Abstract
In multicellular organisms, organogenesis requires tight control and coordination of cell proliferation, cell expansion, and cell differentiation. We have identified Arabidopsis (Arabidopsis thaliana) nucleosome assembly protein 1 (AtNAP1;1) as a component of a regulatory mechanism that connects cell proliferation to cell growth and expansion during Arabidopsis leaf development. Molecular, biochemical, and kinetic studies of AtNAP1;1 gain- or loss-of-function mutants indicate that AtNAP1;1 promotes cell proliferation or cell expansion in a developmental context and as a function of the farnesylation status of the protein. AtNAP1;1 was farnesylated and localized to the nucleus during the cell proliferation phase of leaf development when it promotes cell division. Later in leaf development, nonfarnesylated AtNAP1;1 accumulates in the cytoplasm when it promotes cell expansion. Ectopic expression of nonfarnesylated AtNAP1;1, which localized to the cytoplasm, disrupts this developmental program by promoting unscheduled cell expansion during the proliferation phase.
Genetic analysis of eukaryotic protein prenyl transferases showed that modification of target proteins by farnesyl or geranylgeranyl is critical for control of development, growth, and signaling. Prenyl transferases covalently attach a 15-carbon farnesyl diphosphate (FPP) or the 20-carbon geranylgeranyl diphosphate (GGPP) isoprenoids to a Cys acceptor, which is part of a C-terminal CaaX box motif in the substrate proteins. Protein farnesyl transferase (PFT) and geranylgeranyl transferase (PGGT-I) share a common α-subunit but have distinct β-subunits. Specificity of PFT and PGGT-I is determined by the β-subunits of each enzyme through sequence-specific recognition of the C-terminal CaaX box motif in substrate proteins (Yalovsky et al., 1999; Sinensky, 2000). Specific inhibitors of PFT or PGGT-I inhibit progression of the cell cycle in plant and animal cells, indicating that prenylation is required for the function of proteins involved in regulating cell division (Morehead et al., 1995; Qian et al., 1996; Tamanoi et al., 2001). For example, Ras, LKB1, CENP, or TC10, which are involved in cell proliferation and differentiation or cytoskeletal functions, are prenylated proteins. Prenylation modulates their function by facilitating membrane association as well as protein-protein interaction (Tamanoi et al., 2001; Hussein and Taylor, 2002; Martin and St Johnston, 2003; Elam et al., 2005). Protein prenylation is conserved in animals and plants (Yalovsky et al., 1999), but, unlike mice in which PFT is essential for early embryonic proliferation (Mijimolle et al., 2005), loss of Arabidopsis (Arabidopsis thaliana) PFTβ (era1) or PFT/PGGT-Iα (plp) gene functions is not lethal, although the mutants are affected in their development (Yalovsky et al., 2000a; Running et al., 2004; Mijimolle et al., 2005). This could be due to the specific types of proteins that are prenylated in plants, because most plant candidate protein prenyl transferase protein substrates differ from those identified in yeast (Saccharomyces cerevisiae) and animals (Galichet and Gruissem, 2003).
Among plant candidate PFT protein substrates, nucleosome assembly protein 1 (NAP1) is conserved among eukaryotes and has been identified in soybean (Glycine max), pea (Pisum sativum), rice (Oryza sativa), and tobacco (Nicotiana tabacum) BY2 cells, human, yeast, murine, and Drosophila melanogaster cells (Ishimi et al., 1984; Ishimi and Kikuchi, 1991; Simon et al., 1994; Yoon et al., 1995; Hu et al., 1996; Ito et al., 1996; Rougeulle and Avner, 1996; Rodriguez et al., 1997; Shen et al., 2001; Dong et al., 2003). Most NAP1 proteins contain a typical CaaX box recognition motif for PFT, and the human NAP1-like1 was shown to be in vivo farnesylated in COS-1 cells (Kho et al., 2004). NAP1 was first identified from HeLa cells by its ability to facilitate in vitro assembly of nucleosomes under physiological conditions. The current model suggests that NAP1 is part of a multifactorial chromatin assembly machinery, which mediates the ATP-facilitated assembly of regularly spaced nucleosomes (Ishimi et al., 1984, 1987; Ishimi and Kikuchi, 1991; Walter et al., 1995; Yoon et al., 1995; Hu et al., 1996; Ito et al., 1996; McQuibban et al., 1998; Nakagawa et al., 2001). In addition to their proposed histone chaperone activity, NAP1 proteins may be involved in transcriptional regulation through chromatin remodeling (Kawase et al., 1996; Ito et al., 2000; Shikama et al., 2000; Asahara et al., 2002; Levchenko and Jackson, 2004; Rehtanz et al., 2004). Recent genetic studies also revealed functions of NAP1 in the control of mitosis and during development. Xenopus and yeast NAP1 interact specifically with B-type cyclin (Clb). Deletion of the NAP1 gene in a yeast strain that is dependent upon Clb2 function results in a prolonged delay of mitosis with normal levels of Clb2/p34CDC28-associated kinase activity, and yeast cells are unable to induce events required for assembly or proper function of the mitotic spindle. Yeast NAP1 was also shown to regulate the cell cycle in combination with the Gin4 kinase, NBP1, and SDA1 (Kellogg et al., 1995; Kellogg and Murray, 1995; Altman and Kellogg, 1997; Shimizu et al., 2000; Zimmerman and Kellogg, 2001). In addition, several mammalian NAP1 homologs appear to regulate cell proliferation and differentiation (von Lindern et al., 1992; Simon et al., 1994; Abu-Daya et al., 2005). A role of NAP1 during development was revealed through the construction of knockout mutants in mice and Drosophila. The inactivation of Drosophila NAP1 is embryo lethal, and a null mutation of the brain-specific mouse NAP1l2 gene results in embryonic lethality beginning at midgestation. Surviving mice mutant embryos showed extensive surface ectoderm defects, open neural tubes, and exposed brain, suggesting a tissue-specific role for NAP1L2 in the regulation of neuron proliferation (Rogner et al., 2000; Lankenau et al., 2003). But despite this extensive information, the biochemical and molecular functions of NAP1 proteins and the role of the prenyl modification is still poorly understood.
Arabidopsis has four NAP1-related genes whose function is currently not known. We investigated Arabidopsis nucleosome assembly protein 1 (AtNAP1;1), which has a predicted CaaX motif, to understand the function of the protein and the potential role of its farnesylation during Arabidopsis development. Characterization of gain- and loss-of-function mutants demonstrated that AtNAP1;1 contributes to the regulation of cell proliferation and cell expansion. The developmental function of AtNAP1;1 appears to be regulated by the differential farnesylation of the protein during specific stages of leaf development.
RESULTS
AtNAP1;1 Is a Farnesylated Protein
The presence of the CKQQ motif at the C-terminal end of AtNAP1;1 suggested that the protein is a substrate of PFT. To test if AtNAP1;1 can be prenylated by PFT, we incubated the purified protein with recombinant Arabidopsis PFT and [3H]FPP. AtNAP1;1 was labeled strongly in the presence of both PFT and [3H]FPP but not with PGGT-I using either [3H]FPP or [3H]GGPP (Fig. 1A). AtNAP1;1 was also labeled weakly by PFT using [3H]GGPP, because the enzyme is somewhat promiscuous for GGPP (Trueblood et al., 1993; Lane and Beese, 2006). Mutation of the conserved Cys farnesyl acceptor in the CKQQ motif to Ser (AtNAP1;1C369S) confirmed that farnesylation required a functional farnesylation motif. Among the three other Arabidopsis NAP1 proteins, AtNAP1;2 and AtNAP1;3 have a similar CKQQ motif and are likely prenylated as well. Similarly, NAP1 is encoded by small gene families in tobacco and rice (Dong et al., 2003). The two OsNAP1 and three of the NtNAP1 proteins have CaaX boxes that can be prenylated in vitro by Arabidopsis PFT (A. Galichet and W. Gruissem, unpublished data).
Figure 1.
AtNAP1 is prenylated in vitro and in vivo. A, Wild type (containing an intact CKQQ CaaX box) and C369S (CKQQ CaaX box mutated to SKQQ) versions of AtNAP1;1 are used as substrates for plant protein prenyltransferases. Symbols + and − indicate the presence or absence of purified AtNAP1;1, purified prenyltransferase, FPP, or GGPP. After electrophoresis and fluorography, exposure was carried out for 5 d. B, Wild-type and era1-1 Arabidopsis flower soluble extracts were used as prenyltransferase sources. Prenylation reactions were carried out with 50 μg of crude extract, and + and − symbols indicate the presence or absence of AtNAP1;1, AtNAP1;1C369S, and FPP. Exposure was carried out for 15 d. C, In vivo prenylation assay. Fluorography and immunoblots of protein extracts from tobacco BY-2 cells expressing GFP-AtNAP1;1 or GFP-AtNAP1;1C369S fusion proteins and labeled with 3H-mevelonic acid. Protein extracts were separated by SDS-PAGE in 10% gel, followed by western blotting. Fluorography was carried out for 3 weeks.
