starch, and Pla-0 has high Suc and low reducing sugars. This indicates specific changes in the regulation of starch or Suc synthesis, and invertase activity. Most accessions also followed the general trend of a positive correlation between carbohydrates and amino acids and the amino acids to nitrate ratio. There were four exceptions; Bur, Lip-0, Rsch-0, and Bch-1 had high carbohydrates but relatively low amino acid levels. All of these accessions had below-average nitrate, and three (Bur, Lip-0, and Rsch-0) had a relatively large rosette.
Variation of Individual Amino Acids
Measurements of the total free amino acid pool might mask changes of individual amino acids. The immediate product of nitrate and ammonium assimilation is Gln, followed by Glu. The amino group from Glu is transferred by AlaAT and AspAT to form Ala and Asp, using C skeletons provided via PEPC. Glu, Asp, and Ala provide the amino groups for most other amino acids. In C3 leaves, photorespiration leads to the rapid formation of Gly, which typically increases in the light (Matt et al., 2001a, 2001b).
Individual free amino acids were determined by OPA-HPLC in 10 accessions (Bch-1, Bla-3, Bur, C24, Col-0, Lip-0, Nd-0, Wil, Ws, and Ze-0) in one experiment. The levels and variation of the individual amino acid levels are provided in Supplemental Table S6. The majority of the total amino acid pool (approximately 86%) is composed of the central amino acids Gln, Glu, Ala, Asp, Gly, and Ser (Supplemental Table S6, last column; see also Stitt and Krapp, 1999). Figure 5 summarizes the relations between individual amino acids and other metabolic parameters in these 10 accessions. Significant correlations (P < 0.05) are bolded. Gln is positively correlated with Glc6P and sugars, as expected if the initial steps of nitrate assimilation are activated by sugars (see introduction). Gln is also positively correlated with Gly. The latter is the most variable amino acid (Supplemental Table S6). Glu, Asp, and Ala are positively related to each other (especially Asp and Glu) but only weakly positively related to Gln. Most minor amino acids are weakly but positively related with each other, and with Glu, Asp, and Ala. Some minor amino acids are also related to Gln (Leu, Val, Phe), nitrate (Ile, Trp), or Glc6P and sugar (Leu, Val, Phe) levels. Some are related to the protein content, but none with FW. The major central amino acids (Glu, Ala, Asp) are unrelated or even negatively correlated with PEPC, AspAT, and AlaAT activity (Fig. 5). Minor amino acids were also unrelated to these enzyme activities.
Figure 5.
Individual amino acids. Individual amino acid levels were determined by HPLC in 10 accessions (Bch-1, Bla-3, Bur, C24, Col-0, Lip-0, Nd-0, Wil, Ws, and Ze-0) in one sample experiment. Pearson correlation coefficients were calculated for each pairwise comparison of the metabolic parameters. Significant correlations (0.65 or more, P < 0.05) are bolded. Color coding is as follows: very light blue, correlations of 0.5 to 0.65; blue, correlations of 0.65 to 0.8; dark blue, correlations above 0.8; pale yellow, correlations of −0.65 to −0.5; and orange, correlations of −0.8 to −0.65. The original data are provided in Supplemental Table S6.
DISCUSSION
Natural Variation of Metabolites, Enzyme Activities, and Rosette Size
Natural diversity provides a complementary approach to uncover features of regulatory networks, which may have been overlooked in studies of physiological responses or individual mutants. This article investigates the variation of soluble sugars and starch, nitrate and amino acids, structural components, plant weight, and the activities of seven representative enzymes from central metabolism across 24 Arabidopsis accessions. The plants were grown in short-day conditions and low light with excess nitrate to characterize the response of metabolism and growth in conditions where C is limiting. Metabolites and enzymes from N metabolism were included because (see introduction) it is very responsive to changes in the C supply. The accessions were genotyped using a set of 115 SNP markers evenly distributed through the Arabidopsis genome. Taking every marker as a separate point in the genome, the accessions represented most of the global diversity present in a larger set of 406 accessions.
The first aim was to identify metabolic parameters that show large genetic variation. These will be useful for future studies that exploit natural diversity to identify genes and dissect regulatory networks that regulate C allocation. The most variable parameters included rosette weight, reducing sugars, starch at the end of the night, and, maybe unexpectedly, the activities of several enzymes. The least variable parameters included starch at the end of the day, nitrate, total protein content, and phenolics. Two-way ANOVA revealed that 20% to 50% of the variation in rosette weight and several metabolites and enzyme activities were genetically determined. The low variation and heritability for nitrate and protein may reflect the growth conditions, which were selected to ensure N was in excess.
