Abstract
For decades, numerous ex vivo studies have documented that latent herpes simplex virus (HSV) reactivates efficiently from ganglia, but rarely from the central nervous systems (CNS), of mice when assayed by mincing tissues before explant culture, despite the presence of viral genomes in both sites. Here we show that 88% of mouse brain stems reactivated latent virus when they were dissociated into cell suspensions before ex vivo explant culture. The efficient reactivation of HSV from the mouse CNS was demonstrated with more than one viral strain, viral serotype, and mouse strain, further indicating that the CNS can be an authentic latency site for HSV with the potential to cause recurrent disease.
Herpes simplex virus (HSV) infects approximately 90% of the world's population (13, 27). After infection, two distinct life cycles can be distinguished. The first is a productive phase of 1 to 2 weeks, during which virus replicates and can be recovered from cell-free homogenates of infected tissues, such as peripheral sensory ganglia and the central nervous system (CNS). This is followed by a latent phase, during which infectious virus is no longer detectable but viral genomes can be detected in the neurons of both ganglia and CNS (3, 8, 20, 24, 27, 28). Subsequently, latent virus can reactivate to cause lesions in the periphery and CNS (13, 27). HSV-induced encephalitis has been the most common cause of sporadic, fatal encephalitis, and two-thirds of cases are likely due to viral reactivation from latency (13, 27). In addition, HSV has been suspected to be involved in a number of neurological disorders, such as epilepsy, multiple sclerosis, Alzheimer's disease, Parkinson's disease, and autism (1, 2, 9, 11, 12, 21).
The mouse model, in which HSV establishes latency and then reactivates in neural tissues upon stimulation in a manner similar to that in human infection, is used extensively for studying HSV latency (14, 27, 29). For decades, studies using the mouse model found that reactivation of latent HSV occurred efficiently and consistently in ganglia, but extremely rarely in the CNS, when assayed ex vivo by mincing tissues before explant culture (3, 5, 7, 15, 16, 20, 22-24, 30). This procedure has been the most sensitive and reproducible technique for assaying HSV reactivation. As latency involves establishment, maintenance, and reactivation, the failure to detect viral reactivation from the CNS per se in ex vivo studies has cast doubt on the authenticity of the CNS as a “latently infected tissue” (20, 22) and has raised the question of whether recurrent herpes encephalitis is a reactivation event in the CNS. Consequently, the detection of virus in latently infected mouse brains in vivo following immunosuppression was interpreted as the spread of reactivated virus from ganglia (14, 27). However, investigations into the nature of latency in ganglia and the CNS found no obvious qualitative difference between these two sites with respect to the cell types in which virus is latent, the structure and levels of latent viral genomes, or latent viral gene expression (3, 6, 7, 20, 22, 24). In this study, we sought to address the unresolved issue of HSV reactivation from the CNS by using an alternative approach.
BALB/c mice were infected with 2 × 107 PFU of HSV type 1 (HSV-1) strain KOS via both eyes following corneal scarification. The infected mice did not show obvious signs of CNS infection during acute infection, and all infected mice survived. At 30 days postinfection (p.i.), when virus had established latency, mouse brains, spinal cords (cervical and thoracic segments), and trigeminal ganglia were excised. The brain was further dissected into brain stem (pons-medulla), cerebellum, olfactory bulb, frontal cortex, and hippocampus. The weights of neural tissues from three mice were measured. The mean (± standard error of the mean) weight of one spinal cord, trigeminal ganglion, brain stem, cerebellum, olfactory bulb, frontal cortex, or hippocampus specimen was 40 ± 10, 10 ± 1, 110 ± 10, 80 ± 3, 40 ± 3, 90 ± 3, or 10 ± 3 mg/tissue, respectively. When 20 trigeminal ganglia, 20 spinal cords, and 20 brain stems were frozen and homogenized to assay for the presence of infectious virus by plaque assay on Vero cells as previously described (30), virus could not be recovered from any of these tissues. These results are consistent with previous studies (3, 6, 7, 16, 24, 30) and confirmed that persistent infection was not present in the neural tissues used for our study.