To substantiate that AtNAP1;1 is a substrate for PFT, protein extracts from wild-type or era1-1 flowers were tested as a source for the enzyme (Fig. 1B). AtNAP1;1 but not AtNAP1;1C369S was labeled in the presence of protein extracts from wild-type flowers, confirming that the protein extract had PFT activity and that AtNAP1;1 could be correctly farnesylated at the Cys acceptor in the CKQQ prenylation motif. The protein extract from era1-1 flowers was unable to farnesylate AtNAP1;1, confirming the lack of PFT activity in the mutant and establishing that AtNAP1;1 is a substrate of PFT.
To confirm that AtNAP1;1 is also farnesylated in vivo, we expressed green fluorescent protein (GFP)-AtNAP1;1 and GFP-AtNAP1;1C369S in tobacco BY-2 cells under control of the cauliflower mosaic virus (CaMV) 35S promoter. Soluble proteins from cells labeled with [3H]mevalonic acid were extracted and separated on SDS-polyacrylamide gels, which were then used either for immunoblot analysis with a polyclonal NAP1 antibody or for fluorography to detect labeled GFP-AtNAP1;1 (Fig. 1C). GFP-AtNAP1;1 and GFP-AtNAP1;1C369S were expressed to a similar level in BY-2 cells. A labeled protein corresponding to the size of GFP-AtNAP1;1 was detected only in extracts from cells expressing GFP-AtNAP1;1 but not in cells expressing GFP-AtNAP1;1C369S or in control BY-2 cells. Together, these results establish that AtNAP1;1 is efficiently farnesylated in vivo as well.
AtNAP1;1 Subcellular Localization during the Cell Cycle Is Regulated But Does Not Depend on Farnesylation in Heterologous Cell Systems
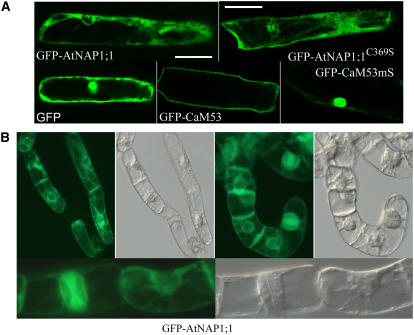
Most farnesylated proteins in yeast and animal cells are targeted to the plasma membrane (Sinensky, 2000), although in plants, farnesylated APETALA1 has also been found in the nucleus (Yalovsky et al., 2000b). To investigate the subcellular localization of AtNAP1;1, GFP-AtNAP1;1 and GFP-AtNAP1;1C369S were transiently expressed in onion (Allium cepa) epidermal cells. GFP alone was distributed uniformly in the nucleus and the cytoplasm, whereas GFP-AtNAP1;1 and GFP-AtNAP1;1C369S were restricted to the cytoplasm (Fig. 2A). As controls, we also expressed GFP-CaM53 and GFP-CaM53mS. Their previously reported respective localization to the membrane and the nucleus (Rodriguez-Concepcion et al., 1999) was also observed in onion epidermal cells, suggesting that expression of PFT substrate proteins using the 35S promoter did not exceed the prenylation capacity of the cell that could result in a nonspecific localization of the protein.
Figure 2.
Farnesylation does not affect subcellular localization of AtNAP1;1. A, GFP-AtNAP1;1, GFP-AtNAP1;1C369S, GFP-CaM53, GFP-CaM53mS, and GFP proteins were detected by green fluorescence using confocal laser scanning microscopy in epidermal onion cells 38 h after bombardment. Bars correspond to 50 μm. B, Localization of GFP-AtNAP1;1 and GFP- AtNAP1;1C369S fusion proteins in tobacco BY-2 tobacco cells. Transgenic suspension of BY-2 tobacco cells were established and used for localization.
To obtain further insights into the potential role of farnesylation for AtNAP1;1 subcellular localization, GFP-AtNAP1;1 and GFP-AtNAP1;1C369S were stably expressed in transgenic tobacco BY-2 cells (Fig. 2B). GFP-AtNAP1;1 was localized in both the cytoplasm and the nucleoplasm of interphase cells. During mitosis, GFP-AtNAP1;1 was colocalized with the phragmoplast in telophase. Localization of GFP-AtNAP1;1C369S was similar (data not shown). Together, we conclude that the farnesylation status of AtNAP1;1 has no immediate role in the subcellular localization of the protein during the cell cycle.
Farnesylation Is Necessary for AtNAP1;1 Function in Yeast
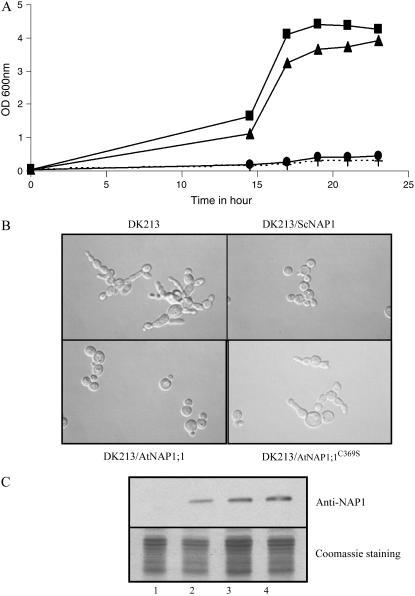
We examined the physiological role of AtNAP1;1 farnesylation by functional complementation assays using the yeast nap1 mutant strain DK213. This mutant has a reduced growth rate at 37°C and a delay in mitosis, resulting in cells that are elongated and form clumps (Kellogg et al., 1995). Transformants of DK213 expressing AtNAP1;1, AtNAP1;1C369S, or ScNAP1 were analyzed for their growth and their cell phenotypes at 37°C (Fig. 3, A and B). ScNAP1 and AtNAP1;1, but not AtNAP1;1C369S, rescued the elongated bud phenotype and the temperature sensitivity, demonstrating that AtNAP1;1 could complement the yeast nap1 mutation. Western-blot analysis of yeast protein lysates with an anti-NAP1 antibody revealed that the three NAP1 proteins were expressed at similar levels (Fig. 3C). Therefore, the farnesyl-Cys acceptor of AtNAP1;1 is required for its function during cell cycle progression in yeast.
Figure 3.
AtNAP1;1 complements the yeast nap1 mutant. DK213 yeast nap1 cells were transformed with pJR1133 vector alone (+), ScNAP1 (▴), AtNAP1-1 (▪), or AtNAP1;1C369S (•), and cells were arrested in G2/M and then released in fresh media at the permissive temperature (30°C) or at the restrictive temperature (37°C). A, Cell proliferation was monitored at 37°C by measuring the OD600 nm of the culture over a period of 24 h. B, Nomarski images after 16 h at 30°C. C, Immunoblot analysis of wild-type DK213 yeast cells (1) or DK213 cells expressing ScNAP1 (2), AtNAP1;1 (3), or AtNAP1;1C369S proteins. Protein extracts were separated by SDS-PAGE in 10% gel followed by western blotting.
Cell Proliferation Is Reduced in Atnap1;1 Leaves
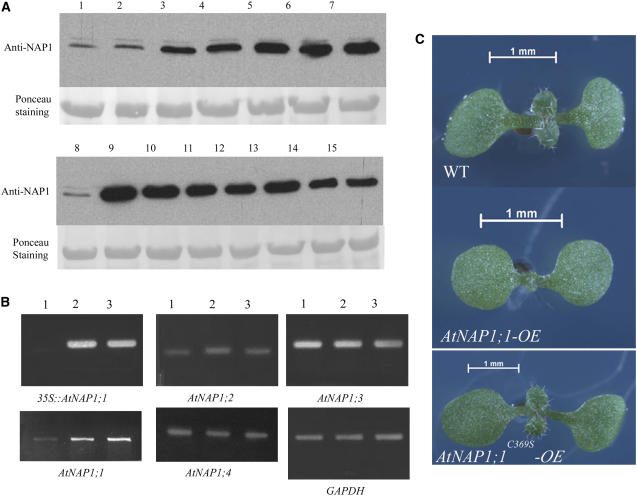
To gain further insight into AtNAP1;1 function, we identified two independent Atnap1;1 mutant alleles in the SALK T-DNA insertion collection (SALK_013610 and SALK_095311; Alonso et al., 2003). These alleles have a T-DNA insertion in the seventh or the 10th exon of AtNAP1;1 that we named Atnap1;1-1 and Atnap1;1-2 (Fig. 4A). Western-blot analysis of extracts from the two Atnap1;1 mutant alleles and control plants together with extracts from Atnap1;2 and Atnap1;3 mutants (T-DNA insertion lines SALK_002892 [T-DNA insertion in the second intron] and SAIL_373_H11 [T-DNA insertion in the 10th exon], respectively; Sessions et al., 2002; Alonso et al., 2003) established the lack of AtNAP1;1 in Atnap1;1-1 and Atnap1;1-2 (Fig. 4B). This was consistent with quantitative reverse transcription (qRT)-PCR data that showed no accumulation of the different NAP1 mRNAs in the respective mutants (data not shown). The results also confirmed that the major protein detected by the antibody corresponds to AtNAP1;1, but that the antibody cross reacted with AtNAP1;2 and AtNAP1;3 as well. The observed differences in band intensity could result from a difference in expression of the AtNAP1 proteins or from the higher specificity of the antibody for AtNAP1;1.
