Abstract
CD46 is used by human group B adenoviruses (Ads) as a high-affinity attachment receptor. Here we show evidence that several group B Ads utilize an additional receptor for infection of human cells, which is different from CD46. We tentatively named this receptor receptor X. Competition studies with unlabeled and labeled Ads, recombinant Ad fiber knobs, and soluble CD46 and CD46 antibodies revealed three different subgroups of group B Ads, in terms of their receptor usage. Group I (Ad16, -21, -35, and -50) nearly exclusively uses CD46. Group II (Ad3, -7p, and -14) utilizes receptor X and not CD46. Group III (Ad11p) uses both CD46 and the alternative receptor X. Interaction of group II and III Ads with receptor X occurs via the fiber knob. Receptor X is an abundantly expressed glycoprotein that interacts with group II and III Ads at relatively low affinity in a Ca2+-dependent manner. This receptor is expressed at high levels on human mesenchymal and undifferentiated embryonic stem cells, as well as on human cancer cell lines. These findings have practical implications for stem cell and gene therapy.
Human adenoviruses (Ads) have been classified into six subgroups (A to F) currently containing 51 serotypes. Group B Ads form two genetic clusters, B1 (Ad3, Ad7, Ad16, Ad21, and Ad50) and B2 (Ad11, Ad14, Ad34, and Ad35) (44). Most B1 Ads are mainly associated with acute respiratory disease and, unlike the species C Ads (e.g., Ad5), do not establish persistence (43). The B2 serotypes 11p, 34, and 35 have mainly been associated with infections of the kidneys and urinary tract. Recently, gene transfer vectors based on group B Ads have shown promise for cell and gene therapy. Vectors containing fibers from group B Ads efficiently transduce human cell types that are relatively refractory to infection with classical serotype Ad5 vectors, including malignant tumor cells (30, 39), hematopoietic stem cells (26, 35, 46), mesenchymal stem (MES) cells (9, 18), dendritic cells (DC) (5, 27, 28), lymphocytes (33), chorion villus cells, and endothelial cells (13).
CD46 has been identified as a cellular receptor for group B Ads (7, 32, 37) whereby the two distal extracellular domains of CD46 are involved in Ad binding (6, 7). In humans, CD46 is a ubiquitously expressed membrane protein with complement regulatory functions.
A series of data suggest the existence of an additional group B Ad receptor(s). (i) Several groups found that Ad3 and Ad7 do not use CD46 for infection (7, 21), (ii) Ad3 and Ad7 (group B1) and Ad35 (group B2) do not compete for binding on HeLa cells (31, 35), and (iii) Ad11p fiber knob can completely block binding of wild-type Ad35 to A549 cells, while recombinant Ad35 fiber knob cannot completely block Ad11p binding (22, 41). While these data suggest that Ad3 and Ad7 and probably Ad11 can use a receptor that is different from CD46, the nature of this receptor(s) remains elusive. In this study, we investigated receptor usage by group B Ads on human cells and laid the groundwork for identification of the receptor. Also, the data obtained indicate that group B Ads are useful tools for gene transfer into human MES and embryonic stem (ES) cells.
MATERIALS AND METHODS
Cell lines.
293 (Microbix, Toronto, Ontario, Canada), HeLa, Huh7, K562, and Hep-2 cells (all from the American Type Culture Collection) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). Y79 cells were maintained in RPMI 1640 medium supplemented with 20% FCS, 1 mM sodium pyruvate, and 10 mM HEPES. SKOV3.ip1 cells (provided by David Curiel, University of Alabama at Birmingham) were cultured in DMEM-F12 supplemented with 10% FCS. CHO-K1 and CHO-C2 cells (provided by John Atkinson, Washington University, St. Louis, MO) were cultured in minimal essential medium (MEM) supplemented with 10% FCS, 200 μM asparagine, and 200 μM proline. All of the media described above were additionally supplemented with 2 mM l-glutamine, 100 U penicillin/ml, and 100 μg streptomycin/ml (Pen-Strep).
Primary cells.
MHF2 cells (human fibroblasts) were maintained in DMEM supplemented with 10% FCS, 2 mM l-glutamine, and Pen-Strep. Human MES cells and CD34+-enriched cells were isolated from human umbilical-cord blood (UCB). Human MES cells and CD34+-enriched cells were isolated from human UCB. Nucleated cells were then obtained by Ficoll density gradient centrifugation and washed twice with sterilized phosphate-buffered saline (PBS). To generate MES cells, isolated UCB cells were cultured in DMEM supplemented with 20% FCS, 2 mM l-glutamine, and Pen-Strep. Cells were seeded at a density of 1 × 106 to 107/cm2. Medium was changed after 5 days, and nonadherent cells were discarded. Thereafter, half of the medium was changed at weekly intervals. CD34+-enriched cells were purified from UCB directly after Ficoll density gradient centrifugation with MiniMACS VS+ separation columns (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's instructions and immediately processed for analysis. Monkey, dog, and mouse CD34+ cells were purified from bone marrow cells as described elsewhere (25). The purity of CD34+ preparations was verified by flow cytometry and was consistently greater than 90%. Immature human DC were prepared as described elsewhere (41). hSF6 (NIH code UC06) cells, human ES cells, were obtained from the University of California San Francisco. ES cells were maintained as previously described (45), except that the base medium was DMEM-F12 rather than DMEM (Invitrogen, Carlsbad, CA). Other supplements were as described. Essentially, the cells were cultured in medium containing serum replacer (Invitrogen) on gamma-irradiated primary mouse embryonic fibroblasts. Cultures were passaged with dispase. Cells were passaged 1 day prior to being exposed to virus. They were either plated onto feeders in unconditioned medium or onto Matrigel (Becton Dickinson, Franklin Lakes, NJ)-coated plates in filtered (0.22-μm-pore-size filter) human ES cell medium that had been conditioned by unirradiated mouse embryonic fibroblasts for 24 h.
Viruses.
Ad3 (GB strain), Ad7p (Gomen strain), Ad11p (Slobitzki strain), Ad14 (DeWit strain), Ad16 (Ch.79 strain), Ad21 (AV-1645 strain), Ad35 (Holden strain), and Ad50 (Wan strain) were all obtained from the American Type Culture Collection. Chimeric Ads, based on serotype 5 but possessing the fiber shaft and knob domains of serotype 35 (Ad5/35) or serotype 11 (Ad5/11), were previously constructed and express green fluorescent protein (GFP) as a transgene under the control of the cytomegalovirus (CMV) promoter (Ad5/35-CMV-GFP, Ad5/11-CMV-GFP) (35, 40) or express the Escherichia coli lacZ gene under the control of the Rous sarcoma virus (RSV) promoter (Ad5/35-RSV-β-Gal, Ad5/11-RSV-β-Gal) (25). The Ad5/3-CMV-GFP vector was generously provided by David Curiel (University of Alabama at Birmingham). Ads were propagated in 293 cells, methyl-3H thymidine labeled, and purified, and titers of genomes and viral particles (VP) and PFU were determined as described elsewhere (35). Ad3 and Ad35 were labeled with Cy3 by using Fluorolink Cy3 reactive dye according to the manufacturer's (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) instructions.
Antibodies, recombinant fiber knobs, soluble CD46, and siRNA.
Monoclonal antibodies (MAbs) directed against the CD46 CCP1 domain (J4-48; Research Diagnostics), the CD46 CCP2 domain (MEM-258; Serotec), the CD46 CCP3/4 domain (GB-24) (8), integrin α2 (DX5; Immunotech), integrin α3 (P1B5; Gibco), integrin α4 (9F10; Pharmingen), integrin α5 (IIA1; Pharmingen), integrin α6 (GoH3; Pharmingen), integrin αv (L230; ATTC), integrin β1 (P4C10; Chemicon), integrin β3 (AB1932; Chemicon), integrin β4 (439-9B; Pharmingen), and integrin αvβ5 (P1F6; Chemicon) were used for flow cytometry and to compete Ad binding in attachment and/or cytopathic effect (CPE) studies. The final antibody concentration was 10 μg/ml for CPE and attachment assays. Small interfering RNA (siRNA) oligonucleotides were obtained from QIAGEN. CD46 siRNA, control siRNA, and transfection of siRNA into HeLa cells were described previously (7). Integrin siRNAs were directed against the following sequences: integrin αv, 5′-AGCAACTTTATTATAGATTTA-3′; integrin β1, catalog no. SI00300573, target sequence not provided. Ad3, Ad11p, and Ad35 recombinant fiber knob domain proteins were prepared as described elsewhere (7). The fiber knob proteins were dialyzed against 5 mM KCl, 17% glycerol, and 10 mM MgCl2. Soluble CD46 was prepared as described elsewhere (7).
