Abstract
The hepatitis B virus (HBV) envelope proteins have the ability to assemble three types of viral particles, (i) the empty subviral particles (SVPs), (ii) the mature HBV virions, and (iii) the hepatitis delta virus (HDV) particles, in cells that are coinfected with HBV and HDV. To gain insight into the function of the HBV envelope proteins in morphogenesis of HBV or HDV virions, we have investigated subdomains of the envelope proteins that have been shown or predicted to lie at the cytosolic face of the endoplasmic reticulum membrane during synthesis, a position prone to interaction with the inner core structure. These domains, referred to here as cytosolic loops I and II (CYL-I and -II, respectively), were subjected to mutagenesis. The mutations were introduced in the three HBV envelope proteins, designated small, middle, and large (S-HBsAg, M-HBsAg, and L-HBsAg, respectively). The mutants were expressed in HuH-7 cells to evaluate their capacity for self-assembly and formation of HBV or HDV virions when HBV nucleocapsid or HDV ribonucleoprotein, respectively, was provided. We found that SVP-competent CYL-I mutations between positions 23 and 78 of the S domain were permissive to HBV or HDV virion assembly. One mutation (P29A) was permissive for synthesis of the S- and M-HBsAg but adversely affected the synthesis or stability of L-HBsAg, thereby preventing the assembly of HBV virions. Furthermore, using an in vitro infection assay based on the HepaRG cells and the HDV model, we have shown that particles coated with envelope proteins bearing CYL-I mutations were fully infectious, hence indicating the absence of an infectivity determinant in this region. Finally, we demonstrated that the tryptophan residues at positions 196, 199, and 201 in CYL-II, which were shown to exert a matrix function for assembly of HDV particles (I. Komla-Soukha and C. Sureau, J. Virol. 80:4648-4655, 2006), were dispensable for both assembly and infectivity of HBV virions.
The hepatitis B virus (HBV) is characterized by a most peculiar budding mechanism, which is nucleocapsid independent and driven by its viral envelope proteins at a cellular internal membrane (17, 35, 36). The three envelope proteins, encoded by a unique open reading frame on the HBV genome, bear the hepatitis B virus surface antigen (HBsAg) and are referred to as large, middle, and small (L-HBsAg, M-HBsAg, and S-HBsAg, respectively) because they differ in the sizes of their respective amino-terminal ends (16). Interestingly, the driving force of the viral particle budding process is provided by the sole S-HBsAg protein (32), which is produced in abundance in infected cells. All three envelope proteins are synthesized at the endoplasmic reticulum (ER) membrane, where they aggregate through protein-protein interactions leading primarily to the secretion of empty S-HBsAg-coated subviral particles (SVPs) (16). It is only when L-HBsAg is present in the envelope protein aggregates at the ER membrane that the HBV nucleocapsid can be recruited in the budding complex and released as a mature virion (4). Owing to the overwhelming activity of S-HBsAg for self-assembly, in comparison to that of L-HBsAg, HBV virion formation occurs only on rare occasions.
In addition to the formation of SVPs and mature HBV virions, the HBV envelope proteins can also assist in the assembly of hepatitis delta virus (HDV) particles (2, 49). HDV is an occasional satellite of HBV. Its genome consists of a circular single-stranded RNA molecule with only one open reading frame from which a protein is known to be translated (50). The latter is synthesized in two isoforms, the small and large hepatitis delta antigens (S-HDAg and L-HDAg, respectively). HDV RNA replicates without any assistance from the helper HBV, and it assembles with multiple copies of S- and L-HDAg to form a ribonucleoprotein (RNP). However, the RNP can exit the cell only under the condition of HBV envelope proteins being present to assemble the transport vesicles (46, 49).
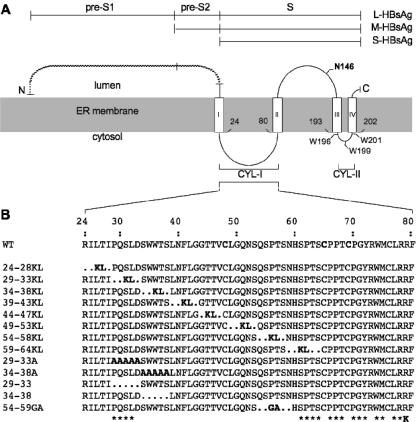
As mentioned above, the dynamics of HBV particle assembly and secretion is provided by S-HBsAg. This protein is 226 amino acid residues in length. It is an integral membrane glycoprotein (42), which is anchored in the ER lipid bilayer through an amino-terminal transmembrane domain (TMD-I) between residues 4 and 24 (11, 12). It comprises a downstream cytosolic loop (CYL-I) between residues 24 and 80, a second transmembrane domain (TMD-II) between residues 80 and 100, and an antigenic loop (AGL) encompassing residues 101 to 164, facing the ER lumen (or the surface of extracellular particles). The carboxyl terminus (residues 165 to 226) is predicted to contain two TMDs (TMD-III and -IV), located at positions 173 to 193 and 202 to 222, respectively (38), separated by a short sequence (residues 194 to 201) referred to here as cytosolic loop II (CYL-II) because it is predicted to reside at the cytosolic side of the ER membrane. The M-HBsAg protein sequence is longer than that of S-HBsAg by 55 residues (the pre-S2 domain) at its amino terminus; it is coassembled with the latter in the viral envelope but is dispensable for both morphogenesis and in vitro infectivity (4, 44). L-HBsAg comprises the entire M polypeptide with an additional amino-terminal extension (pre-S1) of 108 to 119 residues depending on the HBV genotype. It has been described with two topologies, with the amino-terminal pre-S domain (pre-S1 plus pre-S2) being either cytosolic at the ER membrane (internal on secreted virions) or luminal (exposed at the virion surface) (5, 24, 34, 41). The internal conformation is involved in recruiting the nucleocapsid for virion assembly, whereas the external position corresponds to a receptor-binding function at viral entry (6, 25, 26).
SVP assembly was shown to be impaired by large deletions of the S-HBsAg sequence, including deletions of TMD-I or TMD-II or carboxyl-terminal truncations (20, 29, 40). A number of small deletions in the carboxyl part of CYL-I or that of the AGL were also detrimental for SVP production, but surprisingly, deletion of the entire AGL was tolerated for SVP secretion and seemed to affect only the mutant stability or its expression level (33). Mutational analysis of cysteine residues revealed that those located at positions 48, 65, and 69 in CYL-I and 107 in the AGL were essential for SVP secretion (30, 31).
With respect to HBV virion assembly, L-HBsAg, along with S-HBsAg, is clearly indispensable, because it exerts a matrix function mediated by a short linear sequence (residues 92 to 113, positions in the genotype D, ayw3 subtype) in the pre-S domain (3, 27). In a recent study by Löffler-Mary et al. (28), a second HBV assembly domain has been proposed to lie in CYL-I between residues 35 and 59.
S-HBsAg is sufficient for the envelopment and secretion of the HDV RNP, and in this respect, the HDV virion assembly process contrasts with that of HBV, which requires L-HBsAg in addition to S-HBsAg (4, 49). In a recent study, we have shown that CYL-II, including tryptophan residues at positions 196, 199, and 201, constitutes a major determinant of HDV assembly and hence could represent a matrix domain for HDV (22). Additional S-HBsAg determinants, such as the 24-28 region in CYL-I and the glycosylation site (Asn-146) in the AGL, contribute, to a lesser extent, to the assembly or stability of HDV particles (19, 43). Since there are subdomains of S-HBsAg, such as the carboxyl half of CYL-I, which have not been fully explored, additional determinants of the RNP envelopment process may exist.
With regard to the initial phase of viral entry, a unique receptor on the hepatocyte is likely to be utilized by both HBV and HDV particles, based on the reasonable assumption that their outer envelope structures are indistinguishable (1, 2). The prime role of the L-HBsAg pre-S domain is sustained by a series of reports on both the HBV and HDV models. Recent studies have identified the very amino-terminal end of the pre-S1 domain, including the myristate group attached to it, as a putative primary receptor-binding domain (1, 9, 14, 26). More recently, we have identified a new infectivity determinant in the envelope protein AGL, whose essential function may relate to a postbinding event during disassembly or internalization of the viral particle (18).