Meanwhile, neural tissues were assayed for reactivation of latent virus by a conventional mincing method. Briefly, tissues were chopped finely (∼1 mm3) and directly incubated with monolayers of Vero cells, which support viral replication, in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum, 2 mM l-glutamine, 0.075% HCO3, 200 U/ml penicillin, 200 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Fungizone). The culture medium was changed every 3 days. Cultures were inspected daily for cytopathic effect in Vero cells. Any culture that did not show cytopathic effect after 10 days was harvested, frozen, thawed, sonicated, and replated onto fresh monolayers of Vero cells. The next day, the culture medium was aspirated, and medium containing 1.5% methylcellulose was overlaid. Cultures were incubated for 4 more days and then stained with crystal violet. Consistent with previous studies (3, 7, 16, 24, 30), virus was recovered from ganglia, but not from any brain stem or cerebellum, of 16 mice.
In addition, neural tissues were assayed for reactivation of latent virus by an alternative, dissociation method. Briefly, tissues were chopped finely and incubated in serum-free DMEM containing 0.08% trypsin, 0.013% collagenase type I-A (Sigma), 20 mM HEPES, l-glutamine, HCO3, penicillin, streptomycin, and amphotericin B. After constant agitation for 40 min at 37°C, the suspension was centrifuged at 300 × g for 10 min at 4°C, and then the pellet was resuspended in DMEM containing serum and supplements as described above for the mincing method. Subsequently, the suspension was transferred to confluent monolayers of Vero cells (6 × 105 cells/well in six-well plates seeded the day before). Dissociated cells from the brain stem, cerebellum, and frontal cortex were transferred to three wells, and those from the olfactory bulb, hippocampus, and trigeminal ganglion were transferred to one well. The explant cultures were incubated at 37°C in 5% CO2 undisturbed for the first 3 days. The culture medium was changed every 3 days. During the first medium change, care was taken to avoid disruption of the attachment between dissociated neural tissues and Vero cell monolayers. The cultures were maintained and observed for cytopathic effect as described for the mincing method. Any well in which virus was recovered was scored positive for the specimen assayed. Using the dissociation method, virus was recovered from spinal cords (33%) and brains (Table 1). In the brain, the rate of recovery of virus from brain stem (71%) was much higher than that from cerebellum (20%), olfactory bulb (12%), frontal cortex (12%), and hippocampus (0%).
TABLE 1.
Recovery of HSV from neural tissues of latently infected micea
| Virus | Inoculum (PFU/eye) | Mouse strain | Tissue examined | No. of samples reactivating/total no. of samples (% reactivating) |
|---|---|---|---|---|
| HSV-1 (KOS) | 2 × 107 | BALB/c | Brain stem | 15/21 (71) |
| Cerebellum | 4/20 (20) | |||
| Olfactory bulbs | 1/9 (12) | |||
| Frontal cortex | 1/9 (12) | |||
| Hippocampus | 0/9 (0) | |||
| Spinal cord | 3/9 (33) | |||
| Trigeminal ganglion | 9/9 (100) | |||
| HSV-1 (McKrae) | 5 × 105 | C57BL/6 | Brain stem | 8/10 (80) |
| Trigeminal ganglionb | 9/10 (90) | |||
| HSV-2 (333) | 2 × 104 | C57BL/6 | Brain stem | 7/8 (88) |
| Trigeminal ganglionb | 7/8 (88) |
Mice were infected with various strains of HSV at the indicated dose via the right eye (strains McKrae and 333) or both eyes (strain KOS) following corneal scarification. At 30 days postinfection, neural tissues were harvested and assayed for reactivation of latent virus by the dissociation method. Data were collected from at least two independent experiments.
The ipsilateral trigeminal ganglion was used for study.
