Abstract
Neonatal Borna disease virus (BDV) infection of the rat brain is associated with microglial activation and damage to the certain neuronal populations. Since persistent BDV infection of neurons in vitro is noncytolytic and noncytopathic, activated microglia have been suggested to be responsible for neuronal cell death in vivo. However, the mechanisms of activation of microglia in neonatally BDV-infected rat brain have not been investigated. To address these issues, activation of primary rat microglial cells was studied following exposure to purified BDV or to persistently BDV-infected primary cortical neurons or after BDV infection of primary mixed neuron-glial cultures. Neither purified virus nor BDV-infected neurons alone activated primary microglia as assessed by the changes in cell shape or production of the proinflammatory cytokines. In contrast, in the BDV-infected primary mixed cultures, we observed proliferation of microglia cells that acquired the round morphology and expressed major histocompatibility complex molecules of classes I and II. These manifestations of microglia activation were observed in the absence of direct BDV infection of microglia or overt neuronal toxicity. In addition, compared to uninfected mixed cultures, activation of microglia in BDV-infected mixed cultures was associated with a significantly greater lipopolysaccharide-induced release of tumor necrosis factor alpha, interleukin 1β, and interleukin 10. Taken together, the present data are the first in vitro evidence that persistent BDV infection of neurons and astrocytes rather than direct exposure to the virus or dying neurons is critical for activating microglia.
Borna disease virus (BDV) is a nonsegmented, negative-strand RNA virus that persistently infects the central nervous system (CNS) and causes behavioral abnormalities in a broad spectrum of warm-blooded animals (3, 12, 22). In neonatally infected rats, BDV causes a life-long persistent infection of the CNS with minimal signs of classical inflammatory cell infiltration (e.g., encephalitis and meningitis) and the absence of overt clinical disease. Nonetheless, neonatal BDV infection is associated with a progressive loss of granule cells in the dentate gyrus of the hippocampus, Purkinje cells in the cerebellum, and GABA-ergic neurons in the neocortex (5, 16, 18, 45).
Because BDV establishes a persistent noncytolytic infection in various cell lines and primary neurons and astrocytes in vitro (6, 20, 34), the mechanisms of neuronal degeneration in vivo remain unclear. Previous studies have indicated that even if the virus does not infect microglia in vivo, neonatal BDV infection is associated with strong microgliosis (6, 22, 38). Intriguingly, BDV-associated microgliosis has been found predominantly in areas of significant neuronal loss, i.e., cortex, hippocampus, and cerebellum (22, 33, 38, 44), leading to the hypothesis that microglia activation plays a central role in BDV-associated neuronal damage (38, 44).
Microglia, the resident macrophage population in the brain, play a central role in inflammatory processes and acute and chronic neurodegenerative diseases of the CNS (36). Once stimulated, microglia undergo several transformations from the resting state (i.e., ramified cell morphology) to the reactive state (i.e., amoeboid morphology) (43). This process is generally termed “activation” and includes proliferation, expression of microphage-specific and activation markers, changes in cell shape, and secretion of a variety of cytokines and free radicals (43) that are believed to contribute to neurodegeneration (2, 36 ).
It has been shown that in vitro and in vivo microglia can be activated by several factors, including double-stranded RNA (29), viral proteins (32), bacterial membranes (40), pathogen-stimulated neurons or astrocytes (37, 41), and apoptotic neurons (11, 13). However, the mechanisms of microglia activation during neonatal BDV infection have not been adequately investigated. In the present study, a number of different possible mechanisms of microglia activation in the context of BDV infection were explored, including activation via direct incubation with purified virus and persistently BDV-infected primary neurons or via BDV infection of mixed neuron-glial cultures. We found that neither direct exposure to purified BDV nor coculturing with BDV-infected neurons led to activation of the primary microglia cultures as evaluated by changes in the cell morphology or the production of the proinflammatory cytokines. In contrast, BDV infection of the mixed neuron-glial cultures resulted in a significant increase in the number of round-shaped major histocompatibility complex class I (MHC-I)- and MHC-II-positive microglia cells without any signs of overt neurotoxicity. In addition, compared to the uninfected mixed cultures, the morphological changes in microglia in the BDV-infected mixed cultures were associated with a significantly greater release of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-10 following lipopolysaccharide (LPS) treatment. These results provide the first in vitro evidence that persistent BDV infection of neurons and astrocytes rather than direct exposure to the virus or dying neurons is important for activating microglia.
MATERIALS AND METHODS
Reagents.
LPS from Escherichia coli 026:B6, staurosporine, Hoechst 33258, DNase, poly-l-lysine, laminin, and fluorescein isothiocyanate (FITC)-labeled isolectin I-B4 from Griffonia simplicifolia seeds (lectin IB4) were obtained from Sigma Chemical Co. (St. Louis, MO). Mouse anti-rat CD11b/c (clone OX42) monoclonal antibody was purchased from BD Biosciences (San Diego, CA). Monoclonal anti-MHC-I (clone OX18) and anti-MHC-II (clone OX6) antibodies were from GeneTex (San Antonio, TX). Rabbit anti-activated caspase-3 antibody was obtained from Promega (Madison, WI). Rabbit anti-ionized calcium binding adapter molecule 1 (Iba1) antibody was obtained from Wako Chemicals (Richmond, VA). Chicken anti-microtubule-associated protein 2 (MAP2) polyclonal antibody, rabbit anti-glial fibrillary acidic protein (GFAP), and mouse anti-ED1 glycoprotein (rat macrophage lysosomal membrane antigen antibody; clone ED1) and the secondary antibodies carbocyanin (Cy) 3, Cy 5, or FITC-conjugated donkey anti-mouse, anti-rabbit, and anti-chicken immunoglobulin G antibodies were obtained from Chemicon (Temecula, CA). Monoclonal antibody directed against BDV nucleoprotein protein (anti-BDV N; clone Bo18) was prepared as described previously (19). Dulbecco's modified Eagle medium (DMEM) with high glucose (4,500 mg/liter), DMEM/F12 (1:1) nutritional supplemented medium, neurobasal A medium with serum-free B-27 supplement (NBM), heat-inactivated horse serum, HEPES buffer solution, Hank's balanced salt solution, l-glutamine solution, penicillin-streptomycin solution (P/S; 50 U/50 μg per ml), trypsin (0.25%)-EDTA (1 mM), and trypan blue were purchased from Invitrogen/GIBCO-BRL (Carlsbad, CA). Certified heat-inactivated fetal bovine serum (FBS) was obtained from HyClone (Logan, UT). Recombinant human immunodeficiency virus type 1 (HIV-1) Tat protein was prepared as described previously (9). LPS and Tat stocks of 1 mg/ml were prepared in DMEM.