High heritability has been reported for traits like root architecture 55% to 63% (Loudet et al., 2005), flowering time 86% to 92% (el-Lithy et al., 2006), and seed germination (Clerkx et al., 2004). Heritability is often lower for metabolic traits. This may be partly due to the smaller variation of these traits, the precision of scoring, or a larger dependence on environmental factors. In analyses of Arabidopsis recombinant inbred line (RIL) populations, a heritability of 20% to 60% was found for metabolic traits in leaves like the fractional contribution of N to DW, and phosphate, nitrate, and amino acid (Loudet et al., 2003a, 2003b), and 14% to 49% for starch, Suc, and reducing sugar levels (Calenge et al., 2006). The slightly lower values in our studies may be because we analyzed a large range of genotypically diverse accessions, rather than a RIL population. Heritabilities were 15% to 32% for total plant and leaf mass and leaf area, 0% to 23% for photosynthesis, C, and N content (reported in RILs generated by crossing two Hordeum spontaneum lines; Poorter et al., 2005), and 69% for nitrate in a maize (Zea mays) RIL population (Hirel et al., 2001). Less information is available with respect to the heritability of variation for enzyme activity. A similar range to ours (25%–55%) was reported for 10 enzyme activities in an Arabidopsis RIL population (Mitchell-Olds and Pedersen, 1998). Higher heritability (75%–85%) was obtained for nitrate reductase and Gln synthetase in maize RIL lines (Hirel et al., 2001).
Epistatic interactions can mask phenotypic variation between accessions. This becomes evident as transgressive segregation in progeny from crosses between populations (e.g. Koornneef et al., 1998, 2004). In three pilot crosses between C24 and Bch-1, Col-0, or Nd-0, no evidence was found for transgressive segregation for sugar, starch, amino acid, protein, and nitrate levels in the F2 generation (data not shown). However, we cannot exclude the possibility that more phenotypic diversity would emerge in segregating populations from other parents. Calenge et al. (2006) recently reported transgression for starch in a Bay-0 × Sha RIL population.
Coordinated Responses of Metabolites
The major aim was to identify metabolic parameters that correlate with each other across the 24 accessions. Such correlations could provide insights into the structure of metabolic and regulatory networks. In addition to searching for general trends across the entire set of metabolites, we also compared the responses in individual genotypes. Interpretation could be complicated if differences in biomass are due to secondary changes, for example, increasing exhaustion of nutrients (Matt et al., 2002). Such problems are minimized by the present experimental design, where plants were grown in short-day conditions under relatively low light and supplied with ample nutrients. All of the accessions accumulated large amounts of nitrate and underwent almost near-complete diurnal turnover of starch, confirming that growth was not limited by N and probably also not by other nutrients.
Different soluble sugars correlated with each other and, less strongly, with starch across the 24 accessions, revealing that some accessions maintain higher levels of carbohydrates than others. The weaker correlation to starch might be due to the relatively low variation for starch at the end of the day. Starch also correlated with sugars at the end of the night. Plants accumulate starch during the day and remobilize it at night (Geiger and Servaites, 1994). Rapidly growing plants exhaust almost all their starch during the night. Extension of the night by a few hours leads to complete exhaustion of starch and triggers major changes in metabolism and gene expression (Thimm et al., 2004; data not shown), including an inhibition of growth that is not immediately reversed when the plants are reilluminated (Gibon et al., 2004b). Our results indicate that different Arabidopsis accessions differ with respect to the extent to which they exhaust starch in a regular light/dark cycle (see below for further discussion). In a small number of accessions (e.g. Yo-0, Ei, Bch-1, Mt-0, and Pla-0), shifts between individual carbohydrates were found, indicating changes in the allocation strategy.
Sugars and, to a lesser extent, starch show a weak negative trend with nitrate and a positive trend with total amino acids. As a result, carbohydrate levels are positively related to the amino acid to nitrate ratio. This relation was found in the majority of the accessions. A small number of accessions (Bur, Lip-0, Rsch-0, and Bch-1) had high carbohydrate and low amino acid levels, indicating that poising of C and N metabolism might be shifted in these accessions. In one experiment, the levels of individual amino acids were analyzed in 10 accessions to provide a more differentiated picture. Sugar levels were positively related with Gln, unrelated or negatively related to Glu, Asp, and Ala, and had no consistent relation with most of the minor amino acids. The correlation between carbohydrates and Gln is consistent with a stimulation of nitrate assimilation by sugar (see introduction). The unaltered or lower levels of Glu, Asp, and Ala and the absence of a clear trend to higher levels of minor amino acids in accessions with higher sugar levels indicate that high sugar stimulates amino acid utilization more strongly than the first steps of inorganic N assimilation (see below for further discussion). This notion is supported by the observation that sugar addition to C-depleted seedlings leads to the coordinated induction of many genes that are required for protein synthesis after 3 h (Price et al., 2004; Thimm et al., 2004) and a decrease of minor amino acid levels after 3 to 8 h (Osuna et al., 2006).
Coordinated Changes of Enzyme Activities
Mitchell-Olds and Pedersen (1998) reported positive genetic correlations between activities of five enzymes of central C metabolism (total activity of phosphoglucose isomerase, phosphoglucomutase, Fru-bisphosphatase, Glc6P dehydrogenase, and Glc-6-phosphatase) in an Arabidopsis RIL population. Our results strengthen the evidence that natural variation can generate coordinated changes of enzyme activities. They also allow an integration of information changes of enzyme activities, metabolites, and rosette composition and size (see below).