In the nervous system, the bipolar axon of trigeminal neurons continues past the ganglion and connects to the brain stem via a synapse (3, 7, 30, 31). The observations that none of 16 latently infected brain stems yielded reactivated virus by the mincing method and that spinal cords and other brain regions that are not connected to trigeminal ganglia were able to yield reactivated virus by the dissociation method (Table 1) indicate that the CNS tissues assayed were not contaminated with tissue from trigeminal ganglia. It is impossible to disconnect the trigeminal ganglia from the brain stem and investigate viral reactivation from only the brain in living mice or rabbits without damaging the brain stem and inducing death. In our studies, HSV was recovered from explant cultures of dissociated brain tissues in close contact with Vero cells. To rule out the possibility that the close contact between brain tissues and Vero cells was critical for reactivation (perhaps by allowing transfer of latent viral genomes from brain tissues to Vero cells), dissociated brain stems were cultured without contact with Vero cells by plating on top of transwells during explant. Under these conditions, infectious virus was recovered from 4 of 11 brain stem cultures, demonstrating that brain tissues were the source of reactivated virus.
In the CNS, the brain stem had the highest reactivation frequency. To investigate whether this was correlated with viral replication during acute infection and subsequent establishment of latency in this region, brain tissues were harvested from infected mice during acute infection and assayed for infectious virus. There was hardly any virus in brain tissues at days 1 and 2 p.i. (data not shown), which was consistent with a previous study (16). Viral growth in brain tissues peaked around day 5 p.i. (Table 2). The peak viral titer in brain stems was more than 100-fold higher than that in other brain regions.
TABLE 2.
Levels of acute viral replication and numbers of latent viral genomes in brain stems and other brain regions
| Tissue examined | Viral titer in tissue at day p.i.a:
|
No. of latent viral genomes at day 30 p.i.b | ||
|---|---|---|---|---|
| 3 | 5 | 7 | ||
| Brain stem | 2.2 ± 0.3 (5) | 4.1 ± 0.1 (5) | 1.6 ± 0.1 (5) | 5.9 ± 0.1 (10) |
| Cerebellum | 0.5 ± 0.3 (5) | 1.6 ± 0.3 (5) | 0.7 ± 0.3 (5) | 1.6 ± 0.4 (10) |
| Olfactory bulbs | 0 (5) | 1.3 ± 0.5 (5) | 0 (5) | 3.9 ± 0.3 (6) |
| Frontal cortex | 0 (5) | 0.5 ± 0.3 (5) | 0.3 ± 0.3 (5) | 4.2 ± 0.4 (6) |
| Hippocampus | 0 (5) | 0.7 ± 0.5 (5) | 0 (5) | 3.2 ± 1.1 (6) |
BALB/c mice were infected with 2 × 107 PFU of HSV-1 strain KOS via both eyes, and brain tissues were harvested at indicated times p.i. for titration of virus. Results are expressed as the log10 mean PFU ± standard error of the mean/100 mg tissue for each group. Data were collected from at least two independent experiments, and the number of samples assayed for each group is indicated in parentheses.
At day 30 p.i., brain tissues were harvested and processed to quantify viral genomes by quantitative real-time PCR. Results are expressed as the log10 mean number of genomes ± standard error of the mean/μg DNA for each group. Data were collected from at least two independent experiments, and the number of samples assayed for each group is indicated in parentheses.
Subsequently, brain tissues were harvested from latently infected mice at day 30 p.i. to investigate the establishment of latency by using quantitative real-time PCR to determine the amount of viral genomes present. Briefly, neural tissues were homogenized in guanidine thiocyanate and processed as previously described (17). DNA in the homogenate was extracted with 2% sodium dodecyl sulfate and then with phenol-chloroform. The amounts of viral and cellular (adipsin) DNA in tissues were quantified by real-time PCR using SYBR green PCR master mix and an ABI Prism 7900 sequence detection system (Applied Biosystems) according to the manufacturer's protocols. The primers tk1 (300 nM), tk reverse (5′-CCAAAGAGGTGCGGGAGTTT-3′; 900 nM), MT1 (100 nM), and MT2 (100 nM), were used to amplify viral (thymidine kinase) and adipsin genes as previously described (17). The PCR conditions were 95°C for 15 seconds and 60°C for 1 min for 40 cycles. Quantification standards composed of HSV genomes and cellular DNA were used to determine the amounts of viral genomes and cellular DNA in tissue samples, and then the amount of viral genomes was normalized to the amount of cellular DNA as previously described (17). Table 2 shows that the average number of latent viral genomes in the brain stem was more than 10-fold higher than those in other brain regions. Our results, which demonstrate that mouse brain stems support a higher level of acute viral replication and harbor more latent viral genomes than other brain regions, are consistent with previous studies (3, 7, 16, 24) and provide an explanation for the high reactivation frequency found in this region. This may also explain previous reports of brain stem encephalitis observed in humans (18, 26).
