Abstract
The unique region of the capsid protein VP1 (VP1u) of human parvovirus B19 (B19) elicits a dominant immune response and has a phospholipase A2 (PLA2) activity, which is necessary for the infection. In contrast to the rest of the parvoviruses, the VP1u of B19 is thought to occupy an external position in the virion, making this region a promising candidate for vaccine development. By using a monoclonal antibody against the most-N-terminal portion of VP1u, we revealed that this region rich in neutralizing epitopes is not accessible in native capsids. However, exposure of capsids to increasing temperatures or low pH led to its progressive accessibility without particle disassembly. Although unable to bind free virus or to block virus attachment to the cell, the anti-VP1u antibody was neutralizing, suggesting that the exposure of the epitope and the subsequent virus neutralization occur only after receptor attachment. The measurement of the VP1u-associated PLA2 activity of B19 capsids revealed that this region is also internal but becomes exposed in heat- and in low-pH-treated particles. In sharp contrast to native virions, the VP1u of baculovirus-derived B19 capsids was readily accessible in the absence of any treatment. These results indicate that stretches of VP1u of native B19 capsids harboring neutralizing epitopes and essential functional motifs are not external to the capsid. However, a conformational change renders these regions accessible and triggers the PLA2 potential of the virus. The results also emphasize major differences in the VP1u conformation between natural and recombinant particles.
Human parvovirus B19 (B19) is the only well-documented member of the Parvoviridae causing disease in humans. It is generally associated with the mild and frequent childhood disease erythema infectiosum, or fifth disease (1). In some cases and depending on the physiological conditions of the host, other more severe clinical symptoms can develop, such as acute and chronic arthropathies (28), hemolytic disorders (32), and hydrops fetalis and fetal death (6, 10).
The single-stranded DNA genome of B19 is packaged into a nonenveloped, icosahedral capsid consisting of 60 structural subunits, of which approximately 95% are VP2 (58 kDa) and 5% are VP1 (83 kDa). VP1 differs from VP2 only in an N-terminal “unique region” (VP1u) composed of 227 additional amino acids (8, 27). Following the infection, antibodies against VP1 and VP2 are produced resulting in the rapid elimination of the virus from the peripheral blood. The dominant immune response against B19 is mainly elicited by the VP1-unique region, which harbors strong neutralizing epitopes (2, 31, 39). A poor immune response against VP1u has been linked to persistent infections (21).
The immunodominance of VP1u, the presence of neutralizing epitopes and experimental evidence suggest that in contrast to other parvoviruses, VP1u of B19 occupies an external position in the capsid and therefore is accessible to antibody binding. Baculovirus-derived empty capsids and a proportion of human plasma-derived virions could be immunoprecipitated by using antisera raised against the entire VP1u (30). Baculovirus-expressed B19 capsids containing truncated Flag-VP1 were recognized by an anti-Flag monoclonal antibody (MAb) (19). Similarly, baculovirus-derived B19 capsids in which VP1u was replaced with lysozyme were enzymatically active and immunogenic (26). These results suggest that VP1u occupies an external position on the capsid. In other studies, antibodies raised against peptides spanning the whole VP1u were highly neutralizing, but surprisingly, the neutralizing activity of the antisera did not correlate with binding activity to recombinant empty capsids, which was low or absent (2, 31), suggesting that stretches of VP1u might be internal and not accessible. The position occupied by VP1u in the native capsid is of considerable importance for several reasons. The immunodominance, presence of neutralizing epitopes, and accessibility make VP1u a promising target for the development of vaccines. VP1u has also important functions in the virus life cycle. It harbors a phospholipase A2 (PLA2) motif (12) that is required for the infection (16, 37). It has been recently shown that capsids without the entire VP1 are not infectious and are unable to be exported from the nuclei (38). The presumed external position of VP1u PLA2 has led to the assumption that extracellular B19 capsids are enzymatically active. The PLA2 activity of B19 capsids is thought to play a role in the pathogenesis of the virus and in particular in the induction of autoimmune reactions and inflammatory processes (22, 24, 36).
Most of the studies conducted to examine the external conformation of VP1u have been performed using baculovirus-derived capsids, which does not necessarily correspond to the structure of VP1u in native particles. In order to identify the position of VP1u in natural B19 capsids, we have investigated the accessibility of two distant regions of the protein playing a role in virus infection and immunology. One is situated at the most-amino-terminal portion of VP1u where various neutralizing epitopes have been previously identified (2), and the other is situated near the junction between VP1 and VP2 where the PLA2 enzymatic core is located (12). The results showed that while these critical regions of VP1u are accessible in recombinant capsids, they are not exposed in the native particles. However, after an in vitro or cell-mediated stimulus, they become accessible, leading to antibody binding and subsequent virus neutralization or leading to the activation of the viral PLA2 potential.
MATERIALS AND METHODS
Cells and viruses.
UT7/Epo cells were cultured in RPMI with 10% FCS and 2 U/ml of recombinant human erythropoietin (Janssen-Cilag, Midrand, South Africa) at 37°C and 7.5% CO2. B19-containing serum samples were obtained from different individuals: Mar-54 (genotype 1; Marburg, Germany); Char-89 (genotype 1; Charlotte, NC); and Char-07 (genotype 1; Charlotte, NC). The serum samples did not contain B19-specific immunoglobulin M (IgM) or IgG antibodies. B19 virus was concentrated from infected serum by ultracentrifugation through 20% sucrose. The viral pellets were washed and resuspended in phosphate-buffered saline (PBS). Baculovirus-expressed B19 capsids were kindly provided by R. Franssila (Helsinki, Finland) and were produced in High-5 cells as described elsewhere (14).
