Abstract
The human polyomavirus JC virus (JCV) infects 70% of the population worldwide. In immunosuppressed patients, JCV infection can lead to progressive multifocal leukoencephalopathy (PML), a fatal demyelinating disease of the central nervous system (CNS). The majority of PML cases occur in the setting of human immunodeficiency virus (HIV) infection, and it has been suggested that the link between HIV and the development of PML is in part related to the production of numerous cytokines in the CNS during HIV infection. To examine the link between the expression of inflammatory cytokines and JCV infection, we tested an anti-inflammatory compound, cyclosporine A (CsA), for its ability to block JCV infection of glial cells. We found that CsA inhibited JCV infection by preventing the activation of the transcription factor nuclear factor of activated T cells 4 (NFAT4). Luciferase reporter assays and chromatin immunoprecipitation assays revealed that NFAT4 directly bound the JCV promoter during infection and was important for the activation of both early and late transcription. In addition, the expression of the JCV early viral gene products increased NFAT activity to further aid viral transcription. The necessity of NFAT for JCV infection suggests that calcium signaling and the activation of NFAT in glial cells are required for JCV infection of the CNS.
The human polyomavirus JC virus (JCV) is the causative agent of a fatal central nervous system (CNS)-demyelinating disease known as progressive multifocal leukoencephalopathy (PML) (28). JCV is a common virus, with 70% of the adult population testing serologically positive for JCV antibodies (16, 27). Initial infection is not related to clinical disease but is followed by the establishment of a lifelong, persistent infection. PML occurs predominately in immunosuppressed patients, with the majority of cases occurring in the setting of human immunodeficiency virus (HIV) infection. Recently, PML has been reported in patients being treated with natalizumab, a drug designed to inhibit trafficking of leukocytes into inflamed tissue (15, 18, 37). PML is thought to develop following reactivation of the virus and dissemination from peripheral sites to the CNS, where the primary targets are astrocytes and oligodendrocytes. The mechanism by which JCV becomes reactivated and traffics to the CNS is unclear. The relationship between HIV infection and the development of PML is an obscure one, but the higher incidence of PML in HIV patients indicates a scenario that occurs specifically in HIV patients and is more conducive to JCV infection of the CNS. One possible explanation for this is the increase in the levels of inflammatory cytokines in the CNS during HIV infection.
Cyclosporine A (CsA) is an anti-inflammatory compound that is typically used as an immunosuppressant. CsA is an inhibitor of the Ca2+-calmodulin-dependent serine phosphatase calcineurin, a crucial component of the calcium-signaling pathway that can stimulate the production of inflammatory cytokines in response to extracellular stimuli (9). To test whether the activation of inflammatory cytokines triggered by calcium signaling was required for JCV infection, we treated glial cells with increasing amounts of CsA and then infected the cells with JCV. Treatment of glial cells with CsA inhibited JCV infection. The primary target of calcineurin is the transcription factor family nuclear factor of activated T cells (NFAT) (10). Treatment of glial cells with an inhibitor of the NFAT family also inhibited JCV infection, implicating NFAT involvement in the life cycle of JCV. Reporter assays and chromatin immunoprecipitation (ChIP) assays show that NFAT4 (NFATc3, NFATx) directly binds to a site in the JCV promoter region, activates both early and late viral transcription, and is essential for productive infection of glial cells by JCV. Finally, the expression of early viral gene products helps to activate NFAT, aiding viral transcription.
MATERIALS AND METHODS
Cells and virus.
SVG-A and U-87MG cells were cultured in media supplemented with 10% fetal calf serum (Mediatech, Inc.) and maintained in a humidified 37°C CO2 incubator. The Mad-4 strain of JCV was used in these experiments, and its production has been previously described (20).
Cell proliferation.
SVG-A cells were plated in a 96-well dish with 1,000 cells per well. They were treated with the appropriate concentration of CsA and maintained in a humidified 37°C CO2 incubator for 48 h. Cell proliferation was then assayed using Promega's CellTiter 96 nonradioactive cell proliferation assay {MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]} according to the manufacturer's protocol. Absorbance was measured using a SpectraMax Plus plate reader (Molecular Probes).
Infection and indirect immunofluorescence.
Cells were grown to ∼70% confluence in a six-well dish and infected for 1 h with 512 hemagglutinating units of JCV in the presence of Eagle's minimal essential medium (EMEM) plus 2% serum in a total volume of 250 μl. At 3 days postinfection (p.i.), cells were fixed in ice-cold acetone for 10 min. Infected cells were detected using a monoclonal antibody (PAB597) against the major capsid protein VP1. PAB597 targets the simian virus 40 (SV40) major capsid protein VP1 and has been previously shown to cross-react with JCV VP1 (4). JCV T antigen (T-Ag) was detected by the monoclonal antibody PAB962, which is specific for JCV T-Ag and does not cross-react with SV40 T-Ag (34). Primary antibody was detected with an Alexa-Fluor 488-labeled goat anti-mouse secondary antibody (Molecular Probes). Cells were counterstained with 0.02% Evans blue. Positive cells were visualized on a Nikon epifluorescence microscope (Eclipse E800; Nikon Inc.).
Western blots.