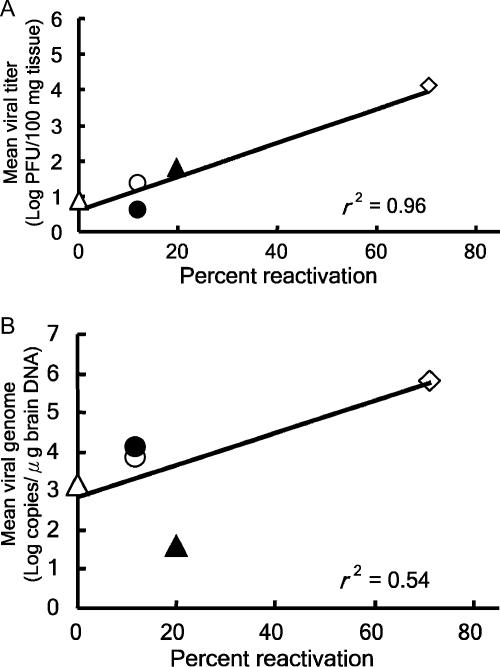
By comparing the reactivation frequencies (Table 1) and average viral titers at day 5 p.i. (Table 2) in different brain regions, a correlation between the two can been seen. When we applied linear regression analysis for these values, the correlation was very strong (r2 = 0.96) (Fig. 1A). Surprisingly, quite a few latent viral genomes were detected in the hippocampus, where no viral reactivation could be observed (Tables 1 and 2). Using linear regression analysis, we found that the correlation between the reactivation frequencies and average numbers of latent viral genomes in different brain regions was indeed weaker (r2 = 0.54) (Fig. 1B). Future studies are needed to elucidate the relationship between latency and reactivation potential in these tissues. Nevertheless, these data indicate that the presence of viral genomes might not be a good index to predict the ability of HSV reactivation to cause recurrent disease in the CNS. Indeed, previous studies using the presence of viral genomes in patient specimens as an index for investigating the involvement of HSV in neurological diseases found contradictory results (1, 12). Thus, besides detecting the presence of infectious virus, our study suggests that testing the potential of viral reactivation from specific regions of brain specimens of patients might be a better indicator for investigating the association between HSV and neurological diseases.
FIG. 1.
Correlation between reactivation frequency and acute replication or number of latent viral genomes in brain regions. The reactivation frequencies were plotted against the average viral titers at day 5 p.i. (A) or against the average number of latent viral genomes (B) in brain stems (open diamond), cerebellums (solid triangle), olfactory bulbs (open circle), frontal cortices (solid circle), and hippocampi (open triangle). The best-fit lines were generated by linear regression of values, and the correlation coefficients (r2) are displayed in each panel.
