Abstract
Lentiviruses, human immunodeficiency viruses (HIVs), and simian immunodeficiency viruses (SIVs) are distinguished from oncoretroviruses by their ability to infect nondividing cells such as macrophages. Retroviruses must gain access to the host cell nucleus for replication and propagation. HIV and SIV preintegration complexes (PIC) enter nuclei after traversing the central aqueous channel of the limiting nuclear pore complex without membrane breakdown. Among the nucleophilic proteins, namely, matrix, integrase, Vpx, and Vpr, present in HIV type 2/SIV PIC, Vpx is implicated in nuclear targeting and is also available for incorporation into budding virions at the plasma membrane. The mechanisms of these two opposite functions are not known. We demonstrate that Vpx is a nucleocytoplasmic shuttling protein and contains two novel noncanonical nuclear import signals and a leptomycin B-sensitive nuclear export signal. In addition, Vpx interacts with the cellular tyrosine kinase Fyn through its C-terminal proline-rich motif. Furthermore, our data indicate that Fyn kinase phosphorylates Vpx and regulates its export from nucleus. Replacement of conserved tryptophan residues within domain 41 to 63 and tyrosine residues at positions 66, 69, and 71 in Vpx impairs its nuclear export, virion incorporation, and SIV replication in macrophages. Nuclear export is essential to ensure the availability of Vpx in the cytoplasm for incorporation into virions, leading to efficient viral replication within nondividing cells.
Human and simian immunodeficiency viruses (HIV and SIV) are able to infect terminally differentiated macrophages and memory T cells (6, 12, 14, 17, 29, 51, 52). This biological feature is necessary for viral dissemination and persistence and distinguishes lentiviruses from oncoretroviruses (30). Efficient uncoating of the viral core plays a critical role in lentivirus replication (54). Viral reverse transcription complex transcribes RNA into DNA, which forms the viral preintegration complex (PIC) in the cytoplasm and is then imported into the nucleus through the nuclear envelope via an active mechanism within 4 to 6 h of infection. The nuclear envelope is studded with nuclear pore complexes that form conduits for bidirectional transport of many macromolecules (7, 9, 15). During active transport, the central aqueous channel can accommodate protein complexes as large as 25 nm in diameter (9, 37, 38, 40). However, the HIV/SIV PIC, with a stoke diameter of 56 nm, represents one of the largest known examples of cargo successfully transported across the nuclear pore complex by a mechanism yet unknown.
Lentiviruses contain genes for regulatory (rev and tat) and accessory (vif, vpx, vpr, vpu, and nef) proteins in addition to the structural proteins (gag, pol, and env) that are found in all retroviruses (8, 51). The highly conserved (18, 25) viral protein X (Vpx) is predominantly found in the nuclei of HIV type 2 (HIV-2)- and SIV-infected cells, indicating the strength of its nuclear targeting signal (5, 26, 31, 39). This nucleophilic property of Vpx, coupled with its presence in the viral PIC, facilitates more efficient HIV/SIV replication in macrophages (12, 19, 31, 39, 41, 42). Vpx is packaged efficiently in progeny virions (1, 12, 26, 31) and localizes within the viral core (27). Immediately following entry of a new virion into the target cell Vpx becomes available during early replication events even before de novo viral protein synthesis starts. Late expression during virus production and early availability during initial infection enables Vpx to participate in the early stages of the viral life cycle.
Phosphorylation plays a critical role in controlling nuclear transport of proteins in eukaryotic cells from yeasts and plants to higher mammals (11, 14, 23, 33, 42, 49). Phosphorylation of HIV-1 MA, Vpr, and Vpx is essential for their association with viral PIC (2, 14, 24, 42). However, it is unclear how these nucleophilic proteins are directed into budding virions. The present study was designed to define the mechanism of Vpx nuclear export and its contribution to HIV-2/SIV replication in nondividing cells.
In this study, we have employed SIV molecular clones containing mutations that selectively compromise the nuclear export phenotype of Vpx while maintaining its import into the nucleus. We showed that export of SIV Vpx from the nucleus is mediated by a novel tryptophan-rich leptomycin B (LMB)-sensitive nuclear export signal (NES) located at the N terminus of Vpx. Furthermore, we showed that nuclear export is critical for the availability of Vpx in the cytoplasm for its efficient incorporation into budding virus particles. In addition, our data suggest that phosphorylation of Vpx by a cellular Src-like tyrosine kinase, Fyn, modulates its export from nucleus. Finally, by investigating the role of nuclear export of Vpx during viral infection, we demonstrated that it was directly implicated in SIV replication in macrophages.
MATERIALS AND METHODS
Construction of SIVsm(Pbj1.9) Vpx mutant proviral clones.
A QuikChange site-directed mutagenesis kit (Stratagene, United States) was used to introduce mutations into the vpx gene of infectious molecular clone SIVsm(PBj1.9). Mutagenized vpx genes were PCR amplified and inserted into the mammalian expression vector pCDNA3 (Invitrogen Life Technology, United States). None of the introduced nucleotide substitutions resulted in amino acid changes in the overlapping Vif open reading frame. All introduced mutations were confirmed by DNA sequence analysis.
Cell culture and infection.
293T, Cos-7, HeLa, CEMx174, and Jurkat cells were maintained in either Dulbecco's modified Eagle's medium or RPMI 1640 medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum. Macaque peripheral blood mononuclear cells (PBMCs) were obtained using heparin-treated whole blood and lymphocyte separation medium (Organon Teknika, United States). Macrophages were purified from unstimulated macaque PBMCs as described previously (31). Virus stocks were generated in 293T cells and used for infection of macaque PBMCs and macrophages as described previously (31).
Metabolic labeling and immunoprecipitation.
The infection-transfection protocol for the vaccinia virus expression system was as described previously (31). Briefly, Cos-7 cells were infected with vTF7-3, a vaccinia virus expressing T7 RNA polymerase (13), and transfected using wild-type Vpx or relevant Vpx mutant constructs using Lipofectin (Invitrogen life Technology, United States). Transfected cells were labeled with phosphate-free Dulbecco's modified Eagle's medium containing 1.0 mCi of 32Pi (Bhabha Atomic Research Centre, India). The labeled cells were lysed with lysis buffer without sodium dodecyl sulfate (SDS) (1% [vol/vol] Triton X-100, 0.5% [wt/vol] deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride in phosphate-buffered saline, and 0.2 mM Na2VO4). Labeled Vpx proteins were immunoprecipitated with anti-Vpx monoclonal antibody and resolved on SDS-8 to 15% polyacrylamide gel electrophoresis (SDS-8 to 15% PAGE) followed by autoradiography.
Western blot analysis.
Cos-7 cells in 60-mm-diameter dishes were infected with vTF7-3 and transfected with various Vpx expression plasmids as described previously (31). Expression of all the Vpx mutant proteins was determined by Western blot analysis using anti-Vpx monoclonal antibody. For determining the characteristics of Vpx packaging into virus particles, virions from the culture supernatants (cells transfected with Vpx wild-type or mutant proviral clones) were concentrated by ultracentrifugation (125,000 × g) through a 20% sucrose cushion for 2 h or virus-like particles (variants of Vpx cotransfected with Gag expression plasmids) were concentrated through a Centricon 30 apparatus and were resolved on SDS-12% PAGE followed by Western blot analysis using anti-Vpx and anti-Gag monoclonal antibodies.
Fluorescence microscopy.
Cos-7 cells in chamber culture slides (Becton Dickinson, United States) were infected with vTF7-3 and transfected using Vpx expression plasmids using Lipofectin as described previously (31). Transfected cells were fixed with 3% paraformaldehyde and probed with anti-Vpx monoclonal antibody (1:250). Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin G (Molecular Probes, The Netherlands) was used as a secondary antibody to visualize the subcellular localization of Vpx proteins. Vectors containing green fluorescent protein (GFP) fusion proteins were visualized directly. The cells were mounted in mounting medium (Vector Laboratories, United States) containing 4′,6′-diamidino-2-phenylindole (DAPI) to stain nuclei. Samples were viewed with an upright Nikon E800 microscope (Nikon, Japan) and photographed with a DXM1200 camera using Image Pro-Plus 4.5 software.