Antibodies.
Two monoclonal antibodies, one specific for the unique region of minor capsid protein VP1 (MAb 1418-1) and the other directed to the major capsid protein VP2 (MAb 860-55D), were kindly provided by S. Modrow (Regensburg, Germany). The two MAbs were produced from peripheral blood mononuclear cells from normal, healthy individuals with high titers of serum antibodies against B19 virus proteins (15). MAb 1418-1 recognizes an epitope spanning VP1u residues 30 to 42, while MAb 850-55D is directed to a conformational epitope of VP2 and only recognizes intact capsids (11). For immunoblotting, a mouse anti-B19 VP antibody was used (US Biologicals, Swampscott, MA). For immunofluorescent detection of B19 proteins, a mouse antibody against human parvovirus B19 was applied (Novocastra, Newcastle, United Kindom).
Exposure of viral particles to heat and low pH.
Before any treatment, B19 capsids diluted in PBSA (PBS with 1% bovine serum albumin [BSA]) were precleared by incubation with protein L and G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h to eliminate nonspecific binding and remove traces of immunoglobulins and immunocomplexes that might be present. The cleared supernatants were exposed to heat in thin-walled tubes for 3 min in a preheated thermoblock. After temperature treatment, the samples were cooled on ice and immediately used for subsequent reactions. When total particle disintegration was intended, the viral suspensions were heated to 85°C for 10 min. For pH treatments, the viral suspension was acidified by adding HCl and the pH was monitored. After the treatment, the pH of the viral suspension was neutralized by dilution in PBSA, for immunoprecipitation experiments, or in assay buffer (Cayman Chemical, Ann Arbor, MI), for measurement of PLA2 activity. To study the reversibility of the VP1u rearrangement, the temperature or the pH of the viral suspension was restored in a gradual fashion to the original values and further incubated at 37°C for 1 h.
Immunoprecipitation.
Following heat or low-pH treatments, B19 capsids were immunoprecipitated with MAb 1418-1 (against VP1u) or with MAb 850-55D (against intact capsids). After overnight incubation at 4°C in the presence of 20 μl of protein G-agarose beads, the supernatant was discarded or used for a further immunoprecipitation. The beads were washed four times with PBSA. Immunoprecipitated viral capsids were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. After transfer to a polyvinylidene difluoride membrane, the blot was probed with a mouse anti-B19 VP antibody (1:500; US Biologicals, Swampscott, MA), followed by a horseradish peroxidase-conjugated secondary antibody (1:20,000 dilution). The viral structural proteins were visualized with a chemiluminescence system (Pierce, Rockford, IL).
Quantitative PCR.
Amplification and real-time detection of PCR products were performed by using the Lightcycler system (Roche Diagnostics, Rotkreuz, Switzerland) with SYBR green (Roche). PCR was carried out using the FastStart DNA SYBR green kit (Roche) following the manufacturer's instructions. The primers used for B19 DNA amplification were B19-forward (5′-GGGCAGCCATTTTAAGTGTTT-3′) and B19-reverse (5′-GCACCACCAGTTATCGTTAGC-3′).
Infectivity assay.
UT7/Epo cells were seeded onto 96-well plates at a concentration of 7 × 104 cells/well. Cells were infected with B19 at 500 DNA-containing particles per cell in RPMI without serum. After 1 h at 4°C, the cells were transferred to 37°C and further incubated for 4 days in the presence of 10% FCS and 2 U/ml of erythropoietin. The cells were fixed with acetone-methanol (1/1 [vol/vol]) for 5 min at −20°C. After fixation, the cells were air dried and washed with PBSA. Subsequently, a mouse antibody against human parvovirus B19 was applied (1:40 diluted in PBSA, clone R92F6 IgG1; Novocastra) for 1 h at room temperature. The cells were washed three times with PBSA for 5 min, and as a secondary antibody, a conjugated F(ab′)2 fragment of goat anti-mouse immunoglobulins was added (1:50 dilution; DakoCytomation, Glostrup, Denmark) for 1 h at room temperature. After final washings with PBSA, the cells were overlaid with 50 μl glycerol-PBS solution (1:1) and examined under a fluorescence microscope.
Binding assay.
UT7/Epo cells were infected with 50 DNA-containing particles per cell for 1 h at 4°C to allow virus attachment but not internalization in the presence or absence of antibody against VP1u. Cells were then washed four times with ice-cold PBSA to remove unbound virus. Subsequently, the cell pellet was resuspended in 200 μl of PBSA and total DNA was extracted by using the DNeasy tissue kit (QIAGEN, Valencia, CA). Cell-associated viral DNA was quantified by using real-time PCR as specified above.
Phospholipase A2 activity assay.
Native and baculovirus-expressed B19 capsids were assayed for PLA2 activity before and after heat or manoalide treatments by using a colorimetric assay (sPLA2 assay kit; Cayman Chemical, Ann Arbor, MI), following the manufacturer's instructions. The absorbance at 414 nm was determined every minute. The PLA2 activity was expressed as micromoles of substrate hydrolyzed per minute per milliliter.
RESULTS
Accessibility of VP1u in native capsids.