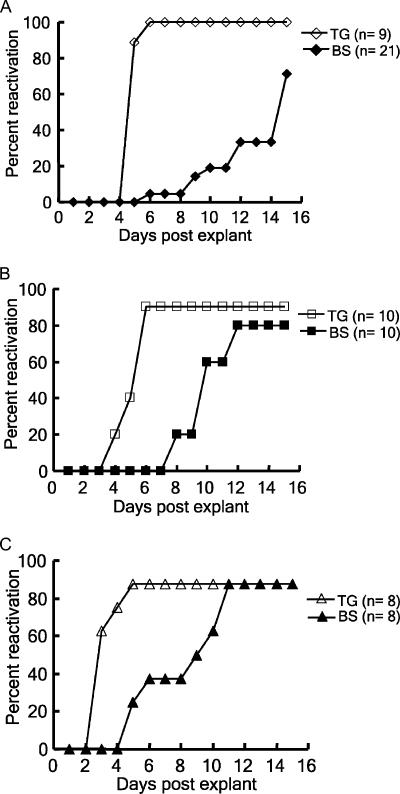
HSV-1 strain KOS and BALB/c mice were used for the studies described above. We next repeated our studies using HSV-1 strain McKrae and a mouse strain (C57BL/6) which is relatively resistant to HSV infection. When C57BL/6 mice were infected with 5 × 105 PFU of McKrae via the right eye following corneal scarification, most infected mice showed signs of encephalitis, manifested by hunched posture, lethargy, ataxia, and anorexia during acute infection. Only 50% of the infected mice survived. When the neural tissues of these remaining mice were harvested at day 30 p.i. and assayed for reactivation by the dissociation method, 80% of the brain stems yielded reactivated virus (Table 1). There are two serotypes of HSV, types 1 and 2. When HSV-2 (strain 333) was used for study, which resulted in a 50% death rate for C57BL/6 mice infected with 2 × 104 PFU of virus via the right eye, 88% of latently infected brain stems yielded reactivated virus, at a level equal to that in latently infected ganglia (Table 1). These results demonstrate that the efficient reactivation of HSV from mouse CNS is not a phenomenon specific to a viral strain, viral serotype, or mouse strain. Figure 2 shows the reactivation kinetics of HSV-1 KOS, HSV-1 McKrae, and HSV-1 333 from latently infected mouse brain stems and ganglia. We calculated the average days for detecting the cytopathic effect induced by virus reactivated from brain stems of mice latently infected with HSV-1 KOS, HSV-1 McKrae, and HSV-2 333 (12.5 ± 3.1, 10.0 ± 0.5, and 8.5 ± 1.0 days, respectively) and found that virulent viral strain HSV-1 McKrae reactivated from latently infected mouse brain stems earlier than avirulent strain HSV-1 KOS.
FIG. 2.
Kinetics of reactivation of HSV from mouse neural tissues. Brain stems (BS) (filled symbols) and trigeminal ganglia (TG) (open symbols) of mice inoculated with (A) HSV-1 KOS, (B) HSV-1 McKrae, or (C) HSV-2 333 were harvested at day 30 p.i. to assay for viral reactivation by the dissociation method.
In this study, the efficient reactivation of HSV was demonstrated in the brain stems of mice latently infected with avirulent HSV-1 strain KOS, which did not display severe signs of CNS infection during acute phase. Thus, inducing clinically evident encephalitis during primary infection is not a requirement for the capacity of an HSV strain to reactivate from the CNS. However, we did find that virulent HSV-1 strain McKrae reactivated more efficiently than KOS from latently infected mouse brain stems, as demonstrated by a higher reactivation frequency and earlier appearance of reactivated virus, despite the fact that a 40-fold-lower inoculum was used to infect a relatively resistant mouse strain, C57BL/6. This suggests that viral neurovirulence may play an important role in HSV reactivation from the CNS. Thus, we are currently investigating the influence of viral virulence on HSV reactivation from the CNS. Besides viral factors, immunosuppression also influences HSV reactivation from the CNS (14, 27). Collectively, these results indicate that using the presence of viral genomes in patient specimens as the only index to investigate the association between HSV and neurological diseases is not adequate, because critical viral and host factors may be overlooked.