Figure 4.
Arabidopsis Atnap1;1 mutant alleles are affected in cell proliferation during leaf development. A, Atnap1;1 gene structure and location of T-DNA insertions in the Atnap1;1-1 and Atnap1;1-2 mutant alleles. B, Western-blot analysis of AtNAP1 proteins in wild type (lane 1), Atnap1;1-1 (lane 2), Atnap1;1-2 (lane 3), Atnap1;2 (lane 4), and Atnap1;3 (lane 5) mutant alleles. Samples were collected from 9-d-old plants, 25 μg of total protein was loaded per well, and membrane was probed with polyclonal AtNAP1 antibody. C, Nine-day-old sterile-grown plants showing the differences in leaf and cotyledon size. D, Atnap1;1-1 and Atnap1;1-2 average first leaf blade area and average number of cells (E). Twenty individual leaves and 40 palisade mesophyll cells were analyzed in duplicate.
Homozygous Atnap1;1-1 and Atnap1;1-2 plants were enlarged compared to wild type early in development but then became reduced in size (Fig. 4, C and D). In contrast, Atnap1;2 and Atnap1;3 plants had no obvious phenotype (data not shown). Interestingly, by day 7, Atnap1;1-1 and Atnap1;1-2 leaves had more than twice as many cells compared to wild type (Fig. 4E). Similarly, 9-d-old Atnap1;1-1 and Atnap1;1-2 cotyledons were enlarged with more cells (data not shown). Between day 7 and 15, cell proliferation slowed in mutant leaves, while cell number continued to increase in wild-type leaves, therefore resulting in approximately 17% fewer cells in mature mutant leaves (Fig. 4E). In contrast, cell size was not significantly different in the two mutant lines compared to wild type during leaf development (data not shown). Together, these results suggest that loss of AtNAP1;1 function alters the normal cell proliferation during leaf development.
Gain of Function of AtNAP1;1 Alters Leaf Growth and Size
To examine the effects of AtNAP1;1 gain of function and the potential role of AtNAP1;1 farnesylation in vivo, we generated Arabidopsis transgenic plants in which the AtNAP1;1 or AtNAP1;1C369S cDNA were expressed under the control of the constitutive CaMV 35S promoter. Nine AtNAP1;1 and 11 AtNAP1;1C369S T1 hygromycin-resistant plants with single T-DNA insertions were selected for further analysis. Six independent T3 homozygous AtNAP1;1 and seven AtNAP1;1C369S lines were analyzed by western blot using the anti-NAP1 antibody. Except one AtNAP1;1 line, all transgenic lines highly expressed the transgene (Fig. 5A) without any detectable change in the RNA levels expressed from the endogenous members of the AtNAP1 gene family (Fig. 5B). Lines AtNAP1;1 3.5.3 (AtNAP1;1-OE) and AtNAP1;1C369S 2.2.5 (AtNAP1;1C369S-OE) had similar levels of transgene expression and were therefore selected for a full analysis (Fig. 5A, lanes 7 and 10). There was no difference in germination and in the timing of leaf primordia initiation between AtNAP1;1-OE, AtNAP1;1C369S-OE, and wild-type lines. Plants expressing AtNAP1;1 developed smaller leaves and cotyledons, whereas these organs were enlarged in plants expressing AtNAP1;1C369S (Fig. 5C). In contrast, there was no difference in petal size, indicating that AtNAP1;1 overexpression preferentially affected the development of vegetative organs. Because the difference in leaf size was the most striking phenotype, we analyzed leaf development in more detail in the two gain-of-function mutants.
Figure 5.
Phenotypic analysis of AtNAP1;1-OE and AtNAP1;1C369S-OE plants. A, Western-blot analysis of 20-d-old wild-type (lanes 1 and 8), AtNAP1;1-OE (lanes 2–7), and AtNAP1;1C369S-OE (lanes 9–15) T3 homozygous plant crude extracts. Twenty-five micrograms of total protein was loaded per well and membrane was probed with polyclonal AtNAP1 antibody. B, Expression analysis of AtNAP1;1 genes was monitored in wild-type (lane 1), AtNAP1;1-OE (lane 2), and AtNAP1;1C369S-OE (lane 3) 20-d-old plants using semiquantitative RT-mediated PCR. C, Nine-day-old sterile-grown AtNAP1;1-OE and AtNAP1;1C369S-OE plants show altered plant organ size.
AtNAP1;1 Gain of Function Modulates Cell Number and Size during Leaf Development
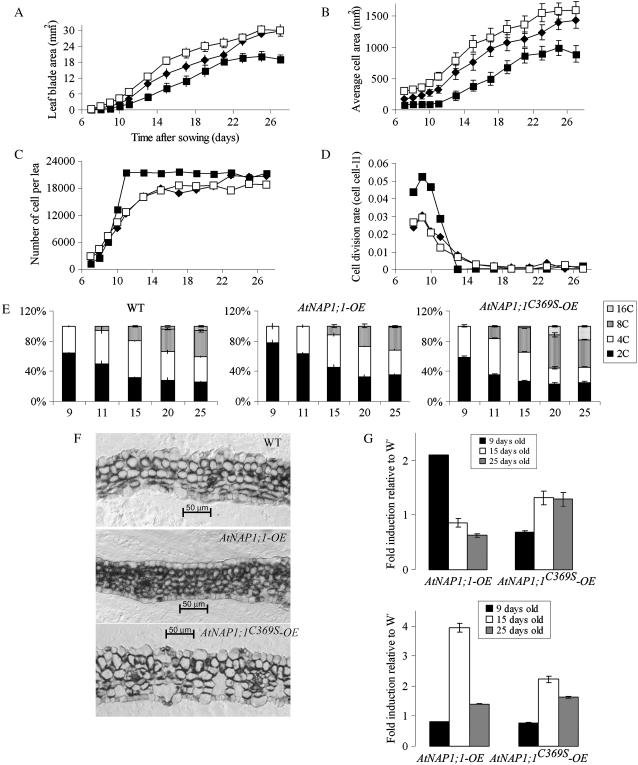
A change in organ size can reflect an alteration in cell size or number or both. To address these possibilities, we performed a kinematic analysis of the first leaf using the method reported by De Veylder and colleagues (De Veylder et al., 2001). Between inception and maturity, Arabidopsis leaf development can be categorized into three stages: proliferation (until day 12 after sowing), expansion (days 12–19), and maturity (after day 19; Beemster et al., 2005). The first leaf of AtNAP1;1-OE, AtNAP1;1C369S-OE, and control lines was harvested between 7 and 27 d after sowing and average leaf area and adaxial palisade cell size were determined (Fig. 6, A and B). From these data, the average palisade cell number was calculated (Fig. 6C). We found that 9 d after sowing the AtNAP1;1-OE plants, first leaf size was reduced 65%, which was mainly the result of decreased cell size (63%). In 15-d-old AtNAP1;1-OE plants, the first leaf had approximately 20% more cells than the wild-type leaf. These cells were 50% reduced in size, which reduced the leaf blade area by approximately 40%. This trend continued in AtNAP1;1-OE mature first leaf, resulting in a 35% decrease of leaf size after 26 d. In contrast, the 9-d-old AtNAP1;1C369S-OE first leaf had an increased cell size and number (55% and 16%, respectively), which resulted in an enlarged leaf area of approximately 78% relative to wild-type control plants. After 15 d, the AtNAP1;1C369S-OE first leaf blade size was still 36% larger than control leaves, but at 26 d the AtNAP1;1C369S-OE first leaf was comparable in size to wild-type first leaves. We then calculated the relative cell division rate, and the results in Figure 6D show that the cell division rate in AtNAP1;1C369S-OE and wild-type plants increased until day 9 and then rapidly declined during the following 5 d. In contrast, the rate of cell division was increased in AtNAP1;1-OE. Based on these results, we calculated the average cell cycle duration as the inverse of the relative cell division rate (Beemster et al., 2005). On day 9, the average cell cycle duration in the first leaf was 32.7 h in wild type, 33.8 h in AtNAP1;1C369S-OE, and 19.2 h in AtNAP1;1-OE plants.