Several of the enzyme activities varied by >2-fold. These changes were positively correlated across most of the 24 accessions, irrespective of whether activity is compared on a FW, DW, or total protein basis. The strongest correlation is between PEPC and AspAT. The correlation was weaker for AlaAT, GluDH, fumarase, and PGIcy, while PGIch behaved in a rather independent manner. The strongly correlated enzymes have related functions. PEPC is involved in the production of C skeletons for the synthesis of amino acids and other metabolites that are derived from the tricarboxylic acid cycle, and AspAT and AlaAT are involved in the exchange of amino groups between different organic acids and central amino acids that act as amino donors during amino acid metabolism.
Enzyme activity was assayed in optimized assays with saturating concentrations of substrates and, where known, activators (Gibon et al., 2004a). An increase of activity in these conditions might be due to a posttranslational modification that is stable after extraction, a change in the protein structure leading to a higher kcat, or an increase of expression. The first possibility is unlikely because the assays were designed to avoid changes in activity due to known mechanisms for posttranslational regulation and because most posttranslational modifications are reversed quite quickly after preparing extracts (see Gibon et al., 2004a). The second also appears unlikely because it would require that polymorphisms in the structural genes of several enzymes are coordinately inherited across large numbers of accessions. The most likely explanation is that there is variation in a signaling pathway that coordinately regulates the expression of these enzymes.
There are several reports that a QTL for an enzyme activity is located in a genomic region that contains a structural gene for the enzyme (Mitchell-Olds and Pedersen, 1998; Prioul et al., 1999; Hirel et al., 2001; Obara et al., 2001). Many enzymes are involved in a particular process and most enzymes are encoded by small gene families. There is therefore a high probability that a large genomic region contains a potential candidate. Proof that an enzyme activity QTL is due to a polymorphism in a gene for a pathway enzyme requires cloning of the variant genes and analysis of the expression and characteristics of the encoded proteins (Fridman et al., 2004). In some cases, the QTL for an enzyme activity clearly does not map to the vicinity of a structural gene for an enzyme (Rauh et al., 2002; Loudet et al., 2003a; Krapp et al., 2005). In a study of six glycolytic enzymes, Mitchell-Olds and Pedersen (1998) reported that a QTL mapped close to the position of a structural gene in three cases, whereas for three other enzymes the activity changed in a correlated manner and the QTL was not located in the vicinity of a potential structural gene, indicating that it might be a shared regulatory locus.
Relationship between Enzyme Activities, Metabolite Levels, and Rosette Size
The rosette FW correlated with rosette DW and, for a large subset of the accessions, with RGR in the week before harvest, showing that the rosette FW is an indirect indicator of the rate of growth. Rosette FW showed considerable variation between accessions. It was negatively correlated to starch, unrelated to sugars, amino acids, and organic acids, and was positively correlated to several enzyme activities in central metabolism.
If increased growth is due to a higher rate of photosynthesis, a lower rate of respiration, or faster N assimilation, rosette size should correlate positively with the levels of carbohydrates or amino acids. This was seen for some accessions (Bur, Lip-0, Rsch-0, and Ze-0). However, when all 24 accessions are analyzed, larger rosette size is associated with a trend to slightly decreased levels of carbohydrates and amino acids, and smaller rosette size with higher carbohydrates and amino acids. Calenge et al. (2006) recently reported 39 QTLs for starch and sugar levels in a Bay-0 × Sha RIL population, several of which colocalized with each other. They found a positive correlation between rosette DW and starch, and more weakly with sugars. This apparently differs from our results. Carbohydrates were measured at the beginning and end of the light period in our study, when they are at their diurnal minimum and maximum, whereas they were measured 2 h into the photoperiod by Calenge et al. (2006). It is unclear at present if their result reflects a specific aspect of the Bay-0 × Sha RIL population or if faster growth is linked with especially rapid starch accumulation in the first hours of the light period.
Leaf area is known to be an important determinant of growth rates in low irradiance growth regimes (Poorter and Remkes, 1990; Poorter et al., 2005). Investing less protein per unit of leaf biomass allows a larger leaf area, which increases light interception and photosynthesis on a whole-plant basis. Pla-0 had the highest leaf protein content of the 24 accessions we studied but a small rosette. However, when the entire set is analyzed, leaf protein did not vary greatly, and was only weakly and nonsignificantly related to rosette size.
Rosette size would be increased if a larger proportion of the photosynthate was invested in shoot growth. Our data do not allow us to directly assess this possibility. However, the shoot to root ratio will be high in these N-replete plants, and it appears unlikely that changes in shoot-root allocation could be wholly responsible for the differences in rosette size.
Rosette size was positively correlated with the activities of several enzymes in central metabolism, especially PEPC and AspAT. These enzymes are involved in the generation of C skeletons and the transfer of amino groups for amino acid synthesis. Increased enzymatic capacity will allow fluxes to be increased, without this requiring higher levels of metabolites; indeed, it may even lead to decreased levels of metabolites that are upstream of the enzymes in the metabolic network. This is consistent with our observation that rosette weight is often unrelated or weakly negatively correlated with carbohydrate levels.
