Abstract
A new nodulation-defective mutant of Lotus japonicus does not initiate nodule cortical cell division in response to Mesorhizobium loti, but induces root hair deformation, Nod factor-induced calcium spiking, and mycorrhization. This phenotype, together with mapping data, suggested that the mutation could be in the ortholog of the Medicago truncatula NSP1 gene (MtNSP1). The sequence of the orthologous gene (LjNSP1) in the L. japonicus mutant (Ljnsp1-1) revealed a mutation causing a premature stop resulting in loss of the C-terminal 23 amino acids. We also sequenced the NSP2 gene from L. japonicus (LjNSP2). A mutant (Ljnsp2-3) with a premature stop codon was identified by TILLING showing a similar phenotype to Ljnsp1-1. Both LjNSP1 and LjNSP2 are predicted GRAS (GAI, RGA, SCR) domain transcriptional regulators. Transcript steady-state levels of LjNSP1 and LjNSP2 initially decreased and then increased following infection by M. loti. In hairy root transformations, LjNSP1 and MtNSP1 complemented both Mtnsp1-1 and Ljnsp1-1 mutants, demonstrating that these orthologous proteins have a conserved biochemical function. A Nicotiana benthamiana NSP1-like gene (NbNSP1) was shown to restore nodule formation in both Ljnsp1-1 and Mtnsp1-1 mutants, indicating that NSP1 regulators from legumes and non-legumes can propagate the Nod factor-induced signal, activating appropriate downstream targets. The L. japonicus nodules complemented with NbNSP1 contained some cells with abnormal bacteroids and could fix nitrogen. However, the NbNSP1-complemented M. truncatula nodules did not fix nitrogen and contained very few bacteria released from infection threads. These observations suggest that NSP1 is also involved in infection, bacterial release, and normal bacteroid formation in nodule cells.
Legumes produce root nodules in response to Nod factors secreted by rhizobia. These Nod factor signals are essential for root hair deformation, induction of early nodulation genes, formation of nodule primordia, and infection by rhizobia. The earliest plant responses to Nod factors include an influx of calcium, plasma-membrane depolarizations, and then induction of cytosolic calcium spiking around the nucleus of epidermal root cells (Oldroyd and Downie, 2004). Purified Nod factors are sufficient to cause a range of early responses involved in the host developmental program (Hirsch and Fang, 1994; Schultze and Kondorosi, 1998; Downie and Walker, 1999). The Nod factors are the principle determinants by which legumes can be nodulated by specific rhizobia; the basis for this host specificity is the structure of the Nod factor, suggesting that highly specific plant receptors perceive Nod factor signals, thereby initiating the plant developmental response.
Nod factor-induced root hair deformation is associated with reorganization of actin filaments in preparation for infection (Cardenas et al., 1998; Sieberer et al., 2005). The root hairs bend back, entrapping bacteria and thereby allowing infection foci to form as entrapped microcolonies. The infection thread, initiated by invagination of the plant cell wall and membrane, grows down through the root hair cell by external deposition of cell wall material, allowing colonization by bacteria of the developing nodule (Brewin, 2004).
Several legume genes required for nodule morphogenesis have been placed on a pathway based on the phenotypes of the mutants (Oldroyd and Downie, 2004). Mutations in the predicted Nod factor receptor Lotus japonicus genes LjNFR1 and LjNFR5 (Madsen et al., 2003; Radutoiu et al., 2003) and the Medicago truncatula MtNFP1 (Ben Amor et al., 2003) block Nod factor-induced root hair deformation, the calcium influx, and calcium spiking (Ben Amor et al., 2003; Miwa et al., 2006). Mutations in the L. japonicus genes LjSYMRK (Stracke et al., 2002), CASTOR, POLLUX (Imaizumi-Anraku et al., 2005), LjNUP133 (Kanamori et al., 2006), and LjSYM24 (Miwa et al., 2006) and M. truncatula MtDMI1 and MtDMI2 (Catoira et al., 2000) block calcium spiking but not root hair deformation or the calcium influx (Shaw and Long, 2003; Miwa et al., 2006). L. japonicus mutants that retain Nod factor-induced calcium spiking, but lack nodule morphogenesis, include Ljccamk and its M. truncatula ortholog Mtdmi3 (Wais et al., 2000; Tirichine et al., 2006), Ljsym6 (Schauser et al., 1998; Harris et al., 2003; Kistner et al., 2005), and Ljnin (Schauser et al., 1998). All of these mutants can also be split into two classes based on their ability to form mycorrhizal associations. Thus, seven loci in L. japonicus (SYMRK, CASTOR, POLLUX, NUP133, SYM24, SYM6, and CCaMK) and three in M. truncatula (DMI1, DMI2, and DMI3) are required for both nodulation and mycorrhization (Catoira et al., 2000; Kistner et al., 2005). Three loci not required for mycorrhization are LjNFR1, LjNFR5, and MtNFP, which are thought to be specifically involved in Nod factor recognition (Ben Amor et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003). The L. japonicus LjNIN gene product, which is thought to act downstream of calcium spiking, is also not required for mycorrhization (Schauser et al., 1998). The CCaMK is a chimeric calcium/calmodulin-dependent protein kinase that is thought to integrate the calcium-spiking signal resulting in the activation of downstream regulatory proteins (Gleason et al., 2006; Tirichine et al., 2006).
In M. truncatula, two additional genes required for nodule morphogenesis have been described as acting downstream of MtDMI3 encoding CCaMK (Levy et al., 2004; Mitra et al., 2004a). These two genes, MtNSP1 (M. truncatula NSP1 gene) and MtNSP2 (Catoira et al., 2000; Oldroyd and Long, 2003), are not required for the mycorrhizal symbiosis. Plants carrying mutations in MtNSP1 or MtNSP2 are blocked for Nod factor-induced gene expression but have root hair deformation (Catoira et al., 2000; Kalo et al., 2005; Smit et al., 2005) and normal Nod factor-induced calcium influx and spiking (Oldroyd and Long, 2003). Both NSP genes are essential for all known Nod factor-induced changes in M. truncatula gene expression (Mitra et al., 2004b), and Mtnsp1 and Mtnsp2 mutants completely lack infection threads and cortical cell division after inoculation with Sinorhizobium meliloti (Catoira et al., 2000; Oldroyd and Long, 2003; Mitra et al., 2004b). The predicted NSP1 and NSP2 proteins both belong to the GRAS (GAI, RGA, SCR) domain proteins, which contain a variable N-terminal region and a conserved C-terminal GRAS domain (Kalo et al., 2005; Smit et al., 2005). The function of these genes and their similarity to GRAS domain proteins suggests that they are Nod factor-activated transcription regulators (Kalo et al., 2005; Smit et al., 2005).
The GRAS domain protein family is exclusive to plants, with homologs in many higher plants, such as barley (Hordeum vulgare), rice (Oryza sativa), Arabidopsis (Arabidopsis thaliana), tomato (Lycopersicon esculentum), petunia (Petunia hybrida), and lily (Lilium longiflorum; Bolle, 2004). The GRAS domain proteins are described as transcriptional regulators showing significant sequence homology in their C-terminal regions. This region is composed of five domains: two Leu heptad repeats flanking a VHIID domain (named after the most conserved amino acids); a PFYRE domain that is located after the second Leu-rich domain, and a C-terminal SAW domain (Pysh et al., 1999; Bolle, 2004; Tian et al., 2004). In Arabidopsis, 33 GRAS domain proteins have been isolated, and these have been divided into eight subfamilies, each with distinct conserved domains and functions (Bolle, 2004).
In this study, we report the identification of the L. japonicus GRAS-type LjNSP1 (NSP1 gene from L. japonicus) and LjNSP2 genes, which are important for nodulation. We have investigated the potential conservation of function of a related NSP1-like gene from Nicotiana benthamiana and have shown that it is able to restore nodulation to nsp1 mutants of M. truncatula and L. japonicus although infection and formation of normal bacteroids were not fully complemented.
RESULTS
Identification of a Novel Mutation in L. japonicus Affecting Early Stages of Nodulation
Approximately 45,600 ethyl methanesulfonate-mutagenized M2 L. japonicus Gifu B-129 plants from 3,843 families were screened for absence of nodules (Nod−) or presence of small white nodules (Perry et al., 2003). Selected symbiotically defective mutants were then screened for defects in infection thread formation as described previously (Lombardo et al., 2006) using Mesorhizobium loti strain R7A (pXLGD4) constitutively expressing lacZ.