Virus stock preparation and titration.
Purified virus stock was prepared from human oligodendroglia cells (kindly provided by G. Pauli, Berlin, Germany) (30) persistently infected with BDV strain He/80 (10) as described previously (31). Briefly, confluent 175-cm2 culture flasks were washed with 20 mM HEPES (pH 7.4) and incubated with 20 ml of 20 mM HEPES containing 250 mM MgCl2 and 1% FBS for 1.5 h at 37°C to lyse the cells. Subsequently, supernatants were harvested and centrifuged twice at 2,500 × g for 15 min to remove cell debris. Virus particles were concentrated by ultracentrifugation for 1 h at 20°C at 80,000 × g onto a 20% sucrose cushion containing 20 mM HEPES and 1% FBS. Virus-containing pellets were resuspended in phosphate-buffered saline (PBS) to approximately 106 focus-forming units/ml. Determination of viral titers was carried out on C6 rat glioma cells (ATCC, Manassas, VA) by an immunofocus assay with monoclonal anti-BDV N antibody as described previously (7, 21). Mock stock was prepared from noninfected oligodendroglia cells as described above for BDV stock. The protein concentration of virus stocks was determined using a bicinchoninic acid protein assay from Pierce Biotechnology, Inc. (Rockford, IL).
Rat cell culture. (i) Neonatal microglia.
All experiments were performed with adherence to the National Institutes of Health guidelines on the use of experimental animals and with protocols approved by the Johns Hopkins Medical Institution's Research and Animal Care Committee. To isolate rat neonatal microglia, cerebral cortices from postnatal day 1 to 3 Lewis rats (Harlan, Indianapolis, IN) were surgically removed and placed in cold DMEM; the meninges were carefully separated, and cortices were minced and dissociated with trypsin-EDTA at 37°C for 15 min. After tituration with DNase (3 μg/ml) and washing, the mixed glial cell suspension from two brains was plated in a poly-l-lysine-coated 75-cm2 vented cell culture flask with DMEM/F12 medium supplemented with 10% FBS and 1% (vol/vol) P/S and grown in a humidified 5% CO2 incubator at 37°C. On days 14 to 21 in vitro, microglia were detached using an orbital shaker (150 rpm for 7 h at 37°C with airtight caps on) and centrifuged (150 × g for 15 min), and microglia number and viability were assessed by trypan blue exclusion. Microglia were plated either alone or over neurons in DMEM supplemented with 10% FBS and 1% (vol/vol) P/S (see below).
(ii) Cortical neuron-rich cultures.
Neuron-rich cultures were prepared from cortices of embryonic day 19 (E19) or E20 Lewis rats using standard techniques (27). Briefly, meninges-free cortices were isolated, trypsinized, and mechanically dissociated by passage through fire-polished Pasteur pipettes. Washed cells were plated (200,000 cells/cm2) onto poly-l-lysine (0.05 mg/ml) and laminin (0.1 mg/ml)-coated tissue culture coverslips (Fisher Brand; Fisher Scientific) in NBM with l-glutamine, B-27, and P/S supplements (complete NBM). On day 2 in vitro, cells were infected with BDV or mock stocks diluted with fresh medium (multiplicity of infection [MOI] of 0.02), and medium was partially (50%) replaced every fourth day thereafter.
(iii) Cortical mixed neuron-glial cultures.
For preparation of mixed neuron and glia cultures, cortices from E15 to E17 Lewis rats were used. Dissociated cells (350,000 cells/cm2) were cultured in DMEM/F12 supplemented with 5% FBS, 5% horse serum, and 1% P/S (vol/vol) and otherwise treated as described above for neuron-rich cultures.
(iv) Coculture and treatment protocols.
Activation of microglia by BDV infection was evaluated either by exposing microglia to purified virus or BDV-infected neurons or in the mixed BDV-infected and uninfected primary neuron-glial cultures. Microglial cells (microglia seeding density, 50,000 cells/cm2 in DMEM supplemented with 10% FBS and 1% P/S) were seeded over primary cortical neurons grown at an initial seeding density of 200,000 cells/cm2 for 14 days in vitro, 12 days postinfection (p.i.). To further purify microglial cultures from loosely attached astrocytes and dead cells, medium was replaced 2 h later with fresh NBM (cocultures with primary neurons) or with DMEM-10% FBS (all other cocultures). Twelve hours later, medium was replaced again with NBM or DMEM-1% FBS-1% P/S (vol/vol) supplemented with either LPS (0, 1, 10, and 100 ng/ml; dilution of culture medium with LPS was <1% [vol/vol]), Tat (0, 1, 10, and 100 ng/ml; <1% [vol/vol]), the BDV stock (MOI up to 0.1; up to 5% [vol/vol]), or the mock stock. At the end of incubations, cell supernatants were collected and stored at −70°C until tested.
Immunocytochemistry.
