Abstract
Despite their evolutionary distance, the Saccharomyces cerevisiae retrotransposon Ty1 and retroviruses use similar strategies for replication, integration, and interactions with their hosts. Here we examine the formation of circular Ty1 DNA, which is comparable to the dead-end circular products that arise during retroviral infection. Appreciable levels of circular Ty1 DNA are present with one-long terminal repeat (LTR) circles and deleted circles comprising major classes, while two-LTR circles are enriched when integration is defective. One-LTR circles persist when homologous recombination pathways are blocked by mutation, suggesting that they result from reverse transcription. Ty1 autointegration events readily occur, and many are coincident with and dependent upon DNA flap structures that result from DNA synthesis initiated at the central polypurine tract. These results suggest that Ty1-specific mechanisms minimize copy number and raise the possibility that special DNA structures are a targeting determinant.
In addition to undergoing transpositional integration, the linear DNA synthesized by reverse transcription of the long terminal repeat (LTR) retrotransposon Ty1 and retrovirus RNA has multiple fates which can modulate the efficiency of integration into the host genome (75, 76, 80, 82). Reverse transcription is a sequential process with specific requirements for priming and strand transfer steps. Specific host tRNAs prime minus-strand reverse transcription, and purine-rich fragments of retroelement RNA (polypurine tracts [PPTs]), which are relatively resistant to the RNase H activity of reverse transcriptase (RT), prime plus-strand synthesis. The minus strand is synthesized first, followed by plus-strand synthesis using the minus strand as a template. Reverse transcription generates a linear double-stranded cDNA that is a substrate for the element-encoded integrase (IN) protein, which inserts the linear DNA into the host genome and creates a target site duplication (TSD) in the process. Both reverse transcription and integration are essential steps in the life cycle of retroelements.
Several lentiviruses, including human immunodeficiency virus type 1 (HIV-1) (13-15) and equine infectious anemia virus (71), as well as the retrotransposon Ty1 (30, 31), have two PPTs, one located just upstream of the 3′ LTR boundary (PPT) and the second near the center of the element (cPPT) (Fig. 1A). A central DNA flap is created when the 5′ end of the plus strand initiated at the cPPT is displaced by the 3′ end of plus-strand DNA after strand transfer (Fig. 1B). The size and position of the DNA flaps are determined by the placement of a central termination sequence (CTS) downstream of the cPPT. The position of the HIV-1 CTS creates an 88- or 98-nucleotide DNA flap downstream of the cPPT, while the Ty1 CTS creates flaps of up to 130 nucleotides with 30% of the flaps clustered 30 to 50 nucleotides downstream of the cPPT. The essential role of the PPT in reverse transcription is well established; however, the role of the cPPT is somewhat controversial. Mutations in the cPPT have been reported to decrease the efficiency of HIV-1 replication or Ty1 retrotransposition, though defects in HIV-1 replication are not always observed (48). There are also reports that the central DNA flap of HIV-1 is important for nuclear import of the preintegration complex in nondividing cells (2, 87). In addition, inclusion of the cPPT and CTS usually stimulates the transduction efficiency of lentiviral vectors severalfold (4, 18, 69, 88).
FIG. 1.
Organization of the Ty1 polypurine tracts. (A) A Ty1 element showing the locations of the long terminal repeats (triangles), the GAG and POL genes, the integrase-coding domain (IN), the central polypurine tract (cPPT), the central termination sequence (CTS), and the polypurine tract (PPT). The sequences of the wild-type cPPT and the cppt-74 mutant (30) are shown below. (B) The Ty1 central DNA flap is created when the 5′ end of the plus strand initiated at the cPPT is displaced by the 3′ end of plus-strand DNA. The size and position of the DNA flaps are determined by the CTS.
As has been established for unincorporated retroviral DNAs (75, 80), there may be nonproductive fates for unincorporated Ty1 DNA that limit retrotransposition, including the formation of one- or two-LTR circles, and intramolecular integration of the element into itself, termed autointegration (Fig. 2). Retroviral one-LTR circles can form by homologous recombination (HR) between the directly repeated LTRs (21, 44) or by errors in reverse transcription (51, 75) (Fig. 2A). Retroviral two-LTR circles form by nonhomologous end joining (NHEJ) (34, 38, 45) and contain a characteristic sequence called the circle junction (CJ) (74), where the ends of the unincorporated linear DNA are joined. Retroviral CJ sequences have been used to determine the status of the linear DNA termini prior to integration (40, 70, 81). Ty1 LTR junction molecules, putative autointegration events, and addition of nontemplated nucleotides to the termini of linear DNA have been detected in virus-like particle preparations (20, 30, 58).
FIG. 2.
Circle formation by Ty1. (A) Ty1 circles may form by host homologous recombination (HR) and nonhomologous end joining (NHEJ) functions or by reverse transcription (RT). Ty1 elements may also undergo autointegration mediated by IN. (B) Characteristics of Ty1 circles formed by autointegration. TSD+, 5-bp target site duplication created upon IN-catalyzed integration.
Conserved genes involved in HR (RAD51 and RAD52) and NHEJ (RAD50) modulate the levels of retroviral and Ty1 DNA and the level of retroviral two-LTR circles (41, 61). RAD52 is required for LTR-LTR recombination of chromosomal Ty1 elements and between unincorporated Ty1 cDNA and chromosomal Ty1 elements (47, 59, 65, 84). DNL4 (ligase 4) encodes a DNA ligase required for NHEJ in mammalian cells and Saccharomyces cerevisiae (39) and may be required for forming Ty1 two-LTR circles.
Autointegration has the potential to divert a significant fraction of transposition intermediates into dead-end products and occurs with diverse mobile genetic elements, including Tn5, Tn10, bacteriophage Mu, and retroviruses (5, 50, 55, 67, 79). Two types of DNA circles result from IN-mediated autointegration, and each has defining characteristics (Fig. 2B); “inversion circles” are produced when IN joins opposite DNA strands, and “deletion circles” arise from joining of the same strands.
Here we explore the formation of circular DNA by Ty1. Our results suggest that more than half of the unincorporated Ty1 cDNA accumulates as circular forms. Ty1 autointegration occurs preferentially in a region adjacent to, and dependent upon, a functional cPPT. These autointegration events may reveal new targeting determinants, because for Ty1, chromosomal integration usually occurs upstream of genes actively transcribed by RNA polymerase III (76).
MATERIALS AND METHODS
Genetic techniques, media, and strains.
Saccharomyces cerevisiae genetic techniques and media were used as described previously (29, 66). The Ty1-less strain DG2203 (MATα his3-Δ200hisG trp1 ura3) was derived by crossing strains DG2196 and DG1768 (24). Targeted integrative transformation was carried out after linearization of the TRP1-integrating plasmids pBDG1085 (pGTy1, wild type [WT]), pBDG1095 (pGTy1, frameshift [FS]), pBDG1242 (pGTy1, in-2600) (9), and pRS404 (vector) with Bsu36I and linearization of the URA3-integrating plasmids pJef1229 (pGTy1, WT), pBDG1285 (pGTy1Zeo, WT), pBDG1288 (pGTy1Zeo, in-2600), pBDG1289 (pGTy1, cppt-74), pBDG1295 (pGTy1Zeo, cppt-74), and pBDG1299 (pGTy1, in-K596,597G) with StuI. DG2203 derivatives containing an integrated pGTy1 element at TRP1 or URA3 were identified by Southern analysis using a 32P-labeled Ty1 probe. DNL4 and RAD50-52 deletion derivatives of DG2203 were constructed by microhomologous recombination (49) using drug resistance genes designed for gene replacement (27, 77) and the genome sequence of Saccharomyces paradoxus (36). The dnl4-ΔnatMX rad50-ΔkanMX rad52-ΔkanMX triple mutant was constructed by crossing the Ty1-less strains DG2792 [MATa his3-Δ200hisG Ty1his3-AI(96) trp1 ura3 pBDG1085/TRP1 dnl4-ΔnatMX rad52-ΔkanMX] and DG2642 (DG2203 rad50-ΔkanMX). The dnl4-ΔnatMX rad51-ΔkanMX rad52-ΔkanMX triple mutant was constructed by crossing the Ty1-less strains DG2792 [MATa his3-Δ200hisG Ty1his3-AI(96) trp1 ura3 pBDG1085/TRP1 dnl4-ΔnatMX rad52-ΔkanMX] and DG2632 (DG2203 rad51-ΔkanMX). In each cross, G418r (nonparental ditype configuration; 2:2 segregation for G418r), nourseothricin-resistant Trp+ Ura− His− segregants were analyzed by genetic complementation tests for sensitivity to the mutagen methyl methanesulfonate (0.0125% [vol/vol] in yeast extract-peptone-dextrose [YEPD]) to confirm the presence of the rad50, rad51, and rad52 mutations. A 2μm-URA3-based pGTy1 plasmid pBDG1278 (pGTy1Zeo/2μ-URA3) was introduced into DG2203 containing pBDG1085 or the triple mutants for replicon rescue. Quantitative Ty1his3-AI insertion rates were determined as described previously (17).
Plasmids.