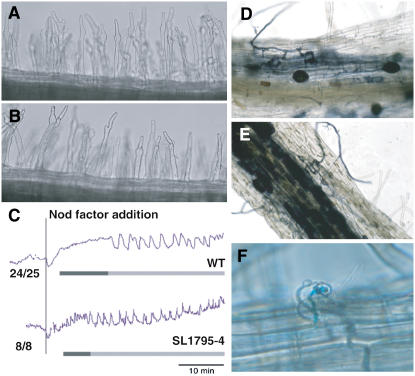
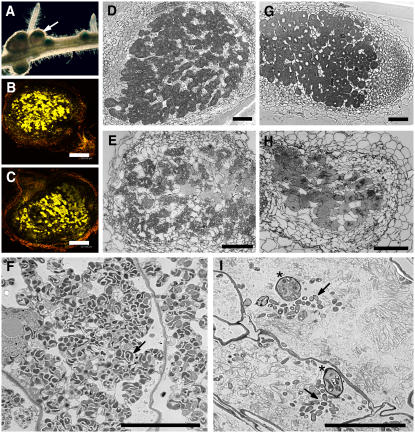
A mutant, SL1795-4, was identified that lacked nodules and M. loti-induced cortical cell division but retained root hair deformation. The mutant lacked infection threads and infection pockets, although very occasionally infection pockets were observed (about 1% of wild-type levels; Fig. 1F). However, the mutant did have normal infection by the arbuscular mycorrhizal fungus Glomus intraradices (Fig. 1, D and E), Nod factor (10 nm) from M. loti-induced root hair deformation (Fig. 1, A and B), and normal cytosolic calcium spiking, as well as a calcium influx (Fig. 1C). No nodules were observed on SL1795-4 roots after 4 weeks growth on agar, whereas the wild type typically formed five to 20 nodules under these conditions. The mutant showed clear signs of nitrogen limitation (yellowing of leaves and stunted growth) but grew normally if the agar was supplemented with nitrate.
Figure 1.
Symbiotic phenotype of L. japonicus SL1795-4. A, Root hair deformation in wild-type roots was normal in the root hair elongation zone after addition of Nod factor to a final concentration of 10 nm. B, Root hair deformation in SL1795-4 was indistinguishable from the wild type. C, Analysis of calcium spiking in root hairs showed normal Nod factor-induced calcium spiking in SL1795-4. Each trace originates from a single root hair cell, either from the wild type (previously shown in Miwa et al., 2006) or the mutant carrying the SL1795-4 allele. The variability between the traces shown is typical of the variation seen within wild type (Miwa et al., 2006). The numbers refer to the number of cells showing calcium spiking relative to the total number of cells assayed. Root hairs were injected with a calcium-sensitive dye Oregon green-dextran plus the reference dye Texas-red. After about 20 min, Nod factor was added to a final concentration of 10 nm. A calcium influx was observed in both the wild type and the SL1795-4 mutant and was followed by calcium spiking. The dark bar underlines the calcium flux, whereas the light bar is drawn under the calcium spiking. D and E, Mycorrhizal infection of SL1795-4 (E) was indistinguishable from that seen with wild-type plants after colonization with G. intraradices (D). F, Very rare infection threads (1% of wild-type level) could be found in the SL1795-4 mutant; however, no cortical cell division was observed. [See online article for color version of this figure.]
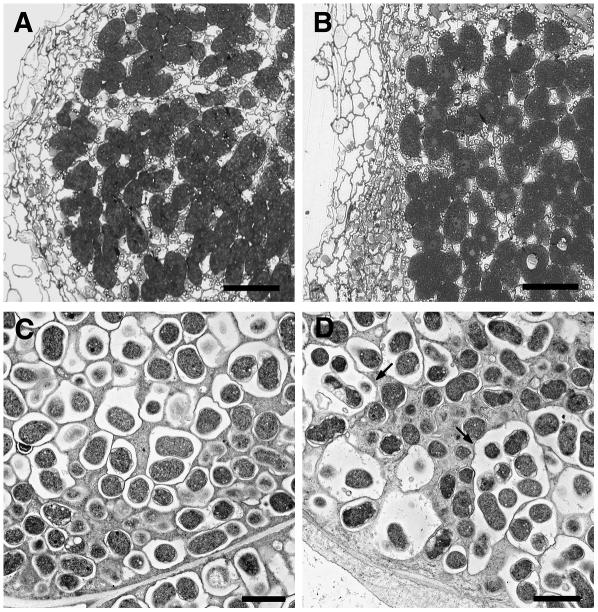
After growth in nitrogen-limited soil for 2 months, the SL1795-4 mutant usually formed a few nodules. Of 26 plants tested, 17 formed nodules producing an overall average of 1.7 ± 0.4 nodules, whereas equivalent wild-type plants had an average of 35 ± 5.5 nodules per plant. Light microscopy showed that the nodules formed on the mutant were infected similarly to wild-type (Fig. 2, A and B), but electron microscopy revealed that in some nodule sections, the number of bacteroids (3–25) contained within the symbiosome membrane of the mutant (Fig. 2D) was much higher than that seen in the wild type (a maximum of four; Fig. 2C). This phenotype was only observed in the central region of the nodule, whereas the symbiosomes in the surrounding cells looked normal. This unusual symbiosome structure was observed in about one-half of the SL1795-4 nodules investigated and is similar to that seen in the Fix− mutant Ljsym105 (Hossain et al., 2006). To measure the nitrogen-fixation potential of these nodules, measurements of acetylene reduction were made using individual nodules from both wild type and mutant. Nodules were matched for size, and acetylene reduction assays with SL1795-4 produced 4.7 ± 1.8 nmol ethylene h−1 compared with wild-type levels of 6.3 ± 3.0 nmol ethylene h−1. The nodulation and infection phenotype defects scored in the original mutant were indistinguishable from those observed in symbiotically defective seedlings from backcrossed progeny (one backcross).
Figure 2.
Structure of SL1795-4 mutant nodules after growth for 60 d. A, Light micrograph of a mature wild-type nodule (60 d after inoculation) showing infected cells packed with bacteria. B, Light micrograph of an SL1795-4 nodule showing infected cells similar to those in wild-type nodules. C and D, Transmission electron micrographs of mature wild-type (C) and mutant (D) nodules. Within SL1795-4 (D), many bacteroids were found surrounded by one symbiosomal membrane (arrow), whereas in the wild type symbiosomes contained only one to four bacteroids (C). Bars = 50 μm (A and B) and 2 μm (C and D).
Mapping of the Nodulation Mutation in SL1795-4
SL1795-4 was crossed to its parent and to the wild-type ecotype Miyakojima (MG-20). The segregation ratios observed in the F2 progeny from both crosses indicated recessive monogenic inheritance (Table I). Bulked segregants using two groups of 12 mutant F2 progeny from the cross with MG-20 were used initially to map the mutation. Using microsatellite markers representing all chromosomes, only TM0049 at 56.7 cM on chromosome 3 cosegregated. Further mapping with 111 non-nodulating individuals from 462 F2 plants localized the mutation between the flanking markers TM0416 (55.5 cM) and TM0049 (56.7 cM). No recombination events were seen with the marker TM0115 (56.3 cM; Fig. 3A).
Table I.
Segregation analysis of the nodulation defect in L. japonicus SL1795-4
| Parental Lines | F2 Progeny Analyzed | No. of Plants
|
Observed
|
||
|---|---|---|---|---|---|
| Nod+ | Nod− | χ2 Valuea | P Value | ||
| SL1795-4 × MG-20 | 462 | 351 | 111 | 0.234 | P > 0.05 |
| SL1795-4 × B-129 | 47 | 36 | 11 | 0.064 | P > 0.05 |
χ2 value, P > 0.05 when χ2 < 3.84.
Figure 3.