Figure 6.
Increased AtNAP1;1 level in Arabidopsis leaves influences cell proliferation, cell size, and differentiation. A to D, Kinematic analysis of AtNAP1;1-OE and AtNAP1;1C369S-OE leaf development. Kinematic analysis was performed on the first leaf pair of wild-type (♦), AtNAP1;1-OE (▪), and AtNAP1;1C369S-OE (□) plants. Twenty individual leaves and 40 palisade mesophyll cells were analyzed in duplicate. A, Average leaf blade area; B, average cell area; C, average number of cells; and D, division rate. E, Quantification of ploidy distribution as mean ± sd of two independent measurements involving different sets of plants. F, Transverse sections through the central part of 9-d-old plastic-embedded first leaves stained with toluidine blue. Scale bars are 50 μm. G, Expression analyses of CYCB1 (top) and EXP5 (bottom) genes by qRT-PCR in AtNAP1;1-OE and AtNAP1;1C369S-OE 9-, 15-, and 25-d-old first leaves. Results of quantification using GAPDH as a reference are relative to wild-type expression level.
The kinematic analysis of leaf development revealed that ectopic expression of wild-type and mutant NAP1 resulted in alterations of cell size and number in the first leaf. Interestingly, ectopic expression of AtNAP1;1C369S primarily increased cell size, but the comparable size of mature AtNAP1;1C369S-OE and wild-type first leaves suggested that a compensatory mechanism corrects for the increased cell size in AtNAP1;1C369S-OE. Such compensation was not apparent in AtNAP1;1-OE plants, suggesting that the farnesylation of AtNAP1;1 has a profound effect on the function of the protein in this mechanism.
Timing of Cell Differentiation Is Altered by Ectopic AtNAP1;1C369S Expression
Cell proliferation, expansion, and differentiation are closely linked during leaf development (Beemster et al., 2005). Since the ectopic expression of AtNAP1;1 or AtNAP1;1C369S affected cell division rate and cell expansion, we investigated the effect of the wild-type and mutant proteins on cell differentiation. We first analyzed DNA endoreplication as an early marker of leaf cell differentiation (Melaragno et al., 1993) by measuring DNA content of nuclei from primary leaves of wild-type, AtNAP1;1-OE, and AtNAP1;1C369S-OE plants beginning at day 9 after sowing until day 25. In wild-type leaves, all leaf nuclei had a DNA content of 2C and 4C at day 9 (Fig. 6E, left), but beginning at day 11, the population of cells with 8C nuclei increased, reaching a maximum of 34% of the cells on day 25. Cells with 16C nuclei were first detected at day 20, and this population of cells increased to 6% of total leaf cells after 25 d. Together, the results confirm a correlation between DNA endoreplication and cell expansion (Beemster et al., 2005). Leaves of plants expressing AtNAP1;1 had an increased population of cells with 2C nuclei after 9 d (78% compared to 64% in wild type), which suggests that the G2 phase in AtNAP1;1-OE plants may be shortened relative to G1 during the proliferation phase (Fig. 6E, middle). In addition, cells with 8C and 16C nuclei appeared later in AtNAP1;1-OE plants, suggesting that DNA endoreplication was delayed. The ploidy of nuclei in AtNAP1;1C369S-OE leaves at day 9 was similar to wild-type leaves (Fig. 6E, right), indicating that the mutant protein had little effect during the proliferation phase. In contrast, after the proliferation phase, cells with 8C and 16 C nuclei were detectable earlier in AtNAP1;1C369S-OE plants, and their population was significantly increased at day 25, suggesting that the increased size of AtNAP1;1C369S-OE leaf cells (Fig. 6B) was correlated with earlier and increased DNA endoreplication.
We next examined the effects of ectopic AtNAP1;1 and AtNAP1;1C369S expression on leaf structure and cell differentiation. Dicotyledonous leaves have a distinct tissue organization with specific cell types between the adaxial and abaxial leaf surfaces. The palisade layer below the adaxial epidermis consists of tightly packed elongated cells arranged with their long axes perpendicular to the leaf surface. The spongy mesophyll cells between the palisade cell layer and the abaxial epidermis are smaller and more rounded (Donnelly et al., 1999). Primary 9-d-old AtNAP1;1-OE leaves did not show the typical dorsoventral organization of the leaf parenchyma into palisade and spongy mesophyll observed in wild-type leaves (Fig. 6F, top) but instead had a larger number of smaller spherical cells (Fig. 6F, center). Palisade and spongy mesophyll layers became more distinct in older AtNAP1;1-OE first leaves, but they remained smaller than cells in wild-type leaves (data not shown). Microscopic analysis of the AtNAP1;1C369S-OE first leaf at day 9 revealed that all tissue layers had significantly larger and morphologically more differentiated cells than the wild-type first leaf (Fig. 6F, bottom). Together, the results indicate that ectopic expression of AtNAP1;1 or AtNAP1;1C369S had opposing effects on cell differentiation and elongation, which correlated with the changes in nuclear ploidy.
Ectopic Expression of AtNAP1;1 Affects the Expression of Cell Division and Cell Expansion-Related Genes
To begin to understand how ectopic AtNAP1;1 expression may modulate cell proliferation, expansion, and differentiation at the molecular level, we analyzed the accumulation of mRNAs for cell cycle and cell expansion-related genes during leaf development. qRT-PCR revealed that the CycB1;1 mRNA level was increased in AtNAP1;1-OE leaves at day 9 but was not significantly changed in AtNAP1;1C369S-OE leaves compared to wild type (Fig. 6G, top). No difference was found in the expression of CycD1;1 and CycD1;3 genes (data not shown). EXP5 represents a member of the gene family for expansin proteins that induce extension of the plant cell wall. The mRNA of this gene was strongly increased in leaves expressing AtNAP1;1C369S, consistent with the increased cell size (Fig. 6G, bottom). Interestingly, EXP5 mRNA also accumulated to higher levels in leaves expressing AtNAP1;1 despite their smaller cell size, suggesting that EXP5 function is not entirely restricted to cell elongation.
Farnesylation of AtNAP1;1 Is Specific to the Cell Proliferation Phase during Leaf Development
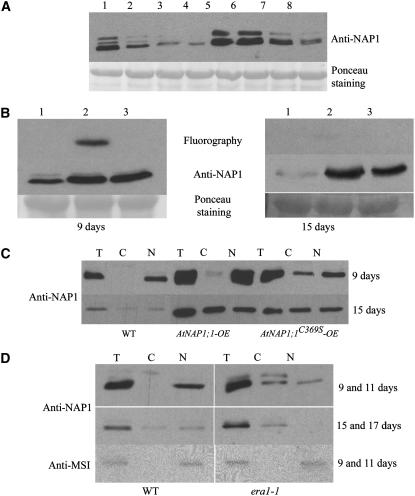
Given the effects of ectopic AtNAP1;1 or AtNAP1;1C369S expression at the cellular and molecular levels, we next asked whether posttranslational farnesylation of AtNAP1;1 was altered during leaf development, which in turn could modulate the function of AtNAP1;1 to affect cell proliferation, expansion, and differentiation. For this work, we also took advantage of the era1-1 mutant, which lacks PFT activity (Yalovsky et al., 2000a). Immunoblot analysis of protein extracts isolated from wild-type and era1-1 Arabidopsis primary leaves during development using the anti-NAP1;1 antibody revealed three bands, with AtNAP1;1 being the major band (Figs. 7A and 4B). In the wild-type first leaf, NAP1 proteins were highly expressed during the cell proliferation phase but declined later in development (Fig. 7A, lanes 1–4). Attachment of farnesyl increases protein mobility in SDS-polyacrylamide gels (Zhu et al., 1993). Interestingly, AtNAP1;1 migrated faster at day 9 as compared to days 15 and 21 (Fig. 7A, lanes 2 and 3) or to AtNAP1;1 protein in era1-1 protein extract (Fig. 7A, lanes 5–8). The shift in mobility was reproducible and could indicate that AtNAP1;1 expressed later in development does not become farnesylated or that farnesyl is removed from the existing protein. To investigate this possibility, we incubated primary leaves isolated from 9- and 15-d-old AtNAP1;1-OE and AtNAP1;1C369S-OE plants with [3H]mevalonic acid and analyzed the labeled proteins. Figure 7B shows that AtNAP1;1, but not AtNAP1;1C369S, was actively farnesylated in 9-d-old primary leaves. AtNAP1;1 farnesylation was not detectable in 15-d-old leaves, although the protein accumulated to similar levels at both developmental stages. No labeled protein was detected in wild-type leaves at either developmental stage. This was not unexpected considering the high levels of AtNAP1;1 expression in the transgenic line that was needed to detect labeled protein and the low efficiency of farnesylation during the short labeling time.
