Abstract
The NAD(P)H dehydrogenase (NDH) complex in chloroplasts mediates photosystem I cyclic and chlororespiratory electron transport. Eleven chloroplast genes and three nuclear genes have been identified as encoding Ndh subunits, but the entire subunit composition is still unknown. An Arabidopsis (Arabidopsis thaliana) chlororespiratory reduction (crr3) mutant was isolated based on its lack of transient increase in chlorophyll fluorescence after actinic light illumination; this was due to a specific defect in accumulation of the NDH complex. The CRR3 gene (At2g01590) encodes a novel protein containing a putative plastid-targeting signal and a transmembrane domain. Consistent with the gene structure, CRR3 localized to the membrane fraction of chloroplasts. In addition to the essential function of CRR3 in stabilizing the NDH complex, the NDH complex is also required for the accumulation of CRR3. These results suggest that CRR3 interacts with the NDH complex in the thylakoid membrane. In contrast to other subunits in the chloroplast NDH complex, CRR3 is not conserved in cyanobacteria from which the chloroplast NDH complex is believed to have originated. We propose that CRR3 is a subunit of the NDH complex, which is specific to the chloroplast.
Chloroplasts are derived from the integration of prokaryotic cyanobacteria into eukaryotic cells via endosymbiosis. Consequently, chloroplasts consist of proteomes that originated from both prokaryotes and eukaryotes. The basic machinery for photosynthesis and housekeeping functions exhibits high similarity with the prokaryotic versions, whereas the regulatory machinery is often specific to eukaryotes. For example, whereas the system of chloroplast gene expression is similar to that of prokaryotes (Sugiura et al., 1998), it is regulated by members of the pentatricopeptide repeat family, which is specific to eukaryotes (Lurin et al., 2004).
Eleven subunits of the chloroplast NAD(P)H dehydrogenase (NDH) complex, which is involved in PSI cyclic electron transport (Munekage and Shikanai, 2005) and chlororespiration (Peltier and Cournac, 2002) are encoded in the chloroplast genome. Although the chloroplast NDH complex is functionally and structurally homologous to the mitochondrial NADH dehydrogenase complex (complex I), chloroplast NDH is more similar to the cyanobacterial NDH complex than to the mitochondrial complex in the same species. In addition to 11 chloroplast-encoded subunits (NdhA–NdhK), three nuclear-encoded subunits (NdhM–NdhO) were recently identified in chloroplasts using a biochemical approach (Rumeau et al., 2005). Because their orthologs were discovered in cyanobacteria (Prommeenate et al., 2004; Battchikova et al., 2005), three nucleus-encoded ndh genes are also likely to have originated in cyanobacteria and to have transferred from the chloroplast genome to the nuclear genome. Thus, it is generally accepted that the chloroplast NDH complex originated in cyanobacteria.
In the cyanobacterium Synechocystis PCC 6803, the NdhD and NdhF subunits are encoded by six and three genes, respectively. As a result of modification of the subunit composition, the cyanobacterial NDH complex is involved in multiple functions in respiration, PSI cyclic electron transport, and CO2 uptake (Ohkawa et al., 2000; Shibata et al., 2001; Zhang et al., 2004, 2005). In higher plants, however, both subunits are encoded by single-copy genes in the chloroplast genome (Matsubayashi et al., 1987). Chloroplasts NdhD and NdhF are most similar to cyanobacterial NdhD1, NdhD2, and NdhF1, respectively. These subunits are included in the NDH1-L complex that is involved in respiration and probably also in PSI cyclic electron transport. This is consistent with the fact that the NDH complex is involved in PSI cyclic electron transport and chlororespiration in higher plants (Burrows et al., 1998; Shikanai et al., 1998).
Although the chloroplast NDH complex originated in cyanobacteria, it is not involved in CO2 uptake. Consistent with their difference in function, the genes specifically involved in this process (ndhD3, ndhD4, ndhF3, cupA, and cupB) are absent from the Arabidopsis (Arabidopsis thaliana) genome. Although the chloroplast NDH complex mediates PSI cyclic electron transport, its contribution is much lower in higher plants than in cyanobacteria. Instead of the NDH-mediated pathway, the PROTON GRADIENT REGULATION 5 (PGR5)-dependent pathway significantly contributes to PSI cyclic electron transport in higher plants (Munekage et al., 2002, 2004).
