Abstract
Although studies have established that innate and adaptive immune responses are important in controlling West Nile virus (WNV) infection, the function of CD4+ T lymphocytes in modulating viral pathogenesis is less well characterized. Using a mouse model, we examined the role of CD4+ T cells in coordinating protection against WNV infection. A genetic or acquired deficiency of CD4+ T cells resulted in a protracted WNV infection in the central nervous system (CNS) that culminated in uniform lethality by 50 days after infection. Mice surviving past day 10 had high-level persistent WNV infection in the CNS compared to wild-type mice, even 45 days following infection. The absence of CD4+ T-cell help did not affect the kinetics of WNV infection in the spleen and serum, suggesting a role for CD4-independent clearance mechanisms in peripheral tissues. WNV-specific immunoglobulin M (IgM) levels were similar to those of wild-type mice in CD4-deficient mice early during infection but dropped ∼20-fold at day 15 postinfection, whereas IgG levels in CD4-deficient mice were ∼100- to 1,000-fold lower than in wild-type mice throughout the course of infection. WNV-specific CD8+ T-cell activation and trafficking to the CNS were unaffected by the absence of CD4+ T cells at day 9 postinfection but were markedly compromised at day 15. Our experiments suggest that the dominant protective role of CD4+ T cells during primary WNV infection is to provide help for antibody responses and sustain WNV-specific CD8+ T-cell responses in the CNS that enable viral clearance.
West Nile virus (WNV) is a single-stranded, positive-sense, enveloped RNA virus and is a member of the Flaviviridae family. WNV is endemic in Africa, the Middle East, North America, and parts of Europe and cycles enzootically between birds and Culex mosquitoes, with humans, horses, and other animals as dead-end hosts (6, 18, 74). WNV infection in humans is usually asymptomatic or self-limiting, with a mild febrile illness, but may progress to meningitis, encephalitis, paralysis, and death. Severe neuroinvasive disease occurs more frequently in the elderly and immunocompromised, and an intact immune system is required for control of WNV infection (38, 46, 55).
Several groups have established that mice deficient in particular aspects of the immune response have increased tissue viral loads and mortality after WNV infection compared to congenic wild-type mice (9, 43, 54, 59, 71). Alpha interferon (IFN-α), IFN-β, and IFN-γ and γδ T cells have an early antiviral role and control initial WNV infection in peripheral tissues, limiting viremia and dissemination to the central nervous system (CNS) (54, 70). Complement activation protects mice from WNV infection primarily by enhancing antibody and T-cell responses (42, 43). The induction of WNV-specific immunoglobulin M (IgM) coincides with the clearance of WNV from the bloodstream (8, 9), and CD8+ T cells eliminate WNV from infected cells through cytolytic mechanisms, thus preventing viral persistence in peripheral and CNS tissues (16, 31, 59, 60, 70, 71).
Generally, CD4+ T lymphocytes are believed to control viral infection through several mechanisms, including activation and priming of B- and T-cell responses, production of inflammatory and antiviral cytokines, direct cytotoxic effects of infected cells, and promoting memory responses. However, the particular CD4+ T-cell-dependent mechanisms that control individual viruses may differ significantly. While CD4+ T cells prime essential B-cell responses following infection by measles virus, lymphocytic choriomeningitis virus (LCMV), and rotavirus (13, 45, 51, 68), CD4+ T-cell-independent antibody responses are sufficient to control primary murine cytomegalovirus and influenza virus infections (29, 37). Although CD4+ T cells enhance cytotoxic CD8+ T-cell development through cytokine production and maturation of antigen-presenting cells (25, 44, 75), their requirement for establishing and modulating primary virus-specific CD8+ T-cell responses also varies. CD4+ T cells are required to generate efficient primary effector CD8+ T-cell responses against herpes simplex virus type 2 and mouse hepatitis virus (28, 63) but not against LCMV and influenza virus (2, 3, 64). In contrast, it is well established that CD4+ T cells have a critical role in promoting memory CD8+ T-cell responses for many viruses, including LCMV, influenza virus, and human adenovirus (2, 3, 26, 57, 64, 65). Finally, for some viruses, including human immunodeficiency virus, influenza virus, and herpes simplex virus type 1 (10, 49, 66, 73), CD4+ T cells also have direct cytotoxic activity through Fas-Fas ligand- or perforin-dependent pathways.
Some understanding of the function of CD4+ T cells in WNV infection has been suggested by experiments with related flaviviruses (41). An important priming role of CD4+ T cells for memory CD8+ T cells was observed in a yellow fever virus challenge model (39). Similarly, depletion of CD4+ T cells reduced the effectiveness of adoptively transferred peptide-stimulated splenocytes in the control of Japanese encephalitis virus (JEV) infection (47). The role of CD4+ T cells in primary flavivirus infection is less clear, as mice lacking CD4+ T cells showed no change in susceptibility to dengue viruses (58). In vitro studies of CD4+ T-cell effector and proliferative responses have been characterized for flaviviruses including WNV. CD4+ cytotoxic T cells recognize peptides from structural and nonstructural proteins and lyse infected targets or peptide-pulsed cells (1, 15, 19, 76). The direct contribution of CD4+ T cells to WNV control and clearance in vivo remains unexplored. Herein, using mice with genetic and acquired deficiencies of CD4+ T cells, we assessed their function in controlling WNV infection. Our data suggest that CD4+ T cells contribute to protection by sustaining antibody production and effector CD8+ T cells that allow rapid viral clearance.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were cultured as previously described (8). WNV strain 3000.0259 was isolated in New York in 2000 from mosquitoes and was passaged once in C6/36 Aedes albopictus insect cells (11). Virus was diluted to 102 PFU in 50 μl of Hanks balanced salt solution containing 1% heat-inactivated fetal bovine serum for injection into mice.
Mouse experiments.
All of the mice used in these experiments were of the C57BL/6 (H-2KbDb) genetic background. Wild-type and CD4−/− (14) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Congenic major histocompatibility complex (MHC) class II−/− mice (40) were purchased (Taconic, Germantown, NY), and perforin−/−, IFN-γ−/−, or Fas ligand-deficient (gld) mice were obtained as gifts (T. Ley and H. Virgin, Washington University School of Medicine). Mice were genotyped and bred in the animal facility at the Washington University School of Medicine. Mouse experiments were performed in accordance with Washington University Animal Safety guidelines. Eight- to 10-week-old mice were used for all studies and inoculated subcutaneously with WNV by footpad injection after anesthetization with xylazine and ketamine.
Antibody depletion of CD4+ T cells.
CD4+ T cells were depleted from wild-type mice with a rat monoclonal antibody (MAb; YTS 191.1, IgG2b) specific for mouse CD4+ T cells (gift of H. Virgin, Washington University, St. Louis, MO). A hybridoma (SFR33, rat IgG2b) that produced a MAb against human HLA-DR5 molecules was grown in parallel and used as an isotype control. YTS 191.1 and SFR33 were cultivated in serum-free medium in 1-liter bioreactor flasks according to the manufacturer's (Integra Biosciences, Switzerland) directions. The supernatants were harvested, centrifuged, filtered, and quantified by enzyme-linked immunosorbent assay. Anti-CD4 or anti-HLA-DR5 MAbs (500 μg) were transferred to 8- to 10-week-old wild-type mice via the intraperitoneal route every 5 days, starting 3 days before infection. Depletion of CD4+ T cells was confirmed by flow cytometry after staining of peripheral blood mononuclear cells or splenocytes with a fluorescein isothiocyanate (FITC)-conjugated anti-CD4 MAb (Becton Dickinson Biosciences, San Jose, CA) and found to be ≥99%.