Our results support the hypothesis that one of the factors that contributes to the faster C utilization and growth in Arabidopsis is increased catalytic activity of enzymes in central C and N metabolism. Faster-growing accessions often have lower levels of carbohydrates, including lower starch and sugars at the end of the night, than slower-growing accessions, prompting the second hypothesis that faster-growing accessions are less “conservative” than slower-growing accessions and hold less carbohydrate in reserve as starch to cope with unexpected fluctuations of the conditions. As discussed in Poorter et al. (2005), growth often correlates with high rates of photosynthesis and respiration per unit leaf mass. It is possible that this may be partly due to increased activities of enzymes. It should be stressed that our data do not show that the enzymes investigated in this article are key or “limiting”; it is possible that many other enzymes in the pathways show similar changes of activity (compare with Mitchell-Olds and Pedersen, 1998). Further, higher metabolic fluxes alone will not be sufficient to drive faster growth. The relatively low proportion of the total variation explained by the genetically determined changes (15%–40%) and the strength of the correlations (R = 0.5–0.8) highlight that changes in enzyme activities are probably just one of the outputs from a regulatory network, which acts on metabolism and growth processes.
In conclusion, replicated experiments with a set of 24 genotypically diverse Arabidopsis accessions have revealed that a larger rosette is frequently accompanied by higher activities of enzymes in central C and N metabolism, and unaltered or slightly decreased levels of central C and N metabolites. These results indicate that increased growth is driven by increased fluxes due to higher catalytic capacity, rather than increased levels of metabolites. This underlines the importance of combining transcript and metabolite analyses with measurements of enzyme activities. Enzyme activities might be useful markers for growth potential. Changes in enzyme activity integrate inputs that act at different levels in the regulatory system (Gibon et al., 2004a), including transcription, posttranscriptional processing, translation, and protein turnover. Measurements of enzyme activities also have the advantage, compared to transcript levels or metabolite levels, of being less susceptible to noise caused by short-term changes of the conditions (Gibon et al., 2004a). To test these suggestions, it will be necessary to extend these analyses to a larger range of growth conditions and genotypes, and to link them with strategies to identify genes that generate these coordinated changes of enzyme activities.
MATERIALS AND METHODS
Reagents
Reagents NAD+, NADH, NADP+, and NADPH and all enzymes except invertase were purchased from Roche. All other reagents and enzymes were obtained at Sigma-Aldrich.
Arabidopsis Accessions
Arabidopsis (Arabidopsis thaliana) accessions used in this study are Bch-1, Bla-3, Bur, C24, Col-0, Ei, Eil-0, Est, Ler, Lip-0, Mt-0, Nd-0, Ob-0, Pa-1, Pla-0, Rsch-0, Sol-0, Ta, Te, Tsu-0, Wil, Ws, Yo-0, and Ze-0. They were obtained from various sources: Col-0 from G. Rédei (University of Missouri, Columbia, MO); C24 from J.P. Hernalsteens (Vrije Universiteit, Brussels); Ler from M. Koornneef (Wageningen University, Wageningen, The Netherlands); and Bch-1, Eil-0, Lip-0, Rsch-0, Te, and Yo-0 from S. Misera (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany); all others were retrieved from the Nottingham Arabidopsis Stock Centre, through which all accessions are now available. Accessions were homogenized by single-seed propagation and were bulk amplified prior to the analysis (Törjék et al., 2003).
For analysis of genetic diversity, genotypes of a total of 406 Arabidopsis accessions were analyzed using 115 SNP markers (Törjék et al., 2003; Schmid et al., 2006). Heterozygous genotypes of SNPs were excluded from further analysis (<2%). The genetic diversity of the 24 accessions was compared with that of (1) the Versailles core collection of 24 accessions that was defined based on DNA sequence data from 10 loci analyzed in 265 worldwide accessions (McKhann et al., 2004) and (2) a core collection of 24 accessions defined with the program Mstrat (Gouesnard et al., 2001). All 406 accessions and 115 SNPs were used in Mstrat to generate a core collection of 24 accessions with maximal allelic richness. To do this, 100 core collections were generated independently, and the core set with the highest Nei index was retained. The three core collections were characterized by the number of alleles captured and Nei's diversity index (Nei, 1987) as calculated by Mstrat. Mstrat calculates Nei's diversity index as 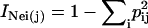 , where pij represents the ith class frequency of the jth variable and the index over all variables is the sum of the individual indices
, where pij represents the ith class frequency of the jth variable and the index over all variables is the sum of the individual indices 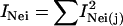 .
.
Plant Growth and Sampling
Seeds were germinated and grown for the first 7 d on a 2:1 (v/v) mix of GS90 soil (composition: peat, clay, coconut fiber, 2 g/L salt, 160 mg/L N, 190 mg/L P2O5, 230 mg/L K2O, pH 6, supplied by Werner Tantau Gmb & Co. KG) and vermiculite (Gebrüder Patzer), with a daylength of 16 h, temperature 6°C at night and 20°C during daytime, humidity 75%, and luminosity 145 μmol m−2 s−1 with color fluorescent lamps HFT 36/830 and HFT 36/840 (Hagemeier). After 7 d, seedlings were transferred to a controlled walk-in growth chamber. Growth was continued in an 8-h-light/16-h-dark regime at temperatures and humidities of 16°C and 75% at night and of 20°C and 60% during the day. Illumination was 145 μmol m−2 s−1 with color fluorescent lights HFT 36/830 and HFT 36/840 (Hagemeier), except for the first and last half hours of the day when intensity was reduced to 50% of the original level. At the age of 2 weeks, plants of average sizes were transferred to separate pots 6 cm in diameter and filled as for germination. Plants were switched to a controlled reach-in growth chamber after 1 further week in the short-day conditions outlined above. Daylength was then 8 h, temperature a constant 20°C, and illumination an average 125 μmol m−2 s−1 with color fluorescent lamp F15T8TL741 (Philips Lighting Company). Plants were watered daily.
