Abstract
The ameliorating effect of nitrate on the acidification of the cytoplasm during short-term anoxia was investigated in maize (Zea mays) root segments. Seedlings were grown in the presence or absence of nitrate, and changes in the cytoplasmic and vacuolar pH in response to the imposition of anoxia were measured by in vivo 31P nuclear magnetic resonance spectroscopy. Soluble ions and metabolites released to the suspending medium by the anoxic root segments were measured by high-performance liquid chromatography and 1H nuclear magnetic resonance spectroscopy, and volatile metabolites were measured by gas chromatography and gas chromatography-mass spectrometry. The beneficial effect of nitrate on cytoplasmic pH regulation under anoxia occurred despite limited metabolism of nitrate under anoxia, and modest effects on the ions and metabolites, including fermentation end products, released from the anoxic root segments. Interestingly, exposing roots grown and treated in the absence of nitrate to micromolar levels of nitrite during anoxia had a beneficial effect on the cytoplasmic pH that was comparable to the effect observed for roots grown and treated in the presence of nitrate. It is argued that nitrate itself is not directly responsible for improved pH regulation under anoxia, contrary to the usual assumption, and that nitrite rather than nitrate should be the focus for further work on the beneficial effect of nitrate on flooding tolerance.
Although nitrate has a beneficial effect on plant survival under anoxia, the relative importance of the mechanisms by which this might be achieved is unclear (Gibbs and Greenway, 2003; Greenway and Gibbs, 2003; Stoimenova and Kaiser, 2004). Recent work has confirmed both the physiological significance of nitrate and/or nitrate reduction in moderating the impact of oxygen deprivation, and the difficulty of providing conclusive mechanistic explanations (Stoimenova et al., 2003; Allègre et al., 2004).
For example, transgenic tobacco (Nicotiana tabacum) plants lacking root nitrate reductase (NR) activity were more sensitive to root anoxia than wild-type control plants, but the metabolic phenotype of the transformants was unexpectedly complicated, with a substantial increase in fermentation under anoxia and a greater acidification of the cytoplasm (Stoimenova et al., 2003). It was concluded that the absence of NR activity in the transgenic roots did not limit the regeneration of NADH, and it was suggested that the beneficial effects of nitrate reduction might lie in a down-regulation of the metabolic rate under anoxia (Stoimenova et al., 2003). Similarly, experiments on hydroponically grown tomato (Lycopersicon esculentum) plants during the development of root anoxia showed that nitrate reduction was beneficial in preventing the onset of wilting and necrosis in the leaves, and that anoxia caused increases in NR activity and nitrite release to the growth medium (Allègre et al., 2004). The effects on NR activity and nitrite production, which were confirmed in parallel experiments on excised root systems (Morard et al., 2004), were in agreement with the current model for NR activation (Kaiser et al., 1999), but again it was not possible to draw firm conclusions about the protective effect of nitrate reduction on the plants. Thus, the impact of nitrate and nitrate reduction on anaerobic metabolism in plants is incompletely understood and merits further investigation.
One major area of uncertainty is the relationship between nitrate and pH regulation during oxygen deprivation. This link is important because cytoplasmic acidosis is a determinant of flooding intolerance (Roberts et al., 1984a), and exposure to nitrate was found to reduce the acidification of the cytoplasm in anoxic maize (Zea mays) root tips (Roberts et al., 1985). Since NR is reversibly activated at low pH (Kaiser and Brendle-Behnisch, 1995) and since nitrate reduction is proton consuming, it has been argued that nitrate reduction could form the basis for a biochemical pH-stat (Botrel et al., 1996; Botrel and Kaiser, 1997; Ratcliffe, 1999; Greenway and Gibbs, 2003). Unfortunately, this proposal is unsustainable because nitrate reduction generates protons when the only source of reducing power is glycolysis (Gerendás and Ratcliffe, 2002; Libourel, 2003). In agreement with this analysis, anaerobic metabolism was demonstrated to be more acidifying in wild-type tobacco roots capable of reducing nitrate to nitrite than in transformants lacking NR (Stoimenova et al., 2003). Thus, the reason for the beneficial effect of nitrate on pH regulation under anoxia is unknown.
In principle, the improvement in pH regulation could reflect either a direct effect of nitrate metabolism on cytoplasmic pH itself, or an indirect nitrate-induced change in carbon metabolism or ion transport with consequential changes in pH regulation (Libourel, 2003). To assess the contribution of these mechanisms, the response of maize root segments to short-term anoxia was examined in the presence and absence of nitrate by: (1) measuring changes in cytoplasmic and vacuolar pH using in vivo 31P NMR (Ratcliffe, 1997), and (2) profiling the soluble and volatile ions and metabolites released by anoxic root segments into the surrounding medium. NR is substrate inducible, and a comparison was made between: (1) roots grown on nitrate and subjected to anoxia in the presence of nitrate; (2) roots grown on chloride, switching to nitrate during the anoxic experiment; and (3) roots grown and treated with chloride only. The most striking difference between the root exudates turned out to be in the level of nitrite, and the subsequent demonstration that a low level of nitrite itself is sufficient to reduce the acidification of the cytoplasm under anoxia focuses attention on a primary role for nitrite rather than nitrate in the anaerobic response.
