Abstract
Gamma-2 herpesviruses encode homologues of mammalian D-type cyclins (v-cyclins), which likely function to manipulate the cell cycle, thereby providing a cellular environment conducive to virus replication and/or reactivation from latency. We have previously shown that the v-cyclin of murine gammaherpesvirus 68 is an oncogene that binds and activates cellular cyclin-dependent kinases (CDKs) and is required for efficient reactivation from latency. To determine the contribution of v-cyclin-mediated cell cycle regulation to the viral life cycle, recombinant viruses in which specific point mutations (E133V or K104E) were introduced into the v-cyclin open reading frame were generated, resulting in the disruption of CDK binding and activation. While in vitro growth of these mutant viruses was unaffected, lytic replication in the lungs following low-dose intranasal inoculation was attenuated for both mutants deficient in CDK binding as well as virus in which the entire v-cyclin open reading frame was disrupted by the insertion of a translation termination codon. This replication defect was not apparent in spleens of mice following intraperitoneal inoculation, suggesting a cell type- and/or route-specific dependence on v-cyclin-CDK interactions during the acute phase of virus infection. Notably, although a v-cyclin-null virus was highly attenuated for reactivation from latency, the E133V v-cyclin CDK-binding mutant exhibited only a modest defect in virus reactivation from splenocytes, and neither the E133V nor K104E v-cyclin mutants were compromised in reactivation from peritoneal exudate cells. Taken together, these data suggest that lytic replication and reactivation in vivo are differentially regulated by CDK-dependent and CDK-independent functions of v-cyclin, respectively.
The eukaryotic cell cycle comprises a series of coordinated events leading to cellular DNA and the segregation of the replicated DNA to daughter cells. The transitions through this cycle are controlled by the temporal and spatial activation of highly conserved holoenzymes consisting of a short-lived regulatory cyclin subunit and a cyclin-dependent kinase (CDK) (5, 54, 55, 71). Quiescent (G0) cells stimulated by mitogens up-regulate the highly redundant D-type cyclins (cyclins D1, D2, and/or D3), which bind and activate CDK4 and/or CDK6 to mediate progression of the cell cycle into and through the G1 phase. Cyclin D/CDK activity allows for the expression of E-type cyclins, which bind and activate CDK2 to drive progression through the restriction point from the G1 phase into the S phase. Cyclin A expression allows for the continued activation of CDK2 and subsequently binds and activates CDK1 as S phase progresses and the G2/M boundary is reached, at which stage cyclin B levels are maximal for the regulation of mitosis in conjunction with CDK1. The activities, localization, and stability of these holoenzymes are further affected by protein-protein interactions and posttranslational modifications that act to positively or negatively modulate cyclin-CDK functions in a sequential manner, highlighting the complex regulatory network that ensures faithful cellular replication.
Manipulation of the host cell cycle is a common strategy employed by many DNA viruses to ensure a cellular environment conducive to viral replication (64). Among the gammaherpesviruses, rhadinoviruses such as Kaposi's sarcoma-associated herpesvirus (KSHV), herpesvirus saimiri, rhesus rhadinovirus, and murine gammaherpesvirus 68 (γHV68) all encode homologues of cellular D-type cyclins (10, 32, 38, 49, 73). While these viral cyclins have been extensively studied in a cellular context, their functions in relation to host-pathogen interactions remain poorly understood. Due to its association with human disease, KSHV v-cyclin has received significant attention. KSHV v-cyclin preferentially binds CDK6 but also binds CDK2 and CDK4 and is able to mediate the phosphorylation of many substrates traditionally associated with CDK2, thereby deregulating normal cell cycle progression in numerous cell culture models (34, 43, 64, 71). Previously, we demonstrated that when expressed as a transgene in mice, γHV68 v-cyclin promotes cell cycle progression in primary lymphocytes and is a potent oncogene (68). γHV68 v-cyclin binds and activates CDK2 both in vitro and in vivo, binds CDK1 in vitro, and mediates the phosphorylation of numerous G1- and S-phase substrates (9, 66), all of which likely contribute to the oncogenic potential of v-cyclin.
As one of the few identified rhadinoviruses naturally infecting murid rodents, γHV68 has become the tool of choice for analyses of gammaherpesvirus pathogenesis (23, 51, 62, 63, 72). Previously, we and others have shown that the v-cyclin of γHV68 is a critical regulator of reactivation, as recombinant viruses lacking v-cyclin do not efficiently reactivate from latency (25, 70). Additionally, v-cyclin is required for the maintenance of latency in the absence of B cells, highlighting both the need for v-cyclin-dependent reactivation and reseeding of the latent non-B-cell compartment and the distinct genetic programs required in different cell types (69). The infrequent detection of v-cyclin transcripts during latency (4, 42, 74) further suggests a specific temporal role of v-cyclin, strengthening the hypothesis that v-cyclin expression is a critical event in γHV68 reactivation from latency.
When the mechanisms of v-cyclin-mediated reactivation are considered, one attractive possibility is that the same functions required for cell cycle manipulation are required to regulate reactivation. However, previous findings demonstrated that providing ex vivo cell cycle stimulation by multiple methods does not fully ameliorate the reactivation defect of B cells latently infected with v-cyclin-deficient virus (25, 47), suggesting that cell cycle manipulation alone is insufficient to explain the role of v-cyclin in regulating reactivation. To conclusively determine the CDK-dependent functions of v-cyclin during viral infection, we have generated two independent single-amino-acid-substitution viral mutants, v-cyc[K104E] and v-cyc[E133V], in which the v-cyclin does not bind or activate CDKs. Previous characterization of these mutations by transient expression in cultured cells indicated that these mutants do not bind or activate cellular CDKs (66). Analysis of these mutants in the context of the viral infection reveals that v-cyclin-CDK interactions are required for acute infection in the lungs of mice following intranasal infection but are at least partially dispensable for virus reactivation from latency. These data provide the first evidence that the γHV68 v-cyclin is a multifunctional protein encoding both CDK-dependent and CDK-independent mechanisms of action that regulate virus replication and persistence in the host.
MATERIALS AND METHODS
Tissue culture.
NIH 3T12 (CCL-164), NIH 3T3 (CCL-163), and LA-4 (CCL-196) cells were obtained from the ATCC. Cre-recombinase-expressing Vero cells (Vero-CRE cells) were obtained from David Leib (Washington University School of Medicine, St. Louis, MO). C57BL/6 mouse embryonic fibroblasts (MEFs) were derived as previously described (76). LA-4 cells were maintained in Ham's F-12K medium supplemented with 15% fetal calf serum, 100 U penicillin/ml, 100 mg of streptomycin/ml, and 2 mM l-glutamine. All other cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 2 mM l-glutamine, unless otherwise specified. Vero-CRE cells were supplemented with 300 μg/ml hygromycin. All cells were maintained in tissue culture incubators at 5% CO2 and 37°C.
Generation of viral mutants.
All recombinant viruses were generated from the γHV68 bacterial artificial chromosome (BAC), a kind gift of Ulrich Kozinowski (3). Initially, the kanamycin (Kan) cassette and flanking Flip recombinase (FRT) sites from pCP15 (11) were amplified by PCR with primers adding an NcoI site to the 5′ end of the cassette and an NsiI site to the 3′ end and subcloned into pL3700 (70) cut with NcoI and NsiI. The resulting plasmid was cut with HinDIII and BsrGI. The 3.2-kb fragment was gel purified twice and used to generate the v-cycKAN BAC as previously described (50). v-cyc[K104E] and v-cyc[E133V] mutants were generated by allelic exchange essentially as previously described (60). pCMV-TAG2B-K104E and pCMV-TAG2B-E133V (66) were each digested with NcoI and NsiI, and the 475-bp fragments were isolated by gel extraction. These fragments were subcloned into pL3700 cut with NcoI and NsiI to generate pL3700-K104E and pL3700-E133V, respectively. The resulting plasmids were cut with NheI and SalI, and the 3.7-kb fragment was gel purified and cloned into the suicide targeting vector pGS824 cut with NheI and SalI. O72.Stop was generated by digesting pL7300.Stop (70) with NheI and SalI, and the 3.7-kb fragment was cloned into the same sites of pGS284. All resulting plasmids were confirmed for authenticity and orientation by restriction enzyme mapping. Bacterially mediated allelic exchange was performed as previously described (18, 44, 60) by using v-cycKAN BAC as the target for recombination. Potential mutant clones were screened by PCR, restriction mapping, and Southern blotting. Marker rescue viruses were generated by cloning the NheI-SalI fragment of pL3700 containing wild-type sequences into the same sites of pGS284, and allelic exchange was performed as described above using each mutant BAC as the target for recombination. To generate viral stocks, it was necessary to remove BAC vector sequences (2). Recombinant BAC DNA was isolated and transfected into Vero-CRE cells, and viral supernatants were passaged through Vero-CRE cells a second time and then used to generate large viral stocks in NIH 3T12 cells. Wells were observed over time via fluorescence microscopy, and no green fluorescent protein expression was observed, indicating the excision of BAC sequences. The titers of all stocks were determined by plaque assay using NIH 3T12 fibroblasts in three independent experiments.
Southern blotting.
One microgram of BAC DNA of each recombinant was digested with BamHI and EcoRI for diagnostic fragments of the v-cyclin region. Additional diagnostic cuts were performed to assess the introduction, and subsequent rescue, of specific mutations: HpaI for O72.Stop, NgoMIV for K104E, and SpeI for E133V. Digests were electrophoresed on 0.9% agarose gels and transferred by alkaline transfer onto Nytran nylon membranes (Turboblot; Schleicher and Schuell, Keene, NH) according to the manufacturer's recommendations. Southern blots were probed with the BamHI-HinDIII fragment of pL3700 for analysis of the v-cyclin region. The probe was 32P labeled by random primer extension according to the manufacturer's recommendations (Megaprime DNA labeling kit; Amersham International). Blots were hybridized at 68°C and washed with two changes of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate followed by two changes of 0.2× SSC-0.1% sodium dodecyl sulfate prior to exposure to film.
Immunoblotting and kinase assays.