CAT assays.
Chloramphenicol acetyltransferase (CAT) assays were performed as essentially described by Hope et al. (20). Cos-7 cells were transfected with indicated reporter plasmids along with cytomegalovirus-beta-galactosidase expression plasmid. All the cell lysates were normalized for beta-galactosidase activity before CAT analysis, and each experiment was repeated three times.
Construction, expression, and purification of His tag-Vpx fusion proteins.
Vpx from SIVsm(PBj1.9) was cloned into pET16b vector at NdeI and BamHI sites and purified from Escherichia coli strain BL-21(DE3) as described previously (42). Purified proteins were stored at −70°C.
GST pulldown assays.
SH3 domains of Fyn, Src, Hck, full-length Crk, and importin-β as glutathione transferase (GST) fusion proteins were expressed in E. coli BL21(DE3) or M15-prap4 cells and purified as described previously (48). GFP and GFP-Vpx (full length) were synthesized using [35S]methionine and a T7-RNA polymerase-based rabbit reticulocyte lysate-coupled transcription/translation system per the instructions of the manufacturer (Promega, United States) and examined for integrity on SDS-15% PAGE. The binding reaction mixture comprised equal amounts of in vitro-translated GFP or GFP-Vpx with GST alone, GST-Fyn-SH3, GST-Src-SH3, GST-Hck-SH3, GST-Crk, and GST-importin-β bound to 50 μl of glutathione-Sepharose or Fyn SH3/SH2 domain-containing glutathione-agarose beads (Santa Cruz, United States) in a final volume of 300 μl of binding buffer (25 mM HEPES [pH 7.9], 150 mM KCl, 0.1% NP-40, 5% glycerol, 0.5 mM dithiothreitol, 0.4 mM phenylmethylsulfonyl fluoride, 1 mM sodium fluoride, 1 mM sodium orthovanadate, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin). After overnight incubation at 4°C, the beads were washed four times with 1.0 ml of binding buffer and boiled for 5 min in sample buffer containing SDS. Eluted proteins were separated by SDS-15% PAGE and autoradiographed.
In vitro kinase assay.
Purified recombinant Vpx protein (3 μg) was incubated with Fyn as well as mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase 2 (ERK-2) immunoprecipitates from Jurkat cells activated with phorbol myristate acetate (PMA) or recombinant Fyn or MAPK-ERK-2 (Upstate Biotechnology, United States) in 20 μl of kinase reaction buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 20 mM glycerol phosphate, and 0.1 mM sodium vanadate) containing 10 μCi of [γ-32P]ATP (Bhabha Atomic Research Centre, India). Samples were incubated for 30 min at 30°C, and reactions were terminated by adding 7 μl of SDS sample buffer and boiling for 5 min. A portion (5 μl) of the sample was separated on SDS-15% PAGE followed by autoradiography.
RESULTS
Vpx shuttles between nucleus and cytoplasm.
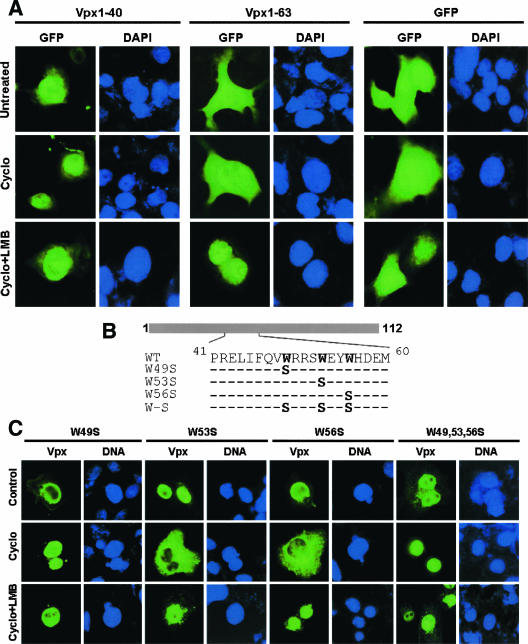
GFP fusions containing different Vpx deletion fragments expressed in either HeLa or Cos-7 cells were found to localize differentially (GFP-Vpx1-63 in the cytoplasm and GFP-Vpx20-40 in the nucleus) (48). This indicated the presence of NES in SIVsm Vpx. GFP-Vpx1-63 fusion proteins were probably shuttling into and out of the nucleus and appeared cytoplasmic due to longer dwell time. We therefore studied the effect of a well-known nuclear export inhibitor, LMB, and/or translational inhibitor, cycloheximide, on the nuclear accumulation of GFP-Vpx fusion proteins. LMB is known to block nuclear export due to a covalent modification at a cysteine residue in the central conserved domain of an export receptor, chromosomal region maintenance 1 (CRM-1) (29). GFP-Vpx was localized in cytoplasm in the presence of cycloheximide alone and in nucleus with both cycloheximide and LMB (Fig. 1A). This suggests the presence of both nuclear localization signal and NES in Vpx. As a positive control, we examined the effect of the same doses of cycloheximide and LMB on shuttling of a GFP-HIV-1-Rev fusion protein and observed a similar pattern of localization (Fig. 1A). This assay was further validated with the observation of unchanged nuclear localization for the nuclear export-defective Rev, GFP-Revmt, in the presence of cycloheximide with or without LMB (Fig. 1A). In summary, these data provide evidence that Vpx is a nucleocytoplasmic shuttling protein and that its export from nucleus is sensitive to LMB.
FIG. 1.
Assessment of nuclear export properties of Vpx. (A) Cos-7 cells were transfected with GFP-Vpx, GFP-HIV-1 Revwt, and GFP-HIV-1 Revmt expression plasmids and treated with the translational inhibitor cycloheximide (5 μg/ml) alone or in combination with 20 ng/ml of LMB. LMB inhibits exportin 1-CRM-1-mediated nuclear export of proteins. Immunofluorescence analysis results suggest that Vpx is a nuclear export protein and that its export is sensitive to LMB, similar to those seen with HIV-1 Rev. Revmt is the nuclear export-defective mutant (the conserved Leu residues within the nuclear export signal were exchanged with Ala), and the pattern of this mutant protein localization was not changed in the presence or absence of the indicated drugs. (B) Structure of HIV-1 rev-vpx fusion gene. (C) SIV Vpx encodes a fully functional NES. Cos-7 cells were transfected with pDM128 alone or in combination with the indicated Rev or Rev-Vpx fusion constructs, and the CAT activity was determined as described by Hope et al. (20).
Conserved tryptophan and tyrosine residues of Vpx play a critical role in its nuclear export.
A series of plasmids that encode variants of Vpx with substitutions in different regions as shown in Table 1 were used to identify the functional nuclear export signal (NES) in Vpx. Multiple sequence analysis showed that the residues targeted for mutagenesis are conserved across distinct HIV-2 and SIV isolates derived from different consensus groups. Wild-type and mutant Vpx expression plasmids were transfected into Cos-7 cells to evaluate the abilities of various Vpx mutants to be exported to the cytoplasm in the presence of cycloheximide. Specific Vpx signal was detected for all the mutants tested, but their ability to perform nuclear export differed dramatically from that of the wild-type protein (Table 1). Three different patterns of protein localization were observed in the presence of cycloheximide: localization to the cytoplasm (S2, S13, T17, W53, Y66, Y69, G86, C87, and W99); localization to both the nucleus and cytoplasm (W56 and K68); and localization to the nucleus predominantly (W49S, W49, 53, 56S, Y71A, and Y66, 69, and 71A) (Table 1; Fig. 2C). These results indicate that replacement of tryptophan and tyrosine residues selectively impaired export of Vpx from nucleus without altering its nuclear import. It is unlikely that the effect on localization resulted from global misfolding or altered protein stability of Vpx. For example, Vpx mutants H82S and P103 and 106S that are defective for nuclear import and export are efficiently incorporated into virus particles, as seen with the wild type. These mutant proteins retain the ability to interact with the structural protein Gag. Cycloheximide-LMB treatment did not alter the localization of H82S mutant protein (Table 1). Taken together, these results strongly suggest that the conserved tryptophan and tyrosine residues play an important role in export of Vpx from nucleus and that its cytoplasmic localization is critical for virion incorporation.