RESULTS
Improved Cytoplasmic pH Regulation in the Presence of Nitrate
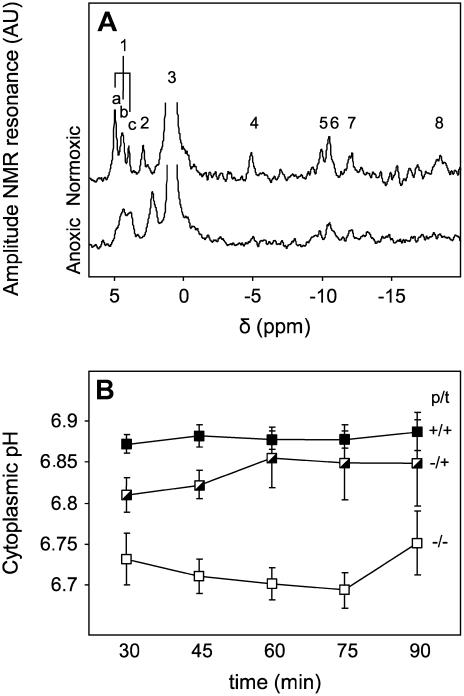
In vivo 31P NMR spectroscopy was used to determine the effect of nitrate on the cytoplasmic acidification that occurs in response to anoxia (Fig. 1). The 31P NMR spectra of the maize root segments showed the expected features for a highly vacuolated tissue (Gerendás et al., 1990; Ratcliffe, 1994), with a dominant signal from the vacuolar inorganic phosphate (Pi) pool as well as weaker signals from a range of cytoplasmic metabolites (Fig. 1A). The chemical shift of the cytoplasmic Pi signal indicated that the cytoplasmic pH was 7.52 ± 0.02 under normoxic conditions, irrespective of whether the roots had been exposed to nitrate before or during the measurement (data not shown). Imposition of anoxia reduced the cytoplasmic pH by up to 0.8 pH units, with roots that were pretreated and treated with KCl showing the largest drop, and roots pretreated and treated with KNO3 showing the smallest (Fig. 1B). Roots pretreated with KCl and transferred to KNO3 during the NMR experiment showed an intermediate acidification. The cytoplasmic pH values were stable over a 2-h period under anoxia and recovered to a normal aerobic value within 1 h of reinstating the oxygen supply (data not shown).
Figure 1.
Effect of nitrate on cytoplasmic pH in maize root segments following a switch to anoxia. A, In vivo 31P NMR spectra of maize root segments recorded over 1 h under aerobic or anoxic conditions. The labeled peaks correspond to: 1, several phosphomonoesters, including (a) Glc-6-P and (c) phosphocholine, with (b) unassigned; 2, cytoplasmic Pi; 3, vacuolar Pi; 4, 5, and 8, the γ-, α-, and β-phosphates, respectively, of nucleoside triphosphate; 6, UDP-Glc and NAD(P)(H); and 7, UDP-Glc. The position of peak 2 is sensitive to the cytoplasmic pH, moving to lower chemical shift values as the cytoplasm acidifies. B, Cytoplasmic pH values in maize root segments following a switch to anoxia at time zero. Roots were either grown and then incubated during the NMR experiment with nitrate (p/t: +/+; ▪), or grown with chloride and then incubated with nitrate (−/+;  ), or grown and incubated with chloride only (−/−; □). The pH values prior to the imposition of anoxia were: (+/+), pH 7.54 ± 0.02; (−/+), pH 7.51 ± 0.01; and (−/−), pH 7.51 ± 0.01. Each value is the mean of between three and six replicate measurements ±se.
), or grown and incubated with chloride only (−/−; □). The pH values prior to the imposition of anoxia were: (+/+), pH 7.54 ± 0.02; (−/+), pH 7.51 ± 0.01; and (−/−), pH 7.51 ± 0.01. Each value is the mean of between three and six replicate measurements ±se.
Limited Metabolism of Nitrate under Anoxia
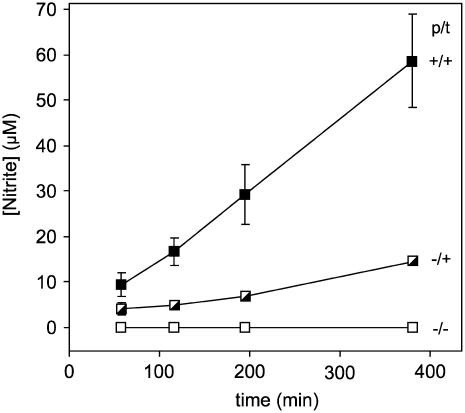
Roots pretreated with KNO3 or incubated with KNO3 during the anoxic treatment released nitrite to the medium under anoxia (Fig. 2). NR is substrate inducible in maize roots (Li and Oaks, 1993), and Figure 2 shows that root segments from seedlings induced with 10 mm nitrate during the pretreatment period excreted more than 3 times as much nitrite as the segments that were only transferred into the same concentration of nitrate at the beginning of anoxia. No nitrite was released from the segments taken from seedlings that had not been treated with nitrate at all.
Figure 2.
Effect of nitrate on nitrite release from maize root segments following a switch to anoxia. The growth and incubation conditions are defined in the legend for Figure 1, and each value is the mean of between three and eight replicate measurements ±se.
No nitrogen-containing volatiles, such as nitrous or nitric oxides, were detected by gas chromatography (GC) during a 6-h anoxic time course, and GC-mass spectrometry (MS) analysis of the gas phase during incubations with [15N]labeled nitrate or nitrite failed to reveal 15N-labeled fragments (data not shown).
Similarly, no evidence for metabolism beyond nitrite was found when NMR was used to monitor root segments during incubations with 0.5 mm K15NO2 under anoxia. Nitrite, rather than nitrate, was used in these experiments to avoid dilution of the label by endogenous pools (nitrate is stored in vacuoles, whereas nitrite is not) and to facilitate delivery of the label to the cytoplasm (the anion of a weak acid will tend to accumulate in the neutral cytoplasm rather than the vacuole). In vivo 15N NMR showed no accumulation of 15N-labeled metabolites in the root segments over a 7-h time course, and NMR analysis of the suspending medium from experiments in which the incubation was extended to 24 h only showed the presence of a very weak amino signal, most probably from Ala, corresponding to a likely concentration of no more than 10 μm after a 6-h incubation (data not shown).