NIH 3T12 cells were infected at a multiplicity of 10 for 1 h and washed twice with phosphate-buffered saline (PBS), and medium wasreplaced with complete medium for 24 h. Infected cells were harvested by scraping and centrifugation, and cell lysates were made in ELB with protease and phosphatase inhibitors (66). One hundred micrograms of total protein was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes. Membranes were incubated in blocking buffer (PBS with 0.05% Tween-20 [PBS-T] and 5% nonfat milk) for 1 h at room temperature and then incubated with the indicated antibody in blocking buffer overnight at 4°C. Blots were washed three times in PBS-T and incubated with horseradish peroxidase-conjugated donkey anti-rabbit, anti-mouse (Jackson Immunochemicals), or anti-chicken (Gallus Immunotech) secondary antibodies at a 1:5,000 dilution in blocking buffer for 1 h at room temperature. Blots were washed three times in PBS-T, developed with ECL-Plus reagent (Amersham Biosciences), and exposed to film. Primary antibodies used were rabbit polyclonal anti-v-cyclin antiserum (68), chicken polyclonal anti-ORF59 (66), and mouse anti-β-actin (Sigma). Kinase assays were performed essentially as previously described (66). Briefly, NIH 3T3 cells were plated and allowed to grow to confluence. Cells were then serum starved for 72 h in low-serum media containing 0.05% fetal calf serum. Cells were infected in low-serum medium at a multiplicity of 10 for 1 h, washed twice with PBS, and replaced with low-serum medium. Twenty-four hours postinfection, cells were harvested, and lysates were made as described above. Three hundred micrograms of total protein was subjected to immunoprecipitation with agarose bead-conjugated rabbit anti-CDK2 antibodies (M2; Santa Cruz) overnight at 4°C with gentle orbital agitation. Beads were collected by centrifugation, washed four times in ice-cold ELB with protease and phosphatase inhibitors, and washed twice in kinase buffer. In vitro kinase reactions were performed using glutathione S-transferase-C-terminal retinoblastoma protein (GST-pRb) fusion protein (Santa Cruz) as an exogenous substrate. Reactions were terminated by the addition of SDS running buffer and boiling for 10 min. Samples were subjected to SDS-PAGE, and the gels were fixed and dried. Samples were then analyzed by autoradiography using phosphorscreens and visualized using a Typhoon9410 apparatus (Amersham Biosciences). Relative kinase activity was determined using ImageQuant software (Amersham Biosciences) by spot intensity analysis normalized to mock infection. All lysate protein concentrations were calculated from a standard curve of bovine serum albumin in lysis buffer with the DC protein assay (Bio-Rad).
Plaque assays, in vitro growth, and determination of viral titers.
Plaque assays were performed on NIH 3T12 fibroblasts. Viral growth in vitro was determined by infection of NIH 3T12 cells at a multiplicity of infection (MOI) of 10 PFU per cell, with removal of the inoculum after 1 h of infection to measure a single cycle of virus replication, or at 0.01 PFU per cell (NIH 3T12) or 0.05 PFU per cell (LA-4) to measure multiple cycles of virus replication. Seventy-two hours prior to infection, 2 × 105 LA-4 cells were plated in medium containing 1% fetal calf serum, which allowed the growth of cells to confluence, followed by a cessation of division as assessed by cell counting up to 10 days postplating (data not shown). Cells and supernatants were collected at various times postinfection and frozen at −80°C. Samples were then subjected to two cycles of freezing and thawing, and virus was then quantitated by plaque assay (sensitivity, 50 PFU/ml). Organs for which virus titers were to be determined were thawed and homogenized by mechanical disruption with four 1-min pulses in the presence of 1.0-mm zirconia/silica beads (Biospec Products) in a Mini-Beadbeater-8 (Biospec Products). Samples were then diluted in cMEM and plated onto NIH 3T12 cells. Infections were allowed to adsorb at 37°C for 1 h with occasional agitation and overlaid with complete medium containing 20 g/liter methylcellulose (Sigma). Plaques were visualized 6 to 7 days postinoculation by staining with neutral red (Sigma) overnight.
Infections of mice and organ harvests.
Female C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) were used at between 8 and 12 weeks of age. Mice were placed under isofluorane anesthesia prior to both infection and sacrifice. Intranasal inoculations were performed with 103 PFU of the indicated virus in a volume of 20 μl of complete medium. Intraperitoneal inoculations were performed by injection with 103 PFU in a volume of 0.5 ml of complete medium. Upon sacrifice, spleens and lungs were harvested by sterile dissection. Organs for determinations of titers were placed in 1 ml complete medium and frozen at −80°C. Spleens for latency analysis were weighed and then placed into complete medium, homogenized, and filtered, and erythrocytes were removed by ammonium chloride lysis. Resident peritoneal exudate cells (PECs) were harvested by peritoneal lavage with 10 ml of incomplete medium. For analysis of acute infection, each animal was analyzed independently. For analysis of latency parameters, pooled splenocytes or PECs from four to five infected mice were used for each independent experiment.
LD ex vivo reactivation analyses.
The frequency of cells capable of reactivation from latency was determined by limiting-dilution (LD) analysis as previously described (12, 65, 70, 76-78). Briefly, PECs and splenocytes were harvested from infected mice at the times indicated, and single-cell suspensions were generated. Erythrocytes were removed from spleen samples by ammonium chloride lysis. Cells were resuspended in cMEM and plated in serial twofold dilutions, starting at 105 cells per well, onto MEF monolayers in 96-well tissue culture plates. Twenty-four wells were plated per dilution, with a total of 12 dilutions per sample per experiment. Wells were microscopically scored for cytopathic effect 21 days postplating, after which samples were replated to confirm reactivation of infectious virus. To detect preformed infectious virus, parallel samples were resuspended in hypotonic medium and subjected to mechanical disruption in the presence of 0.5-mm-diameter silica beads. Disruption was carried out by four 1-min pulses in a Mini-Beadbeater-8 and was previously shown to kill >99% of cells, with no significant effect on titers of preformed virus (76). Ex vivo stimulation of splenocytes with anti-CD40 and anti-immunoglobulin G (IgG)/IgM antibodies was performed as previously described (47).
Statistical methods.
All data were analyzed using GraphPad Prism software (GraphPad Software). Titer, spleen weight, and spleen cell number data were statistically analyzed with the nonparametric Mann-Whitney test. Frequencies of reactivation and viral-genome-positive cells were obtained from the cell number at which 63% of wells scored positive for either reactivating virus or the presence of the viral genome based on Poisson distribution; data were subjected to nonlinear regression analysis to obtain the single-cell frequency for each limiting-dilution analysis.
RESULTS
Generation of v-cyclin mutant viruses.
Previously, we and others have shown that a disruption of the γHV68 v-cyclin open reading frame (ORF), by either a large insertion or the introduction of a premature in-frame translation termination codon, severely attenuates the ability of recombinant viruses to reactivate efficiently from latency (25, 70). To determine the contribution of v-cyclin-CDK interactions and thus the contribution of cell cycle regulation by v-cyclin to this process, specific loss-of-function mutations previously characterized in vitro by our laboratory (66) were introduced into the v-cyclin locus using the γHV68 genome cloned as a BAC and utilizing methodologies and strategies described previously (1-3, 11, 44, 50, 60, 70). In addition to these loss-of-function mutants, we regenerated the O72.Stop mutation, previously described by our laboratory (70), to control for any phenotypic discrepancies between BAC-derived mutants and those previously generated by homologous recombination in mammalian cells. Each mutant was generated by the “rescue” of the v-cycKAN BAC as described in Materials and Methods to yield a v-cyclin allele containing either the O72.Stop, K104E, or E133V mutation (v-cyc[STOP], v-cyc[K104], and v-cyc[E133V], respectively). Multiple recombinants from independent bacterial matings were identified by Kan sensitivity and screened by PCR and Southern blot analysis to confirm proper recombination. Each isolate was then rescued to the wild-type sequence by allelic exchange. For each mutant, specific isolates and corresponding marker rescues were selected at random from the panel of confirmed isolates, and DNA was prepared for further analysis and viral stock generation.
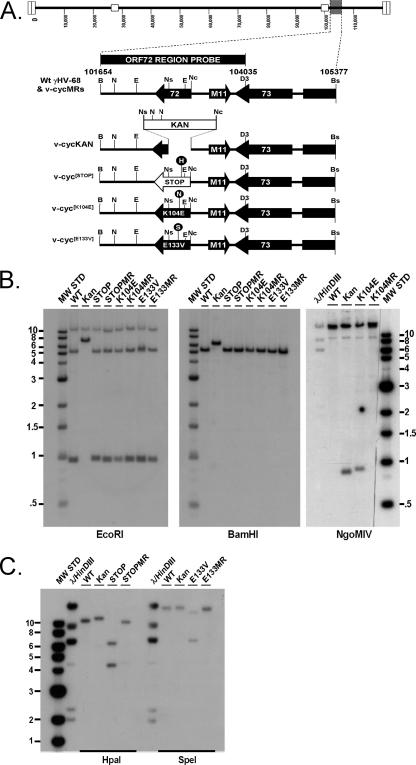
The structures of all mutant and marker rescue γHV68-BACs were confirmed by Southern blot analysis on EcoRI- and BamHI-digested DNA (Fig. 1). As expected, the ORF72 region probe hybridized to 10.8-, 5.2-, and 0.9-kb EcoRI fragments of all DNAs analyzed except v-cycKAN, which hybridized to 10.8-kb and 6.5-kb EcoRI fragments, indicating the insertion-deletion caused by the addition of the Kan cassette. Similarly, the probe hybridized a 6.2-kb BamHI fragment of v-cycKAN, while the remaining DNAs hybridized only a 5.2-kb BamHI fragment. To confirm the specific mutations introduced into each individual mutant, separate Southern blots were performed with DNAs digested with HpaI (STOP), NgoMIV (K104E), or SpeI (E133V). As expected, digestion with each enzyme yielded hybridization patterns indicating the introduction of the unique diagnostic restriction enzyme sites and linked mutations and the absence of this pattern in marker rescue isolates. DNAs were then transfected into Cre-recombinase-expressing Vero cells for Cre-mediated excision of BAC sequences, followed by the generation of viral stocks as described in Materials and Methods. Wild-type γHV68-BAC DNA was used to generate the wild-type control virus used in these studies.
FIG. 1.