Passive antibody transfer experiments.
Immune serum was isolated from WNV-infected convalescent wild-type C57BL/6 mice 4 to 5 weeks after infection, heat inactivated for 30 min at 56°C, and stored at −80°C. Naive serum was also collected and used as a control. One milliliter of immune or naive serum was passively transferred to MHC class II−/− mice by an intraperitoneal route on the same day or 10 days after WNV infection, and survival kinetics were monitored.
Adoptive transfer of CD4+ T cells.
Spleens were isolated from uninfected wild-type mice or WNV-infected wild-type, perforin−/−, IFN-γ−/−, or Fas ligand-deficient mice 9 days after WNV infection. Single-cell suspensions were obtained after disruption of tissue through a 40-μm-pore-size filter. After erythrocyte lysis in ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA), splenocytes were washed into Dulbecco modified Eagle medium containing 5% fetal bovine serum and counted and CD4+ T cells were purified (≥93% purity) by negative selection with magnetic beads (Miltenyi Biotec, Auburn, CA). One day after footpad infection with 102 PFU of WNV, 107 CD4+ T cells in phosphate-buffered saline (PBS) were adoptively transferred to CD4−/− mice via an intraperitoneal route. CD4−/− mice were used as recipients of CD4+ T cells (rather than MHC class II−/− mice) in the adoptive-transfer studies because of a requirement for interaction between CD4 and MHC class II molecules during antigen presentation.
Quantification of viral burden.
For analysis of WNV in tissues of infected mice, organs were recovered after cardiac perfusion with PBS (20 ml) and dissection. Tissues were placed on ice, weighed, and stored at −80°C. To determine viral titers, tissues were homogenized with a bead beater apparatus and titrated for virus by plaque assay on BHK-21 cells as previously described (8). Serum was obtained from whole blood by phlebotomy of the axillary vein immediately prior to euthanasia. Viral RNA was extracted from 50 μl of serum with the QIA-AMP viral RNA extraction kit (QIAGEN, Palo Alto, CA). Primers corresponding to the nucleotide 1160 to 1229 region of the WNV E protein were used to perform real-time reverse transcription-PCR according to a previously described protocol (8, 35).
Measurement of WNV-specific antibodies.
The levels of WNV-specific IgM and IgG were determined with an enzyme-linked immunosorbent assay against purified WNV E protein as previously described (9). Neutralization titers were determined with a flow cytometry assay that measured inhibition of infection with a green fluorescent protein-expressing pseudotyped reporter virus particle (RVP) (53). Serum was serially diluted and incubated with WNV RVPs in duplicate for 1 h at room temperature, and the mixture was added to Raji DC-SIGNR cells and incubated at 37°C for 48 h. Reduction of RVP infectivity was measured as a function of green fluorescent protein fluorescence by flow cytometry. Results were plotted with GraphPad Prism, and the titer of 50% inhibition was determined.
Immunohistochemistry.
Tissue staining was performed according to a previously described protocol (54). Briefly, 20 days after WNV infection, moribund CD4-depleted or wild-type mice were perfused extensively with PBS. Brains were harvested and bisected; half of each brain was homogenized for viral titer determination, and the remainder was fixed in paraformaldehyde, embedded in paraffin, and sectioned. After deparaffinization, tissue sections were stained for WNV with a combination of MAbs (E18, E22, and E31) (50) against the WNV E protein and an antigen-enhancing staining kit (DakoCytomation). After staining, slides were photographed with a light microscope with a digital camera attached (Nikon Eclipse E400).
Intracellular IFN-γ staining.
Intracellular staining for IFN-γ was performed 7 or 15 days after WNV infection on erythrocyte-depleted splenocytes. We added 106 splenocytes (100 μl) to a 96-well round-bottom immunoassay plate (TPP, Switzerland) and stimulated them with 0.1 μg/ml of an immunodominant Db-restricted NS4B peptide (W. Purtha, B. Shrestha, E. Sitati, T. Hansen, and M. Diamond, unpublished data) for 4 h at 37°C with the addition of Golgi plug (1 μl/ml; BD Biosciences). Splenocytes were then cooled to 4°C and incubated with FITC-conjugated CD8 or an isotype FITC-conjugated antibody (Becton Dickinson Biosciences) for 30 min at 4°C. After being washed three times in PBS containing 5% goat serum, splenocytes were fixed with 1% paraformaldehyde in PBS, permeabilized with saponin, and stained with an allophycocyanin (APC)-conjugated anti-IFN-γ antibody or an APC-conjugated isotype control (BD Biosciences) for 30 min at 4°C. After final washing, staining was assessed by flow cytometry and the percentage of CD8+ lymphocytes that expressed IFN-γ was determined with CellQuest software (Becton Dickinson).
Quantification of CNS leukocytes.
Immunostaining of brain leukocytes was performed as previously described (59). Briefly, whole brains were harvested from wild-type and MHC class II−/− mice 9 or 15 days after infection with WNV. Prior to harvest, extensive cardiac perfusion was performed with PBS (30 ml) to deplete intravascular leukocytes. Brains were homogenized through a 70-μm-pore-size filter, and CNS leukocytes were isolated by Percoll gradient centrifugation. After determination of the total number of cells, leukocytes were incubated with FITC-conjugated anti-CD3 antibody and APC-conjugated anti-CD8 or isotype control antibody or FITC-conjugated anti-CD45 antibody (BD Biosciences) at 4°C for 30 min, fixed with 1% paraformaldehyde in PBS, and visualized by flow cytometry. IFN-γ production by CD8+ brain leukocytes was also determined.
Statistical analysis.
All data were analyzed with Prism software (GraphPad Software, San Diego, CA). For survival analysis, Kaplan-Meier curves were analyzed by the log-rank test. For virus burden, antibody titer, IFN-γ, and leukocyte staining experiments, statistical significance was determined with the Mann-Whitney test.
RESULTS
MHC class II−/− and CD4-depleted mice are more susceptible to WNV infection.