Flow cytometry.
Adherent cells were detached by treatment with Versene (Gibco). After being washed, cells were resuspended in 120 μl of wash buffer (WB; phosphate-buffered saline-1% fetal bovine serum) and incubated for 45 min at 4°C with MAbs (final concentration, 5 μg/ml). Subsequently, cells were washed with WB two times and incubated with Alexa Fluor 488 goat anti-mouse antibody (1/100 dilution; Molecular Probes) or with Alexa Fluor 488 goat anti-rat antibody (1/100 dilution; Molecular Probes) for 30 min at 4°C. After incubation with the secondary antibody, cells were washed two times with WB and 104 cells were analyzed in duplicate by flow cytometry.
β-Galactosidase (β-Gal) and alkaline phosphatase expression.
Ad5/35-RSV-β-Gal and Ad5/11-RSV-β-Gal transduction was detected by X-Gal staining. Cells were fixed with a solution containing PBS, 0.5% glutaraldehyde, and 1 mM MgCl2 for 20 min at room temperature (RT). Staining for β-Gal was performed with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (7). Alkaline phosphatase expression in ES cells was detected as described elsewhere (3).
Attachment assays.
Unless otherwise indicated, cells were detached from culture dishes by incubation with Versene and washed with PBS (Gibco). A total of 105 cells/tube were resuspended in 100 μl of ice-cold adhesion buffer (DMEM supplemented with 2 mM MgCl2, 1% FCS, and 20 mM HEPES) containing 3H-labeled Ad at a multiplicity of infection (MOI) of 8,000 VP per cell. After 1 h of incubation at 4°C, cells were pelleted and washed twice with 0.5 ml of ice-cold PBS. After the last wash, the supernatant was removed and the cell-associated radioactivity was determined with a scintillation counter. The number of VP bound per cell was calculated by using the virion specific radioactivity and the number of cells. Competition assays included the following modifications from the protocol described above. (i) For knob competition, various concentrations (0.01 to 20 μg/ml) of fiber knob in 50 μl adhesion buffer were added and allowed to attach for 45 min at 4°C before 3H-labeled Ad was added to a final volume of 100 μl. (ii) For antibody competition, 10 μg/ml of antibody in 50 μl adhesion buffer was added and allowed to attach for 30 min at 4°C before 3H-labeled Ad was added to a final volume of 100 μl. (iii) For cross competition, a 2- to 100-fold excess of unlabeled Ad in 50 μl adhesion buffer was added and allowed to attach for 1 h at 4°C before 3H-labeled Ad was added to a final volume of 100 μl. (iv) For C3b competition, various concentrations (0.01 to 25 μg/ml) of human purified C3b fragment (Antigen Site, San Diego, CA) in 50 μl adhesion buffer were incubated with cells for 30 min at room temperature before 3H-labeled Ad was added to a final volume of 100 μl. (v) For soluble CD46 competition, 3H-labeled Ad was incubated at a total volume of 100 μl CD46 supernatant (diluted 1:1 with adhesion buffer) at room temperature for 1 h before addition to cells. Assays of attachment to HeLa cells were also performed as described above after detachment of cells from culture dishes with 0.05% trypsin-0.5 mM EDTA solution for 15 min or after deglycosylation by incubation with tunicamycin (Sigma) at a concentration of 0.1 or 0.5 μg/ml for 48 h as described previously (8).
To analyze the affinity of binding of the different Ads to their receptor(s), different amounts of 3H-labeled Ad particles ranging from 2,000 to 800,000 VP per cell were incubated with 105 cells in the presence and absence of CD46 blockade (with or without preincubation of cells with the MEM-258 MAb for 30 min on ice). Numbers of attached VP per cell were determined, and Scatchard plots were made. The binding affinities (Ka)S of individual Ads were calculated on the basis of the slope with standard Excel software. The number of receptor sites was extrapolated from the intercept of the lines with the x axis.
Ad-Cy3 attachment studies.
MES cells were detached from culture dishes by incubation with Versene (Gibco), and 1 × 104 cells/chamber were plated on eight-well Tissue-Tek chamber slides (Nalge Nunc International) and incubated at 37°C overnight. The next day, cells were incubated with Ad3 or Ad35 labeled with Cy3 at an MOI of 8,000 VP/cell at 4°C for 45 min in 200 μl adhesion buffer. hSF6 and CD34+ cells were incubated in suspension with 8,000 VP/cell Cy-3 labeled Ad3 or Ad35 at 4°C for 45 min in 100 μl adhesion buffer. Cells were then washed with PBS, fixed for 15 min with acetone-methanol at 4°C, washed two times with PBS, and air dried. Cytospins of hSF6 and CD34+ cells onto glass slides were done, and cells were analyzed by fluorescence microscopy.
CPE assay.
A total of 2 × 105 HeLa cells per well of a 24-well plate were incubated for 30 min at 4°C with 300 μl DMEM-10% FCS-glutamine-Pen-Strep containing 10 μg/ml MEM-258 antibody (Serotec) or J4-48 (Research Diagnostics). Thereafter, 700 μl DMEM-10% FCS-glutamine-Pen-Strep containing unlabeled Ad at an MOI of 100 PFU/cell was added and cells were incubated at 37°C. After 48 h, the cells were washed with PBS and fixed with 4% paraformaldehyde for 3 min at room temperature. Fixed cells were washed with PBS and incubated for 3 min in 1% crystal violet in 70% ethanol, followed by three rinses with water. Air-dried cells were photographed.
RESULTS
Binding of group B Ads in the presence of CD46 blocking antibodies.
To assess the differential utilization of CD46 and the new receptor X, we performed attachment studies with 3H-labeled group B Ads in the presence and absence of anti-CD46 antibodies on a series of transformed and primary human cell cultures. We used an anti-CD46 MAb against CCP2 (MEM-258) that has been previously shown to specifically block binding of and infection of Ad35 fiber-containing Ad vectors (6). We performed studies with Ad3, -7p, -14, -11p, -35, -16, -21, and -50 on human tumor cell lines of different tissue origins on the basis of the speculation that the expression levels of receptor X might vary between cell lines. The following cell lines were used: HeLa (cervical carcinoma), K562 (erythroleukemia), Hep-2 (larynx carcinoma), SKOV3 (ovarian cancer), 293 (neuronal origin), and Huh7 (hepatoma). Because CD46 is upregulated on tumors (12, 17, 24, 42) and tumor cell lines are thought to express high levels of CD46 (1), which might complicate the study of alternative receptors, a second set of binding studies was performed with primary human cells. We focused on cell types for potential use in ex vivo gene therapy such as human stem cells, DC, and skin fibroblasts (MHF2). Stem cells used in this study included hematopoietic stem cells residing in CD34+ cord blood-derived cells (CD34+), MES cells, and ES cells (line hSF6). Our previous work (7, 34) and Scatchard blot analyses described in this study (see Fig. 4) provided information on the density of CD46 on key cell lines used in this study. CD46 density on HeLa cells was ∼8 × 104 molecules per cell; on K562 cells, it was ∼6 × 104 molecules per cell; and on MES cells, it was ∼3 × 104 molecules per cell.
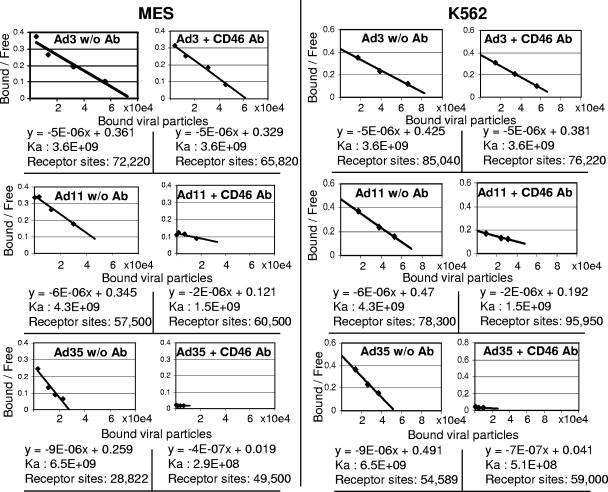
FIG. 4.
Affinity and receptor numbers. Ka values and receptor sites for Ad3, -11p, and -35 on MES cells and K562 cells are shown. w/o Ab, without antibody.