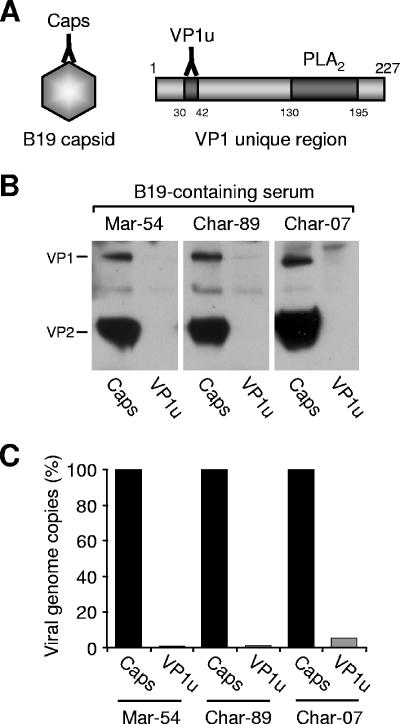
Two MAbs, one specific for VP1u (MAb 1418-1), and the other recognizing only intact capsids (MAb 860-55D), were used to immunoprecipitate B19 capsids from various B19-containing serum samples (Fig. 1A). While MAb 860-55D recognized and efficiently immunoprecipitated the capsids present in the serum, no reactivity was observed with MAb 1418-1 (Fig. 1B). In order to increase the sensitivity of the assay, the amount of viral DNA present in the immunoprecipitated fraction was amplified and quantified by real-time PCR. The results showed that MAb 1418-1 immunoprecipitated only 1 to 5% of the viral DNA immunoprecipitated with MAb 860-55D (Fig. 1C), indicating that in the vast majority of native capsids, the VP1u epitope recognized by MAb 1418-1 is not accessible.
FIG. 1.
Accessibility of VP1u in native B19 capsids. (A) Schematic representation indicating the localization of the epitopes recognized by MAb 860-55D (intact capsid) and MAb 1418-1 (VP1u) and the position of the PLA2 motif within the VP1u. (B) Immunoprecipitation of B19 capsids from three different B19-containing human serum samples by MAb 1418-1 (VP1u) and MAb 860-55D (Caps). (C) Quantification of B19 DNA in the immunoprecipitated fractions.
VP1u becomes progressively accessible at increasing temperatures.
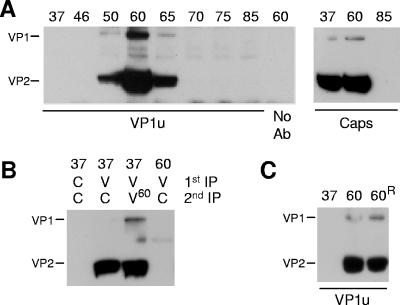
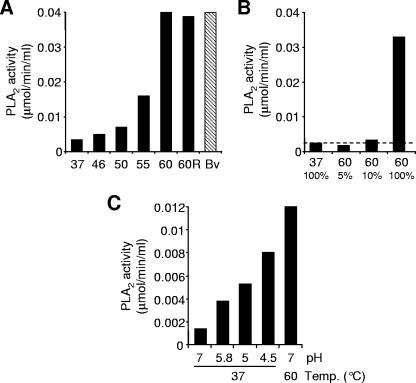
It has been previously shown for several parvoviruses that mild heat treatments mimic cell-mediated capsid conformational changes leading to the externalization of the internal VP1u sequences (4, 9, 20, 35). In an attempt to investigate whether a similar rearrangement can be induced in B19 capsids, viral suspensions were briefly exposed to increasing temperatures. As shown in Fig. 2A, the reactivity of MAb 1418-1 increased drastically following the heat treatment and was maximal at 60°C. The amount of viral capsids immunoprecipitated with MAb 1418-1 at 60°C was similar to the amount immunoprecipitated at 37°C with the MAb 860-55D (Fig. 2A). In both cases, no more viral proteins were detectable in the supernatants (Fig. 2B), indicating that VP1u is exposed in virions as well as in empty capsids. The progressive lower reactivity of MAb 1418-1 with VP1 protein at temperatures above 60°C suggests that MAb 1418-1 recognizes a conformationally defined epitope.
FIG. 2.
Accessibility of VP1u after heat treatment. (A) B19 capsids were treated at increasing temperatures (from 37 to 85°C) for 3 min, cooled on ice, and immunoprecipitated with MAb 1418-1 (VP1u) or MAb 860-55D (Caps). (B) Supernatants from previous immunoprecipitation (1st IP) were again immunoprecipitated (2nd IP). (C) Reversibility of VP1u externalization. Capsids were treated at 60°C for 3 min and rapidly cooled on ice (lane 60) or slowly cooled to 37°C and further incubated at 37°C for 1 h (60R). The capsids were subsequently immunoprecipitated with MAb 1418-1 (VP1u). C, Caps; V, VP1u; V60, supernatant heated at 60°C for 3 min before immunoprecipitation.
Irreversibility of heat-induced VP1u exposure.
We further investigated the capability of B19 capsids to restore the original internal VP1u conformation after its heat-induced exposure. B19 capsids were heated to 60°C for 3 min to achieve the maximal externalization of VP1u. Subsequently, some viral suspensions were cooled rapidly on ice, while others were slowly cooled to 37°C and further incubated at 37°C for 1 h. The results showed that once externalized, this region of VP1u remained steadily accessible on the capsid surface (Fig. 2C).