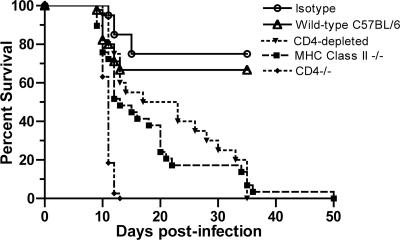
Previous studies with mice have demonstrated that B cells, antibody, and CD8+ T cells were all required to fully protect mice from lethal WNV infection (8, 9, 12, 59, 70, 71). Because of the possible role of CD4+ T cells in priming some of these responses, we examined their function directly. Initial survival analysis was performed with CD4 antibody-depleted wild-type mice. Subsequently, studies were extended to congenic CD4−/− and MHC class II−/− mice. We compared the phenotype of both genetically deficient mice to that of the CD4 antibody-depleted wild-type mice, as each of the knockout models has potential limitations. CD4−/− mice have abnormal development and class restriction of CD8+ T cells (67), and MHC class II−/− mice retain a small number (<1%) of non-MHC class II-restricted CD4+ T cells (21, 40). After subcutaneous infection with 102 PFU of WNV, all mice exhibited signs of morbidity after the first week, including fur ruffling, a hunchback position, and weight loss. Whereas 70% of the wild-type mice survived and recovered fully by 15 days postinfection, all MHC class II−/− and CD4 antibody-depleted mice died by 50 days after infection (P ≤ 0.004) (Fig. 1). Notably, the mean time to death of the CD4-depleted and MHC class II−/− mice was protracted (21 ± 9 days and 18 ± 11 days, respectively, compared to the wild type [11 ± 1 days], P ≤ 0.05). In contrast, CD4−/− mice were more vulnerable to infection, with 100% lethality and a more rapid disease course (mean time to death of 11 ± 1 days). Although there were differences among the models, in all cases an absence of CD4+ T cells led to uncontrolled infection and death. Because of the previously characterized developmental defects in the immune system of the CD4−/− mice, for most of the subsequent virologic and immunologic experiments we used the two models (CD4 antibody-depleted wild-type and MHC class II−/− mice) that yielded similar phenotypes.
FIG. 1.
Survival of CD4-deficient mice after WNV infection. Wild-type (n = 30), isotype control antibody-treated (n = 20), MHC class II−/− (n = 29), CD4 antibody-depleted (n = 20), and CD4−/− (n = 37) C57BL/6 mice were infected with 102 PFU of WNV in at least two independent experiments. Significant decreases in survival compared to that of wild-type mice were as follows: MHC class II−/− mice, P = 0.0003; CD4 antibody-depleted mice, P = 0.0004; CD4−/− mice, P < 0.0001.
Persistent WNV infection in the CNS of MHC class II−/− and CD4-depleted mice.
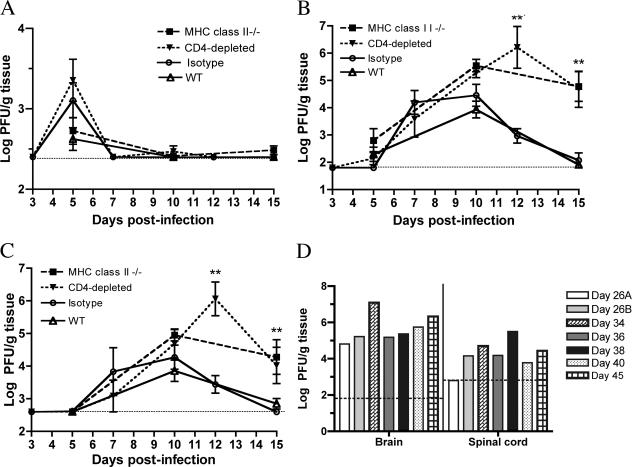
To understand at what stage CD4+ T cells act, the viral-burden kinetics in the periphery and CNS were examined at days 5, 10, and 15 after infection (Fig. 2A to D).
FIG. 2.
WNV burdens in tissues. WNV burdens in the spleens (A), brains (B), and spinal cords (C) of wild-type (WT), isotype control antibody-treated, MHC class II−/−, and CD4 antibody-depleted mice were determined by viral plaque assay of samples from 7 to 10 mice per time point per group at days 5, 10, and 15 after infection. (D) WNV levels in the CNSs of individual MHC class II−/− mice at later times after infection as determined by viral plaque assay. The dotted line indicates the limit of sensitivity of the assay. Asterisks indicate time points at which differences were statistically significant (P ≤ 0.05).
Viremia.
In all wild-type and CD4-deficient mice, WNV levels were below the level of detection by direct plaque assay throughout the time course. However, with a more sensitive quantitative reverse transcription-PCR assay (8), we detected similar levels of WNV RNA in the sera of wild-type, MHC class II−/−, and CD4-depleted mice 5 days after WNV infection (data not shown). By day 10 postinfection, WNV RNA levels in serum had fallen below the detection level in all mice. Thus, the presence of CD4+ T cells was not critical for clearance of WNV from the blood.
Spleen.
The pattern of WNV infection in the spleens of MHC class II−/− and CD4-depleted mice also was largely similar to that in wild-type mice or isotype antibody-depleted mice, respectively, as no statistically significant differences in peak titers were observed (P ≥ 0.5). In all cases, WNV was cleared from the spleen by day 10 postinfection (Fig. 2A).
CNS. (i) Brain.
Through day 7, the levels of viral accumulation in the brain were similar in wild-type and CD4-deficient mice. However, by day 10, higher levels of WNV were measured in the brains of MHC class II−/− (105.5 PFU/g) and CD4-depleted (105.3 PFU/g) mice compared to the wild type (103.7 PFU/g) (P ≤ 0.05), and they persisted at day 15, whereas wild-type mice had virtually cleared the infection (MHC class II −/−, 104.8 PFU/g, and CD4-depleted, 104.7 PFU/g, compared to the wild type [102 PFU/g]; P ≤ 0.001) (Fig. 2B).
(ii) Spinal cord.
Similar results were observed in the spinal cord with increased infection in CD4-deficient mice at day 10 and WNV persistence at day 15 (MHC class II−/−, 104.2 PFU/g, and CD4-depleted 104.1 PFU/g, respectively, compared to the wild type [102.8 PFU/g]; P < 0.05) (Fig. 2C). A more extended course of CNS infection in MHC class II−/− mice showed continued viral replication with an average of 105 PFU of WNV in the brain and 104 PFU of WNV in the spinal cord, even 45 days following infection (Fig. 2D). In comparison, infectious WNV was not detected in the CNS of wild-type mice after day 21 (59, 60).
Histopathology in the brain after WNV infection.
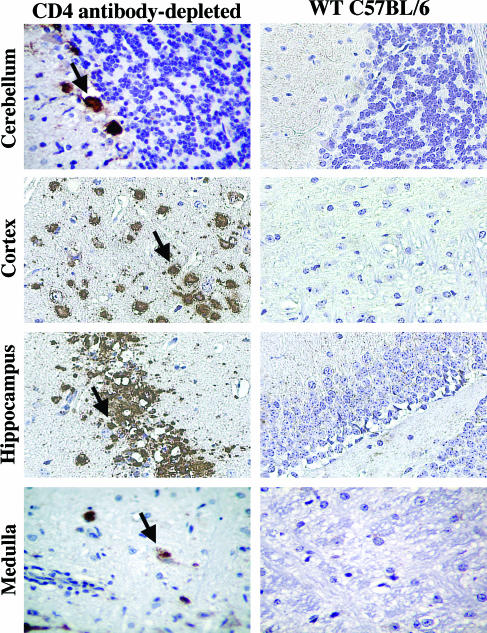
To understand the cellular basis for increased infection and mortality in mice lacking CD4+ T cells, we examined brain tissues for histopathological changes following infection. Brains were harvested from CD4-depleted and wild-type mice 20 days after infection. CD4-depleted mice displayed enhanced WNV antigen staining, particularly in neurons in the cortex and cerebellum, whereas wild-type mice showed little antigen staining at this time (Fig. 3).
FIG. 3.