Here, using a genetic approach and an in vitro model, we have explored the regions of the HBV envelope protein S domain, which are known (CYL-I) or predicted (CYL-II) to lie at the cytosolic side of the ER membrane during assembly, for their function in morphogenesis and infectivity of HBV or HDV virions. Due to their intracellular cytosolic disposition, they have the potential of being instrumental for virion assembly but also of playing a role at viral entry during a postbinding, envelope disassembly process or for internalization of the viral cargo.
MATERIALS AND METHODS
Plasmids.
The plasmid pCIHBenv(−) drives the expression of an HBV pregenomic RNA under the control of a cytomegalovirus (CMV) promoter. The HBV sequence was mutated at the pre-S1, pre-S2, and S start codon (AUG to ACG) to ablate translation of the envelope proteins while preserving that of the viral polymerase. The vector was constructed by cloning a greater-than-unit-length HBV DNA fragment (positions 1820 to 1990 on the genotype D ayw3 viral genome) between positions 701 and 1349 in the pCI vector (Promega) to allow the initiation of pregenomic HBV RNA transcription from a CMV promoter at the authentic site (nucleotide 1820) on the viral DNA. This was achieved using the primer 5′-ggccgagccgagctcgtttagtgaaccgAACTTTTTCACCTCTGCC-3′ and its complement in a PCR fusion reaction (the 5′ half of the primer is specific for the 3′ end of the CMV promoter, and the 3′ half, indicated in capital letters, represents the 5′ end of the HBV pregenome). The HDV recombinant plasmid pSVLD3 (23) drives the replication of HDV RNA and the expression of the HDV RNP. The HBV expression vector pT7HB2.7 is used for production of L-, M-, and S-HBsAg proteins under the control of the endogenous HBV promoters (45). Envelope protein mutants have been constructed as pT7HB2.7 derivatives. Mutants were generated using the PCR technique with two complementary mutagenic oligonucleotides as described previously (20), and all mutant plasmids were sequenced using the Big Dye terminator sequencing protocol (Applied Biosystems). A series of mutants contained a five- or six-residue deletion, combined or not with an insertion of Lys-Leu, Gly-Ala, or five-alanine. The Lys-Leu or Gly-Ala dipeptide corresponded to the Hind-III or Kas-I restriction site used in a previous study to provide easy detection and tracking of the mutant plasmids (19). Mutants were designated by the positions in the S domain of the first and last residues of the deleted sequence and, when applicable, the one-letter code of the substituted residues (Fig. 1). Single amino acid substitutions were also carried out using the PCR overlap extension method (19). The mutations were designated by the one-letter code of the wild-type (wt) amino acid, followed by its position in the S domain of the envelope proteins and the one-letter code of the substituted amino acid.
FIG. 1.
Schematic representations of envelope protein mutants. (A) L-, M-, and S-HBsAg proteins are depicted by horizontal lines. The topology of the envelope protein S domains at the ER membrane is represented. Open boxes represent transmembrane regions in the envelope proteins S domain, and the shaded area corresponds to the ER membrane. Experimentally defined (I and II) or putative (III and IV) transmembrane signals are indicated. (B) CYL-I sequences of the envelope protein mutants are indicated. Mutants are designated by the positions of the first and last deleted amino acids followed by the one-letter codes of the inserted residues. Asterisks indicate the positions of amino acids that were substituted with alanine in mutants presenting single amino acid substitutions. The R79K mutation (K) is indicated.
Virus particle production in HuH-7 cells.
HuH-7 cells were maintained in Williams' medium E supplemented with 10% fetal bovine serum. Production of HBV and HDV particles was achieved by transfection of 106 cells with 1 μg of pCIHBenv(−) or pSVLD3, respectively, and 1 μg of pT7HB2.7 (positive control) or derivatives. Transfections were conducted using the FuGENE 6 reagent (Roche) as described previously (43). For HBV virions analysis, culture medium was harvested at days 3, 6, 9, and 11 posttransfection. Cells were harvested at day 11 in lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 1% NP-40) complemented with Complete reagent (Roche). The lysate was centrifuged at 13,000 rpm for 1 min to separate the cytoplasm from the nuclei, and DNA extraction was conducted on the cytoplasmic fraction. For HDV virion analysis, culture medium was collected at days 5, 7, 9, and 12 posttransfection. Transfected cells were harvested at day 12 posttransfection in RLT buffer (QIAGEN RNeasy minikit) for extraction of viral RNA.
Analysis of HBV envelope proteins.
Proteins from the cytoplasmic fraction prepared as described above were precipitated in the presence of 8 volumes of 100% ethanol and subjected to centrifugation for 1 min at 13,000 rpm. The pellet was resuspended in an appropriate volume of Laemmli buffer complemented with 2% β-mercaptoethanol. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane (Roche). The membrane was blocked in Tris-buffered saline (TBS) (20 mM Tris-HCl [pH 7.4], 0.5 M NaCl)-1% casein for 1 h. Immunodetection was achieved by incubating the membrane for 2 h with rabbit anti-S (R247) and anti-preS2 (R257) antibodies at 1:500 and 1:1,000 dilutions, respectively, in TBS-casein (19). After four washes in TBS-0.3% Tween 20 for 15 min, the membrane was incubated for 1 h with TBS-casein containing mouse antirabbit antibodies coupled to horseradish peroxidase at a 1:5,000 dilution. After five washes in TBS-0.3% Tween 20 and five washes in TBS, the immunoblot was developed using the ECL reagents (Amersham Pharmacia Biotech), followed by exposure to Kodak film for signal detection. For analysis of secreted viral particles, culture medium from transfected cells was clarified by centrifugation at 5,000 × g for 30 min. Clarified medium was then subjected to ultracentrifugation on a 20% sucrose cushion in phosphate-buffered saline for 2 h at 50,000 rpm in an SW50 rotor (Beckman). Sedimented particles were resuspended in an appropriate volume of Laemmli buffer containing 2% β-mercaptoethanol, and samples were assayed by immunodetection for the presence of HBV envelope proteins as described above.
Analysis of HBV virions.
Virions from clarified supernatants were immunoprecipitated with anti-preS1 antibodies by overnight incubation at 4°C, followed by incubation in the presence of protein A-agarose beads (Roche) for 2 h at 4°C. Beads were subjected to sedimentation by centrifugation for 1 min at 13,000 rpm and washed five times in PBS. Bound particles were disrupted with sodium dodecyl sulfate (SDS)-containing buffer (QIAamp DNA minikit; QIAGEN). After centrifugation for 1 min at 13,000 rpm, supernatants were collected and DNA was extracted using the QIAamp DNA minikit (QIAGEN). DNA was precipitated in the presence of 0.3 M sodium acetate, 10 μg of glycogen (Roche), and 2 volumes of 100% ethanol. DNA was subjected to sedimentation by centrifugation at 14,000 rpm for 15 min, washed in 75% ethanol, dried at room temperature, and resuspended in an appropriate volume of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) buffer. Purified DNA samples were subjected to electrophoresis through a 1% agarose gel. After electrophoresis, the agarose gel was washed twice in alkaline buffer (0.4 M NaOH, 1 M NaCl), followed by direct capillary transfer overnight to a positively charged nylon membrane (Zeta-Probe; Bio-Rad). The membrane was then incubated in neutralization buffer (0.5 M Tris-HCl [pH 7.2], 1 M NaCl) for 15 min. Hybridization of the membrane-bound DNA to a 32P-labeled, negative-strand HBV DNA-specific RNA probe was conducted according to the membrane manufacturer's specifications. The membrane was dried before exposure to a Biomax MR Kodak film. Phosphorimager scanning was used for quantitative analysis of the signals. Cells harvested at day 11 posttransfection were analyzed for the presence of HBV DNA as described above. Quantitative analysis of secreted particles was achieved using a commercial anti-HBsAg enzyme-linked immunosorbent assay (ELISA) kit (Diasorin).
Analysis of HDV virions.