Plasmids were constructed using standard techniques (32, 63). Briefly, pBDG1085 (pGTy1/TRP1 WT) was constructed by ligating an EagI-EcoRI fragment from pRS404 (YIp/TRP1), a BstEII-EagI fragment from pBDG202 (pGTy1/2μ-URA3), and a BstEII-EcoRI fragment from pBDG919 (pGTy1/2μ-TRP1). pBDG1095 (pGTy1/TRP1 FS) was constructed by ligating an EagI-EcoRI fragment from pRS404, a BstEII-EagI fragment from pBDG202, and a BstEII-EcoRI fragment from pJW117 (pGTy1 frameshift [+1, BamHI]/2μ-URA3). pBDG1242 (pGTy1/TRP1 in-2600) was constructed by subcloning an AflII-BstEII fragment from pBDG720 (pGTy1 in-2600/2μ-URA3) into pBDG1085. pBDG1278 was constructed by introducing a cassette containing the zeocin resistance gene (Zeo), a lac operator, and a ColE1 plasmid replication origin that was PCR amplified from the RSVPCA12 plasmid (60) using primers Ty-ACC1 (5′-GCGATAGTCGACCCGTAGAAAAGATC-3′) and Ty-ACC2 (5′-GCATGCGTCGACTGTTGACAATTAATCATCGGCA-3′). The resulting PCR fragment was digested with AccI and cloned into the ClaI site of pBDG202. The orientation in which Ty1 and Zeo were transcribed in the same direction was used in the present study (BDG/Cas). pBDG1285 was constructed by subcloning the PshAI-HindIII fragments from pBDG1278 into pBDG725 (pGTy1/YIp5), pBDG1288 was constructed by subcloning a HindIII-XhoI fragment from pBDG720 into pBDG1285, and pBDG1289 was constructed by subcloning a HindIII-XhoI fragment from pJef1105-74 (pGTy1 cppt-74/2μ-URA3; kindly provided by M. Wilhelm) (Fig. 1A) into pJef1229 (pGTy1/YIp5; kindly provided by J. Boeke). pBDG1295 was constructed by subcloning a HindIII-XhoI fragment from pJef1105-74 into pBDG1288, and pBDG1299 was constructed by subcloning a HindIII-XhoI fragment from pDSM27 (pGTy1 K596,597G/2μ-URA3) into pJef1229. pBDG1198 (5,848 nucleotides) was constructed by subcloning a BglII-XhoI fragment from TyA1-GAG into pRS406 and served as a size marker for circular Ty1 DNA. pBDG1088 was constructed by subcloning the IN-coding domain (nucleotides 2041 to 3945) as an XhoI-BamHI PCR fragment into pRS426. pBDG996 is a centromere-based URA3 plasmid containing Ty1his3-AI(d1) and will be described in detail elsewhere. pDSM207 was constructed by subcloning an XhoI-SnaBI fragment from pJef1105-74 into pBDG996. Plasmid constructs were verified by drug resistance phenotypes, DNA sequencing, and restriction enzyme analysis. Plasmids pRS404, pRS406, pRS426 (68), pBDG202 (pGTy1-H3Cla) (23), pJW117 (24), pJef1105-74 (30), and pDSM27 (54) have been described previously.
Galactose induction and genomic DNA isolation.
Cells were grown to saturation in liquid selective medium plus raffinose (2%) at 20oC. The cultures were diluted at least 40-fold into yeast extract-peptone (YEP) plus galactose (2%) and grown to mid-log phase (1 × 107 cells/ml) at 20oC. Genomic DNA was isolated from 10 ml of cells after spheroplasting with zymolyase 20T (ICN Pharmaceuticals, Costa Mesa, CA) as described previously (8).
Southern analysis.
DNA samples digested with PstI or SnaBI were separated by 0.8% (wt/vol) agarose gel electrophoresis and capillary blotted to Hybond N membrane according to the supplier's recommendations (Amersham Biosciences, Piscataway, NJ). Undigested genomic DNA samples were also separated by 0.8% (wt/vol) agarose gel electrophoresis in Tris-acetate running buffer. The gel was stained with ethidium bromide, photographed, and processed for Southern filter hybridization as described above. Fifty nanograms of undigested or linearized marker plasmid pBDG1198 was usually included on gels containing undigested genomic DNA. 32P-labeled hybridization probes were generated by random-prime labeling (Amersham Biosciences). Probe I from GAG was generated by digesting pBDG202 with PstI and PvuII and purifying the 663-bp fragment, probe II from TyB1-POL was generated by digesting pBDG1088 with XhoI and EcoRV and purifying the 738-bp fragment, and probe III from POL was generated by digesting pBDG202 with HindIII and SnaBI and purifying the 834-bp fragment. Hybridization signals were assigned on the basis of the patterns obtained with mutant Ty1 elements, and fragment sizes were predicted from restriction enzyme analysis or marker plasmids and from the map locations of different hybridization probes. Hybridization signals were quantitated by phosphorimage analysis and normalized to the integrated pGTy1 element present in the Ty1-less strains using protocols suggested by the manufacturer (GE Healthcare, Piscataway, NJ).
PCR.
Ty1 junction molecules and CJs were detected by PCR using about 1 μg genomic DNA per 50-μl reaction mixture volume, Titanium Taq DNA polymerase and conditions recommended by the supplier (Clontech, Mountain View, CA), primers CJxba5784w-2 (5′-GCCATTCTAGAGTGTAGAATTGCAGATTCC-3′) and CJ1125c (5′-CGATGAATTCGCGTCATCTTCTAACACCGT-3′), and the following cycling conditions: 2 min at 95oC (1 cycle); 30 cycles, with 1 cycle consisting of 30 s at 95oC, 15 s at 56oC, and 1 min at 72oC; and 7 min at 72oC (1 cycle). PCR products were separated by 2% agarose gel electrophoresis, and bands of 200 to 300 bp were purified using QIAquick (QIAGEN, Valencia, CA), followed by TOPO TA cloning (Invitrogen, Carlsbad, CA). An M13Reverse primer (5′-CAGGAAACAGCTATGAC-3′) was used for DNA sequencing. PCRs to detect the autointegration events adjacent to the cPPT were performed as described above except the cycling conditions were 2 min at 95oC (1 cycle); 30 cycles, with 1 cycle consisting of 15 s at 95oC, 15 s at 58oC, and 15 s at 72oC; and 72oC 7 min (1 cycle), and the PCR primers were TYB4203c (5′-GATGCTGAATATCACCTCTTGC-3′), TYB5130c (5′-ACGAAGCATCACTTATTGCGAC-3′), TYB5483c (5′-CTTGGTCTCGATGTAGTATACGT-3′), and TYBout (5′-GAACATTGCTGATGTGATGACA-3′). DNA preparations were analyzed by PCR using primers specific to LEU2 as described previously (24).
Ty1Zeo circles.
Aliquots of genomic DNA were digested with PvuI to minimize the possibility of recovering the pGTy1 expression vector, followed by electroporation into Escherichia coli DH5α and selection for zeocin resistance. Plasmids isolated from zeocin-resistant, ampicillin-sensitive transformants were subjected to restriction enzyme analysis and then sequenced using a primer complementary to the Zeo cassette (Ori-2 [5′-GCAAGCAGCAGATTACGCGCA-3′]). Target site duplications from inversion circles were verified by DNA sequence analysis with a primer from the 5′ end of Ty1 (TyAout2 [5′-GCCTTCTCACATTCTTCTGTT-3′]). BLAST 2 sequences (http://www.ncbi.nlm.nih.gov/BLAST/bl2seq/wblast2.cgi) was used to map the LTR junctions by comparing Ty1Zeo sequences with Ty1-H3 (GenBank accession number M18706). Similar distributions of joining events were obtained from several independent experiments using wild-type and cppt-74 pGTy1Zeo elements; therefore, the data were pooled. WebLogo (http://weblogo.berkeley.edu/logo.cgi) (16) was used to derive consensus 5-bp TSDs inferred from the sequence adjacent to the Ty1 LTR.
RESULTS
Ty1 circles.
To determine the overall level of circular Ty1 DNA present in cells undergoing retrotransposition, Southern analysis was performed using genomic DNA and a 32P-labeled probe derived from TYA1-GAG (Fig. 3). The strains contained a wild-type or mutant GAL1-promoted Ty1 element integrated into the genome to minimize Ty1 copy number without sacrificing the ability of a GAL1-promoted Ty1 to induce high levels of retrotransposition in the presence of galactose (8). In addition, the strains have no other chromosomal Ty1 elements (24), which would complicate the hybridization patterns.
FIG. 3.