Genomic structures of mutant viruses. (A) Schematic representation of γHV68 region encoding v-cyclin (ORF72) and surrounding genes from bp 101654 to bp 105377, based on the γHV68 WUMS clone sequence (73). The ORF72 region probe is depicted above the wild-type (Wt) γHV68 schematic and includes bp 101654 to bp 104035. The restriction enzyme sites shown were used for the generation of genomic clones or probes, mutagenesis, or diagnostics and are represented as follows: B, BamHI; Bs, BsrGI; D3, HinDIII; E, EcoRI; H, HpaI; N, NgoMIV; Nc, NcoI; Ns, NsiI; S, SpeI. (B and C) Southern blot analysis of wild-type (WT), mutant, and marker rescue viruses. BAC DNAs were purified, digested with the indicated restriction enzyme, electrophoresed, blotted, and hybridized with the ORF72 region probe described above. Panels labeled BamHI and EcoRI represent general diagnostic Southern analyses for all recombinants, while those labeled NgoMIV, HpaI, and SpeI are diagnostic Southern blots for the specific mutants v-cyc[K104E], v-cyc[STOP], and v-cyc[E133V], respectively. MW STD, molecular weight standard.
Functional analysis of v-cyclin mutant viruses.
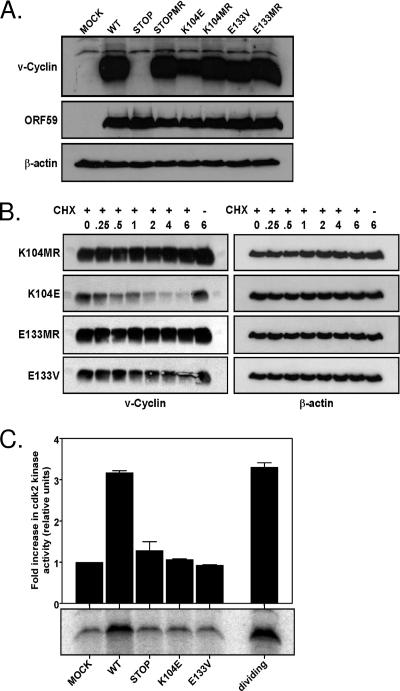
To confirm expression of the mutant v-cyclins, NIH 3T12 fibroblast were infected at an MOI of 10 PFU/cell and harvested 24 h postinfection for immunoblot analyses. As expected, robust v-cyclin expression was observed 24 h postinfection in samples infected with the wild type, marker rescue, or CDK-binding-deficient v-cyclin mutants, and this expression was absent from cells either mock infected or infected with the v-cyc[STOP] mutant virus (Fig. 2A). Expression of the early gene ORF59 was comparable among all infected samples, and β-actin confirmed the equivalent loading of cell extracts. Cells infected with the v-cyc[K104E] mutant appeared to express less v-cyclin protein than did the wild type, marker rescue, or v-cyc[E133V].
FIG. 2.
v-Cyclin protein expression and function. (A) Immunoblot analysis of v-cyclin protein expression. NIH 3T12 fibroblasts were lytically infected with wild-type γHV68 (WT), v-cyc[STOP], v-cyc[STOPMR],v-cyc[K104E], v-cyc[K104MR], v-cyc[E133V], or v-cyc[E133MR] or were mock infected. Cell lysates were collected at 24 h postinfection and subjected to SDS-PAGE and Western analysis. Blots were sequentially probed with rabbit polyclonal anti-v-cyclin antiserum (top panel), chicken polyclonal anti-ORF59 (middle panel), and mouse anti-β-actin (bottom panel). (B) Protein stability of mutant v-cyclin alleles. NIH 3T12 fibroblasts were lytically infected with v-cyc[K104E], v-cyc[K104MR], v-cyc[E133V], or v-cyc[E133MR]. Twenty-four hours postinfection, 50 mg/ml cycloheximide (CHX) (+) or vehicle control (−) was added to each well, and cell lysates were collected at the indicated times, in hours, posttreatment. Equivalent protein amounts were subjected to SDS-PAGE and Western analysis. Blots were probed sequentially with rabbit polyclonal anti-v-cyclin antiserum (left panels) or mouse anti-β-actin (right panels). (C) v-Cyclin mutants do not activate CDK2 activity during infection. Contact-inhibited, serum-starved NIH 3T3 fibroblasts were infected with wild-type γHV68, v-cyc[STOP], v-cyc[K104E], or v-cyc[E133V] or were mock infected in low-serum medium. Cell lysates were collected 24 h postinfection and subjected to immunoprecipitation with rabbit polyclonal anti-CDK2 directly conjugated to agarose beads. Uninfected, actively dividing NIH 3T12 fibroblasts were similarly processed as a positive control. Immunoprecipitates were used as active enzymes for in vitro kinase assays with glutathione S-transferase-pRb as an exogenous substrate. Reactions were terminated by the addition of loading buffer and boiling followed by SDS-PAGE and autoradiography. Analysis (top panel) was performed with ImageQuant software, with data normalized to mock infection. Also shown (bottom panel) is a representative experiment (one of two independent analyses).
Although the v-cyclin K104E and E133V mutations (as well as many other analogous mutations) have been previously characterized as having little or no difference from the wild type with regard expression or stability, no v-cyclin point mutants have been investigated in the context of an ongoing viral infection, which could influence the stability of these mutants. To investigate the expression and stability of the v-cyclin CDK mutants, NIH 3T12 fibroblasts were infected at an MOI of 10 PFU/cell for 24 h, followed by treatment with cycloheximide to halt protein synthesis. Samples were harvested for immunoblot analyses at the indicated times posttreatment (Fig. 2B). These analyses demonstrated that within 2 h of cycloheximide treatment, there was a marked decrease in the protein level of the v-cyc[K104E] mutant, which was not seen with the other CDK-binding mutant v-cyclin or v-cyclin expression from the marker rescue virus infections. This indicates that in the absence of new protein synthesis, the pool of K104E v-cyclin is rapidly degraded, leading to the decreased steady-state protein levels observed in Fig. 2A.
In addition to expression, it was necessary to confirm that the K104E and E133V mutations were, in fact, loss-of-function mutants during an infection. To show that the v-cyclin proteins expressed by v-cyc[K104E] and v-cyc[E133V] viruses do not bind and activate CDK2, contact-inhibited, serum-starved NIH 3T3 fibroblasts were infected, and cell lysates were made at 24 h postinfection. Equivalent amounts of cell extracts were subjected to immunoprecipitation with anti-CDK2-agarose beads, and the precipitates were analyzed in an in vitro kinase assay utilizing GST-pRb as an exogenous substrate. An equivalent amount of protein from uninfected, actively dividing NIH 3T3 cells was used as a positive control for CDK2 kinase activity. As shown in Fig. 2C, wild-type γHV68 was able to induce a nearly threefold increase in relative kinase activity compared to uninfected cells, whereas v-cyc[STOP], v-cyc[K104E], and v-cyc[E133V] exhibited no significant increase in kinase activity above the background level, indicating that these v-cyclin mutants do not activate CDK2 during infection. This result confirms and extends our previous in vitro analyses of the v-cyclin CDK-binding mutants (66).
v-Cyclin interaction with cellular CDKs is not required for viral replication in vitro but is required for acute replication in the lungs in vivo following low-dose intranasal inoculation.
Although previous studies have shown no in vitro growth defect of v-cyclin-null viruses (25, 70), we sought to confirm this finding and extend it to the viruses harboring v-cyclin mutants that are unable to bind or activate cellular CDKs. Single-step (MOI = 10) and multistep (MOI = 0.01) infections of NIH 3T12 fibroblasts demonstrated that, as expected, there was no discernible kinetic defect in virus replication for the v-cyc[STOP], v-cyc[K104E], or v-cyc[E133V] mutant, and all reached titers comparable to those of the wild-type virus (Fig. 3A and B). Similar results were obtained in contact-inhibited, serum-starved LA-4 lung epithelial cells (Fig. 3C) (discussed below) as well as in NIH 3T3 fibroblasts and quiescent MEFs (data not shown).
FIG. 3.
Growth of v-cyclin mutants is similar to that of wild-type (WT) γHV68 in vitro. NIH 3T12 fibroblasts were infected with 10 PFU (A) or 0.01 PFU (B) per cell of wild-type γHV68 (closed diamonds), v-cyc[STOP] (closed squares), v-cyc[STOPMR] (open squares), v-cyc[K104E] (closed circles), v-cyc[K104MR] (open circles), v-cyc[E133V] (closed triangles), or v-cyc[E133MR] (open triangles). Inocula were removed, and samples were washed with PBS and repleted with fresh medium 1 h postinfection for determinations of single-step growth (A) or left for multistep growth analysis (B). Samples were harvested at the indicated times, and viral titers were determined by plaque assay. (C) Growth of wild-type γHV68 and v-cyclin mutants in lung epithelial cells in vitro. LA-4 lung epithelial cells were plated in low-serum medium, allowed to grow to contact inhibition, and rested for 72 h. Cells were then infected with 0.05 PFU wild-type γHV68 (closed diamonds), v-cyc[STOP] (closed squares), v-cyc[K104E] (closed circles), or v-cyc[E133V] (closed triangles) per cell in low-serum medium. Samples were harvested at the indicated times, and viral titers were determined by plaque assay. All data (A to C) are representative of two independent experiments, with titers of each sample determined in duplicate.
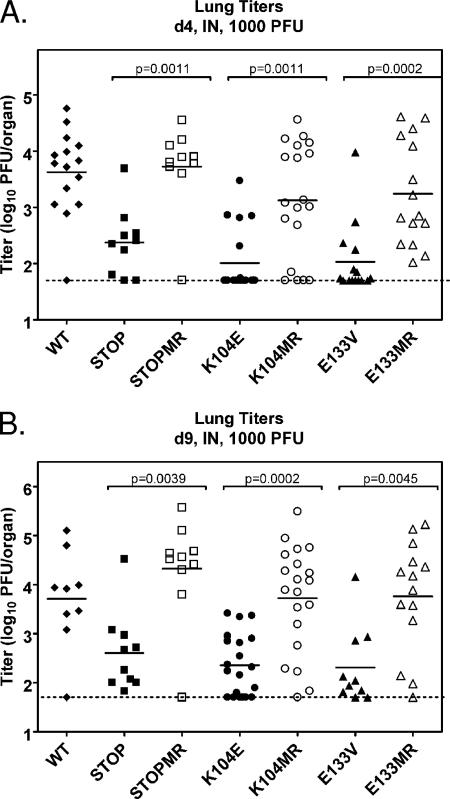
Previous studies that investigated the role of the γHV68 v-cyclin during acute infection in vivo yielded conflicting results (25, 70). We have previously shown that v-cyclin function is dispensable for acute virus replication in the liver, lungs, and spleen of C57BL/6 mice following high-dose (1 × 106 PFU) intraperitoneal inoculation (70). However, another group (25), using the same dose and route of infection, observed a replication defect in multiple tissues in BALB/c mice. We sought to clarify and extend these findings by analyzing the replication of v-cyc[STOP], v-cyc[K104E], and v-cyc[E133V] during the acute phase of virus infection following intranasal or intraperitoneal inoculation. Initially, C57BL/6 mice were infected with 1,000 PFU of the indicated virus via intranasal inoculation, and acute replication in the lung was assessed. Notably, viruses expressing mutant v-cyclin alleles displayed a severe, but not absolute, defect in acute replication at multiple times postinfection (Fig. 4A and B). Importantly, the titers of all diluted viral inocula were redetermined by plaque assay to ensure proper dosage (data not shown). Statistical analyses revealed that differences between each mutant virus and their specific marker rescue viruses were significant (P values of <0.05, as indicated in figures). In addition, a comparison of the respective marker rescue virus replication to wild-type virus replication demonstrated that there were no statistically significant differences. These results indicate that v-cyclin and, specifically, binding and activation of CDKs by v-cyclin are required for efficient replication in the lungs of infected mice following intranasal inoculation. However, this replication defect was not evident following high-dose (1 × 106 PFU) intranasal inoculation with v-cyc[STOP] or v-cyc[K104E] (data not shown), indicating that the dependence on v-cyclin function for acute virus replication in the lungs is dependent on dose.