Viral particles from clarified supernatants were subjected to sedimentation by centrifugation on 1 ml of a TNE (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA)-20% sucrose cushion for 2 h at 50,000 rpm in an SW50 rotor (Beckman). Sedimented particles were resuspended in TNE. RNA was extracted using the QIAamp Viral RNA minikit (QIAGEN) and precipitated in the presence of 0.3 M sodium acetate, 10 μg of glycogen (Roche), and 50% isopropanol. RNA was subjected to sedimentation by centrifugation at 14,000 rpm for 15 min, and the RNA pellet was washed in 75% ethanol, dried at room temperature, and resuspended in an appropriate volume of RNase-free water. Purified RNA was subjected to electrophoresis through a 2.2 M formaldehyde, 1.2% agarose gel and transferred to a nylon membrane (Roche). Membrane-bound RNA was hybridized to a 32P-labeled genomic HDV RNA-specific probe at 59°C in hybridization buffer (6× SSPE [0.9 M NaCl, 60 mM NaH2PO4 {pH 7.4}, 6 mM EDTA], 10% SDS, 200 μg/ml single-stranded DNA, 50% formamide). The membrane was washed twice in low-stringency wash buffer (1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% SDS) for 30 min at room temperature and twice in high-stringency wash buffer (0.1× SSC, 0.1% SDS) for 30 min at 65°C. The membrane was dried before exposure to Biomax MR Kodak film. Quantitative analysis of HDV RNA was achieved by phosphorimager scanning. Cells harvested in RLT buffer (QIAGEN) at day 12 posttransfection were analyzed for the presence of intracellular HDV RNA using the RNeasy minikit (QIAGEN). RNA was analyzed by Northern blotting as described above. Quantitative analysis of secreted particles was achieved using a commercial anti-HBsAg ELISA kit (Diasorin).
In vitro infection assays.
HepaRG cells were maintained in Williams' medium E supplemented with 10% fetal bovine serum, 5 μg/ml insulin, and 5 × 10−5 M hydrocortisone hemisuccinate (15). Prior to inoculation they were treated with 2% dimethyl sulfoxide for at least 2 weeks as described previously (15). For infection with HDV virions, clarified supernatants containing wt or mutant virions were used as inocula after normalization of their titers based on HDV RNA quantification. HepaRG cells (3.3 × 105 cells/20-mm-diameter well) were exposed to HDV virions for 16 h in the presence of 4% polyethylene glycol (PEG) 8000. Cells were harvested at day 8 postinoculation in RLT buffer (QIAGEN), and total cellular RNA was purified and tested for the presence of genomic or antigenomic HDV RNA (18). For infection with HBV virions, inocula consisted of wt or mutant virions adjusted to approximately 1.5 × 107 genome equivalents (ge)/ml by precipitation of viral particles from culture medium with PEG 8000. HepaRG cells (3.3 × 105 cells/20-mm-diameter well) were exposed to undiluted inocula and to a two-, four- and eightfold dilution for 16 h in the presence of 5% PEG 8000. Cells were harvested at day 12 postinoculation in RLT buffer (QIAGEN). Total cellular RNA was purified using the QIAGEN-RNeasy minikit, and mRNAs were selected using the Oligotex kit (QIAGEN). They were tested for the presence of newly synthesized HBV mRNA (marker of infection) by Northern blot analysis by hybridization to a 32P-labeled HBV mRNA-specific probe under the conditions described above.
RESULTS
Effects of CYL-I mutations on SVL assembly and secretion.
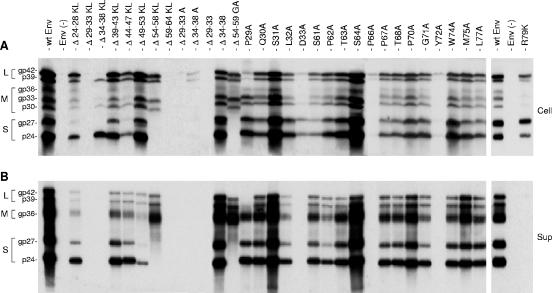
The function of HBV envelope protein CYL-I in the morphogenesis of HBV or HDV particles has been previously investigated (19, 28). However, its activity in HDV morphogenesis was evaluated with the S-HBsAg protein in the absence of M- and L-HBsAg, and the sequence was not fully explored due to the limitations of the mutagenesis approach, which did not allow the production of stable mutants carrying deletions in the carboxyl moiety of CYL-I. Here we have attempted the construction of stable SVP-competent mutants for S-, M- and L-HBsAg (SML mutants) that cover the entire CYL-I domain (Fig. 1), and we have investigated their capacity for HDV or HBV maturation. Combined deletion/insertion mutations (19) and single amino acid substitutions were introduced in a vector (pT7HB2.7) coding for the three envelope proteins (SML plasmid). They were designed such that Cys-48, Cys-65, and Cys-69, previously shown to be essential to SVP secretion, were not affected (30). Mutant plasmids were then introduced by transfection into HuH-7 cells for expression. Following transfection, cell culture supernatants were collected at days 2 and 4, and cells were harvested at day 4. Both cell lysates and culture medium samples were analyzed for the presence of envelope proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with a mixture of anti-S (R247) and anti-preS2 (R257) antibodies. As shown in Fig. 2, the SML Δ29-33KL and Δ59-64KL mutants were not detected in either cells or supernatants (Fig. 2A). This is in agreement with previous results obtained with S-HBsAg mutants carrying the same mutations (19). The Δ34-38KL mutant was detected only in cell lysate but solely in the nonglycosylated form. This was due to an unwanted, PCR-induced mutation of the glycosylation site, Asn-146 to His. In contrast to the Δ34-38KL mutation, the Δ24-28KL, Δ39-43KL, Δ44-47KL, and Δ49-53KL mutations were tolerated for protein synthesis and secretion, as evidenced by the presence of all three envelope proteins in both cell lysates and supernatants (Fig. 2A and B, respectively). Δ54-58KL and Δ54-59GA exhibited a distinctive profile, since only the L-HBsAg and M-HBsAg proteins were detected. This is explained by the fact that anti-S antibodies (R247) recognize a linear epitope encompassing the deleted residues (19). Because immunoblotting also included anti-preS2 antibodies (R257), L-HBsAg and M-HBsAg mutants could be detected. Since L-HBsAg and M-HBsAg could not have been secreted in the absence of S-HBsAg, we concluded that the Δ54-58KL and Δ54-59GA S-HBsAg proteins were correctly synthesized and secreted. It has to be noted that the use of an alternative anti-S antibody (Calbiochem), no longer available in our laboratory, allowed the proper detection of the mutants to levels equivalent to that of the wt (19).
FIG. 2.
Effects of CYL-I mutations on envelope protein synthesis and secretion. (A) Cellular proteins harvested at day 4 posttransfection (Cell) were separated on an acrylamide gel, transferred to a PVDF membrane, and probed with a mixture of anti-S and anti-preS2 antibodies (1:500 and 1:1,000, respectively). Signals are from 105 cells. (B) A pool of culture medium collected at days 2 and 4 posttransfection (Sup) was subjected to ultracentrifugation. Sedimented particles were disrupted in Laemmli buffer and analyzed as described above. Signals are from 1 ml of culture medium. Env(−), envelope protein negative; wt SML, wt S-, M-, and L-HBsAg. Molecular weights of the glycosylated (gp) and unglycosylated (p) forms of the HBV envelope proteins are indicated.
Due to the deleterious effect of the Δ29-33KL, Δ34-38KL, and Δ59-64KL combined deletions/insertions on SVP secretion, we constructed additional mutants to complete CYL-I mapping. A set of mutations consisted in the substitution of five alanine residues for the 29-to-33 or the 34-to-38 sequence. Unfortunately, the resulting Δ29-33A and Δ34-38A mutants were poorly expressed and remained undetectable in culture medium. An additional set of mutants was then prepared, consisting in the simple deletion of residues 29 to 33 or 34 to 38 (Δ29-33 or Δ34-38, respectively). The Δ34-38 mutant was properly synthesized and competent for SVP assembly, but the Δ29-33 mutant remained undetectable. We then constructed single amino acid substitutions with alanine between positions 29 and 33. Among the resulting mutants, the Q30A, S31A, and L32A mutants were expressed and secreted to near-wt levels, whereas the D33A mutant failed to be secreted. Interestingly, the P29A mutation appeared to exert an adverse effect only on the L-HBsAg protein, which was barely detectable in the cell lysate and absent from the supernatant, even though the M-HBsAg and S-HBsAg mutants were present at wt levels.