Impact of Nitrate and Nitrate Reduction on Anoxic Carbon Metabolism and Ion Transport
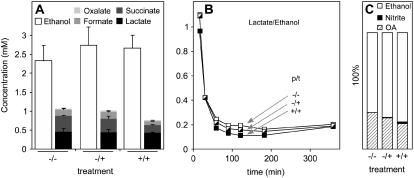
Lactate and ethanol were released into the suspending medium in response to anoxia (Fig. 3A), with an initial burst of lactate production giving way to a largely ethanolic fermentation process (Fig. 3B). Pretreatment with 10 mm KNO3 or addition of nitrate to the incubation medium at the start of the anoxic incubation did not alter the release of ethanol and lactate significantly, although the proportion of ethanol released during the experiment increased in the presence of nitrate (Fig. 3C).
Figure 3.
Effect of nitrate on the release of organic acids and ethanol from maize root segments following a switch to anoxia. A, Ethanol, lactate, succinate, formate, and oxalate concentrations at the end of a 6-h anoxic experiment. The growth and incubation conditions are defined in the legend for Figure 1, and each value is the mean of between three and eight replicate measurements ±se. B, Ratio of lactate to ethanol accumulation in the suspending medium over the 6-h time course of an anoxic experiment. C, Relative accumulation of ethanol, nitrite, and combined organic acids (OA) based on the data in A. The differences between OA and ethanol were significant.
Anoxic maize root segments also released smaller quantities of succinate, formate, and oxalate into the suspending medium (Fig. 3A), and although the amounts decreased for segments taken from seedlings pretreated with nitrate, none of the changes were significant. Succinate accumulation is a common response to short-term anoxia in roots (Roberts et al., 1992; Good and Muench, 1993), and in the absence of nitrate, the level of succinate in the suspending medium was the same at the end of a 6-h anoxic incubation as the level of lactate. The bulk of the succinate in the suspending medium was released in the first hour of the experiment (data not shown), in agreement with the effect of anoxia on carbon metabolism in maize root tips (Roberts et al., 1992). Changes in overall carbon metabolism were apparent from the significantly increased ratio of ethanol to combined organic acids for roots treated and grown in the presence of nitrate in comparison with untreated plants grown in the absence of nitrate (Fig. 3C).
Glu, Suc, and, to a lesser extent, Fru leaked into the suspending medium under anoxia, reaching millimolar concentrations over a 6-h time course (Table I), and there was also a release of chloride, sulfate, and phosphate (Table II). In general, treatments showed only small differences, with the most obvious changes being the reduced chloride efflux from roots pretreated and incubated with nitrate, and the negligible nitrate efflux from roots pretreated and incubated with chloride.
Table I.
Accumulation of carbohydrates in the medium of maize root segments during anoxia
The carbohydrate content of the suspending medium was measured after a 6-h anoxic experiment in which root segments (1.5 g) were incubated in 10 mL of 2 mm KH2PO4, 0.1 mm CaCl2 (pH 6.0). Roots were pretreated and/or incubated with 10 mm KCl or KNO3. Each value is the mean of between three and eight replicate measurements ±se. There were no significant differences between the three treatments.
| Concentration
|
|||
|---|---|---|---|
| KCl + KCl | KCl + KNO3 | KNO3 + KNO3 | |
| mm | |||
| Fru | 0.26 ± 0.04 | 0.34 ± 0.04 | 0.31 ± 0.01 |
| Glu | 0.99 ± 0.14 | 1.09 ± 0.12 | 0.80 ± 0.04 |
| Suc | 1.85 ± 0.07 | 1.75 ± 0.04 | 1.68 ± 0.04 |
Table II.
Accumulation of inorganic anions in the medium of maize root segments during anoxia
The inorganic anions in the suspending medium were measured after a 6-h anoxic experiment in which root segments (1.5 g) were incubated in 10 mL of 2 mm KH2PO4, 0.1 mm CaCl2 (pH 6.0). Roots were pretreated and/or incubated with 10 mm KCl or KNO3. Each value is the mean of between three and eight replicate measurements ±se. Superscripts indicate significant differences between treatments.