Acidification leads to VP1u accessibility.
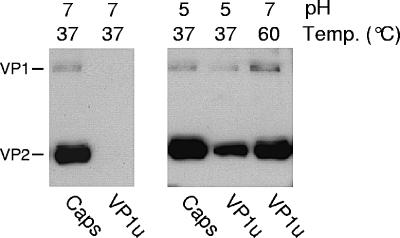
Parvoviruses enter the host cells via receptor-mediated endocytosis, where they encounter an acidified environment. We have previously shown conformational changes in the capsid of minute virus of mice (MVM) during the endosomal trafficking of the virus (25, 29). One of these capsid rearrangements, which depend on the low endosomal pH, is the externalization of the VP1u originally buried inside the capsid. It was therefore of interest to investigate the effect of low pH on B19 VP1u rearrangement. As shown in Fig. 3, exposure of viral capsids to mild endosome-like acidic conditions also induced a conformational change leading to the accessibility of the VP1u.
FIG. 3.
Accessibility of VP1u after low-pH treatment. B19 capsids were treated at pH 7 or 5 for 1 h at 37°C. After the treatment, the pH of the viral suspension was neutralized by dilution in PBSA and the capsids were immunoprecipitated with MAb 1418-1 (VP1u) or MAb 860-55D (Caps).
The VP1u epitope recognized by MAb 1418-1 becomes accessible only after cell receptor attachment.
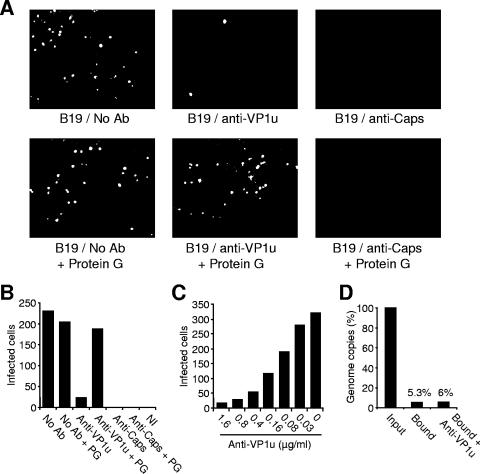
By using a human erythroid cell colony-forming assay, it has been previously shown that MAb 1418-1 and MAb 860-55D both have a strong neutralizing activity (15). We evaluated the neutralization capacity of the MAbs by using an immunofluorescence infectivity assay, as described above. The results confirmed the strong neutralizing activity of both MAbs (Fig. 4A and B), which was dose dependent (Fig. 4C). Giving the lack of reactivity of MAb 1418-1 with B19 capsids under natural cell-free conditions, we hypothesized that the MAb binds and neutralizes the virus following putative capsid rearrangements induced by cell receptor attachment. To verify this hypothesis, following the incubation of the virus suspension with MAb 1418-1 or MAb 860-55D, the MAbs were removed by adding protein G-agarose beads (Santa Cruz Biotechnology). After overnight incubation, the supernatant was added to the cells. The results showed that the infectivity could be rescued in the samples incubated with MAb 1418-1 but not with MAb 860-55D (Fig. 4A and B), indicating that binding and subsequent virus neutralization by MAb 1418-1 occur only after cell-induced conformational changes.
FIG. 4.
Neutralization activity of MAb 1418-1 (VP1u) and MAb 860-55D (Caps). (A) B19 was preincubated with the MAbs or PBS (no antibody [No Ab]) for 1 h at 4°C. Subsequently, protein G-agarose beads or PBS was added, as indicated, and the mixture was further incubated overnight. After a short centrifugation to pellet the beads, the supernatants were added to UT7 cells at a multiplicity of infection of 500 DNA-containing particles per cell and incubated at 37°C for 4 days. Cells were fixed and stained as specified in Materials and Methods and visualized under a fluorescence microscope. A representative field is shown. (B) Cells showing specific B19 immunofluorescence staining indicative of infection. (C) Cells infected with B19 in the presence of increasing concentrations of MAb 1418-1 (VP1u). (D) Attachment of B19 to UT7 cells in the presence or absence of MAb 1418-1 (VP1u). Cells were infected as specified above in the presence or absence of MAb 1418-1 and incubated at 4°C for 1 h to allow virus attachment. Subsequently, cells were washed four times with PBSA and the amount of cell-associated viral DNA was quantified by real-time PCR as described in Materials and Methods. PG, protein G-agarose beads.
The mechanism of virus neutralization by anti-VP1u MAb was further investigated. Typically, binding of B19 to UT7/Epo cells is poor (5 to 6% of the input). As shown in Fig. 4D, virus attachment was unaffected in the presence of MAb 1418-1, indicating that the MAb disrupts a step(s) following virus attachment to the cell.
The VP1u PLA2 motif is internal in native B19 capsid but can be externalized upon heat or low-pH treatments.
Parvoviruses have a PLA2 activity associated with VP1u (4, 7, 12, 13, 23, 24, 34, 37). The VP1u PLA2 activity in native extracellular capsids is undetectable, but it increases after heat or pH treatments (4, 13, 34). The reason is that VP1u PLA2 is internal but becomes externalized after such treatments. We have quantified and compared the PLA2 activities of untreated and heat- or low-pH-treated B19 native capsids. The results showed that similarly to other parvoviruses, the PLA2 activity of untreated B19 particles is barely detectable but increases drastically upon mild heat or low-pH treatments (Fig. 5). B19 capsids were unable to restore the internal position of the PLA2 motif under all condition tested, indicating that once exposed, the external position of the enzymatic motif is stable (Fig. 5A). The PLA2 activity of native untreated capsids was approximately 7% the activity of the heat-treated capsids (Fig. 5B).