WNV antigen staining in the brains of wild-type (WT) and CD4-depleted mice. The brains of wild-type and CD4 antibody-depleted mice were harvested 20 days after WNV infection, sectioned, and stained for WNV antigen. Examples of infected cells are indicated by arrows. Representative images from the cerebellum, cortex, hippocampus, and medulla are shown after a review of brains from at least three independent wild-type or CD4 antibody-depleted mice.
Reduced WNV-specific antibody levels in MHC class II−/− and CD4-depleted mice.
Our virologic experiments established that MHC class II−/− and CD4-depleted mice were unable to clear WNV infection from the CNS. Because CD4+ T-cell-dependent responses are critical for B-cell activation, isotype class switching, affinity maturation, and antibody production (23, 44, 48), we evaluated how the absence of CD4+ T cells altered antibody responses to WNV. We measured the WNV-specific IgM and IgG responses at 5, 10, and 15 days following infection.
(i) IgM.
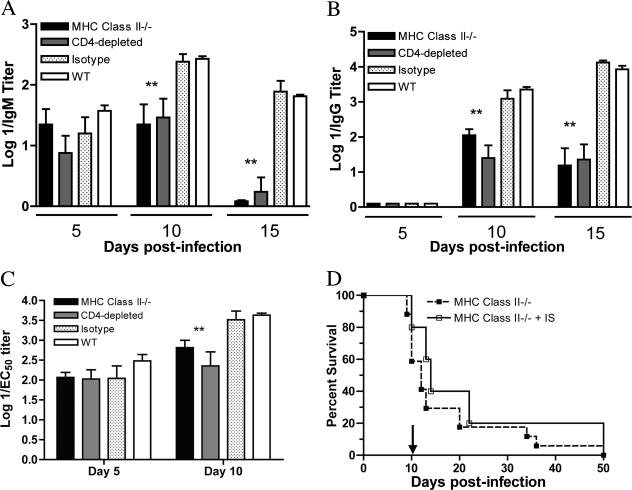
Similar levels of WNV-specific IgM were observed in CD4-depleted and wild-type mice 5 days after WNV infection (P ≥ 0.1). However, in CD4-depleted mice, WNV-specific IgM decreased at day 10 postinfection by four- to fivefold (mean titers: MHC class II−/−, 1/60, and CD4-depleted, 1/60, compared to the wild type [1/280]; P ≤ 0.005) and more precipitously dropped at day 15 (mean titers: MHC class II−/−, <1/10, and CD4-depleted, 1/10, compared to the wild type [1/65]; P ≤ 0.05), perhaps because of consumption of specific IgM and clearance of virus-immune complexes (Fig. 4A).
FIG. 4.
Antibody responses against WNV. Serum samples from wild-type (WT), MHC class II−/−, and CD4 antibody-depleted mice were collected at the indicated time points. The development of WNV-specific IgM (A) or IgG (B) antibodies was determined after incubation of serum with adsorbed control or purified WNV E protein. Neutralizing activity of serum samples (C) from wild-type and CD4-deficient mice on days 5 and 10 after infection was determined by a flow cytometry-based neutralization assay. Asterisks indicate significant differences between wild-type and CD4-deficient mice (P < 0.05). Data are an average of at least three independent experiments performed in duplicate and reflect 5 to 10 mice per group. (D) Passive administration of immune or naive serum from wild-type mice to MHC class II−/− mice (n = 5) 10 days after WNV infection. The arrow indicates the time at which immune or naive serum was administered. The difference in survival was not statistically significant (P > 0.2).
(ii) IgG.
Throughout the time course, WNV-specific IgG production was blunted in MHC class II−/− and CD4-deficient mice (Fig. 4B). Approximately 10- to 30-fold lower levels were observed at day 10 (mean titers: MHC class II−/−, 1/280, and CD4-depleted 1/70, compared to the wild type [1/2,400]; P ≤ 0.05) and 15- to 100-fold lower levels on day 15 (mean titers: MHC class II−/−, 1/64, and CD4-depleted, 1/593, compared to the wild type [1/9,400]; P ≤ 0.05) after WNV infection.
(iii) Neutralizing activity.
To assess the qualitative effects of a deficiency of CD4+ T cells on the WNV-specific antibody response, we examined the neutralizing activity of serum antibodies from wild-type and CD4-deficient mice. Notably, comparable levels of neutralization were observed at day 5 after infection, likely because the neutralizing activity of antibodies in serum at this time reflects the equivalent T-cell-dependent IgM response (Fig. 4C and reference 9). However, by day 10, four- to eightfold lower levels of neutralization (mean titer of 50% inhibition: MHC class II−/−, 1/1,000, and CD4-depleted, 1/450, compared to the wild type [1/4,100]; P ≤ 0.005) were observed in serum antibodies from CD4-deficient mice.
Passive transfer of immune antibody to MHC class II−/− mice.
Previous studies demonstrated that passive transfer of WNV immune serum prior to WNV infection protected B-cell-deficient and RAG B- and T-cell-deficient mice for several weeks (8, 9). Analogously, passive transfer of immune serum on the day of infection completely protected MHC class II−/− mice from death (100% survival, 10/10 mice) several weeks after infection (data not shown). Because a significant percentage (>75%) of the mortality in the CD4-depleted and MHC class II−/− mice occurred late, after day 10, and correlated with elevated CNS viral burdens, we assessed whether this phenotype was due, in part, to inadequate antibody responses. To test this, we passively transferred 1 ml of immune serum to MHC class II−/− mice at day 10 after infection and evaluated them for an effect on survival. Notably, passive transfer of high-titer immune serum (WNV-specific IgG titer = 1/15,000; 50% plaque reduction neutralization test titer = 1/1,400) at day 10 after infection failed to alter the survival pattern of MHC class II−/− mice (P > 0.1) (Fig. 4D). Thus, immune antibody against WNV was sufficient to prevent but not clear CNS infections in MHC class II−/− mice.
CD8+ T-cell responses.
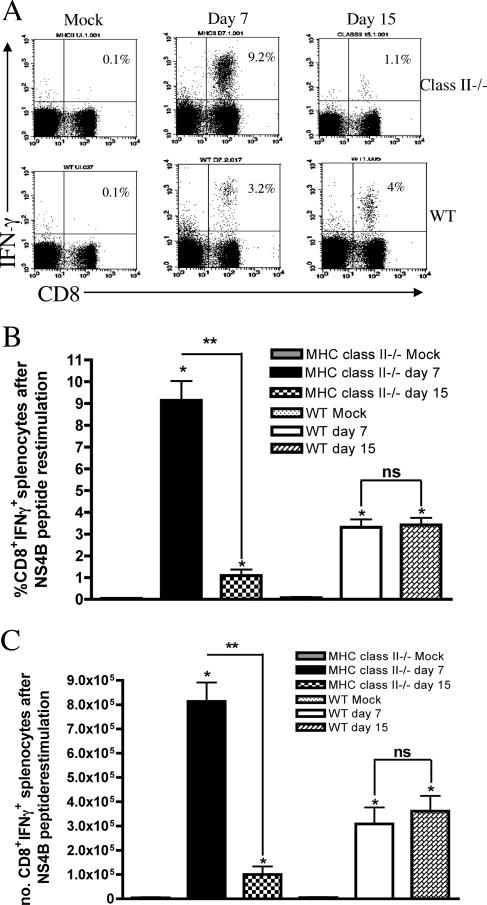
CD8+ T cells are required for clearance of WNV from the CNS (59, 60, 71). The role of CD4+ T-cell responses in activating CD8+ T cells during primary virus infection remains controversial and may be virus specific (3, 26, 28, 63, 65, 69). To determine how CD4+ T cells affected the activation of WNV-specific CD8+ T cells, we examined IFN-γ production after ex vivo stimulation of splenocytes with an immunodominant Db-restricted WNV peptide against the NS4B protein. We observed a significant increase in the levels of IFN-γ production by CD8+ T cells in splenocytes from both wild-type and MHC class II −/− mice at day 7 after infection (3- and 10-fold, respectively, compared to mock-infected mice; P ≤ 0.01), suggesting a lack of requirement for CD4+ T cells in the initial priming of WNV-specific CD8+ T cells (Fig. 5A to C). This result is consistent with our virologic results, which showed relatively equivalent kinetics of clearance from the spleen; previous studies have established a requirement of CD8+ T cells for clearance from this organ (59, 60). Because prior studies had also demonstrated viral persistence in mice deficient in CD8+ T cells, we assessed how a CD4+ T-cell deficiency affected the numbers of WNV-specific CD8+ T cells in the spleen at a later time point. In contrast to day 7, at day 15 the number of WNV-specific CD8+ T cells that produced IFN-γ decreased by 10-fold in MHC class II−/− mice (1% versus 10% at day 7; P < 0.005) while IFN-γ production remained constant in wild-type CD8+ T cells (P > 0.3) (Fig. 5).