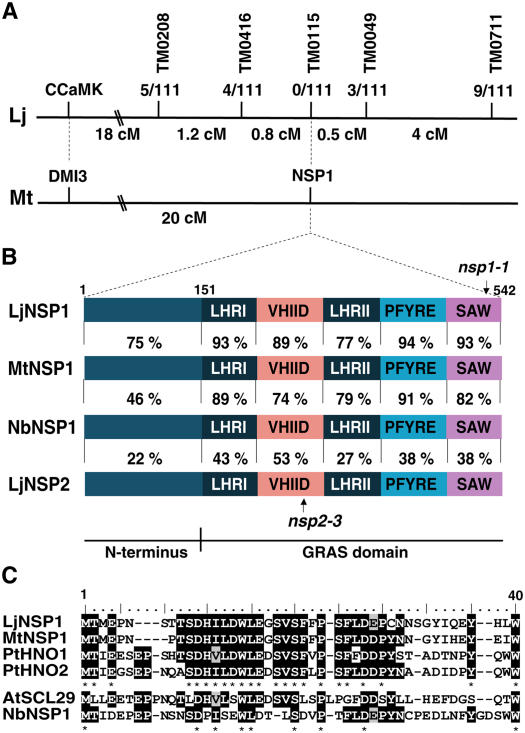
Identification of the L. japonicus NSP1 gene. A, Genetic map of the Ljnsp1-1 (SL1795-4) region with markers and numbers of recombinant events above the line. The genetic distance in centimorgans is indicated below the line showing the distance between the markers. The corresponding syntenic region of M. truncatula chromosome 8 (Mt) is shown. B, Similarities between the predicted L. japonicus NSP1 and the predicted orthologs M. truncatula NSP1 or the N. benthamiana NSP1-like proteins. The percentage of similarity between L. japonicus NSP1 and NSP2 is also shown. The GRAS domain consists of two Leu-rich regions (LHRI and LHRII) and three conserved regions called VHIID, PFYRE, and SAW. All the proteins, except for LjNSP2, show similarity across the entire protein including the variable N-terminal region, indicating that they are probably orthologous. PCR amplification of the Ljnsp1-1 and Ljnsp2-3 alleles revealed premature stop codons in the SAW and VHIID domains, respectively (Supplemental Fig. S1). C, Alignment of the first 40 residues found in the N termini of LjNSP1, MtNSP1, P. trichocarpa PtHNO1, PtHNO2, Arabidopsis SCL29, and NbNSP1. A conserved region from position 9 to 33 is present in the most closely related proteins (LjNSP1, MtNSP1, PtHNO1, and PtHNO2), whereas the conserved region is less apparent in the more distant proteins (AtSCL29 and NbNSP1). [See online article for color version of this figure.]
Four mutations affecting the nodule symbiosis have been mapped on the lower part of chromosome 3, namely, HAR1, srh1, vrh1, and ccamk (Solaiman et al., 2000; Krusell et al., 2002; Nishimura et al., 2002; Karas et al., 2005; Sandal et al., 2006; Tirichine et al., 2006). However, the locations of these mutations (Sandal et al., 2006) and the phenotypes of the HAR1, srh1, and vrh1 mutants are different from the SL1795-4 mutant. The phenotype of the Ljccamk mutant is similar, but LjCCaMK maps between TM0110 and TM0005 (Tirichine et al., 2006), approximately 20 cM from the nodulation mutation in SL1795-4. We noted that MtNSP1 is located approximately 20 cM from DMI3 encoding the CCaMK in M. truncatula (Ané et al., 2002; Smit et al., 2005). The Mtnsp1 mutant has normal root hair deformation and calcium spiking in response to Nod factor, but lacks cortical cell division and is colonized normally by mycorrhizal fungi (Catoira et al., 2000) and thus appears phenotypically similar to the L. japonicus SL1795-4 mutant. The possible synteny between M. truncatula and L. japonicus indicated that the gene mutated in SL1795-4 could be an ortholog of M. truncatula NSP1 (Fig. 3A).
Cloning of LjNSP1
No expressed sequence tag (EST) sequences homologous to MtNSP1 could be found in the L. japonicus database. DNA hybridization using the MtNSP1 GRAS-encoding domain as probe indicated that a single band was present in L. japonicus. NSP1 was amplified from L. japonicus genomic DNA using several different primers spanning the whole MtNSP1 region. Some of the primer combinations produced several bands and/or smears, and in these cases nested PCR was used to produce single bands that could be used for DNA sequencing. The 3′ and 5′ ends were obtained by 3′ RACE and using GenomeWalker. The assembled LjNSP1 DNA sequence of 1,632 bp showed 75% identity to the M. truncatula gene at the nucleotide level. PCR-based amplification of LjNSP1 and DNA sequencing from wild type was carried out on both genomic DNA and cDNA using a primer set spanning the whole coding region. The sequence obtained confirmed the assembled sequence and revealed the lack of any introns.
LjNSP1 encodes a predicted protein of 542 amino acids belonging to the GRAS domain proteins. The GRAS protein family contains five recognizable GRAS motifs, which are identifiable in the region from amino acids 151 to 542 of LjNSP1 (Fig. 3B). Several amino acids are relatively invariant in most members of the GRAS protein family. These include the domain containing the PFYRE motif, designated after the respective amino acids (Pysh et al., 1999), and the conserved residues within the conserved domain illustrated in Figure 3B were clearly identified in LjNSP1 (Supplemental Fig. S1). The VHIID sequence is present in all members of the family, but only the His and the Asp residues are absolutely conserved, and in LjNSP1 the LHILD residues are found starting at position 269 (consensus position 292 on Supplemental Fig. S1). The C-terminal SAW motif in LjNSP1 is SLW at position 529 (consensus position 557 on Supplemental Fig. S1), and the SAW domain contains three pairs of conserved residues: R(x)4E, W(x)7G, and W(x)10W.
Alignment of LjNSP1 and MtNSP1 revealed high overall identity/similarity (77%/83%; Fig. 3B). The overall identity of the GRAS domain was 81%, whereas the identity in the N-terminal third of the proteins was 68%. The N-terminal 40 residues in LjNSP1 and MtNSP1 showed higher identity/similarity (85%/88%) compared to the N-terminal region overall (68%/75%), particularly over residues 9 to 33 (SDHILDWLEGSVSFFPSFLDDPYN), suggesting a functionally conserved domain (Fig. 3C). A putative nuclear localization site present in MtNSP1 (PKKR) was also found in an exact match in the LjNSP1 protein starting at position 93 (consensus position 103 on Supplemental Fig. S1).
SL1795-4 Contains a Mutation in Ljnsp1 and Can Be Complemented by Cloned MtNSP1
Sequencing of LjNSP1 from SL1795-4 revealed a G to A nucleotide substitution at position 1,560, changing Trp 520 (TGG) to a stop codon (TGA; consensus position 547 on Supplemental Fig. S1). This resulted in a predicted protein lacking the C-terminal 23 amino acids, which include the residues [W(x)10W] that are part of the SAW domain (Fig. 3B; Supplemental Fig. S1). This indicates that the SAW motif is very important for the function of the NSP1 protein.
We verified that the Ljnsp1-1 mutation in SL1795-4 caused the nodulation defect by Agrobacterium rhizogenes hairy root transformation (Table II). LjNSP1 inserted into p-KGW-RR under control of the M. truncatula NSP1 promoter (pMtNSP1) restored nodulation in transformed hairy roots inoculated with M. loti (Fig. 4, B and C), whereas no nodules were observed with the vector lacking LjNSP1. The LjNSP1 construct also complemented the M. truncatula Mtnsp1-1 mutant (Table II). Conversely, MtNSP1 under the control of its own promoter (Smit et al., 2005) complemented the Ljnsp1-1 mutant SL1795-4 in hairy root transformations (Table II). These reciprocal complementation experiments demonstrate that these orthologous genes have a conserved biochemical function.
Table II.
Hairy root complementation tests for nodulation with L. japonicus, M. truncatula, and N. benthamiana NSP1 genes
| Plant Background | Transforming Binary Plasmid | Transformed Plants | No. of Plants Nodulated |
|---|---|---|---|
| L. japonicus nsp1-1 | pKGW-pMtNSP1 vector | 81 | 0 |
| L. japonicus nsp1-1 | pKGW-pMtNSP1-LjNSP1 | 130 | 104 |
| L. japonicus nsp1-1 | pKGW-pMtNSP1-MtNSP1 | 17 | 16 |
| L. japonicus nsp1-1 | pKGW-pMtNSP1-NbNSP1 | 88 | 43 |
| M. truncatula nsp1-1 | pKGW-pMtNSP1 vector | 58 | 0 |
| M. truncatula nsp1-1 | pKGW-pMtNSP1-LjNSP1 | 71 | 53 |
| M. truncatula nsp1-1 | pKGW-pMtNSP1-NbNSP1 | 56 | 56 |
Figure 4.