| Concentration
|
|||
|---|---|---|---|
| KCl + KCl | KCl + KNO3 | KNO3 + KNO3 | |
| mm | |||
| Chloride | 11.99 ± 0.31a | 1.76 ± 0.03b | 0.45 ± 0.05c |
| Nitrate | 0.00 ± 0.00a | 9.04 ± 0.16b | 9.46 ± 0.12b |
| Phosphate | 3.20 ± 0.05 | 3.34 ± 0.05 | 3.37 ± 0.10 |
| Sulfate | 0.35 ± 0.03 | 0.35 ± 0.03 | 0.46 ± 0.01 |
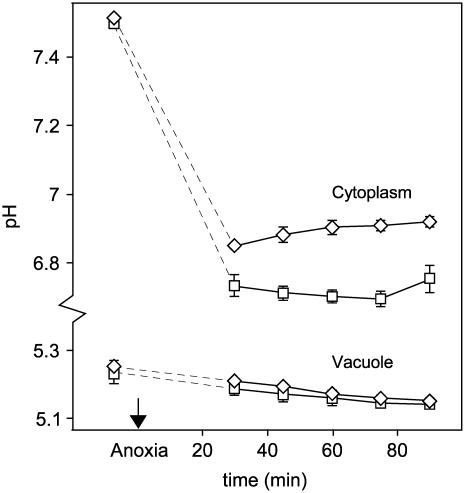
Impact of Nitrate and Nitrate Reduction on Vacuolar pH
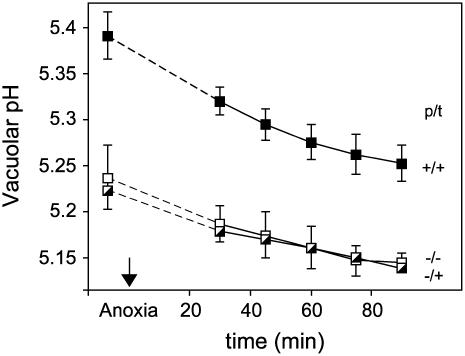
In vivo 31P NMR spectroscopy was also used to determine the effect of nitrate on the vacuolar pH during anoxia (Fig. 4). Estimates of vacuolar pH obtained from the chemical shift of the vacuolar Pi signal indicated: (1) that the pH in the nitrate pretreated samples was offset to a more alkaline pH value, and (2) that a similar acidification occurred irrespective of the growth and treatment conditions. These conclusions need to be treated with caution because inferences about the vacuolar pH are complicated by the influence of ionic strength and ions other than H+, particularly Mg2+, Ca2+, and K+, on the Pi chemical shift in the vacuolar pH range (Roberts et al., 1981). For example, the change in the vacuolar Pi chemical shift observed in the nitrate pretreated roots might reflect an increase in vacuolar ionic strength arising from the storage of nitrate, rather than an increase in pH. However, the changes in chemical shift observed over a short-term anoxic experiment are more likely to reflect proton accumulation from the cytoplasm than a change in vacuolar ionic strength, and the similarity of the time courses in Figure 4 indicates that the initial difference in vacuolar pH cannot be responsible for the differences observed in the cytoplasmic response to anoxia (Fig. 1B).
Figure 4.
Effect of nitrate on vacuolar pH in maize root segments following a switch to anoxia. A series of in vivo 31P NMR spectra were recorded before, during, and after a 2-h period of oxygen deprivation, and the vacuolar pH was inferred from the chemical shift of the vacuolar Pi signal. The growth and incubation conditions are defined in the legend for Figure 1, and each pH value is the mean of between three and six replicate measurements ±se. The switch to anoxia occurred at time zero, the dotted lines connect the normoxic and anoxic pH values, and the se values for the −/+ experiment are omitted for reasons of clarity.
Impact of Nitrite on Cytoplasmic and Vacuolar pH under Anoxia
The nitrate-induced improvement in cytoplasmic pH regulation under anoxia (Fig. 1B) could be caused by a metabolite derived from nitrate, rather than by nitrate itself, and the most obvious candidate is nitrite. Nitrite was released during anoxia by roots pretreated and/or incubated with nitrate (Fig. 2), and it accumulated to a concentration of 2 to 3 μm in the anoxic suspending medium during 31P NMR experiments on root segments pretreated and treated with KNO3 (data not shown). As nitrous acid is a permeable weak acid that can readily equilibrate across cell membranes, simply adding nitrite to the external medium provides a method for generating a cytoplasmic nitrite pool that should be comparable to the concentration present in the nitrate-treated roots under anoxia. In fact, adding 5 μm nitrite to the incubation medium during experiments on roots grown and incubated in the absence of nitrate reduced the acidification of the cytoplasm under anoxia (Fig. 5). Without nitrite, such roots showed the most severe acidification (Fig. 1B), but in the presence of 5 μm nitrite the cytoplasmic pH recovered under anoxia to a value comparable to that observed for roots that were grown and incubated with nitrate. In contrast, nitrite had no effect on the time course for the vacuolar pH (Fig. 5), showing that there was no link between the impact of nitrate/nitrite on cytoplasmic pH regulation and the vacuolar pH response. This also suggests that the more alkaline vacuolar pH value for roots grown and incubated with nitrate (Fig. 4) is not a factor in the improved cytoplasmic pH regulation.
Figure 5.
Effect of adding 5 μm nitrite to the incubation medium on the cytoplasmic and the vacuolar pH values of root segments grown and incubated without nitrate. The switch to anoxia occurred at time zero, the dotted lines connect the normoxic and anoxic pH values, and each pH value is the mean of at least three replicate measurements ±se. □, No nitrite; ⋄, 5 μm nitrite.
DISCUSSION
Nitrate Reduction Is Not Responsible for Improved pH Regulation under Anoxia
It is difficult to provide a convincing explanation for the beneficial effect of nitrate on cytoplasmic pH regulation under anoxia (Fig. 1) in terms of a direct effect of nitrate reduction on cytoplasmic pH. Nitrate metabolism is very limited under anoxia, typically proceeding only as far as nitrite in anoxia-intolerant tissues (Fig. 2; Lee, 1979; Botrel et al., 1996), and although nitrite production has the potential to regenerate NAD+, the concomitant H+ production is as acidifying as fermentation to lactate (Gerendás and Ratcliffe, 2002; Stoimenova et al., 2003). Thus, under anoxia the balanced equation for nitrite production is:
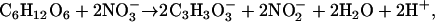 |
assuming glycolysis to be the source of the reducing power, whereas under aerobic conditions, where the electrons are supplied by the complete oxidation of Glc to carbon dioxide, the production of nitrite is H+ neutral:
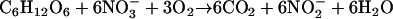 |
and the complete reduction to ammonium is H+ consuming:
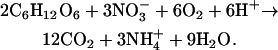 |
It follows that the role of nitrate in the amelioration of cytoplasmic acidosis cannot be explained on the basis of a biochemical pH-stat in which NR is reversibly activated by the fall in cytoplasmic pH under anoxia (Botrel et al., 1996; Botrel and Kaiser, 1997).