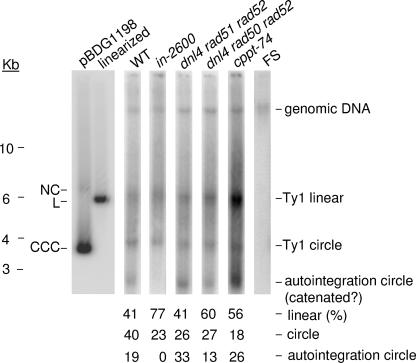
Presence of Ty1 circles. Undigested genomic DNAs were isolated from cells expressing wild-type Ty1 (WT), mutant Ty1 elements defective in IN (in-2600) or in the cPPT (cppt-74), or GAG-POL due to a +1 frameshift mutation early in GAG (FS) and cellular mutants defective in HR and NHEJ (dnl4Δ rad51Δ rad52Δ and dnl4Δ rad50Δ rad52Δ). Undigested genomic DNAs isolated from various cells were separated by agarose gel electrophoresis and analyzed by Southern hybridization using a 32P-labeled TyA1-GAG probe. pBDG1198 (5,848 bp; refer to Materials and Methods) was used as a size marker for Ty1 (5,918-bp) linear and circular molecules. The positions of circular DNA containing single-strand breaks (NC), covalently closed circles (CCC), and linear DNA (L) and of linear size standards (in kilobases) are shown to the left. The relative amounts of linear and circular DNA were determined using phosphorimage analysis and normalized to the amount of integrated pGTy1 element present in genomic DNA.
Putative circular Ty1 DNA molecules comprised at least half of the unincorporated Ty1 DNA in cells expressing a wild-type element, as determined by phosphorimage analysis (Fig. 3). In addition to linear Ty1 DNA (5,918 bp), two types of circular Ty1 DNA were detected by Southern hybridization. The first had an electrophoretic mobility in agreement with that expected for Ty1 circles containing one or two LTRs. This band was independent of functional IN, because its intensity was not altered in an in-2600 mutant, which encodes a defective IN protein resulting from the insertion of five additional codons (52). The second, faster-migrating band was dependent on the presence of functional IN, suggesting that it contains circular Ty1 DNA produced by autointegration and, if so, the circular DNA could also be catenated (21, 44). Heterogeneously sized circles may also be present but would not be visible by this type of analysis.
We initially determined that Ty1 circle formation was not appreciably affected in a rad52Δ mutant (data not shown), suggesting the possibility that Ty1 circles are produced using multiple recombination pathways. To address this possibility, two triple deletion mutants were constructed that should block HR (by deleting RAD50, the recA homolog RAD51, and RAD52) and NHEJ (by deleting RAD50 and the DNA ligase gene DNL4). Both mutants contained deletions for DNL4 and RAD52. One of the mutants also had a RAD50 deletion (dnl4Δ rad50Δ rad52Δ), while the other mutant had a RAD51 deletion (dnl4Δ rad51Δ rad52Δ). However, one-LTR and two-LTR circles, as well as autointegration circles, were present at wild-type levels in dnl4Δ rad51Δ rad52Δ and dnl4Δ rad50Δ rad52Δ mutants (Fig. 3). We also determined that both types of Ty1 circles were present in a cppt-74 mutant (Fig. 1A), which is defective for plus-strand DNA synthesis from the cPPT (30). As expected, unincorporated Ty1 cDNA was not detected when the pGTy1 element contained a +1 frameshift mutation early in the GAG gene (FS).
LTR junction molecules and circle junctions.
Although putative circular forms of Ty1 DNA were reproducibly detected in genomic DNA by Southern analysis, we were unsuccessful in isolating enough circular DNA for direct physical analyses of catenated molecules. However, the presence of Ty1 circles allowed us to investigate the nature of the LTR-LTR joining event using the following assays: PCR analysis using nonoverlapping primers that span the circle junction (Fig. 4; see file S1 in the supplemental material), replicon rescue using Ty1 elements that contain a Zeor gene and a bacterial plasmid origin of replication (Fig. 5; see Table 2; also see file S2 in the supplemental material), and additional Southern analyses (Fig. 6). PCR amplification was carried out using DNA from galactose-induced cells expressing wild-type Ty1, from the in-2600 mutant, or from glucose-grown cells (Fig. 4A). The CJ5784w and CJ125c primers amplified a broad band of 200 to 250 bp, which is in the size range expected for the CJ product containing two LTRs (259 bp). When the in-2600 mutant was analyzed, a minor band (B band) was present in addition to a major band (T band) that was closer to the size expected for a canonical CJ. PCR products were not detected when cells were grown in the repressing carbon source glucose or when template DNA was omitted from the reaction mixture. All of the DNA preparations were competent for PCR as shown by amplification of the LEU2 gene.
FIG.4.
Ty1 LTR junction formation. (A) The structure of a Ty1 two-LTR junction molecule containing a circle junction (CJ). The LTR domains unique 3′ (U3), repeated (R), and unique 5′ (U5) are shown (6, 75). The positions of the PCR primers CJ5784w and CJ125c used to amplify LTR junction molecules and the positions of PPT (polypurine tract) (used to initiate plus-strand DNA synthesis) and the primer binding site (PBS) (tRNA-Met anneals to initiate minus-strand reverse transcription) are shown. Below are PCR products, separated by agarose gel electrophoresis, in the size range expected for a canonical circle junction (CJ = 259 bp) amplified with primers CJ5784w and CJ125c and genomic DNA from cells expressing wild-type Ty1 (WT, galactose), an in-2600 mutant (in-2600, galactose), cells grown under repressing conditions (WT, glucose), or a reaction mixture lacking yeast DNA (− template). When the in-2600 mutant was analyzed, a minor, bottom band (labeled B) was present in addition to a major, top band (T) that was closer to the size expected for a canonical CJ. Primers specific to the LEU2 gene were used to show that genomic DNA was PCR competent. (B) LTR junction molecules obtained from wild-type Ty1. Unidirectional deletions into U5 or U3 are at the top and designated by the arrow. A canonical CJ is depicted by the striped bar, and a canonical CJ with intervening DNA inserted between the LTR ends is shown by the open bar and asterisk. LTR junction molecules with deletions into U5 (PPT-proximal LTR; also refer to panel A) are on the left (filled bars, minus numbering), while those with deletions into U3 (PBS-proximal LTR) are on the right (filled bars, plus numbering). LTR junction molecules with intervening DNA are designated by asterisks (see file S1 in the supplemental material). (C) LTR junction molecules obtained from an in-2600 mutant enriched for T-band sequences (Fig. 4A). The resolution of the figure is about 5 bp.
FIG. 5.
Autointegration events recovered using wild-type or cPPT-defective (cppt-74) Ty1 elements containing the Zeo cassette. (A) The positions of the Ty1 LTRs (triangles), the cPPT, and autointegration events resulting in deletion circles (short solid lines) and inversion circles (short dotted lines with diamonds) are shown. Ty1 sequence coordinates are shown below. The resolution of the figure is about 10 bp (see file S2 in the supplemental material for nucleotide positions of the autointegration events). (B) Consensus sequence of the 5-bp target site duplication for Ty1 autointegration events constructed using WebLogo (16). The size of the nucleotide at each position reflects the frequency with which that nucleotide occurs in the target site. Target sites are oriented in the 5′ to 3′ direction from the end of the LTR adjacent to the Zeo cassette. Error bars are twice the small sample correction.
TABLE 2.
The cPPT and endogenous Ty1his3-AI retrotranspositiona
| Strain genotype | Mean Ty1his3-AI retrotransposition rate (SD) (10−6)
|
Fold decrease | |
|---|---|---|---|
| WT | cppt-74 | ||
| WT | 6.7 (1.0) | 2.7 (0.7) | 2.5 |
| rad52Δ | 8.75 (1.0) | 5.0 (0.5) | 1.75 |
The centromere-based plasmid pBDG996 containing a wild-type Ty1his3-AI or the cppt-74 mutant were introduced into wild-type and rad52Δ Ty1-less strains, and the rate of His+ colony formation was determined as previously described (17). The rate of His+ formation was the average of two independent experiments.
FIG. 6.
Southern analysis of unincorporated Ty1 DNA. (A) Organization of the Ty1 element. The positions of regions I (black rectangle), II (rectangle with brickwork pattern), and III (shaded rectangle) that were used as hybridization probes, positions of restriction endonuclease cleavage sites for PstI (P) and SnaBI (S), and positions of the cPPT, the cPPT-associated autointegration hot spot (filled and unfilled circles), and resulting deletion circles (dotted lines) are shown. Probes I and II hybridized with PstI fragments of 3 to 3.3 kb (filled circle). (B) Genomic DNAs from cells expressing wild-type Ty1, the +1 frameshift mutant (FS), and in-2600 were digested with PstI, separated by agarose gel electrophoresis, and subjected to Southern analysis with 32P-labeled probe I (black rectangle) or II (rectangle with brickwork pattern) from GAG or the proximal segment of POL, respectively. Hybridization signals from the integrated pGTy1 element (pGTy1), LTR junctions, left-end (LE) and right-end (RE) cDNA, and the cPPT-associated autointegration hot spot (filled circle) are shown alongside the blots. (C) Genomic DNA from cells expressing wild-type Ty1 or the cppt-74 mutant was digested with PstI and analyzed with probe I, as described above for panel B, or with SnaBI and probe III (shaded rectangle) from the end of POL. Signals from the cPPT-associated autointegration hot spot (filled and unfilled circles) and cPPT-initiated plus-strand DNA (cPPT) are shown alongside the blots. The positions of the autointegration band adjacent to the cPPT (filled circle) and the autointegration hot spot fragment derived from one deletion circle (2.1 kb; unfilled circle) are indicated.