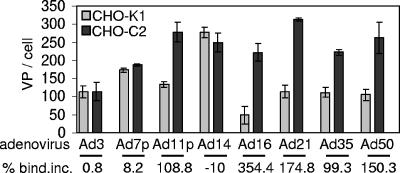
In studies with tumor cell lines, with regard to inhibition by the MEM-258 MAb, we found three groups of group B Ad serotypes (Table 1). In group I, binding of Ad35, -16, -21, and -50 could be blocked >80%, indicating that these serotypes preferentially use CD46 for binding. Differences between serotypes in the magnitude of binding are most likely due to different Kas to CD46 (for example, when measured on CD46-expressing CHO cells [CHO-C2], the Kas for Ad35, Ad11p, Ad21, and Ad50 were 2.5 × 109, 4.9 × 109, 4.9 × 109, and 1.0 × 1010 M−1, respectively [A.G., unpublished data]). Differences in the attachment of a given serotype to different cell lines might be due to different cell sizes and CD46 densities. In group II, binding of Ad3, Ad7, and Ad14 was blocked by MEM-258 MAb less than 48%, indicating that these serotypes preferentially use receptor X. The impact of binding through receptor X was different for different cell lines (potentially as a result of different receptor levels). Interestingly, interaction of Ad3 (and Ad7 and Ad14; data not shown) with 293 cells was nearly independent of CD46. In group III, attachment of Ad11p was blocked by the MEM-258 MAb between 40 and 80%, indicating that this serotype uses both CD46 and receptor X. The outcome of studies of binding to primary cells was similar to that observed for transformed cell lines. For group I Ads such as Ad35, as in tumor cell lines, binding could be efficiently blocked by the MEM-258 MAb. Binding of group II Ads such as Ad3 was not decreased in the presence of the MEM-258 MAb. Group III Ad11p binding to primary cells was partially inhibited by the MEM-258 MAb. Our data on the usage of CD46 by group B Ads were corroborated by studies with CHO cells that do (CHO-C2) or do not (CHO-K1) express CD46 (Fig. 1). We found that significantly more Ad11p, -16, -21, -35, and -50 particles bound to CHO-C2 than to CHO-K1, while binding of Ad3, Ad7, and Ad14 was not significantly different between the two cell lines. These findings corroborate our data obtained with the MEM-258 MAb and show that Ad3, -7, and -14 binding is largely independent of CD46.
TABLE 1.
Inhibition of Ad attachment by CD46 antibodiesa
| Cell type and name | % Inhibition of binding by:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7p | Ad11p | Ad14 | Ad16 | Ad21 | Ad35 | Ad50 | |
| Tumor | ||||||||
| HeLa | 42.8 | 34.6 | 57.4 | 44.1 | 92.4 | 89.8 | 90.6 | 89.6 |
| K562 | 33 | 33.8 | 72.4 | 38.7 | 95.4 | 92.2 | 93.4 | 92.8 |
| Hep-2 | 39 | 64.8 | 40.9 | 84.5 | ||||
| Skov3 | 28.5 | 76 | 83.1 | |||||
| 293 LP | 0.4 | 41.7 | 89.4 | |||||
| Huh7 | 47.7 | 79.9 | 93.7 | |||||
| Stem | ||||||||
| MES | 11.2 | 57.1 | 90.7 | 90.2 | ||||
| hSF6 | 20.7 | 41.2 | 95.9 | 81.1 | 82.2 | |||
| CD34+ | 10.3 | 11.9 | 56.7 | 62.9 | ||||
| Normal | ||||||||
| MHF2 | 6.1 | 81.4 | 21.4 | 89.3 | ||||
Cells were preincubated with anti-CD46 antibody MEM-258 or control antibody. 3H-labeled Ads were then added at an MOI of 8,000 VP per cell, and the number of VP bound per cell was determined after 1 h of incubation at 4°C as described in Materials and Methods. Cell types are grouped as transformed or tumor cell lines, primary stem cells, and primary normal cells. Shown is the percentage of inhibition of binding after preincubation with control versus CD46 antibody. All samples were analyzed in triplicate. Standard deviation was less than 10%.
FIG. 1.
Attachment study with group B Ads on CHO-K1 versus CHO-C2 cells. 3H-labeled Ads were added, and the number of VP bound per cell was determined after 1 h of incubation on ice.
Level of Ad attachment to different cell lines.
For group I Ads such as Ad35, overall, fewer particles bound to primary cells compared to tumor cell lines (Table 2). Group II Ad3 bound more efficiently than group I and III Ads to ES cells. In contrast, binding of group I and III Ads to CD34+ cells, DC, and MHF2 was more efficient than that of group II Ads. In agreement with a recent study (33), we found that Ad3 binds less to DC than does Ad11p or Ad35 (whose binding is most likely mediated by CD46). Importantly, compared to skin fibroblasts, six out of the seven tumor cell lines we investigated showed elevated binding levels of Ad3 (differences: HeLa, 5.7-fold; Huh7, 4.5-fold; K562, 3.1-fold; Hep2, 2.8-fold; SKOV3, 1.8-fold). This indicates that receptor X might be upregulated in tumor cells.
TABLE 2.
Group B Ad attachment to cell linesa
| Cell type and name | No. of VP bound/cell
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7p | Ad11p | Ad14 | Ad16 | Ad21 | Ad35 | Ad50 | |
| Tumor | ||||||||
| HeLa | 2,563 | 1,512 | 1,605 | 1,434 | 1,950 | 2,014 | 1,888 | 1,658 |
| K562 | 1,364 | 906 | 1,112 | 1,547 | 3,937 | 3,721 | 1,352 | 3,533 |
| Hep-2 | 1,250 | 921 | 955 | 959 | ||||
| Skov3 | 807 | 965 | 812 | |||||
| 293 LP | 557 | 402 | 559 | |||||
| Huh7 | 2,018 | 2,518 | 2,012 | |||||
| Stem | ||||||||
| MES | 960 | 887 | 737 | 748 | 720 | |||
| hSF6 | 1,175 | 740 | 585 | 542 | ||||
| CD34+ | 41 | 80 | 71 | 93 | ||||
| Normal | ||||||||
| MHF2 | 444 | 881 | 449 | 1,013 | ||||
| DC | 232 | 524 | 1,980 | 2,097 | 1,972 | 1,142 | ||
3H-labeled Ads were added to cells at an MOI of 8,000 VP per cell, and the number of VP bound per cell was determined after 1 h of incubation at 4°C as described in Materials and Methods. All samples were analyzed in triplicate. Standard deviation was less than 10%.
Transduction of tumor and stem cells by group B Ads in the presence of CD46 blocking antibodies.
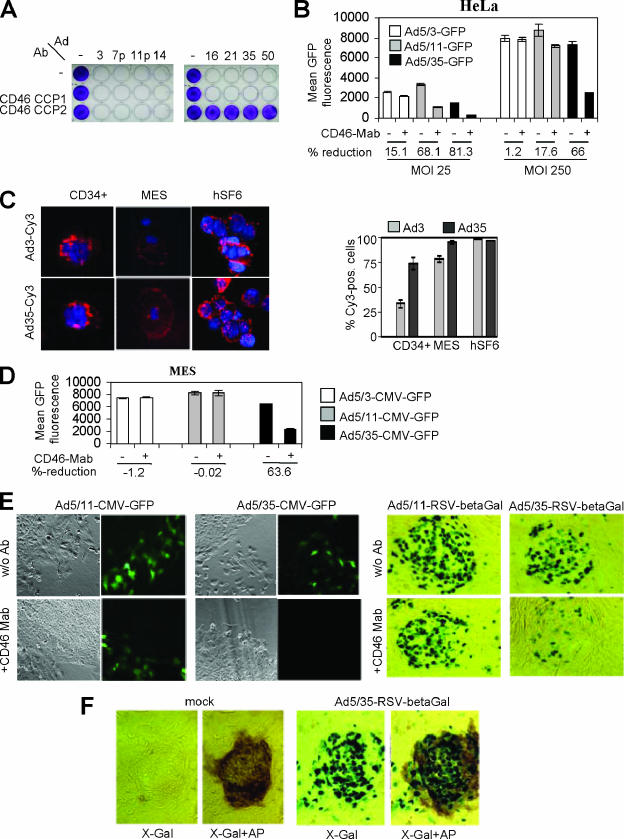
Although it is thought that the degree of attachment largely determines the tropism and infection efficiency of Ads, other steps such as internalization, intracellular trafficking, and gene expression are factors that can impact the infection efficiency of Ads. To validate our finding about the role of CD46 and receptor X in Ad infection, we performed competition studies with the MEM-258 MAb as in Table 1 but measured the formation of CPE or Ad-mediated transgene expression as a surrogate for infection efficiency. The CPE assays (Fig. 2A) showed that infection by Ad16, -21, -35, and -50 can be blocked by the anti-CCP2 antibody but not by an antibody against a CD46 domain that is not directly involved in group B Ad binding (anti-CCP1). In contrast, the anti-CCP2 antibody did not inhibit the infection and CPE of Ad3, -7p, -11p, and -14. To study the impact of the MEM-258 MAb on Ad3, Ad35, and Ad11p infection in more detail, we used chimeric Ad5 vectors that possess the Ad3, Ad11, or Ad35 fibers and express GFP (Ad5/3-GFP, Ad5/35-GFP, and Ad5/11-GFP) (Fig. 2B). In the presence of the MEM-258 MAb, GFP expression levels after infection of HeLa cells with Ad5/3-GFP at MOIs of 25 and 250 PFU/cell decreased only 15.1 and 1.2%, respectively. In contrast, for Ad5/35-infected cells, MEM-258 MAb incubation reduced gene expression levels 81.3% and 66% (at MOIs of 25 and 250 PFU/cell). Ad5/11-GFP-mediated gene expression decreased 68.1 and 17.6% in the presence of MEM-258. Again, this shows that infection via the Ad35 fiber depends to a larger degree on CD46 than infection via the Ad11 fiber (particularly at higher MOIs), while infection via the Ad3 fiber knob is largely independent of CD46.