At 5 to 16 days in vitro (or as indicated in the text) cocultures were washed two times with PBS and then fixed for 15 min with PBS plus 4% (wt/vol) paraformaldehyde at room temperature. After permeabilization with 0.1% Triton X-100 (Sigma), cultures were blocked for 2 h in PBS with 2% donkey serum (Chemicon) and further incubated with primary antibody mixtures overnight at 4°C in blocking solution. After extensive washing, the cultures were incubated with secondary antibodies in blocking solution for 1 h at room temperature. The following antibodies were used: anti-MAP2 (1:1,000 dilution) as a neuronal marker; anti-GFAP (1:800) as an astrocyte marker; anti-CD11b/c (1:50) and anti-Iba1 (1:800) as microglia markers; anti-ED1 (1:100), anti-MHC-I (1:100), and anti-MHC-II (1:100) as markers of microglia activation; anti-activated caspase-3 (1:200) as an apoptotic marker; and anti-BDV N (1:50) to identify infected cells (34). In some experiments microglia were visualized with FITC-lectin IB4 (5 μg/ml) that was added to the primary antibody mixture (42). Immunostaining without primary antibodies was used as a negative control. Following mounting with Gel/Mount medium (Electron Microscopy Sciences, Hatfield, PA), the samples were allowed to dry in the dark and were viewed using a 25×, 40×, or 63× objective lenses on an LSM 5 PASCAL confocal microscope from Carl Zeiss (Melville, NY). Digital images of the cells were captured and processed by using the software supplied with the microscope.
Microglial activation assays.
Microglial activation was assessed by (i) counting round (activated) microglia cells; (ii) immunostaining for the markers of activation, i.e., MHC-I and -II or ED1 (see above); and (iii) measuring the levels of NO and interleukins TNF-α, IL-1β, and IL-10 in the culture medium.
Microglia stained for specific cell markers (anti-CD11b/c or lectin-IB4) show two subpopulations of cells: flat ramified and round amoeboid cells. The round morphology as well as intensive CD11b/c staining are the typical hallmarks of reactive microglia in vivo and in vitro (43). These cells were clearly distinguishable from resting microglia. Counting round microglia cells was performed manually in 10 random fields (digital confocal images at ×25 magnification) per well of the experimental plate. The results from 2 to 4 wells per condition were used for statistical analyses.
The production of NO was determined indirectly through the assay of nitrite (NO2), a stable metabolite of NO, based on the Griess reaction (24). Briefly, a 50-μl aliquot of conditioned medium was mixed with 200 μl of distilled H20 and 20 μl of Griess reagent [0.1% N-(1-naphthyl)ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% phosphoric acid; Invitrogen/Molecular Probes, Carlsbad, CA] and incubated for 30 min at room temperature, and the absorbance was read at 540 nm on a microtiter plate reader (Spectra MAX 250; Molecular Devices, Sunnyvale, CA). Nitrite concentrations were calculated from a standard curve of sodium nitrite (Sigma) ranging from 0.1 to 100 μM.
TNF-α, IL-1β, and IL-10 levels were determined using sandwich enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN). Twenty-five microliters of conditioned medium was incubated with 50 μl of assay diluent RD1-41 in the assay plate for 2 h at room temperature. After any unbound substances were washed away, horseradish peroxidase-linked polyclonal antibody specific for rat TNF-α, IL-1β, or IL-10 was added to the wells. Following the addition of the peroxidase substrate solution, the enzyme-reactive color product was detected by a microplate reader (Spectra MAX 250) set to 450 nm, with the wavelength correction set to 540 nm.
Cytotoxicity assays.
A colorimetric assay (Cell Proliferation Kit II; Roche Diagnostics, Indianapolis, IN) was used to assess BDV-mediated toxicity in pure cortical neuron cultures. The assay is based on the measurement of a soluble formazan dye which is formed upon cleavage of the tetrazolium salt XTT {sodium 3′-[l-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro)benzene sulfonic acid hydrate} by the mitochondrial succinate-tetrazolium reductase system that is active only in viable cells. XTT reagent was added at 50 μl per 100 μl of conditioned medium per well of cocultures in 96-well plates or at 250 μl per 500 μl of conditioned medium per well of 24-well plates. Subsequently, plates were incubated at 37°C for 2 h. Dye absorbance was estimated in 100-μl samples from experimental 24-well plates. Measurements were performed at 490 nm with the reference wavelength of 690 nm using a Spectra MAX 250 plate reader.
Viability of neurons in mixed neuron-glial cultures was assessed by means of trypan blue exclusion. After incubations, cell cultures were stained with 1.5% trypan blue at 37°C for 5 min. Cultures were washed once with DMEM-1% FBS (without phenol red) and examined under light microscopy. Cells stained with trypan blue were regarded as nonviable. The viability of the cultures was calculated as the percentage of the ratio of the number of unstained cells (viable cells) against the total number of cells counted (viable cells plus nonviable cells).
Statistical analysis.
The data were analyzed by a Student's t test and are presented as means ± standard deviations. A P value of <0.05 was considered the criterion for statistical significance. All experiments were repeated two or three times with 2 to 4 wells per each condition used in each experiment.
RESULTS
Purified BDV does not activate rat primary microglia.