TABLE 1.
Effect of cycloheximide and LMB on SIV Vpx subcellular localization
| Vpx mutant | Subcellular localization of Vpxa
|
||
|---|---|---|---|
| Control | + Cyclo | + Cyclo and LMB | |
| Wild type | Nuc | Cyt | Nuc |
| ΔVpxb | |||
| S2L | Nuc/Cyt | Cyt | Cyt |
| S13N | Nuc/Cyt | Cyt | Cyt |
| T17I | Nuc/Cyt | Cyt | Cyt |
| W24S | Nuc | Cyt | Nuc |
| T28I | Nuc/Cyt | Cyt | Cyt |
| E30P | Nuc | Cyt | Nuc |
| H39L | Cyt | Cyt | Nuc/Cyt |
| W49S | Nuc | Nuc | Nuc |
| W53S | Nuc | Cyt | Nuc |
| W56S | Nuc | Nuc/Cyt | Nuc |
| W49 53 56S | Nuc | Nuc | Nuc |
| S52A | Cyt | Cyt | Cyt |
| S63 65A | Cyt | Cyt | Cyt |
| T67A | Cyt | Cyt | Cyt |
| K68A | Nuc | Nuc/Cyt | Nuc |
| R70A | Cyt | Cyt | Cyt |
| Y66 69 71A | Nuc | Nuc | Nuc |
| Y66 69 71S | Nuc | Nuc | Nuc |
| Y66A | Nuc | Cyt | Nuc |
| Y69A | Nuc | Cyt | Nuc |
| Y71A | Nuc | Nuc | Nuc |
| Y66 69A | Nuc | Cyt | Nuc |
| Y66 71A | Nuc | Nuc | Nuc |
| Y69 71A | Nuc | Nuc | Nuc |
| L74 I75S | Cyt | Cyt | Cyt |
| H82S | Cyt | Cyt | Cyt |
| G86C87S | Nuc/Cyt | Cyt | Nuc/Cyt |
| W99S | Nuc/Cyt | Cyt | Nuc/Cyt |
| P103 106S | Cyt | Cyt | Cyt |
Subcellular localization of Vpx was detected by immunofluorescence staining. Cyclo,-cycloheximide; Nuc, nuclear; Cyt, cytoplasmic.
ΔVpx, Vpx null mutant.
FIG. 2.
Vpx encodes a transferable NES. (A) Evidence for the presence of nuclear export activity within residues 41 to 63 of Vpx. Localization of GFP-Vpx fusion proteins in Cos-7 cells was visualized in the presence of cycoheximide (Cyclo) (5 μg/ml) alone or in combination with LMB (20 ng/ml). GFP-Vpx1-63 localizes in the nucleus in cells treated with both cycloheximide and LMB in contrast to its cytoplasmic localization in untreated or cells treated with cycloheximide alone. Nuclear localization of GFP-Vpx1-40 was not altered in the presence of indicated drugs, suggesting that the signal required for nuclear export resides within domain 41 to 63 of Vpx. (B) Schematic representation of Vpx variants containing mutations at conserved tryptophan residues. (C) Subcellular localization of Vpx mutant proteins. Cos-7 cells were transfected with various Vpx mutant expression plasmids and treated with cycloheximide alone or in combination with LMB. Localization of mutant proteins was analyzed by indirect immunofluorescence using anti-Vpx monoclonal antibody. Immunofluorescence analyses suggest that conserved tryptophan residues within domain 41 to 63 are essential for nuclear export of Vpx.
Nuclear export of SIVsm(Pbj1.9) Vpx can complement effector domain function of HIV-1 Rev.
A transient transfection system with reporter plasmid pDM128 derived from the env region of HIV-1 (20, 28) was used to characterize the nuclear export of Vpx. The transcript produced by pDM128 harbors a single intron containing a CAT coding sequence which is excised when the RNA is spliced. Cells transfected with pDM128 alone expressed the spliced transcripts in the cytoplasm and yielded very low levels of CAT activity (Fig. 1C, lane 1). In contrast, cotransfection with a functional HIV-1 Rev expression vector (pRevwt) permitted the unspliced transcripts to enter the cytoplasm, thus increasing the CAT activity (Fig. 1C, lane 3). Rev mutant (Revmt) was created by exchanging two of the hydrophobic leucine with alanine residues in the effector domain (Fig. 1B); these residues are known to abrogate the export of Rev from nucleus (28, 35). The results in Fig. 1C indicate the low levels of CAT activity in Revmt (lane 2)-transfected cells compared with Revwt (lane 3)-transfected cells, suggesting that mutation in the Rev effector domain blocks the nuclear export of CAT mRNA (unspliced RNA) to the cytoplasm. A fusion protein was generated by fusing full-length Vpx to the carboxyl terminus of HIV-1 Revmt to assay the activity of Vpx nuclear export signal in the above-mentioned system (Fig. 1B). Interestingly, Vpx could complement the defects of HIV-1 Rev effector domain and increase the CAT activity in the Revmt-Vpx-transfected cell (Fig. 1C, lane 4). These results provide evidence for the presence of a functional NES in Vpx and further suggest that Vpx NES is able to complement export activity of heterologous proteins.
Signal sequence within residues 41 to 63 of Vpx is essential for nuclear export.
Deletion analysis of Vpx showed that amino acid residues 1 to 40 of Vpx were able to transport the heterologous protein GFP to the nucleus, whereas residues 1 to 63 localized it to the cytoplasm (48). It appears that GFP-Vpx1-63 shuttles into and out of the nucleus. To this end, GFP fusion constructs containing Vpx1-40 and Vpx1-63 were transfected into Cos-7 cells and treated with cycloheximide in the presence or absence of LMB. The results in Fig. 2A indicate more nuclear accumulation of the GFP-Vpx1-63 protein upon cycloheximide and LMB treatment compared to the results seen with untreated as well as cycloheximide-treated cells. Identical doses of the indicated drugs did not alter the nuclear localization of GFP-Vpx1-40 (Fig. 2A). These results suggest the possibility that export signal resides between residues 41 and 63 of Vpx.
All the conserved amino acid residues (Table 1) in Vpx were exchanged by quick-change mutagenesis to further characterize the signal for Vpx nuclear export. Nuclear export activity for all Vpx mutant proteins was examined with Cos-7 cells in the presence of cycloheximide with or without LMB by indirect immunofluorescence using anti-Vpx monoclonal antibody. Cycloheximide (with or without LMB) did not alter the nuclear localization of Vpx protein containing a mutation at tryptophan 49 (W49S) alone or in combination with tryptophan residues at positions 53 and 56 (W49, 53, 56S) (Fig. 2B and C; Table 1). In addition, replacement of tryptophan residues abrogated the nuclear export activity of Vpx1-63 as well (data not shown). Conservation of tryptophan residues in all HIV-2 and SIV isolates suggests the importance of these residues in Vpx function. These data provide evidence for the presence of nuclear export signal within residues 41 to 63 of Vpx. Surprisingly, a similar pattern of localization for mutant Vpx protein with tyrosine residues exchanged to alanine (Y66, 69, 71A) (Table 1) was observed. Cycloheximide treatment did not alter the nuclear localization of these mutant proteins. Phosphorylation of tyrosine residues may therefore play an important role in Vpx nuclear export. Collectively, these data suggest that tryptophan residues within domain 41 to 63 may play a critical role in export of Vpx from nucleus.
Vpx interacts with Fyn SH3 domain through its C-terminal proline-rich domain.