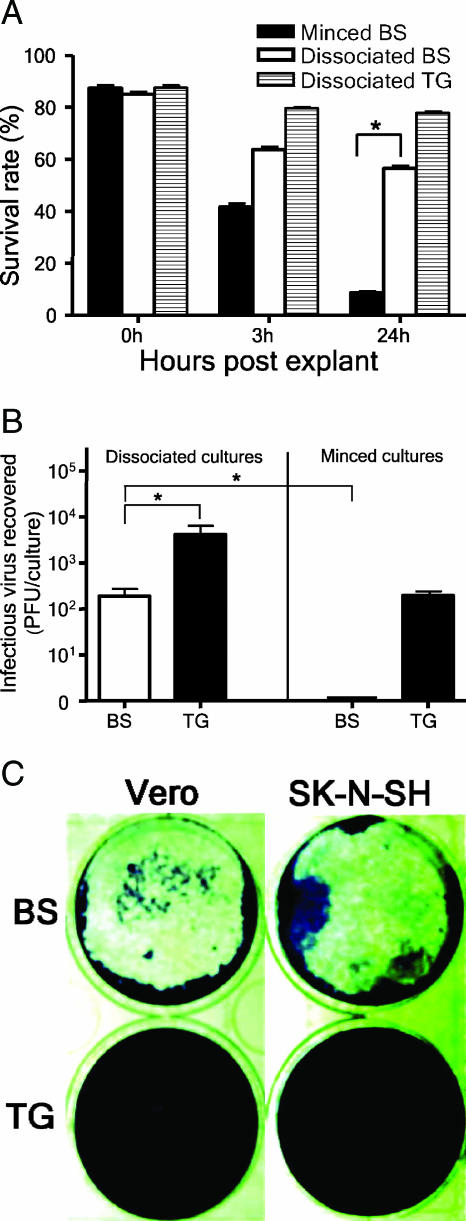
The key to our successful reactivation of latent HSV from mouse brains was the dissociation method, which was modified from the protocol used first by Leib et al. (19) and later by several laboratories to assay for viral reactivation from ganglia (4). Previously, cellular factors, such as the ability of ganglionic and CNS tissues to survive in explant culture, have been proposed as a possible explanation for the efficient reactivation of latent HSV from ganglia but not from the CNS (6). Thus, we next determined whether the dissociation method supports viral reactivation from mouse CNS by affecting the survival of fragile brain cells. Brain stems removed from 10-week-old uninfected BALB/c mice were minced or dissociated and then cultured. The minced brain stems in cultures were gently passed through Pasteur pipettes several times to release cells from tissues before the cell viabilities of minced and dissociated cultures were evaluated by trypan blue exclusion. Figure 3A shows that the brain stem cells processed by the mincing method lost viability much more rapidly than those processed by the dissociation method. The percentage of viable brain stem cells in dissociated cultures was significantly higher than that in minced cultures at 24 h postexplant (P < 0.05 by Student's t test), indicating that the dissociation method sustained the viability of brain stem cells. When 2,000 PFU of virus was added to cultures of brain stems from uninfected mice and left for 24 h, the brain stem cultures processed by the dissociation method, but not those processed by the mincing method, sustained viral infectivity (Fig. 3B). These data showed that the dissociation method greatly enhanced the cell viability and viral infectivity of brain stem cultures compared to the mincing method, and this may explain why the dissociation method supports efficient reactivation of latent HSV from mouse CNS.
FIG. 3.
The dissociation method sustains the cell viability and viral infectivity of brain stem cultures. (A) Brain stems (BS) and trigeminal ganglia (TG) harvested from uninfected BALB/c mice were minced or dissociated and then cultured before the cell viabilities of minced and dissociated cultures were evaluated by trypan blue exclusion. One hundred nucleated cells were counted, and the unstained (viable) cells were recorded. (B) BS and TG harvested from uninfected BALB/c mice were minced or dissociated and cultured. HSV-1 KOS (2,000 PFU) was added to cultures, and cultures were harvested for titration of infectious virus at 24 h p.i. Data shown in panels A and B are the means ± standard errors of the means from three independent experiments, each done in duplicate. *, P < 0.05 by Student's t test. (C) Dissociated BS, but not TG, preparations damaged less confluent Vero and human neuronal (SK-N-SH) cell monolayers. Dissociated cells from one-third of a BS or one TG were added to monolayers of Vero or SK-N-SH cells and cultured for 48 h before being stained with crystal violet. The data shown represent two experiments.