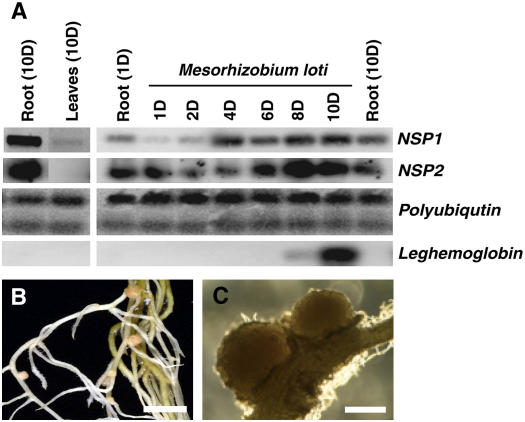
Expression patterns of LjNSP genes and complementation of Ljnsp1-1 (SL1795-4) by NSP1. A, LjNSP1 and LjNSP2 expression was investigated by semiquantitative RT-PCR before and after M. loti inoculation using polyubiquitin as an internal control. The leghemoglobin gene used is induced early during nodulation (Stracke et al., 2002) and functions as a positive control for the M. loti treatment. Uninoculated roots (1D and 10D) are shown as controls to compare with the expression seen over a period of 1 to 10 d (1D–10D) postinoculation with M. loti. A low level of LjNSP1 but not LjNSP2 expression was detected in leaves following prolonged exposure of the film. This is shown relative to the level of expression seen in uninoculated roots. B, Complementation of the nsp1-1 mutant by the MtNSP1 gene was revealed by the formation of nodules on several plants. Several pink nodules could be observed in the complemented plants, indicating that they were functional nodules (bar = 2 mm). Similar nodules were found when the nsp1-1 mutant was complemented using LjNSP1 (data not shown). C, The nodules showed a normal size and presence of lenticels (bar = 0.5 mm). [See online article for color version of this figure.]
TILLING for Mutations in nsp2
The L. japonicus NSP2 gene was amplified from genomic and cDNA using primers from the M. truncatula NSP2 gene sequence and no introns were present. LjNSP2 encodes a predicted protein of 499 amino acids showing 73% identity to M. truncatula NSP2 at the nucleotide level. The LjNSP1 and LjNSP2 proteins show low similarity (20% identity/33% similarity), although some domains showed higher similarity (Fig. 3B).
A mutant, SL781-3, carrying an allele of LjNSP2 found by TILLING (Perry et al., 2003), had a phenotype similar to the Ljnsp1-1 mutant, in that it retained Nod factor-induced root hair deformation, calcium spiking, and had normal mycorrhization. However, SL781-3 lacked infection threads and formed no nodules or associated cortical cell divisions even after prolonged growth with M. loti (data not shown). The SL781-3 mutant carried a nucleotide substitution, changing Gln-244 (CAA) to a stop codon (TAA) in LjNSP2. The predicted protein lacked part of the VHIID domain and the rest of the C-terminal domain (Fig. 3B). The previously described Ljsym35 (also called Ljsym70) mutant also has a similar phenotype (Miwa et al., 2006; Sandal et al., 2006), and genetic crosses revealed the mutations in Ljsym35 and SL781-3 to be allelic because F1 progeny from the cross formed no nodules. The Ljsym35/Ljsym70 locus maps to the top of chromosome 1 (Sandal et al., 2006), which is syntenic with the region carrying the MtNSP2 gene (Choi et al., 2004). When we tried to amplify the LjNSP2 gene from the Ljsym35 mutant, we obtained no product, and DNA hybridization experiments using the LjNSP2 gene as a probe revealed that the hybridizing band seen in the wild type was completely absent (data not shown). These results show that LjNSP2 is deleted in the Ljsym35 mutant. The identical symbiotic phenotypes of Ljsym35 and SL781-3 are consistent with a complete loss of function of LjNSP2 in both mutants. In parallel work, another mutant has been identified as carrying a mutation allelic to Ljsym35. In that work, the mutant was called Ljnsp2-1 and the mutation in Ljsym35 was renamed Ljnsp2-2 (M. Kawaguchi, personal communication). We have therefore called the mutation in SL781-3 Ljnsp2-3.
Analysis of LjNSP1 and LjNSP2 Expression
Expression of LjNSP1 during the early stages of symbiosis was investigated by semiquantitative reverse transcription (RT)-PCR. A slight reduction of expression occurred in the first 2 d; such a reduction in expression has also been observed for CASTOR and POLLUX, which function earlier in the pathway (Imaizumi-Anraku et al., 2005). Increased expression of LjNSP1 was observed in roots 4 d after inoculation (Fig. 4A), and this increase occurred well before the expression of the leghemoglobin gene (Fig. 4A), which is induced early in symbiosis (Stracke et al., 2002). LjNSP1 was further induced during nodule formation, and this points to NSP1 possibly playing a role during nodule morphogenesis and/or infection. LjNSP2 showed an expression pattern very similar to LjNSP1 (Fig. 4A), with the difference that the up-regulation of the LjNSP2 might be somewhat delayed compared with LjNSP1. The expression patterns of both LjNSP genes in roots were similar to that reported for the MtNSP2 in M. truncatula (Kalo et al., 2005).
Even though there were no obvious defects in growth and development under nonsymbiotic conditions, we investigated whether LjNSP1 and LjNSP2 were expressed in leaf tissue as was described for both MtNSP1 (Smit et al., 2005) and MtNSP2 (Kalo et al., 2005). Overexposure of the film revealed the presence of the LjNSP1 transcript at very low levels in leaves (Fig. 4). However, we observed no expression of LjNSP2 in leaves. Both MtNSP1 and MtNSP2 are expressed mainly in roots with very low expression in other tissue types (Kalo et al., 2005; Smit et al., 2005).
Phylogeny of the GRAS Domain Gene Family Identifies a Putative NSP1 Ortholog in N. benthamiana
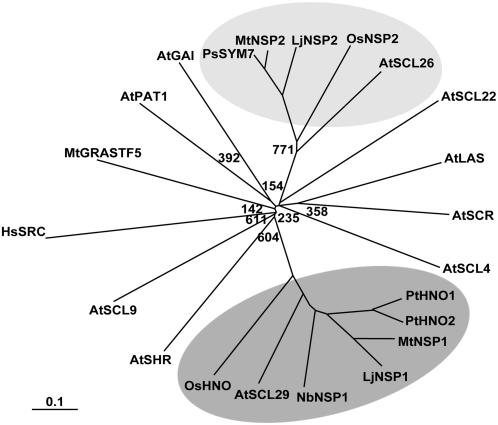
The GRAS domain proteins can be divided into eight subfamilies based on similarities of their N-terminal regions (Bolle, 2004). LjNSP1 is very similar to MtNSP1 and is also very similar to two predicted proteins in Populus trichocarpa (PtHNO1, PtHNO2). The closest Arabidopsis homolog is AtSCL29 (Smit et al., 2005; Fig. 5). The sequence similarity was particularly high in the first 40 residues that were noted above as being highly conserved between LjNSP1 and MtNSP1 (Fig. 3C). This could indicate that this N-terminal domain may be important for a specific function of these NSP1-like proteins from different plants.
Figure 5.
Phylogenetic analysis of GRAS proteins. This unrooted tree shows the separation of the two NSP branches and their relationship with other GRAS proteins from Arabidopsis (At), rice (Os), M. truncatula (Mt), L. japonicus (Lj), P. trichocarpa (Pt), and N. benthamiana (Nb). Full-length amino acid sequences were aligned using ClustalX, and the bootstrapped tree was visualized in TreeView. All bootstrap values above 800 have been removed. The NSP1 subclade is marked in dark gray and the NSP2 subclade in light gray. Based on this analysis of Arabidopsis GRAS domain proteins and searches of the entire Arabidopsis protein database, it appears unlikely that there is an ortholog of NSP2 in Arabidopsis. To see the full tree and accession numbers, see Supplemental Figure S2. Scale: 0.1 nucleotide substitutions per site.
Comparisons revealed a very clear separation between the NSP1 and NSP2 families (Fig. 5). LjNSP2 is very similar to MtNSP2 and Pisum sativa SYM7; the closest Arabidopsis homolog is AtSCL26 (Fig. 5). A predicted protein from rice (OsNSP2) also groups with the NSP2 branch. Phylogenetic analysis of the NSP1 protein group, containing the MtNSP1, LjNSP1, PtHNO1, PtHNO2, AtSCL29, and rice OsHNO, showed a weak relationship with the Arabidopsis SHR transcription factor (see Supplemental Fig. S2). The NSP2 protein group, containing the MtNSP2, LjNSP2, PsSYM7, AtSCL26, and OsNSP2, had a weak relationship with the Arabidopsis SCL4/7 and HAM branches (see Supplemental Fig. S2).