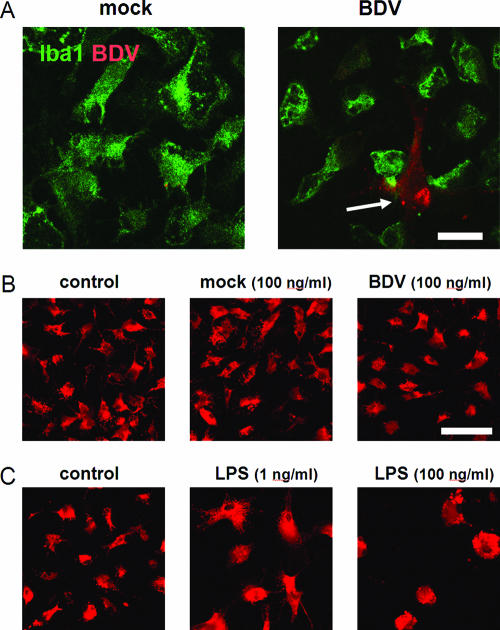
To investigate if BDV RNA, viral proteins, or virus particles activate microglia, rat primary microglia cultures were exposed to 40 to 200 μg/ml (which is equivalent to a MOI of 0.1) of purified BDV stocks that contain all of the above components. Five-day exposure of the cultured microglia to BDV did not result in their infection, as was evidenced by the absence of specific anti-BDV immunostaining in Iba1-positive microglia (Fig. 1A). In contrast, BDV antigen was found in Iba1-negative cells that were typically present in primary microglia cultures at low numbers (<2%) (Fig. 1, white arrow). Double staining of the microglia cultures revealed that the majority of Iba1-negative cells were GFAP-positive astrocytes (data not shown).
FIG. 1.
Cultured microglia are neither infected nor activated by purified BDV. (A) Microglia were exposed to the purified mock stock (left) or BDV stocks at a MOI of 0.1 (right) for 5 days. Images show immunostaining for a microglial marker, Iba1 (green), and BDV N (red). Note the lack of colocalization of the Iba1- and BDV-positive cells. The arrow points to an infected nonmicroglial cell occasionally present in the microglia cultures. (B) Exposure of microglia to the mock- or BDV-infected stocks did not resulted in change of the cell morphology or the ED1 expression. (C) LPS treatment induced a dose-dependent change in the cell shape. In panels B and C immunostaining for activated the macrophage/microglia activation marker ED1 (red) is shown. The data shown represent the 5-day cultures. Scale bar, 40 μm.
Immunostaining for the ED1 glycoprotein, a marker of activated macrophage/microglia, revealed no change in the ED1 expression and cell morphology by microglia after 5 days of exposure to BDV stock (Fig. 1B). In contrast, treatments with LPS (used as a positive control) were associated with a typical “reactive” microglia appearance, i.e., an increase in the cell size and a transition from the star-shaped to round morphology (Fig. 1C). As expected, exposure of microglia to 1 to 100 ng/ml of LPS or HIV-encoded Tat (the known activators of microglia [9, 39]) induced a dose-dependent release of NO and TNF-α in culture medium. In contrast, exposure of microglia to purified BDV did not result in changes in NO and TNF-α release compared to microglia exposed to mock stock (Fig. 2).
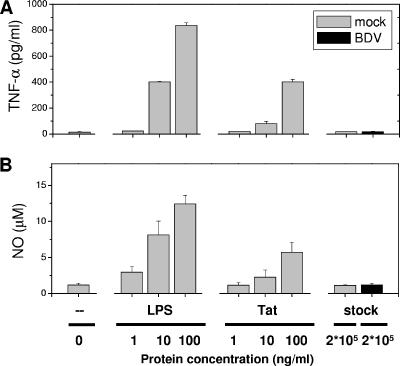
FIG. 2.
LPS and HIV-Tat but not purified BDV activate microglia to produce TNF-α and NO. Microglia cultures (1-day in vitro) were exposed to cell-free purified BDV or mock stock for 2 days in the presence or absence of LPS (1, 10, 100 ng/ml) or HIV-Tat protein (1, 10, 100 ng/ml). The concentrations of the proinflammatory molecules TNF-α (A) and NO (B) released by the microglia were measured following incubation with LPS (1, 10, 100 ng/ml), Tat (1, 10, 100 ng/ml), the cell-free purified BDV (200 μg/ml which is equivalent to a MOI of 0.1) or mock stocks (200 μg/ml). Untreated cultures (zero) served as the negative control. The results of a representative experiment are shown, and data are the means ± standard error of the means for two wells of one 24-well plate. Duplicate experiments yielded similar results. Note the elevated secretion of NO and TNF-α following treatments with LPS or the Tat protein but not following incubation with the purified virus or BDV-infected neuronal cells.
Persistent BDV infection of primary neurons alone does not activate microglia.
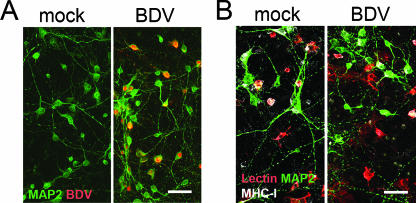
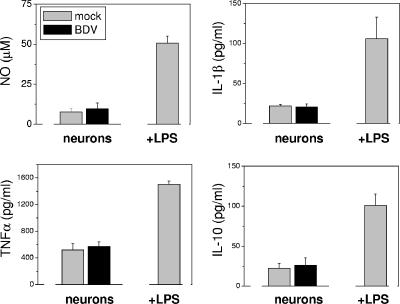
During the first 2 weeks after neonatal BDV infection in vivo, viral RNA and nucleoprotein are present predominantly in neurons (44). Thus, we studied whether BDV-infected neurons were able to activate microglia in cell culture. Freshly harvested microglia were seeded over BDV- and mock-infected cortical neurons 12 days p.i., and cocultures were observed for 1 to 7 days. About 60 to 90% of cortical neurons were infected at the time of cocultures (Fig. 3A). Coculturing of microglia with BDV-infected primary neurons did not result in changes in microglia morphology or the expression of MHC-I (Fig. 3 B) and MHC-II (data not shown) compared to mock cultures. Additionally, there were no significant alterations in the secretion of NO, proinflammatory cytokines (TNF-α and IL-1β), or the anti-inflammatory cytokine IL-10 (Fig. 4 for 3-day cocultures; also data not shown). In contrast, LPS treatment (100 ng/ml) of the cocultures gave rise to a significant elevation of the levels of NO and the cytokines (Fig. 4), with no difference between mock- and BDV-infected neurons (data not shown). This LPS-induced increase in the secretion of the cytokines was most likely due to the cultured microglia because the LPS treatment of the primary neurons alone did not lead to an up-regulation of NO or the cytokines (data not shown).