Figure 7.
Stage-specific farnesylation and subcellular localization of AtNAP1;1 directs its functions. A, Western-blot analysis of AtNAP1 proteins during wild-type and era1-1 primary leaf development. Samples were collected after 9, 14, 19, and 25 d for wild type (lanes 1–4) and 11, 16, 21, and 27 d for era1-1 (lanes 5–8). The difference of harvesting time originates from a 2-d delay in era1-1 germination. Twenty-five micrograms of total protein was loaded per well and separated by SDS-PAGE in 7.5% gel followed by western blotting. Membrane was probed with polyclonal AtNAP1 antibody. B, In vivo prenylation assay of AtNAP1;1 during leaf development. The 9- and 15-d-old wild-type (lane 1), AtNAP1;1-OE (lane 2), and AtNAP1;1C369S-OE (lane 3) first leaves were labeled with [3H]mevalonic acid, and protein extracts were subjected to immunoblot analysis and fluorography. Fluorography was carried out for 3 weeks. C, Subcellular fractionation of 9- and 15-d-old wild-type, AtNAP1;1-OE, and AtNAP1;1C369S-OE first leaf protein extracts. Twenty micrograms of total protein (T) and equivalent volumes of cytoplasmic (C) and nuclear (N) fractions were loaded. D, Subcellular fractionation of 9- and 15-d-old wild-type and 11- and 17-d-old era1-1 primary leaf protein extracts. The experiment was carried out as described for C. As control, 50 μg of total protein and equivalent volumes of the two other fractions were used for detection using the antibody raised against the MSI nuclear protein.
Because era1-1 lacks PFT activity, NAP1;1 is not farnesylated in this mutant (Fig. 1, A and B) or modified by PGGT-I, which is somewhat promiscuous for the CaaX motif (Trueblood et al., 1993; Lane and Beese, 2006). Interestingly, AtNAP1 proteins accumulated to significantly higher levels in the era1-1 first leaf early in development (Fig. 7A, lanes 5 and 6) and remained at higher levels in older leaves (Fig. 7A, lanes 7 and 8) as wild type at comparable stages. This result suggests that the lack of AtNAP1 farnesylation in era1-1 may affect the regulation of NAP1 gene expression. Alternatively, nonfarnesylated NAP1;1 may be more stable in era1-1.
Different Subcellular Localization of AtNAP1;1 Is Correlated with the Different Phases of Leaf Development
Subcellular analysis of AtNAP1;1 expressed in BY-2 tobacco cells had revealed that the protein was located in the cytoplasm or the nucleus depending on stage of the cell cycle, but this was not depending on farnesylation (Fig. 2). Considering the opposing effects of ectopically expressed AtNAP1;1 and AtNAP1;1C369S on cell proliferation and expansion (Fig. 6), it was still possible that the farnesylation status of NAP1;1 during leaf development (Fig. 7B) was important to direct the subcellular localization of the protein. We therefore analyzed the subcellular localization of AtNAP1 proteins during development of the first leaf. In leaves from control and AtNAP1;1-OE lines, the AtNAP1 proteins detected by the antibody were found exclusively in the nuclear fraction on day 9, whereas they were equally distributed between the nuclear and cytoplasmic fractions on day 15 (Fig. 7C). In contrast, AtNAP1 proteins were equally distributed between the nucleus and the cytoplasm in leaves of AtNAP1;1C369S expressing lines at both 9 and 15 d. A parallel analysis of the first leaf of era1-1 showed that AtNAP1 proteins were found both in the nucleus and cytoplasm at day 11, whereas they were exclusively present in the cytoplasm after 17 d (Fig. 7D). Together, we conclude that farnesylation of AtNAP1;1 directs the protein to the nucleus early in leaf development, perhaps in concert with other localization mechanisms. The results also suggest that farnesylation and nuclear localization of AtNAP1;1 facilitate cell proliferation during early leaf development.
DISCUSSION
The analysis of mutants has provided significant new insights into leaf development during the last several years. Typically, leaf growth is tightly regulated by the control of cell number, cell size, and differentiation. The genetic and biochemical networks that integrate these cellular processes, however, are still largely unknown. Our analysis established that AtNAP1;1 function is required for correct cell proliferation control during Arabidopsis leaf development and that this function is dependent on the temporal farnesylation of the protein.
Organ development is tightly coordinated at the cellular level by cell division, expansion, and differentiation such that each organ reaches its appropriate size relative to the size of the organism. In determinate plant organs, particularly leaves, final organ cell number is regulated by the initial number of cells recruited into the primodium, the timing of cell division, and the rate of proliferation. After mitotic activity ceases, cell differentiation and expansion establish the regular pattern of tissue layers in the leaf blade (Donnelly et al., 1999). In Arabidopsis, loss of AINTEGUMENTA function or ectopic expression of KRP2, an inhibitor of cell division, reduces leaf cell number, which is largely compensated by increase in cell size (Mizukami and Fischer, 2000; De Veylder et al., 2001). These observations suggest that, at the organ level, both cell division and expansion are coordinated. They follow patterns dependent on the anatomical and developmental context to generate leaves of normal size and shape. The balanced regulation of cell division and expansion may also ensure the completion of a basic morphogenesis program in case either one of the processes is impaired. Several mutations have been reported, however, that affect the balance between cell number and size, resulting in modifications of leaf growth and shape. The Arabidopsis angustifolia and rotundifolia mutants have reduced polar cell expansion but no alteration in cell number, resulting in the production of long, narrow leaves and shorter, wider leaves, respectively (Kim et al., 1998, 2002). In contrast, the Arabidopsis struwwelpeter mutant has smaller leaves with fewer cells but maintains cell size. Overexpression of both E2Fa and DPa, two transcription factors that are required for cell cycle regulation, strongly induces cell proliferation in Arabidopsis but also severely reduces cell expansion and plant growth (Autran et al., 2002; De Veylder et al., 2002). Together, cell division or cell expansion can independently influence leaf morphogenesis by affecting leaves to maintain compensatory mechanisms during growth.
AtNAP1;1 Is a Possible Regulatory Link That Controls Cell Proliferation during Leaf Development
The reduced leaf growth in Atnap1;1 is the consequence of a decreased rate of cell division. In contrast, ectopic AtNAP1;1 expression in Arabidopsis increased cell proliferation but did not affect the time window of cell cycle activity during leaf development. Because the effect of either loss or gain of AtNAP1;1 function appears to be restricted primarily to the phase of cell proliferation, we suggest that AtNAP1;1 functions as a stage-specific positive regulator of cell proliferation. The high AtNAP1 protein level that we detected in leaves during the cell proliferation phase is also consistent with a role of AtNAP1 in the control of cell division. In humans, HsNAP1L1 gene is expressed in all tissues but its expression is increased in proliferating cells. Moreover, the HsNAP1L1 protein level increases in cultured T-lymphocytes induced to proliferate (Simon et al., 1994). Similarly, mouse NAP1L2 is mainly expressed in the nervous system and is necessary for normal proliferation of neuronal precursor cells (Rogner et al., 2000). Interestingly, ectopic AtNAP1;1C369S expression did not have a significant affect on cell proliferation, suggesting that prenylation of NAP1;1 is required for its function during the cell proliferation phase of leaf development.
How does AtNAP1;1 influence cell proliferation rate? Our analysis has revealed that AtNAP1;1 overexpression increases the expression of CYCB1;1, which functions in late G2 and M phases (Donnelly et al., 1999). Most of the targets regulated by CYCB1;1-CDK complexes are currently unknown, but unscheduled activation of CYCB1;1 expression by AtNAP1;1 may result in a shortened G2 phase or interfere with developmentally regulated exit from the cell cycle.