Assessing the impact of nitrite production on cytoplasmic pH is further complicated by the pH changes that would occur if a proportion of the nitrite were to be removed from the cytoplasm through transport or further metabolism. Passive diffusion of nitrous acid across the plasma membrane or a 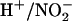 symport would tend to alkalinize the cytoplasm, whereas channel-mediated efflux of K+ and
symport would tend to alkalinize the cytoplasm, whereas channel-mediated efflux of K+ and 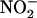 would acidify the cytoplasm. Alternatively, removing
would acidify the cytoplasm. Alternatively, removing 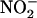 by conversion to nitric oxide (NO), N2O, or N2, none of which were detected here, would all consume protons to some extent. Passive efflux of nitrous acid has the potential to remove all the protons associated with nitrite synthesis, but the extent to which it can occur is limited by the pH difference across the plasma membrane, and it would not have been favored by the external pH of 6.0 used in these experiments. Thus, while nitrite certainly accumulated in the external medium (Fig. 2), the extent to which nitrite was removed from the cytoplasm and the mechanisms involved are unknown. However, the conclusion that nitrite production would tend to acidify the cytoplasm under anoxia remains true.
by conversion to nitric oxide (NO), N2O, or N2, none of which were detected here, would all consume protons to some extent. Passive efflux of nitrous acid has the potential to remove all the protons associated with nitrite synthesis, but the extent to which it can occur is limited by the pH difference across the plasma membrane, and it would not have been favored by the external pH of 6.0 used in these experiments. Thus, while nitrite certainly accumulated in the external medium (Fig. 2), the extent to which nitrite was removed from the cytoplasm and the mechanisms involved are unknown. However, the conclusion that nitrite production would tend to acidify the cytoplasm under anoxia remains true.
Nitrate Pretreatment Causes Only Limited Changes in Anoxic Carbon Metabolism
The metabolite profile of the suspending medium did not reveal clear changes in anoxic carbon metabolism that might point to an indirect mechanism for the nitrate-induced amelioration of cytoplasmic acidosis (Fig. 3; Table I). An overview of the pathways leading to fermentation end products was obtained by measuring the release of metabolites to the suspending medium, and it was found that nitrate pretreatment had little effect on the release of the dominant end products ethanol and lactate (Fig. 3A), although it did increase the proportion of ethanol relative to the combined release of organic acids (Fig. 3C).
The balance between lactate and ethanol production in maize roots under anoxia is determined by the cytoplasmic pH (Roberts et al., 1984b; Fox et al., 1995), and the higher cytoplasmic pH in the nitrate pretreated roots (Fig. 1) might be expected to lead to a slower and/or less complete switch to ethanol fermentation in the nitrate-treated roots. However, nitrate treatment did not lead to an increase in lactate release, suggesting that one effect of the nitrate pretreatment may be to facilitate the switch to the less acidifying ethanolic fermentation at higher pH, perhaps through a change in the pH optimum for pyruvate decarboxylase or lactate dehydrogenase.
The reduction in the proportion of organic acids released to the suspending medium (Fig. 3C), which reflected small but individually insignificant reductions in formate and succinate (Fig. 3A), correlated with the improvement in the cytoplasmic pH. However, the interpretation of the changes in the minor products is complicated by uncertainties about the pathways involved and the mechanisms of metabolite release. For example, consider the release of succinate, which showed a significant decrease for the nitrate pretreated roots. De novo synthesis from Glc might be possible, but in maize root tips the initial burst in succinate production has been attributed to the conversion of Asp and Glc to succinate and Ala, with the release of 2H+ per molecule of Glc consumed (Roberts et al., 1992). Subsequently, this cytoplasmic acidifying process could be either reinforced or reduced by the efflux of succinate, the net result being dependent on the efflux mechanism, or, alternatively, there could be further metabolism by the nitrate-reducing, succinate-oxidizing complex in the plasma membrane (Stöhr and Ullrich, 1997).
Nitrite and NO Are Candidates for Explaining the Beneficial Effect of Nitrate
The beneficial effect of nitrate acquires a different perspective with the striking observation that exposure to micromolar levels of nitrite is sufficient to confer the same effect as nitrate itself (Fig. 5). This alkalinizing effect is the opposite of the pH change that would be expected for the influx of a permeable weak acid from the relatively acidic suspending medium and thus points to the need for a more subtle interpretation of the data. The reduction of nitrite to NO would remove H+ from the cytoplasm:
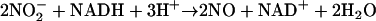 |
and would be alkalinizing when coupled with glycolysis:
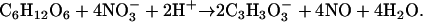 |
However, the lack of significant metabolism beyond nitrite, coupled with the low concentration of nitrite, appears to rule out a direct metabolic effect on the proton balance in the cytoplasm and is more suggestive of a signaling effect. Thus, a possible explanation is that the availability of nitrite under anoxia gives rise to an NO-mediated reduction in metabolic rate, with a consequent decrease in acidification, as previously suggested from an analysis of the anoxic response of tobacco roots lacking NR activity (Stoimenova et al., 2003).