FIG. 3.
Microglia are not activated in cocultures with BDV-infected primary cortical neurons. (A) Primary cortical neurons were mock- or BDV-infected and immunostained for a neuronal marker, MAP2 (green), and BDV (red) on day 12 p.i. About 60% to 90% of neurons were infected on day 12 in vitro. (B) BDV- or mock-infected primary cortical neurons were cocultured with microglia on day 12 p.i. and immunostained for a microglia marker after 3 days of coculturing (lectin-IB4; red), neurons (MAP2; green) and for MHC-I (white). Cocultures of cortical neurons with microglia were not associated with activation of microglia by BDV-infected neurons. Note the absence of differences in the microglia morphology between the infected and control cultures. Scale bar, 40 μm.
FIG. 4.
Infected neurons do not induce release of the soluble factors by microglia. Concentrations of proinflammatory (TNF-α, IL-1β, and NO) and anti-inflammatory (IL-10) molecules released by microglia in supernatants of 2-day cocultures of microglia with mock- or BDV-infected cortical neurons (day 14 p.i.) were determined as described in Materials and Methods. LPS (100 ng/ml; 2 days)-treated cocultures of microglia with mock-infected neurons served as the positive control. Note the increased secretion of the soluble factors following LPS treatment and the low and comparable secretion of these factors in the infected and control cultures. Data are means ± standard deviations of three independent experiments.
Activation of microglia in mixed brain neuron-glial cultures.
The microglia functions have been shown to be dependent on astrocytes, which release a broad spectrum of cytokines and growth factors (15). In particular, astrocytes have been shown to mediate activation of microglia under pathological conditions (37, 41). Since astrocytes are also susceptible to BDV infection in vitro and in vivo (7, 34, 44), we studied microglia activation in mixed cortical neuron-glial cultures consisting of neurons, astroglia, and microglia (8, 25) from E15 to E17 rats. On day 10 p.i., BDV infection was found mostly in astrocytes (GFAP-positive cells, 47 ± 8% of all BDV-infected cells) and neurons (MAP2-positive cells, 39 ± 7%) but not in microglia (data not shown). Immunofluorescent microscopic analysis of the mixed cultures revealed high numbers of amoeboid, round microglia cells in the BDV-infected mixed cultures as early as 10 days p.i. (Fig. 5A and B, filled arrowheads). These round microglia were characterized by more intensive CD11b/c and lectin IB4 staining than the resting, star-shaped microglia population (Fig. 5A and B; open arrowheads). Both subpopulations of microglia were present in the BDV- or mock-treated cultures; however, there were significantly more round microglia cells in the BDV-infected cultures compared to the control ones (P < 0.001) (Fig. 5C). In addition, unlike star-shaped microglia, round microglia coexpressed MHC-I and -II molecules, indicative of microglial activation (Fig. 6). Notably, the changes in the cell morphology of activated microglia were not associated with neuronal cytotoxicity as assessed by the trypan blue exclusion assay and activated caspase-3 (a marker for apoptotic cells) immunostaining (data not shown). Furthermore, there was no difference in the accumulation of NO, TNF-α, IL-1β, or IL-10 between the mock- or BDV-infected mixed cultures at 1, 2, and 4 weeks p.i. (data not shown).
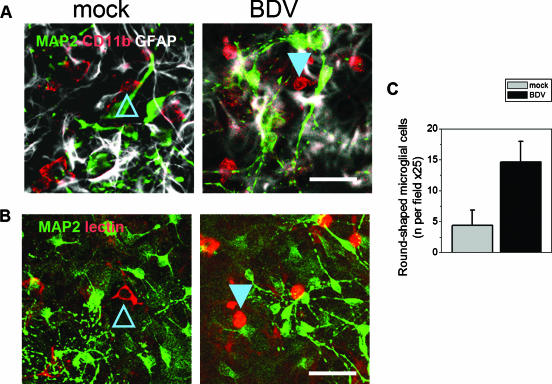
FIG. 5.
BDV infection of mixed cultures leads to formation of round reactive microglia. Mock- or BDV-infected mixed cortical neurons prepared from E15 to E17 rats were stained on day 9 p.i. for microglia markers CD11b/c (A, red) and lectin IB4 (B, red), as well as neuronal marker MAP2 (green) and the astrocyte marker GFAP (white). Compared to the control cultures (left frames), the mixed BDV-infected cultures (right frames) had greater numbers of amoeboid, round microglia cells (filled arrowheads). These round microglia were characterized by more intensive CD11b/c and lectin IB4 staining than the resting, star-shaped microglia population (open arrowheads). Scale bar, 40 μm. (C) Quantitation of the numbers of round (activated) microglia cells in mock- and BDV-infected mixed cultures. There were significantly more round microglia cells in the BDV-infected cultures compared to the control cultures. Data are means ± standard deviations of three independent experiments (P < 0.001 versus the control cultures; Student t test).
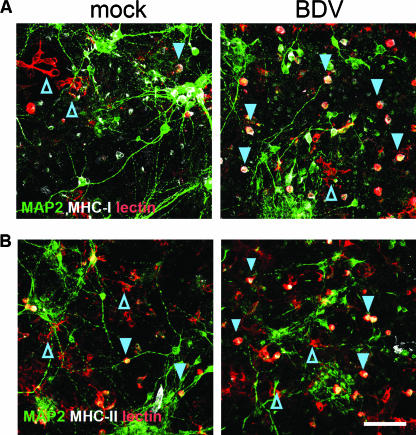
FIG. 6.
Expression of MHC-I and -II by activated round microglia. Mock- or BDV-infected mixed cortical neurons prepared from E15 to E17 rats were stained on day 9 p.i. for microglia marker lectin-IB4 (red), neuronal marker MAP2 (green), and activation markers MHC-I and -II (white). Note that the majority of round microglia cells in the BDV-infected mixed cultures are positive for MHC-I (A) or MHC-II (B) (filled arrowheads) compared to star-shaped microglia in the mock-infected cultures (open arrowheads). Scale bar, 80 μm.