We cloned and sequenced 82 PCR products from cells expressing wild-type Ty1 (Fig. 4B; see file S1 in the supplemental material) and 47 products from the in-2600 mutant (Fig. 4C; see file S1 in the supplemental material). The sequence analysis revealed only one PCR product containing a canonical CJ from wild-type Ty1, while two others contained intervening sequence inserted between two complete LTRs. The remaining PCR products from wild-type Ty1 contained one complete LTR U3 or U5 segment and deletions of various lengths extending into the other LTR. Several of the junctions that contained deletions also contained additional sequences of various lengths inserted between the complete LTR and a deleted U3 or U5 segment.
The T band from the in-2600 mutant element yielded a different pattern of LTR junction molecules than the broad band from wild-type Ty1 (Fig. 4C), while the B band resembled the pattern obtained with wild type (data not shown). Canonical CJs recovered from the in-2600 mutant comprised about 21% of the PCR products (10 of 47), and those containing additional DNA made up another 47% (22 of 47). The remaining 15 PCR products contained unidirectional deletions at the CJ similar to those obtained with wild-type Ty1. Most of the intervening DNA sequences obtained with wild-type Ty1 or the in-2600 mutant were too short to identify by database searches (see file S1 in the supplemental material); however, many of the intervening sequences contained 5′-CCA-3′ or its complement 5′-TGG-3′ at one terminus. Since CCA is added to the termini of tRNAs, it is likely that the intervening DNA segments are derived from tRNAs, or from the addition of CCA to Ty1 RNA by ATP (CTP):tRNA nucleotidyltransferase (1). In addition, five of the longer DNA segments inserted at the CJ were derived from the 3′ ends of Thr-tRNA (UGU). Together these results suggest that the unidirectional deletions are largely dependent on active IN, and those recovered from the in-2600 mutant may result from leaky IN activity and were detected because of the sensitivity of the PCR assay. As will become evident below, the unidirectional deletions probably are autointegration events within the LTRs, resulting in deletion circles.
Recovery of Ty1Zeo circles by replicon rescue.
Genomic DNA was prepared from galactose-induced cells containing an integrated pGTy1 element tagged with the Zeo cassette, which includes a bacterial plasmid replication origin and a zeocin resistance gene (60). The DNA was digested with PvuI to minimize the recovery of pGTy1Zeo circles that might form in yeast. The pGTy1Zeo plasmid contains a single PvuI restriction site in the ampicillin resistance gene, whereas Ty1Zeo does not contain any PvuI sites. The DNA was introduced into the recA Escherichia coli strain DH5α by electroporation followed by selection for zeocin resistance. The resulting transformants were screened for ampicillin resistance, and plasmid DNA from zeocin-resistant, ampicillin-sensitive colonies were analyzed (Fig. 5 and Table 1; see file S2 in the supplemental material). In a typical experiment using a wild-type Ty1Zeo element, 75% (18 of 24) of the transformants were Zeor Amps, and the defective pGTy1FSZeo element yielded only Zeor Ampr transformants. Plasmids recovered from Zeor Ampr colonies usually resembled pGTy1Zeo.
TABLE 1.
Ty1Zeo circles
| Ty1Zeo circle or deletion | No. (%) of Ty1Zeo circles recovered from the following plasmid-carrying cells:
|
||||
|---|---|---|---|---|---|
| WT (pGTy1Zeo)a | in-2600 (pGTy1Zeo)a | WT (pGTy1, pGTy1Zeo)b | dnl4Δ rad51Δ rad52Δ (pGTy1, pGTy1Zeo)b | cppt-74 (pGTy1Zeo)a | |
| Deletion circle | 60 (45.4) | 0 | 5 (41.7) | 4 (26.7) | 18 (14) |
| Inversion circle | 8 (6.8) | 0 | 1 (8.3) | 2 (13.3) | 4 (3) |
| Solo LTR | 33 (25) | 3 (27.3) | 6 (50) | 5 (33.3) | 43 (33.3) |
| Simple bidirectional deletion | 24 (18.2) | 7 (63.6) | 0 | 4 (26.7) | 48 (37.3) |
| Complex bidirectional deletion | 6 (4.6) | 1 (9.1) | 0 | 0 | 16 (12.4) |
pGTy1Zeo integrated at URA3.
pGTy1 integrated at TRP1, pGTy1Zeo episomal.
The 131 Ty1Zeo circles recovered from cells expressing wild-type Ty1 were subjected to DNA sequence analysis using a primer from the 3′ end of the Zeo cassette to determine the sequence across the LTR junction (Fig. 5A and Table 1; see file S2 in the supplemental material). The most common type of Ty1Zeo circle recovered (45.8% [60 of 131]) was from an autointegration event joining the same strands, forming deletion circles. These circular Ty1Zeo elements contained a complete LTR joined to Ty1 sequences located elsewhere in the element. Surprisingly, there was a possible “hot spot” for autointegration events generating deletion circles starting about 30 nucleotides downstream of the cPPT (nucleotides 3782 to 3791). The other type of autointegration event, called an inversion circle, occurs when IN catalyzes joining of opposite DNA strains. About 6% (8 of 131) of the recovered plasmids were Ty1Zeo inversion circles; these circles were the same size as a complete element and contained an inversion of Ty1 sequences at the insertion site. Another prominent class of Ty1Zeo circles (25.2% [33 of 131]) contained a single LTR. Consistent with the PCR analysis, we did not recover any circle junctions where the two LTRs were joined. We also recovered 24 (18.3%) bidirectional deletions, most of which removed one LTR completely but did not delete much of the LTR downstream of the Zeo cassette, as would be expected from the way the Ty1Zeo circles were selected. Six (4.6%) of the deletions were complex in that they contained additional inversions or insertions of non-Ty1 sequences. Bidirectional deletions were sometimes bracketed by perfect or imperfect homologies of less than 15 nucleotides and therefore may have been formed by recombination.
Ty1Zeo circles recovered from an in-2600 mutant and a dnl4Δ rad51Δ rad52Δ mutant were compared with the Ty1 circles obtained from a wild-type element (Table 1). The types of Ty1Zeo circles recovered were in general agreement with the results of Southern analyses of undigested genomic DNA from cells expressing an unmarked Ty1 element (Fig. 3). Notably, autointegration circles were not obtained with the in-2600 mutant, perhaps because the Ty1Zeo assay is not sensitive enough to detect the residual IN activity present in the in-2600 mutant. Ty1Zeo circles containing a single LTR were also recovered from the dnl4Δ rad51Δ rad52Δ triple mutant, suggesting that these events are not the result of homologous recombination between the LTRs present in linear Ty1 DNA.
To determine whether the autointegration events adjacent to the cPPT observed with wild-type Ty1Zeo were dependent on the cPPT, 129 Ty1Zeo circles were recovered from cells expressing the cppt-74 mutant (Fig. 5A and Table 1; see file S2 in the supplemental material). The number of Ty1 autointegration events decreased about threefold, and the number of bidirectional deletions increased twofold in the absence of the cPPT compared with those of a wild-type Ty1Zeo element. Although autointegration events were recovered at a number of positions in the cppt-74 mutant, none were close to the cPPT.
Autointegration consensus sequence.
Four of the Ty1Zeo inversion circles recovered from cells expressing wild-type Ty1Zeo were sequenced from both LTRs and showed 5-bp TSDs typical of Ty1 transposition events in that they did not show a strong consensus sequence (see file S2 in the supplemental material): 5′-ATTAG-3′ (insertion site 1514), 5′-CATCT-3′ (insertion site 12906), 5′-CAATC-3′ (insertion site 13116), and 5′-GAAAT-3′ (insertion site 14736). Therefore, we derived a consensus target site from a larger collection of Ty1Zeo deletion and inversion circles recovered from cells expressing wild-type Ty1 (74 circles) and the cppt-74 mutant (22 circles), assuming that the TSD occurred normally during autointegration (Fig. 5B). The consensus sequence obtained by analyzing autointegration events using WebLogo (16) showed a preference for AT-rich sequences for both wild-type Ty1 and the cppt-74 mutant, which is similar to previous studies where large numbers of Ty1 integration sites have been determined at the CAN1 locus (46, 62, 83).
Southern analysis of unincorporated Ty1 DNA.
To gain independent evidence for an autointegration preference near the cPPT and confirm the presence of circular Ty1 DNA, we performed Southern hybridizations using 32P-labeled probes from GAG (probe I), the center of the element (probe II), and from the RT region (probe III) (Fig. 6). Autointegrations adjacent to the cPPT form deletion circles of about 3.8 and 2.1 kb; therefore, subsequent restriction enzyme digestion results in fragments of predicted sizes (Fig. 6A). Genomic DNA was digested with PstI and processed for Southern analysis, and the resulting filters were hybridized with probes I and II (Fig. 6B). PstI cuts twice in Ty1 and yields fragments of 1,138, 754, and 4,053 bp. Both probes I and II hybridized with PstI fragments of 3 to 3.3 kb from cells expressing wild-type Ty1, which is the expected size for fragments generated if autointegration events occurred near the cPPT. The autointegration band adjacent to the cPPT was not detected in the in-2600 or FS mutant. PstI fragments of 5.1 to 5.4 kb from circular LTR junction molecules containing one LTR and two LTRs and from LTR-LTR autointegration events (Fig. 4B) were also detected. As expected, a genomic DNA fragment containing the integrated pGTy1 element, and unincorporated Ty1 cDNA fragments of about 1.1 and 4 kb from the left end (LE) and right end (RE) of Ty1 were present. The integrated pGTy1 band was present in all samples, and the LE and RE bands were present in the wild-type and in-2600 strains.