FIG. 5.
CD8+ T-cell activation after WNV infection. Mock- or WNV-infected splenocytes from wild-type (WT) and MHC class II−/− mice were harvested and stimulated ex vivo with a Db-restricted NS4B peptide. Cells were stained with antibodies against CD8 and IFN-γ and analyzed by flow cytometry on days 7 and 15. (A) Representative flow cytometry profiles showing intracellular IFN-γ staining of splenic CD8+ T cells after WNV infection in wild-type or MHC class II−/− mice. The percentage of CD8+ IFN-γ+ cells divided by the total number of CD8+ T cells is indicated in the top right corner. Percentages (B) and total numbers (C) of CD8+ IFN-γ+ splenocytes are also shown. Data are an average of at least three independent experiments and reflect 5 to 10 mice per group. Asterisks indicate statistically significant differences from mock-infected mice (*, P < 0.05) or between days 7 and 15 (**, P < 0.005).
CD8+ T-cell trafficking to the brain.
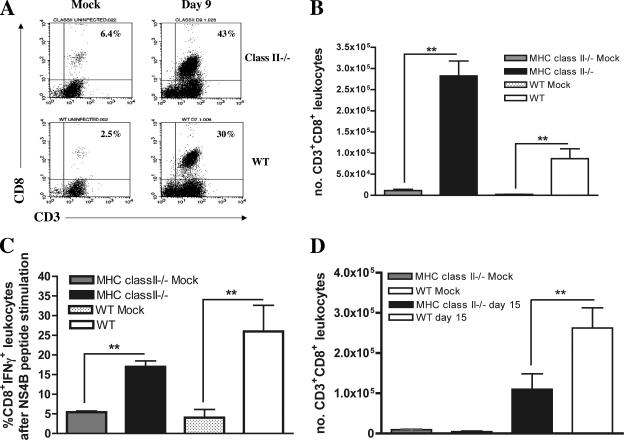
An absence of CD8+ T cells results in viral persistence in the CNS of WNV-infected mice (59, 60, 71). Although no defect in early splenic activation of CD8+ T cells was observed, we speculated that the increased and sustained WNV levels in the brains of CD4-deficient mice could be due to impaired recruitment of activated CD8+ T cells to the brain. CD4+ T-cell help modulates chemokine receptor expression (4, 7), including CXCR3 and CCR5, two receptors that are essential for leukocyte trafficking to the CNS after WNV infection (17, 31). To address this, we compared the levels of CD8+ cells in the brains of MHC class II−/− and wild-type mice after infection with WNV (Fig. 6A to D). Nine days following infection, brain leukocytes were isolated and the number of CD8+ and CD45+ leukocytes was measured by flow cytometry. Although similar numbers of CD45+ leukocytes were observed in the brains of MHC class II−/− and wild-type mice (data not shown), a significantly higher number (MHC class II −/−, 2.8 × 105; wild type, 9.5 × 104; P < 0.005) of CD3+ CD8+ T cells was present in the MHC class II −/− mice (Fig. 6A and B), perhaps because these mice have a higher baseline level of CD8+ T cells in the spleen because of the absence of CD4 (20). To determine if the CD8+ T cells were WNV specific, we measured the IFN-γ production of brain CD8+ T cells after ex vivo restimulation of leukocytes with a Db-restricted NS4B peptide. No significant difference was observed in the number or percentage of WNV-specific CD8+ T cells, as ∼15 to 20% of the brain CD8+ leukocytes from MHC class II−/− and wild-type mice produced IFN-γ (Fig. 6C, P > 0.5). This suggests that the inefficient clearance of WNV from the CNS in CD4-deficient mice was not due to impaired trafficking of antigen-specific CD8+ T cells. However, by day 15 a distinct picture emerged (Fig. 6D); although MHC class II−/− mice had quantities of CD45+ leukocytes in the brain similar to those of wild-type mice (data not shown), they had significantly fewer CD3+ CD8+ T cells in the brain (1 × 105 compared to 2.5 ×105 in wild-type mice; P < 0.05). Taken together, these experiments suggest that CD4+ T cells sustain the numbers of antigen-specific CD8+ T cells in the CNS.
FIG. 6.
Infiltrating brain leukocytes. Brains were harvested from WNV-infected wild-type (WT) and MHC class II−/− mice on days 9 and 15. Leukocytes were isolated by Percoll gradient centrifugation and double stained for CD3 and CD8. (A) Representative flow cytometry profiles showing CD3+ CD8+ T cells in the brain on day 9 after WNV infection in wild-type and MHC class II−/− mice. (B) Total number of CD3+ CD8+ leukocytes on day 9 postinfection. (C) Percent CD8+ IFN-γ+ staining of brain CD8+ leukocytes on day 9 after ex vivo restimulation with a Db-restricted WNV NS4B peptide. Significant differences from mock-infected mice are noted by asterisks. (D) CD3+ CD8+ leukocyte numbers in the brain on day 15 postinfection. Significant differences in the number of leukocytes between wild-type and MHC class II−/− mice are noted by asterisks (P < 0.05). Data are an average of at least three independent experiments and reflect five to eight mice per group.
CD4+ T-cell effector function during WNV infection.