Incomplete sequences similar to LjNSP1 were found in translated EST libraries from lettuce (Lactuca sativa), potato (Solanum tuberosum), and N. benthamiana. Sequence EST fragments from lettuce (BQ857799, 52% identity/69% similarity) and potato (CK861723, 67% identity/82% similarity) were found covering 221 residues of the midregion and 150 residues of the GRAS domain, respectively. Due to the incomplete sequences their precise position in the phylogenetic tree cannot be defined unambiguously and so they were not included in Figure 5. Two predicted protein sequences from N. benthamiana covered the N terminus (48% identity/60% similarity) and GRAS domain (64% identity/79% similarity). Using cDNA and PCR primers, we confirmed that the two EST sequences found in N. benthamiana spanned a single gene, expected not to have any introns. When compared to LjNSP1, the N. benthamiana NSP1-like sequence we generated seemed to be complete. The sequence encodes a predicted protein with 56% identity/70% similarity to both LjNSP1 and MtNSP1, clearly placing it in the same phylogenetic grouping (Fig. 5). The highest similarity was in the LHRI, PFYRE, and SAW motifs, whereas the N-terminal region showed only 46% similarity (Fig. 3B). Again, as observed for the other NSP1-like proteins, NbNSP1 showed higher similarity in the first 40 amino acids (Fig. 3C). We decided to determine if NbNSP1 was functionally equivalent to LjNSP1 and MtNSP1 by complementation. We chose the N. benthamiana gene because it belongs to the asterid clade and is therefore outside the rosid clade, which contains all known nodulating plants (Doyle, 1994). Furthermore, N. benthamiana is readily transformable and so the function of this gene could possibly be analyzed using gene silencing.
The N. benthamiana NSP1-like gene was cloned such that the open reading frame was inserted into the p-KGW-RR vector under the control of the pMtNSP1 promoter. The resulting plasmid in A. rhizogenes was used to produce transformed hairy roots of both the Ljnsp1-1 (SL1795-4) and the Mtnsp1-1 (B85) mutants. In transformed roots of both species, nodule-like structures could be observed 2 weeks after inoculation. No nodule-like structures were observed in hairy root transformations with the vector containing the MtNSP1 promoter but lacking the inserted gene. To test for nitrogenase activity, acetylene reduction was assayed using individual nodules on hairy roots of the L. japonicus (SL1795-4) and M. truncatula (B85) nsp1 mutants complemented for nodulation with the NbNSP1 transgene. Acetylene reduction was observed with the complemented L. japonicus nodules (4.0 ± 2.2 nmol ethylene h−1 compared to 6.3 ± 1.7 nmol ethylene h−1 with matched wild-type nodules). In contrast, no acetylene reduction was observed with any of the 20 tested nodules of the M. truncatula nsp1-1 mutant (B85) complemented with NbNSP1, whereas wild-type nodules produced 2.8 ± 0.7 nmol ethylene h−1.
To measure infection in the nodules formed on the NbNSP1-transformed hairy roots of the M. truncatula nsp1-1 mutant, we inoculated with S. meliloti carrying a constitutively expressed lacZ reporter gene and stained the nodules for LacZ activity (Fig. 6A). Several of the nodules stained at the tips, suggesting that bacteria were at the tips of nodules where the infection zone is found. Light and electron microscopy of similar nodules revealed that there were infection threads present in these M. truncatula nodules, but very few bacteria were present within the cells (Fig. 6I). Some bacteria were present within cells, but these were seldom surrounded by a peribacteroid membrane, and in those few cases where bacteria appeared to have a peribacteroid membrane, there seemed to be degradation of the bacteria. These data suggest that NbNSP1 complementation allowed infection thread growth, but the release of the bacteria into nodule cells and/or possibly symbiosome function was not fully restored.
Figure 6.
Roots of SL1795-4 (Ljnsp1-1) and B85 (Mtnsp1-1) were transformed with A. rhizogenes carrying an N. benthamiana NSP1-like gene cloned behind the MtNSP1 promoter to produce hairy roots. A, Nodules formed on Mtnsp1-1 complemented with NbNSP1 were stained with X-Gal to reveal bacteria inside the nodules (arrow). B, L. japonicus nsp1-1 hairy roots containing NbNSP1 produced nodules containing bacteria revealed by staining with Syto13 (bar = 288.65 μm). C, A wild-type L. japonicus nodule stained with Syto13 is shown for comparison (bar = 237.89 μm). D and E, Light micrographs of stained L. japonicus nodule sections of wild type (D) and Ljnsp1-1 complemented with NbNSP1 (E). F, Electron micrograph section of an Ljnsp1-1 nodule complemented with NbNSP1, showing abnormal bacteroids within a symbiosome membrane (arrow). G and H, Light micrographs of stained M. truncatula nodule sections of wild type (G) and Mtnsp1-1 complemented with NbNSP1 (H) showing few infected cells. I, Electron micrograph section of an Mtnsp1-1 nodule complemented with NbNSP1, showing several infection threads (star) and only some bacteria in nodule cells but not contained in a symbiosome membrane (arrow). Bar = 100 μm (D, E, G, and H) and 10 μm (F and I). [See online article for color version of this figure.]
To determine if infection was normal in the nodules formed on the NbNSP1-transformed hairy roots of the L. japonicus nsp1-1 mutant, we initially cut nodules in half and stained them with Syto13, which can be used to visualize bacteria within plants (Haynes et al., 2004). This revealed that there were many bacteria within most of the nodules (Fig. 6, B and C). Light and electron microscopy of similar nodules revealed that about half of the cells in the L. japonicus nodules were infected (Fig. 6, E and F). Electron microscopy revealed the presence of many bacteria within peribacteroid membranes, although many of these bacteroids appeared to be at a lower population density and were clearly misshapen (Fig. 6F) compared with wild-type controls (Fig. 2C). Therefore, although the NbNSP1 gene can restore nodulation, it cannot restore normal release of rhizobia to a M. truncatula nsp1-1 mutant. Although the release of bacteria can be restored, albeit relatively inefficiently, in complemented nodules of the L. japonicus nsp1-1 mutant, the bacteroid shape appeared abnormal, indicating a possible role in the formation of normal symbiosomes.
DISCUSSION
The L. japonicus LjNSP1 and LjNSP2 genes identified here encode two very divergent GRAS domain regulators orthologous to those encoded by the M. truncatula MtNSP1 and MtNSP2 genes described previously (Kalo et al., 2005; Smit et al., 2005). In both legumes, mutations in the genes affect the induction of cortical cell divisions associated with nodule morphogenesis and also block the formation of infection threads required for the root infection that occurs concomitantly with nodule morphogenesis during legume nodulation (Catoira et al., 2000; Oldroyd and Long, 2003; Mitra et al., 2004b). The Ljnsp2-3 mutation causes a more severe phenotype than the Ljnsp1-1 mutation.
Based on different lines of evidence, it is clear that the NSP1 and NSP2 gene products act downstream of the calcium-calmodulin-dependent kinase that is thought to be activated by Nod factor-induced calcium spiking (Oldroyd and Downie, 2004). Mutations in the two genes appear to have a less severe effect on root hair deformation than does mutation of the CCaMK gene (Catoira et al., 2000; Oldroyd and Long, 2003; Miwa et al., 2006), and the mutant plants retain normal mycorrhization, suggesting a role downstream of the common signaling pathway components shared by the rhizobial and mycorrhizal symbioses (Catoira et al., 2000; Kistner et al., 2005). However, the strongest evidence for NSP1 and NSP2 proteins acting downstream of the CCaMK comes from the experiments with transformed hairy roots expressing a constitutively activated form of the CCaMK. A deleted form of the CCaMK lacking an inhibitory domain could induce nodule morphogenesis in transgenic roots of M. truncatula even in the absence of rhizobia or Nod factors. However, this did not occur in Mtnsp1 or Mtnsp2 mutants (Gleason et al., 2006), demonstrating that these two gene products must be required to link the activated CCaMK to the induction of downstream genes required for the activation of cell division associated with nodule morphogenesis. Such a role for these NSP1 and NSP2 GRAS domain proteins fits well with previous observations about related GRAS domain regulators, which constitute a family of plant-specific proteins that play roles in various developmental processes such as signal transduction, meristem maintenance, and development (Bolle, 2004). Thirty-three members of the GRAS domain family have been identified in Arabidopsis (Bolle, 2004). These have been characterized as transcriptional regulatory proteins, and consistent with this role, most GRAS proteins have been found to be located in the nuclei (Tian et al., 2004). In line with these observations, MtNSP1 was shown to be present in the nucleus (Smit et al., 2005), whereas MtNSP2 was found associated with the endoplasmic reticulum/nuclear envelope but relocalized into the nucleus after Nod factor addition (Kalo et al., 2005).