Southern analysis was used to determine whether the autointegration preference adjacent to the cPPT was maintained in the cppt-74 mutant (Fig. 6C). The autointegration band adjacent to the cPPT detected after PstI digestion and hybridization with probe I was not detected in the cppt-74 mutant. Genomic DNA was also digested with SnaBI, which cleaves once in Ty1 at nucleotide 5463, and hybridized with probe III to detect an autointegration hot spot fragment derived from one deletion circle (2.1 kb) and the cPPT-initiated plus-strand fragment (cPPT; 1.7 kb). The difference in size between these two SnaBI fragments results from the complete LTR (334 bp) present in the deletion circle. Both restriction fragments were detected in cells expressing wild-type Ty1, but not in the cppt-74 mutant. In contrast, the LTR junction molecules derived from circular Ty1 DNA containing one or two LTRs and LE cDNA remained at wild-type levels in the cppt-74 mutant.
Detecting autointegration events by PCR.
The results of Ty1Zeo and Southern analyses encouraged us to develop a PCR-based assay to detect autointegration events adjacent to the cPPT (Fig. 7). Oligonucleotide primers that annealed to sequences at various distances downstream of the cPPT (4203c, 5130c, and 5483c) were used in PCRs with a primer adjacent to the 3′ LTR of Ty1 (TyBout, nucleotide 5486) and with DNA from strains expressing wild-type Ty1, several defective elements (in-2600, cppt-74, in-K596,597G, and the FS mutant), and an empty GAL1 vector (Fig. 7A). If the cPPT mediates a preference for autointegration events, PCR products of ∼827 bp (TyBout plus 4203c), ∼1,754 bp (TyBout plus 5130c), and ∼2,107 bp (TyBout plus 5483c) should be amplified on the basis of the closest Ty1Zeo insertion at nucleotide 3808 (see file S2 in the supplemental material). The ability to amplify these products should require an expression-competent Ty1 element with a functional IN and a cPPT. PCR products in the expected size range (Fig. 7B) were obtained using DNA from cells expressing wild-type Ty1 but not from the in-2600, cppt-74, or FS mutant. We also determined that nuclear entry of the Ty1 preintegration complex was not required for autointegration activity adjacent to the cPPT by analyzing an element that contained a defective nuclear localization signal (NLS) in IN (in-K596,597G) (54). The NLS-defective mutant had wild-type levels of autointegration activity adjacent to the cPPT, as shown by amplification of the ∼827-bp product. All DNA samples were competent for PCR, as determined by amplification of the chromosomal LEU2 gene (data not shown).
FIG. 7.
PCR analysis of the autointegration hot spot mediated by the cPPT. (A) The autointegration hot spot region adjacent to the cPPT (nucleotide 3782) is shown along with primers 4203c, 52130c, 5483c, and TyBout (5486) and one deletion circle (dotted line). DNA from strains expressing wild-type Ty1, several defective elements (in-2600, cppt-74, in-K596,597G, and the FS mutant), and an empty GAL1 vector was used. If the hot spot were adjacent to the cPPT and begins at Ty1 nucleotide ∼3808 (inferred from analyzing Ty1Zeo circles), PCR products of ∼827 bp (TyBout plus 4203c), ∼1,754 bp (TyBout plus 5130c), ∼2,107 bp (TyBout plus 5483c) should be amplified. (B) Genomic DNA from cells expressing wild-type, in-2600, cppt-74, FS, nuclear-localization-defective (in-K596,597G) Ty1 elements or an empty vector were used in PCRs with the primer pairs shown below, and the amplified fragments were separated by agarose gel electrophoresis. PCR products resulting from autointegration events associated with the cPPT are noted. The asterisks indicate PCR products in the expected size range. The positions of molecular size standards (in base pairs) are noted to the left of the leftmost gel. (C) The ∼827-bp PCR product resulting from amplification with primers TyBout plus 4203c was sequenced, and the autointegration insertion sites were compared with those obtained from sequencing Ty1Zeo circles (Fig. 5). The resolution is about 5 bp (see file S2 in the supplemental material for insertion sites). Also shown are the central Ty1 DNA flaps resulting from cPPT-initiated DNA synthesis and variable termination of the incoming plus strand at the CTS (rectangle), with 30% of strands terminating 30 to 50 nucleotides downstream of the cPPT (filled rectangle) (30).
Central DNA flap and autointegration.
The ∼827-bp amplification products obtained by PCR with primers TyBout plus 4203c were cloned and sequenced to determine the autointegration sites (see file S2 in the supplemental material). We then aligned the Ty1 autointegration events obtained from analyzing Ty1Zeo circles and PCR products described above with the position of the Ty1 central DNA flap structures mapped previously (30) (Fig. 7C). There was a striking correspondence between the autointegration activity adjacent to the cPPT using two independent approaches and the positions of the central DNA flaps present in Ty1. The Ty1 autointegration events in this preferred region started 26 nucleotides downstream of the cPPT, occurred in a region of about 80 nucleotides, and were coincident with the positions of the central DNA flaps.
Ty1his3-AI retrotransposition.
To determine whether additional defects in Ty1 retrotransposition could be detected in the cppt-74 mutant under more stringent genetic conditions (25, 53), we introduced the cppt-74 mutation into a Ty1 element marked with the his3-AI indicator gene (17) and determined the rate of His+ colony formation in wild-type and rad52Δ Ty1-less backgrounds. Retrotransposition generates most of the His+ colonies obtained with wild-type Ty1his3-AI. Following splicing of the artificial intron (AI) and reverse transcription, recombination events involving Ty1HIS3 cDNA are also detected by this assay and become more prevalent when Ty1 cannot complete reverse transcription or undergo integration (65). The cppt-74 mutation decreased Ty1his3-AI integration 2.5-fold in a wild-type Ty1-less background (Table 2) and 1.75-fold in a rad52Δ mutant, where Ty1 DNA recombination is blocked, which is comparable to previous results obtained using a GAL1-promoted Ty1 element (30, 31).
DISCUSSION
We show that at least half the unincorporated Ty1 DNA present in cells undergoing retrotransposition is diverted into dead-end joining reactions, resulting in circular DNA. Accumulation of Ty1 circles does not completely depend on the activities of yeast HR and NHEJ pathways. Instead, Ty1 one-LTR circles may arise by errors in reverse transcription and by IN-mediated autointegration events. Wild-type Ty1 produces few two-LTR circles. However, two-LTR circles are greatly enriched when IN is defective, suggesting that integration-competent Ty1 DNA is short-lived because Ty1 DNA is efficiently integrated or degraded. Analysis of Ty1 autointegration events revealed a hot spot adjacent to, and dependent upon, the cPPT near the center of the element. Because the length of this Ty1 autointegration hot spot corresponds to the variation in the positions and accompanying sizes of the central DNA flaps formed by variable termination of plus-strand synthesis, Ty1 target specificity may be enhanced by the presence of special DNA structures.
Formation of solo LTRs from chromosomal Ty1 elements can occur by HR or single-strand annealing pathways, which require RAD52 (39, 47, 84). For these reasons, we expected that RAD52 would also be required for one-LTR circle formation. Nonetheless, Southern analyses of undigested genomic DNA show that wild-type levels of Ty1 circles containing one or two LTRs are present not only in rad52Δ mutants but also when NHEJ is blocked in dnl4Δ rad50Δ rad52Δ or dnl4Δ rad51Δ rad52Δ host mutants. The following results suggest that the hybridization signal from undigested genomic DNA is primarily from one-LTR circles. (i) Ty1 circles are present when IN is defective. (ii) CJs characteristic of two-LTR circles are present at a low level when Ty1 produces active IN. (iii) LTR junction molecules of the size expected for a single LTR are present when genomic DNA is digested with PstI or SnaBI and analyzed by Southern blotting. (iv) Ty1Zeo circles containing a single LTR are recovered from a dnl4Δ rad51Δ rad52Δ mutant. However, the recovery of Ty1Zeo circles containing bidirectional deletions ending in microhomologies extends previous results indicating that linear Ty1 cDNA undergoes degradation (43) and suggests a role for HR and NHEJ in forming circular Ty1 DNA in wild-type cells (39).
In contrast, cell lines defective for HR and NHEJ genes contain much lower levels of retroviral two-LTR circles (34, 38, 45). The cellular factors required for forming retroviral one-LTR circles have not been completely defined. Results from one study suggest that the RAD50/MRE11/NBS1 nuclease is involved in retroviral one-LTR circle formation, perhaps by exposing single strands of the repeated LTRs for subsequent HR (38).