FIG. 5.
PLA2 activity of heat- and low-pH-treated B19 native capsids. (A) PLA2 activity of B19 capsids (1.8 μg) was measured following exposure to increasing temperatures for 3 min and rapidly cooled on ice. The bar labeled “60R” represents capsids treated at 60°C for 3 min, slowly cooled to 37°C, and further incubated at 37°C for 1 h. Bv, bee venom (1 ng). (B) Quantification of the basal PLA2 activity of native untreated B19 capsids. Viral capsids (1.2 μg) were treated for 3 min at 60°C to achieve maximal PLA2 activity. Following the heat treatment, the viral suspension was diluted 10- and 20-fold and the PLA2 activity was measured and compared to that of untreated capsids. (C) PLA2 activity of low-pH-treated B19 capsids. After the treatment, the pH of the viral suspension was neutralized by dilution in assay buffer (Cayman Chemical, Ann Arbor, MI). A final amount of 0.5 μg of B19 capsids was used to measure the PLA2 activity.
The externalization of the amino-terminal part of VP1u (amino acid positions 30 to 42) and the PLA2 motif (amino acid positions 130 to 195) had common features. The maximal exposure of both regions was achieved at the same temperature (Fig. 2A and 5A) and in both cases was irreversible (Fig. 2C and 5A). The reactivity of MAb 1418-1 decreased at temperatures above 60°C (Fig. 2A), as did the enzymatic activity of the capsids (data not shown). Exposure to heat induced the externalization of both VP1u regions more efficiently than did low pH (Fig. 3 and Fig. 5C).
Stability of the internal conformation of VP1u in native capsids.
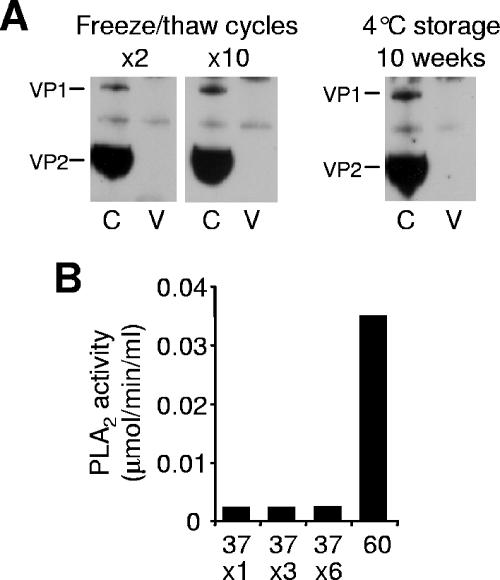
Standard laboratory manipulations, like freeze/thaw cycles or long-term storage of viruses at 4°C, might trigger capsid structural modifications and in some cases a reduction of the viral infectivity (17). With the aim of investigating the influence of standard laboratory manipulations on the VP1u native conformation, we subjected viral suspensions to freeze-thaw cycles (2 to 10) or to a long period of storage (10 weeks) at 4°C. These treatments failed to render VP1u accessible to antibody binding (Fig. 6A) or to increase the PLA2 activity (Fig. 6B), indicating that the native VP1u conformation within the viral capsid is highly stable.
FIG. 6.
Stability of the internal conformation of VP1u in native capsids. B19 capsids were subjected to freeze-thaw cycles (2 and 10) or to a long period of storage (10 weeks) at 4°C. (A) Capsids were immunoprecipitated with MAb 1418-1 (V; against VP1u) or 860-55D (C; against intact capsids). (B) Following various freeze-thaw cycles, the PLA2 activity of B19 capsids (1.2 μg) was measured and compared to the activity of heat-treated (37 and 60°C) particles.
Comparison of levels of VP1u accessibility in native and baculovirus-derived capsids.
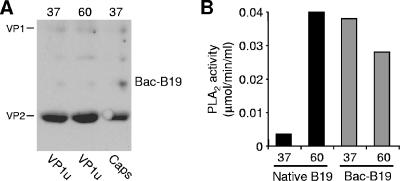
Our results with natural capsids are different from those of previous studies using baculovirus-derived capsids in which it was shown that VP1u is exposed on the capsid surface (19, 26, 30). In order to identify possible differences in the VP1u conformation between natural and recombinant capsids, the accessibility of VP1u in baculovirus-derived capsids was also investigated. In clear contrast to native untreated capsids, the VP1u epitope recognized by MAb 1418-1 was readily accessible in the untreated recombinant capsids and exposure to heat did not increase its reactivity (Fig. 7A).
FIG. 7.
VP1u accessibility in baculovirus-derived B19 capsids before (37°C) and after treatment of capsids at 60°C for 3 min. (A) Immunoprecipitation of baculovirus-expressed B19 capsids (Bac-B19) by MAb 1418-1 (VP1u) and MAb 860-55D (Caps). (B) PLA2 activity of native and baculovirus-derived B19 capsids (1.8 and 3.5 μg, respectively).