It has been proposed (Pysh et al., 1999) that the N-terminal region of GRAS proteins could function as an activator, and that the higher level of divergence in this region compared to the GRAS domain region could reflect requirements for specific activation following interactions with different signaling proteins acting in various developmental pathways. The conserved C-terminal region could be involved in interactions with downstream proteins such as the transcriptional machinery and/or accessory proteins (Bolle, 2004). The Leu heptad repeats in the conserved LHRI-VHIID-LHRII domains have been suggested to be involved in protein-protein interactions, and the VHIID domain might mediate protein-DNA interactions (Pysh et al., 1999). The PFYRE and SAW motifs are also important because mutations in these motifs cause very severe phenotypes as observed for SLR1 (SLENDER RICE1; Itoh et al., 2002), RGA (Silverstone et al., 1998; Itoh et al., 2002), and LjNSP1 (this study).
A GRAS protein from lily, designated LiSCL (L. longiflorum Scarecrow-like), is expressed specifically at the premeiotic phase within anthers. The protein has two highly basic regions, and transient expression analyses of dissected GFP-LiSCL fusion proteins show that both basic regions are important for the nuclear localization (Morohashi et al., 2003). Truncated LiSCL proteins demonstrate that the amino terminus of the LiSCL protein strongly induces transcriptional activation in yeast as well as in plant cells (Morohashi et al., 2003). The DELLA GRAS domain proteins are repressors of GA signaling in plants (Bolle, 2004). Deletion analysis of the rice DELLA protein, SLR1, revealed that SLR1 can be divided into four parts: a GA signal perception domain located at the N terminus; a regulatory domain for its repression activity; a dimer-formation domain containing LHRI essential for signal perception and repression activity; and a repression domain at the C terminus containing the PFYRE and SAW conserved motifs (Itoh et al., 2002). A mutation 16 nucleotides upstream of the translation stop of SLR1 resulted in a loss-of-function phenotype (Ikeda et al., 2001); based on this comparison, it seems unlikely that the occasional nodules produced by the Ljnsp1-1 mutant could be due to the mutation affecting only the C-terminal region of the protein. SLR1 is phosphorylated on an N-terminal Ser residue(s) within the DELLA/TVHYNP and polyS/T/V domain (Itoh et al., 2005). Possibly the N terminus of one or both of the NSP proteins could contain an N-terminal signal perception domain as described for SLR1 and act to regulate transcription in vivo as with LiSCL (Morohashi et al., 2003).
At this stage, it is not clear how activation of NSP1 and NSP2 occurs. One theoretical possibility is phosphorylation by the CCaMK, which is nuclear located and acts upstream of the NSP proteins. However, such a direct interaction seems unlikely because the CCaMK is presumably activated by mycorrhizal signaling, but this does not lead to induction of many of the genes whose expression requires NSP1 and NSP2. It is likely that the NSP proteins fulfill similar but nonredundant roles, as nsp1 and nsp2 mutants have very similar phenotypes (Catoira et al., 2000; Oldroyd and Long, 2003). This suggests that the proteins could work together in a cooperative manner (Kalo et al., 2005; Smit et al., 2005). In this regard, it appears that the pattern of expression of both genes in L. japonicus is similar following rhizobial inoculation. Thus, there appeared to be a decrease in gene expression 2 d after inoculation followed by a steady increase in expression over time. This is similar to what was observed for MtNSP2 expression in M. truncatula, suggesting that NSP1 plays a continuing role that goes beyond early signaling, possibly being required for maintenance of nodule development and/or infection. This may be somewhat different from the apparent lack of change in expression observed for MtNSP1 following the addition of Nod factor. However, in those experiments, expression was only followed for 2 d after addition of Nod factor (Smit et al., 2005).
The identification of ESTs with sequence similarity to GRAS proteins found in bryophytes indicates that this family of proteins arose before the emergence of land plants over 400 million years ago (Nishiyama et al., 2003). The presence of putative orthologs of NSP1 in a variety of plants, such as Arabidopsis, P. trichocarpa (Smit et al., 2005), N. benthamiana, potato, and lettuce (this study), indicates a more ancient function other than symbiosis. Our observation that the N. benthamiana NSP1-like gene can function in the Nod factor-signaling pathway indicates the conserved domains necessary for perception and activation in the NSP1 protein have been conserved among legumes and non-legumes. However, the nsp1 mutants of L. japonicus and M. truncatula develop normally when grown with a nitrogen source, showing that NSP1 is not required for normal growth. The increased mRNA levels in roots after rhizobial inoculation suggest a specialized role in nodule formation, but it does not exclude other functions. The NSP genes could have been recruited for a nodule-specific function from a preexisting common gene. Other key genes involved in nodulation, such as leghemoglobin and the genes required for both the rhizobial and mycorrhizal symbioses, seem to have been recruited this way from preexisting genes involved in coordination of plant development (Andersson et al., 1996; Kistner and Parniske, 2002).
The data presented here and elsewhere (Kalo et al., 2005; Smit et al., 2005) suggest that legumes have recruited preexisting genes to make a functional signaling pathway leading to nodule organogenesis. A surprise is that a signaling protein such as NSP1 from N. benthamiana has retained the ability to activate the nodule morphogenesis pathway in legumes because clearly that cannot be its normal function. The relatively better complementation of the L. japonicus compared with the M. truncatula nsp1 mutants could be due to differences between nodulation and infection in these legumes, but we cannot rule out the possibility that a truncated form of the L. japonicus NSP1 protein from the Ljnsp1-1 mutant might interact with NbNSP1, enhancing its function. To try to determine a possible function for NbNSP1, we tried virus-induced gene silencing, which is relatively effective in N. benthamiana (Ratcliff et al., 2001). However, we were unable to detect any abnormalities in the growth of the plants carrying the N. benthamiana RNAi construct compared with controls lacking the NbRNAi construct. Further work with stably expressed NbRNAi will be required to understand the role of this gene in N. benthamiana. The ability of NbNSP1 to complement nodulation in legumes implies that, if NSP1 proteins are activated by nodulation signaling, the N. benthamiana NSP1-like protein has retained the activation and/or protein interaction site(s) required for activation. It also implies that its ability to activate downstream gene expression is unlikely to be direct in terms of transcriptional activation of nodulation-specific promoters. Instead, it seems more probable that it acts to activate some aspect of the gene induction pathway that is common to several genes. If this is the case, then it may be that some other nodulation signaling component has a more specific effect in the induction of early nodulation genes in legumes responding to rhizobia. An additional consideration is that the timing and location of expression of the NSP1-like gene may be important for induction of early nodulation gene expression, and it is important to note that we expressed the NbNSP1 gene from the MtNSP1 promoter. Future work on the expression and activation of the NSP genes in legumes may give an insight into how related GRAS domain regulators can propagate the nodulation signals activated by rhizobia.
MATERIALS AND METHODS
Plant Growth and Bacterial Strains
Lotus japonicus genotype gifu B-129 was used as wild-type control for phenotypic and genotypic analysis. For nodulation studies in compost, seeds were scarified for 16 min in sulfuric acid. Plants were grown in the greenhouse where seeds were planted into small plastic pots in Scotts Levington F1 compost or terragreen sand. For growth on FP agar medium, the scarified seeds were sterilized with 10% bleach for 15 min. Filter paper (grade 0860; Schleicher and Schull) was placed between the agar and the roots to prevent the roots growing into the agar. The roots were then covered by another filter paper to keep the roots moist. The plants were grown in a vertical position in a growth chamber (day/night cycles of 18 h/6 h; temperature 20°C/15°C). Mesorhizobium loti strain R7A (pXLG4) carrying lacZ was used for screening purposes. Inoculation of plants in the greenhouse was done using a wild-type strain (R7A) of M. loti. Nodule numbers were scored on at least 10 plants, and data on nodulation and acetylene reduction tests are shown with ses. Medicago truncatula nsp1-1 (B85) seeds were kindly provided by Giles Oldroyd.