In support of this suggestion, nitrite is readily converted to NO under anoxia (Rockel et al., 2002; Crawford, 2006; Stöhr and Stremlau, 2006). The level and activation state of cytosolic NR, which is one of the enzymes that catalyzes the formation of NO in plant roots, increase under anoxia (Botrel et al., 1996; Botrel and Kaiser, 1997; Stoimenova et al., 2003), and the increase in nitrite that occurs under anoxia could favor the conversion of nitrite to NO. There is also evidence that root mitochondria can reduce nitrite to NO via mitochondrial electron transport, and that this process is strongly favored in the absence of oxygen (Gupta et al., 2005; Planchet et al., 2005). This latter process may well have been dominant under the conditions of the experiment reported in Figure 5 because the cytosolic NR activity would have been low in root segments grown and incubated without nitrate. Finally, NO can also be synthesized from nitrite via the activity of the root-specific plasma membrane-bound nitrite:NO reductase (Stöhr et al., 2001). None of these processes is likely to generate NO at levels that would have been sufficient for detection by the analytical methods used here, indicating that NO production is more likely to function as part of a signaling mechanism rather than as a mechanism for facilitating fermentation.
Hemoglobin-based nitrate recycling has recently been proposed as an alternative to the classical fermentation pathways (Igamberdiev and Hill, 2004). In this scheme, nitrate is recycled through nitrite and NO, consuming oxygen and regenerating NAD+, and although the cycle must fail under true anoxia, the very high affinity of the hypoxia-induced class 1 hemoglobin molecule for oxygen is expected to permit the cycle to operate at the low oxygen concentrations in oxygen-deprived tissues. The cycle provides a potential role for nitrate, nitrite, or NO in sustaining anaerobic metabolism, and it has been argued that it would allow redox and energy status to be maintained while reducing the production of ethanol and lactate (Igamberdiev and Hill, 2004). The net effect of the cycle is to reduce oxygen to water according to the equation:
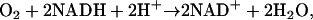 |
and if the reducing power is provided by glycolysis, then the balanced equation for this alternative fermentation pathway is:
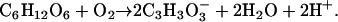 |
This confers no immediate benefit in terms of pH regulation since the process is as acidifying as lactate production, or the conversion of nitrate to nitrite, and like the latter it would also need to operate in parallel with a pathway to remove pyruvate since pyruvate does not usually accumulate in oxygen-deprived tissues. Thus, while the hemoglobin cycle could be driven by inputs of either nitrate or nitrite in the presence of residual oxygen, it does not provide the key to improved pH regulation.
CONCLUSION
In summary, it is nitrite that appears to be responsible for the nitrate-associated amelioration of the acidification of the cytoplasm following the imposition of anoxia on maize root tips. Nitrate reduction did not increase fermentation metabolism, the metabolic contribution of nitrate reduction itself was modest compared to the major fermentation products, and neither nitrate nor nitrate reduction were required for the improvement in the cytoplasmic pH response caused by nitrite. These results are in line with the previous findings on tobacco roots (Stoimenova et al., 2003), and they provide no support for an explanation in terms of a biochemical pH-stat based on nitrate reduction or for the hypothesis that fermentation is limited by the ability to recycle NADH.
The explanation for the nitrite-induced amelioration of the cytoplasmic acidification during anoxia remains to be established, but the possible involvement of NO in anoxic signaling would be an attractive subject for further investigation. Experiments with NO donors and scavengers, as well as more sensitive NO measurements, will be key to distinguishing the potential contribution of NO from the effects of nitrite itself. From a mechanistic point of view, the increased ratio in the release of ethanol to combined organic acids warrants further investigation into the influence that nitrite, or a metabolite downstream of nitrite, might have on the pH sensitivity of the shift from lactate to ethanol production. Finally, from an environmental perspective, future experiments might also address the extent to which nitrite-mediated processes contribute to the acclimation that occurs during the slow onset of oxygen deprivation that is characteristic of natural flooding events.
MATERIALS AND METHODS
Plant Material
Maize seeds (Zea mays L. cv B73; Pioneer) were washed with tap water for 30 min and germinated in the dark at 28°C between sheets of absorbent paper soaked in 0.1 mm CaSO4. After 52 h, approximately 100 seedlings with 1-cm roots were transferred to a hydroponic (5-L) culture containing 2 mm KH2PO4, 0.1 mm CaSO4 (pH adjusted to 6.0 with KOH). The growth medium was replaced on the third, fifth, and seventh days after the transfer, and experiments were carried out on the eighth day. KCl (10 mm) or KNO3 (10 mm) pretreatments were started on the sixth day, and the seedlings were kept in the dark throughout.
Root Preparation for HPLC and GC Measurements
Roots were excised 1 cm below the seed, rinsed with distilled water, and 5-mm segments were cut into fresh aerated growth medium. The segments were washed for 1 min with a 1% SDS solution to remove microbes stuck to the roots, rinsed extensively with growth medium, given a final rinse with distilled water, and blotted. The root segments (1.5 g fresh weight) were transferred to capped bottles and incubated at 25°C in 10 mL of either 2 mm KH2PO4, 0.1 mm CaSO4 (pH 6.0) or 50 mm Suc, 10 mm MES, 0.1 mm CaSO4 (pH 6.0), both supplemented with either 10 mm KCl or 10 mm KNO3. All experimental media contained kanamycin (10 mg/L) and amphotericin B (5.6 mg/L) to minimize microbial contamination.
Capped bottles were coupled to a gas exchange device, through syringe needles, that replaced the gas phase in the bottles with nitrogen by alternately creating under-pressure, thereby removing gas from the bottles (0.2 bar), and producing over-pressure with nitrogen gas (1.7 bar). The bottles were disconnected at a pressure of approximately 1.4 bar to minimize oxygen influx during sampling. The gas exchange procedure, which typically took 5 min, also caused the root segments to be vacuum infiltrated.