Plants were harvested 5 weeks after germination. Five independent samples of three whole rosettes per sample were gathered per accession for metabolite or enzymatic measurements. Those were immediately frozen in liquid N. Sampling was performed in the last hour of the day or the night, and was completed within 30 min. DW evaluation involved five samples of two whole rosettes per accession. The experiment was repeated three times for end-of-day measurements (end-of-day experiments 1–3), twice for end-of-night evaluations (end-of-night experiments 1 and 2), and twice for DW determination (DW experiments 1 and 2), over a period of 18 months. Plants were randomized to distribute individuals of a given accession with respect to growth chambers, levels, and position on the shelf. A different randomization pattern was used in each experiment. Each repeat was performed in a different set of reach-in growth chambers.
Growth Analysis
On two time points (8 and 1 d before the harvest), leaf area plant was measured. For each genotype, leaf area was determined for 15 individual plants. Pictures were taken using a digital camera (Canon IXY65) and analyzed using MRI Cell Image analyzer software (Montpellier RIO Imaging; see http://www.mri.cnrs.fr). From these measurements, RGR (mg g−1 d−1) was calculated using the classical approach (Hunt, 1982):
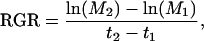 |
where M1 and M2 are the plant mass at times t1 and t2, respectively.
Metabolite and Enzyme Extraction and Measurements
Metabolites were extracted twice with 80% ethanol and once with 50% ethanol. Starch and protein were extracted as described previously (Hendriks et al., 2003). Enzymes were extracted as described previously (Gibon et al., 2004a), except that vortexing was avoided. For all assays, standards were assayed on each microplate. Each parameter was assayed in duplicate with extracts randomized across microplates. Samples were randomized during extraction and analysis to avoid evaluating a given parameter for all extracts of a particular accession on a single microplate. Standards were included on each microplate as measuring references.
Protein amounts were assessed with the Bio-Rad Bradford reagent (Bio-Rad Laboratories) according to the manufacturer's instructions. Starch and Glc6P were measured as described previously (Gibon et al., 2002; Hendriks et al., 2003). Sugars were detected as described by Stitt et al. (1989) with volumes adapted to a microplate format. Reaction mix included 1.14 mg of extract, 80 mm HEPES, pH 7.0, 2.5 mm magnesium chloride, 0.95 mg/mL NADP, 1.6 mg/mL ATP, and 0.6 units Glc6P dehydrogenase grade I in a final volume of 190 μL. Hexokinase (0.9 units), phosphoglucose isomerase (0.2 units), and invertase (1 μL of the enzyme powder redissolved to saturation) were successively added. Starch and total sugar detections were modified for samples harvested at the end of the night to increase the sensitivity of the measurements. One reaction for total sugar measurements contained 1.14 mg of extract, 3 mm magnesium chloride, 1.25 mm ATP, 0.75 mm NADP, 0.7 units of Glc6P dehydrogenase grade I, 0.5 mm MTT, 0.25 units of diaphorase, and 0.1% Triton X in a total volume of 200 μL. Optical density was read at 570 nm. Hexokinase, phosphoglucose isomerase, and invertase diluted as above were added simultaneously after stabilization of the reading. Starch detection was performed with the same reaction mix as for total sugars with 0.6 mg of digested extract (Hendriks et al., 2003). Hexokinase was added to the reaction after stabilization of the optical density at 570 nm. Each end-of-night starch value was subtracted from the starch content at the end of the day to calculate starch turnover. Therefore, each end-of-day experiment contains two starch turnover values (labeled 1 and 2 in Supplemental Tables S1 and S2), one for each of the end-of-night experiment. Likewise, starch turnover values were divided by starch content at the end of the day to derive two relative starch turnover values for each end-of-day experiment (labeled 1 and 2 in Supplemental Table S1).
Assay of free amino acids was based on the reaction of amino acids with fluorescamine (Bantan-Polak et al., 2001). Reaction mix harbored 0.038 mg of extract, 7.2 mm sodium borate, pH 8.0, and 0.043% fluorescamine in a final volume of 207 μL. Individual amino acids were measured by HPLC after OPA derivatization exactly as described previously (Geigenberger et al., 1996). Asp measurements were adapted to a microplate format from previously described coupled enzymatic assays (Moellering, 1986). Reaction mix contained 0.95 mg of extract, 44 mm Tricine, pH 8.0, 0.32 mm NADH, 3.2 mm oxoglutarate, and 3 units of malate dehydrogenase in a total volume of 125 μL. Reaction was triggered with 0.4 units of AspAT. Nitrate detection was adapted to microplates from previously described coupled enzymatic assays (Mori, 2000). A 100-μL reaction included 9.5 μg of extract, 0.1 m potassium phosphate, pH 7.5, 0.25 mm NADPH, and 0.005 units of nitrate reductase. After a 30-min incubation at room temperature in darkness, nitrite detection was triggered with the addition of 30 μL of sulfanilamide 2% and 30 μL of N-(1-naphtyl)-ethylenediamine dihydrochloride 0.04% (w/v).