FIG. 4.
v-Cyclin interaction with CDKs is required for acute replication in the lung following intranasal (IN) inoculation. C57BL/6 mice were infected with 1,000 PFU of wild-type (WT) γHV68 (closed diamonds), v-cyc[STOP] (closed squares), v-cyc[STOPMR] (open squares), v-cyc[K104E] (closed circles) v-cyc[K104MR] (open circles), v-cyc[E133V] (closed triangles), or v-cyc[E133MR] (open triangles) via intranasal inoculation. Lungs were harvested at 4 (A) and 9 (B) days postinfection, and titers were determined by plaque assay. Data shown are compiled from two to three independent experiments with four to five mice each. Solid bars represent the means of each group, and the dashed line indicates the limit of detection of the assay (50 PFU/organ). Statistically significant differences (P < 0.05), as determined by nonparametric Mann-Whitney analysis, are indicated.
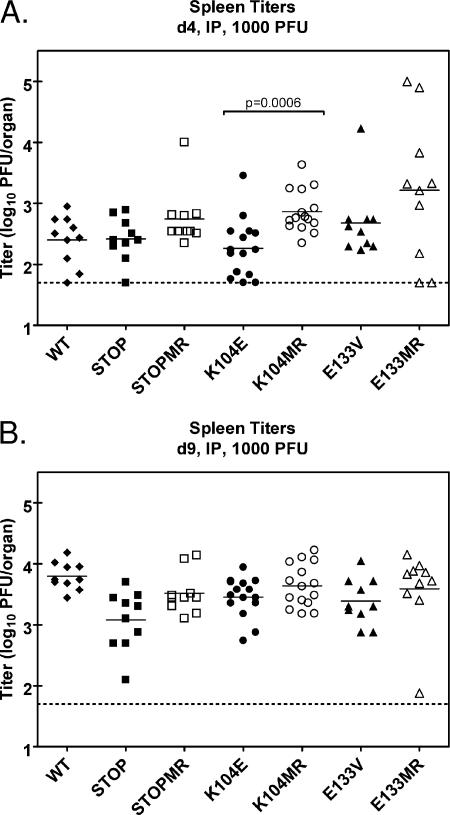
Additionally, mice were inoculated with 1,000 PFU via intraperitoneal injection and assayed for acute virus replication in the spleen at days 4 and 9 postinfection. In these analyses, only v-cyc[K104E] displayed a significant phenotype at day 4 compared to its marker rescue (Fig. 5A), suggesting a possible modest defect at early times postinfection, although the replication of the v-cyc[STOP] and v-cyc[E133V] mutants was not significantly different from that of marker rescue controls. The observed differences between the behaviors of the two CDK-binding mutant v-cyclins may be directly related to the decreased stability of the K104E mutant (Fig. 2B). Splenic titers of mutant and marker rescue viruses were not significantly different at day 9 postinfection (Fig. 5) or following high-dose intraperitoneal inoculation (data not shown), as previously suggested (70). Taken together, these data indicate that the requirement for v-cyclin function during acute infection is both dose dependent and route and/or cell type specific.
FIG. 5.
v-Cyclin interaction with CDKs is dispensable for acute replication in the spleen following intraperitoneal (IP) inoculation. C57BL/6 mice were infected with 1,000 PFU of wild-type (WT) γHV68 (closed diamonds), v-cyc[STOP] (closed squares), v-cyc[STOPMR] (open squares), v-cyc[K104E] (closed circles), v-cyc[K104MR] (open circles), v-cyc[E133V] (closed triangles), or v-cyc[E133MR] (open triangles) via intraperitoneal injection. Spleens were harvested at 4 (A) and 9 (B) days postinfection, and lytic titers were determined by plaque assay. Data shown are compiled from two to three independent experiments with five mice each. Solid bars represent the means of each group, and the dashed line indicates the limit of detection of the assay (50 PFU/organ). Statistically significant differences (P < 0.05) as determined by nonparametric Mann-Whitney analysis are indicated.
Since v-cyclin mutants deficient for CDK binding are defective in acute replication in the lungs but not the spleen, there may be a cell-type-specific requirement for v-cyclin function. To further investigate this, we assessed virus replication in the lung epithelial cell line LA-4. LA-4 cells were contact inhibited, growth arrested in low-serum medium, and then infected to assay for multistep growth (MOI = 0.05). As shown in Fig. 3C, there was no defect in the kinetics of replication of the v-cyc[STOP], v-cyc[K104E], or v-cyc[E133V] mutant in quiescent LA-4 cells in vitro. Thus, replication in the LA-4 epithelial cell line does not recapitulate the growth defect observed in vivo.
v-Cyclin mutant viruses do not exhibit splenomegaly in immunocompetent mice.
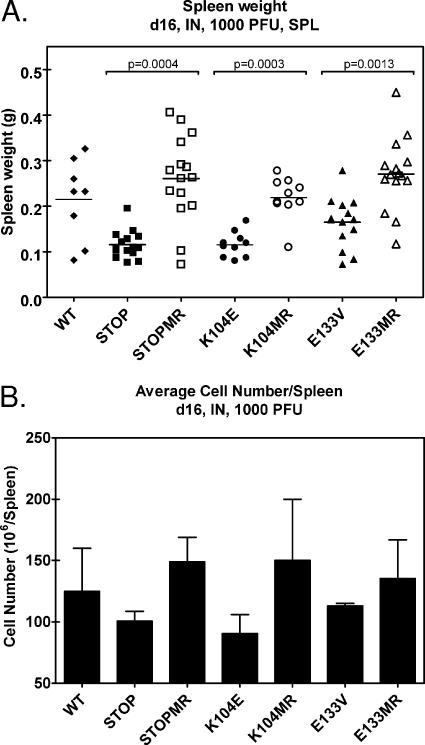
Inoculation of immunocompetent mice with γHV68 results in a pronounced, transient splenomegaly characterized by an enlargement of the spleen and elevated numbers of lymphocytes within the spleen. Although this response to infection is not well understood, it has been shown to be CD4-T-cell dependent (67). At day 16 after intranasal infection, we noted a striking difference in the sizes of spleens infected with wild-type or marker rescue control viruses compared to those of spleens infected with the mutant v-cyclin viruses (Fig. 6). Determination of spleen weights revealed a significant difference in spleen weight in mice infected with v-cyc[STOP], v-cyc[K104E], or v-cyc[E133V] compared to that of mice infected with marker rescue controls (Fig. 6A). Cell numbers per spleen were determined (in triplicate) from pools of five spleens following disruption and red blood cell lysis. Similar to spleen weights, the number of cells/spleen was lower in mice infected with v-cyc[STOP], v-cyc[K104E], or v-cyc[E133V] than in mice infected with the marker rescue controls and wild-type virus (Fig. 6B). These data demonstrate that the development of splenomegaly following low-dose intranasal infection with v-cyclin mutant viruses is impaired and correlate with diminished acute virus replication in the lungs at earlier times postinfection.
FIG. 6.
v-Cyclin mutant viruses do not induce splenomegaly by 16 days after intranasal infection. (A) Spleen weights of C57BL/6 mice 16 days after intranasal infection with 1,000 PFU of wild-type (WT) γHV68 (n = 8), v-cyc[STOP] (n = 15), v-cyc[STOPMR] (n = 15), v-cyc[K104E] (n = 10), v-cyc[K104MR] (n = 10), v-cyc[E133V] (n = 13), or v-cyc[E133MR] (n = 14). Statistically significant differences (P < 0.05), as determined by nonparametric Mann-Whitney analysis, are indicated. SPL, splenocytes. (B) Cell number in spleens of infected mice. Pooled samples of three to five spleens were analyzed for cell number per spleen following organ disruption and erythrocyte lysis. Data represent two to three independent experiments. Error bars represent the standard errors of the means.
Analysis of splenic latency following intranasal inoculation provides evidence for a CDK-independent function(s) of the γHV68 v-cyclin.