The accessibility of the VP1u region where the PLA2 motif is located was also examined. For this purpose, the PLA2 activity of natural and baculovirus-derived B19 capsids was measured and compared. As shown in Fig. 7B, the PLA2 activity from baculovirus-derived particles was already detectable in the absence of any treatment. Exposure of capsids to heat drastically increased the PLA2 activity in the native particles but not in the recombinant capsids (Fig. 7B).
Inhibition of B19 PLA2 by manoalide.
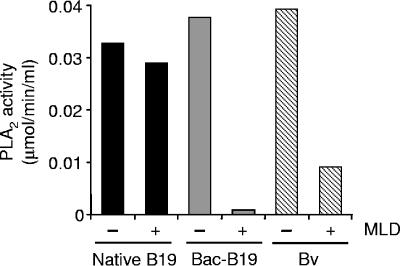
The phospholipase enzymes of bee venom and parvoviruses have been classified as group III and XIII PLA2 enzymes, respectively (3, 7). We have examined the inhibitory effect of manoalide, a potent inhibitor of PLA2 enzymes on native and baculovirus-expressed capsids. Interestingly, while manoalide efficiently inhibited the PLA2 activity from bee venom and baculovirus-derived B19 capsids, the PLA2 activity of native B19 capsids was basically unaffected (Fig. 8), indicating the existence of critical conformational differences in the PLA2 enzymatic core between recombinant and native capsids.
FIG. 8.
Inhibition of the PLA2 enzymes from native B19 capsids, baculovirus-derived particles, and bee venom by manoalide. Heat-treated B19 native capsids (1.2 μg), untreated recombinant B19 capsids (3.5 μg), and bee venom (1 ng) were incubated with the specific PLA2 inhibitor manoalide (50 μM) or with dimethyl sulfoxide (the solvent used to resuspend manoalide) for 3 h. Subsequently, the PLA2 activity was measured. Bv, bee venom; MLD, manoalide.
DISCUSSION
The immune response to parvovirus B19 infection is directed against VP1 and VP2. The unique region of the VP1 (VP1u) of B19 elicits a dominant immune response, and it has been shown to harbor efficient neutralizing epitopes (2, 31, 39). These findings suggest that VP1u is located externally to the capsid, a feature in sharp contrast to those of other parvoviruses, whose VP1u has been shown to be internal and not accessible. The accessibility of VP1u in B19 capsids was first examined by using antisera raised against the entire VP1u protein. By using these antisera, it was possible to fully immunoprecipitate baculovirus-derived particles and a proportion of virions (30). However, since the antisera used in this study contained antibodies spanning the whole VP1u, it was not possible to identify the accessible region(s). Antibodies originated from small peptides spanning the entire VP1 were shown to be neutralizing. Surprisingly, binding of these antibodies to recombinant capsids was irregular and particularly low for antibodies targeting the most-amino-terminal region of VP1u (2, 31). In another study, it has been shown that recombinant B19 capsids containing progressively truncated VP1u sequences fused to a Flag peptide at the amino terminus could be recognized by an anti-Flag antibody (19). However, as stated by the authors, the results obtained from recombinant B19 with truncated VP1u cannot be extrapolated to the structure of VP1u in the native virions.
Our understanding of the B19 structure has largely depended on recombinant capsids. The extensive characterization of B19 recombinant particles contrasts with the limited studies performed with natural virions. This is mainly due to the lack of a highly permissive cell line where natural B19 could easily propagate to large quantities. Although B19 recombinant and natural capsids have been shown to be similar (5, 18), the particular position occupied by VP1u in the capsid structure has not been systematically studied and compared. In a study performed with MVM, it was observed that in the absence of treatments, baculovirus-derived capsids showed already significant levels of PLA2 activity while natural capsids did not (13). It was concluded that baculovirus-expressed MVM capsids might assemble slightly differently in insect cells, such that some VP1us are exposed on the particle surface. In order to better understand the natural position of VP1u in native B19 virions, we have examined the accessibility of two distant regions of the protein playing a role in the virus infection and immunology. One of the regions examined is the most-amino-terminal portion of VP1u, in which a cluster of neutralizing epitopes has been previously identified (2). The other region examined is positioned near the junction between VP1 and VP2 where the PLA2 enzymatic core is located. Our results indicate that VP1u of native B19 or at least these important regions of the protein are not accessible as previously suggested.
Treatments of capsids with heat or low pH have been shown to render VP1u on the surface of the particles in all parvoviruses examined (4, 9, 13, 20, 34, 35). VP1u can also be exposed during cell entry (25, 29, 33, 35). Similarly, the B19 VP1u regions examined in the present studies became exposed upon heat or low-pH treatments. Additionally, exposure of VP1u was also observed after B19 incubation with susceptible cells, as judged by the neutralization of the virus with MAb 1418-1 (Fig. 4).
The position occupied by VP1u in the B19 capsid is particularly important. VP1u elicits a dominant immune response in humans, and it contains highly neutralizing epitopes, making this region of VP1 a promising candidate for vaccine development. The neutralizing effect of antibodies can be achieved by interference of the antibody with the attachment of the virion to the cell surface or subsequent processes. Despite the fact that MAb against VP1u did not bind the virus (Fig. 1B) and did not block virus attachment (Fig. 4D), the antibody was still able to neutralize the infection (Fig. 4A, B, and C), indicating that the epitope, originally not accessible, became exposed after receptor attachment. These results are in agreement with another study in which antibodies against VP1u were highly neutralizing but surprisingly poorly reactive against free virus (31). These observations would imply that there are neutralizing antibodies against VP1u which do not target the free viral capsids but exclusively target the cell-associated capsids. This is particularly important since antibodies directed to the VP1u of B19 play a critical role in the resolution of the infection.