Phenolic compounds were assayed as described previously (Singleton and Rossi, 1965) with volumes adapted to microplates. One reaction mix contained 88 μg of extract, 3.8% sodium carbonate, and 5 μL of Folin Ciocalteu's phenol reagent (Sigma-Aldrich) in a final volume of 100 μL. Optical density was read at 630 nm until completion of the reaction.
Chlorophyll was evaluated as described previously (Arnon, 1949) with 1.5 mg of extract diluted in 120 μL of 98% ethanol.
PEPC, AlaAT, AspAT, and GluDH were measured as described previously (Gibon et al., 2004a). Phosphoglucose isomerase activities were measured as described previously (Jones et al., 1986) with volumes adapted to microplates. Reaction mix included 0.5 mg of extract, 0.5% (w/v) BSA, 0.1 m Tricine, pH 7.8, 1.2 mm NADP, 0.1 units Glc6P dehydrogenase grade I, and 10 mm magnesium chloride in a total volume of 100 μL. Reaction was started with 2 mm Fru-6-P. Fumarase detection was adapted to microplates from the coupled enzymatic assay described previously (Hatch, 1978). Reaction mix harbored 0.1 m HEPES, pH 7.5, 0.8 mm NADP, 4 mm magnesium chloride, 5 mm potassium phosphate, pH 7.5, 0.02 units malic enzyme, and 0.5 mg of extract in a final volume of 100 μL. Mixture was incubated at room temperature for 20 min, after which 10 mm fumarate was added to start the reaction.
Statistical Analysis
Statistical analyses were carried out with SAS Version 9.1.2 (SAS Institute 2003). A 2-factorial ANOVA was used to partition the variance into genetic and experimental variance with the following mixed model in the GLM procedure:
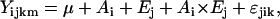 |
where μ is the general mean, Ai is the random effect of the ith accession genotype, Ej is the fixed effect of the jth experiment, Ai × Ej is the random interaction effect of the ith accession genotype with the jth experiment, and ɛjik is the error of Yijk.
The explained genetic variances of a genotype main effect (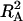 ) and of a A × E interaction [
) and of a A × E interaction [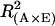 ] were calculated as follows:
] were calculated as follows:
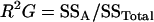 |
 |
where SSA, SSA × E, and SSTotal are the sum of squares of the factor accession, A × E interaction, and the total sum of squares, respectively, which were obtained from the GLM procedure. Genetic correlation (Pearson coefficients) between trait values were calculated with the least-square means of accessions averaged across experiments. As an additional tool to examine and visualize relationships between biomass, metabolites, and enzymes, a principal components analysis based on the correlation matrix was calculated.
Outliers in Supplemental Tables S1, S2, and S6 were determined and plotted with the program Analyze-it for Microsoft Excel (Version 1.71; see http://www.analyse-it.com/).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Data for the levels of all measured parameters in all experiments (three end-of-day, two end-of-night, and two DW experiments).
Supplemental Table S2. Data for RGR and leaf area for 17 ecotypes.
Supplemental Table S3. Data for all measured parameters expressed per gram DW (left) and per milligram protein (right).
Supplemental Table S4. Two-way ANOVA analysis.
Supplemental Table S5. Least-square mean averages for each parameter and accession, averaged across the entire data set.
Supplemental Table S6. Individual amino acid analysis data sheet.
Acknowledgments
We thank Oliver Bläsing, Manuela Guenther, Melanie Höhne, Inmaculada Castro-Marin, and Christina Fritz for technical help, Rhonda Meyer for supplying Arabidopsis seeds and information, Joachim Fisahn for use of lab equipment, Karin Köhl and the “green team” for the excellent cultivation and care of the plants, and Curt Hannah for editorial help.
This work was supported by the Max Planck Society, the European Commission (RTN project PLUSN to J.M.C. and contract no. QLK1–CT–2001–01080 to N.P.), and the German Federal Ministry for Education and Research (GABI-EVAST grant no. 0313122B to M.v.K., T.A., and M.S., GABI-BMBF project no. 0312277A to Y.G. and R.S., and two joint GABI-Genoplante Projects on Functional Genomics of Nitrogen Metabolism and Studies of Natural Diversity, 0312853 and 0313062, to L.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Joanna M. Cross (jomafrcr1970@yahoo.co.uk).
The online version of this article contains Web-only data.