Finally, to analyze the CYL-I carboxyl moiety that was not amenable to a deletion/insertion approach, single amino acid substitutions with alanine were carried out at the positions indicated in Fig. 1. We observed that every alanine substitution was compatible with synthesis and secretion, with the exception of P66A and Y72A, which significantly affected protein synthesis or stability (Fig. 2). Moreover, the R79K mutation, which was reported as permissive for SVP secretion by Löffler-Mary et al. (28), was found here to block secretion. R79K mutant proteins were detected in the cell lysates to near-wt levels but remained undetectable in the culture supernatant (Fig. 2).
In conclusion, we have identified a series of SVP-competent envelope protein mutations throughout the CYL-I sequence between positions 23 and 78, except for Asp-33, Cys-48, His-60, Cys-65, Pro-66, Cys-69, Tyr-72, Arg-73, and Cys-76. HBV envelope protein mutants (designated SML mutants) carrying the resulting SVP-permissive mutations were thus amenable to further investigation of their activity in assembly and secretion of HBV or HDV virions.
Effects of CYL-I mutations on the maturation of HBV virions.
To measure the effect of CYL-I mutations on HBV virion morphogenesis, HuH-7 cells were cotransfected with a plasmid containing a greater-than-genome-length HBV DNA fragment (pCIHBenv−) in which mutations were introduced to ablate the expression of the envelope proteins and a plasmid coding for either the wt or the above-mentioned SVP-competent SML mutants (pT7HB2.7 or derivatives, respectively). Culture medium was collected between days 3 and 11 posttransfection, and cells were harvested at day 11. The amounts of HBV envelope proteins in the supernatant were measured using an HBsAg-specific ELISA (Fig. 3C). Both cell lysate and culture medium were analyzed for the presence of HBV DNA. Mutations were considered permissive for HBV maturation when viral DNA was detected in the culture medium in proportion to the amounts of envelope proteins. Values for envelopment efficiency were obtained by dividing phosphorimager values for HBV DNA by ELISA values for HBsAg. A mutant was considered deficient for virion formation when HBV DNA was not detectable (<3 × 105 genome equivalents/ml measured by Southern blot analysis and phosphorimager scanning) in culture medium normalized to 1011 SVPs/ml (measured by HBsAg-specific ELISA).
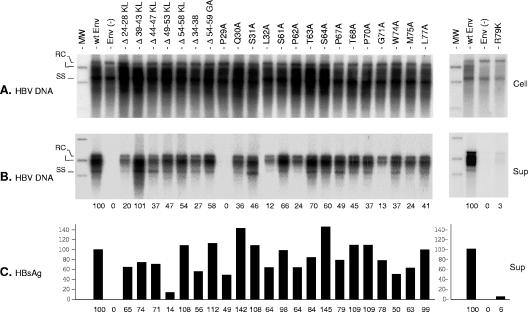
FIG. 3.
Effects of envelope protein CYL-I mutations on secretion of HBV virions. (A) Southern blot analysis of intracellular HBV DNA. Total DNA from HuH-7 cells (Cell) cotransfected with pCIHBenv(−) and pT7HB2.7 or derivatives was extracted at day 11, separated on a 1% agarose gel, transferred to a nylon membrane, and probed with a 32P-RNA probe specific for negative-strand HBV DNA. Signals are from 105 cells. Molecular weight markers (MW) include 10 pg of cloned linear (L) HBV DNA, corresponding to 3 × 106 ge. (B) Culture media collected at days 3, 6, 9, and 11 posttransfection (Sup) were pooled and subjected to immunoprecipitation using an anti-preS1 antibody. Precipitates were disrupted, and DNA was extracted and analyzed by Southern blot hybridization as described above. Signals are from 1 ml of culture medium. For a positive control (wt Env), the 100% values correspond to 200 pg/ml of HBV DNA (left panel) or 300 pg/ml (right panel), approximately 6 × 107 or 9 × 107 ge/ml, respectively. (C) Histogram showing the relative amounts of secreted HBV envelope proteins measured with an anti-HBsAg ELISA (DiaSorin). For a positive control (wt Env), the 100% value corresponds to approximately 400 ng/ml of HBsAg (left panel) or 150 ng/ml (right panel), equivalent to approximately 1011 or 3.7 × 1010 SVPs, respectively. RC, relax circular; L, linear; SS, single stranded; Env(−), envelope protein negative; wt SML, wt S-, M-, and L-HBsAg. Numbers below each panel are percentages of the wt value.
Transfection efficiency was shown to be homogenous, as demonstrated by the detection in cell lysates of comparable levels of the replicative forms of HBV DNA at day 11 posttransfection (Fig. 3A). For an arbitrary value of 100 for intracellular HBV DNA in the positive control, the mean value and standard deviation were 107 ± 9.8. For detection of secreted virions, cell culture supernatants were incubated with an anti-preS1 antibody to selectively immunoprecipitate enveloped HBV virions and exclude nonenveloped capsids that are known to be released in substantial amounts under such experimental conditions (28). Immunoprecipitates were then analyzed for the presence of viral DNA, which served as a marker of virion assembly. As shown in Fig. 3, the immunoprecipitation protocol allowed a selective isolation of mature HBV virions, excluding naked nucleocapsids [Env(−)]. Mutants that were permissive for secretion of SVPs carrying the L-HBsAg protein were competent for assembly of HBV virions, even though to various degrees (Fig. 3B). As expected, the P29A mutation, which was deleterious to the synthesis or stability of L-HBsAg without affecting M- or S-HBsAg, prevented HBV morphogenesis, as demonstrated by the absence of viral DNA in the immunoprecipitate.
In two independent experiments, the Δ24-28KL, Q30A, L32A, and G71A mutants presented with a slight defect for HBV virion assembly. For the most affected mutant (G71A), we estimated the ratio of HBV virions to total particles to be 17% of that of the wt, which corresponds to a concentration of 7.8 × 106 ge/ml and 3.1 × 1010 SVPs/ml, as opposed to 6 × 107 ge/ml and 3.7 × 1010 SVPs/ml for the wt. Although the R79K mutation severely affected SVP secretion, small amounts of HBsAg (9 ng/ml) could be detected in the culture medium by ELISA. It was thus tested for virion assembly, because the mutation was reported by Löffler-Mary et al. (28) to be detrimental to virion formation. The presence of trace amounts of HBV DNA in the particles immunoprecipitated from the culture medium with anti-preS1 antibodies suggested that the conservative R79K mutation was tolerated for nucleocapsid envelopment and virion secretion.
Overall, our results lead us to the conclusion that the CYL-I sequence is not critical to HBV virion assembly, and hence it is considered to be devoid of a matrix domain. However, a number of mutations within this domain (Δ24-28, L32A, P62A, and G71) partially affected the efficiency of the nucleocapsid envelopment (30%, 19%, 37%, and 17% of that of the wt, respectively), indicating that CYL-I may participate in HBV maturation, as previously suggested (39). Some mutants, such as the Δ39-43KL and Δ49-53KL mutants, appear more efficient than the wt for virion formation or stabilization of the particle. Regarding the R79K mutation, our results are in contradiction with those obtained by Löffler-Mary et al. (28), who reported that R79K or deletions between residues 35 and 59 were tolerated for SVP secretion but were detrimental to virion formation.
Effects of CYL-I mutations on the maturation of HDV virions.