Although 14 subunits have been identified in the chloroplast NDH complex, the subunits involved in electron donor binding are still unclear (for review, see Shikanai and Endo, 2000). This is closely related to the long debate on the electron donor to the NDH complex. The NDH complex purified from cyanobacteria accepts electrons from NADPH, but not from NADH (Mi et al., 1995; Matsuo et al., 1998). In contrast, the purified chloroplast NDH complex favors NADH over NADPH as an electron donor (Sazanov et al., 1998; Casano et al., 2000; Rumeau et al., 2005). Despite extensive efforts to identify additional subunits, the entire subunit composition remains unclear. The difficulty is partially due to the fragile nature of the complex. Our genetic approach focusing on the chlorophyll fluorescence change depending on NDH activity has led to the identification of chlororespiratory reduction (crr) mutants that are specifically defective in NDH activity (Hashimoto et al., 2003). Here, we report the characterization of crr3 in which a novel chloroplast protein is impaired. Although CRR3 is not found in cyanobacteria, the nature of CRR3 is similar to the subunits of the NDH complex.
RESULTS
crr3 Is Specifically Defective in the Accumulation of the Chloroplast NDH Complex
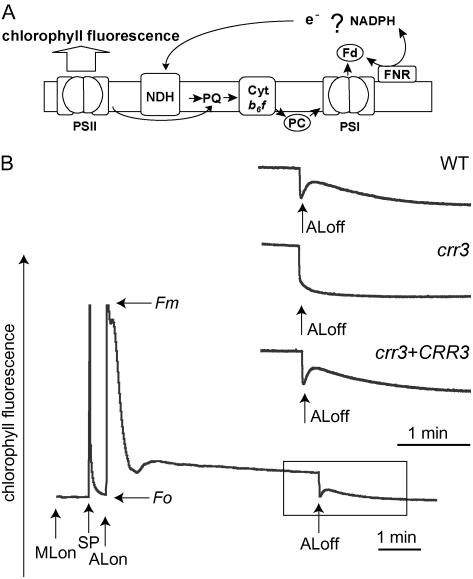
The chloroplast NDH complex mediates electron transport from the stromal reducing pool to plastoquinone (PQ; Fig. 1A). After actinic light (AL) illumination, the NDH complex still donates electrons to PQ in the dark to the extent that the reducing equivalents are available, resulting in a transient increase in chlorophyll fluorescence in the wild type (Fig. 1B). The fluorescence level is roughly proportional to the reduction of the PQ pool, which depends on the activity of the chloroplast NDH complex. Arabidopsis crr mutants were isolated based on a lack of this chlorophyll fluorescence change using two-dimensional fluorescence imaging (Hashimoto et al., 2003). crr3 is a recessive mutant defective at a single locus (data not shown) and did not show any increase in chlorophyll fluorescence after AL illumination (Fig. 1B).
Figure 1.
Monitoring of NDH activity using chlorophyll fluorescence analysis. A, Schematic model of NDH function. The NDH complex functions in electron transport from an unidentified electron donor, possibly NAD(P)H or ferredoxin (Fd) to PQ. PQ reduction was monitored by chlorophyll fluorescence emitted from PSII. PQ reduction in the dark depends on NDH activity and can be monitored as a transient increase in chlorophyll fluorescence after AL illumination. PC, Plastocyanin; FNR, Fd-NADP+ oxidoreductase. B, Analysis of the transient increase in chlorophyll fluorescence after turning off AL. The bottom curve indicates a typical trace of chlorophyll fluorescence in the wild type (WT). Leaves were exposed to AL (50 μmol photons m−2 s−1) for 5 min. AL was turned off and the subsequent change in chlorophyll fluorescence level was monitored. Insets are magnified traces from the boxed area. The fluorescence levels were normalized by Fm levels. ML, Measuring light; SP, saturating pulse of white light; crr3 + CRR3, crr3 complemented by introduction of wild-type genomic CRR3.
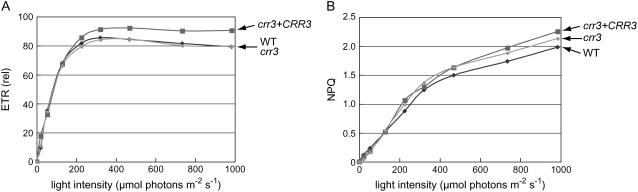
The contribution of the chloroplast NDH complex in photosynthetic electron transport is minor and the Arabidopsis mutants specifically defective in NDH activity do not show any distinct phenotype under mild growth conditions in a growth chamber (Hashimoto et al., 2003; Kotera et al., 2005; Munshi et al., 2005, 2006). To assess whether the crr3 defect is specific to the NDH complex, the light intensity dependence of two chlorophyll fluorescence parameters were compared between crr3 and the wild type (Fig. 2). Whereas the electron transport rate (ETR) reflects the relative rate of electron transport through PSII, nonphotochemical quenching (NPQ) is mainly related to dissipation of excessive absorbed light energy as heat from PSII (Niyogi et al., 2005). These parameters reflect rather subtle defects in photosynthetic electron transport and have often been used for primary characterization of mutants defective in photosynthesis. In crr3, both parameters showed the same light-intensity dependence as the wild type (Fig. 2). We conclude that the crr3 defect is specific to the NDH complex.