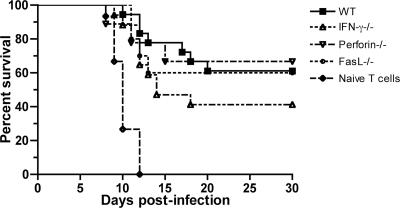
Beyond their role in priming, CD4+ T cells also have direct effector functions and modulate some viral infections through perforin- or Fas ligand-mediated cytolytic mechanisms (10, 15, 73) or through the production of antiviral cytokines, such as IFN-γ (24, 72). To evaluate whether CD4+ effector function contributed to protection against WNV infection, adoptive-transfer studies were performed. To generate WNV-primed CD4+ T cells, wild-type, perforin−/−, IFN-γ−/−, or Fas ligand-deficient C57BL/6 mice were infected with WNV, and at day 9, spleens were harvested and CD4+ T cells were purified by negative selection to greater than 93% purity with antibody-coated magnetic beads (data not shown). These cells were adoptively transferred into congenic CD4−/− mice 1 day after WNV infection, and survival was analyzed. Notably, WNV-primed wild-type CD4+ T cells significantly rescued CD4−/− mice, resulting in a 70% survival rate compared to the 0% survival observed when naive or no CD4+ T cells were transferred (P < 0.0001) (Fig. 7). Nonetheless, in recipient mice, WNV-specific IgM and IgG levels at day 8 were similar to those of CD4−/− mice (mean IgM titer, 1/150 compared to 1/80 [P > 0.2]; mean IgG titer, 1/30 compared to 1/5 [P > 0.2]) yet significantly lower than those of wild-type mice (mean IgM titer, 1/450 [P < 0.01]; mean IgG titer, 1/700 [P < 0.005]); thus, the protection generated by CD4+ T-cell transfer appeared to occur through an antibody-independent mechanism. Interestingly, transfer of WNV-primed CD4+ T cells deficient in perforin, Fas ligand, or IFN-γ had no significant effect on survival rates compared to WNV-primed wild-type CD4+ T cells (P ≥ 0.1) (Fig. 7). Overall, in the adoptive-transfer model, the effector and antibody priming functions of CD4+ T cells did not have a dominant role in protection against WNV infection.
FIG. 7.
Effector CD4+ T-cell responses. CD4+ T cells were purified from WNV-primed wild-type (WT), IFN-γ−/−, perforin−/−, or Fas ligand-deficient mice or uninfected wild-type mice and transferred 24 h after infection into CD4−/− mice (n = 9 to 18 mice for each CD4+ T-cell transfer). Significant changes were noted for mice receiving wild-type primed CD4+ T cells compared to naive CD4+ T cells (P < 0.0001). No significant difference was observed after adoptive transfer of primed wild-type or primed effector molecule-deficient CD4+ T cells (P > 0.1).
DISCUSSION
Because of the possible role of CD4+ T cells in priming protective adaptive immune responses against WNV, we examined their function directly following infection. Using mice with genetic or acquired deficiencies in CD4+ T-cell function, we demonstrate their requirement for survival after WNV infection. An absence of CD4+ T-cell function resulted in persistent WNV levels in the CNS, ultimately leading to uniform mortality. CD4+ T-cell-deficient mice had blunted antibody responses and a failure to sustain T-cell responses in the CNS.
CD4+ T cells and primary flavivirus infection.
Previous experiments established that CD4+ T cells proliferate after exposure to WNV and other flavivirus-infected targets in vitro (1, 19, 33, 34, 41, 52, 76). Our study demonstrates an essential role for CD4+ T cells in limiting pathogenesis in vivo during primary infection. These results are consistent with adoptive-transfer studies showing that depletion of CD4+ T cells from ex vivo peptide-stimulated splenocytes diminished protection after JEV infection (47). In contrast, no increase in lethality was observed in CD4−/− mice after primary infection with yellow fever virus (39) or dengue virus (58). Thus, although the models vary, it appears that even among flaviviruses there is a virus-specific requirement for CD4+ T-cell-mediated protection during primary infection.
CD4+ T cells and the antibody response.
The initial antibody response against WNV, the development of specific IgM by day 5 after infection, was similar in wild-type and CD4-deficient mice. Accordingly, viral clearance from the serum, a process that depends on the production of WNV-specific IgM (9), was unaffected. In contrast, as infection progressed, WNV-specific IgM levels rapidly fell in CD4-deficient mice, compared to those in wild-type mice. We suspect that the fall in WNV-specific IgM in CD4-deficient mice is due to the clearance of virus-IgM immune complexes and the lack of CD4+ T-cell-dependent production of IgM (48). In comparison, WNV-specific IgG levels were blunted throughout the course of infection. These results are consistent with studies that show that CD4+ T cells are required for the efficient production of antiviral IgG (45, 51, 52, 56). Somewhat surprisingly, despite impaired IgM and IgG production, neutralizing activity was retained until relatively late in the time course. In agreement with this, in a mouse model of JEV infection, depletion of CD4+ T cells did not affect the neutralizing activity of serum antibodies at day 5 postinfection (47). These results are also consistent with our recent studies, which show that DIII-specific neutralizing IgG is not detected in mouse serum until day 15 postinfection (T. Oliphant and M. Diamond, unpublished data). Thus, WNV-specific neutralizing IgG may have a more significant role during the memory response to a secondary challenge.
CD4+ T cells and priming of CD8+ T-cell responses.
CD4+ T cells have been shown to prime CD8+ T-cell responses following primary or secondary viral infection (3, 26, 28, 63, 65). In our studies, antigen-specific restimulation of splenic CD8+ T cells at day 7 after infection was unaffected by an absence of CD4+ T cells. Thus, CD4+ T cells are not required to establish the initial antigen-specific CD8+ T-cell response against WNV. These results are consistent with recent studies with LCMV that show direct priming of CD8+ T cells by type I interferon (36). Nonetheless, only 1 week later, restimulation of splenic CD8+ T cells from MHC class II−/− mice was markedly reduced compared to that of cells from wild-type mice. Thus, our data suggest that CD4+ T cells are required to sustain primary CD8+ T-cell responses against WNV.
As CD8+ T cells clear WNV infection in the CNS (59, 71), we hypothesized that the viral persistence observed in CD4-deficient mice could be due to a decrease in CD8+ T-cell trafficking in the brain. CD8+ T cells utilize the chemokine receptors CXCR3 and CCR5 to efficiently cross the blood-brain barrier (16, 31), and CD4+ T cells modulate the expression levels of chemokines (4, 7, 32). Surprisingly, at day 9 after infection, CD8+ T cells migrated into the brains of MHC class II−/− mice at normal levels. However, by day 15, the number of WNV-specific CD8+ T cells had significantly fallen compared to that in wild-type mice, despite sustained levels of infectious virus. Thus, CD4+ T cells may be required not for CD8+ T-cell trafficking but rather to maintain the levels of CD8+ T cells in the CNS. These findings agree with a previous study with mouse hepatitis virus in which CD8+ T cells rapidly underwent apoptosis in CD4-deficient mice (63). Alternatively, CD4+ T cells may provide signals or secrete cytokines to retain or proliferate WNV-specific CD8+ T cells in the CNS (30).
Effector CD4+ T-cell role.
For some viruses, CD4+ T cells control infection in part through their direct cytotoxic activity or by secreting antiviral cytokines (5, 10, 23, 27, 72, 73). Although adoptive transfer of WNV-primed CD4+ T cells significantly improved the survival of CD4−/− mice after WNV infection, no significant decrease in protection was observed when primed CD4+ T cells deficient in IFN-γ, perforin, or Fas ligand expression were transferred. Thus, in contrast to studies with gammaherpesvirus 68, where an important effector role for CD4+ T cells was observed (61, 62), a direct effector role for CD4+ T cells was not sufficient to control WNV infection.