To evaluate the effects of CYL-I mutations on HDV assembly and thereby directly compare the HBV and HDV assembly processes, we cotransfected HuH-7 cells with an HDV recombinant plasmid (pSVLD3) for replication of HDV RNA and production of HDV RNP and the plasmids described above, coding for wt or SVP secretion-permissive envelope protein mutants (pT7HB2.7 or derivatives, respectively). Following transfection, culture medium was collected every 2 days, and cells were harvested at day 12. Both cell lysate and culture medium were analyzed for the presence of HDV RNA and envelope proteins. The transfection efficiency was shown to be homogenous by the detection at similar levels of genomic HDV RNA in cell lysates collected at day 12 posttransfection (Fig. 4A). For an arbitrary value of 100 for intracellular HDV RNA in the positive control, the mean value and standard deviation were 105 ± 6.9.
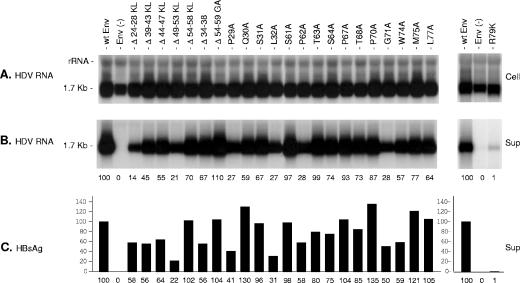
FIG. 4.
Effects of envelope protein CYL-I mutations on secretion of HDV virions. (A) Northern blot analysis of intracellular HDV RNA. Total RNA from HuH-7 cells (Cell) cotransfected with pSVLD3 and pT7HB2.7 or derivatives was extracted at day 12, separated on a 1.2% agarose gel, transferred to a nylon membrane, and probed with a genomic strand-specific, 32P-labeled RNA probe. Signals were obtained from 105 cells. (B) Northern blot analysis of extra cellular HDV RNA. Culture media collected at days 5, 7, 9, and 12 posttransfection (Sup) were pooled and subjected to ultracentrifugation on a sucrose cushion. RNA was extracted and analyzed as described above. Signals are from 0.5 ml of culture medium. For positive control (wt Env), the 100% values correspond to 800 pg/ml of HDV RNA (left panel) or 400 pg/ml (right panel), which approximate 8 × 108 or 4 × 108 ge, respectively. (C) Histogram showing the relative amounts of secreted HBV envelope proteins measured with an anti-HBsAg ELISA (DiaSorin). For a positive control (wt Env), the 100% value corresponds to approximately 700 ng/ml of HBsAg (left panel) or 300 ng/ml (right panel), equivalent to approximately 1.75 1011 or 7.4 × 1010 SVPs, respectively. Env(−), envelope protein negative; wt Env, wt S-, M-, and L-HBsAg. Numbers below each panel are percentages of the wt value.
For detection of HDV virions in the culture medium, supernatants harvested between days 5 and 12 were pooled and subjected to ultracentrifugation on a sucrose cushion as described in Material and Methods. RNA was extracted from sedimented particles and assayed by Northern blot hybridization (Fig. 4B). The amounts of HBV envelope proteins in the supernatant were measured using an HBsAg-specific ELISA (Fig. 4C). Mutations were considered permissive for HDV maturation when viral RNA was detected in the culture medium in proportion to the amounts of envelope proteins. Values for envelopment efficiency were obtained by dividing phosphorimager values for HDV RNA by ELISA values for HBsAg. As shown in Fig. 4B, none of the mutations seemed to drastically affect the assembly of HDV virions, as evidenced by the amounts of HDV RNA, which in most cases were proportional to those of secreted HBsAg. As previously reported (19), the Δ24-28KL mutant was the most impaired for HDV maturation (estimated here at 24% of that of the wt) (Fig. 4). As expected, P29A, which was found to be detrimental to L-HBsAg synthesis but permissive for S-HBsAg secretion, did not affect HDV morphogenesis (Fig. 4). This is in agreement with the absence of an L-HBsAg function in HDV formation. With regard to the R79K mutant, which is severely impaired in its capacity for SVP secretion, the presence of trace amounts of HDV RNA in the culture medium of transfected cells suggested that the mutation was tolerated for HDV RNP envelopment and budding. From these experiments, we concluded that CYL-I does not contain a major HDV envelopment determinant (matrix domain) but, as suggested by the phenotype of some mutants, such as Δ24-28, it may contain elements that facilitate the HDV maturation process or stabilize the virion envelope.
Effects of CYL-I mutations on infectivity.
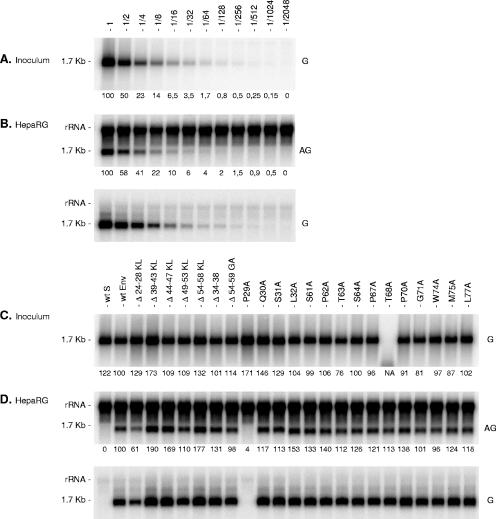
To analyze the effect of CYL-I mutations on infectivity, we chose to use the HDV model combined with susceptible HepaRG cells (15, 18). The in vitro HDV infection system has been shown to constitute a robust surrogate model for analyzing the activity of the HBV envelope proteins at viral entry (18). We first evaluated the sensitivity of the in vitro infection assay by measuring the effect of the inoculum titer on the cell response. HepaRG cells were inoculated with 1 ml of supernatant containing approximately 2 × 108 genome equivalents of wt HDV particles and decreasing twofold dilutions of the inoculum. At day 8 postinoculation, the amounts of intracellular HDV RNA (marker of infection) were measured. Figure 5A shows a strong correlation between inoculum titers, ranging from 2 × 108 to 1.5 × 106 ge per ml, and the amounts of intracellular genomic or antigenomic HDV RNA postinoculation.
FIG. 5.
Effects of envelope protein CYL-I mutations on the infectivity of HDV virions. (A) Supernatant (1 ml) containing approximately 2 × 108 ge of wt HDV virions was serially diluted prior to analysis by Northern blot hybridization with a genomic-strand-specific, 32P-labeled RNA probe (G). One milliliter of undiluted or 2- to 2,048-fold diluted wt HDV-containing medium was added to HepaRG cells in the presence of 4% PEG 8000. (B) Cells were harvested 8 days after inoculation and analyzed for the presence of HDV RNA by Northern blot hybridization with an antigenomic (AG) or a genomic (G) strand-specific, 32P-labeled RNA probe. Dilution factor is indicated. (C) Supernatants (1 ml) from HuH-7 cell cultures were normalized for their genomic HDV RNA concentration. One ml of normalized inoculum was added to HepaRG cells in the presence of 4% PEG 8000. Inocula were recovered after overnight exposure to cells, and their genomic HDV RNA content was controlled by Northern blot hybridization using a genomic strand-specific, 32P-labeled RNA probe. Signals are from 0.5 ml of postexposure inocula. Cells were harvested 8 days after exposure and analyzed for the presence of HDV RNA as described above. rRNA which hybridized nonspecifically to antigenomic-specific HDV RNA probes served as a loading control. The size in kilobases of HDV RNA is indicated. wt S, HDV particles coated with the wt S-HBsAg only. wt Env, HDV particles coated with wt S-, M-, and L-HBsAg. Numbers below each panel are percentages of the wt value. NA, not applicable.
Since all of the CYL-I envelope protein mutants were permissive for HDV virion assembly, though to various degrees, most of the CYL-I region could thus be evaluated for function at viral entry. HDV particles carrying wt or mutant envelope proteins were produced in HuH-7 cells and inoculated into differentiated HepaRG cells after virion titers were normalized on the basis of the HDV RNA concentration. Noninfectious HDV particles coated with the S-HBsAg protein only (wt S) were used as a negative control. Normalization was further controlled by measuring the levels of HDV RNA in the inocula that were recovered after overnight exposure to the target cells (Fig. 5C). At day 8 postinoculation, total cellular RNA was extracted and subjected to Northern blot analysis using probes specific for antigenomic or genomic HDV RNA (Fig. 5D). All mutants were infectious, as demonstrated by the detection of both genomic and antigenomic HDV RNA (HDV particles carrying the P29A mutation were not infectious due to their L-HBsAg-lacking envelope). Interestingly, signal from cells exposed to the Δ24-28KL mutant was slightly lower than that of cells inoculated with wt HDV, an indication that the mutation, previously shown to partially alter HDV assembly, may also adversely affect disassembly at viral entry. Note that the absence of an HDV RNA signal in the T68A inoculum (Fig. 5C) was due to a loss of RNA during the extraction process, since wt levels of both genomic and antigenomic RNA were detected in cells that had been exposed to the mutant (Fig. 5D).