In this study, we also compared the trigeminal ganglion and brain stem cultures processed by the dissociation method with regard to levels of viable cells and viral infectivity. We found that the percentage of viable cells in ganglionic cultures was higher than that in brain stem cultures at 24 h postexplant (Fig. 3A). Interestingly, after the addition of 2,000 PFU of virus to cultures for 24 h, viral amplification was observed in ganglionic but not in brain stem cultures, as shown by the recovery of 5,100 ± 1,528 and 193 ± 29 PFU of virus from ganglionic and brain stem cultures, respectively (Fig. 3B). There was more than a 25-fold difference in viral yield between these two types of cultures, and the difference was statistically significant (P < 0.05 by Student's t test). In our study, confluent monolayers (6 × 105 cells/well in six-well plates seeded the day before) of Vero cells were used for reactivation assay, because we observed that the dissociated preparations of brain stem, but not of trigeminal ganglion, damaged less confluent monolayers (3 × 105 cells/well in six-well plates seeded the day before) of Vero cells and human neuronal (SK-N-SH) cells, a result that may be caused by the release of toxic substances from dead brain tissues (Fig. 3C). These data indicate that the dissociation method provides more favorable conditions for HSV reactivation from ganglia than from the CNS.
More than 70 years ago, Goodpasture (10) suggested that HSV was latent in neural tissues. Subsequent studies led to the assumption that latent HSV genomes in the CNS were defective for reactivation (20, 22, 25). This long-standing concept and the lack of an assay impaired studies on HSV reactivation from the CNS for more than two decades, even though HSV was suspected of being involved in neurological diseases, such as epilepsy, multiple sclerosis, Alzheimer's disease, and Parkinson's disease (1, 9, 11, 12, 21). Our current findings of efficient reactivation of latent HSV from mouse CNS strongly argue against this concept and are consistent with investigations showing no obvious qualitative differences in the latent infections of ganglia and the CNS (3, 6, 7, 20, 22, 24). Thus, the presence of infectious virus in latently infected mouse brains following immunosuppression (14, 27) is likely due to the reactivation of latent virus in the brain in situ and not necessarily to the spread of reactivated virus from ganglia.
The observation that the CNS is an authentic latency site broadens our understanding of the nature of HSV latency and recurrent disease in the CNS. This also has implications for the use of HSV as a vector for gene therapy and the controversy regarding HSV as a possible etiological agent for several human neurological diseases (1, 2, 9, 11, 12, 21). Most importantly, this study establishes a very sensitive and reproducible technique for assaying HSV reactivation from the CNS and will provide impetus to reinitiate studies on the possible associations between HSV and devastating neurological diseases.
Acknowledgments
We thank D. Coen, R. Lausch, N. Fraser, J. Pesola, J. Everly, and S. Flahive for critical reading of the manuscript. HSV strains KOS, McKrae, and 333 were kindly provided by D. Coen, G.-C. Perng, and S.-L. Hung.
This work was supported by a grant from the Taiwan National Science Council (93-2320-B-006-38).
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Beffert, U., P. Bertrand, D. Champagne, S. Gauthier, and J. Poirier. 1998. HSV-1 in brain and risk of Alzheimer's disease. Lancet 351:1330-1331. [DOI] [PubMed] [Google Scholar]
- 2.Binstock, T. 2001. Anterior insular cortex: linking intestinal pathology and brain function in autism-spectrum subgroups. Med. Hypoth. 57:714-717. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera, C. V., C. Wohlenberg, H. Openshaw, M. Rey-Mendez, A. Puga, and A. L. Notkins. 1980. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature 288:288-290. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S.-H., A. Pearson, D. M. Coen, and S.-H. Chen. 2004. Failure of thymidine kinase-negative herpes simplex virus to reactivate from latency following efficient establishment. J. Virol. 78:520-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook, M. L., and J. G. Stevens. 1976. Latent herpetic infections following experimental viraemia. J. Gen. Virol. 31:75-80. [DOI] [PubMed] [Google Scholar]