FIG. 2.
Role of CD46 in group B Ad infection. (A) CPE assay with group B Ads on HeLa cells. A total of 2 × 105 HeLa cells/well were seeded in 24-well plates. Twenty-four hours later, cells were preincubated with antibodies directed against the CCP1 or CCP2 domain of CD46 for 30 min (10 μg/ml). Ads were then added at an MOI of 100 PFU/cell (∼2,000 VP/cell). Forty-eight hours after infection, cells were washed with PBS, fixed with 4% paraformaldehyde, and stained with crystal violet as described in Materials and Methods. (B) Fiber chimeric Ad infection blocking study. HeLa cells were preincubated with the MEM-258 MAb and then infected with Ad5/3-CMV-GFP, Ad5/11-CMV-GFP, or Ad5/35-CMV-GFP at an MOI of 25 or 250 PFU/cell, and GFP fluorescence was assessed by flow cytometry 16 h after infection. (C) Ad-Cy3 binding to primary cells. Primary cells were incubated with Ad3-Cy3 or Ad35-Cy3 either in suspension (CD34+, hSF6) or attached to chamber slides (MES). Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Cy3-labeled VP appear as red dots on the cell surface. Magnifications were ×20 (MES), ×40 (hSF6), and ×100 (CD34+). The graph on the right shows the average percentage of Cy3-positive cells from 10 random viewing fields (magnification, ×40). (D) MES cells were preincubated with the MEM-258 MAb for 30 min at RT (10 μg/ml). Cells were then infected with Ad5/3-CMV-GFP, Ad5/11-CMV-GFP, or Ad5/35-CMV-GFP at an MOI of 25 PFU/cell. GFP fluorescence was assessed by flow cytometry 24 h after infection. (E) hSF6 cells were preincubated with the MEM-258 MAb for 30 min at RT (10 μg/ml). Cells were then infected with Ad5/11-CMV-GFP or Ad5/35-CMV-GFP (left side). Representative samples of GFP fluorescence 24 h after infection and the corresponding cell morphology are shown. w/o Ab, without antibody. (Right side) Infection was performed as described above, with Ad5/11-RSV-β-Gal and Ad5/35-RSV-β-Gal. At 24 h after infection, cells were fixed and stained for β-Gal expression. Magnification, ×20. (F) hSF6 cells were mock infected or infected with Ad5/35-RSV-β-Gal at an MOI of 250 PFU/cell. At 24 h after infection, cells were fixed and stained for β-Gal expression (X-Gal) and then for alkaline phosphatase (X-Gal+AP) as described in Materials and Methods. β-Gal-positive cells show a green-blue nucleus and slightly green cytoplasm. AP-positive cells show brown cytoplasm. Representative samples are shown.
The efficient interaction of Ad3 with stem cells (see Table 2) was corroborated by studies with Cy-3-labeled particles (Fig. 2C). While Cy3-Ad3 bound to human ES cells as efficiently as Cy3-Ad35, fewer Cy3-Ad3 than Cy3-Ad35 particles bound to CD34+ cells and MES cells. To study the involvement of CD46 and receptor X in transduction of human stem cells, we infected MES and ES cells with Ad5/35 (which uses CD46), Ad5/3 (which uses receptor X), and Ad5/11 (which uses CD46 and receptor X) vectors in the absence and presence of CD46 blocking antibodies. In MES cells, the MEM-258 MAb significantly reduced Ad5/35-CMV-GFP infection, while Ad5/3 and Ad5/11 infection was not affected (Fig. 2D). This indicates that Ad5/3 and Ad5/11 infection of MES cells can occur via an alternative receptor, in a CD46-independent manner, whereas Ad5/35 transduction of MES cells is predominantly CD46 dependent. The effect of MEM-258 on infection of hSF6 cells was similar (Fig. 2E). While the number of transgene-expressing cells decreased more than 70% for Ad5/35 vector-infected cells in the presence of MEM-258, antibody preincubation reduced transduction of Ad5/11 vectors only 13%. Interestingly, when infected with Ad5/11 and Ad5/35.CMV-GFP (at an MOI of 250 PFU/cell), only cells in the periphery of hSF6 clones that had undergone differentiation expressed GFP (Fig. 2E, left side). Apparently, the CMV promoter was not active in undifferentiated ES cells. In contrast, Ad5/11 and Ad5/35 vectors that contained the lacZ gene under the control of the RSV promoter conferred efficient transgene expression at the same MOI used for the GFP-expressing vectors in all ES cell subsets, including undifferentiated ES cells (Fig. 2E, right side). Undifferentiated ES cells can be identified by positive staining for alkaline phosphatase (Fig. 2F).
Cross competition with Ads and fiber knobs.
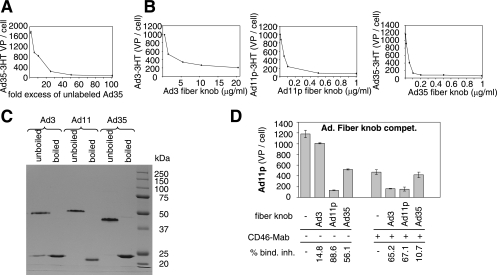
To further investigate the use of receptor X by group B Ads, we performed cross-competition studies with HeLa cells with and without the MEM-258 MAb. In these studies, we used an excess of unlabeled virus that allowed for ≥85% inhibition of the corresponding 3H-labeled virus binding to HeLa cells in the absence of the MEM-258 MAb, as shown, for example, in Fig. 3A for Ad35. Table 3 shows that Ad3, Ad7p, Ad11p, and Ad14 cross compete for CD46-independent binding, while Ad35 does not block the binding of these serotypes.
FIG. 3.
Group B Ad-cell interaction occurs via fiber knob. (A) Cross competition of Ad35 for attachment to HeLa cells. Cells were incubated with a 2- to 100-fold excess of unlabeled Ad35. Thereafter, 3H-labeled Ad35 was added at an MOI of 8,000 VP per cell and the number of VP bound per cell was determined. (B) Fiber knob competition of Ad3, Ad11p, and Ad35 attachment to HeLa cells. Cells were preincubated with various concentrations of the recombinant fiber knob proteins of Ad3 (1 to 20 μg/ml), Ad11p (0.01 to 1 μg/ml), and Ad35 (0.01 to 1 μg/ml). Corresponding 3H-labeled Ads were then added at an MOI of 8,000 VP per cell, and numbers of VP bound per cell were determined. (C) Analysis of recombinant Ad3, Ad11p, and Ad35 fiber knob proteins by electrophoresis (unboiled versus boiled for 3 min at 100°C) on a 10% polyacrylamide gel. The bands were visualized by brilliant blue R-250 (Fisher Biotech) stain reagent. The values on the right are molecular masses of marker proteins in kilodaltons. (D) Fiber knob competition of Ad11p attachment to HeLa cells. Cells were preincubated with the MEM-258 MAb, and subsequently recombinant fiber knob proteins of Ad3 (10 μg/ml), Ad11p (1 μg/ml), and Ad35 (1 μg/ml) were added. Thereafter, 3H-labeled Ad11p was added at an MOI of 8,000 VP per cell and attachment was analyzed. bind. inh., binding inhibition.