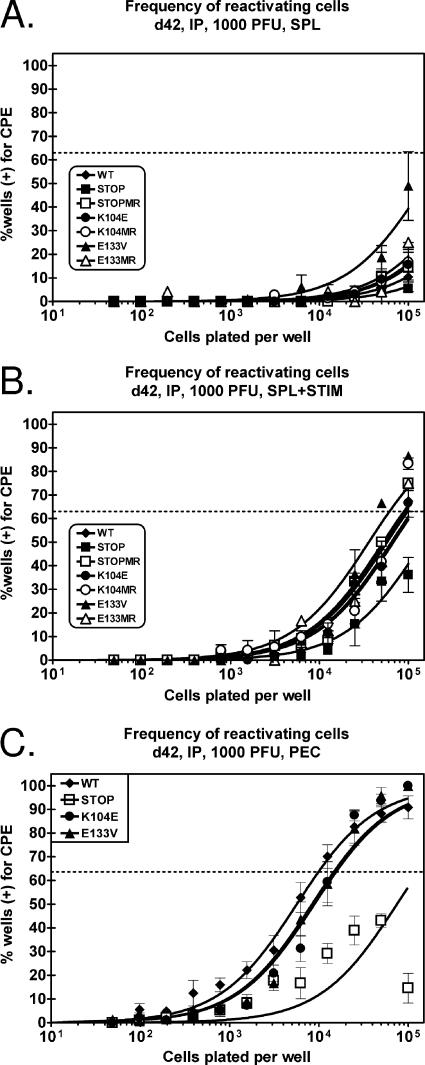
Previous studies have not addressed a role for v-cyclin in the establishment of, and reactivation from, latency following intranasal inoculation. Here, we have shown a dose-dependent replication defect of v-cyclin mutant viruses (Fig. 4) that correlates with differences in splenomegaly (Fig. 6) following inoculation via this route. To determine the capacity of v-cyclin mutant viruses to reactivate 16 days after intranasal infection, splenocytes were analyzed by employing a limiting-dilution reactivation assay. Figure 7 depicts the frequency of splenocytes 16 days postinfection (1,000 PFU via intranasal inoculation) that spontaneously reactivate virus upon explant into tissue culture. As expected, wild-type γHV68 (∼1 in 2,000), v-cyc[STOPMR] (∼1 in 2,000), v-cyc[K104MR] (∼1 in 4,000), and v-cyc[E133MR] (∼ 1 in 1,800) all exhibited similar reactivation frequencies. The frequency of reactivation for v-cyc[E133V] (∼ 1 in 7,400) was approximately fourfold lower than that for the control viruses, while v-cyc[STOP] (∼1 in 85,000) and v-cyc[K104E] (∼1 in 80,000) exhibited severe defects in virus reactivation (ca. 40-fold reduced frequency of cells reactivating virus). The discrepancy between the results obtained with the v-cyc[E133V] and those obtained with the v-cyc[K104E] mutant are discussed below (see Discussion).
FIG. 7.
Analysis of splenocyte reactivation following intranasal infection provides evidence for a CDK-independent function(s) of the γHV68 v-cyclin. C57BL/6 mice were infected with 1,000 PFU of (A) v-cyc[STOP], (B) v-cyc[K104E], (C) v-cyc[E133V], or the corresponding marker rescue virus via intranasal (IN) inoculation. Sixteen days postinfection, splenocytes (SPL) were harvested, made into single-cell suspensions, and subjected to twofold limiting dilutions on MEF indicator monolayers for determinations of the ex vivo frequencies of cells reactivating from latency. Mechanically disrupted cells were plated in parallel. Curve fit lines were derived by nonlinear regression analysis, and symbols represent means and standard errors of the means (error bars) of data from individual experiments as indicated. The dashed line (63%) represents the value used to calculate the frequency of reactivating cells as indicated by a Poisson distribution. Data represent three independent experiments, with each experiment containing cells pooled from four to five mice. (D) Graphical representation of preformed infectious virus from limiting-dilution assays (A to C). WT, wild type; CPE, cytopathic effect. Bars for each sample represent twofold dilutions of mechanically disrupted splenocytes.
In addition to analyzing virus reactivation from intact splenocytes, we also analyzed mechanically disrupted splenocytes (which eliminates any contribution of virus reactivation) for the presence of preformed infectious virus in these samples. Notably, we detected the presence of preformed infectious virus in many of these samples (Fig. 7). The origin of virus at day 16 postinfection is unclear but could reflect either delayed clearance of acute virus replication and/or virus replication arising from reactivation events. On the basis of the ability of the limiting-dilution assay to detect 0.2 PFU of γHV68 (76, 77), coupled with a very conservative estimate of 1 PFU per productively infected cell (65), the presence of preformed infectious virus in splenocytes of mice infected with wild-type γHV68 corresponds to productive infection in at most 1 in 420,000 cells. Infections with v-cyc[STOPMR], v-cyc[K104MR], and v-cyc[E133MR] all yielded similar levels of preformed infectious virus (at most 1 in 470,000, 1 in 525,000, and 1 in 170,000 infected cells producing virus, respectively). Importantly, these conservative estimates demonstrate that the frequency of cells that were productively infected did not contribute significantly to the measurements of the frequency of cells reactivating virus in these analyses. Extrapolating the best-fit curve of the nonlinear regression analyses indicates that v-cyclin CDK-binding mutant viruses displayed slightly lower levels of preformed infectious virus than wild-type or marker rescue control viruses (Fig. 7D). However, there were nearly undetectable levels of preformed infectious virus present in v-cyc[STOP] mutant-infected spleens, which was strikingly different than what was observed with the CDK-binding mutants (v-cyc[K104E], 1 in 1.9 × 106; v-cyc[E133V], 1 in 9 × 105; and v-cyc[STOP], 1 in 3 × 107). It is notable that the v-cyc[STOP] mutant, which is highly compromised for virus reactivation, is also highly compromised for the presence of preformed infectious virus in the spleen at day 16 compared to either wild-type virus or the CDK-binding mutant viruses (Fig. 7D), even though the CDK-binding mutant viruses exhibited a nearly identical defect in acute virus replication in the lungs (Fig. 4). Thus, there may be a role for virus reactivation in the ongoing virus replication detected in the spleen at day 16 postinfection following low-dose intranasal inoculation.
While the levels of preformed infectious virus do not significantly influence the results of ex vivo reactivation analyses (Fig. 7), they severely interfered with determining the frequency of splenocytes harboring viral genomes as determined by LD-PCR analysis. Notably, attempts to remove cell-surface-associated virus by treating splenocytes with acid and/or trypsin, followed by washing input cells, prior to LD-PCR analysis did not remedy this problem (data not shown). Only splenocytes recovered from v-cyc[STOP]-infected mice, which had nearly undetectable levels of preformed infectious virus, yielded reliable LD-PCR results (ca. 1 in 1,000 viral-genome-positive cells) (data not shown). Based on historical data, the frequency of splenocytes harboring v-cyc[STOP] at day 16 postinfection was ca. three- to fivefold lower than that routinely observed with wild-type virus infection at day 16 postinfection by this route and dose (18, 46, 79), indicating a minor defect in the establishment of viral latency that does not account for the observed severe defect in virus reactivation (Fig. 7 and Table 1).
TABLE 1.
Summary of acute virus replication and reactivation from latency
| Virus | Mean log10 titer± SEMa
|
Frequency of ex vivo reactivationb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Following i.n. administration in lung
|
Following i.p. administration in spleen
|
Day 16 splenocytes | Day 42
|
|||||
| Day 4 | Day 9 | Day 4 | Day 9 | Splenocytes | Stimulated splenocytes | PECs | ||
| Wild-type γHV68 | 3.63 ± 0.20 | 3.71 ± 0.33 | 2.40 ± 0.13 | 3.80 ± 0.07 | 1/2,000 | LDc | 1/109,000d | 1/9,500 |
| STOP | 2.37 ± 0.19 | 2.61 ± 0.25 | 2.42 ± 0.11 | 3.08 ± 0.15 | 1/85,000 | LD | 1/250,000d | 1/120,000d |
| STOPMR | 3.72 ± 0.24 | 4.33 ± 0.33 | 2.75 ± 0.15 | 3.51 ± 0.11 | 1/2,000 | LD | 1/87,000 | NDe |
| K104E | 2.01 ± 0.13 | 2.36 ± 0.14 | 2.27 ± 0.12 | 3.45 ± 0.08 | 1/80,000 | LD | 1/105,000d | 1/14,700 |
| K104MR | 3.12 ± 0.23 | 3.72 ± 0.25 | 2.86 ± 0.09 | 3.64 ± 0.09 | 1/4,000 | LD | 1/90,000 | ND |
| E133V | 2.03 ± 0.16 | 2.31 ± 0.25 | 2.68 ± 0.18 | 3.38 ± 0.12 | 1/7,400 | 1/260,000d | 1/61,000 | 1/13,800 |
| E133MR | 3.24 ± 0.24 | 3.76 ± 0.30 | 3.21 ± 0.37 | 3.59 ± 0.20 | 1/1,800 | LD | 1/94,000 | ND |
i.n., intranasal; i.p., intraperitoneal.
Determined from the mean of three to six independent experiments with splenocytes or PECs pooled from four to five mice per experimental group.
LD, below the limit of detection of the assay (cannot be reliably extrapolated).
Value extrapolated from the best-fit curve of nonlinear regression.
ND, not determined.
v-Cyclin-CDK interaction is not required for reactivation from latently infected PECs.
To avoid issues associated with diminished acute virus replication, we assessed virus reactivation following the establishment of latency in C57BL/6 mice that were inoculated with 1,000 PFU of wild-type or mutant viruses via intraperitoneal injection. As shown in Fig. 5, there is at most only a modest impact of the v-cyclin mutants on acute virus replication in the spleen following this dose and this route of virus inoculation. Virus reactivation from latency was assessed from splenocytes and PECs at day 42 postinfection (Fig. 8). In the absence of stimulation, we observed only a very low frequency of splenocytes spontaneously reactivating virus (Fig. 8A). However, of interest, the v-cyc[E133V] mutant exhibited the highest frequency of spontaneously reactivating splenocytes, while the v-cyc[STOP] mutant exhibited nearly undetectable levels of virus reactivation. This observation provides further support for the presence of a CDK-independent function(s) of v-cyclin. We have previous demonstrated that splenocyte reactivation can be augmented by treating latently infected splenocytes with anti-CD40 and anti-IgG/IgM (47). Using this stimulation protocol, we did not observe any defect of CDK-binding mutants in reactivation from splenocytes, while v-cyc[STOP] still exhibited a reactivation defect (Fig. 8B).
FIG. 8.