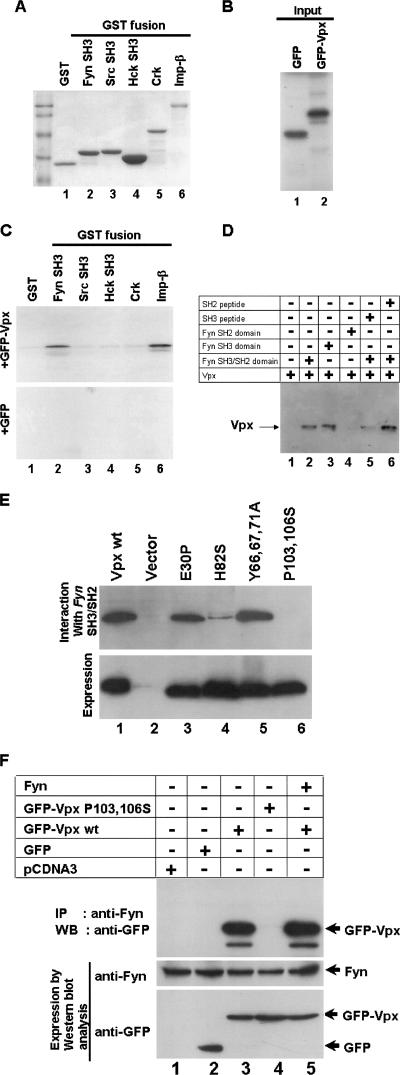
Proline-rich motifs are known to interact with SH3 domains of Src-like tyrosine kinases as well as of proteins that are involved in signal transduction pathways (34, 44, 45). To understand the role of the C-terminal proline-rich domain (RPGP7GLA) in Vpx function, we first tested whether Vpx interacts with any host Src-like kinases. SH3 domains of Fyn, Src, Hck, and full-length Crk were used as an affinity matrix to identify the Vpx-interacting partner in an in vitro GST pulldown assay. Equal amounts of GST, GST-Fyn-SH3, GST-Src-SH3, GST-Hck-SH3, and GST-Crk (Fig. 3A) bound with glutathione-Sepharose beads were incubated with in vitro-translated GFP or GFP-Vpx (Fig. 3B), and the bound proteins were analyzed by autoradiography. Vpx was found to specifically associate with Fyn SH3 domain (Fig. 3C, lane 2). Importin-β was used as a positive control in this assay (Fig. 3C, lane 6). The specificity of the interaction between the Vpx and Fyn SH3 domains was further confirmed by competition experiments with SH3 and SH2 domain peptides. Cos-7 cell lysates expressing Vpx protein were incubated with glutathione-agarose-bound Fyn SH3/SH2 domains in the presence or absence of peptides corresponding to the SH3 or SH2 domain of Fyn and probed with anti-Vpx monoclonal antibody. Vpx interaction was observed only with the SH3 domain (Fig. 3D, lane 3) and not with the SH2 domain (Fig. 3D, lane 4). Furthermore, interaction between Vpx and SH3 domain was selectively blocked by SH3 domain peptide (Fig. 3D, lane 5) but not by SH2 peptide (Fig. 3D, lane 6). In addition, peptides derived from SH3 and SH2 domains of another Src-like tyrosine kinase, Lck, did not compete for Vpx binding with the Fyn SH3 domain (data not shown), suggesting the specific interaction between the Vpx and the Fyn SH3 domains.
FIG. 3.
Evidence for Vpx interaction with the cellular Src-like tyrosine kinase Fyn. (A) SH3 domains of various kinases were expressed and purified as GST fusions. Glutathione-Sepharose beads containing equal amounts of GST and GST-SH3 fusion proteins as well as GST-importin-beta (GST-Imp-β) were used in the in vitro pulldown assays as indicated by the results of Coomassie blue staining. (B) [35S]methionine-labeled GFP and GFP-Vpx proteins (10%) were used in the GST pulldown assays. (C) Vpx interacts with Fyn SH3 domain. The results of GST pulldown assays using Fyn, Src, Hck, and full-length Crk as GST fusion proteins suggest that Vpx specifically interacts with the SH3 domain of Fyn. GST-importin-β was used as a positive control in this reaction. (D). Fyn peptides corresponding to the SH3 but not the SH2 domain block its interaction with Vpx. Cos-7 cell lysates containing Vpx were incubated with SH3 or SH2 domain peptides and mixed with glutathione-agarose beads containing Fyn SH3/SH2 domain. Bound proteins were resolved on SDS-15% PAGE followed by Western blot analysis using anti-Vpx monoclonal antibody. (E) Mutations within the COOH-terminus proline-rich motif abrogate Vpx interaction with the Fyn SH3 domain. Cos-7 cell lysates containing various mutant Vpx proteins were incubated with glutathione-agarose beads containing th e Fyn SH3/SH2 domain. Bound proteins were resolved on SDS-15% PAGE followed by Western blot analysis with anti-Vpx monoclonal antibody. (F) Vpx interaction with Fyn in vivo. GFP and GFP-Vpx (wild-type and P103 and 106S) expression plasmids were transfected in Cos-7 cells. After 16 h transfection, cell lysates were subjected to immunoprecipitation (IP) with anti-Fyn polyclonal antibody followed by Western blot analysis (WB) using anti-GFP monoclonal antibody. Expression of GFP-Vpx fusion proteins (5% input) and Fyn (10% input) was determined by Western blot analysis using anti-GFP monoclonal and anti-Fyn polyclonal antibodies, respectively.
Involvement of C-terminal proline-rich motif (RPGP7GLA) in the interaction of Vpx with the Fyn SH3 domain was next examined. Cos-7 cell lysates containing wild-type or mutant Vpx proteins were incubated with glutathione-agarose bead-bound Fyn SH3/SH2 domain fusion proteins. The bound proteins were resolved on SDS-12% PAGE and probed with anti-Vpx monoclonal antibody. The results in Fig. 3E indicate that exchange of proline residues at position 103 and 106 with serine in the C-terminal proline-rich domain completely blocked Vpx binding with the Fyn SH3 domain (lane 6). In contrast, exchange of the glutamic acid residue at position 30 with proline as well as tyrosine residues at positions 66, 69, and 71 with alanine resulted in a wild-type interaction (Fig. 3E, lanes 3 and 5). However, replacement of conserved histidine 82 with serine severely affected Vpx interaction with the Fyn SH3 domain. Together, these data suggest that the C-terminal proline-rich motif plays a critical role in Vpx interaction with SH3 domain of Fyn.
We performed coimmunoprecipitation assays in which the interaction between Vpx and Fyn was carried out in vivo. Cell lysates from GFP and GFP fusions containing wild-type as well as P103 and 106S mutant Vpx expression vectors were transfected in Cos-7 cells and immunoprecipitated with anti-Fyn polyclonal antibody followed by Western blot analysis using anti-GFP monoclonal antibody. Vpx specifically interacts and coprecipitates with endogenous Fyn kinase (Fig. 3F, upper panel, lane 3), and the exchange of proline 103 and 106 with serine in Vpx prevented this interaction with Fyn (Fig. 3F, upper panel, lane 4). Vpx interacts with both endogenous and ectopically expressed Fyn (Fig. 3F, upper panel, lanes 3 and 5). Interestingly, more binding of Vpx was noticed in cells cotransfected with Vpx and Fyn (upper panel, lane 5); this correlates with Fyn expression in the cotransfected cells (Fig. 3F, middle panel, lane 5). As expected, the correct molecular masses of Fyn proteins (Fig. 3F, middle panel) and GFP-Vpx fusion proteins (Fig. 3F, lower panel) were observed. GFP was used as a negative control in this assay (Fig. 3F, lane 2). These results reconfirmed the specific binding of Vpx to the cellular tyrosine kinase Fyn.
Vpx is a substrate for cellular tyrosine kinase Fyn.