TABLE 3.
Cross competition of groups Ads for attachment to HeLa cellsa
| Unlabeled Ad | % Inhibition of binding by 3H-labeled Ad:
|
||||
|---|---|---|---|---|---|
| Ad3 | Ad7p | Ad11p | Ad14 | Ad35 | |
| Ad3 | 83.8 | 69.5 | 72.7 | 65.9 | −14.1 |
| Ad7p | 59.9 | 62.9 | 69.7 | 45 | −9.9 |
| Ad11p | 75.7 | 64.7 | 79 | 63.4 | 8 |
| Ad14 | 77.6 | 76.5 | 65 | 70.5 | −9 |
| Ad35 | 4 | −3.4 | −1.1 | −5.3 | 11.9 |
Cells were preincubated with MAb MEM-258 followed by a 100-fold excess of unlabeled Ad. Control cells were incubated with MAb MEM-258 only. Thereafter, 3H-labeled Ads were added at an MOI of 8,000 VP per cell and the number of VP bound per cell was determined after 1 h of incubation at 4°C. Percentage of binding inhibition compared to control settings was calculated. All samples were analyzed in triplicate. Standard deviation was less than 10%.
Furthermore, we carried out competition studies with recombinant fiber knob domains on HeLa cells. Ad3, Ad35, and Ad11 fiber knob domains were used at concentrations that inhibited binding of the corresponding virus more than 90% (Fig. 3B). Notably, in contrast to Ad11 and Ad35, not all recombinant Ad3 knob protein was present in trimeric form (Fig. 3C), which might affect the affinity of interaction with receptor X and might explain why 10-fold more Ad3 knob than Ad35 knob was needed to achieve the same inhibition of binding of the parental Ad. Importantly, the fact that Ad3 knob blocks Ad3 binding indicates that interaction with receptor X is fiber knob mediated. Cross-competition studies with fiber knobs revealed that Ad3 knob inhibits Ad7p and Ad14 binding but does not significantly block Ad11p binding (Table 4). It was surprising that Ad3 knob was unable to compete with Ad11p binding, in contrast to Ad3 virus. Only when interaction of Ad11p with CD46 is blocked by MEM-258 MAb does Ad3 knob block Ad11p binding (Fig. 3D). Also, in agreement with earlier data (41), while Ad11 fiber knob blocked binding of Ad35 virus more than 90%, Ad35 knob blocked Ad11p binding only 70%. Ad35 knob decreased Ad3, 7p, and 14 binding by ∼42%. A similar outcome was seen when unlabeled Ad35 virus or MEM-258 MAb was used as a competitor (data not shown). When taking the cross-competition data together, one can conclude that group II Ad3, -7p, -11p, and -14 compete for the same receptor, which is different from CD46. Group III Ad11 uses both CD46 and receptor X and can block binding of group I and II Ads. Ad11p appears to have a higher affinity toward CD46 than toward receptor X, as Ad11p only binds to receptor X when CD46 is blocked. Group I Ads use CD46. Binding of Ad35knob/virus to CD46 appears to affect interaction of Ad3, -7p, and -14 with receptor X. Along this line, CD46 antibodies specific to the Ad35 binding domain of CD46 partially inhibit Ad3 binding (Table 4, lower section), suggesting a potential physical juxtaposition of the two receptors.
TABLE 4.
Ad binding competition assay with fiber knob, CD46 MAb, and soluble CD46
| Competitor | % Inhibition of binding by 3H-labeled Ad:
|
||||
|---|---|---|---|---|---|
| Ad3 | Ad7p | Ad11p | Ad14 | Ad35 | |
| Ad3 fibera | 82.3 | 81.5 | 13.3 | 80.9 | 13.9 |
| Ad11p fibera | 82.8 | 74.6 | 87.2 | 69.5 | 91.7 |
| Ad35 fibera | 42.3 | 42.3 | 70.2 | 42.5 | 94.1 |
| CD46 Abb | 42.8 | 34.6 | 57.4 | 44.1 | 90.6 |
| Soluble CD46c | 13.4 | 7.2 | 40 | 7.5 | 50.9 |
| Soluble CD46c | −0.1 | 3.1 | 41.4 | −5.4 | 43.6 |
HeLa cells were preincubated with recombinant fiber knob proteins from Ad3 (10 μg/ml), Ad11p (1 μg/ml), or Ad35 (1 μg/ml). 3H-labeled Ads were then added at an MOI of 8,000 VP per cell, and attachment was analyzed.
An antibody (Ab) competition assay was carried out as described for Table 1, with MAb MEM-258 (10 μg/ml), before addition of 3H-labeled Ads.
Soluble CD46 competition of group B Ad attachment to HeLa or MES cells (bottom-most row). 3H-labeled Ads were preincubated with soluble CD46 before addition to cells. Control cells were incubated with 3H-labeled Ad only (without competitor). Percentages of 3H-labeled Ad binding inhibition compared to control cells are shown. All samples were analyzed in triplicate. Standard deviation was less than 10%.
Receptor number and affinity.
Binding studies in combination with Scatchard blot analyses were performed to measure the affinity (Ka) of Ads for receptor X and receptor numbers on two representative cell cultures, K562 cells and MES cells. MES cells that efficiently bind Ad3 were used as target cells (Fig. 4). To assess the impact of CD46 on Ka, binding studies were performed in the presence and absence of the MEM-258 MAb. On MES cells, more Ad3 binding sites (72,220) than Ad35 binding sites (28,822) were found. While the Ka of Ad3 did not change in the presence of the MEM-258 MAb, the Ka for Ad35 binding decreased by more than 1 order of magnitude, indicating that Ad35 is unlikely to bind with sufficient affinity to a cellular receptor other than CD46. The affinity of Ad3 toward receptor X was lower than the affinity of Ad35 toward CD46. The outcome of studies with K562 cells was similar, although the numbers of both Ad3 and Ad35 receptors were higher than on MES cells (85,040 and 54,589, respectively). This study and Fig. 3D also support our earlier observation that Ad11p has a higher affinity toward CD46 than toward receptor X. MEM-258 reduced the Ka of A11p to a higher degree than the Ka of Ad3, suggesting that Ad3 has a higher affinity for receptor X than Ad11p does. Taken together, this indicates that, on both MES and K562 cells, receptor X is more abundantly expressed but has less Ad binding affinity than CD46 does.
Characterization of receptor X.
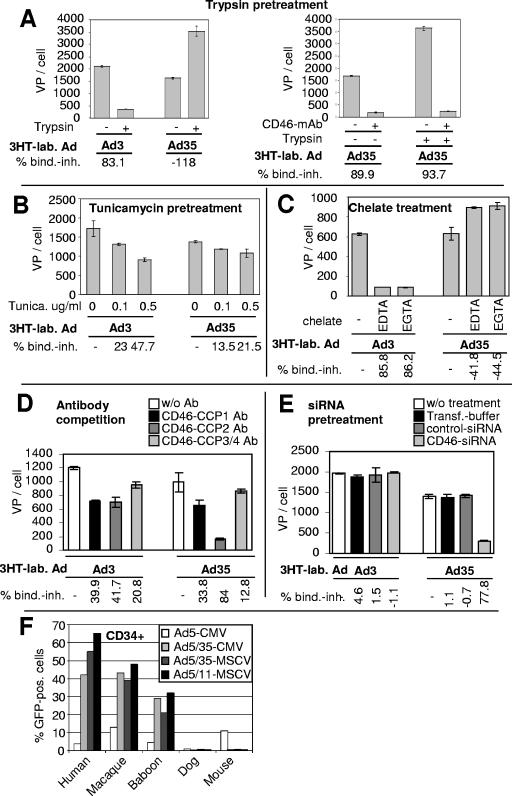
We next attempted to better characterize receptor X, which is used by group II and III Ads. Attachment studies were performed with group I Ad3 and HeLa cells after pretreatment of cells with trypsin (Fig. 5A). Trypsin pretreatment inhibited Ad3 binding, suggesting that receptor X is a protein. In contrast, trypsin pretreatment enhanced Ad35 binding to CD46, which can be blocked by the MEM-258 MAb. This indicates that the Ad35 binding domain(s) of CD46 is relatively resistant to trypsin. We also speculate that removal of other cell surface proteins opens access to CD46 or reduces electrostatic repulsion between Ad35 and CD46. Furthermore, pretreatment of cells with tunicamycin at a concentration that effectively inhibits N-glycosylation of CD46 (8) significantly reduced binding of Ad3 to HeLa cells, suggesting that carbohydrate side chains might be involved in Ad3 interaction with receptor X (Fig. 5B). (In agreement with earlier observations, N-glycosylation of CD46 is not critical for Ad35 binding [8].) Binding of Ad3 was almost entirely dependent on divalent cations, while EDTA and EGTA pretreatment had no effect on Ad35 binding (Fig. 5C). Importantly, none of the antibodies against other CD46 domains (CCP1, -3, and -4) blocked Ad3 binding more than the CCP2-specific antibody (Fig. 5D). Furthermore, preincubation of HeLa cells with the complement factor C3b, at a concentration that inhibited binding of an anti-CCP3/4 antibody, did not decrease attachment of Ad3 (data not shown). Taking these finding together, it is highly unlikely that other domains of CD46 are involved in Ad3 binding. To ultimately show that Ad3 does not use CD46, we transfected HeLa cells with CD46 siRNA to knock down CD46 expression (7). Figure 5E shows that in CD46 siRNA-transfected cells but not in cells treated with control siRNA, Ad35 binding decreased 77.8% while Ad3 binding increased 1.1%. This finding also provides further evidence that the reduction of Ad3 binding by the MEM-258 MAb and Ad35/Ad35 knob is unspecific and probably due to steric hindrance.