Reactivation from latently infected splenocytes and PECs does not require v-cyclin-CDK interactions following intraperitoneal inoculation. C57BL/6 mice were infected with 1,000 PFU of wild-type (WT) γHV68, v-cyc[STOP], v-cyc[STOPMR], v-cyc[K104E], v-cyc[K104MR], v-cyc[E133V], or v-cyc[E133MR] via intraperitoneal (IP) injection. (A and B) Forty-two days postinfection, splenocytes (SPL) were harvested and subjected to twofold limiting dilutions on MEF indicator monolayers for determinations of the ex vivo frequency of cells reactivating from latency in the absence (A) or presence (B) of anti-IgG/IgM and anti-CD40 stimulation. Mechanically disrupted cells were plated in parallel, and preformed infectious virus was undetected in all cases (data not shown). C57BL/6 mice were infected with 1,000 PFU of wild-type γHV68 (closed diamonds), v-cyc[STOP] (closed squares), v-cyc[K104E] (closed circles), and v-cyc[E133V] (closed triangles) via intraperitoneal injection. Data represent three to four independent experiments, with each experiment containing cells pooled from four to five mice. (C) Forty-two days postinfection, PECs were harvested and subjected to twofold limiting dilutions on MEF indicator monolayers for determinations of the ex vivo frequency of cells reactivating from latency. Mechanically disrupted cells were plated in parallel, and preformed infectious virus was undetected in all cases (data not shown). Curve fit lines were derived by nonlinear regression analysis, and symbols represent means and standard errors of the means (error bars) of data from individual experiments as indicated. The dashed line (63%) represents the value used to calculate the frequency of reactivating cells as indicated by a Poisson distribution. Data represent independent experiments (wild-type γHV68, n = 6; v-cyc[STOP], n = 4; v-cyc[K104E], n = 6; v-cyc[E133V], n = 4), with each experiment containing cells pooled from four to five mice. CPE, cytopathic effect.
Analysis of the reactivation of PECs 42 days postinfection revealed a defect in the reactivation of v-cyc[STOP] (∼1 in 120,000) compared to wild-type γHV68 (∼1 in 9,500), consistent with previous findings (25, 70). However, there was little or no effect on the frequency of PECs reactivating virus from mice infected with either CDK-binding-deficient mutant viruses (v-cyc[K104E], ∼1 in 14,700; v-cyc[E133V], ∼1 in 13,800). Taken together, these data suggest that while v-cyclin expression plays a critical role in virus reactivation, the ability of v-cyclin to bind and activate CDKs is largely dispensable for efficient reactivation from latency.
DISCUSSION
All known gamma-2 herpesviruses encode a v-cyclin that possesses an expanded repertoire of CDK binding compared to cellular homologues (34, 43, 64, 71). We and others previously demonstrated that the v-cyclin of γHV68 is required for efficient reactivation from latency (25, 70), is required for the maintenance of latency in the absence of B cells (69), and serves as a potent oncogene in transgenic mice (68). In this report, we demonstrate that the CDK-dependent functions of the γHV68 v-cyclin are critical for acute replication in the lungs of infected mice following intranasal inoculation (Fig. 4) but are largely dispensable for reactivation from latency (Fig. 7 and 8).
It is interesting that the K104E mutation generated here is analogous to the mutations previously used in numerous studies of cellular and viral cyclins (15, 20, 24, 35, 36, 52, 66). Generally, these mutants are utilized as loss-of-function mutants or to elucidate CDK-independent functions. However, none of these mutants, including the γHV68 v-cyclin, have been studied in the context of an ongoing viral infection, when the protein synthesis, processing, and degradation machinery of the cell is likely usurped by the virus. The K104E mutation comprises a lysine-to-glutamate substitution at a highly conserved residue within the cyclin box, the region of cyclins critical for CDK engagement (37). Structural determination of the γHV68 v-cyclin (9) indicates that this residue participates in protein-protein interactions between v-cyclin and CDK2 by forming several hydrogen bonds between the two proteins but that it also forms an intramolecular salt bridge with the glutamate at amino acid 133 (E133) within the CDK-interacting surface of the protein. Although both K104E and E133V mutations disrupt the salt bridge between residues 104 and 133, our analyses demonstrate that a substitution of a negatively charged residue for K104 is more deleterious to the stability of the protein during infection than the substitution of a valine for E133 (Fig. 2B). Notably, infection of contact-inhibited, growth-arrested fibroblasts with v-cyc[K104E] or v-cyc[E133V] demonstrated a failure to activate CDK2 kinase activity above background levels (Fig. 2C), consistent with our previous demonstration that these mutant v-cyclins fail to bind either CDK2 or CDC2/CDK1 (66).
CDK-dependent v-cyclin function during acute virus replication.
We show here that efficient acute replication in the lungs requires CDK-dependent functions of the γHV68 v-cyclin following low-dose intranasal inoculation (Fig. 4). Previous analyses of v-cyclin-deficient viruses yielded conflicting results with respect to the role of v-cyclin in acute virus replication. Hoge and colleagues (25) previously concluded that v-cyclin is required for acute replication in the spleen following intraperitoneal inoculation and is necessary for efficient replication in multiple tissues, with the latter being based on a competition assay with wild-type γHV68 in severe combined immunodeficiency (SCID) mice. However, we have previously shown that acute replication of v-cyclin-deficient virus is comparable to that of wild-type virus in liver, spleen, and lungs following high-dose (1 × 106 PFU) intraperitoneal inoculation of immunocompetent mice (70). In addition, the latter studies also demonstrated that the kinetics of lethality, at multiple doses, in SCID mice were identical for v-cyc[STOP] and wild-type virus following intraperitoneal inoculation (70). Based on our recent results, we think it is likely that the absence of a replication defect of v-cyclin null virus in our previous studies was the result of employing a high inoculating dose of virus, which masked a replication defect. Indeed, we also failed to observe a replication defect in the lungs following intranasal infection or spleens following intraperitoneal infection with v-cyc[STOP] or v-cyc[K104E] at high doses (1 × 106 PFU) (data not shown). However, this makes reconciling the findings of Hoge et al. (25) problematic, since those studies also used a high inoculating dose of virus (1 × 106 PFU).
Several experimental differences between the studies of Hoge et al. (25) and those of van Dyk et al. (70) may have contributed to the reported discrepancy with regard to the role of v-cyclin in acute virus replication. First, the studies of Hoge et al. (25) were carried using 4- to 6-week-old BALB/c mice, while the studies of van Dyk et al. (70) were carried out using 7- to 10-week-old C57BL/6 mice. Thus, both mouse age and strain may impact acute γHV68 replication. Second, the mutation strategies used by Hoge et al. (25) and van Dyk et al. (70) to ablate v-cyclin expression differed in important ways. Hoge and colleagues (25) inserted a β-galactosidase expression cassette between the EcoRI sites within and downstream of the v-cyclin open reading frame, an insertion/deletion that not only disrupted the v-cyclin ORF but also deleted 208 bp downstream of the v-cyclin ORF and placed the expression cassette in the opposite orientation of the v-cyclin gene. The latter gives rise to transcripts that are antisense to the adjacent ORF73 (encoding the LANA homologue of γHV68). This is notable since we have recently reported the existence of ORF72 (v-cyclin) and ORF73 (LANA homologue) transcripts that are generated from a shared promoter distal to both ORFs (4). As such, the mutation generated previously by Hoge et al. (25) may alter the polyadenylation of the ORF73 (LANA) transcript, interfere with transcription of ORF73 via an antisense mechanism, and/or affect the splicing of transcripts generated in this region of the viral genome. Finally, the insertion/deletion introduced by Hoge et al. (25) may have impacted the function of the adjacent lytic origin of replication (OriLyt). The region of the γHV68 genome from bp 100723 to 101974 has been defined as containing the minimal cis elements required for OriLyt-directed plasmid replication, although near-maximal levels of replication were achieved by the inclusion of sequences from bp 100723 to 103120 (17). While the insertion engineered by Hoge and colleagues does not disrupt the minimal sequence, use of the EcoRI site at bp 102216 places the cassette within 250 bp of the minimal origin and well within the sequences required for maximal replication. In summary, the deletion introduced by Hoge and colleagues ablates the v-cyclin poly(A) site and sequences proximal to OriLyt and as such may have phenotypic consequences beyond that of deleting the v-cyclin gene. The v-cyclin mutant used by van Dyk et al. (70) to analyze acute virus replication also contained an inserted β-galactosidase expression cassette. However, in contrast to the v-cyclin mutant described previously by Hoge et al. (25), this insertion was completely contained within the v-cyclin gene, and the expression cassette was oriented in the same direction as the v-cyclin gene.
Notwithstanding the differences in the role of v-cyclin in acute virus replication following high-dose intraperitoneal inoculation discussed above, our current analyses of v-cyc[STOP], v-cyc[K104E], and v-cyc[E133V] viruses described here, utilizing a lower inoculating dose (1,000 PFU) and alternate route of administration (intranasal inoculation), conclusively demonstrate that a CDK-dependent mechanism(s) of v-cyclin is required for efficient acute virus replication in the lungs of mice (Fig. 4). However, this requirement for v-cyclin function was not required for acute virus replication in the spleens of mice infected at the same dose administered via intraperitoneal inoculation (Fig. 5).
The v-cyclin mutant viruses are not the only γHV68 mutants that replicate normally in tissue culture, but they exhibit altered replication in vivo under some experimental conditions. A significant difference in γHV68 growth in vitro and in vivo has been previously reported for a γHV68 mutant lacking ORF21, the thymidine kinase gene (13). While there is no indication that thymidine kinase and v-cyclin participate in a shared signaling pathway, they are both postulated to be necessary for viral replication in quiescent, nondividing cells. Both are defective for replication in the lungs of infected mice following intranasal inoculation but exhibit normal growth in vitro, even when infecting quiescent fibroblasts or late-passage MEFs. This result is strikingly different from those obtained for infections performed with recombinant viruses defective for essential γHV68 genes (e.g., ORF50 [45, 50], ORF31 [19, 30], and ORF45 [28, 29]), which display severe growth attenuation in vitro and in vivo and require trans complementation for lytic growth. Thus, genes carried by γHV68 that manipulate cellular processes benefiting the virus may often be required only in specific cell types and/or during specific stages of infection in vivo.