Figure 2.
In vivo analysis of electron transport activity. A, Light-intensity dependence of ETR. ETR was depicted relative to ΦPSII × light intensity (μmol photons m−2 s−1). sds < 15% of values at the maximal light intensity (n = 5). The curves of wild type and crr3 almost overlap. B, Light-intensity dependence of NPQ of chlorophyll fluorescence. sds < 10% of values at the maximal light intensity (n = 5). crr3 + CRR3, crr3 transformed with genomic wild-type CRR3.
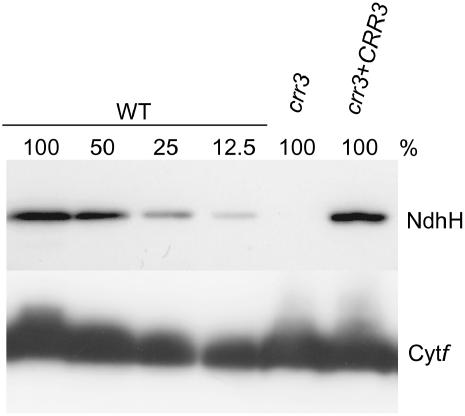
The crr3 phenotype specifically defective in activity of the NDH complex may be due to impaired accumulation of the complex. To assess this possibility, the protein level of NdhH, a subunit of the NDH complex, was evaluated in crr3 (Fig. 3). In crr3, the NdhH level was drastically reduced to below the detection limit (at least <12.5% of the wild type). In contrast, the level of cytochrome f, a subunit of the cytochrome b6f complex, was not affected in crr3. These data are consistent with the results of chlorophyll fluorescence analysis, which suggests a specific loss of NDH activity in crr3. We conclude that the accumulation of the NDH complex is specifically impaired in crr3.
Figure 3.
Protein-blot analysis of the NDH complex. Immunodetection of an NDH subunit, NdhH, and a subunit of the cyt b6f complex, Cytf. Proteins were extracted from the thylakoid membrane fraction of the chloroplasts. Lanes were loaded with the protein samples corresponding to 0.5 μg chlorophyll for Cytf and 5 μg chlorophyll for NdhH (100%) and the series of dilutions indicated. crr3 + CRR3, crr3 transformed by wild-type genomic CRR3.
CRR3 Encodes a Novel Membrane Protein That Is Specific to Higher Plants
The gene responsible for the crr3 phenotype was identified based on the genetic map. The crr3 mutant (Columbia gl1 background) was crossed with the polymorphic wild type (Landsberg erecta). Fine mapping using approximately 150 F2 plants pinpointed the 190-kb region between markers RGA and F14H20 at the top of chromosome 2. Because the crr3 phenotype is specific to chloroplast NDH activity, the nucleotide sequences of candidate genes that encode proteins with predicted target signal to plastids (Predotar [http://urgi.infobiogen.fr/predotar/predotar.html] and TargetP [http://www.cbs.dtu.dk/services/TargetP]) were determined. Finally, one nucleotide substitution from C to T was discovered in At2g01590.
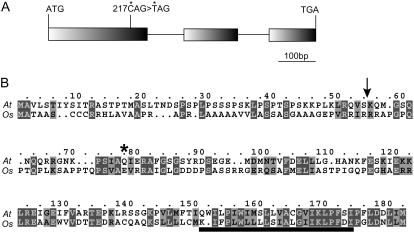
Direct sequencing of the reverse transcription-PCR products showed that At2g01590 consists of three exons and two introns (Fig. 4A). A single-nucleotide substitution in crr3 generates a stop codon in the first exon, strongly suggesting that crr3 completely lacks the function of At2g01590. To verify that the mutation is responsible for the defect in the NDH complex, the wild-type genomic sequence containing At2g01590 was introduced into crr3. This transformation fully complemented the transient increase in chlorophyll fluorescence after AL illumination (Fig. 1B) and also the accumulation of NdhH protein (Fig. 3). We thus concluded that the crr3 phenotype is caused by the mutation in At2g01590 (CRR3).
Figure 4.