FIG. 5.
Characterization of receptor X. All data shown were acquired with HeLa cells. (A) Cells were detached from culture dishes with trypsin (+) or EDTA (−). Cells were preincubated with MEM-258 MAb (right side). 3H-labeled (3HT-lab.) Ad3 or Ad35 was then added at an MOI of 8,000 VP per cell. (B) Cells were pretreated with tunicamycin (Tunica.) at the concentrations shown for 48 h. Then, 3H-labeled Ad3 or Ad35 was added at an MOI of 8,000 VP per cell and the numbers of VP per cell were determined. (C) Cells were incubated with 3H-labeled Ad3 or Ad35 (MOI, 8,000 VP/cell) in the presence of 10 mM EDTA or EGTA. Numbers of VP bound per cell were determined. (D) Antibody competition of Ad attachment. Cells were preincubated with CD46-CCP1-, CD46-CCP2-, or CD46-CCP3/4-specific MAbs as described in Materials and Methods. Thereafter 3H-labeled Ad3 or Ad35 (MOI, 8,000 VP/cell) was added and the numbers of VP bound per cell were measured. (E) Cells were mock transfected or transfected with control siRNA or CD46 siRNA as described in Materials and Methods. At 48 h after transfection, 3H-labeled Ad3 or Ad35 (8,000 VP/cell) was added and the numbers of VP bound per cell were determined. (F) Fiber chimeric Ad infection study on CD34+ cells of different species. A total of 105 CD34+ cells were infected with GFP-expressing viruses (Ad5-CMV-GFP, Ad5/35-CMV-GFP, Ad5/11-CMV-GFP, Ad5/11-MSCV-GFP) at an MOI of 25 PFU/cell in suspension in 300 μl. GFP fluorescence intensities were assessed by flow cytometry 24 h after infection. MSCV, murine stem cell virus. w/o Ab, without antibody; bind. inh., binding inhibition.
To analyze the presence of group B Ad receptors in different species, we infected CD34+ cells with Ad5/11 and Ad5/35, which express the gene for GFP under the control of the murine stem cell virus or CMV promoter. Interestingly, Ad5/11 vectors cannot transduce CD34+ cells from dogs and mice (Fig. 5F), which argues against the presence of receptor X in these species. In contrast, CD34+ cells from primates (humans, macaques, and baboons) are efficiently transduced by Ad5/35 or Ad5/11 vectors, indicating that both group B Ad receptors, CD46 and receptor X, are well conserved within different species of primates.
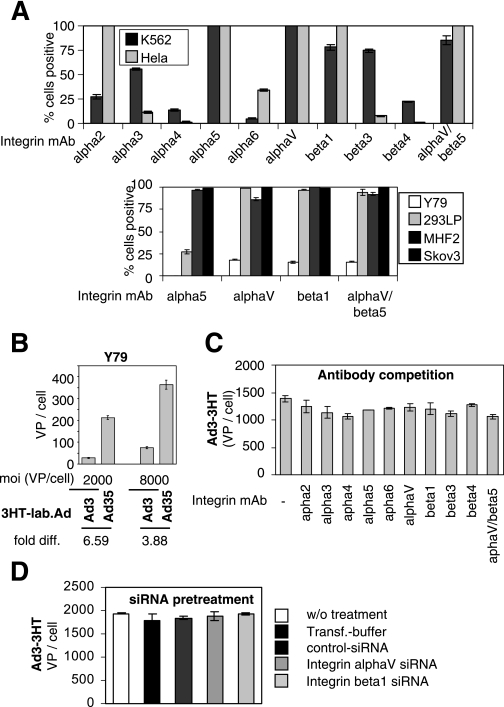
A number of lines of data suggest that receptor X is an integrin. (i) Cations (Ca2+) are important in maintaining the structure of integrins (11), (ii) integrins are expressed at a high density on the surface of most cells including stem cells (4), (iii) integrins form complexes with CD46 (20), and (iv) integrins are functionally involved in tumor invasion and metastasis and are therefore upregulated during tumor progression (10). To assess whether receptor X is an integrin, we analyzed surface expression of the most common integrins on HeLa and K562 cells, cell lines that both efficiently bind Ad3 (Table 1). Integrins that are significantly expressed at high levels on both cell lines include α5, αv, β1, and αvβ5 (Fig. 6A). In further studies, we therefore focused on those integrins. Other cell lines (293, MHF2, and SKOV3) that bind Ad3 also showed significant integrin αv, β1, and αvβ5 expression, whereas integrin α5 was less detectable in 293 cells. Of interest, Y79 cells, a CD46high cell line that expresses no or only very low levels of integrins (38), does not efficiently bind Ad3 (Fig. 6B). Despite indications that αv, β1, and β5 are candidates for receptor X, we were not able to confirm a critical role for these integrins in Ad3 binding. Antibodies against the most widely expressed α and β integrin subunits, including αv, β1, and β5, did not significantly block Ad3 binding to HeLa cells (Fig. 6C). Previous studies showed that these antibodies block Ad5 vector uptake and infection at the concentration used in our competition experiments (19). More importantly, knockdown of critical integrin subunit β1 and αv expression by siRNA did not affect Ad3 binding (Fig. 6D). (In the present study, siRNA transfection resulted in a >25% decrease in β1 and αv integrin levels on the surface of HeLa cells, as determined by flow cytometry.) Notably, integrins can only be displayed at the cell surface as αβ heterodimers and knockdown of one the subunits prevents presentation of the corresponding integrin (11). Finally, ectopic expression of αv and β1 integrins in CHO cells did not increase Ad3 binding compared to that in naive CHO cells (data not shown).
FIG. 6.
Assessment of integrins as receptor X candidates. (A) Flow cytometry analysis of integrin expression on K562, HeLa, Y79, 293, MHF2, and SKOV3 cells was carried out as described in Materials and Methods. Percentages of Alexa Fluor 488-positive cells are shown. (B) Studies of Ad3 and Ad35 attachment to Y79 cells. Y79 cells were incubated with 3H-labeled (3HT-lab.) Ads at an MOI of 2,000 or 8,000 VP per cell, and the numbers of VP bound per cell were determined. diff., difference. (C) Antibody competition of Ad3 binding to HeLa cells. Cells were preincubated with MAbs directed toward integrins (10 μg/ml) as described in Materials and Methods. 3H-labeled Ads (8,000 VP/cell) were then added, and the numbers of VP bound per cell were determined. (D) HeLa cells were mock transfected or transfected with control siRNA or siRNA specific for αv and β1 integrins as described in Materials and Methods. At 48 h after transfection, 3H-labeled Ad3 (8,000 VP/cell) was added and the number of VP bound per cell was determined. Transf., transfer.
In conclusion, our data indicate that receptor X is an abundantly expressed glycoprotein that interacts with group II and III Ads at relatively low affinity in a bivalent-cation-dependent manner. Our finding that this receptor is apparently expressed at high levels and with uniformity on stem cells and tumor cell lines has practical implications for gene therapy.