In conclusion, with the exception of residues at positions 33, 48, 60, 65, 66, 69, 72, 73, and 76, whose function could not be tested using a mutagenesis approach, the CYL-I sequence does not contain any discrete domain essential for infectivity.
Effects of CYL-II mutations on HBV assembly.
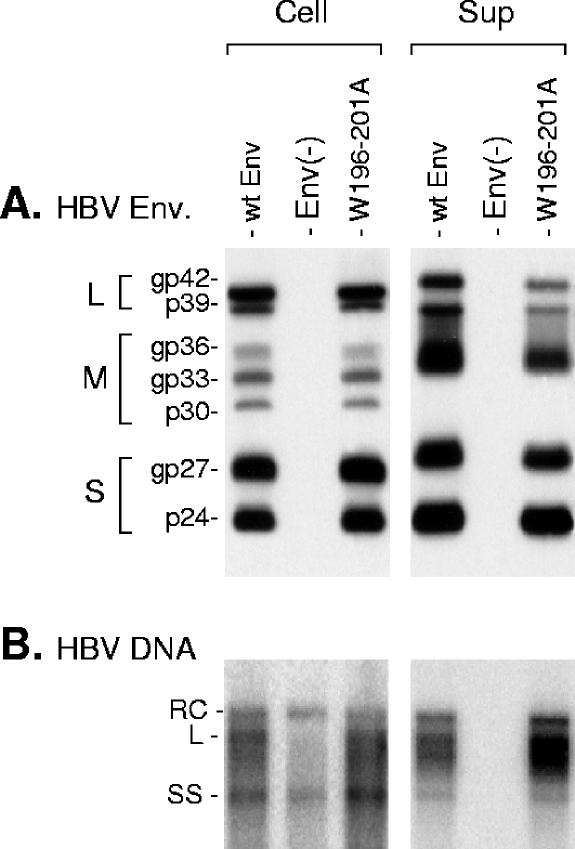
Recently, we demonstrated that CYL-II fulfilled a pivotal role in assembly of HDV particles (22). In fact, this activity was shown to depend on tryptophan residues at positions −196, −199, and −201, with the most conservative substitution of Phe for either Trp being sufficient to ablate HDV virion assembly. Since Trp-196, -199, and -201 are conserved among all orthohepadnaviruses and yet are dispensable for SVP secretion, their contribution, if any, to the HBV life cycle may thus reside in the virion assembly process or at viral entry. To study the effect of CYL-II mutations on HBV virion assembly, HuH-7 cells were cotransfected with pCIHBenv(−) and either pT7HB2.7 or a derivative carrying a triple substitution of Ala for Trp-196, -199, and -201 (W196-201A). Detection of envelope proteins and HBV DNA in the transfected-cell supernatant was conducted as described above (Fig. 6). The W196-201A mutation was permissive for envelope protein secretion and for HBV assembly, as demonstrated by the presence of secreted envelope proteins and that of HBV DNA in particles immunoprecipitated from the culture medium with anti-preS1 antibodies (Fig. 6B).
FIG. 6.
Effects of CYL-II envelope protein mutations on assembly of HBV virions. Production of HBV particles by transfection of 106 cells with 1 μg of pCIHBenv(−) and 1 μg of pT7HB2.7 (wt Env), an empty plasmid, Env(−), or a W196-201A mutant plasmid. (A) Cellular proteins (Cell) harvested at day 4 posttransfection were separated on an acrylamide gel, transferred to a PVDF membrane, and probed with a mixture of anti-S and anti-preS2 antibodies. Signals are from 105 cells. A pool of culture medium collected at days 3, 6, 9, and 12 posttransfection was subjected to ultracentrifugation. Sedimented particles were disrupted in Laemmli buffer and analyzed as described above. Signals (Sup) are from 1 ml of culture medium. (B) Total DNA from transfected cells (Cell) was extracted, separated on a 1% agarose gel, transferred to a nylon membrane, and probed with a 32P-RNA probe specific for negative-strand HBV DNA. Signals are from 105 cells. Culture medium (Sup) was subjected to immunoprecipitation using an anti-preS1 antibody. Precipitates were disrupted, and DNA was extracted and analyzed by Southern blot hybridization as described above. Signals are from 1 ml of culture medium. The L-HBsAg, M-HBsAg, and S-HBsAg proteins are indicated (L, M, and S respectively). Env(−), envelope proteins negative; wt Env, wt S-, M-, and L-HBsAg. Molecular weights of the glycosylated (gp) and unglycosylated (p) forms of the HBV envelope proteins are indicated. RC, relax circular; L, linear; SS, single stranded.
Effects of CYL-II mutations on HBV infectivity.
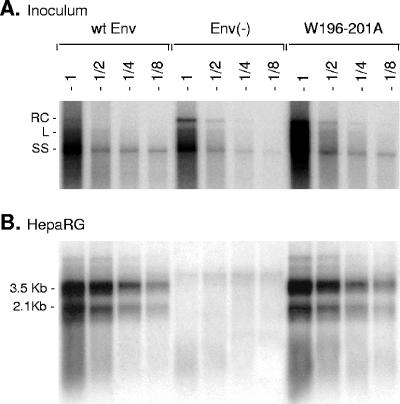
To evaluate the effect of Trp substitution on infectivity, we could not use the HDV infection model, because CYL-II Trp mutations ablate the matrix domain involved in HDV virion formation (22). Hence, differentiated HepaRG cells were inoculated with wt and W196-201A HBV particles, and the evaluation of infection was performed as described by Gripon et al. (15). Supernatant collected from cells transfected with pCIHBenv(−) in the absence of an envelope protein expression vector was used as a negative control. As shown in Fig. 7, the W196-201A HBV mutant was fully infectious, since newly synthesized intracellular HBV mRNAs, which served as a marker of infection, were detected in HepaRG cells at day 12 after inoculation. This result demonstrated that the CYL-II tryptophan-rich motif, which is essential to HDV assembly, has no function in HBV infectivity.
FIG. 7.
Effects of CYL-II envelope protein mutations on infectivity of HBV virions. (A) Culture medium from cells transfected with pCIHBenv(−) and 1 μg of pT7HB2.7 (wt Env), an empty plasmid, Env(−), or W196-201A mutant plasmid, collected at days 3, 6, 9, and 11 posttransfection (Sup), were pooled and subjected to a 20× concentration by precipitation with PEG 8000. One hundred microliters of undiluted (1) or a two- (1/2), four- (1/4), or eightfold (1/8) dilution of each inoculum was examined for the presence of HBV DNA. HBV DNA signals in the inoculum derived from cells transfected in the absence of envelope protein coding plasmid [Env(−)] are from extracellular nonenveloped nucleocapsids, whereas signals from wt Env and W196-201A samples are from both naked nucleocapsids and virions. (B) HepaRG cells (3.3 × 105 cells) were exposed to 2 ml of inoculum for 16 h in the presence of 5% PEG 8000. HepaRG cells were harvested at day 12 postinoculation, and mRNA was purified and tested for the presence of newly synthesized HBV mRNA by Northern blot hybridization using a negative-strand-specific, 32P-labeled RNA probe. RC, relax circular; L, linear; SS, single strand. The sizes in kilobases of HBV mRNAs are indicated.