- 6.Deatly, A. M., J. G. Spivack, E. Lavi, D. R. O'Boyle II, and N. W. Fraser. 1988. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J. Virol. 62:749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou, S., A. C. Minson, H. J. Field, J. R. Anderson, and P. Wildy. 1986. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J. Virol. 57:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, N. W., W. C. Lawrence, Z. Wroblewska, D. H. Gilden, and H. Koprowski. 1981. Herpes simplex type 1 DNA in human brain tissue. Proc. Natl. Acad. Sci. USA 78:6461-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannicliffe, A., J. A. Saldanha, R. F. Itzhaki, and R. N. Sutton. 1985. Herpes simplex viral DNA in temporal lobe epilepsy. Lancet i:214-215. [DOI] [PubMed] [Google Scholar]
- 10.Goodpasture, E. W. 1929. Herpetic infection, with especial reference to involvement of the nervous system. Medicine (Baltimore). 8:233-243. [DOI] [PubMed] [Google Scholar]
- 11.Hemling, N., M. Roytta, J. Rinne, P. Pollanen, E. Broberg, V. Tapio, T. Vahlberg, and V. Hukkanen. 2003. Herpesviruses in brains in Alzheimer's and Parkinson's diseases. Ann. Neurol. 54:267-271. [DOI] [PubMed] [Google Scholar]
- 12.Itzhaki, R. F., W. R. Lin, D. Shang, G. K. Wilcock, B. Faragher, and G. A. Jamieson. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241-244. [DOI] [PubMed] [Google Scholar]
- 13.Jones, C., M. Inman, W. Peng, G. Henderson, A. Doster, G. C. Perng, and A. K. Angeletti. 2005. The herpes simplex virus type 1 locus that encodes the latency-associated transcript enhances the frequency of encephalitis in male BALB/c mice. J. Virol. 79:14465-14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastrukoff, L., C. Long, P. C. Doherty, Z. Wroblewska, and H. Koprowski. 1981. Isolation of virus from brain after immunosuppression of mice with latent herpes simplex. Nature 291:432-433. [DOI] [PubMed] [Google Scholar]
- 15.Knotts, F. B., M. L. Cook, and J. G. Stevens. 1973. Latent herpes simplex virus in the central nervous system of rabbits and mice. J. Exp. Med. 138:740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knotts, F. B., M. L. Cook, and J. G. Stevens. 1974. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J. Infect. Dis. 130:16-27. [DOI] [PubMed] [Google Scholar]
- 17.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latronico, N., and A. Candiani. 1987. Brainstem herpes virus encephalitis. Lancet ii:690-691. [DOI] [PubMed] [Google Scholar]
- 19.Leib, D. A., K. C. Nadeau, S. A. Rundle, and P. A. Schaffer. 1991. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc. Natl. Acad. Sci. USA 88:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsden, H. 1980. Herpes simplex virus in latent infection. Nature 288:212-213. [DOI] [PubMed] [Google Scholar]
- 21.Martin, J. R. 1981. Herpes simplex virus types 1 and 2 and multiple sclerosis. Lancet ii:777-781. [DOI] [PubMed] [Google Scholar]
- 22.Minson, A. C. 1983. The state of the herpes genome. Nature 302:477. [DOI] [PubMed] [Google Scholar]
- 23.Price, R. W., B. J. Katz, and A. L. Notkins. 1975. Latent infection of the peripheral ANS with herpes simplex virus. Nature 257:686-688. [DOI] [PubMed] [Google Scholar]
- 24.Rock, D. L., and N. W. Fraser. 1983. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature 302:523-525. [DOI] [PubMed] [Google Scholar]
- 25.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, G. D. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 26.Schmidbauer, M., H. Budka, and P. Ambros. 1989. Herpes simplex virus (HSV) DNA in microglial nodular brainstem encephalitis. J. Neuropathol. Exp. Neurol. 48:645-652. [DOI] [PubMed] [Google Scholar]
- 27.Sekizawa, T., and H. Openshaw. 1984. Encephalitis resulting from reactiva-tion of latent herpes simplex virus in mice. J. Virol. 50:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sequiera, L. W., L. C. Jennings, L. H. Carrasco, M. A. Lord, A. Curry, and R. N. Sutton. 1979. Detection of herpes-simplex viral genome in brain tissue. Lancet ii:609-612. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, J. G., and M. L. Cook. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843-845. [DOI] [PubMed] [Google Scholar]
- 30.Tullo, A. B., C. Shimeld, W. A. Blyth, T. J. Hill, and D. L. Easty. 1982. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J. Gen. Virol. 63:95-101. [DOI] [PubMed] [Google Scholar]
- 31.Ugolini, G., H. G. Kuypers, and P. L. Strick. 1989. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science 243:89-91. [DOI] [PubMed] [Google Scholar]