References
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Phenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantan-Polak T, Kassai M, Grant KB (2001) A comparison of fluorescamine and naphthalene-2,3-dicarboxaldehyde fluorogenic reagents for microplate-based detection of amino acids. Anal Biochem 297: 128–136 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenge F, Saliba-Colombani V, Mathieu S, Loudet O, Daniel-Vedele F, Krapp A (2006) Natural variation for carbohydrate content in Arabidopsis. Interaction with complex traits dissected by quantitative genetics. Plant Physiol 141: 1630–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causse M, Rocher JP, Henry AM, Charcosset A, Prioul JL, de Vienne D (1995) Genetic dissection of the relationship between carbon metabolism and early growth in maize, with emphasis on key enzyme loci. Mol Breed 1: 259–272 [Google Scholar]
- Chatterton NJ, Silvius JE (1979) Photosynthate partitioning into starch in soybean leaves. I. Effects of photoperiod versus photosynthetic period duration. Plant Physiol 64: 749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton NJ, Silvius JE (1981) Photosynthate partitioning into starch in soybean leaves. II. Irradiance level and daily photosynthetic period duration effects. Plant Physiol 67: 257–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Salamini F, Gebhardt C (2001) A potato molecular-function map for carbohydrate metabolism and transport. Theor Appl Genet 102: 284–295 [Google Scholar]
- Cheng CL, Acedo GN, Cristinsin M, Conkling MA (1992) Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA 89: 1861–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter WD, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751 [DOI] [PubMed] [Google Scholar]
- Clerkx EJ, El-Lithy ME, Vierling E, Ruys GJ, Blankestijn-De Vries H, Groot SP, Vreugdenhil D, Koornneef M (2004) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135: 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Lithy ME, Bentsink L, Hanhart CJ, Ruys GJ, Rovito D, Broekhof JL, van der Poel HJ, van Eijk MJ, Vreugdenhil D, Koornneef M (2006) New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics 172: 1867–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and mapping of yield-associated QTL. Genetics 141: 1147–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D (2004) Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Lerchl J, Stitt M, Sonnewald U (1996) Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ 19: 43–55 [Google Scholar]
- Geiger DR, Servaites JC (1994) Diurnal regulation of photosynthetic C metabolism in C3 plants. Annu Rev Plant Biol 45: 235–256 [Google Scholar]
- Gibon Y, Bläsing OE, Hannemann J, Carillo P, Höhne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M (2004. a) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günter M, Stitt M (2004. b) Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilisation, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Gouesnard B, Bataillon TM, Decoux G, Rozale C, Schoen DJ, David JL (2001) MSTRAT: an algorithm for building germplasm core collections by maximising allelic or phenotypic richness. J Hered 92: 93–94 [DOI] [PubMed] [Google Scholar]
- Harrison J, Hirel B, Limani AM (2004) Variation in nitrate uptake and assimilation between two accessions of Lotus japonicus and their recombinant inbred lines. Physiol Plant 120: 124–131 [DOI] [PubMed] [Google Scholar]
- Hatch MD (1978) A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem 85: 271–275 [DOI] [PubMed] [Google Scholar]
- Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Bertin P, Quillere I, Bourdoncle W, Attagnant C, Dellay C, Gouy A, Cadiou S, Retailliau C, Falque M, et al (2001) Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol 125: 1258–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R, editor (1982) Plant Growth Curves. Arnold Publishers, London
- Jones TWA, Gottlieb LD, Pichersky E (1986) Reduced enzyme activity and starch level in an induced mutant of chloroplastic phosphoglucose isomerase. Plant Physiol 81: 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Weiner H, Huber SC (1999) Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol Plant 105: 384–389 [Google Scholar]
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P (2005) Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 102: 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJ, Soppe W (1998) Genetic control of flowering time in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 49: 345–370 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55: 141–172 [DOI] [PubMed] [Google Scholar]
- Krapp A, Saliba-Colombani V, Daniel-Vedele F (2005) Analysis of C and N metabolisms and of C/N interactions using quantitative genetics. Photosynth Res 83: 251–263 [DOI] [PubMed] [Google Scholar]
- Lejay L, Gansel X, Cerezo M, Tillard P, Muller C, Krapp A, von Wiren N, Daniel-Vedele F, Gojon A (2003) Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15: 2218–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Lejay L, Tillard P, Lepetit M, Olive F, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two
 uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar]
uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar] - Loudet O, Chaillou S, Krapp A, Daniel-Vedele F (2003. b) Quantitative trait loci analysis of water and anion contents in interaction with nitrogen availability in Arabidopsis thaliana. Genetics 163: 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O, Chaillou S, Merigout P, Talbotec J, Daniel-Vedele F (2003. a) Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol 131: 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O, Gaudon V, Trubuil A, Daniel-Vedele F (2005) Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor Appl Genet 110: 742–753 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J, Feil R, Hendriks JH, Gibon Y, Morcuende R, Scheible WR, Osuna D, Carillo P, Hajirezaei M, Stitt M (2006) Sugars lead to large changes of trehalose-6-phosphate in Arabidopsis, which are correlated with changes in the post-translational activation of AGPase and the rate of starch synthesis. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001. a) The diurnal changes of nitrogen metabolism in leaves of nitrate-replete tobacco are driven by an imbalance between nitrate reductase activity and the rate of nitrate uptake and ammonium and glutamine metabolism during the first part of the light period. Plant Cell Environ 24: 177–190 [Google Scholar]
- Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001. b) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ 24: 1119–1137 [Google Scholar]
- Matt P, Krapp A, Haake V, Mock HP, Stitt M (2002) Decreased Rubisco activity leads to dramatic changes of nitrate metabolism, amino acid metabolism and the levels of phenylpropanoids and nicotine in tobacco antisense RBCS transformants. Plant J 30: 663–677 [DOI] [PubMed] [Google Scholar]
- Matt P, Schurr U, Krapp A, Stitt M (1998) Growth of tobacco in short day conditions leads to high starch, low sugars, altered diurnal changes of the Nia transcript and low nitrate reductase activity, and an inhibition of amino acid synthesis. Planta 207: 27–41 [DOI] [PubMed] [Google Scholar]
- McKhann HI, Camilleri C, Bérard A, Bataillon T, David JL, Reboud X, Le Corre V, Caloustian C, Gut IG, Brunel D (2004) Nested core collections maximising genetic diversity in Arabidopsis thaliana. Plant J 38: 193–202 [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Pedersen D (1998) The molecular basis of quantitative genetic variation in central and secondary metabolism in Arabidopsis. Genetics 149: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering H (1986) L-Aspartate and L-asparagine. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Ed 3, Vol 8. Verlag-Chemie, Deerfield Beach, FL, pp 350–355
- Moorhead G, Douglas P, Cotelle V, Harthill J, Morrice N, Meek S, Deiting U, Stitt M, Scarabel M, Aitken A, et al (1999) Phosphorylation-dependent interactions between enzymes of plant metabolism and 14-3-3 proteins. Plant J 18: 1–12 [DOI] [PubMed] [Google Scholar]
- Mori H (2000) Direct determination of nitrate using nitrate reductase in a flow system. J Health Sci 46: 385–388 [Google Scholar]
- Nei M, editor (1987) Molecular Evolutionary Genetics. Columbia University Press, New York
- Obara M, Kajiura M, Fukuta Y, Yano M, Hayashi M, Yamaya T, Sato T (2001) Mapping of QTLs associated with cytosolic glutamine synthetase and NADH-glutamate synthase in rice (Oryza sativa L.). J Exp Bot 52: 1209–1217 [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Scheible W-R, Gibon Y, Bläsing O-E, Thimm O, Höhne M, Günter M, Udvardi MK, et al (2006) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J (in press) [DOI] [PubMed]
- Peters JL, Cnudde F, Gerats T (2003) Forward genetics and map-based cloning approaches. Trends Plant Sci 8: 484–491 [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559 [DOI] [PubMed] [Google Scholar]
- Poorter H, van Rijn CP, Vanhala TK, Verhoeven KJ, de Jong YE, Stam P, Lambers H (2005) A genetic analysis of relative growth rate and underlying components in Hordeum spontaneum. Oecologia 142: 360–377 [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16: 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul JL, Pelleschi S, Sene M, Thevenot C, Causse M, de Vienne D, Leonardi C (1999) From QTLs to candidate genes in maize. J Exp Bot 50: 1281–1288 [Google Scholar]
- Purves DW, Law R (2002) Experimental derivation of functions relating growth of Arabidopsis thaliana to neighbour size and distance. J Ecol 90: 882–894 [Google Scholar]
- Rauh BL, Basten C, Buckler ES IV (2002) Quantitative trait loci analysis of growth response to varying nitrogen sources in Arabidopsis thaliana. Theor Appl Genet 104: 743–750 [DOI] [PubMed] [Google Scholar]
- Rolland F, Sheen J (2005) Sugar sensing and signaling networks in plants. Biochem Soc Trans 33: 269–271 [DOI] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29: 426–434 [DOI] [PubMed] [Google Scholar]
- Schmid KJ, Törjék O, Meyer RC, Schmuths H, Hoffmann MH, Altmann T (2006) Evidence for large-scale population structure of Arabidopsis thaliana from genome-wide SNP markers. Theor Appl Genet 112: 1104–1114 [DOI] [PubMed] [Google Scholar]
- Sergeeva LI, Vonk J, Keurentjes JJ, van der Plas LH, Koornneef M, Vreugdenhil D (2004) Histochemical analysis reveals organ-specific quantitative traits loci for enzyme activities in Arabidopsis. Plant Physiol 134: 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144–158 [Google Scholar]
- Stitt M, Krapp A (1999) The molecular physiological basis for the interaction between elevated carbon dioxide and nutrients. Plant Cell Environ 22: 583–622 [Google Scholar]
- Stitt M, Lilley RMcC, Gerhardt R, Heldt HW (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174: 518–552 [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Tholen D, Voesenek LA, Poorter H (2004) Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia x hybrida. Plant Physiol 134: 1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum KE, Shin MJ, Palenchar PM, Kouranov A, Coruzzi GM (2004) Genome-wide investigation of light and carbon signaling interactions in Arabidopsis. Genome Biol 5: R10 [DOI] [PMC free article] [PubMed]
- Törjék O, Berger D, Meyer RC, Muessig C, Schmid KJ, Rosleff Sörensen TR, Weisshaar B, Mitchell-Olds T, Altmann T (2003) Establishment of a high-efficiency SNP-based framework marker set for Arabidopsis. Plant J 36: 122–140 [DOI] [PubMed] [Google Scholar]
- Vincentz M, Moureaux T, Leydecker MT, Vaucheret H, Caboche M (1993) Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginifolia leaves by nitrogen and carbon metabolites. Plant J 3: 315–324 [DOI] [PubMed] [Google Scholar]