Direct evidence of Vpx phosphorylation was obtained by an in vitro kinase assay using various cellular kinases (Nik, MAPK-ERK-1, Lck, and Fyn) immunoprecipitated from PMA-activated Jurkat cell lysate by specific antibodies (Fig. 4A). Immunoaffinity-purified Fyn was able to selectively phosphorylate recombinant Vpx (Fig. 4B, lane 4). Recombinant Fyn was also able to phosphorylate Vpx in vitro (Fig. 4C, lane 3). This provides evidence that Vpx is a substrate for Fyn. Vpx phosphorylation by Fyn kinase was sensitive to its inhibitor, PP2 (16) (Fig. 4C, lane 4), but not to a MAPK pathway inhibitor, PD98059 (Fig. 4C, lane 5). These data provide evidence that PP2 specifically inhibits Fyn activity. Results in Fig. 4D show that Vpx is also phosphorylated by MAPK-ERK-2 (lane 2) and that this is selectively inhibited by hypericin (lane 3) but not by PP2 (lane 4). SIVsm Vpx protein was expressed in E. coli BL-21(DE3) and purified using Ni-nitrilotriacetic acid chromatography (Fig. 4E) and was used for in vitro kinase assay. Vpx phosphorylation by MAPK plays an important role in Vpx nuclear import (42). It is possible that Vpx phosphorylation by Fyn may modulate export of Vpx from nucleus.
FIG. 4.
Vpx is a substrate for the cellular tyrosine kinase Fyn. (A) Expression levels of the various cellular kinases indicated were determined with PMA-activated Jurkat cells by Western blot analysis using the respective antibodies. (B to D) Immunoaffinity-purified Fyn kinase (B), recombinant Fyn kinase (C), and MAPK-ERK-2 (D) activity was examined by in vitro kinase assays using bacterially purified recombinant SIVsm(Pbj1.9) Vpx. Phosphorylated Vpx was visualized by electrophoresis using SDS-15% PAGE followed by autoradiography. The Fyn kinase inhibitor PP2, but not the MEK inhibitor PD98059, selectively blocks Fyn-mediated Vpx phosphorylation. The MAPK inhibitor hypericin selectively prevents ERK-2-mediated phosphorylation of Vpx. (E) Vpx was expressed in E. coli BL21(DE3) and purified by Ni-nitrilotriacetic acid affinity chromatography.
Fyn kinase modulates nuclear export of Vpx.
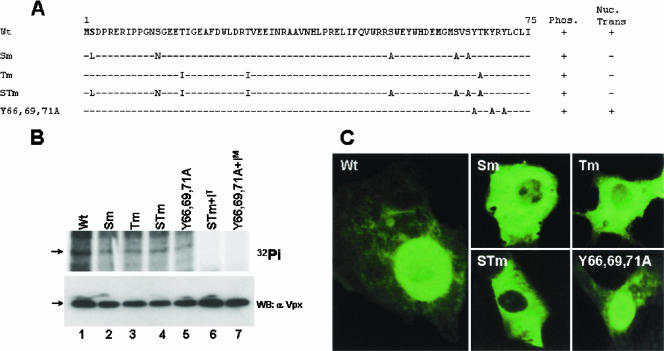
All the potential phosphorylation residues such as serines, threonines, and tyrosines in Vpx were exchanged by site-directed mutagenesis (Fig. 5A) to determine the role of phosphorylation in export of Vpx from nucleus. The phosphorylation status and expression status of all Vpx mutant proteins were ascertained by expressing them using a vaccinia virus expression system and labeling with 32Pi in Cos-7 cells. The lysates from the labeled cells were immunoprecipitated with anti-Vpx monoclonal antibodies and separated by SDS-15% PAGE. Equal levels of phosphorylation were found for all the Vpx mutants in the absence of kinase inhibitors (Fig. 5B, upper panel, lanes 1 to 5). Analysis of Vpx mutants in the presence of various kinase inhibitors indicated that phosphorylation of STm (both serine and threonine residues were mutated) was completely inhibited by the tyrosine kinase inhibitor PP2 (Fig. 5B, upper panel, lane 6) and tyrosine mutant (Y66, Y69, Y71) by the MAPK pathway inhibitor hypericin (Fig. 5B, upper panel, lane 7) despite equal amounts of mutant proteins, as observed in the transfected cells (Fig. 5B, lower panel). These results suggest that Vpx is phosphorylated by two different cellular kinases, MAPK-ERK-2 and Fyn. Using immunofluorescence analysis, Vpx-specific signal was observed for all the mutants in Cos-7 cells. Cytoplasmic localization for serine as well as threonine mutants and wild-type nuclear localization for tyrosine mutants confirms the role for serine and threonine residues in Vpx import into the nucleus (Fig. 5C).
FIG.5.
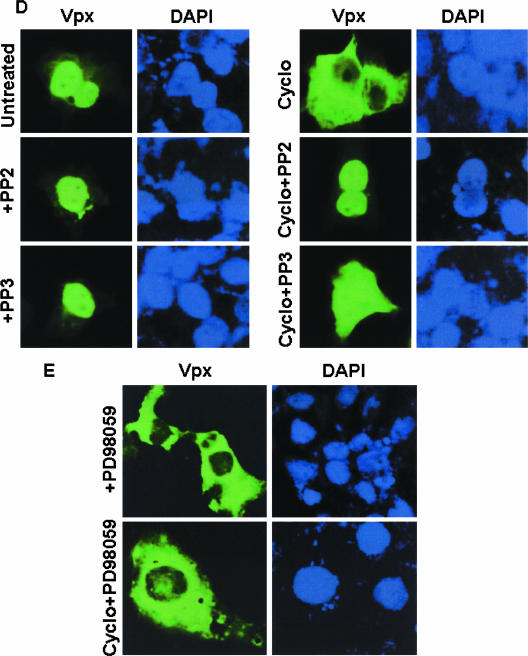
Phosphorylation plays an important role in Vpx nuclear transport. (A) Summary of intracellular localization and phosphorylation status of different Vpx mutants. Wt, wild type; Sm, all serine residues were replaced; Tm, all threonine residues were replaced; STm, all serine and threonine residues were replaced; Y66, 69, 71A, tyrosine residues at positions 66, 69, and 71 were replaced. (B) Various Vpx mutant constructs were transfected in Cos-7 cells and labeled with 32Pi in the presence or absence of the indicated kinase inhibitors. Results suggest that the tyrosine kinase inhibitor PP2 blocks the phosphorylation of serine/threonine mutant protein and that the MAPK inhibitor hypericin inhibits the phosphorylation of tyrosine mutant protein. Introduced mutations and kinase inhibitors did not alter the expression of various Vpx mutant proteins (lower panel). Expression of Vpx mutant proteins was determined by Western blot analysis using anti-Vpx monoclonal antibody. S/Tm+IT, serine/threonine mutant with the tyrosine kinase inhibitor PP2; Y66,69,71A+IM, tyrosine mutant with the MAPK inhibitor hypericin. (C) Replacement of serine and threonine residues abrogates Vpx nuclear import whereas tyrosine mutant protein (Y66,69,71A) retains wild-type localization as evidenced by immunofluorescence analysis. (D) Tyrosine phosphorylation plays a critical role in Vpx nuclear export. Cos-7 cells were transfected with Vpx expression plasmids and treated with cycloheximide alone or in combination with the indicated kinase inhibitors. Vpx localization was determined by indirect immunofluorescence using anti-Vpx monoclonal antibody followed by an anti-mouse Alexa Fluor 488-conjugated secondary antibody. Nuclei were stained with DAPI. (E) Results suggest that Vpx nuclear export was specifically inhibited by the Fyn kinase inhibitor PP2 and not by either the inactive tyrosine kinase inhibitor PP3 or the MAPK pathway inhibitor PD98059.