BDV infection of mixed brain cell cultures potentiates activation with LPS.
The levels of soluble factors secreted by microglia following treatment with LPS have been found to be proportional to the numbers of activated microglia cells in vitro (8, 25) and in vivo (17, 25). Therefore, we studied if the observed increase in the numbers of round microglia in BDV-infected mixed cultures would be associated with an enhanced release of soluble factors following LPS treatment. As expected, exposure of the cultures (14 to 16 days p.i.) to LPS (100 ng/ml) resulted in the significantly higher levels of interleukins TNF-α, IL-1β, and IL-10 in the supernatants from infected cells compared to the uninfected cells (P < 0.01 for IL-1β and IL-10 and P < 0.04 for TNF-α) (Fig. 7). No differences in the levels of NO were found between the BDV-infected and control cultures following LPS treatment (Fig. 7).
FIG. 7.
Activation of microglia in BDV-infected mixed cultures is associated with an increased LPS-stimulated secretion of the cytokines and NO. Mock- or BDV-infected mixed cortical neurons prepared from E15 to E17 rats were exposed at day 14 p.i. to LPS (100 ng/ml) or left untreated. Two days after exposure, supernatants of the mixed cultures were analyzed for release of proinflammatory (TNF-α, IL-1β, and NO) and anti-inflammatory (IL-10) molecules as described in Materials and Methods. Note a significantly greater release of the soluble factors in the BDV-infected cultures compared to the control cultures. The data are means ± standard deviations of three or four independent experiments. #, P < 0.05 versus the LPS-treated control cultures (Student's t test).
DISCUSSION
The main findings of the present study are that persistent BDV infection of mixed neuron-glial cultures activates microglia, whereas neither direct exposure to purified virus nor persistent BDV infection of neurons alone is able to activate primary microglia in vitro. This is the first report of a casual link between microglia activation and persistent BDV infection of neurons and astrocytes in the absence of overt neurotoxicity.
Microglia activation has been shown to be triggered by several means, such as direct virus infection of microglia (e.g., HIV or visna virus), released virus particles or viral proteins (25), double-stranded RNA (29), neuronal cell death (43), or by infiltrating T cells (4). However, little was known about the mechanism of microglia activation in brains of rats following neonatal BDV infection (44). In contrast to HIV, feline immunodeficiency virus, and visna virus that infect microglia, our study demonstrated the lack of infection of microglia by BDV in the culture. To the best of our knowledge, this is the first evidence that BDV does not infect microglia in vitro, consistent with previous reports of the absence of microglia infection by BDV in vivo (38, 44). We also showed that direct exposure of microglia to BDV did not lead to activation of microglia. This finding may not be completely unexpected from a physiological standpoint, given that persistent BDV infection is noncytolytic in vivo and that virus is only minimally released into extracellular compartments in culture (7, 30).
Neuronal injury is a potent physiological trigger of microglia activation as evidenced by in vivo experiments with selective neurotoxins and axotomy (43). In neonatal BDV infection, the first signs of microglia activation (MHC-I expression and proliferation) and apoptosis of neurons are observed simultaneously, between 1 and 2 weeks p.i. (44). Therefore, it is conceivable that BDV-associated neuropathology might act as a primary trigger of microglia activation. However, our data challenge this hypothesis because (i) we did not observe any signs of neurotoxicity in BDV-infected mixed neuron-glial cultures for up to 3 weeks p.i. and (ii) microglia activation was evident as early as 1 week p.i. Thus, our findings indicate that noncytolytic BDV infection of neurons and glia can trigger microglia activation.
The present data demonstrate that BDV infection induces microglia reaction in mixed cultures of microglia, glia, and neurons but not in cocultures of purified microglia and neurons. This finding is in agreement with several reports on inducible microglia proliferation in mixed versus pure microglia cultures (26, 28). On the one hand, the different outcomes of microglia activation in mixed and pure culture systems could reflect the requirement for the presence of cell-to-cell contacts between microglia and other glial cells (e.g., astrocytes) for proper maturation and reaction to infection in vitro (26, 28). On the other hand, the major glial cell population, astrocytes, has been shown to produce soluble microglia mitogens, such as TNF-α (14, 26), granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5 (35) that might activate microglia in vitro. At present, it remains unclear whether the cell-to-cell contacts or soluble factors secreted by neurons or astrocytes or both play a main role in activating microglia during BDV infection. Future studies will identify the specific mechanisms of how astrocytes contribute to the BDV-induced microglia activation.
The lack of BDV-associated increase in secretion of IL-1β, TNF-α, IL-10, or NO in mixed cultures of microglia, neurons, and astrocytes is inconsistent with previous in vivo findings of upregulation of proinflammatory cytokines in brains of newborn BDV-infected rats (22, 33, 38). One of the reasons for the lack of upregulation of the proinflammatory factors could be that cell interactions normally present in the brain but not included in the in vitro study may be required. For example, effects of T cells may be of a particular interest. Transient infiltrates of low numbers of T cells in brain tissue have been reported in neonatal BDV infection at 2 to 3 weeks p.i. (38), preceding the peak of microglial activation in the rat brain (4 weeks [38, 44]). T cells can be activated by BDV proteins or BDV-infected astrocytes (34) and, in turn, can activate microglia to produce interleukins (4, 23). Alternatively, it is conceivable that microglia in the cultures undergo only the initial state of activation, whereas stronger stimuli would be required to attain the fully activated state with a resulting cytokine secretion. For example, proliferation of MHC-I and -II and immunoglobulin expression by microglia are early manifestations of the CNS response to infection with mouse hepatitis virus (46) and vesicular stomatitis virus (1). Thus, one can hypothesize that stronger activation of microglia could be conferred by infiltrating T cells or by another stimulus. It is noteworthy that an additional stimulation of microglia in the BDV-infected cultures with a strong microglia activator, LPS, resulted in a significantly greater release of TNF-α, IL-1β, and IL-10 compared to the mock-infected LPS-stimulated cell cultures.