In addition to neutralizing epitopes, VP1u has a PLA2 activity, which is necessary for the infection (37). The general assumption that VP1u of B19 is external to the capsids has led to the suggestion that enzymatically active extracellular virions might contribute to the induction of inflammatory responses (24). Our data indicate, however, that the PLA2 motif of B19 is internal in most of the viral particles and therefore not active. It has been recently shown that B19 PLA2 can induce synoviocyte migration without infection (24). This effect might be due to the basal PLA2 activity of B19 (Fig. 5B), which although low, was still higher than that in any other parvovirus (4, 13, 34). In fact, the effect on synoviocytes was only observed when a large amount of virions (exceeding 1011) was incubated with the cells. Another possibility is that the PLA2 potential of B19 is triggered by binding to a cellular receptor. In this way, the receptor-bound B19 capsids would become enzymatically active before internalization. In our studies, we show evidence that at least the most-N-terminal portion of VP1u becomes accessible on the capsid surface following receptor binding; however, whether the receptor-induced VP1u externalization also involves the PLA2 motif region would require further investigation. It has been previously shown for other parvoviruses that VP1u becomes exposed only during the intracellular trafficking (29, 35) and in particular inside the endosomes (25, 33). In this sense, the early conformational change of the B19 VP1u on the cell surface involving a cellular receptor(s) would represent a unique mechanism within the parvovirus family.
The results obtained in previous studies using B19 recombinant capsids and in the present studies using natural capsids suggest major differences in VP1u conformation. We have further confirmed those differences by demonstrating the direct accessibility of VP1u in untreated baculovirus-derived capsids and the distinct susceptibility to the PLA2 inhibitor manoalide. Further studies are in progress to identify the cellular factor that triggers the exposure of these regions in the native capsids, which will help us to understand the role of VP1u in the natural infection and the mechanisms of B19 neutralization in humans by antibodies targeting originally inaccessible VP1u epitopes.
Acknowledgments
The authors thank S. Modrow (Regensburg, Germany) for kindly providing the monoclonal antibodies 1418-1 against VP1u and 860-55D against intact capsids. We gratefully acknowledge the gift of baculovirus-derived B19 capsids from R. Franssila (Helsinki, Finland).
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Anderson, M. J., S. E. Jones, S. P. Fisher-Hoch, E. Lewis, S. M. Hall, C. L. R. Bartlett, B. J. Cohen, P. P. Mortimer, and M. S. Pereira. 1983. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet i:1378. (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S., M. Momoeda, M. Kawase, S. Kajigaya, and N. S. Young. 1995. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicit neutralizing antibodies in rabbits. Virology 206:626-632. [DOI] [PubMed] [Google Scholar]
- 3.Balsinde, J., M. V. Winstead, and E. A. Dennis. 2002. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 531:2-6. [DOI] [PubMed] [Google Scholar]
- 4.Bleker, S., F. Sonntag, and J. A. Kleinschmidt. 2005. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J. Virol. 79:2528-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, C. S., J. W. M. Van Lent, J. M. Vlak, and W. J. M. Spaan. 1991. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J. Virol. 65:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, T., A. Anand, L. D. Ritchie, J. P. Clewley, and T. M. Reid. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033-1034. [DOI] [PubMed] [Google Scholar]
- 7.Canaan, S., Z. Zadori, F. Ghomashchi, J. Bollinger, M. Sadilek, M. E. Moreau, P. Tijssen, and M. H. Gelb. 2004. Interfacial enzymology of parvovirus phospholipases A2. J. Biol. Chem. 279:14502-14508. [DOI] [PubMed] [Google Scholar]
- 8.Cotmore, S. F., V. C. McKie, L. J. Anderson, C. R. Astell, and P. Tattersall. 1986. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J. Virol. 60:548-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotmore, S. F., A. M. D'abramo, Jr., C. M. Ticknor, and P. Tattersall. 1999. Controlled conformational transitions in the MVM virion expose the VP1 N-terminus and viral genome without particle disassembly. Virology 254:169-181. [DOI] [PubMed] [Google Scholar]
- 10.Crane, J., et al. 2002. Parvovirus B19 infection in pregnancy. J. Obstet. Gynaecol. Can. 24:727-743. [PubMed] [Google Scholar]
- 11.Dorsch, S., B. Kaufmann, U. Schaible, E. Prohaska, H. Wolf, and S. Modrow. 2001. The VP1-unique region of parvovirus B19: amino acid variability and antigenic stability. J. Gen. Virol. 82:191-199. [DOI] [PubMed] [Google Scholar]
- 12.Dorsch, S., G. Liebisch, B. Kaufmann, P. von Landenberg, J. H. Hoffmann, W. Drobnik, and S. Modrow. 2002. The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity. J. Virol. 76:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr, G. A., L. G. Zhang, and P. Tattersall. 2005. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc. Natl. Acad. Sci. USA 102:17148-17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franssila, R., K. Hokynar, and K. Hedman. 2001. T helper cell-mediated in vitro responses of recently and remotely infected subjects to a candidate recombinant vaccine for human parvovirus B19. J. Infect. Dis. 183:805-809. [DOI] [PubMed] [Google Scholar]