CD4-depleted and MHC class II−/− mice had a unique phenotype after WNV infection, with high-level viral persistence and a protracted, stepwise lethality curve resulting in uniform lethality by 50 days. Although prior studies with CD8−/− and perforin−/− mice demonstrated viral persistence up to 35 days after infection (59), the levels of WNV were ∼100-fold lower and no significantly increased death rate was observed after day 15. On this basis, we suggest that the CD8+ T-cell defects in the brains of CD4-deficient mice cannot completely explain the lethality phenotype. Analogously, administration of neutralizing immune serum at day 10 after infection by itself did not alter the late phase of mortality in MHC class II−/− mice. We suggest that the combined absence of CD4-dependent antibody responses and help for CD8+ T cells results in high-level viral persistence and protracted mortality.
In summary, our experiments demonstrate that CD4+ T cells have a critical function in the control and resolution of primary WNV infection. Our study expands the current knowledge of the in vivo mechanisms of CD4+ T-cell-mediated antiviral immunity during primary infection and the understanding of the immunologic basis of control of WNV infection. Severe neuroinvasive WNV infection in humans is more common in immunosuppressed patients (22, 46, 55). It is intriguing to consider that this increased risk of severe WNV infection in humans is in part due to dysfunctional CD4+ T-cell responses, such as in those of persons with advanced human immunodeficiency virus disease or recipients of ablative chemotherapy. No large-scale prospective clinical studies of WNV infection have been published that directly address this question. As therapies against WNV become available, it will be important to target high-risk populations. We suggest that clinical studies that evaluate the natural history and risk factors of neuroinvasive WNV infection in humans should include a detailed analysis of CD4+ T-cell function.
Acknowledgments
We thank R. Klein for critical review of the manuscript.
This work was supported by NIH grants AI061373 (M.S.D.) and U54 AI057160 (Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research) and the Washington University Markey Scholar Program (E.M.S.).
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Aihara, H., T. Takasaki, T. Matsutani, R. Suzuki, and I. Kurane. 1998. Establishment and characterization of Japanese encephalitis virus-specific, human CD4+ T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J. Virol. 72:8032-8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., L. Hunziker, R. M. Zinkernagel, T. Storni, and M. Kopf. 2004. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur. J. Immunol. 34:317-326. [DOI] [PubMed] [Google Scholar]
- 3.Belz, G. T., D. Wodarz, G. Diaz, M. A. Nowak, and P. C. Doherty. 2002. Compromised influenza virus-specific CD8+-T-cell memory in CD4+-T-cell-deficient mice. J. Virol. 76:12388-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuneu, H., Z. Garcia, and P. Bousso. 2006. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J. Immunol. 177:1406-1410. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. M., E. Roman, and S. L. Swain. 2004. CD4 T cell responses to influenza infection. Semin. Immunol. 16:171-177. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. J. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 7.Castellino, F., A. Y. Huang, G. Altan-Bonnet, S. Stoll, C. Scheinecker, and R. N. Germain. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440:890-895. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, M. S., E. M. Sitati, L. D. Friend, S. Higgs, B. Shrestha, and M. Engle. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198:1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doymaz, M. Z., C. M. Foster, D. Destephano, and B. T. Rouse. 1991. MHC II-restricted, CD4+ cytotoxic T lymphocytes specific for herpes simplex virus-1: implications for the development of herpetic stromal keratitis in mice. Clin. Immunol. Immunopathol. 61:398-409. [DOI] [PubMed] [Google Scholar]
- 11.Ebel, G. D., A. P. Dupuis II, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle, M. J., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr, T., H. Y. Naim, M. F. Bachmann, A. F. Ochsenbein, P. Spielhofer, E. Bucher, H. Hengartner, M. A. Billeter, and R. M. Zinkernagel. 1998. T-cell independent IgM and enduring protective IgG antibodies induced by chimeric measles viruses. Nat. Med. 4:945-948. [DOI] [PubMed] [Google Scholar]
- 14.Fung-Leung, W. P., M. W. Schilham, A. Rahemtulla, T. M. Kundig, M. Vollenweider, J. Potter, W. van Ewijk, and T. W. Mak. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443-449. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon, S. J., F. A. Ennis, and A. L. Rothman. 1999. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 73:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass, W. G., J. K. Lim, R. Cholera, A. G. Pletnev, J. L. Gao, and P. M. Murphy. 2005. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 202:1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass, W. G., D. H. McDermott, J. K. Lim, S. Lekhong, S. F. Yu, W. A. Frank, J. Pape, R. C. Cheshier, and P. M. Murphy. 2006. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granwehr, B. P., K. M. Lillibridge, S. Higgs, P. W. Mason, J. F. Aronson, G. A. Campbell, and A. D. Barrett. 2004. West Nile virus: where are we now? Lancet Infect. Dis. 4:547-556. [DOI] [PubMed] [Google Scholar]
- 19.Green, S., I. Kurane, S. Pincus, E. Paoletti, and F. A. Ennis. 1997. Recognition of dengue virus NS1-NS2a proteins by human CD4+ cytotoxic T lymphocyte clones. Virology 234:383-386. [DOI] [PubMed] [Google Scholar]
- 20.Grusby, M. J., and L. H. Glimcher. 1995. Immune responses in MHC class II-deficient mice. Annu. Rev. Immunol. 13:417-435. [DOI] [PubMed] [Google Scholar]
- 21.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 22.Hayes, E. B., N. Komar, R. S. Nasci, S. P. Montgomery, D. R. O'Leary, and G. L. Campbell. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr. 2001. How the immune system protects the host from infection. Microbes Infect. 3:1167-1171. [DOI] [PubMed] [Google Scholar]
- 24.Janeway, C. A., Jr., S. Carding, B. Jones, J. Murray, P. Portoles, R. Rasmussen, J. Rojo, K. Saizawa, J. West, and K. Bottomly. 1988. CD4+ T cells: specificity and function. Immunol. Rev. 101:39-80. [DOI] [PubMed] [Google Scholar]
- 25.Janeway, C. A., P. Travers, M. Walport, and M. Shlomick. 2001. Immunobiology: the immune system in health and disease, 5th ed. Garland Publishing, New York, N.Y.