We next investigated whether export of Vpx from nucleus is dependent on Fyn-mediated phosphorylation. To this end, nuclear export of Vpx was determined in the presence of PP2 (inhibitor of Fyn kinase activity) alone or in combination with the translational inhibitor cycloheximide. Vpx localizes into the nucleus in both PP2-treated and untreated cells (Fig. 5D). Interestingly, PP2 inhibits the export of Vpx from nucleus (Fig. 5D). This inhibition was specific, since an inactive Fyn tyrosine kinase inhibitor, PP3 (50) (Fig. 5D), or a MAPK pathway inhibitor, PD98059 (Fig. 5E), did not alter the cytoplasmic localization of Vpx in cells treated with cycloheximide. In addition, treatment with cycloheximide and LMB (alone or in combination) did not change the nuclear localization of Vpx Y66, Y69, 71A mutant protein (Table 1). These data suggest that Fyn-mediated phosphorylation may be important for export of Vpx from nucleus.
Compromise of Vpx export from nucleus results in impaired incorporation of Vpx into virions.
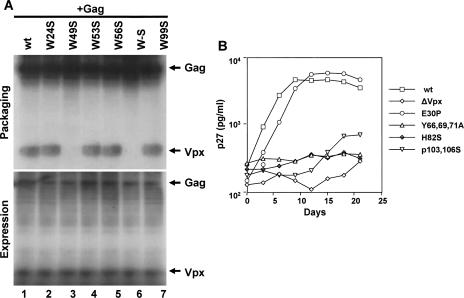
Export of Vpx from nucleus ensures its availability in the cytoplasm for incorporation into new virions. This can be verified by the presence of Vpx mutant proteins in the virus particles. vpx mutant SIVsm(PBj1.9) proviral clones were transfected into 293T cells, and the virus particles were collected by centrifuging the cell culture supernatants over a 20% sucrose cushion. Equal amounts of viral pellets (normalized by p27gag content) were examined by Western blot analysis. Probing with anti-Vpx monoclonal antibody revealed the absence of Vpx from Y66, Y69, 71A mutant virus particles (data not shown), with results similar to those seen with viruses lacking the entire vpx gene. Probing with an anti-Gag monoclonal antibody showed that expression, processing, and assembly of Gag was not affected by the various vpx mutations (data not shown). In addition, replacement of tryptophan residues at position 49, alone or in combination with those at positions 53 and 56, also abrogated Vpx packaging into virus-like particles (Fig. 6A, upper panel, lane 3 and 6) under conditions in which equal amounts of proteins were found in the transfected cell lysates (Fig. 6A, lower panel). Interestingly, exchange of histidine 82 and proline 103 and 106 residues with serine did not abrogate Vpx packaging (data not shown) despite defective nuclear import (Table 2). Importantly, Y66, 69, 71A and W49, 53, 56S Vpx mutants were compromised for nuclear export activity (Fig. 2C and Table 2) as well as virion packaging (Fig. 6A) but retained wild-type nuclear import (Table 2). These data suggest that cytoplasmic localization of Vpx is essential for its incorporation into virus particles. To further understand the importance of phosphorylation, nuclear import, and export for Vpx virion incorporation, the characteristics of Vpx packaging were determined in the presence of kinase inhibitors. Virion packaging of Vpx was observed in the presence of both MAPK and the tyrosine kinase inhibitors hypericin and PP2 (data not shown). These data provide evidence that phosphorylation may be a transient event to bring Vpx out of the nucleus. Once in the cytoplasm, Vpx is readily packaged into virus particles. It is noted that both phosphorylated and unphosphorylated forms of Vpx are packaged into the virus particles (data not shown), indicating that phosphorylation may not be essential for efficient incorporation of Vpx into virus particles. Together, these results suggest that the tyrosine as well as tryptophan residues are essential for efficient export of Vpx from nucleus and that this is critical for its incorporation into virus particles.
FIG. 6.
Nuclear export is essential for virion incorporation of Vpx and SIV replication in nondividing macaque macrophages. (A) Tryptophan residues within domain 41 to 63 play a critical role in Vpx virion incorporation. Vpx mutants were cotransfected with polyprotein Gag pr55 expression vector in Cos-7 cells. The presence of Vpx mutant proteins in the virus-like particles was determined from the culture media after labeling the transfected cells with [35S]methionine. The labeled Vpx and Gag proteins from culture supernatant (virus-like particles) and cell lysates were immunoprecipitated with anti-Vpx and anti-Gag monoclonal antibodies. The immunoprecipitates were resolved on SDS-15%PAGE followed by autoradiography. (B) Replication kinetics of wild-type and vpx mutant PBj1.9 proviruses. Terminally differentiated macaque macrophages were infected with the indicated SIVsm(PBj1.9) virus constructs equilibrated by p27gag content (10 ng of p27gag per 106 cells). Virus replication was assessed by quantifying the amounts of p27gag antigen in culture supernatants at 3-day intervals postinfection. wt, wild type; ΔVpx, a control construct lacking a functional vpx open reading frame.
TABLE 2.
Nuclear export is essential for Vpx incorporation into virus particles for subsequent virus replication in macrophages
| SIVsmPBj1.9 Vpx mutant | Nuclear importa | Nuclear exporta | Packaging into virus particlesb | Viral replicationd
|
|
|---|---|---|---|---|---|
| PBMC | Macrophages | ||||
| Wild type | + | + | +++ | +++ | +++ |
| ΔVpxc | − | +++ | ± | ||
| E30P | + | + | +++ | +++ | +++ |
| W46 53 56S | + | − | − | ND | ND |
| Y66 69 71A | + | − | − | +++ | ± |
| Y66A | + | + | +++ | ND | ND |
| Y71A | + | + | + | ND | ND |
| L74 I75S | − | − | − | +++ | ± |
| H82S | − | − | ++ | +++ | ± |
| G86C87S | − | − | +++ | +++ | + |
| P103 106S | − | − | +++ | +++ | + |
Nuclear import and export of Vpx was detected by immunofluorescence staining. +, imported into or exported from the nucleus; −, no import or export.
Packaging of Vpx into virus particles was determined by Western blot analysis. +++, Vpx detected; +, extremely low level of Vpx detected; −, no Vpx detected.
ΔVpx, Vpx null mutant.
Replication of vpx mutant viruses was assayed both in the dividing (macaque PBMC) and nondividing (macaque macrophages) target cells by measuring the release of core antigen p27gag in the culture supernatant. +++, high level of replication; +, low level of replication; ±, extremely low level of replication; ND, not determined.
Nuclear export activity of Vpx modulates virus replication in macrophages.
To understand the importance of the nuclear export and virion incorporation properties of Vpx for optimal virus replication in nondividing cells, SIVsm(PBj1.9) proviral clones with the following characteristics were selected: (a) clones with wild-type Vpx virion incorporation and defective Vpx nuclear import and (b) clones with wild-type Vpx nuclear import and defective nuclear export as well as virion incorporation functions. All vpx mutant viruses replicated as efficiently as the wild type and to high titers in macaque PBMCs (Table 2) but not in terminally differentiated monocyte-derived macaque macrophage cultures (Fig. 6B). As expected, mutant Vpx proteins that failed to localize in the nucleus were severely impaired in their ability to support virus replication in macrophages. For example, impairment of virus replication in macrophages was observed for Vpx H82S and P103, 106S mutants that are packaged into virus particles at levels similar to wild-type levels but failed to localize to the nucleus (Table 1 and Table 2). Importantly, failure to replicate in macrophages was also observed for Y66, 69, 71A mutants (Fig. 6B) despite maintaining wild-type nuclear localization. These Vpx mutant proteins failed to be exported into the cytoplasm and therefore could not be packaged into virus particles (Table 2). PBj1.9 mutant viruses with defective nuclear export or virion incorporation of Vpx were found to replicate poorly in macrophages. These results suggest that nuclear export plays a critical role in translocation of Vpx into the cytoplasm for its packaging into virus particles. These findings support the notion that virion-associated Vpx with wild-type nuclear import and export is critical for efficient virus replication in nondividing target cells.