Ty1 one-LTR circles may result from reverse transcription intermediates that stall after plus-strand strong-stop synthesis or from open circular intermediates predicted to be present during reverse transcription, as was first proposed for retroviruses (51, 75). Base pairing between the primer binding site (PBS) sequences on plus-strand strong-stop DNA and the PBS present on the 3′ end of minus-strand DNA would produce a one-LTR circle after displacement synthesis by RT or a host polymerase. Lauermann and Boeke (42) showed that Ty1 PPT-initiated plus-strand strong-stop DNA is a dead-end intermediate because sequence variants within the PBS are not inherited in a subsequent retrotransposition event. Interestingly, these plus-strand reverse transcription intermediates are the ones postulated to anneal with the PBS on minus-strand DNA to form one-LTR circles and therefore are probably involved in the conversion of linear Ty1 DNA into one-LTR circles. Furthermore, if the cPPT-initiated plus-strand DNA displaces PPT-initiated strong-stop DNA and the released primer is utilized to form one-LTR circles, the overall level of circular Ty1 DNA should decrease in the cppt-74 mutant. In support of this idea, we observed a decrease in the level of circular Ty1 DNA and a modest increase in linear Ty1 DNA in the cppt-74 mutant (Fig. 3).
Several studies reported that HIV-1 two-LTR circles are relatively abundant and can be long-lived (10, 12, 26, 56). Conversely, we show that Ty1 two-LTR circles, as monitored by the presence of a CJ, are difficult to detect when IN is functional. A total of 82 PCR products have been sequenced; only 1 contains a canonical CJ, and 2 others contain small intervening DNA segments between the complete LTRs. The remaining 79 products probably result from Ty1 autointegration events within the LTRs because most of these products contain the sequence arrangement expected for a deletion circle. Furthermore, almost 70% of the LTR junction molecules have either canonical CJs or exogenous DNA inserted between the complete LTRs when IN is defective. These results suggest that the fraction of Ty1 DNA which can undergo either genomic integration or autointegration does so rapidly, while the remaining Ty1 DNA is degraded or modified by cellular or element-encoded factors (43, 58). If so, the rapid removal of integration-competent DNA from the total pool may result in an overestimate of the fraction of circles, since this signal is compared to the remaining unincorporated Ty1 DNA rather than the total pool.
Mules et al. (58) reported that the ends of Ty1 and Ty2 linear DNAs can be altered by the addition of nontemplated bases, which probably interferes with IN-mediated integration. A variety of appended sequences have been found at the ends of linear Ty1 extracted from virus-like particles, including C and A residues, 5′-CCA-3′ or 5′-GGT-3′, and sequences from tRNAs for Lys (CUU and UUU), Asp (GUC), Arg (UCU and ACG), Gln (UUG), and Ser (GCU). We have also recovered CJs from unfractionated genomic DNA with sequences at the LTR-LTR junctions indicative of CCA addition, and two partial tRNA Thr (UGU) sequences. These results taken together suggest that linear Ty1 DNA with modified ends can circularize to generate two-LTR circles.
Ty1 undergoes high levels of autointegration compared with other mobile genetic elements. Autointegration events comprise 19% of the Ty1 circles that accumulate in cells expressing wild-type Ty1, as determined by Southern analysis of undigested genomic DNA, and almost 70% of the recovered Ty1Zeo circles. Comparable results have been obtained by characterizing molecular clones of unincorporated circular Moloney murine leukemia virus (Mo-MuLV) cDNA where about 20% of the clones appeared to derive from autointegration events (67). In experiments involving plasmid substrates in vivo, Tn10 autointegration events occur at frequencies similar to that of intermolecular transposition (5). Interestingly, Mo-MuLV produces about the same number of inversion and deletion autointegration events (67), while Tn10 forms only inversion circles (5) and Tn5 forms mostly deletion circles (79). In our analyses using Ty1Zeo, deletion circles are recovered much more frequently than inversion circles. These results suggest that the level and type of autointegration events reflect the different interactions each mobile genetic element has during the process of integration. For example, the bacteriophage Mu B protein, enhances intermolecular transposition in vitro by activating DNA strand transfer (50), while the global cellular regulator H-NS promotes intermolecular transposition of Tn10 by helping maintain the preintegration complex in an unfolded state (78). Retroviruses have coopted the nuclear envelope proteins BAF, LAP2α, and emerin that act to compact retroviral DNA, reduce autointegration, and stimulate the association between the preintegration complex and target DNA to promote intermolecular integration (33, 73). Ty1 autointegration is also likely to have special requirements, since the Ty1 preintegration complex normally recognizes features of chromatin extending from genes transcribed by RNA polymerase III (7), and as is evident from our work, there is an autointegration preference associated with the cPPT.
What promotes Ty1 autointegration downstream of the cPPT? We show that many autointegration events overlap the Ty1 central termination sequence, which contains signals that cause plus-strand synthesis to terminate over a 130-nucleotide A+T-rich region (30). Moreover, Ty1 autointegration events show the same weak preference for AT-rich sequences as do insertions at “cold” chromosomal targets, such as the CAN1 gene (46, 62, 83). We propose that the Ty1 preintegration complex recognizes the DNA flap structures resulting from cPPT-primed DNA synthesis and autointegrates at, or just downstream of, the displaced plus strand within the CTS (Fig. 1). In addition, compilations of large numbers of retroviral insertion sites from several studies have identified weak DNA palindromes as a targeting determinant for HIV-1, simian immunodeficiency virus, Mo-MuLV, and avian sarcoma-leukosis virus (reference 85 and references therein), and the unpaired stems of a DNA cruciform, which are structurally related to palindromes, are hot spots for retroviral integration in vitro using INs from HIV-1 and avian sarcoma virus (35). It will be interesting to determine whether DNA flap structures also create hot spots for Ty1 integration in vitro.
Could DNA flap structures influence Ty1 integration elsewhere in the genome? Ty1 integrates at preferred locations at sites near active RNA polymerase III transcription and is influenced by alterations in the TFIIIB subunit Bdp1 and the Isw2 and Set3 chromatin-remodeling complexes (3, 57). There are less favored sites for Ty1 integration in the promoter regions of genes transcribed by RNA polymerase II and within coding sequences (6). If DNA structure is a component of Ty1 target site specificity, then specialized DNA structures, such as transcription bubbles and DNA flaps created by replication, could also be targeting determinants. However, additional nucleolytic processing would be required to complete Ty1 integration if the branch point of a DNA flap is a targeting determinant (Fig. 1). In addition, cellular Ty1 modulators involved in processing special DNA structures, such as the RecQ family helicase SGS1 (11) and the Fen-1 endonuclease RAD27 (72), or in transcription elongation, such as the Elongator complex subunits ELP2-4, ELP6, and IKI3 may alter target site specificity throughout the genome (28, 64).
Alternatively, a link between the central DNA flap and nuclear import of the HIV-1 preintegration complex has been reported, where HIV-1 mutants lacking the central DNA flap exhibit a nuclear import defect (87). One idea put forth by Zennou et al. (87) is that the central DNA flap allows the retroviral DNA to fold during IN multimerization and this configuration facilitates nuclear entry. If linear Ty1 DNA is folded in a similar way, then the central DNA flap, IN, and the element's termini may be brought together, creating an autointegration preference adjacent to the cPPT. An attractive feature of this model is that Ty1 IN contains the nuclear localization signal required for nuclear entry and retrotransposition (37, 54). Two predictions of this type of nuclear import model are that cPPT and NLS mutations should both confer similar defects in the level of Ty1 retrotransposition and that nuclear import may be required for autointegration hot spot activity. Our results show that the cppt-74 mutant does not phenocopy the NLS-defective in-K596,597G mutant, which has a transposition defect of over 200-fold (54). The autointegration preference adjacent to the cPPT also is not epistatic to nuclear import, since this hot spot persists in the in-K596,597G mutant. Therefore, our results do not support a role for the central DNA flap in nuclear import of the Ty1 preintegration complex. Along these lines, other reports suggest that the HIV-1 central DNA flap is not required for nuclear import (19, 48).
In addition to the cPPT region and associated DNA flap structures, preferential sites for autointegration may exist elsewhere in Ty1 that have escaped detection by replicon rescue of Ty1Zeo elements. If the cPPT is responsible for most of the Ty1 autointegration circles detected by Southern analysis of undigested genomic DNA (Fig. 3), then the cppt-74 mutant should contain fewer autointegration circles. However, the level of overall autointegration events remains about the same in the WT (19%) and cppt-74 mutant (26%) (Fig. 3), even though Ty1Zeo autointegration events decrease threefold in the cppt-74 mutant (Table 1). One class of autointegration events that may be underrepresented using the Ty1Zeo recovery assay includes those that occur in the LTRs, which are detected by PCR in cells containing functional IN (Fig. 4). More-detailed analysis of the autointegration circles will be required to uncover additional preferential integration sites.