Positional cloning of crr3. A, Structure of CRR3. Exons (boxes) and introns (horizontal lines) were determined by direct sequencing of the reverse transcription-PCR products. Position of the crr3 mutation (asterisks) is indicated. B, Alignment of CRR3 homolog sequences. Predicted cleavage site of the target signal (TargetP) is indicated by a vertical arrow. Arabidopsis CRR6 (At) and its rice homolog (Os) are aligned. The rice sequence was predicted from the genome information. The position of the crr3 mutation is indicated by an asterisk. A horizontal bar indicates the transmembrane domain.
The CRR3 gene encodes a protein consisting of 174 amino acids. The first 54 amino acids were predicted to be a target signal to plastids by TargetP (Fig. 4B). CRR3 contains one transmembrane domain at the C-terminal region, suggesting that CRR3 anchors the thylakoid membranes. Although CRR3 is conserved in rice (Oryza sativa), their homologs have not been identified in cyanobacteria from which the chloroplast NDH complex is believed to have originated. Furthermore, CRR3 was not detected in Chlamydomonas reinhardtii in which the chloroplast NDH complex is absent (http://genome.jgi-psf.org/chlre2/chlre2.home.html). These results suggest that CRR3 is a novel factor required for the accumulation of the NDH complex that is specific to higher plants.
CRR3 Localizes to Chloroplast Thylakoids
For the biochemical characterization of CRR3, antibody was raised against it. Trials to fuse the T7 epitope tag to the C-terminal end and also prior to the transmembrane domain resulted in failure to complement the CRR3 function in the mutant, suggesting the essential function of the C-terminal region of CRR3. The mature CRR3 without the C-terminal end containing the transmembrane domain was therefore expressed as a fusion protein with NusA and His tags in Escherichia coli. The recombinant CRR3 was purified, released from the tags, and then used as the antigen.
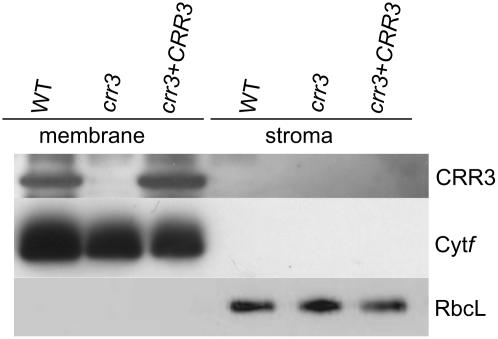
The obtained antibody recognized a protein whose mobility in gel was consistent with the predicted molecular mass of CRR3 (13.7 kD; Fig. 5). The signal was absent in crr3, supporting our conclusion that crr3 is a null allele. Furthermore, the signal was detected in crr3 transformed with the wild-type genomic CRR3 in which NDH activity (Fig. 1B) and the accumulation of NDH complex (Fig. 3) were restored. We conclude that the antibody specifically recognizes CRR3. Consistent with the fact that CRR3 contains a transmembrane domain, CRR3 was detected in the membrane fraction of the chloroplast, but not in the stroma. We conclude that CRR3 localizes to thylakoid membranes, although we cannot eliminate the possibility that CRR3 is a plastid envelope protein.
Figure 5.
Protein-blot analysis of CRR3. A, Immunodetection of CRR3 protein using a polyclonal antibody against recombinant CRR3. Chloroplast preparations were further fractionated to obtain a membrane fraction and a stromal fraction. Large subunits of ribulose 1,5-bisphosphate carboxylase/oxygenase (RbcL) and the Cytf were detected as the control for fractionation. Lanes were loaded with protein samples corresponding to 2.5 μg (CRR3), 0.5 μg (Cytf), and 0.01 μg (RbcL) chlorophyll. crr3 + CRR3, crr3 transformed by genomic CRR3.