DISCUSSION
The HBV envelope proteins have the capacity to form spherical or filamentous SVPs and to assemble HBV or HDV virions (2, 16). In all cases the dynamics of the budding process is sustained by the S-HBsAg protein (13). For HBV virion assembly, the recruitment of preformed nucleocapsid in the envelope is driven by the L-HBsAg amino-terminal domain (pre-S) between residues 92 and 113, which is positioned at the cytosolic face of the ER membrane prior to budding (3). CYL-I, also oriented toward the cytosol at the ER membrane, has been proposed to participate in this process (28), but as discussed below, the experiments reported here do not support this conclusion. With regard to HDV assembly, CYL-II, and more precisely the cluster of tryptophan side chains at positions 196, 199, and 201, is central to the envelopment of the HDV RNP (22). This Trp cluster is likely to exert its function by binding to L-HDAg, the major protein component of the HDV RNP.
In the study presented here, we wished to further investigate the respective contributions of CYL-I and CYL-II in virion assembly, because the former had not been fully explored in our previous study of HDV maturation due to the lack of stable mutants bearing lesions in the carboxyl-terminal half of the loop (19). As mentioned above, CYL-II contains a putative matrix domain (Trp-196, -199, and -201) for HDV RNP envelopment (22). Since this motif is conserved among all orthohepadnaviruses, it could play a functional role in the HBV replication cycle, which remains to be determined. Therefore, parallel analyses of the HBV and HDV maturation processes were carried out to sort CYL elements that are specific for the envelopment of HDV RNP from those involved in that of the HBV nucleocapsid. Using a mutagenesis approach, we could not identify, in CYL-I, motifs (matrix domains) that were dispensable for SVP secretion and essential for the assembly of HDV or HBV virions. As indicated above, this observation was in contradiction with the results obtained by Löffler-Mary et al. (28), who reported that HBV virion assembly was sensitive to changes in the 35-to-59 sequence. The explanations for this discrepancy may lie in the experimental approaches, which differ slightly from one study to the other even though the same genotype of HBV DNA (genotype D ayw3) was utilized. In the Löffler-Mary study, the amounts of envelope protein mutants were not measured in the context of HBV virion production but in a prior experiment where S-HBsAg mutants were singly expressed in COS-7 cells under a heterologous promoter. When virion production was attempted, L-HBsAg and S-HBsAg mutants were expressed from distinct vectors, and synthesis of the envelope protein mRNAs was driven by the potent human metallothionein promoter. This might have changed the efficiency of particle secretion, because transcription of L-HBsAg mRNA from a strong promoter may lead to overproduction of L-HBsAg and thereby to an ER retention of the envelope proteins. The adverse effect of L-HBsAg on SVP secretion has been well documented (8, 37). It is quite possible that some of the CYL-I mutations might have been permissive for SVP secretion when tested in the context of singly expressed S-HBsAg but detrimental when present on both S- and L-HBsAg in a coexpression experiment. Therefore, it was not clear whether the apparent lack of HBV maturation was due to a reduced capacity of the envelope protein mutants for secretion or to a specific deficiency in nucleocapsid envelopment.
In the study described here, we measured the amounts of secreted HBV envelope proteins and viral genome in a single experiment in order to directly relate a mutant's ability for SVP secretion to its efficiency for virion assembly. In addition, to ensure wt ratios between the three types of envelope proteins, their mRNAs were transcribed under the control of their respective HBV promoters from a single recombinant vector. It is also worthwhile to note that under our experimental conditions, the R79K mutation, which was claimed by Löffler-Mary et al. (28) to be deleterious to HBV nucleocapsid envelopment while competent for SVP assembly and secretion, displayed the opposite phenotype when present on the three HBV envelope proteins: the R79K substitution severely impaired SVP secretion but did not seem to have a major impact on the envelopment of HBV nucleocapsid or HDV RNP. Therefore, both Arg-78 (28) and Arg-79 (this study), which are positioned at the cytosolic boundary of TMD-II, are strictly required for SVP secretion and cannot be substituted by the positively charged Lys residue. In addition, a precise positioning of the Arg residues at the ER membrane interface seems to be required for secretion, since the naturally occurring L77R mutation is also inhibitory to SVP secretion (10).
As indicated above, most of the mutations within CYL-I partially affected the efficiency of HBV or HDV maturation, suggesting that CYL-I participates in the packaging efficiency or stability of the virions. However, this function does not reach the level of that exerted by the pre-S matrix domain for HBV maturation or by the CYL-II matrix domain for HDV assembly. Hence, our conclusion is that CYL-I does not bear a matrix domain for HBV or HDV assembly.
In a recent study by Kluge et al. (21), large deletions in L-HBsAg encompassing the region between Leu-42 and His-60 (positions are numbered from Met-1 of the S domain) were shown to be permissive for HBV virion assembly and secretion. This is in agreement with our present observations.
With regard to CYL-II, its cytosolic orientation at the ER membrane is predicted by most of the protein analysis algorithms (38). However, it was also described as being accessible at the surface of particles to monoclonal antibodies that had been raised against HBV particles (7). Until it is experimentally examined, the overall topology of the S-HBsAg carboxyl terminus remains unclear. The two topologies (external and internal on viral particles) might coexist, or the external localization might arise as a consequence of a translocation phenomenon. Within CYL-II, Trp-196, -199, and -201 are critical to HDV maturation while dispensable for SVP assembly and secretion (22). Interestingly, the fact that this tryptophan cluster and numerous aromatic residues are conserved in the carboxyl termini of all orthohepadnavirus envelope proteins suggests that they might fulfill an essential function in the HBV life cycle. Yet as demonstrated here and in a previous study (22), Trp-196, -199, and -201 appear dispensable to the HBV life cycle, since their mutation to alanine is permissive to SVP secretion, virion formation, and infectivity. This observation thus raises the possibility that the Trp motif is conserved as a consequence of essential HBV polymerase (Pol) domains being encoded by the same DNA sequence in the minus-one reading frame. As previously reported, this is in fact the case for Trp-196, whose codon is included in the DNA sequence for the YMDD motif of the Pol catalytic domain. The strict requirement for the presence of a YMDD motif in Pol offers no possibility other than Trp at position 196 in S-HBsAg (22, 47, 48). Therefore, it is reasonable to assume that a few S-HBsAg residues, in addition to Trp-196, might be conserved solely because they share a DNA coding sequence with Pol. To test this hypothesis, a precise mapping of the Pol functional domains in the region that shares a coding sequence with S-HBsAg should be carried out.
The studies presented here, taken together with previous observations (3), indicate that assembly of HDV or HBV virions does not depend on specific CYL-I motifs. HBV virion formation relies mostly on the pre-S matrix domain of L-HBsAg (3), and the contribution of S-HBsAg or that of the S domain of M- or L-HBsAg appears limited (10, 39). In contrast, HDV assembly depends on a CYL-II tryptophan cluster in the carboxyl terminus of the envelope proteins (22). Hence, HDV RNP would not compete with the HBV nucleocapsid for a matrix domain on the envelope proteins during morphogenesis. In addition, the fact that mutations in CYL-I or CYL-II did not affect infectivity suggests that these regions do not directly participate in viral entry, in particular in a receptor binding activity, and they are unlikely to modulate the functions of surface-exposed domains associated with viral entry.
Acknowledgments
We are grateful to C. Trépo and O. Hantz for providing the HepaRG cell line. We thank G. Abou Jaoudé for assistance with the HepaRG cell culture and R. Caparros for the HBsAg ELISA.