The presence of active cPPT priming and CTS termination sites in diverse retroelements, such as HIV-1 and Ty1, strongly suggests that producing two distinct DNA segments separated by a DNA flap may offer a selective advantage. cPPT/CTS mutants replicate or retrotranspose at lower levels than wild-type elements do, but the mechanism through which replication of these mutants is impaired is not fully understood. One problem is that cPPT mutations can apparently cause relatively modest defects on HIV-1 replication (48), which is similar to our results with endogenous Ty1 retrotransposition or with more profound defects on HIV-1 replication (13, 15). Lentiviral vectors appear to respond more uniformly to the presence of the cPPT, where an increase in efficiency is usually observed (4, 18, 69, 88). A further complication is that the cPPT may provide more than a single advantage for viral or Ty1 replication. For example, the cPPT may protect HIV-1 from DNA editing (86), and the cPPT also promotes DNA-DNA recombination by HIV-1 RT displacement synthesis in vitro (22). Whether the cPPT is actually involved in retroviral or Ty1 recombination in vivo remains to be determined. Ty1 presents an additional possibility, since any selective advantage provided by the cPPT can be offset by autointegration via the cPPT, which is a suicidal pathway for Ty1 in the absence of yeast barrier-to-autointegration factors.
Supplementary Material
Acknowledgments
We thank J. Boeke and M. Wilhelm for plasmids and M. Chandler, A. Rattray, and J. Westpheling for helpful discussions.
This work was sponsored by the Center for Cancer Research of the National Cancer Institute, Department of Health and Human Services.
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, and mentioning trade names, commercial products, or organizations does not imply endorsement by the U.S. government.
Footnotes
Published ahead of print on 27 September 2006.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aebi, M., G. Kirchner, J. Y. Chen, U. Vijayraghavan, A. Jacobson, N. C. Martin, and J. Abelson. 1990. Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 265:16216-16220. [PubMed] [Google Scholar]
- 2.Ao, Z., X. Yao, and E. A. Cohen. 2004. Assessment of the role of the central DNA flap in human immunodeficiency virus type 1 replication by using a single-cycle replication system. J. Virol. 78:3170-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachman, N., M. E. Gelbart, T. Tsukiyama, and J. D. Boeke. 2005. TFIIIB subunit Bdp1p is required for periodic integration of the Ty1 retrotransposon and targeting of Isw2p to S. cerevisiae tDNAs. Genes Dev. 19:955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry, S. C., B. Harder, M. Brzezinski, L. Y. Flint, J. Seppen, and W. R. Osborne. 2001. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum. Gene Ther. 12:1103-1108. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin, H. W., and N. Kleckner. 1989. Intramolecular transposition by Tn10. Cell 59:373-383. [DOI] [PubMed] [Google Scholar]
- 6.Boeke, J. D. 1989. Transposable elements in Saccharomyces cerevisiae, p. 335-374. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 7.Boeke, J. D., and S. E. Devine. 1998. Yeast retrotransposons: finding a nice quiet neighborhood. Cell 93:1087-1089. [DOI] [PubMed] [Google Scholar]
- 8.Boeke, J. D., D. J. Garfinkel, C. A. Styles, and G. R. Fink. 1985. Ty elements transpose through an RNA intermediate. Cell 40:491-500. [DOI] [PubMed] [Google Scholar]
- 9.Braiterman, L. T., and J. D. Boeke. 1994. In vitro integration of retrotransposon Ty1: a direct physical assay. Mol. Cell. Biol. 14:5719-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brussel, A., D. Mathez, S. Broche-Pierre, R. Lancar, T. Calvez, P. Sonigo, and J. Leibowitch. 2003. Longitudinal monitoring of 2-long terminal repeat circles in peripheral blood mononuclear cells from patients with chronic HIV-1 infection. AIDS 17:645-652. [DOI] [PubMed] [Google Scholar]
- 11.Bryk, M., M. Banerjee, D. Conte, Jr., and M. J. Curcio. 2001. The Sgs1 helicase of Saccharomyces cerevisiae inhibits retrotransposition of Ty1 multimeric arrays. Mol. Cell. Biol. 21:5374-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler, S. L., E. P. Johnson, and F. D. Bushman. 2002. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J. Virol. 76:3739-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charneau, P., M. Alizon, and F. Clavel. 1992. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J. Virol. 66:2814-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charneau, P., and F. Clavel. 1991. A single-stranded gap in human immunodeficiency virus unintegrated linear DNA defined by a central copy of the polypurine tract. J. Virol. 65:2415-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 16.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curcio, M. J., and D. J. Garfinkel. 1991. Single-step selection for Ty1 element retrotransposition. Proc. Natl. Acad. Sci. USA 88:936-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dardalhon, V., B. Herpers, N. Noraz, F. Pflumio, D. Guetard, C. Leveau, A. Dubart-Kupperschmitt, P. Charneau, and N. Taylor. 2001. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 8:190-198. [DOI] [PubMed] [Google Scholar]
- 19.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichinger, D. J., and J. D. Boeke. 1988. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell 54:955-966. [DOI] [PubMed] [Google Scholar]
- 21.Farnet, C. M., and W. A. Haseltine. 1991. Circularization of human immunodeficiency virus type 1 DNA in vitro. J. Virol. 65:6942-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuentes, G. M., C. Palaniappan, P. J. Fay, and R. A. Bambara. 1996. Strand displacement synthesis in the central polypurine tract region of HIV-1 promotes DNA to DNA strand transfer recombination. J. Biol. Chem. 271:29605-29611. [DOI] [PubMed] [Google Scholar]
- 23.Garfinkel, D. J., M. F. Mastrangelo, N. J. Sanders, B. K. Shafer, and J. N. Strathern. 1988. Transposon tagging using Ty elements in yeast. Genetics 120:95-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garfinkel, D. J., K. Nyswaner, J. Wang, and J. Y. Cho. 2003. Post-transcriptional cosuppression of Ty1 retrotransposition. Genetics 165:83-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garfinkel, D. J., K. M. Nyswaner, K. M. Stefanisko, C. Chang, and S. P. Moore. 2005. Ty1 copy number dynamics in Saccharomyces. Genetics 169:1845-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillim-Ross, L., A. Cara, and M. E. Klotman. 2005. HIV-1 extrachromosomal 2-LTR circular DNA is long-lived in human macrophages. Viral Immunol. 18:190-196. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 28.Griffith, J. L., L. E. Coleman, A. S. Raymond, S. G. Goodson, W. S. Pittard, C. Tsui, and S. E. Devine. 2003. Functional genomics reveals relationships between the retrovirus-like Ty1 element and its host Saccharomyces cerevisiae. Genetics 164:867-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, Inc., San Diego, Calif.
- 30.Heyman, T., B. Agoutin, S. Friant, F. X. Wilhelm, and M. L. Wilhelm. 1995. Plus-strand DNA synthesis of the yeast retrotransposon Ty1 is initiated at two sites, PPT1 next to the 3′ LTR and PPT2 within the pol gene. PPT1 is sufficient for Ty1 transposition. J. Mol. Biol. 253:291-303. [DOI] [PubMed] [Google Scholar]
- 31.Heyman, T., M. Wilhelm, and F. X. Wilhelm. 2003. The central PPT of the yeast retrotransposon Ty1 is not essential for transposition. J. Mol. Biol. 331:315-320. [DOI] [PubMed] [Google Scholar]
- 32.Innis, M. A., D. H. Gelfand, J. J. Sninsky, and T. J. White. 1990. PCR protocols. Academic Press, Inc., San Diego, Calif.