The NDH Complex Is Essential for CRR3 Accumulation
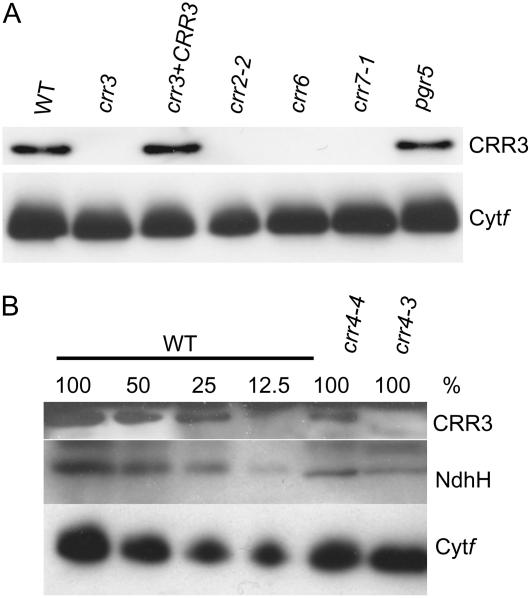
CRR3 is essential for the accumulation of the chloroplast NDH complex (Fig. 3), but is not conserved in cyanobacteria from which the chloroplast NDH complex is believed to have originated (Fig. 4). This is in contrast to the nucleus-encoded subunit genes, ndhM, ndhN, and ndhO (Rumeau et al., 2005), which are common in cyanobacteria. CRR3 may be a regulatory factor that is essential for the expression or assembly of the NDH complex. If this were true, the accumulation of CRR3 would be independent of the NDH complex. To study this possibility, the CRR3 level was analyzed in the mutant backgrounds defective in the accumulation of the NDH complex (Fig. 6A). CRR2 is involved in the intergenic RNA cleavage between rps7 and ndhB in the chloroplast and is possibly essential for the translation of ndhB encoding an Ndh subunit (Hashimoto et al., 2003). CRR7 is a candidate for a novel Ndh subunit and is essential for stabilizing the NDH complex (Munshi et al., 2005). In contrast to CRR7, CRR6 is stable against a mutant background lacking the NDH complex, but is essential to the accumulation of the NDH complex (Munshi et al., 2006). Under these three mutant backgrounds, the CRR3 level was drastically reduced below the detection limit (Fig. 6A). These results indicate that the NDH complex is essential for stabilizing CRR3.
Figure 6.
Immunodetection of CRR3 against mutant backgrounds lacking the NDH complex. A, Analysis of CRR3 stability in crr and pgr5 mutants. B, Analysis of CRR3 and NdhH levels in crr4-3 and crr4-4. Lanes were loaded with the thylakoid membrane protein samples corresponding to 0.5 μg chlorophyll for Cytf, 2.5 μg chlorophyll for CRR3, and 5 μg chlorophyll for NdhH as 100% and a series of dilutions indicated.
In higher plants, PSI cyclic electron transport consists of two partially redundant pathways (Munekage et al., 2004). PGR5 is an essential factor in the non-NDH PSI cyclic electron transport (Munekage et al., 2002). Although the NDH complex somehow complements the function of PGR5-dependent PSI cyclic electron transport in the pgr5 mutant (Munekage et al., 2004), the CRR3 level was not altered in pgr5 (Fig. 6A).
To confirm that the NDH complex is directly required to stabilize CRR3 as a partner that coaccumulates in the thylakoid membranes, we analyzed the CRR3 level in two alleles of crr4. Whereas crr4-3 is a null allele defective in the accumulation of the NDH complex, crr4-4 is a weak allele in which the level of the NDH complex is mildly affected (Kotera et al., 2005). Protein-blot analysis indicated that the CRR3 levels were reduced to approximately 25% in crr4-4 and less than 12.5% in crr4-3 of that in the wild type (Fig. 6B). These were proportional to the NdhH levels in crr4-4 (50%–25%) and crr4-3 (25%–12.5%). Taking all the results together, we propose that CRR3 is a subunit of the NDH complex, which is specific to the chloroplast.
DISCUSSION
crr3 was isolated based on its lack of transient increase in chlorophyll fluorescence after AL illumination (Hashimoto et al., 2003). The fluorescence change strictly depends on NDH activity under nonstress conditions in the air (Hashimoto et al., 2003), facilitating the isolation of mutants specifically defective in the NDH complex (crr mutants). Because the contribution of the NDH complex to PSI cyclic electron transport is rather minor in higher plants, a specific mutation in the NDH complex has little effect on overall electron transport, which can be monitored according to the chlorophyll fluorescence parameters of ETR and NPQ (Fig. 2). Combined with conventional chlorophyll fluorescence analyses, our genetic strategy clarified the genes specifically involved in NDH activity.