DISCUSSION
A classification of group B Ad receptors was previously introduced by Segerman et al. in 2003 (31). They suggested that all group Ads interact with a common receptor (species B Ad receptor). In addition, species B2 Ads (Ad11, -14, -34, -35, and -50) were thought to interact with an additional receptor, designated the species B2 Ad receptor, which is identical to CD46. We propose a revised classification of group B Ads on the basis of the usage of attachment receptors. Our classification is not in agreement with the subdivision into species B1 and B2 Ads (which focuses on genetic differences). Group I Ads (Ad16 [B1], Ad21 [B1], Ad35 [B2], and Ad50 [B2]) use CD46 for infection. Group II Ads (Ad3 [B1], Ad7p [B1], and Ad14 [B2]) use receptor X for infection. Group III Ad11p (B2) uses both CD46 and receptor X. The classification is supported by studies on six established human cells lines and four primary cultures of different tissue origins.
Although it has been reported that Ad3 uses CD46 as a receptor (37), our findings (particularly the studies with CD46 siRNA [Fig. 5E]) and studies by Marttila et al. (21) rather support the conclusion that CD46 is not a high-affinity receptor for either Ad3 or Ad7p. The discrepancies with respect to Sirena's finding could be attributed to different Ad3 isolates, target cells (BHK-CD46 cells versus human cells), and readout parameters.
Our data also argue against a suggestion made by Segerman et al. (32) that different group B Ads might use different domains within CD46 for binding. We have shown earlier that at least two group B Ads, Ad35 and Ad11p, interact with the same CCP2 domain of CD46 (8). Importantly, downregulation of CD46 expression via siRNA inhibited Ad35 binding, but not Ad3 binding, to HeLa cells. Blocking with antibodies against CD46 domains other than CCP2 or by C3b (which binds to CCP3/4) did not significantly affect the binding of all group B Ads. In addition, ectopic expression of CD46 on CHO cells did not increase the binding levels of Ad3, -7p, or -14, whereas Ad11p, -16, -21, -35, and -50 attachment was significantly increased (Fig. 1A). Finally, incubation of Ad11p and Ad35 with soluble CD46 inhibited their binding to HeLa cells and MES cells (Table 4), whereas Ad3, -7p, and -14 binding levels were not significantly affected. These observations support our conclusion that receptor X is different from CD46.
The finding that the MEM-258 MAb partially inhibits the binding of Ad3, -7, and -14 to several CD46high cell lines suggests that the cellular receptor for Ad3 is in close physical proximity to CD46 rather than being CD46 itself. Interestingly, this effect is not as pronounced on primary cells, which have lower CD46 densities. Inhibition of Ad3 binding by CD46 antibodies could be simply due to a high density of receptor X and CD46 on the cell surface. On the other hand, this could indicate that these molecules are coreceptors, implying that binding to one receptor transfers Ad to the other receptor, which, for example, might mediate endocytosis. This is a strategy used by other viruses, including human immunodeficiency virus and reovirus (2). However, our finding that efficient infection of Ad3, -7p, -11p, and -14 occurs via receptor X in the presence of the MEM-258 MAb, as well as the CD46 knockdown and soluble CD46 blocking data, argues against such a coreceptor relationship. The nature of Ad3 blocking by CD46 antibodies remains to be investigated.
The identification of receptor X has important implications for adenovirology and gene therapy. Early studies showed that the Ad3 fiber interacted with a 130-kDa protein in a divalent-cation-dependent manner, while a recent study suggested that CD80 (B7-1) and CD86 (B7-2) function as cellular attachment receptors for Ad3 (36). However, because CD80 and CD86 are mainly expressed on antigen-presenting cells and not on HeLa or MES cells (data not shown), it is unlikely that these two molecules represent receptor X. We found that receptor X is an abundantly expressed protein that is apparently primate specific. The Ka for binding to receptor X is less than that for binding to CD46. This might be due in part to protein-carbohydrate interactions, which are typically of a weak nature. Our finding that chelation of divalent cations had a negative effect on Ad3 binding but not Ad35 binding is in agreement with earlier studies (32). Although a series of data were indicative of a role for integrins in Ad group II and III binding, blocking studies with integrin-specific antibodies and siRNA did not support this indication. Furthermore, despite the fact that quiescent CD34+ cells express very small amounts of αv integrins, Ad3 binding to and infection of hematopoietic cells were efficient (23). While receptor X appears to be expressed on hematopoietic cells, it seem to be lost upon differentiation as Ad3 does not efficiently bind anymore to peripheral blood mononuclear cells (33) or DC (see Table 2). Notably, α5, αv, and β1 integrins are expressed at high levels on DC (14, 29). Taken together, these findings appear to argue against integrins as a candidate for receptor X. We are currently performing more systematic studies with recombinant Ad3 and Ad11 fiber knob domains to purify virus-interacting proteins from MES cell membrane lysates, essentially as we have done before for the identification of CD46 (7).
In this study, we showed that receptor X is expressed at high levels on MES cells and undifferentiated ES cells, derived from line hSF6, and that an Ad11 fiber-containing Ad vector efficiently transduced these cells when CD46 was blocked. This is, to our knowledge, the first study that demonstrates efficient transduction of undifferentiated human ES cells by Ad vectors. In transduction studies, we also found that the CMV promoter, in the context of a first-generation Ad5/35 or Ad5/11 vector, is not active in undifferentiated cells, which is in conflict with a recent study by Kim et al. that found efficient CMV promoter-driven transgene expression in ES cells after plasmid transfection (16). Notably, these authors did not confirm the undifferentiated stage of ES cells. We also demonstrated that an RSV promoter can confer efficient transgene expression in ES cells after infection with Ad5/35 or Ad5/11 vectors at low MOIs.
Furthermore, our binding studies show that Ad3, -7p, -14, and -11p bind more efficiently to cancer cell lines than to primary human fibroblasts or DC, which indicates that receptor X is expressed at higher levels on tumor cell lines than on normal tissue. Our data are supported by a recent study showing that a chimeric virus that utilizes the Ad3 fiber (Ad5/3) preferentially and efficiently infects primary ovarian cancer cells in vitro (15). Of particular interest is also that Ad11 can use two receptors (CD46 and receptor X) present at high levels on tumor cells, which potentially reduces the risk of development of escape mutants by downregulation of Ad attachment receptors in gene therapy trials with oncolytic Ads. Furthermore, Ad11 fiber interaction with receptor X does not increase transduction of normal tissue, as vector biodistribution in baboons after intravenous injection of Ad5/35 and Ad5/11 is similar (25).
In conclusion, our studies indicate that a subset of group B Ads bind to important gene therapy targets independent of CD46. This finding is important for both understanding the molecular mechanisms of Ad pathogenicity and developing group B Ads as a tool for gene therapy.
Acknowledgments
We thank Steve Roffler for critical discussions and are grateful to John Atkinson, Pamela Becker, David Curiel, Angel Nelson, Thalia Papayannopoulou, and Hartmut Stecher for providing valuable materials.