Our results provide valuable insights into the mechanisms of BDV-mediated neuropathology. While a role of microglia in mediating BDV-associated neurodegeneration has been suggested based on the observation of microgliosis and the detection of upregulated expression of mRNAs coding for proinflammatory cytokines in the areas of a significant neuronal loss (22, 33, 38, 44), it remained unclear if activation of microglia was a secondary response to an early pathology of infected neurons or if BDV infection itself triggers microgliosis that affects neuronal survival. We believe that our results provide a missing casual link between BDV infection and microglia activation. Specifically, we hypothesize that in vivo BDV infection of neurons or glia activates microglia that trigger neuronal injury or facilitate ongoing cell damage due to direct effects of BDV. Although we did not find evidence for activation of microglia by cell death in the infected mixed cultures, it is plausible that neuronal death in the BDV-infected brain may further activate already BDV-(pre)activated microglia, leading to secretion of the proinflammatory cytokines and continuation of neuronal death. This scenario might be particularly applicable to a gradual loss of neurons in such brain regions as cerebellum (i.e., Purkinje cells) and striatum (GABA neurons) associated with chronic neuroinflammation.
In conclusion, activation of microglia in BDV-infected mixed neuron-glial cultures provides evidence of infection-driven microglia activation and represents a model for studies of neurotropic infection-mediated microglia activation in vitro.
Acknowledgments
The opinions expressed in this study are those of the authors and do not necessarily reflect the official positions of the U.S. Food and Drug Administration, the U.S. Department of Agriculture, or the U.S. Government.
The study was supported by grant R01MH048948 (M.V.P.) from NIH.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Bi, Z., M. Barna, T. Komatsu, and C. S. Reiss. 1995. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 69:6466-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block, M. L., and J. S. Hong. 2005. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 76:77-98. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L., and H. Ludwig. 2003. Borna disease virus infection, a human mental-health risk. Clin. Microbiol. Rev. 16:534-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabarrocas, J., J. Bauer, E. Piaggio, R. Liblau, and H. Lassmann. 2003. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur. J. Immunol. 33:1174-1182. [DOI] [PubMed] [Google Scholar]
- 5.Carbone, K. M., B. D. Trapp, J. W. Griffin, C. S. Duchala, and O. Narayan. 1989. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J. Neuropathol. Exp. Neurol. 48:631-644. [DOI] [PubMed] [Google Scholar]
- 6.Carbone, K. M., S. W. Park, S. A. Rubin, R. W. Waltrip II, and G. B. Vogelsang. 1991. Borna disease: association with a maturation defect in the cellular immune response. J. Virol. 65:6154-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone, K. M., S. A. Rubin, A. M. Sierra-Honigmann, and H. M. Lederman. 1993. Characterization of a glial cell line persistently infected with borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J. Virol. 67:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J., Y. Zhou, S. Mueller-Steiner, L. F. Chen, H. Kwon, S. Yi, L. Mucke, and L. Gan. 2005. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 280:40364-40374. [DOI] [PubMed] [Google Scholar]
- 9.Conant, K., M. Ma, A. Nath, and E. O. Major. 1996. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-κB binding and protein kinase C activity in primary human astrocytes. J. Virol. 70:1384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubitt, B., C. Oldstone, and J. C. de la Torre. 1994. Sequence and genome organization of Borna disease virus. J. Virol. 68:1382-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De, S. R., M. A. Ajmone-Cat, A. Nicolini, and L. Minghetti. 2002. Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J. Neuropathol. Exp. Neurol. 61:237-244. [DOI] [PubMed] [Google Scholar]
- 12.de la Torre, J. C. 2002. Bornavirus and the brain. J. Infect. Dis. 186(Suppl. 2):S241-S247. [DOI] [PubMed] [Google Scholar]
- 13.De Simone, R., M. A. Ajmone-Cat, P. Tirassa, and L. Minghetti. 2003. Apoptotic PC12 cells exposing phosphatidylserine promote the production of anti-inflammatory and neuroprotective molecules by microglial cells. J. Neuropathol. Exp. Neurol. 62:208-216. [DOI] [PubMed] [Google Scholar]
- 14.Dopp, J. M., A. Mackenzie-Graham, G. C. Otero, and J. E. Merrill. 1997. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J. Neuroimmunol. 75:104-112. [DOI] [PubMed] [Google Scholar]
- 15.Eddleston, M., and L. Mucke. 1993. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neuroscience 54:15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenman, L. M., R. Brothers, M. H. Tran, R. B. Kean, G. M. Dickson, B. Dietzschold, and D. C. Hooper. 1999. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J. Neurovirol. 5:181-189. [DOI] [PubMed] [Google Scholar]
- 17.Faustmann, P. M., C. G. Haase, S. Romberg, D. Hinkerohe, D. Szlachta, D. Smikalla, D. Krause, and R. Dermietzel. 2003. Microglia activation influences dye coupling and Cx43 expression of the astrocytic network. Glia 42:101-108. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Dunia, D., R. Volmer, D. Mayer, and M. Schwemmle. 2005. Borna disease virus interference with neuronal plasticity. Virus Res. 111:224-234. [DOI] [PubMed] [Google Scholar]