In a typical experiment, the incubation medium and gas space were sampled several times over a time course of up to 6 h. After the last sampling event, 3 mL of experimental medium was taken from the bottles containing root samples and transferred to empty control bottles. The control bottles were gas exchanged and incubated for about 3 h. They were sampled for medium and gas content twice, once at the start and once at the end of the control incubation period. If the content of a control bottle changed significantly, then the original sample was considered to be contaminated and the data from that replicate were excluded from the dataset.
HPLC Methods
The inorganic anion content of the medium was measured with an HPLC system (Dionex) connected to an Ionpac AS9-SC column. Samples were eluted using a 1.8 mm bicarbonate/1.7 mm carbonate solution at 1 mL min−1, accelerated to 2 mL min−1 after 10 min, in 18-min runs. Anions were detected using suppressed conductivity with an anion self-regenerating suppressor unit (Dionex). Variations in conductivity, HPLC retention times, and intensities were corrected for using bromide as an internal reference.
A second HPLC system (Thermoquest) was used to detect organic acids, sugars, and ethanol. The HPLC was mounted with a column for organic acid separation (Polyspher OA HY; Merck) eluted with 5 mm H2SO4 at a flow rate of 0.6 mL min−1. The working temperature was 60°C and detection was done by differential refractometry. The assignments of HPLC signals were based on the retention times of calibration standards.
GC and GC-MS Methods
Volatile components were detected using three different GC systems and a GC-MS optimized for the detection of different groups of molecules. CO2, air, N2O, and NO were measured using a Finnigan Model 9001 chromatograph equipped with a capillary plot-fused silica column (Poraplot Q; Chrompack) at 40°C coupled to a thermal conductivity detector using argon as carrier gas. H2, N2, and O2 were measured on a Shimadzu Model 14 chromatograph equipped with a molecular sieve column 5A (Chrompack), at 100°C coupled to a thermal conductivity detector using argon as carrier gas. CH4, C2H4, and C2H2 were measured on a Packard Model 417 chromatograph equipped with flame ionization detector and a molecular sieve 5A column (Chrompack). The column temperature was 70°C and the carrier gas was nitrogen at a flow rate of 20 mL min−1. Mass fragments were analyzed using a GC-MS (Hewlett-Packard Model 5890/5971A) equipped with a mass selective detector (ionization energy, 2,970 eV) with a capillary column (Innowax, 30 m × 0.25 mm; Hewlett-Packard). Samples (2 μL) were introduced via a splitless injection port (300°C) at a column temperature of 160°C.
Root Preparation for 1H NMR Analysis
Root segments, prepared as above but without the SDS washing step, were vacuum infiltrated and transferred to tubes containing the experimental medium. Normoxia or anoxia was imposed by purging with either oxygen or oxygen-free nitrogen gas. Samples (0.5 mL) were taken from the medium at 5, 15, 30, 60, 90, 120, 180, and 360 min after the start of the experiment. Samples were kept on ice until the 1H NMR spectrum was run.
1H NMR Spectroscopy
After adding 0.05 mL of deuterium oxide for the spectrometer lock, spectra were recorded at 6°C on a Varian Unity Inova 500 spectrometer using a presaturation pulse to suppress the water signal.
Root Preparation for in Vivo NMR Spectroscopy
Roots were excised 1 cm below the seed, rinsed with distilled water, and 5-mm segments were cut into a continuously aerated medium containing 50 mm Suc, 10 mm MES, 0.1 mm CaSO4 (pH 6.0) plus either 10 mm KCl or 10 mm KNO3. Root segments were vacuum infiltrated for up to 5 min to eliminate intercellular air spaces that would otherwise have caused a detrimental line-broadening effect on the NMR signals, and then approximately 1 g fresh weight of root tissue was transferred to a 10-mm-diameter NMR tube containing the same medium. A combined air-lift and circulation system was used to circulate the suspending medium through the NMR tube and to supply the tissue with either oxygen or nitrogen (Fox et al., 1989, 1995).
In Vivo NMR Spectroscopy
In vivo NMR spectra were recorded at 25°C using a Bruker CXP 300 spectrometer (Bruker Analytische Messtechnik) with an Oxford Instruments 7.05 T superconducting magnet.
1H-decoupled 31P NMR spectra were acquired in blocks of 1,800 scans at 121.49 MHz using a double-tuned 13C/31P 10-mm-diameter probehead, a 45°-pulse angle, a spectral width of 8,064.52 Hz, and a recycle time of 0.5 s. Chemical shift values were measured relative to the signal at 22.49 ppm from a capillary containing a 2% (v/v) aqueous solution of the tetraethyl ester of methylene diphosphonic acid. In a typical experiment, 31P NMR spectra were recorded for 1 h under normoxia, followed by 2 h under anoxia and a further 1 h under normoxia. Cytoplasmic and vacuolar pH values were determined from the chemical shifts of the cytoplasmic and vacuolar Pi signals using calibration curves similar to those described elsewhere (Spickett et al., 1993). Consecutive 15-min spectra were added to give a reliable estimate of the chemical shift value of the cytoplasmic Pi signal at a particular time point.
1H-decoupled 15N NMR spectra were acquired at 30.42 MHz using a 10-mm broadband probehead, a 60°-pulse angle, a spectral width of 4,500 Hz, a recycle time of 1 s with a low power broadband decoupling during acquisition, and an accumulation time of 1 h (3,600 scans). Chemical shift values were measured relative to the signal at −298.8 ppm from a capillary containing 0.25 mol dm−3 [15N]urea.
Statistical Analysis
Statistical significance was evaluated at the P = 0.05 level using the two-tailed t test in Microsoft Excel assuming equal variances.
Acknowledgments
We thank Dr. M.R. Wormald (University of Oxford) for access to the Varian NMR spectrometer, and the group of Dr. F. Stams (Wageningen University) for facilitating the GC and HPLC experiments. We also acknowledge the preliminary work of C. Drummie.