Calcium Spiking Investigations and Root Hair Deformation Assay
Oregon Green-dextran Mr 10,000 (Molecular Probes) was dissolved in sterile water and Texas Red-dextran Mr 10,000 (Molecular Probes) was used as a reference. Nod factors were purified and analyzed by reverse-phase chromatography (Miwa et al., 2006). Fluorescence was imaged as determined previously using a Nikon TE2000U inverted microscope coupled to a Hamamatsu Photonics digital CCD camera. Root hair deformation was assayed using seedlings grown on plates between filter paper with high humidity for several days. The seedlings were transferred to Faharus slides containing 1 mL of buffered nodulation medium 1 h before Nod factor addition to a final concentration of 10 nm. Nod factor was added and this was left in the dark for approximately 24 h before being analyzed.
Mycorrhization Investigation
Scarified seeds were sown into sand containing Glomus intraradices arbuscular mycorrhiza-infected chives and grown for approximately 1 month. The staining protocol used was as described by Vierheilig et al. (1998).
Mapping and Agrobacterium rhizogenes-Mediated Complementation
A mapping population was obtained by crossing the L. japonicus SL1795-4 (B-129) to L. japonicus MG-20. The mutant and F1 seeds were grown for seeds. The F2 seeds were planted into compost, and mutant plants were identified on absence of nodules and cortical cell division.
DNA was prepared using plant leaf material (one to three young leaves) harvested on ice in collection tubes (Qiagen) containing a tungsten carbide bead (Qiagen). A total of 400 μL of extraction buffer (200 mm Tris-HCl, pH 7.5; 250 mm NaCl; 25 mm EDTA; 0.5% v/v SDS) preheated to 65°C was added to each tube. Samples were homogenized on a mixer mill (Retsch MM300) for 2 min at 30 oscillations/s, and incubated at 65°C for 30 min to 1 h. Samples were centrifuged at 6,500 rpm for 10 min, and 300 μL of supernatant was transferred into fresh collection tubes and purified with approximately 0.8 volumes of phenol:chloroform:isoamyalcohol (25:24:1), pH 8.0. DNA was precipitated at −20°C for 1 h with 1/10 volume 3 m NaAc and 1 volume isopropanol. DNA was pelleted by centrifuging at 5,600 rpm for 45 min. DNA was washed overnight in 70% ethanol before air drying and resuspending in 50 to 100 μL distilled water. A 10-fold dilution of this suspension was used for all further work.
Microsatellite markers were used to map the SL1795-4 allele using a population of 111 homozygous mutants to a genetic position on linkage group 3, between the two markers TM0416 and TM0049. For molecular markers, primers were ordered from Sigma Genosys. Oligonucleotides for TM molecular markers (microsatellite) were designed at Kazusa DNA Research Institute (KDRI, www.kazusa.or.jp).
Transgenic hairy roots and nodulation tests were done as previously described (Stougaard, 1995). For in planta complementation, the MtNSP1 construct (p-KGW-NSP1) was used (Smit et al., 2005; supporting material). Two SnaBI restriction sites were inserted in p-KGW-NSP1 vector using Quickchange II site-directed mutagenesis kit (Stratagene). This made it possible to cut out the M. truncatula gene from the construct, thereby still retaining the promoter and insert the L. japonicus NSP1 gene behind. A triparental mating was performed to integrate the construct into A. rhizogenes strain AR1193.
DNA Isolation and TILLING
The L. japonicus NSP1 sequence was amplified using M. truncatula NSP1-specific primers (see Supplemental Materials and Methods S1). These were used in all possible combinations (39 cycles: 94°C for 15 s, 45°C for 30 s, 72°C for 90 s). A partial sequence was obtained from genomic DNA corresponding to roughly 690 bp of the N-terminal region. Additional sequence was obtained by designing primers specific for the LjNSP1 sequence (see Supplemental Materials and Methods S1) in each end of the obtained fragments and running these in combination with the MtNSP1 primers. The missing 3′ and 5′ ends were amplified by 3′RACE system for RACE (Invitrogen) using oligo(dT) primer and BD GenomeWalker Universal kit (BD Bioscience CLONTECH), respectively, following the manufacturer's instructions. This resulted in the identification of the full-length wild-type sequence and 475 bp of the promoter region. The DNA sequence was obtained, and both wild-type and mutant plants were sequenced in the search for any single basepair changes. An additional mutant was found by TILLING as described in Perry et al. (2003) using the primer pair NSP1-TILL Forw1 (IRD700-CATCCAAGAGTACCACTTGTGGGATCAG) and NSP1-TILL Rev1 (IRD800-CATTCCCATCCAGCTTCCACAAAGAAC). TILLING primers with labels was ordered at MWG-Biothech AG.
The L. japonicus NSP2 sequence was amplified using M. truncatula NSP2-specific primers (see Supplemental Materials and Methods S1). The missing 3′ and 5′ ends were amplified by 3′RACE system for RACE (Invitrogen) using oligo(dT) primer. Following the identification of the full sequence, TILLING was performed as described above, using the primer pair NSP2-TILL Forw2 (IRD700-ACTCAACTCAACAACCTCAGGCATGGA) and NSP2-TILL Rev3 (IRD800-GTCCAAAGGGATGCAGAAAGCAAACAC). The Nicotiana benthamiana NSP1-like gene was cloned by making primers using the two GenBank EST sequences, CK281685 and CK281684. The forward primer started at nucleotide 75 in CK281685 and the reverse primer started at nucleotide 7 in CK281684 (for primer information, see Supplemental Materials and Methods S1). The primers, containing added SnaBI restriction sites, were used to amplify the full-length cDNA, and this was cloned using SnaBI into p-KGW-NSP1.
In Silico Analysis
BLAST searches were performed using the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/blast/) and the Institute for Genomic Research Gene Indices database (http://tigrblast.tigr.org/tgi/). The DNA sequence was translated using the EXPASY tools (http://www.expasy.org/cgi-bin/pi_tool). The deduced amino acid sequences were aligned using the ClustalX program with standard parameters, and a phylogenetic bootstrapped tree was generated in TreeView using the neighbor-joining method.
RT-PCR Analysis of NSP1 and NSP2 Expression
Total RNA from uninoculated roots and M. loti inoculated roots (1, 2, 4, 6, 8, and 10 dpi) was extracted using an RNeasy kit (Qiagen). Isolated total RNA (2.5 μg) was treated with DNaseI, reverse transcribed using oligo(dT) by Superscript II reverse transcriptase (Life Technologies), and subjected to semiquantitative RT-PCR. PCR cycle numbers were defined for each primer set, resulting in amplification within the linear phase. Amplification was exponential up to 27 cycles. Samples lacking the RT treatment were used as control for genomic DNA contamination (data not shown).
For the amplification of the NSP1 gene, the primers NSP1 fwd2 CGAGCACTGACACACCACTT and NSP1 rev3 CTGCAAACCCTGCTTCTTTC were used (24 cycles of 94°C for 15 s, 59°C for 30 s, and 72°C for 50 s). For the amplification of the NSP2 gene, the primers NSP2 3RACE3 (AGTTGCTTCGTTTCTAACTGCGGCCAAG) and NSP1 rev2 (CAAGTCCAAAGGGAAGCAGAAAGCA) were used (24 cycles of 94°C for 15 s, 59°C for 30 s, and 72°C for 50 s). To compare cDNA concentrations in the different samples, the amount of fragments was compared with polyubiquitin and leghemoglobin as controls for RNA concentrations and inoculation (22 cycles of 94°C for 15 s, 59°C for 30 s, and 72°C for 50 s). For primer information, see Stracke et al. (2002). PCR fragments were analyzed by agarose gel electrophoresis followed by Southern-blot hybridization using an [α-32P]-labeled probe. Signals were quantified using a Typhoon 8600 variable mode imager and ImageQuanTL (Amersham Bioscience).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers LjNSP1, EF012819; Ljnsp1-1, EF017372; LjNSP2, DQ665943; Ljnsp2-3, EF053276; and NbNSP1, EF032736.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Materials and Methods S1. Oligonucleotides.
Supplemental Figure S1. Alignment of L. japonicus, M. truncatula, and N. benthamiana NSP1.
Supplemental Figure S2. Phylogenetic analysis of GRAS proteins.