- 26.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 27.Jellison, E. R., S. K. Kim, and R. M. Welsh. 2005. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J. Immunol. 174:614-618. [DOI] [PubMed] [Google Scholar]
- 28.Jennings, S. R., R. H. Bonneau, P. M. Smith, R. M. Wolcott, and R. Chervenak. 1991. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell. Immunol. 133:234-252. [DOI] [PubMed] [Google Scholar]
- 29.Karupiah, G., T. E. Sacks, D. M. Klinman, T. N. Fredrickson, J. W. Hartley, J. H. Chen, and H. C. Morse III. 1998. Murine cytomegalovirus infection-induced polyclonal B cell activation is independent of CD4+ T cells and CD40. Virology 240:12-26. [DOI] [PubMed] [Google Scholar]
- 30.Kitchen, S. G., J. K. Whitmire, N. R. Jones, Z. Galic, C. M. Kitchen, R. Ahmed, and J. A. Zack. 2005. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc. Natl. Acad. Sci. USA 102:3794-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knott, P. G., P. R. Gater, P. J. Dunford, M. E. Fuentes, and C. P. Bertrand. 2001. Rapid up-regulation of CXC chemokines in the airways after Ag-specific CD4+ T cell activation. J. Immunol. 166:1233-1240. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni, A. B., A. Mullbacher, and R. V. Blanden. 1991. In vitro T-cell proliferative response to the flavivirus, West Nile. Viral Immunol. 4:73-82. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni, A. B., A. Mullbacher, C. R. Parrish, E. G. Westaway, G. Coia, and R. V. Blanden. 1992. Analysis of murine major histocompatibility complex class II-restricted T-cell responses to the flavivirus Kunjin by using vaccinia virus expression. J. Virol. 66:3583-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 37.Lee, B. O., J. Rangel-Moreno, J. E. Moyron-Quiroz, L. Hartson, M. Makris, F. Sprague, F. E. Lund, and T. D. Randall. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175:5827-5838. [DOI] [PubMed] [Google Scholar]
- 38.Lim, J. K., W. G. Glass, D. H. McDermott, and P. M. Murphy. 2006. CCR5: no longer a ‘good for nothing’ gene—chemokine control of West Nile virus infection. Trends Immunol. 27:308-312. [DOI] [PubMed] [Google Scholar]
- 39.Liu, T., and T. J. Chambers. 2001. Yellow fever virus encephalitis: properties of the brain-associated T-cell response during virus clearance in normal and gamma interferon-deficient mice and requirement for CD4+ lymphocytes. J. Virol. 75:2107-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen, L., N. Labrecque, J. Engberg, A. Dierich, A. Svejgaard, C. Benoist, D. Mathis, and L. Fugger. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA 96:10338-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathews, J. H., J. E. Allan, J. T. Roehrig, J. R. Brubaker, M. F. Uren, and A. R. Hunt. 1991. T-helper cell and associated antibody response to synthetic peptides of the E glycoprotein of Murray Valley encephalitis virus. J. Virol. 65:5141-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehlhop, E., and M. S. Diamond. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 79:7466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchison, N. A. 2004. T-cell-B-cell cooperation. Nat. Rev. Immunol. 4:308-311. [DOI] [PubMed] [Google Scholar]
- 45.Mozdzanowska, K., M. Furchner, D. Zharikova, J. Feng, and W. Gerhard. 2005. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J. Virol. 79:5943-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müllbacher, A., M. Lobigs, and E. Lee. 2003. Immunobiology of mosquito-borne encephalitic flaviviruses. Adv. Virus Res. 60:87-120. [DOI] [PubMed] [Google Scholar]
- 47.Murali-Krishna, K., V. Ravi, and R. Manjunath. 1996. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J. Gen. Virol. 77(Pt. 4):705-714. [DOI] [PubMed] [Google Scholar]
- 48.Noelle, R. J., and E. C. Snow. 1991. T helper cell-dependent B cell activation. FASEB J. 5:2770-2776. [DOI] [PubMed] [Google Scholar]
- 49.Norris, P. J., H. F. Moffett, O. O. Yang, D. E. Kaufmann, M. J. Clark, M. M. Addo, and E. S. Rosenberg. 2004. Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4+ T cells. J. Virol. 78:8844-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliphant, T., M. Engle, G. E. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oxenius, A., K. A. Campbell, C. R. Maliszewski, T. Kishimoto, H. Kikutani, H. Hengartner, R. M. Zinkernagel, and M. F. Bachmann. 1996. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions. J. Exp. Med. 183:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan, C. H., H. W. Chen, H. W. Huang, and M. H. Tao. 2001. Protective mechanisms induced by a Japanese encephalitis virus DNA vaccine: requirement for antibody but not CD8+ cytotoxic T-cell responses. J. Virol. 75:11457-11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierson, T. C., M. D. Sanchez, B. A. Puffer, A. A. Ahmed, B. J. Geiss, L. E. Valentine, L. A. Altamura, M. S. Diamond, and R. W. Doms. 2006. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346:53-65. [DOI] [PubMed] [Google Scholar]
- 54.Samuel, M. A., and M. S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80:9349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangster, M. Y., J. M. Riberdy, M. Gonzalez, D. J. Topham, N. Baumgarth, and P. C. Doherty. 2003. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J. Exp. Med. 198:1011-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 58.Shresta, S., J. L. Kyle, H. M. Snider, M. Basavapatna, P. R. Beatty, and E. Harris. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shrestha, B., and M. S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrestha, B., M. A. Samuel, and M. S. Diamond. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sparks-Thissen, R. L., D. C. Braaten, K. Hildner, T. L. Murphy, K. M. Murphy, and H. W. Virgin IV. 2005. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNγ. Virology 338:201-208. [DOI] [PubMed] [Google Scholar]
- 62.Sparks-Thissen, R. L., D. C. Braaten, S. Kreher, S. H. Speck, and H. W. Virgin IV. 2004. An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J. Virol. 78:6827-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stohlman, S. A., C. C. Bergmann, M. T. Lin, D. J. Cua, and D. R. Hinton. 1998. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol. 160:2896-2904. [PubMed] [Google Scholar]
- 64.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor, S. F., and B. S. Bender. 1995. Beta 2-microglobulin-deficient mice demonstrate class II MHC restricted anti-viral CD4+ but not CD8+ CTL against influenza-sensitized autologous splenocytes. Immunol. Lett. 46:67-73. [DOI] [PubMed] [Google Scholar]
- 67.Tyznik, A. J., J. C. Sun, and M. J. Bevan. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J. Exp. Med. 199:559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.VanCott, J. L., M. M. McNeal, J. Flint, S. A. Bailey, A. H. Choi, and R. L. Ward. 2001. Role for T cell-independent B cell activity in the resolution of primary rotavirus infection in mice. Eur. J. Immunol. 31:3380-3387. [DOI] [PubMed] [Google Scholar]
- 69.Wang, J. C., and A. M. Livingstone. 2003. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 171:6339-6343. [DOI] [PubMed] [Google Scholar]
- 70.Wang, T., E. Scully, Z. Yin, J. H. Kim, S. Wang, J. Yan, M. Mamula, J. F. Anderson, J. Craft, and E. Fikrig. 2003. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J. Immunol. 171:2524-2531. [DOI] [PubMed] [Google Scholar]
- 71.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 77:13323-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitton, J. L., M. K. Slifka, F. Liu, A. K. Nussbaum, and J. K. Whitmire. 2004. The regulation and maturation of antiviral immune responses. Adv. Virus Res. 63:181-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wijburg, O. L., M. H. Heemskerk, A. Sanders, C. J. Boog, and N. Van Rooijen. 1996. Role of virus-specific CD4+ cytotoxic T cells in recovery from mouse hepatitis virus infection. Immunology 87:34-41. [PMC free article] [PubMed] [Google Scholar]
- 74.Williams, K. 2004. Modes of transmission for West Nile virus. Clin. Lab. Sci. 17:56. [PubMed] [Google Scholar]
- 75.Xiang, J., H. Huang, and Y. Liu. 2005. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J. Immunol. 174:7497-7505. [DOI] [PubMed] [Google Scholar]
- 76.Zivny, J., I. Kurane, C. O. Tacket, R. Edelman, and F. A. Ennis. 1993. Dengue virus-specific, human CD4+ cytotoxic T lymphocytes generated in short-term culture. Viral Immunol. 6:143-151. [DOI] [PubMed] [Google Scholar]