This work was supported by the United Kingdom Biotechnology and Biological Sciences Research Council (I.G.L.L., R.G.R.), St. Hugh's College, Oxford (I.G.L.L.), the University of Oxford (I.G.L.L.), and Aventis Crop Science UK (M.D.F., I.G.L.L., R.G.R.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: R.G. Ratcliffe (george.ratcliffe@plants.ox.ac.uk).
References
- Allègre A, Silvestre J, Morard P, Kallerhoff J, Pinelli E (2004) Nitrate reductase regulation in tomato roots by exogenous nitrate: a possible role in tolerance to long-term anoxia. J Exp Bot 55: 2625–2634 [DOI] [PubMed] [Google Scholar]
- Botrel A, Kaiser WM (1997) Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta 201: 496–501 [DOI] [PubMed] [Google Scholar]
- Botrel A, Magné C, Kaiser WM (1996) Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol Biochem 34: 645–652 [Google Scholar]
- Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57: 471–478 [DOI] [PubMed] [Google Scholar]
- Fox GG, McCallan NR, Ratcliffe RG (1995) Manipulating cytoplasmic pH under anoxia: a critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta 195: 324–330 [Google Scholar]
- Fox GG, Ratcliffe RG, Southon TE (1989) Airlift systems for in vivo NMR spectroscopy of plant tissues. J Magn Reson 82: 360–366 [Google Scholar]
- Gerendás J, Ratcliffe RG (2002) Root pH regulation. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots: The Hidden Half, Ed 3. Marcel Dekker, New York, pp 553–570
- Gerendás J, Ratcliffe RG, Sattelmacher B (1990) 31P nuclear magnetic resonance evidence for differences in intracellular pH in the roots of maize seedlings grown with nitrate or ammonium. J Plant Physiol 137: 125–128 [Google Scholar]
- Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30: 1–47 [DOI] [PubMed] [Google Scholar]
- Good AG, Muench DG (1993) Long-term anaerobic metabolism in root tissue. Metabolic products of pyruvate metabolism. Plant Physiol 101: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Gibbs J (2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct Plant Biol 30: 999–1036 [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56: 2601–2609 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55: 2473–2482 [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Brendle-Behnisch E (1995) Acid-base modulation of nitrate reductase in leaf tissues. Planta 196: 1–6 [Google Scholar]
- Kaiser WM, Weiner H, Huber SC (1999) Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol Plant 105: 385–390 [Google Scholar]
- Lee RB (1979) Release of nitrite from barley roots in response to metabolic inhibitors, uncoupling agents, and anoxia. J Exp Bot 30: 119–133 [Google Scholar]
-
Li XZ, Oaks A (1993) Induction and turnover of nitrate reductase in Zea mays. Influence of
 . Plant Physiol 102: 1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
. Plant Physiol 102: 1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar] - Libourel IGL (2003) The role of nitrate in the root response to anoxia. PhD thesis. University of Oxford, Oxford
- Morard P, Silvestre J, Lacoste L, Caumes E, Lamaze T (2004) Nitrate uptake and nitrite release by tomato roots in response to anoxia. J Plant Physiol 161: 855–865 [DOI] [PubMed] [Google Scholar]
- Planchet E, Gupta KJ, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41: 732–743 [DOI] [PubMed] [Google Scholar]
- Ratcliffe RG (1994) In vivo NMR studies of higher plants and algae. Adv Bot Res 20: 43–123 [Google Scholar]
- Ratcliffe RG (1997) In vivo NMR studies of the metabolic response of plant tissues to anoxia. Ann Bot (Lond) (Suppl A) 79: 39–48 [Google Scholar]
- Ratcliffe RG (1999) Intracellular pH regulation in plants under anoxia. In S Egginton, EW Taylor, JA Raven, eds, Regulation of Tissue pH in Plants and Animals: A Reappraisal of Current Techniques. Cambridge University Press, Cambridge, UK, pp 193–213
- Roberts JKM, Andrade FH, Anderson IC (1985) Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiol 77: 492–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M (1984. a) Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci USA 81: 6029–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Wemmer D, Walbot V, Jardetzky O (1984. b) Mechanism of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci USA 81: 3379–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Hooks MA, Miaullis AP, Edwards S, Webster C (1992) Contribution of malate and amino acid metabolism to cytoplasmic pH regulation in hypoxic maize root tips studied using nuclear magnetic resonance spectroscopy. Plant Physiol 98: 480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Wade-Jardetzky N, Jardetzky O (1981) Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry 20: 5389–5394 [DOI] [PubMed] [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53: 103–110 [PubMed] [Google Scholar]
- Spickett CM, Smirnoff N, Ratcliffe RG (1993) An in vivo nuclear magnetic resonance investigation of ion transport in maize (Zea mays) and Spartina anglica roots during exposure to high salt concentrations. Plant Physiol 102: 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57: 463–470 [DOI] [PubMed] [Google Scholar]
- Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P (2001) A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta 212: 835–841 [DOI] [PubMed] [Google Scholar]
- Stöhr C, Ullrich WR (1997) A succinate-oxidising nitrate reductase is located at the plasma membrane of plant roots. Planta 203: 129–132 [Google Scholar]
- Stoimenova M, Kaiser WM (2004) The role of nitrate reduction in flooding survival. Prog Bot 65: 357–371 [Google Scholar]
- Stoimenova M, Libourel IGL, Ratcliffe RG, Kaiser WM (2003) The role of nitrate reduction in the anoxic metabolism of roots. II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 253: 155–167 [Google Scholar]