AtNAP1;1 Promotes Cell Proliferation and Connects It to Cell Expansion
Following the cell division phase, cell enlargement and cellular differentiation contribute to the final size and shape of leaves. It has been long recognized that a correlation exists between DNA endoreplication and leaf cell expansion, suggesting that DNA ploidy level may determine cell size (Melaragno et al., 1993; Folkers et al., 1997). Plants that overexpress KRP2, which inhibits CDK activity, however, have leaves with significantly enlarged cells but reduced DNA endoreplication, suggesting a more complex link between these two processes (De Veylder et al., 2001). We have found that ectopic expression of AtNAP1;1C369S increased cell size and promoted cellular differentiation already early during the proliferation phase of leaf development. This activity of AtNAP1;1C369S was uncoupled from the promotion of DNA endoreplication, which occurred late during the proliferation phase. The results therefore suggest that increased levels of nonfarnesylated AtNAP1;1 are sufficient to promote unscheduled cell expansion. At present, we can only speculate about the mechanism, but one possibility is that the lack of the farnesyl group alters the subcellular distribution of AtNAP1;1C369S and therefore allows interactions between AtNAP1;1C369S and other proteins that would promote cell expansion only later in leaf development. This view is supported by the observation that the level of nonfarnesylated endogenous AtNAP1;1 is increased later in leaf development.
The possibility that AtNAP1;1 can both negatively or positively influence cell growth and expansion dependent on its farnesylation status is also consistent with the result that ectopic expression of AtNAP1;1 reduced normal cell growth during the cell proliferation phase of leaf development. It must be noted, however, that increased cell division can also inhibit cell growth (Fleming, 2002). The reduced cell size, together with delayed cell differentiation and endoreplication that we observed in AtNAP1;1-OE plants could therefore be a consequence of the initially increased cell proliferation triggered by AtNAP1;1 early during leaf growth, rather than a direct effect of the protein on cell growth. This possibility would also be consistent with the observation that following the cell proliferation phase in AtNAP1;1-OE leaves, cell size remains smaller than in wild-type leaves, but the relative increase in cell size between 11 and 21 d is significantly higher in AtNAP1;1-OE leaves than in wild-type leaves. In this case, the increased levels of AtNAP1;1 later in leaf development could partially compensate the reduced growth during the proliferation phase. This interpretation is reasonable, because loss of AtNAP1;1 function reduces cell proliferation, resulting in smaller leaves with fewer cells, but cell size is not affected. It is unlikely that overexpression of AtNAP1;1 titrates out other prenlylation substrates and PFT. Previous experiments showed that overexpression of CaM53, a prenylated Calmodulin-related protein, does not exceed the prenylation capacity of the cells (Rodriguez-Concepcion et al., 1999). This view is also supported by the localization data shown in Figure 2. Our results therefore suggest that the observed effects in AtNAP1;1-OE plants are only related to the overexpression of the protein. Together, AtNAP1;1 promotes cell proliferation and participates in connecting cell proliferation to cell expansion to achieve the correct balance between cell number and cell size that determines normal leaf blade size.
Farnesylation Regulates AtNAP1;1 Subcellular Localization and Function
In plants, protein farnesylation has been shown to affect protein function and subcellular localization (Zhu et al., 1993; Yalovsky et al., 2000b; Suzuki et al., 2002). Most of AtNAP1;1 is farnesylated during the cell proliferation phase of leaf development, but AtNAP1;1 does not appear to be farnesylated during the subsequent leaf expansion phase. Our complementation studies of the yeast nap1 mutant confirmed that AtNAP1;1 farnesylation is necessary to complement the cell cycle phenotype of the mutant. It was also reported that the carboxy-terminal region of ScNAP1 is important to allow mitotic progression of the nap1 yeast mutant in complementation experiments (Miyaji-Yamaguchi et al., 2003). Together, these results suggest that the farnesylation status of NAP1 is of functional relevance. During the cell proliferation phase of leaf development, farnesylated AtNAP1;1 localizes to the nucleus and promotes cell division. Later in leaf development when cells are expanding, most of the NAP1;1 appears to be nonfarnesylated and accumulates both in the nucleus and the cytoplasm, and absence of AtNAP1;1 farnesylation results in cellular growth. Subcellular localization analysis of the rice and tobacco NAP1 proteins also revealed a similar partitioning of the proteins between the nucleus and the cytoplasm (Dong et al., 2003), although this study did not provide information on the developmental context or farnesylation status of the proteins.
If the farnesylation-dependent localization of AtNAP1;1 is altered during leaf development, as was the case during the cell proliferation phase in AtNAP1;1C369S-OE plants, this results in premature cell expansion. It is possible that unscheduled accumulation of AtNAP1;1 may allow the protein to engage interactions with other proteins that control cell growth and expansion in the context of the cell cycle but independent of AtNAP1;1. These interactions could occur in the nucleus or the cytoplasm, but it is currently unknown if they require NtNAP1;1 to be farnesylated. Also, these interactions do not easily explain how the different subcellular localizations of AtNAP1;1 could exert effects on cell proliferation or cell growth and expansion. Additional experiments will be necessary to identify proteins that specifically interact with NAP1;1 during different leaf development phases.
It is reasonable to expect that the farnesylation status of AtNAP1;1 may allow the protein to engage in different protein complexes, which exert specific functions during leaf development. In yeast, NAP1 was shown to specifically interact with a set of proteins, including histones, Clb2, Gin4, Nbp1, and p300/CREB-binding proteins (Ishimi et al., 1987; Kellogg et al., 1995; Altman and Kellogg, 1997; Shikama et al., 2000; Shimizu et al., 2000). These proteins are involved in different regulatory processes, including cell cycle control, chromatin modifications, or transcription control. There is also considerable evidence in plants for regulatory links between cell proliferation, cell expansion, and environmental and physiological signals (Pyke and Lopez-Juez, 1999; Meijer and Murray, 2001; Beemster et al., 2003). Earlier studies revealed that protein prenylation is required during plant development and identified functional associations between protein prenylation and specific biological processes or signal transduction pathways (Pei et al., 1998; Rodriguez-Concepcion et al., 1999; Yalovsky et al., 2000a; Galichet and Gruissem, 2003; Running et al., 2004). The farnesylation status of NAP1 may therefore provide an interesting but currently little-explored mechanism to link cell cycle and cell growth control functions to the metabolic status of the cell. For example, during the cell proliferation phase early in leaf development, the organ functions as a physiological sink, whereas the cell expansion phase later in leaf development coincides with the autotrophic functions of the organ (Paul and Foyer, 2001; Jeong et al., 2004). An important but still unresolved aspect is the mechanism by which cells regulate the dynamic farnesylation of AtNAP1;1. It has been reported that PFT activity is increased during cell cycle progression in tobacco BY-2 cells (Morehead et al., 1995). It is therefore possible that PFT activity itself is regulated during leaf development, which could result in differential farnesylation of AtNAP1;1. Alternatively, the pool of FPP available for protein prenylation may be tightly regulated during development. Finally, developmentally regulated farnesylation of AtNAP1;1 may require additional cofactors, whose expression is also developmentally controlled. Further experiments will be necessary to distinguish between these scenarios.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants used in our study were all derived from the Columbia (Col-0) accession line. Seeds of Atnap1;1-1 (SALK_013610), Atnap1;1-2 (SALK_095311), and Atnap1;2 (SALK_002892) mutants were obtained from the SALK T-DNA insertion collection (http://signal.salk.edu) and Atnap1;3 (SAIL_373_H11) mutant seeds from the Syngenta Arabidopsis insertion library. Seeds were surface sterilized using 5% bleach and germinated on Murashige and Skoog medium. After 2 weeks, the seedlings were transferred to soil and grown in Conviron chambers with a 16-/8-light/-dark cycle at 23°C in 70% humidity.
Protein Expression and Antibody Production
The Arabidopsis AtNAP1;1 cDNA (At4g26110) was amplified by PCR using the primer NAP1-For (5′-ATGAGCAACGACAAGGATAGCTTC-3′), together with either NAP1-Rev1 (5′-GTCGACTTACTGTTGCTTGCATTCGGG-3′, for the wild-type version of the CaaX box, CKQQ) or NAP1-Rev2 (5′-GTCGACTTACTGTTGCTTGCTTTCGGG-3′, for the C369S version, SKQQ). The wild-type and the mS PCR fragments were cloned in the pCR 2.1-TOPO cloning vector (Stratagene) and subsequently in the pRSETa vector for protein expression in Escherichia coli. Recombinant proteins were purified on nickel-nitrilotriacetic acid agarose talon superflow metal affinity resin (CLONTECH). Polyclonal anti-AtNAP1;1 was produced by injecting the purified AtNAP1;1 in mouse.
Immunoblots
Nitrocellulose membranes were first blocked overnight at 4°C with 5% nonfat milk and subsequently incubated for 2 h at room temperature with the AtNAP1 antibody (diluted 1:5,000), washed with Tris-buffered saline containing Tween 20, and incubated 2 h with 5,000-fold diluted goat anti-mouse secondary antibody conjugated with horseradish peroxidase for detection with an ECL kit (Amersham Pharmacia Biotech). Immunodetection using polyclonal antibodies raised against MSI1 was carried out as described (Köhler et al., 2003).