This work was supported by a grant from the ANRS. C.S. is a CNRS investigator. M.B. was supported by a predoctoral fellowship from ANRS.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Barrera, A., B. Guerra, L. Notvall, and R. E. Lanford. 2005. Mapping of the hepatitis B virus pre-S1 domain involved in receptor recognition. J. Virol. 79:9786-9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonino, F., K. H. Heermann, M. Rizzetto, and W. H. Gerlich. 1986. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J. Virol. 58:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss, V. 1997. A short linear sequence in the pre-S domain of the large hepatitis B virus envelope protein required for virion formation. J. Virol. 71:9350-9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss, V., and D. Ganem. 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88:1059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruss, V., X. Lu, R. Thomssen, and W. H. Gerlich. 1994. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 13:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruss, V., and K. Vieluf. 1995. Functions of the internal pre-S domain of the large surface protein in hepatitis B virus particle morphogenesis. J. Virol. 69:6652-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y. C., K. Delbrook, C. Dealwis, L. Mimms, I. K. Mushahwar, and W. Mandecki. 1996. Discontinuous epitopes of hepatitis B surface antigen derived from a filamentous phage peptide library. Proc. Natl. Acad. Sci. USA 93:1997-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chisari, F. V., P. Filippi, A. McLachlan, D. R. Milich, M. Riggs, S. Lee, R. D. Palmiter, C. A. Pinkert, and R. L. Brinster. 1986. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J. Virol. 60:880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouteau, P., J. Le Seyec, I. Cannie, M. Nassal, C. Guguen-Guillouzo, and P. Gripon. 2001. A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus. J. Virol. 75:11565-11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua, P. K., R. Y. Wang, M. H. Lin, T. Masuda, F. M. Suk, and C. Shih. 2005. Reduced secretion of virions and hepatitis B virus (HBV) surface antigen of a naturally occurring HBV variant correlates with the accumulation of the small S envelope protein in the endoplasmic reticulum and Golgi apparatus. J. Virol. 79:13483-13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eble, B. E., V. R. Lingappa, and D. Ganem. 1986. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol. Cell. Biol. 6:1454-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eble, B. E., V. R. Lingappa, and D. Ganem. 1990. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J. Virol. 64:1414-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganem, D. 1991. Assembly of hepadnaviral virions and subviral particles. Curr. Top. Microbiol. Immunol. 168:61-83. [DOI] [PubMed] [Google Scholar]
- 14.Gripon, P., I. Cannie, and S. Urban. 2005. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 79:1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gripon, P., S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo, and C. Guguen-Guillouzo. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 99:15655-15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heermann, K., and W. Gerlich. 1992. Surface proteins of hepatitis B viruses. In A. Maclachlan (ed.), Molecular biology of HBV. CRC Press, Boca Raton, Fla.
- 17.Huovila, A. P., A. M. Eder, and S. D. Fuller. 1992. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaoude, G. A., and C. Sureau. 2005. Role of the antigenic loop of the hepatitis B virus envelope proteins in infectivity of hepatitis delta virus. J. Virol. 79:10460-10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenna, J., S., and C. Sureau. 1998. Effect of mutations in the small envelope protein of hepatitis B virus on assembly and secretion of hepatitis delta virus. Virology 251:176-186. [DOI] [PubMed] [Google Scholar]
- 20.Jenna, S., and C. Sureau. 1999. Mutations in the carboxyl-terminal domain of the small hepatitis B virus envelope protein impair the assembly of hepatitis delta virus particles. J. Virol. 73:3351-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluge, B., M. Schlager, A. Pairan, and V. Bruss. 2005. Determination of the minimal distance between the matrix and transmembrane domains of the large hepatitis B virus envelope protein. J. Virol. 79:7918-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komla-Soukha, I., and C. Sureau. 2006. A tryptophan-rich motif in the carboxyl terminus of the small envelope protein of hepatitis B virus is central to the assembly of hepatitis delta virus particles. J. Virol. 80:4648-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo, M. Y., M. Chao, and J. Taylor. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J. Virol. 63:1945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert, C., and R. Prange. 2001. Dual topology of the hepatitis B virus large envelope protein: determinants influencing post-translational pre-S translocation. J. Biol. Chem. 276:22265-22272. [DOI] [PubMed] [Google Scholar]
- 25.Le Pogam, S., and C. Shih. 2002. Influence of a putative intermolecular interaction between core and the pre-S1 domain of the large envelope protein on hepatitis B virus secretion. J. Virol. 76:6510-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1999. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 73:2052-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1998. Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity. J. Virol. 72:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loffler-Mary, H., J. Dumortier, C. Klentsch-Zimmer, and R. Prange. 2000. Hepatitis B virus assembly is sensitive to changes in the cytosolic S loop of the envelope proteins. Virology 270:358-367. [DOI] [PubMed] [Google Scholar]
- 29.Machein, U., R. Nagel, R. Prange, A. Clemen, and R. E. Streeck. 1992. Deletion and insertion mutants of HBsAg particles. Arch. Virol. (Suppl.) 4:133-136. [DOI] [PubMed] [Google Scholar]
- 30.Mangold, C. M., and R. E. Streeck. 1993. Mutational analysis of the cysteine residues in the hepatitis B virus small envelope protein. J. Virol. 67:4588-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangold, C. M., F. Unckell, M. Werr, and R. E. Streeck. 1995. Secretion and antigenicity of hepatitis B virus small envelope proteins lacking cysteines in the major antigenic region. Virology 211:535-543. [DOI] [PubMed] [Google Scholar]
- 32.Nassal, M., and H. Schaller. 1996. Hepatitis B virus replication—an update. J. Viral Hepat. 3:217-226. [DOI] [PubMed] [Google Scholar]
- 33.O'Malley, B., and D. Lazinski. 2002. A hepatitis B surface antigen mutant that lacks the antigenic loop region can self-assemble and interact with the large hepatitis delta antigen. J. Virol. 76:10060-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostapchuk, P., P. Hearing, and D. Ganem. 1994. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 13:1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patzer, E. J., G. R. Nakamura, C. C. Simonsen, A. D. Levinson, and R. Brands. 1986. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J. Virol. 58:884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patzer, E. J., G. R. Nakamura, and A. Yaffe. 1984. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J. Virol. 51:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persing, D. H., H. E. Varmus, and D. Ganem. 1986. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science 234:1388-1391. [DOI] [PubMed] [Google Scholar]
- 38.Persson, B., and P. Argos. 1994. Prediction of transmembrane segments in proteins utilising multiple sequence alignments. J. Mol. Biol. 237:182-192. [DOI] [PubMed] [Google Scholar]
- 39.Poisson, F., A. Severac, C. Hourioux, A. Goudeau, and P. Roingeard. 1997. Both pre-S1 and S domains of hepatitis B virus envelope proteins interact with the core particle. Virology 228:115-120. [DOI] [PubMed] [Google Scholar]
- 40.Prange, R., R. Nagel, and R. E. Streeck. 1992. Deletions in the hepatitis B virus small envelope protein: effect on assembly and secretion of surface antigen particles. J. Virol. 66:5832-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prange, R., and R. E. Streeck. 1995. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 14:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon, K., V. R. Lingappa, and D. Ganem. 1988. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J. Cell Biol. 107:2163-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sureau, C., C. Fournier-Wirth, and P. Maurel. 2003. Role of N glycosylation of hepatitis B virus envelope proteins in morphogenesis and infectivity of hepatitis delta virus. J. Virol. 77:5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sureau, C., B. Guerra, and R. E. Lanford. 1993. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J. Virol. 67:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sureau, C., B. Guerra, and H. Lee. 1994. The middle hepatitis B virus envelope protein is not necessary for infectivity of hepatitis delta virus. J. Virol. 68:4063-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sureau, C., A. M. Moriarty, G. B. Thornton, and R. E. Lanford. 1992. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus pre-S antigens. J. Virol. 66:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torresi, J., L. Earnest-Silveira, G. Deliyannis, K. Edgtton, H. Zhuang, S. A. Locarnini, J. Fyfe, T. Sozzi, and D. C. Jackson. 2002. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology 293:305-313. [DOI] [PubMed] [Google Scholar]
- 48.Vietheer, P. T., H. J. Netter, T. Sozzi, and A. Bartholomeusz. 2005. Failure of the lamivudine-resistant rtM204I hepatitis B virus mutants to efficiently support hepatitis delta virus secretion. J. Virol. 79:6570-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, C. J., P. J. Chen, J. C. Wu, D. Patel, and D. S. Chen. 1991. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J. Virol. 65:6630-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, K. S., Q. L. Choo, A. J. Weiner, J. H. Ou, R. C. Najarian, R. M. Thayer, G. T. Mullenbach, K. J. Denniston, J. L. Gerin, and M. Houghton. 1986. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature 323:508-514. (Erratum, 328:456, 1987.) [DOI] [PubMed] [Google Scholar]