This study was supported by NIH grants CA080192, HLA078836, HL53750, and IP20 GM69983.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Anderson, B. D., T. Nakamura, S. J. Russell, and K. W. Peng. 2004. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64:4919-4926. [DOI] [PubMed] [Google Scholar]
- 2.Barton, E. S., B. E. Youree, D. H. Ebert, J. C. Forrest, J. L. Connolly, T. Valyi-Nagy, K. Washington, J. D. Wetzel, and T. S. Dermody. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 111:1823-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernt, K. M., M. Liang, X. Ye, S. Ni, Z.-Y. Li, S. L. Ye, F. Hu, and A. Lieber. 2002. A new type of adenovirus vector that utilizes homologous recombination to achieve tumor-specific replication. J. Virol. 76:10994-11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czyz, J., and A. Wobus. 2001. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation 68:167-174. [DOI] [PubMed] [Google Scholar]
- 5.DiPaolo, N., S. Ni, A. Gaggar, R. Strauss, S. Tuve, Z. Y. Li, D. Stone, D. Shayakhmetov, N. Kiviat, P. Toure, S. Sow, B. Horvat, and A. Lieber. 2006. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 13:756-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischli, C., S. Verhaagh, M. Havenga, D. Sirena, W. Schaffner, R. Cattaneo, U. F. Greber, and S. Hemmi. 2005. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J. Virol. 79:10013-10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaggar, A., D. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 8.Gaggar, A., D. M. Shayakhmetov, M. K. Liszewski, J. P. Atkinson, and A. Lieber. 2005. Localization of regions in CD46 that interact with adenovirus. J. Virol. 79:7503-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gugala, Z., E. A. Olmsted-Davis, F. H. Gannon, R. W. Lindsey, and A. R. Davis. 2003. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther. 10:1289-1296. [DOI] [PubMed] [Google Scholar]
- 10.Guo, W., and F. G. Giancotti. 2004. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell. Biol. 5:816-826. [DOI] [PubMed] [Google Scholar]
- 11.Haas, T. A., and E. F. Plow. 1994. Integrin-ligand interactions: a year in review. Curr. Opin. Cell Biol. 6:656-662. [DOI] [PubMed] [Google Scholar]
- 12.Hara, T., A. Kojima, H. Fukuda, T. Masaoka, Y. Fukumori, M. Matsumoto, and T. Seya. 1992. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br. J. Haematol. 82:368-373. [DOI] [PubMed] [Google Scholar]
- 13.Havenga, M. J., A. A. Lemckert, O. J. Ophorst, M. van Meijer, W. T. Germeraad, J. Grimbergen, M. A. van Den Doelqq, R. Vogels, J. van Deutekom, A. A. Janson, J. D. de Bruijn, F. Uytdehaag, P. H. Quax, T. Logtenberg, M. Mehtali, and A. Bout. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jancic, C., H. E. Chuluyan, A. Morelli, A. Larregina, E. Kolkowski, M. Saracco, M. Barboza, W. S. Leiva, and L. Fainboim. 1998. Interactions of dendritic cells with fibronectin and endothelial cells. Immunology 95:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanerva, A., G. V. Mikheeva, V. Krasnykh, C. J. Coolidge, J. T. Lam, P. J. Mahasreshti, S. D. Barker, M. Straughn, M. N. Barnes, R. D. Alvarez, A. Hemminki, and D. T. Curiel. 2002. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 8:275-280. [PubMed] [Google Scholar]
- 16.Kim, J. H., H. J. Do, S. J. Choi, H. J. Cho, K. H. Park, H. M. Yang, S. H. Lee, D. K. Kim, K. Kwack, S. K. Oh, S. Y. Moon, K. Y. Cha, and H. M. Chung. 2005. Efficient gene delivery in differentiated human embryonic stem cells. Exp. Mol. Med. 37:36-44. [DOI] [PubMed] [Google Scholar]
- 17.Kinugasa, N., T. Higashi, K. Nouso, H. Nakatsukasa, Y. Kobayashi, M. Ishizaki, N. Toshikuni, K. Yoshida, S. Uematsu, and T. Tsuji. 1999. Expression of membrane cofactor protein (MCP, CD46) in human liver diseases. Br. J. Cancer 80:1820-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaän-Shanzer, S., M. J. van de Watering, I. van der Velde, M. A. Goncalves, D. Valerio, and A. A. de Vries. 2005. Endowing human adenovirus serotype 5 vectors with fiber domains of species B greatly enhances gene transfer into human mesenchymal stem cells. Stem Cells 23:1598-1607. [DOI] [PubMed] [Google Scholar]
- 19.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozahic, S., D. Christiansen, S. Manie, D. Gerlier, M. Billard, C. Boucheix, and E. Rubinstein. 2000. CD46 (membrane cofactor protein) associates with multiple β1 integrins and tetraspans. Eur. J. Immunol. 30:900-907. [DOI] [PubMed] [Google Scholar]
- 21.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 79:14429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei, Y. F., K. Lindman, and G. Wadell. 2002. Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 23.Mei, Y. F., A. Segerman, K. Lindman, P. Hornsten, A. Wahlin, and G. Wadell. 2004. Human hematopoietic (CD34+) stem cells possess high-affinity receptors for adenovirus type 11p. Virology 328:198-207. [DOI] [PubMed] [Google Scholar]
- 24.Murray, K. P., S. Mathure, R. Kaul, S. Khan, L. F. Carson, L. B. Twiggs, M. G. Martens, and A. Kaul. 2000. Expression of complement regulatory proteins—CD 35, CD 46, CD 55, and CD 59—in benign and malignant endometrial tissue. Gynecol. Oncol. 76:176-182. [DOI] [PubMed] [Google Scholar]
- 25.Ni, S., K. Bernt, A. Gaggar, Z. Y. Li, H. P. Kiem, and A. Lieber. 2005. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 16:664-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson, M., J. Ljungberg, J. Richter, T. Kiefer, M. Magnusson, A. Lieber, B. Widegren, S. Karlsson, and X. Fan. 2004. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 6:631-641. [DOI] [PubMed] [Google Scholar]
- 27.Rea, D., M. J. Havenga, M. van Den Assemqq, R. P. Sutmuller, A. Lemckert, R. C. Hoeben, A. Bout, C. J. Melief, and R. Offringa. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 166:5236-5244. [DOI] [PubMed] [Google Scholar]
- 28.Rozis, G., S. de Silva, A. Benlahrech, T. Papagatsias, J. Harris, F. Gotch, G. Dickson, and S. Patterson. 2005. Langerhans cells are more efficiently transduced than dermal dendritic cells by adenovirus vectors expressing either group C or group B fibre protein: implications for mucosal vaccines. Eur. J. Immunol. 35:2617-2626. [DOI] [PubMed] [Google Scholar]
- 29.Sadovnikova, E., E. N. Parovichnikova, E. L. Semikina, E. A. Kopiltsova, D. A. Svinareva, V. M. Belkin, N. A. Torubarova, and V. G. Savchenko. 2004. Adhesion capacity and integrin expression by dendritic-like cells generated from acute myeloid leukemia blasts by calcium ionophore treatment. Exp. Hematol. 32:563-570. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai, F., H. Mizuguchi, T. Yamaguchi, and T. Hayakawa. 2003. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol. Ther. 8:813-821. [DOI] [PubMed] [Google Scholar]
- 31.Segerman, A., N. Arnberg, A. Erikson, K. Lindman, and G. Wadell. 2003. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J. Virol. 77:1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segerman, A., K. Lindman, Y. F. Mei, A. Allard, and G. Wadell. 2006. Adenovirus types 11p and 35 attach to and infect primary lymphocytes and monocytes, but hexon expression in T-cells requires prior activation. Virology 349:96-111. [DOI] [PubMed] [Google Scholar]
- 34.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short, J. J., A. V. Pereboev, Y. Kawakami, C. Vasu, M. J. Holterman, and D. T. Curiel. 2004. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology 322:349-359. [DOI] [PubMed] [Google Scholar]
- 37.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skubitz, A. P., M. D. Grossman, J. B. McCarthy, E. A. Wayner, and J. D. Cameron. 1994. The decreased adhesion of Y79 retinoblastoma cells to extracellular matrix proteins is due to a deficit of integrin receptors. Investig. Ophthalmol. Vis. Sci. 35:2820-2833. [PubMed] [Google Scholar]
- 39.Sova, P., X.-W. Ren, N. Shaoheng, K. M. Bernt, J. Mi, N. Kiviat, and A. Lieber. 2004. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol. Ther. 9:496-509. [DOI] [PubMed] [Google Scholar]
- 40.Stecher, H., D. M. Shayakhmetov, G. Stamatoyannopoulos, and A. Lieber. 2001. A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol. Ther. 4:36-44. [DOI] [PubMed] [Google Scholar]
- 41.Stone, D., S. Ni, Z. Y. Li, A. Gaggar, N. DiPaolo, Q. Feng, V. Sandig, and A. Lieber. 2005. Development and assessment of human adenovirus type 11 as a gene transfer vector. J. Virol. 79:5090-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorsteinsson, L., G. M. O'Dowd, P. M. Harrington, and P. M. Johnson. 1998. The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS 106:869-878. [DOI] [PubMed] [Google Scholar]
- 43.Wadell, G. 1984. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 110:191-220. [DOI] [PubMed] [Google Scholar]
- 44.Wadell, G., M. L. Hammarskjold, G. Winberg, T. M. Varsanyi, and G. Sundell. 1980. Genetic variability of adenoviruses. Ann. N. Y. Acad. Sci. 354:16-42. [DOI] [PubMed] [Google Scholar]
- 45.Ware, C. B., A. M. Nelson, and C. A. Blau. 2005. Controlled-rate freezing of human ES cells. BioTechniques 38:879-880, 882-883. [DOI] [PubMed] [Google Scholar]
- 46.Yotnda, P., H. Onishi, H. E. Heslop, D. Shayakhmetov, A. Lieber, M. Brenner, and A. Davis. 2001. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Ther. 8:930-937. [DOI] [PubMed] [Google Scholar]