- 33.Jacque, J. M., and M. Stevenson. 2006. The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature 441:641-645. [DOI] [PubMed] [Google Scholar]
- 34.Jeanson, L., F. Subra, S. Vaganay, M. Hervy, E. Marangoni, J. Bourhis, and J. F. Mouscadet. 2002. Effect of Ku80 depletion on the preintegrative steps of HIV-1 replication in human cells. Virology 300:100-108. [DOI] [PubMed] [Google Scholar]
- 35.Katz, R. A., K. Gravuer, and A. M. Skalka. 1998. A preferred target DNA structure for retroviral integrase in vitro. J. Biol. Chem. 273:24190-24195. [DOI] [PubMed] [Google Scholar]
- 36.Kellis, M., N. Patterson, M. Endrizzi, B. Birren, and E. S. Lander. 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423:241-254. [DOI] [PubMed] [Google Scholar]
- 37.Kenna, M. A., C. B. Brachmann, S. E. Devine, and J. D. Boeke. 1998. Invading the yeast nucleus: a nuclear localization signal at the C terminus of Ty1 integrase is required for transposition in vivo. Mol. Cell. Biol. 18:1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilzer, J. M., T. Stracker, B. Beitzel, K. Meek, M. Weitzman, and F. D. Bushman. 2003. Roles of host cell factors in circularization of retroviral DNA. Virology 314:460-467. [DOI] [PubMed] [Google Scholar]
- 39.Krogh, B. O., and L. S. Symington. 2004. Recombination proteins in yeast. Annu. Rev. Genet. 38:233-271. [DOI] [PubMed] [Google Scholar]
- 40.Kulkosky, J., R. A. Katz, and A. M. Skalka. 1990. Terminal nucleotides of the preintegrative linear form of HIV-1 DNA deduced from the sequence of circular DNA junctions. J. Acquir. Immune Defic. Syndr. 3:852-858. [PubMed] [Google Scholar]
- 41.Lau, A., R. Kanaar, S. P. Jackson, and M. J. O'Connor. 2004. Suppression of retroviral infection by the RAD52 DNA repair protein. EMBO J. 23:3421-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauermann, V., and J. D. Boeke. 1997. Plus-strand strong-stop DNA transfer in yeast Ty retrotransposons. EMBO J. 16:6603-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, B. S., L. Bi, D. J. Garfinkel, and A. M. Bailis. 2000. Nucleotide excision repair/TFIIH helicases RAD3 and SSL2 inhibit short-sequence recombination and Ty1 retrotransposition by similar mechanisms. Mol. Cell. Biol. 20:2436-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, Y. M., and J. M. Coffin. 1990. Efficient autointegration of avian retrovirus DNA in vitro. J. Virol. 64:5958-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, L., J. M. Olvera, K. E. Yoder, R. S. Mitchell, S. L. Butler, M. Lieber, S. L. Martin, and F. D. Bushman. 2001. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 20:3272-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liebman, S. W., and G. Newnam. 1993. A ubiquitin-conjugating enzyme, RAD6, affects the distribution of Ty1 retrotransposon integration positions. Genetics 133:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liefshitz, B., A. Parket, R. Maya, and M. Kupiec. 1995. The role of DNA repair genes in recombination between repeated sequences in yeast. Genetics 140:1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limón, A., N. Nakajima, R. Lu, H. Z. Ghory, and A. Engelman. 2002. Wild-type levels of nuclear localization and human immunodeficiency virus type 1 replication in the absence of the central DNA flap. J. Virol. 76:12078-12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manivasakam, P., S. C. Weber, J. McElver, and R. H. Schiestl. 1995. Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res. 23:2799-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maxwell, A., R. Craigie, and K. Mizuuchi. 1987. B protein of bacteriophage mu is an ATPase that preferentially stimulates intermolecular DNA strand transfer. Proc. Natl. Acad. Sci. USA 84:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller, M. D., B. Wang, and F. D. Bushman. 1995. Human immunodeficiency virus type 1 preintegration complexes containing discontinuous plus strands are competent to integrate in vitro. J. Virol. 69:3938-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monokian, G. M., L. T. Braiterman, and J. D. Boeke. 1994. In-frame linker insertion mutagenesis of yeast transposon Ty1: mutations, transposition and dominance. Gene 139:9-18. [DOI] [PubMed] [Google Scholar]
- 53.Moore, S. P., G. Liti, K. M. Stefanisko, K. M. Nyswaner, C. Chang, E. J. Louis, and D. J. Garfinkel. 2004. Analysis of a Ty1-less variant of Saccharomyces paradoxus: the gain and loss of Ty1 elements. Yeast 21:649-660. [DOI] [PubMed] [Google Scholar]
- 54.Moore, S. P., L. A. Rinckel, and D. J. Garfinkel. 1998. A Ty1 integrase nuclear localization signal required for retrotransposition. Mol. Cell. Biol. 18:1105-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morisato, D., and N. Kleckner. 1987. Tn10 transposition and circle formation in vitro. Cell 51:101-111. [DOI] [PubMed] [Google Scholar]
- 56.Morlese, J., I. A. Teo, J. W. Choi, B. Gazzard, and S. Shaunak. 2003. Identification of two mutually exclusive groups after long-term monitoring of HIV DNA 2-LTR circle copy number in patients on HAART. AIDS 17:679-683. [DOI] [PubMed] [Google Scholar]
- 57.Mou, Z., A. E. Kenny, and M. J. Curcio. 2006. Hos2 and Set3 promote integration of Ty1 retrotransposons at tRNA genes in Saccharomyces cerevisiae. Genetics 172:2157-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mules, E. H., O. Uzun, and A. Gabriel. 1998. In vivo Ty1 reverse transcription can generate replication intermediates with untidy ends. J. Virol. 72:6490-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nevo-Caspi, Y., and M. Kupiec. 1996. Induction of Ty recombination in yeast by cDNA and transcription: role of the RAD1 and RAD52 genes. Genetics 144:947-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh, J., J. G. Julias, A. L. Ferris, and S. H. Hughes. 2002. Construction and characterization of a replication-competent retroviral shuttle vector plasmid. J. Virol. 76:1762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rattray, A. J., B. K. Shafer, and D. J. Garfinkel. 2000. The Saccharomyces cerevisiae DNA recombination and repair functions of the RAD52 epistasis group inhibit Ty1 transposition. Genetics 154:543-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinckel, L. A., and D. J. Garfinkel. 1996. Influences of histone stoichiometry on the target site preference of retrotransposons Ty1 and Ty2 in Saccharomyces cerevisiae. Genetics 142:761-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambrook, J. E., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 64.Scholes, D. T., M. Banerjee, B. Bowen, and M. J. Curcio. 2001. Multiple regulators of Ty1 transposition in Saccharomyces cerevisiae have conserved roles in genome maintenance. Genetics 159:1449-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharon, G., T. J. Burkett, and D. J. Garfinkel. 1994. Efficient homologous recombination of Ty1 element cDNA when integration is blocked. Mol. Cell. Biol. 14:6540-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 67.Shoemaker, C., J. Hoffman, S. P. Goff, and D. Baltimore. 1981. Intramolecular integration within Moloney murine leukemia virus DNA. J. Virol. 40:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sirven, A., F. Pflumio, V. Zennou, M. Titeux, W. Vainchenker, L. Coulombel, A. Dubart-Kupperschmitt, and P. Charneau. 2000. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96:4103-4110. [PubMed] [Google Scholar]
- 70.Smith, J. S., S. Y. Kim, and M. J. Roth. 1990. Analysis of long terminal repeat circle junctions of human immunodeficiency virus type 1. J. Virol. 64:6286-6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stetor, S. R., J. W. Rausch, M. J. Guo, J. P. Burnham, L. R. Boone, M. J. Waring, and S. F. Le Grice. 1999. Characterization of (+) strand initiation and termination sequences located at the center of the equine infectious anemia virus genome. Biochemistry 38:3656-3667. [DOI] [PubMed] [Google Scholar]
- 72.Sundararajan, A., B. S. Lee, and D. J. Garfinkel. 2003. The Rad27 (Fen-1) nuclease inhibits Ty1 mobility in Saccharomyces cerevisiae. Genetics 163:55-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, Y., H. Yang, and R. Craigie. 2004. LAP2alpha and BAF collaborate to organize the Moloney murine leukemia virus preintegration complex. EMBO J. 23:4670-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swanstrom, R., W. J. DeLorbe, J. M. Bishop, and H. E. Varmus. 1981. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc. Natl. Acad. Sci. USA 78:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varmus, H., and P. Brown. 1989. Retroviruses, p. 53-108. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 76.Voytas, D. F., and J. D. Boeke. 2002. Ty1 and Ty5 of Saccharomyces cerevisiae, p. 614-630. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 77.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 78.Wardle, S. J., M. O'Carroll, K. M. Derbyshire, and D. B. Haniford. 2005. The global regulator H-NS acts directly on the transpososome to promote Tn10 transposition. Genes Dev. 19:2224-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinert, T. A., K. M. Derbyshire, F. M. Hughson, and N. D. Grindley. 1984. Replicative and conservative transpositional recombination of insertion sequences. Cold Spring Harb. Symp. Quant. Biol. 49:251-260. [DOI] [PubMed] [Google Scholar]
- 80.Whitcomb, J. M., and S. H. Hughes. 1992. Retroviral reverse transcription and integration: progress and problems. Annu. Rev. Cell Biol. 8:275-306. [DOI] [PubMed] [Google Scholar]
- 81.Whitcomb, J. M., R. Kumar, and S. H. Hughes. 1990. Sequence of the circle junction of human immunodeficiency virus type 1: implications for reverse transcription and integration. J. Virol. 64:4903-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilhelm, M., and F. X. Wilhelm. 2001. Reverse transcription of retroviruses and LTR retrotransposons. Cell. Mol. Life Sci. 58:1246-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilke, C. M., S. H. Heidler, N. Brown, and S. W. Liebman. 1989. Analysis of yeast retrotransposon Ty insertions at the CAN1 locus. Genetics 123:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winston, F., D. T. Chaleff, B. Valent, and G. R. Fink. 1984. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107:179-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu, X., Y. Li, B. Crise, S. M. Burgess, and D. J. Munroe. 2005. Weak palindromic consensus sequences are a common feature found at the integration target sites of many retroviruses. J. Virol. 79:5211-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wurtzer, S., A. Goubard, F. Mammano, S. Saragosti, D. Lecossier, A. J. Hance, and F. Clavel. 2006. Functional central polypurine tract provides downstream protection of the human immunodeficiency virus type 1 genome from editing by APOBEC3G and APOBEC3B. J. Virol. 80:3679-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 88.Zennou, V., C. Serguera, C. Sarkis, P. Colin, E. Perret, J. Mallet, and P. Charneau. 2001. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol. 19:446-450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.