In Vitro and in Vivo Prenylation Assay
In vitro prenylation assay was performed as previously described (Yalovsky et al., 2000b). In vivo prenylation reactions were carried out as follows. Tobacco (Nicotiana tabacum) BY-2 cells expressing GFP-AtNAP1;1 or AtNAP1;1C369S fusion proteins were treated with 5 μm mevinolin during 12 h and then incubated in the presence of 6 mm [3H]mevalonic acid for 24 h. Cells were homogenized in 100 μL of 1× SDS-loading buffer and boiled for 5 min. Leaves from AtNAP1;1 and AtNAP1;1C369S plants were treated as described (Yalovsky et al., 2000b).
Yeast Complementation Assay
AtNAP1;1, AtNAP1;1C369S, and ScNAP1 genes were directionally cloned in pJR1133 vector, containing the URA3 marker. The resulting plasmids were used to transform the yeast (Saccharomyces cerevisiae) strain DK213 (MATa clb3∷TRP1 clb4∷HIS3 Δclb1 NAP1∷LEU2 leu2-3, 112 ura3-52 can1-100 ade2-1 his3-11 Δbar1). Cell cycle arrest in G2/M was performed with 30 μg/mL benomyl for 3 h at 30°C (Zimmerman and Kellogg, 2001).
GFP Constructs and Confocal and Fluorescence Microscopy
Wild AtNAP1;1 and AtNAP1;1C369S cDNA were cloned in pGFP-MRC (Rodriguez-Concepcion et al., 1999). Onion (Allium cepa) epidermal cells were transformed as described (Scott et al., 1999). Confocal imaging was performed using a Leica confocal laser-scanning microscope. For ectopic expression in BY-2 cells, previous constructs in pGFP-MRC vector were digested with SphI to isolate 35S∷GFP-AtNAP1;1-NOS and 35S∷GFP-AtNAP1;1C369S-NOS fragments. These fragments were cloned in pCAMBIA 1380 vector. BY-2 cells were transformed by particle bombardment. Transgenic cells were cultivated in medium containing hygromycin and examined for GFP fluorescence at 520 nm by using a Zeiss Axioplan2 fluorescence microscope.
Construction of AtNAP1;1 Plants and Kinematic Analysis of Leaf Development
AtNAP1;1 and AtNAP1;1C369S cDNA were cloned in the modified vector pCAMBIA 1380 containing a CaMV 35S promoter (kindly provided by L. Gomez-Gomez). The constructs were introduced into Agrobacterium tumefaciens strain LBA4404. These strains were used to transform Arabidopsis Col-0 plants by floral dip (Clough and Bent, 1998). The kinematic analysis of AtNAP1;1, AtNAP1;1C369S, and Atnap1;1 mutants and wild-type Col-0 leaves growth was performed as described by De Veylder et al. (2001) on the adaxial palisade mesophyll cells.
RNA Isolation and qRT-PCR
RNA was extracted using Qiagen RNeasy columns according to the manufacturer's instructions. For RT-PCR analysis, 5 μg total RNA was treated with DNase I and DNA-free RNA was transcribed using an oligo(dT) primer and Moloney murine leukemia virus reverse transcriptase (CLONTECH). Aliquots of the generated cDNA, which equaled 50 ng total RNA, were used as template for qRT-PCR. Specific primers (temperature, melting, 58°C–63°C) were designed to generate PCR products between 150 and 350 bp. CycB1;1 (At3g11520) forward primer (5′-CCTCAACCAGTTAGAGGTGATCC-3′) and reverse primer (5′-GTTTCCAATGTCGCCAAGAG-3′), AtExp5 (At3g29030) forward primer (5′-GCTCATGCCACTTTTTACGG-3′) and reverse primer (5′-TCTCCAGTCCATAACCTTGG-3′) were used and qRT-PCR of GAPDHA (At3g26650) with forward primer (5′-CTCCCTTGGAAGGAGCTAGG-3′) and reverse primer (5′-TTCTTGGCACCAGCTTCAAT-3′) was performed for standardization. qRT-PCR reactions were monitored using an ABI Prism 7700 Sequence Detection system with the SYBR green PCR Mastermix (Applied Biosystem).
Flow-Cytometry Analysis
After removal of the leaf petioles, leaf blades were chopped with a razor blade and ploidy analysis was carried out as described (Köhler et al., 2003).
Histological Analysis
Histological analyses were performed with samples in Technovit 7100 resin according to the manufacturer's instructions (Kulzer & Co.). For transverse sections, tissue samples were cut at the center of the first leaf. For longitudinal sections, tissue samples were cut halfway between themed rib and leaf margin.
Subcellular Protein Fractioning
Nuclei were isolated from harvested first leaves as described (Köhler et al., 2003).
Acknowledgments
We are grateful to Johannes Fütterer for tobacco BY-2 cell transformation and GFP fluorescence analysis, to Joanna Wyrzykowska, Gerrit T.S. Beemster, and Andrew J. Fleming for helpful discussions and for critical reading of the manuscript. We thank Marzanna Gontarczyk for help with the histological preparations and Chantal Ebel for useful discussions. We thank D.R. Kellog for the DK213 yeast strain, Syngenta for the Atnap1;3 T-DNA line, the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA Atnap1;1-1, Atnap1;1-2, and Atnap1;2 insertion mutants, and Arabidopsis Biological Resource Center for providing us the seeds.
This work was supported by the Swiss Federal Institute of Technology Zurich.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wilhelm Gruissem (wgruissem@ethz.ch).
References
- Abu-Daya A, Steer WM, Trollope AF, Friedeberg CE, Patient RK, Thorne AW, Guille MJ (2005) Zygotic nucleosome assembly protein-like 1 has a specific, non-cell autonomous role in hematopoiesis. Blood 106: 514–520 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Altman R, Kellogg D (1997) Control of mitotic events by Nap1 and the Gin4 kinase. J Cell Biol 138: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara H, Tartare-Deckert S, Nakagawa T, Ikehara T, Hirose F, Hunter T, Ito T, Montminy M (2002) Dual roles of p300 in chromatin assembly and transcriptional activation in cooperation with nucleosome assembly protein 1 in vitro. Mol Cell Biol 22: 2974–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, Inze D, Traas J (2002) Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J 21: 6036–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inze D, Vuylsteke M (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, Fiorani F, Inze D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8: 154–158 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A, Engler G, et al (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J 21: 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inze D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Zhu Y, Yu Y, Cao K, Sun C, Shen WH (2003) Regulation of biosynthesis and intracellular localization of rice and tobacco homologues of nucleosome assembly protein 1. Planta 216: 561–570 [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Elam C, Hesson L, Vos MD, Eckfeld K, Ellis CA, Bell A, Krex D, Birrer MJ, Latif F, Clark GJ (2005) RRP22 is a farnesylated, nucleolar, Ras-related protein with tumor suppressor potential. Cancer Res 65: 3117–3125 [DOI] [PubMed] [Google Scholar]
- Fleming AJ (2002) The mechanism of leaf morphogenesis. Planta 216: 17–22 [DOI] [PubMed] [Google Scholar]
- Folkers U, Berger J, Hulskamp M (1997) Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary branching by branch initiation regulators and cell growth. Development 124: 3779–3786 [DOI] [PubMed] [Google Scholar]
- Galichet A, Gruissem W (2003) Protein farnesylation in plants: conserved mechanisms but different targets. Curr Opin Plant Biol 6: 530–535 [DOI] [PubMed] [Google Scholar]
- Hu RJ, Lee MP, Johnson LA, Feinberg AP (1996) A novel human homologue of yeast nucleosome assembly protein, 65 kb centromeric to the p57KIP2 gene, is biallelically expressed in fetal and adult tissues. Hum Mol Genet 5: 1743–1748 [DOI] [PubMed] [Google Scholar]
- Hussein D, Taylor SS (2002) Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci 115: 3403–3414 [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Hirosumi J, Sato W, Sugasawa K, Yokota S, Hanaoka F, Yamada M (1984) Purification and initial characterization of a protein which facilitates assembly of nucleosome-like structure from mammalian cells. Eur J Biochem 142: 431–439 [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Kikuchi A (1991) Identification and molecular cloning of yeast homolog of nucleosome assembly protein I which facilitates nucleosome assembly in vitro. J Biol Chem 266: 7025–7029 [PubMed] [Google Scholar]
- Ishimi Y, Kojima M, Yamada M, Hanaoka F (1987) Binding mode of nucleosome-assembly protein (AP-I) and histones. Eur J Biochem 162: 19–24 [DOI] [PubMed] [Google Scholar]
- Ito T, Bulger M, Kobayashi R, Kadonaga JT (1996) Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Mol Cell Biol 16: 3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ikehara T, Nakagawa T, Kraus WL, Muramatsu M (2000) p300-mediated acetylation facilitates the transfer of histone H2A-H2B dimers from nucleosomes to a histone chaperone. Genes Dev 14: 1899–1907 [PMC free article] [PubMed] [Google Scholar]