Previously, we characterized four genes, CRR2, CRR4, CRR6, and CRR7, which are specifically required for the accumulation of the NDH complex (Hashimoto et al., 2003; Kotera et al., 2005; Munshi et al., 2005, 2006). Both CRR2 and CRR4 encode the members of the pentatricopeptide repeat family and are specifically involved in RNA maturation of the chloroplast ndh genes (Hashimoto et al., 2003; Kotera et al., 2005). CRR2 and CRR4 were probably acquired by higher plants to control the expression of chloroplast ndh genes. In contrast, CRR6 and CRR7 do not contain any motifs suggesting their function and are conserved in cyanobacteria (Munshi et al., 2005, 2006). As well as CRR2 and CRR4, CRR6 and CRR7 are specifically required for the accumulation of the NDH complex, implying that they were simultaneously transferred with the NDH complex from cyanobacteria to eukaryotic cells. We first assessed the simplest possibility—that the identified genes encode novel subunits of the NDH complex. In photosynthetic protein complexes, a mutation primarily affecting a single subunit results in a pleiotropic defect in the accumulation of other subunits (for review, see Wollman et al., 1999; Choquet and Vallon, 2000). The same story is true for the Ndh subunits (Burrows et al., 1998; Kofer et al., 1998; Hashimoto et al., 2003; Kotera et al., 2005; Rumeau et al., 2005). Whereas the NDH complex is essential for stabilizing CRR7, CRR6 is stable even against a mutant background lacking the NDH complex (Munshi et al., 2005, 2006). Based on all this combined information, we proposed that CRR7 was a candidate for a novel subunit of the NDH complex. In contrast, CRR6 may be a nonsubunit factor required for stabilizing the NDH complex.
CRR3 was required for the accumulation of the NDH complex (Fig. 3) and, conversely, CRR3 was destabilized in crr2, crr6, and crr7 (Fig. 6A). This character of CRR3 was identical to that of CRR7. Furthermore, the CRR3 levels were proportional to the NdhH level in two alleles of crr4 in which the accumulation of the NDH complex was affected to a different extent (Fig. 6B), although the stoichiometry of CRR3 to the NDH complex is unclear. The crr4 defect in NdhD accumulation may affect the accumulation of CRR3 more severely than that of NdhH because both NdhD and CRR3 are membrane proteins. However, NdhH is likely to be a subunit of the connecting subcomplex. As the simplest working model, we propose that CRR3 is a subunit of the chloroplast NDH complex. CRR7 is a soluble protein and may be included in an unidentified subcomplex involved in binding to an electron donor. It is also possible that CRR7 localizes between the subcomplex binding to the electron donor and the membrane subcomplex (NdhA–F and possibly NdhL), forming a connecting subcomplex with NdhG to NdhK and NdhM to NdhO. In contrast, CRR3 contains a transmembrane domain and thus may associate with the membrane subcomplex.
Although the chloroplast NDH complex is structurally similar to the cyanobacterial complex, CRR3 is not conserved in cyanobacteria. However, this fact does not exclude the possibility that CRR3 is a eukaryotic subunit in the chloroplast NDH complex. Although the function of the cytochrome b6f complex has been conserved, higher plants and green algae contain an additional small subunit of PetL, which is not conserved in cyanobacteria (Takahashi et al., 1996). As another example, despite the conserved function of the oxygen-evolving center in PSII, there is a diversity of its subunit composition between cyanobacteria and higher plants (De Las Rivas et al., 2004). Even though the chloroplast NDH complex originated in cyanobacteria, the subunit composition may be slightly divergent between cyanobacteria and higher plants.
Since the discovery of 11 ndh genes in the chloroplast genome, the chloroplast NDH complex has been discussed by analogy with the cyanobacterial complex (Shikanai and Endo, 2000). However, the cyanobacterial complex is involved in multiple functions by modifying the subunit composition, which is unlikely in the chloroplast complex. In chloroplasts, the NDH complex is considered to alleviate various oxidative stresses (Endo et al., 1999; Horváth et al., 2000; Munné-Bosch et al., 2005; Wang et al., 2006), whose exact mechanisms are not yet clear. In contrast to cyanobacteria, PGR5-dependent PSI cyclic electron transport significantly contributes to chloroplast energetics (Munekage et al., 2002, 2004). It may be necessary to take into account the diversity of the NDH complex between cyanobacteria and chloroplasts with respect to structure and even function.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seedlings were grown in soil under growth chamber conditions (50 μmol photons m−2 s−1) for 3 to 4 weeks. crr3 was mutagenized by ethyl methanesulfonate (Hashimoto et al., 2003).
Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence was measured using a MINI-pulse-amplitude modulation portable chlorophyll fluorometer (Walz). Minimal fluorescence at open PSII centers in the dark-adapted state (Fo) was excited by a weak measuring light (650 nm) at a light intensity of 0.05 to 0.1 μmol photons m−2 s−1. A saturating pulse of white light (800 ms, 3,000 μmol photons m−2 s−1) was applied to determine the maximal fluorescence at closed PSII centers in the dark-adapted state (Fm) and during AL illumination (Fm′). The steady-state fluorescence level (Fs) was recorded during AL illumination (15–1,000 μmol photons m−2 s−1). NPQ was calculated as (Fm − Fm′)/Fm′. The quantum yield of PSII (ΦPSII) was calculated as (Fm − Fm′)/Fm′ (Genty et al., 1989). ETR was calculated as ΦPSII × light intensity (μmol photons m−2 s−1). The transient increase in chlorophyll fluorescence after turning off AL was monitored as described previously (Shikanai et al., 1998).