DISCUSSION
In this report, we have shown that SIV Vpx protein contains noncanonical nuclear localization signals and a novel tryptophan-rich NES capable of importing and exporting the heterologous protein into and out of the nucleus. The NES identified in Vpx is distinct from the other known hydrophobic leucine-rich export signals (32). The export activity of Vpx is sensitive to LMB, implicating CRM-1-dependent export from the nucleus. Vpx can functionally replace the effector domain of HIV-1 Rev in CAT-based reporter assays involving the export of RNA transcripts. In addition, Fyn-mediated phosphorylation regulates the export of Vpx from nucleus. Furthermore, the data demonstrate that nuclear export activity ensures the availability of Vpx in the cytoplasm for its incorporation into virus particle to support efficient virus replication in macrophages.
Conservation of novel tryptophan-rich Vpx NES sequences from various HIV-2 and SIV isolates suggests that nuclear export might be critical for Vpx function. Sensitivity of Vpx nuclear export to LMB has led us to consider the possibility of its interaction with the CRM-1 pathway, which is a fundamental nuclear transport system used for the export of RNA-binding proteins and specific subclasses of RNA (4, 22). In addition, HIV-1 Rev, Gag, and Vpr proteins are exported into the cytoplasm by CRM-1-dependent pathways (10, 47). Vpx NES can replace the Rev effector domain in Rev-Vpx fusion proteins, confirming that Vpx contains a functional NES. It should be noted that in this assay system the Rev-RNA binding domain provided the specificity for transport of RRE-containing transcript and that Vpx alone may not be able to promote the nuclear export of unspliced CAT-mRNA. Discrepancy between our findings with respect to SIV Vpx and those of Belshan and Ratner (5) with respect to shuttling of HIV-2 Vpx protein may be due to the different assay systems as well as the viral strains used. Many other viruses utilize CRM-1-mediated nuclear export to enhance virus replication, often by encoding adaptor proteins that facilitate the cytoplasmic localization of genomic and subgenomic RNAs. For example, influenza virus encodes M1 and NES/NS2 proteins that facilitate export of viral ribonucleoprotein complexes from the nucleus in an LMB-sensitive manner (3).
Substitution of tyrosine residues at positions 66, 69, and 71 of Vpx blocked Fyn-mediated phosphorylation without altering Vpx binding with Fyn SH3 domain. Interestingly, replacement of proline residues at positions 103 and 106 within the highly conserved RPGP7GLA motif completely prevented Vpx interaction with the Fyn SH3 domain. SH3 domains are well-established protein modules that recognize proline-rich peptide sequences (34, 44). Conservation of a similar domain in nucleocytoplasmic shuttling proteins such as heterogeneous nuclear ribonucleoprotein K and nuclear protein p62 (53) suggests that the proline-rich domain plays a critical role in protein nuclear transport. Interaction between Vpx and the Fyn SH3 domain may bring Vpx into close proximity to the Fyn catalytic domain, which may be critical for Vpx phosphorylation, thereby regulating its nuclear export. This was further supported by the inhibition of the biological activity of the tyrosine mutant (Y66 69 71A), which indeed correlated with loss of Vpx nuclear export activity (Table 2).
Vpx interacts with CA (21) and localizes to viral core (27). The association of Vpx with virus particles may help in stabilizing the viral core and promoting the uncoating process for efficient replication. A recent report suggests that efficient uncoating of the viral core plays an important role in regulating the nuclear transport of the lentiviral genome in nondividing cells (54). The low level of vpx mutant virus (viruses with nuclear import- or export-defective Vpx proteins) replication in macrophages supports the notion that interaction of Vpx with CA may be required for efficient uncoating of the viral core. This may play a critical role in supporting the transport of viral genome into the nucleus and subsequent virus infection in nondividing cells.
The use of CRM-1 pathway for the export of Vpx may be critical for its availability in the cytoplasm for efficient incorporation into budding virus particles. Although we found that amino acid residues 41 to 63 act as an LMB-sensitive NES and regulate Vpx nuclear export, it should be mentioned that tryptophan residues are present instead of the hydrophobic leucine residues normally present in a typical NES, such as that of HIV-1 Rev (32, 35). However, there are several CRM-1-dependent NESs with other hydrophobic residues, such as methionine, isoleucine, valine, phenylalanine, and tryptophan (36, 43, 46, 49). Not only do many LMB-dependent NESs deviate from the consensus motif, but they also differ with respect to their binding affinities for the CRM-1 export receptor; indeed, NESs are thought to require a relatively weak interaction with CRM-1 to ensure proper dissociation from the receptor and translocation through the nuclear pore. Even though Vpx-NES differs from the consensus sequence, it binds to CRM-1 in vivo (unpublished observations). Replication failure of provirus containing nuclear export-defective Vpx protein together with its previously observed interaction with nucleic acids (18) raises the possibility of Vpx involvement in viral RNA export during the life cycle in nondividing target cells in synergy with other viral proteins such as Gag and Rev, which are known to be involved in RNA export (10). Further experimentation will be needed to test this hypothesis. Understanding the precise relationship between the nuclear transport of Vpx and the viral life cycle will hopefully reveal novel targets for the development of specific and new antiviral agents.
Acknowledgments
This work was supported by grants (BT/PR4154/BRB/10/336/2003 and BT/PR5959/Med/12/239/2005) from the Department of Biotechnology, government of India, to S. M., by support from the Centre for DNA Fingerprinting and Diagnostics, and by graduate fellowships from the Council of Scientific and Industrial Research (CSIR), government of India, to P.K.S., P.R.K., and M.R.K.S.R.
We are indebted to B. H. Hahn (University of Alabama at Birmingham, Alabama) for Vpx mutants, T. Hope (Northwestern University) for pDM128 plasmid, T. Sekimoto (Osaka University, Japan) for importin-beta expression plasmids, S. Jameel, IGCEB, New Delhi, India, for expression vectors of SH3 domains of Fyn, Src, Hck, and full-length Crk, and Minoru Yoshida (University of Tokyo, Japan) for providing LMB. We thank T. Ramasarma for critically reading the manuscript.
Footnotes
Published ahead of print on 20 September 2006.