A further complication in attempting to recapitulate cellular conditions in vitro for monitoring virus growth in quiescent cells has been brought into focus by recent studies on the nature of cellular quiescence, which indicate that analyses of virus replication in growth-arrested fibroblast and tumor cell lines may be naïve. By analyzing the transcriptional profile of fibroblasts forced into quiescence by three different methods, Coller and colleagues (14) previously demonstrated a minimal genetic program of quiescence comprised of a subset of genes similarly up- or down-regulated by each treatment. A major feature of this program is the enforced nondividing state of the cells, which ensures both the reversibility of cell cycle arrest and, perhaps more importantly, the suppression of terminal differentiation. To date, we have repeatedly failed to recapitulate the replication defect observed in vivo in growth arrested fibroblast and epithelial cell lines, indicating a disconnect between viral replication in the lungs of infected mice and in cells arrested in culture. Indeed, as shown here, there was no defect in the growth of γHV68 v-cyclin mutants in quiescent murine lung epithelial cells in vitro (Fig. 3C and 4). A major difference my lie in the differentiation state of these cells and the role that differentiation my play in dictating/determining the cellular requirements for viral replication. As discussed previously by Coleman and colleagues (13), infection via intranasal inoculation exposes γHV68 to superficial surfaces of the lung only and, thus, the most differentiated epithelial layer, where the most stringent genetic and biochemical requirements for viral replication are likely necessary. This hypothesis is supported by evidence that high-dose inoculation with v-cyclin-deficient/defective viruses reveals no defect in lung replication, conditions which may lead to more infected cells as well as a broader spectrum of cell types infected. Furthermore, LA-4 lung epithelial cells, a tumor cell line isolated from a urethane-induced lung adenoma, even when subjected to contact inhibition and serum starvation, two potent inducers of quiescence, are capable of reentering the cell cycle, since their transformation likely blocks terminal differentiation. This capacity to reenter the cell cycle may allow the replication of v-cyclin-deficient/defective viruses in these cells in vitro by complementation with cellular cyclins, whereas these viruses are unable to efficiently replicate in healthy, noncycling, terminally differentiated lung epithelial cells in vivo, where it is likely that there is a complete absence of a CDK-dependent v-cyclin function(s) required to prepare a cellular environment for viral replication. It has been demonstrated that human cytomegalovirus permissiveness in some cell types is dependent on terminal differentiation, and in differentiated cells, the virus is able to drive cell cycle progression from G0 by the manipulation of cell cycle regulators in an immediate-early 1 (IE-1)-dependent manner (56, 57, 61). Taken together, these data suggest that herpesviruses have developed strategies for detecting differences or changes in the cellular differentiation state and likely have differential genetic requirements for replication in, maintenance of, or reactivation from latency in cells of various differentiation states. Thus, we speculate that the requirement for CDK-dependent v-cyclin functions during acute in vivo replication may be dependent on the differentiation status, in addition to the type, of infected cells. Further work will be necessary to determine the role of differentiation in the genetic requirements for γHV68 replication.
CDK-independent function(s) of v-cyclin in virus reactivation from latency.
Analysis of the early establishment of splenic latency was complicated by the presence of relatively high levels of preformed infectious virus at day 16 after low-dose intranasal infection. In mechanically disrupted samples, wild-type and marker rescue viruses showed significantly higher levels of preformed infectious virus than v-cyc[STOP], while v-cyc[K104E] and v-cyc[E133V] displayed intermediate phenotypes (Fig. 7). Since this is not likely due to a defect in viral replication in the spleen (Fig. 5), and the phenotype does not segregate with CDK binding and activation, it is possible that analysis at this time point reveals a transitional phase in splenic infection when wild-type and marker rescue viruses are achieving peak infection and when v-cyclin-deficient viruses are initiating only splenic infection due to diminished acute replication in the lungs. An alternative explanation is that preformed infectious virus at this time arose from recently reactivated virus and contributes to the amplification of latency in the spleen prior to full immune control of the infection. Reactivation from intact samples (Fig. 6) shows that despite detectable levels of preformed virus (which do not appreciably influence reactivation numbers) (see Results), v-cyc[E133V] reactivates similarly to its marker rescue virus, while v-cyc[K104E] demonstrated the same defect in reactivation from latency seen with v-cyc[STOP]. This result was striking for two reasons: (i) v-cyc[E133V] yielded a different result than v-cyc[K104E], and (ii) v-cyc[E133V] reactivation was roughly comparable to that observed with wild-type and marker rescue virus controls. With respect to the first point, it seems likely that the observed differences in reactivation frequency between v-cyc[K104E] and v-cyc[E133V] are due largely to the instability of the v-cyc[K104E] mutant, as demonstrated in Fig. 3. Indeed, in this analysis, the v-cyc[K104E] mutant appears to behave like the v-cyclin null virus mutant (v-cyc[STOP]). However, analysis of PEC latency following intraperitoneal inoculation demonstrated that the v-cyc[K104E] mutant is functional in some settings. With respect to the second point, the ability of the v-cyc[E133V] mutant to reactivate from latency at levels comparable to those of the wild-type and marker rescue viruses provides evidence of a CDK-independent function(s) of the v-cyclin playing a role in virus reactivation from splenocytes. Further support for a CDK-independent function(s) of the v-cyclin in viral reactivation came from the analysis of reactivation from latently infected PECs at day 42 postinfection following low-dose intraperitoneal inoculation (Fig. 8C). In this case, both the cyc[K104E] and v-cyc[E133V] mutants were nearly indistinguishable from wild-type virus.
The finding that the regulation of virus reactivation is dependent largely on a CDK-independent function of the γHV68 v-cyclin provides new insight into the function of v-cyclins and the mechanisms required for reactivation. Previously, we and others showed that stimulating proliferation of infected splenocytes postexplant with lipopolysaccharide (25) or anti-CD40-IgG/IgM (47) does not fully complement the reactivation defect of v-cyclin-deficient viruses, suggesting that cell cycle induction is insufficient to overcome the defect in virus reactivation observed with v-cyclin null virus. Here, we demonstrate that the regulation of reactivation by v-cyclin is a CDK-independent event, as mutant v-cyclin viruses defective for CDK binding (and presumably cell cycle activation) remain reactivation competent at levels similar to those of wild-type- or marker rescue-infected PECs and splenocytes (Fig. 7 and 8). These results strongly support the existence of a CDK-independent function(s) of v-cyclin, which plays a critical role in virus reactivation from latency.
Based on a growing body of evidence, significant precedence for such a role is clear, as cellular D-, E-, and A-type cyclins have been linked to transcriptional regulation (6, 15, 20). Of particular note, cyclin D1 has been shown to act as a transcriptional repressor of Myb-like proteins (22, 26), p300 (21), MyoD (58, 59), DMP1 (27), STAT3 (7, 8), SP1 (53), thyroid (39) and androgen (33) receptors, and peroxisome proliferator-activated receptor gamma (75). It has also been implicated as an activator of the estrogen receptor (48, 80) and a cofactor for P/CAF, NcoA, AIB-1, GRIP-1, histone deacetylase 3, and TAF(II)250 (reviewed in reference 15). Generally, these functions are independent of CDKs, as shown by use of a cyclin D1 K114E mutant (analogous to the v-cyclin K104E mutation), kinase-defective CDKs, or cellular CDK inhibitors. Cyclin D3 is implicated in the modulation of hATF5 in a CDK-independent manner (40) and the vitamin D receptor in a CDK-competitive manner (31). With respect to viral cyclin functions, the KSHV v-cyclin directly represses STAT3 transcriptional activity to overcome growth inhibition caused by oncostatin-M in transiently and stably transfected cells (16, 41). In addition, we have preliminary data indicating that the γHV68 v-cyclin can modulate the activation of reporter constructs that are responsive to specific cellular and viral transcription factors in a CDK-independent manner (our unpublished observations). Finally, a recent study has shown that knock-in mice harboring a cyclin D1 mutant defective in activating CDK4 and CDK6 kinase activity still retain functions necessary for several cyclin D1-dependent compartments, including mammary gland development (36). However, these mice are resistant to transformation by exogenous oncogenes, as are cyclin D1-deficient mice (36). The latter analyses provide compelling evidence for the existence of critical CDK-independent functions of at least some cellular cyclins.
In summary, there appear to be strong parallels between the function of γHV68 v-cyclin and both cellular cyclin D1 and KSHV v-cyclin. Further analyses are necessary to characterize the CDK-independent functions of this v-cyclin and the role that they play in virus reactivation.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111-121. [DOI] [PubMed] [Google Scholar]
- 2.Adler, H., M. Messerle, and U. H. Koszinowski. 2001. Virus reconstituted from infectious bacterial artificial chromosome (BAC)-cloned murine gammaherpesvirus 68 acquires wild-type properties in vivo only after excision of BAC vector sequences. J. Virol. 75:5692-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler, H., M. Messerle, M. Wagner, and U. H. Koszinowski. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74:6964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, R. D., III, S. Dickerson, and S. H. Speck. 2006. Identification of spliced gammaherpesvirus 68 LANA and v-cyclin transcripts and analysis of their expression in vivo during latent infection. J. Virol. 80:2055-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arellano, M., and S. Moreno. 1997. Regulation of CDK/cyclin complexes during the cell cycle. Int. J. Biochem. Cell Biol. 29:559-573. [DOI] [PubMed] [Google Scholar]
- 6.Bernards, R. 1999. CDK-independent activities of D type cyclins. Biochim. Biophys. Acta 1424:M17-M22. [DOI] [PubMed] [Google Scholar]
- 7.Bienvenu, F., B. Barre, S. Giraud, S. Avril, and O. Coqueret. 2005. Transcriptional regulation by a DNA-associated form of cyclin D1. Mol. Biol. Cell 16:1850-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienvenu, F., H. Gascan, and O. Coqueret. 2001. Cyclin D1 represses STAT3 activation through a CDK4-independent mechanism. J. Biol. Chem. 276:16840-16847. [DOI] [PubMed] [Google Scholar]
- 9.Card, G. L., P. Knowles, H. Laman, N. Jones, and N. Q. McDonald. 2000. Crystal structure of a gamma-herpesvirus cyclin-CDK complex. EMBO J. 19:2877-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y., P. S. Moore, S. J. Talbot, C. H. Boshoff, T. Zarkowska, K. Godden, H. Paterson, R. A. Weiss, and S. Mittnacht. 1996. Cyclin encoded by KS herpesvirus. Nature 382:410. [DOI] [PubMed] [Google Scholar]