Map-Based Cloning
The crr3 mutation was mapped with molecular markers based on a cleaved amplified polymorphic sequence (Konieczny and Ausubel, 1993). Primer sequences and the restriction enzymes are 5′-GCTTCTATAGGTGTTGCAACTC-3′ and 5′-GTTGTGCGAGAAGTCCTTGTG-3′, EcoRV for F14H20 and 5′-TTCGATTCAGTTCGGTTTAG-3′ and 5′-GTTTAAGCAAGCGAGTATGC-3′, RsaI for RGA. Genomic DNA was isolated from F2 plants derived from a cross between crr3 and the wild type (Landsberg erecta). The genomic sequences of the candidate genes were amplified by PCR using ExTaq DNA polymerase (TaKaRa). The resulting PCR products were directly sequenced using a dye terminator cycle sequencing kit and an ABI PRISM 3100 sequencer (Applied Biosystems).
For complementation of the crr3 mutation, the 2.1-kb wild-type genomic sequence surrounded by 5′-CTGCCATTACCAATGAATGG-3′ and 5′-AGATTCCTCCCGACGGGCGT-3′ was cloned in pBIN19 and introduced into crr3 via Agrobacterium tumefaciens MP90.
Isolation of Chloroplasts and Protein Analysis
Chloroplasts were isolated from the leaves of 4- to 5-week-old plants as described previously (Munshi et al., 2006). The protein samples were separated by 12.5% SDS-PAGE and used for immunodetection (Hashimoto et al., 2003).
Antibody Preparation
The internal CRR3 sequence that does not encode the transit peptide or membrane-spanning domain was amplified using cDNA synthesized from RNA extracted from Arabidopsis leaves by PCR with the synthetic oligonucleotide primers 5′-GGATCCCAAATGGGAAGTCAAAACC-3′ and 5′-CTCGAGTCACATCAAAACTGGTTTCC-3′. These primers provided BamHI and XhoI sites (underlined) for cloning. The amplified sequence was ligated into the pET-43.1a vector (Novagen), which provided the NusA and hexahistidine tags at the N terminus of CRR3. Escherichia coli BL21 (DE3) cells transformed with the plasmid were incubated at 37°C in 4 L of Luria-Bertani medium. Expression of the recombinant protein was induced by addition of 1 mm isopropylthio-β-galactoside at an OD650 of 0.4 to 0.6 for 3 h. Cells were harvested and resuspended in 4 volumes of buffer A (50 mm Tris-HCl, pH 7.5, 0.3 m NaCl, 7 mm 2-mercaptoethanol, and 1 mm phenylmethylsulfonylfluoride). The following steps were performed at 4°C. The cells were disrupted by sonication and centrifuged at 15,000g for 30 min to remove cell debris. The supernatant was loaded onto a 1-mL HisTrap column (GE Healthcare Bio-Sciences) that had been equilibrated with buffer B (0.01 m Na2HPO4, 0.01 m NaH2PO4, and 0.5 m NaCl) containing 10 mm imidazole. The column was washed with buffer B containing 40 mm imidazole. The recombinant CRR3 protein was eluted with buffer B containing 500 mm imidazole. The purified protein was analyzed by SDS-PAGE. To remove the NusA and His tags, the recombinant protein was treated with thrombin protease and further purified via a nickel-chelating column as a through fraction, then used as an antigen.
Acknowledgments
We thank Momoko Miyata for her excellent technical assistance. We are grateful to Gilles Peltier, Tsuyoshi Endo, Amane Makino, and Akiho Yokota for their gifts of antibodies.
This work was supported by a grant-in-aid for Scientific Research on Priority Areas (grant no. 16085206) and for Creative Scientific Research (grant no. 17GS0316) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Toshiharu Shikanai (shikanai@agr.kyushu-u.ac.jp).