- 15.Gigler, A., S. Dorsch, A. Hemauer, C. Williams, S. Kim, N. S. Young, S. Zolla-Pazner, H. Wolf, M. K. Gorny, and S. Modrow. 1999. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J. Virol. 73:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girod, A., C. E. Wobus, Z. Zadori, M. Ried, K. Leike, P. Tijssen, J. A. Kleinschmidt, and M. Hallek. 2002. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J. Gen. Virol. 83:973-978. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, C. K., J. Leszczynski, R. K. Gupta, and G. R. Siber. 1996. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine 14:1417-1420. [DOI] [PubMed] [Google Scholar]
- 18.Kajigaya, S., H. Fujii, A. Field, S. Anderson, S. Rosenfeld, L. J. Anderson, T. Shimada, and N. S. Young. 1991. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc. Natl. Acad. Sci. USA 88:4646-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawase, M., M. Momoeda, N. S. Young, and S. Kajigaya. 1995. Most of the VP1 unique region of B19 parvovirus is on the capsid surface. Virology 211:359-366. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg, S., B. Böttcher, C. W. von der Lieth, S. Bleker, and J. A. Kleinschmidt. 2005. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J. Virol. 79:5296-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzman, G. J., B. J. Cohen, A. M. Field, R. Oseas, R. M. Blaese, and N. S. Young. 1989. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J. Clin. Investig. 84:1114-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann, H. W., P. von Landenberg, and S. Modrow. 2003. Parvovirus B19 infection and autoimmune disease. Autoimmun. Rev. 2:218-223. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y., Z. Zadori, H. Bando, R. Dubuc, G. Fediere, J. Szelei, and P. Tijssen. 2001. Genome organization of the densovirus from Bombyx mori (BmDNV-1) and enzyme activity of its capsid. J. Gen. Virol. 82:2821-2825. [DOI] [PubMed] [Google Scholar]
- 24.Lu, J., N. Zhi, S. Wong, and K. E. Brown. 2006. Activation of synoviocytes by the secreted phospholipase A2 motif in the VP1-unique region of parvovirus B19 minor capsid protein. J. Infect. Dis. 193:582-590. [DOI] [PubMed] [Google Scholar]
- 25.Mani, B., C. Baltzer, N. Valle, J. M. Almendral, C. Kempf, and C. Ros. 2006. Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome. J. Virol. 80:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamura, K., S. Kajigaya, M. Momoeda, S. J. Smith-Gill, and N. S. Young. 1994. Parvovirus particles as platforms for protein presentation. Proc. Natl. Acad. Sci. USA 91:8507-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa, K., and N. Young. 1987. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J. Virol. 61:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid, D. M., T. M. Reid, T. Brown, J. A. Rennie, and C. J. Eastmond. 1985. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet i:422-425. [DOI] [PubMed] [Google Scholar]
- 29.Ros, C., and C. Kempf. 2004. The ubiquitin-proteasome machinery is essential for nuclear translocation of incoming minute virus of mice. Virology 324:350-360. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld, S. J., K. Yoshimoto, S. Kajigaya, S. Anderson, N. S. Young, A. Field, P. Warrener, G. Bansal, and M. S. Collett. 1992. Unique region of the minor capsid protein of human parvovirus B19 is exposed on the virion surface. J. Clin. Investig. 89:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saikawa, T., S. Anderson, M. Momoeda, S. Kajigaya, and N. S. Young. 1993. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J. Virol. 67:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serjeant, G. R., J. M. Topley, K. Mason, B. E. Serjeant, J. R. Pattison, S. E. Jones, and R. Mohamed. 1981. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet ii:595-597. [DOI] [PubMed] [Google Scholar]
- 33.Sonntag, F., S. Bleker, B. Leuchs, R. Fischer, and J. A. Kleinschmidt. 2006. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J. Virol. 80:11040-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suikkanen, S., M. Antila, A. Jaatinen, M. Vihinen-Ranta, and M. Vuento. 2003. Release of canine parvovirus from endocytic vesicles. Virology 316:267-280. [DOI] [PubMed] [Google Scholar]
- 35.Vihinen-Ranta, M., D. Wang, W. S. Weichert, and C. R. Parrish. 2002. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 76:1884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Landenberg, P., H. W. Lehmann, A. Knoll, S. Dorsch, and S. Modrow. 2003. Antiphospholipid antibodies in pediatric and adult patients with rheumatic disease are associated with parvovirus B19 infection. Arthritis Rheum. 48:1939-1947. [DOI] [PubMed] [Google Scholar]
- 37.Zadori, Z., J. Szelei, M. C. Lacoste, Y. Li, S. Gariepy, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]
- 38.Zhi, N., I. P. Mills, J. Lu, S. Wong, C. Filippone, and K. E. Brown. 2006. Molecular and functional analyses of a human parvovirus B19 infectious clone demonstrates essential roles for NS1, VP1, and the 11-kilodalton protein in virus replication and infectivity. J. Virol. 80:5941-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuffi, E., E. Manaresi, G. Gallinella, G. A. Gentilomi, S. Venturoli, M. Zerbini, and M. Musiani. 2001. Identification of an immunodominant peptide in the parvovirus B19 VP1 unique region able to elicit a long-lasting immune response in humans. Viral Immunol. 14:151-158. [DOI] [PubMed] [Google Scholar]