Acknowledgments
We thank Patrick Smit and Renee Guerts for information prior to publication and for supplying pKGW-NSP1-RR. We also thank Wladimir Tameling and David Baulcombe for N. benthamiana cDNA and advice on virus-induced gene silencing. Moreover, we thank Peter Kalo, Anne Edwards, John Marsh, and Giles Oldroyd for primers, sequence information, and very helpful discussions.
This work was supported by the European Union (Marie Curie Ph.D. fellowship RTN–CT–2003–505227 to A.B.H.) via the INTEGRAL network, by the Biotechnology and Biological Sciences Research Council, by the Gatsby Charitable Foundation (to the Sainsbury Laboratory), by the John Innes Foundation, and by the Universities UK Overseas Research Students Awards Scheme (H.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: J. Allan Downie (allan.downie@bbsrc.ac.uk).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Andersson CR, Jensen EO, Llewellyn DJ, Dennis ES, Peacock WJ (1996) A new hemoglobin gene from soybean: a role for hemoglobin in all plants. Proc Natl Acad Sci USA 93: 5682–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ané JM, Levy J, Thoquet P, Kulikova O, de Billy F, Penmetsa V, Kim DJ, Debelle F, Rosenberg C, Cook DR, et al (2002) Genetic and cytogenetic mapping of DMI1, DMI2, and DMI3 genes of Medicago truncatula involved in nod factor transduction, nodulation, and mycorrhization. Mol Plant Microbe Interact 15: 1108–1118 [DOI] [PubMed] [Google Scholar]
- Ben Amor B, Shaw SL, Oldroyd GED, Maillet F, Penmetsa RV, Cook D, Long SR, Denarie J, Gough C (2003) The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J 34: 495–506 [DOI] [PubMed] [Google Scholar]
- Bolle C (2004) The role of GRAS proteins in plant signal transduction and development. Planta 218: 683–692 [DOI] [PubMed] [Google Scholar]
- Brewin NJ (2004) Plant cell wall remodeling in the rhizobium-legume symbiosis. CRC Crit Rev Plant Sci 23: 293–316 [Google Scholar]
- Cardenas L, Vidali L, Dominguez J, Perez H, Sanchez F, Hepler PK, Quinto C (1998) Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol 116: 871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Denarie J (2000) Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell 12: 1647–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Mun JH, Kim DJ, Zhu HY, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB, et al (2004) Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci USA 101: 15289–15294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie JA, Walker SA (1999) Plant responses to nodulation factors. Curr Opin Plant Biol 2: 483–489 [DOI] [PubMed] [Google Scholar]
- Doyle JJ (1994) Phylogeny of the legume family: an approach to understanding the origins of nodulation. Annu Rev Ecol Syst 25: 325–349 [Google Scholar]
- Gleason C, Chaudhuri S, Yang TB, Munoz A, Poovaiah BW, Oldroyd GED (2006) Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441: 1149–1152 [DOI] [PubMed] [Google Scholar]
- Harris JM, Wais R, Long SR (2003) Rhizobium-induced calcium spiking in Lotus japonicus. Mol Plant Microbe Interact 16: 335–341 [DOI] [PubMed] [Google Scholar]
- Haynes JG, Czymmek KJ, Carlson CA, Veereshlingam H, Dickstein R, Sherrier DJ (2004) Rapid analysis of legume root nodule development using confocal microscopy. New Phytol 163: 661–668 [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Fang YW (1994) Plant hormones and nodulation: what's the connection. Plant Mol Biol 26: 5–9 [DOI] [PubMed] [Google Scholar]
- Hossain MS, Umehara Y, Kouchi H (2006) A novel Fix(-) symbiotic mutant of Lotus japonicus, Ljsym105, shows impaired development and premature deterioration of nodule infected cells and symbiosomes. Mol Plant Microbe Interact 19: 780–788 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Anraku H, Takeda N, Charpentier M, Perry J, Miwa H, Umehara Y, Kouchi H, Murakami Y, Mulder L, Vickers K, et al (2005) Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433: 527–531 [DOI] [PubMed] [Google Scholar]
- Itoh H, Sasaki A, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Hasegawa Y, Minami E, Ashikari M, Matsuoka M (2005) Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol 46: 1392–1399 [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, Jakab J, Sims S, Long SR, Rogers J, et al (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EMH, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, et al (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA 103: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas B, Murray J, Gorzelak M, Smith A, Sato S, Tabata S, Szczyglowski K (2005) Invasion of Lotus japonicus root hairless 1 by Mesorhizobium loti involves the nodulation factor-dependent induction of root hairs. Plant Physiol 137: 1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner C, Parniske M (2002) Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci 7: 511–518 [DOI] [PubMed] [Google Scholar]
- Kistner C, Winzer T, Pitzschke A, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Webb KJ, et al (2005) Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell 17: 2217–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426 [DOI] [PubMed] [Google Scholar]
- Levy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ane JM, Lauber E, Bisseling T, et al (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364 [DOI] [PubMed] [Google Scholar]
- Lombardo F, Heckmann AB, Miwa H, Perry J, Yano K, Parniske M, Wang T, Hayashi M, Downie JA (2006) Identification of symbiotically defective mutants of Lotus japonicus affected for infection thread growth. Mol Plant Microbe Interact (in press) [DOI] [PubMed]
- Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, et al (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640 [DOI] [PubMed] [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GED, Long SR (2004. a) A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc Natl Acad Sci USA 101: 4701–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Shaw SL, Long SR (2004. b) Six nonnodulating plant mutants defective for Nod factor-induced transcriptional changes associated with the legume-rhizobia symbiosis. Proc Natl Acad Sci USA 101: 10217–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Sun J, Oldroyd GED, Downie JA (2006) Analysis of nod-factor-induced calcium signaling in root hairs of symbiotically defective mutants of Lotus japonicus. Mol Plant Microbe Interact 19: 914–923 [DOI] [PubMed] [Google Scholar]
- Morohashi K, Minami M, Takase H, Hotta Y, Hiratsuka K (2003) Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J Biol Chem 278: 20865–20873 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, et al (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100: 8007–8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA (2004) Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol 5: 566–576 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Long SR (2003) Identification and characterization of nodulation-signaling pathway 2, a gene of Medicago truncatula involved in Nod factor signaling. Plant Physiol 131: 1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JA, Wang TL, Welham TJ, Gardner S, Pike JM, Yoshida S, Parniske M (2003) A TILLING reverse genetics tool and a web-accessible collection of mutants of the legume Lotus japonicus. Plant Physiol 131: 866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN (1999) The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J 18: 111–119 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592 [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Sandal N, Petersen TR, Murray J, Umehara Y, Karas B, Yano K, Kumagai H, Yoshikawa M, Saito K, Hayashi M, et al (2006) Genetics of symbiosis in Lotus japonicus: recombinant inbred lines, comparative genetic maps, and map position of 35 symbiotic loci. Mol Plant Microbe Interact 19: 80–91 [DOI] [PubMed] [Google Scholar]
- Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259: 414–423 [DOI] [PubMed] [Google Scholar]
- Schultze M, Kondorosi A (1998) Regulation of symbiotic root nodule development. Annu Rev Genet 32: 33–57 [DOI] [PubMed] [Google Scholar]
- Shaw SL, Long SR (2003) Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol 131: 976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer BJ, Ketelaar T, Esseling JJ, Emons AMC (2005) Microtubules guide root hair tip growth. New Phytol 167: 711–719 [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun TP (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debelle F, Gough C, Bisseling T, Geurts R (2005) NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308: 1789–1791 [DOI] [PubMed] [Google Scholar]
- Solaiman MZ, Senoo K, Kawaguchi M, Imaizumi-Anraku H, Akao S, Tanaka A, Obata H (2000) Characterization of mycorrhizas formed by Glomus sp on roots of hypernodulating mutants of Lotus japonicus. J Plant Res 113: 443–448 [Google Scholar]
- Stougaard J (1995) Agrobacterium rhizogenes as a vector for transforming higher plants. Methods Mol Biol 49: 46–61 [DOI] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, et al (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417: 959–962 [DOI] [PubMed] [Google Scholar]
- Tian CG, Wan P, Sun SH, Li JY, Chen MS (2004) Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol Biol 54: 519–532 [DOI] [PubMed] [Google Scholar]
- Tirichine L, James EK, Sandal N, Stougaard J (2006) Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Mol Plant Microbe Interact 19: 373–382 [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64: 5004–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Denarie J, Long SR (2000) Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA 97: 13407–13412 [DOI] [PMC free article] [PubMed] [Google Scholar]