References
- Battchikova N, Zhang P, Rudd S, Ogawa T, Aro E-M (2005) Identification of NdhL and Ssl1690 (NdhO) in NDH-1L and NDH-1M complexes of Synechocystis sp. PCC 6803. J Biol Chem 280: 2587–2595 [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J 17: 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Zapata JM, Martin M, Sabater B (2000) Chlororespiration and poising of cyclic electron transport: plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J Biol Chem 275: 942–948 [DOI] [PubMed] [Google Scholar]
- Choquet Y, Vallon O (2000) Synthesis, assembly and degradation of thylakoid membrane proteins. Biochimie 82: 615–634 [DOI] [PubMed] [Google Scholar]
- De Las Rivas J, Balsera M, Barber J (2004) Evolution of oxygenic photosynthesis: genome-wide analysis of the OEC extrinsic proteins. Trends Plant Sci 19: 18–25 [DOI] [PubMed] [Google Scholar]
- Endo T, Shikanai T, Takabayashi A, Asada K, Sato F (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett 457: 5–8 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36: 541–549 [DOI] [PubMed] [Google Scholar]
- Horváth EM, Peter SO, Joët T, Rumeau D, Cournac L, Horváth GV, Kavanagh TA, Schäfer C, Peltier G, Medgyesy P (2000) Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol 123: 1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofer W, Koop HU, Wanner G, Steinmüller K (1998) Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet 258: 166–173 [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403–410 [DOI] [PubMed] [Google Scholar]
- Kotera E, Tasaka M, Shikanai T (2005) A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433: 326–330 [DOI] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyere C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi T, Wakasugi T, Shinozaki K, Yamaguchi-Shinozaki K, Zaita N, Hidaka T, Meng BY, Ohto C, Tanaka M, Kato A, et al (1987) Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Mol Gen Genet 210: 385–393 [DOI] [PubMed] [Google Scholar]
- Matsuo M, Endo T, Asada K (1998) Isolation of a novel NAD(P)H-quinone oxidoreductase from the cyanobacterium Synechocystis PCC6803. Plant Cell Physiol 39: 751–755 [DOI] [PubMed] [Google Scholar]
- Mi H, Endo T, Ogawa T, Asada K (1995) Thylakoid membrane-bound pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacteria Synechocystis PCC 6803. Plant Cell Physiol 36: 661–668 [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Shikanai T (2005) Cyclic electron transport through photosystem I. Plant Biotechnol 22: 361–369 [Google Scholar]
- Munné-Bosch S, Shikanai T, Asada K (2005) Enhanced ferredoxin-dependent cyclic electron flow around photosystem I and α-tocopherol quinone accumulation in water-stressed ndhB-inactivated tobacco mutants. Planta 222: 502–511 [DOI] [PubMed] [Google Scholar]
- Munshi MK, Kobayashi H, Shikanai T (2005) Identification of a novel protein, CRR7, required for the stabilization of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant J 44: 1036–1044 [DOI] [PubMed] [Google Scholar]
- Munshi MK, Kobayashi H, Shikanai T (2006) CHLORORESPIRATORY REDUCTION 6 is a novel factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Physiol 141: 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Li X-P, Rosenberg V, Jung H-S (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56: 375–382 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T (2000) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J Biol Chem 275: 31630–31634 [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53: 523–550 [DOI] [PubMed] [Google Scholar]
- Prommeenate P, Lennon AM, Markert C, Hippler M, Nixon PJ (2004) Subunit composition of NDH-1 complexes of Synechocystis sp. PCC 6803: identification of two new ndh gene products with nuclear-encoded homologues in the chloroplast Ndh complex. J Biol Chem 279: 28165–28173 [DOI] [PubMed] [Google Scholar]
- Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 7: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ (1998) The chloroplast Ndh complex mediates the dark reduction of the plastoquinone pool in response to heat stress in tobacco leaves. FEBS Lett 429: 115–118 [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T (2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Endo T (2000) Physiological function of a respiratory complex, NAD(P)H dehydrogenase in chloroplasts: dissection by chloroplast reverse genetics. Plant Biotechnol 17: 79–86 [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95: 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Hirose T, Sugita M (1998) Evolution and mechanism of translation in chloroplasts. Annu Rev Genet 32: 437–459 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Rahire M, Breyton C, Popot JL, Joliot P, Rochaix J-D (1996) The chloroplast ycf7 (petL) open reading frame of Chlamydomonas reinhardtii encodes a small functionally important subunit of the cytochrome b6f complex. EMBO J 15: 3498–3506 [PMC free article] [PubMed] [Google Scholar]
- Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye J-Y, Mi H (2006) Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol 141: 465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F-A, Minai L, Nechushtai R (1999) The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim Biophys Acta 1411: 21–85 [DOI] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, Aro E-M (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16: 3326–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Paakkarinen V, Katoh H, Iwai M, Ikeuchi M, Pakrasi HB, Ogawa T, Aro E-M (2005) Isolation, subunit composition and interaction of the NDH-1 complexes from Thermosynechococcus elongatus BP-1. Biochem J 390: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]