- 11.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 12.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman, H. M., B. de Lima, V. Morton, and P. G. Stevenson. 2003. Murine gammaherpesvirus 68 lacking thymidine kinase shows severe attenuation of lytic cycle replication in vivo but still establishes latency. J. Virol. 77:2410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coller, H. A., L. Sang, and J. M. Roberts. 2006. A new description of cellular quiescence. PLoS Biol. 4:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coqueret, O. 2002. Linking cyclins to transcriptional control. Gene 299:35-55. [DOI] [PubMed] [Google Scholar]
- 16.Coqueret, O. 2003. New targets for viral cyclins. Cell Cycle 2:293-295. [PubMed] [Google Scholar]
- 17.Deng, H., J. T. Chu, N. H. Park, and R. Sun. 2004. Identification of cis sequences required for lytic DNA replication and packaging of murine gammaherpesvirus 68. J. Virol. 78:9123-9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, A. G., N. J. Moorman, D. O. Willer, and S. H. Speck. 2006. The M4 gene of gammaHV68 encodes a secreted glycoprotein and is required for the efficient establishment of splenic latency. Virology 344:520-531. [DOI] [PubMed] [Google Scholar]
- 19.Flano, E., Q. Jia, J. Moore, D. L. Woodland, R. Sun, and M. A. Blackman. 2005. Early establishment of gamma-herpesvirus latency: implications for immune control. J. Immunol. 174:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu, M., C. Wang, Z. Li, T. Sakamaki, and R. G. Pestell. 2004. Cyclin D1: normal and abnormal functions. Endocrinology 145:5439-5447. [DOI] [PubMed] [Google Scholar]
- 21.Fu, M., C. Wang, M. Rao, X. Wu, T. Bouras, X. Zhang, Z. Li, X. Jiao, J. Yang, A. Li, N. D. Perkins, B. Thimmapaya, A. L. Kung, A. Munoz, A. Giordano, M. P. Lisanti, and R. G. Pestell. 2005. Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J. Biol. Chem. 280:29728-29742. [DOI] [PubMed] [Google Scholar]
- 22.Ganter, B., S. Fu, and J. S. Lipsick. 1998. D-type cyclins repress transcriptional activation by the v-Myb but not the c-Myb DNA-binding domain. EMBO J. 17:255-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasper-Smith, N., and K. L. Bost. 2004. Initiation of the host response against murine gammaherpesvirus infection in immunocompetent mice. Viral Immunol. 17:473-480. [DOI] [PubMed] [Google Scholar]
- 24.Hinds, P. W., S. F. Dowdy, E. N. Eaton, A. Arnold, and R. A. Weinberg. 1994. Function of a human cyclin gene as an oncogene. Proc. Natl. Acad. Sci. USA 91:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoge, A. T., S. B. Hendrickson, and W. H. Burns. 2000. Murine gammaherpesvirus 68 cyclin D homologue is required for efficient reactivation from latency. J. Virol. 74:7016-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horstmann, S., S. Ferrari, and K. H. Klempnauer. 2000. Regulation of B-Myb activity by cyclin D1. Oncogene 19:298-306. [DOI] [PubMed] [Google Scholar]
- 27.Inoue, K., and C. J. Sherr. 1998. Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism. Mol. Cell. Biol. 18:1590-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia, Q., V. Chernishof, E. Bortz, I. McHardy, T. T. Wu, H. I. Liao, and R. Sun. 2005. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 79:5129-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia, Q., and R. Sun. 2003. Inhibition of gammaherpesvirus replication by RNA interference. J. Virol. 77:3301-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia, Q., T. T. Wu, H. I. Liao, V. Chernishof, and R. Sun. 2004. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J. Virol. 78:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jian, Y., J. Yan, H. Wang, C. Chen, M. Sun, J. Jiang, J. Lu, Y. Yang, and J. Gu. 2005. Cyclin D3 interacts with vitamin D receptor and regulates its transcription activity. Biochem. Biophys. Res. Commun. 335:739-748. [DOI] [PubMed] [Google Scholar]
- 32.Jung, J. U., M. Stager, and R. C. Desrosiers. 1994. Virus-encoded cyclin. Mol. Cell. Biol. 14:7235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knudsen, K. E., W. K. Cavenee, and K. C. Arden. 1999. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59:2297-2301. [PubMed] [Google Scholar]
- 34.Laman, H., D. J. Mann, and N. C. Jones. 2000. Viral-encoded cyclins. Curr. Opin. Genet. Dev. 10:70-74. [DOI] [PubMed] [Google Scholar]
- 35.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 36.Landis, M. W., B. S. Pawlyk, T. Li, P. Sicinski, and P. W. Hinds. 2006. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9:13-22. [DOI] [PubMed] [Google Scholar]
- 37.Lees, E. M., and E. Harlow. 1993. Sequences within the conserved cyclin box of human cyclin A are sufficient for binding to and activation of cdc2 kinase. Mol. Cell. Biol. 13:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, H. M., L. Zhao, and S. Y. Cheng. 2002. Cyclin D1 is a ligand-independent co-repressor for thyroid hormone receptors. J. Biol. Chem. 277:28733-28741. [DOI] [PubMed] [Google Scholar]
- 40.Liu, W., M. Sun, J. Jiang, X. Shen, Q. Sun, W. Liu, H. Shen, and J. Gu. 2004. Cyclin D3 interacts with human activating transcription factor 5 and potentiates its transcription activity. Biochem. Biophys. Res. Commun. 321:954-960. [DOI] [PubMed] [Google Scholar]
- 41.Lundquist, A., B. Barre, F. Bienvenu, J. Hermann, S. Avril, and O. Coqueret. 2003. Kaposi sarcoma-associated viral cyclin K overrides cell growth inhibition mediated by oncostatin M through STAT3 inhibition. Blood 101:4070-4077. [DOI] [PubMed] [Google Scholar]
- 42.Marques, S., S. Efstathiou, K. G. Smith, M. Haury, and J. P. Simas. 2003. Selective gene expression of latent murine gammaherpesvirus 68 in B lymphocytes. J. Virol. 77:7308-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittnacht, S., and C. Boshoff. 2000. Viral cyclins. Rev. Med. Virol. 10:175-184. [DOI] [PubMed] [Google Scholar]
- 44.Moorman, N. J., D. O. Willer, and S. H. Speck. 2003. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 77:10295-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moser, J. M., M. L. Farrell, L. T. Krug, J. W. Upton, and S. H. Speck. 2006. A gammaherpesvirus 68 gene 50 null mutant establishes long-term latency in the lung but fails to vaccinate against a wild-type virus challenge. J. Virol. 80:1592-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser, J. M., J. W. Upton, R. D. Allen III, C. B. Wilson, and S. H. Speck. 2005. Role of B-cell proliferation in the establishment of gammaherpesvirus latency. J. Virol. 79:9480-9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser, J. M., J. W. Upton, K. S. Gray, and S. H. Speck. 2005. Ex vivo stimulation of B cells latently infected with gammaherpesvirus 68 triggers reactivation from latency. J. Virol. 79:5227-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuman, E., M. H. Ladha, N. Lin, T. M. Upton, S. J. Miller, J. DiRenzo, R. G. Pestell, P. W. Hinds, S. F. Dowdy, M. Brown, and M. E. Ewen. 1997. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of CDK4. Mol. Cell. Biol. 17:5338-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholas, J., K. R. Cameron, and R. W. Honess. 1992. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature 355:362-365. [DOI] [PubMed] [Google Scholar]
- 50.Pavlova, I. V., H. W. Virgin IV, and S. H. Speck. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajcani, J., and M. Kudelova. 2005. Murine herpesvirus pathogenesis: a model for the analysis of molecular mechanisms of human gamma herpesvirus infections. Acta Microbiol. Immunol. Hung. 52:41-71. [DOI] [PubMed] [Google Scholar]
- 52.Rovnak, J., J. W. Casey, and S. L. Quackenbush. 2001. Intracellular targeting of walleye dermal sarcoma virus Orf A (rv-cyclin). Virology 280:31-40. [DOI] [PubMed] [Google Scholar]
- 53.Shao, Z., and P. D. Robbins. 1995. Differential regulation of E2F and Sp1-mediated transcription by G1 cyclins. Oncogene 10:221-228. [PubMed] [Google Scholar]
- 54.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 55.Sherr, C. J. 2000. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 60:3689-3695. [PubMed] [Google Scholar]
- 56.Sinclair, J., J. Baillie, L. Bryant, and R. Caswell. 2000. Human cytomegalovirus mediates cell cycle progression through G(1) into early S phase in terminally differentiated cells. J. Gen. Virol. 81:1553-1565. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair, J., and P. Sissons. 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39:293-301. [DOI] [PubMed] [Google Scholar]
- 58.Skapek, S. X., J. Rhee, P. S. Kim, B. G. Novitch, and A. B. Lassar. 1996. Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol. Cell. Biol. 16:7043-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skapek, S. X., J. Rhee, D. B. Spicer, and A. B. Lassar. 1995. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 267:1022-1024. [DOI] [PubMed] [Google Scholar]
- 60.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 62.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson, P. G., and S. Efstathiou. 2005. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral Immunol. 18:445-456. [DOI] [PubMed] [Google Scholar]
- 64.Swanton, C., and N. Jones. 2001. Strategies in subversion: de-regulation of the mammalian cell cycle by viral gene products. Int. J. Exp. Pathol. 82:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tibbetts, S. A., J. Loh, V. Van Berkel, J. McClellan, M. A. Jacoby, S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2003. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 77:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Upton, J. W., L. F. van Dyk, and S. H. Speck. 2005. Characterization of murine gammaherpesvirus 68 v-cyclin interactions with cellular CDKs. Virology 341:271-283. [DOI] [PubMed] [Google Scholar]
- 67.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 68.van Dyk, L. F., J. L. Hess, J. D. Katz, M. Jacoby, S. H. Speck, and H. Virgin IV. 1999. The murine gammaherpesvirus 68 v-cyclin gene is an oncogene that promotes cell cycle progression in primary lymphocytes. J. Virol. 73:5110-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2003. Maintenance of gammaherpesvirus latency requires viral cyclin in the absence of B lymphocytes. J. Virol. 77:5118-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verschuren, E. W., N. Jones, and G. I. Evan. 2004. The cell cycle and how it is steered by Kaposi's sarcoma-associated herpesvirus cyclin. J. Gen. Virol. 85:1347-1361. [DOI] [PubMed] [Google Scholar]
- 72.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 73.Virgin, H. W., III, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Virgin, H. W., III, R. M. Presti, X. Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, C., N. Pattabiraman, J. N. Zhou, M. Fu, T. Sakamaki, C. Albanese, Z. Li, K. Wu, J. Hulit, P. Neumeister, P. M. Novikoff, M. Brownlee, P. E. Scherer, J. G. Jones, K. D. Whitney, L. A. Donehower, E. L. Harris, T. Rohan, D. C. Johns, and R. G. Pestell. 2003. Cyclin D1 repression of peroxisome proliferator-activated receptor γ expression and transactivation. Mol. Cell. Biol. 23:6159-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. I. Virgin. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willer, D. O., and S. H. Speck. 2003. Long-term latent murine gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J. Virol. 77:8310-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zwijsen, R. M., E. Wientjens, R. Klompmaker, J. van der Sman, R. Bernards, and R. J. Michalides. 1997. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405-415. [DOI] [PubMed] [Google Scholar]