- Jeong ML, Jiang H, Chen HS, Tsai CJ, Harding SA (2004) Metabolic profiling of the sink-to-source transition in developing leaves of quaking aspen. Plant Physiol 136: 3364–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase H, Okuwaki M, Miyaji M, Ohba R, Handa H, Ishimi Y, Fujii-Nakata T, Kikuchi A, Nagata K (1996) NAP-I is a functional homologue of TAF-I that is required for replication and transcription of the adenovirus genome in a chromatin-like structure. Genes Cells 1: 1045–1056 [DOI] [PubMed] [Google Scholar]
- Kellogg DR, Kikuchi A, Fujii-Nakata T, Turck CW, Murray AW (1995) Members of the NAP/SET family of proteins interact specifically with B-type cyclins. J Cell Biol 130: 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Murray AW (1995) NAP1 acts with Clb1 to perform mitotic functions and to suppress polar bud growth in budding yeast. J Cell Biol 130: 675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Cheng J, Jaunbergs J, Weinbaum C, Tamanoi F, Falck J, et al (2004) A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc Natl Acad Sci USA 101: 12479–12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GT, Shoda K, Tsuge T, Cho KH, Uchimiya H, Yokoyama R, Nishitani K, Tsukaya H (2002) The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J 21: 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GT, Tsukaya H, Uchimiya H (1998) The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev 12: 2381–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W (2003) Arabidopsis MSI1 is a component of the MEA/FIE polycomb group complex and required for seed development. EMBO J 22: 4804–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KT, Beese LS (2006) Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res 47: 681–699 [DOI] [PubMed] [Google Scholar]
- Lankenau S, Barnickel T, Marhold J, Lyko F, Mechler BM, Lankenau DH (2003) Knockout targeting of the Drosophila nap1 gene and examination of DNA repair tracts in the recombination products. Genetics 163: 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Jackson V (2004) Histone release during transcription: NAP1 forms a complex with H2A and H2B and facilitates a topologically dependent release of H3 and H4 from the nucleosome. Biochemistry 43: 2359–2372 [DOI] [PubMed] [Google Scholar]
- Martin SG, St Johnston D (2003) A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421: 379–384 [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Commisso-Cappelli CN, Lewis PN (1998) Assembly, remodeling, and histone binding capabilities of yeast nucleosome assembly protein 1. J Biol Chem 273: 6582–6590 [DOI] [PubMed] [Google Scholar]
- Meijer M, Murray JA (2001) Cell cycle controls and the development of plant form. Curr Opin Plant Biol 4: 44–49 [DOI] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW (1993) Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5: 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijimolle N, Velasco J, Dubus P, Guerra C, Weinbaum CA, Casey PJ, Campuzano V, Barbacid M (2005) Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell 7: 313–324 [DOI] [PubMed] [Google Scholar]
- Miyaji-Yamaguchi M, Kato K, Nakano R, Akashi T, Kikuchi A, Nagata K (2003) Involvement of nucleocytoplasmic shuttling of yeast Nap1 in mitotic progression. Mol Cell Biol 23: 6672–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97: 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead TA, Biermann BJ, Crowell DN, Randall SK (1995) Changes in protein isoprenylation during the growth of suspension-cultured tobacco cells. Plant Physiol 109: 277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Bulger M, Muramatsu M, Ito T (2001) Multistep chromatin assembly on supercoiled plasmid DNA by nucleosome assembly protein-1 and ATP-utilizing chromatin assembly and remodeling factor. J Biol Chem 276: 27384–27391 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290 [DOI] [PubMed] [Google Scholar]
- Pyke P, Lopez-Juez E (1999) Cellular differentiation and leaf morphogenesis in Arabidopsis. CRC Crit Rev Plant Sci 18: 527–546 [Google Scholar]
- Qian D, Zhou D, Ju R, Cramer CL, Yang Z (1996) Protein farnesyltransferase in plants: molecular characterization and involvement in cell cycle control. Plant Cell 8: 2381–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehtanz M, Schmidt HM, Warthorst U, Steger G (2004) Direct interaction between nucleosome assembly protein 1 and the papillomavirus E2 proteins involved in activation of transcription. Mol Cell Biol 24: 2153–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Yalovsky S, Zik M, Fromm H, Gruissem W (1999) The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J 18: 1996–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P, Munroe D, Prawitt D, Chu LL, Bric E, Kim J, Reid LH, Davies C, Nakagama H, Loebbert R, et al (1997) Functional characterization of human nucleosome assembly protein-2 (NAP1L4) suggests a role as a histone chaperone. Genomics 44: 253–265 [DOI] [PubMed] [Google Scholar]
- Rogner UC, Spyropoulos DD, Le Novere N, Changeux JP, Avner P (2000) Control of neurulation by the nucleosome assembly protein-1-like 2. Nat Genet 25: 431–435 [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Avner P (1996) Cloning and characterization of a murine brain specific gene Bpx and its human homologue lying within the Xic candidate region. Hum Mol Genet 5: 41–49 [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S (2004) Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA 101: 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Wyatt S, Tsou PL, Robertson D, Allen NS (1999) Model system for plant cell biology: GFP imaging in living onion epidermal cells. Biotechniques 26: 1125, 1128–1132 [DOI] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HH, Huang AM, Hoheisel J, Tsai SF (2001) Identification and characterization of a SET/NAP protein encoded by a brain-specific gene, MB20. Genomics 71: 21–33 [DOI] [PubMed] [Google Scholar]
- Shikama N, Chan HM, Krstic-Demonacos M, Smith L, Lee CW, Cairns W, La Thangue NB (2000) Functional interaction between nucleosome assembly proteins and p300/CREB-binding protein family coactivators. Mol Cell Biol 20: 8933–8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Akashi T, Okuda A, Kikuchi A, Fukui K (2000) NBP1 (Nap1 binding protein 1), an essential gene for G2/M transition of Saccharomyces cerevisiae, encodes a protein of distinct sub-nuclear localization. Gene 246: 395–404 [DOI] [PubMed] [Google Scholar]
- Simon HU, Mills GB, Kozlowski M, Hogg D, Branch D, Ishimi Y, Siminovitch KA (1994) Molecular characterization of hNRP, a cDNA encoding a human nucleosome-assembly-protein-I-related gene product involved in the induction of cell proliferation. Biochem J 297: 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M (2000) Recent advances in the study of prenylated proteins. Biochim Biophys Acta 1484: 93–106 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yamaguchi Y, Koizumi N, Sano H (2002) Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J 32: 165–173 [DOI] [PubMed] [Google Scholar]
- Tamanoi F, Kato-Stankiewicz J, Jiang C, Machado I, Thapar N (2001) Farnesylated proteins and cell cycle progression. J Cell Biochem Suppl (Suppl) 37: 64–70 [DOI] [PubMed] [Google Scholar]
- Trueblood CE, Ohya Y, Rine J (1993) Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol Cell Biol 13: 4260–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lindern M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G (1992) Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol 12: 3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter PP, Owen-Hughes TA, Cote J, Workman JL (1995) Stimulation of transcription factor binding and histone displacement by nucleosome assembly protein 1 and nucleoplasmin requires disruption of the histone octamer. Mol Cell Biol 15: 6178–6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Kulukian A, Rodriguez-Concepcion M, Young CA, Gruissem W (2000. a) Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12: 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Bracha K, Toledo-Ortiz G, Gruissem W (2000. b) Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 12: 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Gruissem W (1999) Lipid modifications of proteins: slipping in and out of membranes. Trends Plant Sci 4: 439–445 [DOI] [PubMed] [Google Scholar]
- Yoon HW, Kim MC, Lee SY, Hwang I, Bahk JD, Hong JC, Ishimi Y, Cho MJ (1995) Molecular cloning and functional characterization of a cDNA encoding nucleosome assembly protein 1 (NAP-1) from soybean. Mol Gen Genet 249: 465–473 [DOI] [PubMed] [Google Scholar]
- Zhu JK, Bressan RA, Hasegawa PM (1993) Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc Natl Acad Sci USA 90: 8557–8561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ZA, Kellogg DR (2001) The Sda1 protein is required for passage through start. Mol Biol Cell 12: 201–219 [DOI] [PMC free article] [PubMed] [Google Scholar]