- 19.Haas, B., H. Becht, and R. Rott. 1986. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J. Gen. Virol. 67:235-241. [DOI] [PubMed] [Google Scholar]
- 20.Hans, A., J. J. Bajramovic, S. Syan, E. Perret, I. Dunia, M. Brahic, and D. Gonzalez-Dunia. 2004. Persistent, noncytolytic infection of neurons by Borna disease virus interferes with ERK 1/2 signaling and abrogates BDNF-induced synaptogenesis. FASEB J. 18:863-865. [DOI] [PubMed] [Google Scholar]
- 21.Herzog, S., and R. Rott. 1980. Replication of Borna disease virus in cell cultures. Med. Microbiol. Immunol. 168:153-158. [DOI] [PubMed] [Google Scholar]
- 22.Hornig, M., H. Weissenbock, N. Horscroft, and W. I. Lipkin. 1999. An infection-based model of neurodevelopmental damage. Proc. Natl. Acad. Sci. USA 96:12102-12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard, L. M., A. J. Miga, C. L. Vanderlugt, M. C. Dal Canto, J. D. Laman, R. J. Noelle, and S. D. Miller. 1999. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Investig. 103:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huygen, I. C. 1970. Reaction of nitrogen dioxide with Griess type reagents. Anal. Chem. 42:407-409. [DOI] [PubMed] [Google Scholar]
- 25.Kim, W. G., R. P. Mohney, B. Wilson, G. H. Jeohn, B. Liu, and J. S. Hong. 2000. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 20:6309-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mander, P. K., A. Jekabsone, and G. C. Brown. 2006. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 176:1046-1052. [DOI] [PubMed] [Google Scholar]
- 27.Mattson, M. P., B. Cheng, D. Davis, K. Bryant, I. Lieberburg, and R. E. Rydel. 1992. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12:376-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker, R. B., Y. Azuma, D. C. Bragg, R. V. English, and M. Tompkins. 1999. Microglial proliferation in cortical neural cultures exposed to feline immunodeficiency virus. J. Neuroimmunol. 101:15-26. [DOI] [PubMed] [Google Scholar]
- 29.Nakamichi, K., M. Saiki, M. Sawada, Y. Yamamuro, K. Morimoto, and I. Kurane. 2005. Double-stranded RNA stimulates chemokine expression in microglia through vacuolar pH-dependent activation of intracellular signaling pathways. J. Neurochem. 95:273-283. [DOI] [PubMed] [Google Scholar]
- 30.Pauli, G., and H. Ludwig. 1985. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 2:29-33. [DOI] [PubMed] [Google Scholar]
- 31.Pleschka, S., P. Staeheli, J. Kolodziejek, J. A. Richt, N. Nowotny, and M. Schwemmle. 2001. Conservation of coding potential and terminal sequences in four different isolates of Borna disease virus. J. Gen. Virol. 82:2681-2690. [DOI] [PubMed] [Google Scholar]
- 32.Polazzi, E., T. Gianni, and A. Contestabile. 2001. Microglial cells protect cerebellar granule neurons from apoptosis: evidence for reciprocal signaling. Glia 36:271-280. [DOI] [PubMed] [Google Scholar]
- 33.Rauer, M., A. Pagenstecher, J. Schulte-Monting, and C. Sauder. 2002. Upregulation of chemokine receptor gene expression in brains of Borna disease virus (BDV)-infected rats in the absence and presence of inflammation. J. Neurovirol. 8:168-179. [DOI] [PubMed] [Google Scholar]
- 34.Richt, J. A., and L. Stitz. 1992. Borna disease virus-infected astrocytes function in vitro as antigen-presenting and target cells for virus-specific CD4-bearing lymphocytes. Arch. Virol. 124:95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringheim, G. E. 1995. Mitogenic effects of interleukin-5 on microglia. Neurosci. Lett. 201:131-134. [DOI] [PubMed] [Google Scholar]
- 36.Rock, R. B., G. Gekker, S. Hu, W. S. Sheng, M. Cheeran, J. R. Lokensgard, and P. K. Peterson. 2004. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17:942-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohl, C., and J. Sievers. 2005. Microglia is activated by astrocytes in trimethyltin intoxication. Toxicol. Appl. Pharmacol. 204:36-45. [DOI] [PubMed] [Google Scholar]
- 38.Sauder, C., and J. C. de la Torre. 1999. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J. Neuroimmunol. 96:29-45. [DOI] [PubMed] [Google Scholar]
- 39.Sawada, M., N. Kondo, A. Suzumura, and T. Marunouchi. 1989. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 491:394-397. [DOI] [PubMed] [Google Scholar]
- 40.Si, Q., Y. Nakamura, and K. Kataoka. 2000. A serum factor enhances production of nitric oxide and tumor necrosis factor-alpha from cultured microglia. Exp. Neurol. 162:89-97. [DOI] [PubMed] [Google Scholar]
- 41.Sola, C., C. Casal, J. M. Tusell, and J. Serratosa. 2002. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur. J. Neurosci. 16:1275-1283. [DOI] [PubMed] [Google Scholar]
- 42.Streit, W. J., and G. W. Kreutzberg. 1987. Lectin binding by resting and reactive microglia. J. Neurocytol. 16:249-260. [DOI] [PubMed] [Google Scholar]
- 43.Streit, W. J. 2002. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133-139. [DOI] [PubMed] [Google Scholar]
- 44.Weissenböck, H., M. Hornig, W. F. Hickey, and W. I. Lipkin. 2000. Microglial activation and neuronal apoptosis in Bornavirus infected neonatal Lewis rats. Brain Pathol. 10:260-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zocher, M., S. Czub, J. Schulte-Monting, J. C. de La Torreqq, and C. Sauder. 2000. Alterations in neurotrophin and neurotrophin receptor gene expression patterns in the rat central nervous system following perinatal Borna disease virus infection. J. Neurovirol. 6:462-477. [DOI] [PubMed] [Google Scholar]
- 46.Zuo, J., S. A. Stohlman, J. B. Hoskin, D. R. Hinton, R. Atkinson, and C. C. Bergmann. 2006. Mouse hepatitis virus pathogenesis in the central nervous system is independent of IL-15 and natural killer cells. Virology 350:206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]