REFERENCES
- 1.Accola, M. A., A. A. Bukovsky, M. S. Jones, and H. G. Göttlinger. 1999. A conserved dileucine-containing motif in p6gag governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIVmac and SIVagm. J. Virol. 73:9992-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostini, I., S. Popov, T. Hao, J. H. Li, L. Dubrosky, O. Chaika, N. Chaika, R. Lewis, and M. Bukrinsky. 2002. Phosphorylation of Vpr regulates HIV type 1 nuclear import and macrophage infection. AIDS Res. Hum. Retrovir. 18:283-288. [DOI] [PubMed] [Google Scholar]
- 3.Akarsu, H., W. P. Burmeister, C. Petosa, I. Petit, C. W. Muller, R. W. Ruigrok, and F. Baudin. 2003. Crystal structure of the M1 protein-binding domain of the influenza A virus nuclear export protein (NEP/NS2). EMBO J. 22:4646-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askjaer, P., T. H. Jensen, J. Nilsson, L. Englmeier, and J. Kjems. 1998. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 273:33414-33422. [DOI] [PubMed] [Google Scholar]
- 5.Belshan, M., and L. Ratner. 2003. Identification of the nuclear localization signal of human immunodeficiency virus type 2 Vpx. Virology 311:7-15. [DOI] [PubMed] [Google Scholar]
- 6.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett, A. H., and P. A. Silver. 1997. Nucleocytoplasmic transport of macromolecules. Microbiol. Mol. Biol. Rev. 61:193-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 9.Dingwall, C., and R. A. Laskey. 1991. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 16:478-481. [DOI] [PubMed] [Google Scholar]
- 10.Dupont, S., N. Sharova, C. DeHoratius, C. M. Virbasius, X. Zhu, A. G. Bukrinskaya, M. Stevenson, and M. R. Green. 1999. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature 402:681-685. [DOI] [PubMed] [Google Scholar]
- 11.Engel, K., A. Kotlyarov, and M. Gaestel. 1998. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher, T. M., B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM). EMBO J. 15:6155-6165. [PMC free article] [PubMed] [Google Scholar]
- 13.Fuerst, T. R., P. L. Earl, and B. Moss. 1987. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol. Cell. Biol. 7:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallay, P., S. Swingler, C. Aiken, and D. Trono. 1995. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell 80:379-388. [DOI] [PubMed] [Google Scholar]
- 15.Görlich, D., and I. W. Mattaj. 1996. Nucleocytoplasmic transport. Science 271:1513-1518. [DOI] [PubMed] [Google Scholar]
- 16.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 17.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson, L. E., R. C. Sowder, T. D. Copeland, R. E. Benveniste, and S. Oroszlan. 1988. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science 241:199-201. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, V. M., M. E. Sharkey, C. R. Brown, B. Brichacek, S. Goldstein, J. Wakefield, R. Byrum, W. R. Elkins, B. H. Hahn, J. D. Lifson, and M. Stevenson. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hope, T. J., X. J. Huang, D. McDonald, and T. G. Parslow. 1990. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev. trans-activator: mapping cryptic functions of the arginine-rich motif. Proc. Natl. Acad. Sci. USA 87:7787-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton, R., P. Spearman, and L. Ratner. 1994. HIV-2 viral protein X association with the GAG p27 capsid protein. Virology 199:453-457. [DOI] [PubMed] [Google Scholar]
- 22.Huffman, K. M., S. J. Arrigo, and M. G. Schmidt. 1999. HIV-1 Rev promotes the nuclear export of unspliced and singly spliced RNAs in a mammalian cell-free export system. J. Biomed. Sci. 6:194-205. [DOI] [PubMed] [Google Scholar]
- 23.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 24.Jacqué, J. M., A. Mann, H. Enslen, N. Sharova, B. Brichacek, R. J. Davis, and M. Stevenson. 1998. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 17:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappes, J. C., C. D. Morrow, S. W. Lee, B. A. Jameson, S. B. Kent, L. E. Hood, G. M. Shaw, and B. H. Hahn. 1988. Identification of a novel retroviral gene unique to human immunodeficiency virus type 2 and simian immunodeficiency virus SIVMAC. J. Virol. 62:3501-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappes, J. C., J. S. Parkin, J. A. Conway, J. Kim, C. G. Brouillette, G. M. Shaw, and B. H. Hahn. 1993. Intracellular transport and virion incorporation of vpx requires interaction with other virus type-specific components. Virology 193:222-233. [DOI] [PubMed] [Google Scholar]
- 27.Kewalramani, V. N., and M. Emerman. 1996. Vpx association with mature core structures of HIV-2. Virology 218:159-168. [DOI] [PubMed] [Google Scholar]
- 28.Kim, F. J., A. A. Beeche, J. J. Hunter, D. J. Chin, and T. J. Hope. 1996. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol. Cell. Biol. 16:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahalingam, S., B. Van Tine, M. L. Santiago, F. Gao, G. M. Shaw, and B. H. Hahn. 2001. Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear targeting domain. J. Virol. 75:362-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malim, M. H., D. F. McCarn, L. S. Tiley, and B. R. Cullen. 1991. Mutational definition of the human immunodeficiency virus type 1 Rev activation domain. J. Virol. 65:4248-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattison, C. P., and I. M. Ota. 2000. Two protein tyrosine phosphatases, Ptp2 and Ptp3, modulate the subcellular localization of the Hog1 MAP kinase in yeast. Genes Dev. 14:1229-1235. [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer, B. J., and D. Baltimore. 1993. Signalling through SH2 and SH3 domains. Trends Cell Biol. 3:8-13. [DOI] [PubMed] [Google Scholar]
- 35.Meyer, B. E., J. L. Meinkoth, and M. H. Malim. 1996. Nuclear transport of human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: identification of a family of transferable nuclear export signals. J. Virol. 70:2350-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan, M., J. Thorburn, P. P. Pandolfi, and A. Thorburn. 2002. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J. Cell Biol. 157:957-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell 99:677-690. [DOI] [PubMed] [Google Scholar]
- 38.Ohno, M., M. Fornerod, and I. W. Mattaj. 1998. Nucleocytoplasmic transport: the last 200 nanometers. Cell 92:327-336. [DOI] [PubMed] [Google Scholar]
- 39.Pancio, H. A., N. Vander Heyden, and L. Ratner. 2000. The C-terminal proline-rich tail of human immunodeficiency virus type 2 Vpx is necessary for nuclear localization of the viral preintegration complex in nondividing cells. J. Virol. 74:6162-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pemberton, L. F., and B. M. Paschal. 2005. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6:187-198. [DOI] [PubMed] [Google Scholar]
- 41.Rajendra Kumar, P., P. K. Singhal, S. S. Vinod, and S. Mahalingam. 2003. A non-canonical transferable signal mediates nuclear import of simian immunodeficiency virus Vpx protein. J. Mol. Biol. 331:1141-1156. [DOI] [PubMed] [Google Scholar]
- 42.Rajendra Kumar, P., P. K. Singhal, M. R. Subba Rao, and S. Mahalingam. 2005. Phosphorylation by MAPK regulates simian immunodeficiency virus Vpx protein nuclear import and virus infectivity. J. Biol. Chem. 280:8553-8563. [DOI] [PubMed] [Google Scholar]
- 43.Rashevsky-Finkel, A., A. Silkov, and R. Dikstein. 2001. A composite nuclear export signal in the TBP-associated factor TAFII105. J. Biol. Chem. 276:44963-44969. [DOI] [PubMed] [Google Scholar]
- 44.Ren, R., B. J. Mayer, P. Cicchetti, and D. Baltimore. 1993. Identification of a ten-amino acid proline-rich SH3 binding site. Science 259:1157-1161. [DOI] [PubMed] [Google Scholar]
- 45.Salazar, E. P., and E. Rozengurt. 1999. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 274:28371-28378. [DOI] [PubMed] [Google Scholar]
- 46.Scheifele, L. Z., E. P. Ryan, and L. J. Parent. 2005. Detailed mapping of the nuclear export signal in the Rous sarcoma virus Gag protein. J. Virol. 79:8732-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman, M. P., C. M. de Noronha, M. I. Heusch, S. Greene, and W. C. Greene. 2001. Nucleocytoplasmic shuttling by human immunodeficiency virus type 1 Vpr. J. Virol. 75:1522-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhal, P. K., P. Rajendra Kumar, M. R. Subba Rao, M. Kyasani, and S. Mahalingam. 2006. Simian immunodeficiency virus Vpx is imported into the nucleus via importin alpha-dependent and -independent pathways. J. Virol. 80:526-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song, J. J., and Y. J. Lee. 2004. Tryptophan 621 and serine 667 residues of Daxx regulate its nuclear export during glucose deprivation. J. Biol. Chem. 279:30573-30578. [DOI] [PubMed] [Google Scholar]
- 50.Traxler, P., G. Bold, J. Frei, M. Lang, N. Lydon, H. Mett, E. Buchdunger, T. Meyer, M. Mueller, and P. Furet. 1997. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo [3,4-d]pyrimidines. J. Med. Chem. 40:3601-3616. [DOI] [PubMed] [Google Scholar]
- 51.Trono, D. 1998. When accessories turn out to be essential. Nat. Med. 4:1368-1369. [DOI] [PubMed] [Google Scholar]
- 52.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng, Z., S. M. Thomas, R. J. Rickles, J. A. Taylor, A. W. Brauer, C. Seidel-Dugan, W. M. Michael, G. Dreyfuss, and J. S. Brugge. 1994. Identification of Src, Fyn, and Lyn SH3-binding proteins: implications for a function of SH3 domains. Mol. Cell. Biol. 14:4509-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita, M., and M. Emerman. 2005. The cell cycle independence of HIV infections is not determined by known karyophilic viral elements. PLoS Pathog. 1:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]