SVG-A and U-87MG lysates were prepared in radioimmunoprecipitation assay buffer (Santa Cruz Biotech Inc.). Ramos cell lysates were purchased from Santa Cruz Biotech Inc. Whole-cell extracts were separated on Tris-HCl-ready gels (Bio-Rad). Proteins were transferred to nitrocellulose membranes and blocked with 1× casein-blocking buffer. Blots were probed with the respective antibodies, all diluted in 1× blocking buffer, and then incubated with goat anti-mouse Alexa-Fluor 680 antibodies (Molecular Probes) diluted 1:1,000 in blocking buffer. Blots were viewed using an infrared scanner from LICOR and analyzed using Odyssey software.
Constructs and mutagenesis.
pJCV-MadE and L-luc are promoter reporter constructs which express the firefly luciferase gene under the control of the JCV promoter in either the early or the late orientation (26). The pGFP-VIVIT construct expresses the NFAT peptide inhibitor fused with green fluorescent protein (GFP) under the control of the cytomegalovirus (CMV) promoter (3). pSG5-T expresses the SV40 T-Ag protein from the respiratory syncytial virus promoter (21). pCMV-JCVE expresses the JCV early region under the control of the CMV promoter (35). pRL-TK, which expresses Renilla luciferase from the thymidine kinase promoter of herpes simplex virus, and phMGFP, which expresses only GFP from the CMV promoter, were both purchased from Promega. The pNFAT-luc reporter plasmid, which expresses firefly luciferase under the control of a promoter that contains four repeats of the interleukin-2 (IL-2) NFAT binding site, and pTA-luc, which is the pNFAT-luc construct minus any NFAT sites, were purchased from Panomics. The pJCV-MadE and L-luc reporter constructs were mutated using a Stratagene QuikChange mutagenesis kit according to the manufacturer's instructions, with the primers 5′-GGGAAAAACAAAAGAATTTCCCTGGCCTCC-3′ and 5′-GGAGGCCAGGGAAATTCTTTTGTTTTTCCC-3′ (the mutated residues are underlined).
Transfection and luciferase assays.
Transient transfections were carried out using Fugene (Roche) as described by the manufacturer's protocol. Vectors were cotransfected with the Renilla luciferase control vector (pRL-TK) (Promega). Renilla and firefly luciferase activity were measured 48 h posttransfection using the Promega dual luciferase reporter assay system. Relative luciferase units were measured using a Berthoid Lumat LB9501 luminometer. Firefly luciferase measurements for each experiment were normalized using the Renilla luciferase measurements. The results for each experiment were confirmed by three independent transfections.
Chromatin immunoprecipitation assays.
SVG-A cells at ∼70% confluence in a 75-cm3 flask were infected with JCV (512 hemagglutinating units/105 cells) in the presence of EMEM plus 2% serum in a total volume of 1 ml for 1 h. The cells were then maintained in EMEM plus 10% serum for 9 days. The ChIP assay was performed according to a protocol obtained from Santa Cruz Biotechnology. Briefly, cells were harvested by scraping and washed twice in 1× phosphate-buffered saline. Cells were then treated for 8 min with 1% formaldehyde. Cells were washed three times in ChIP lysis buffer (Santa Cruz Biotech Inc.) and resuspended in 1.0 ml of ChIP lysis buffer with high salt (Santa Cruz Biotech Inc.). Samples were sonicated using a Branson Sonifier 150 at power setting 2 six times for 1 min. Chromatin was precleared with protein A/G PLUS-agarose (Santa Cruz Biotech Inc.) at 4°C for 30 min. Primary antibody was incubated with chromatin overnight at 4°C. Immune complexes were harvested by protein A/G PLUS-agarose for 2 h at 4°C. Beads washed twice with lysis buffer and four times with wash buffer (Santa Cruz Biotech Inc.). Samples were resuspended in elution buffer (Santa Cruz Biotech Inc.) and incubated at 67°C overnight. DNA samples were extracted using a QIAGEN PCR purification kit according to the manufacturer's protocol. SYBR green quantitative PCR (Applied Biosystems) was performed using 3 μl of the immunoprecipitated DNA. The primers used to detect the JC promoter region were 5′-TCCCTATTCAGCACTTTGTCCA-3′ (Mad-1 nucleotides 4991 to 5012) and 5′-TGGAGGCTTTTTAGGAGGCC-3′ (Mad-1 nucleotides 5091 to 5072).
RESULTS
NFAT activity is required for JCV infection of glial cells.
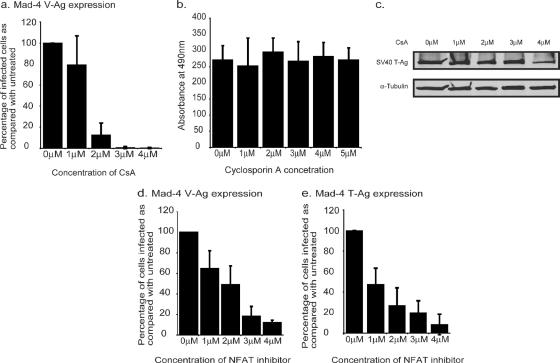
To investigate the relationship between inflammatory cytokine expression and JCV infection of a glial cell line, SVG-A cells were treated with an inhibitor of cytokine gene expression, CsA. Cells were pretreated for 6 h in various concentrations of CsA and then infected with Mad-4, a wild-type strain of JCV. Following infection, the cells were maintained in the appropriate concentration of CsA. After 72 h, the cells were fixed and stained for the late viral protein VP1. Treatment of SVG-A cells with increasing concentrations of CsA during infection with JCV resulted in a dose-dependent decrease in infection as the concentration of CsA increased (Fig. 1a). This result was not due to drug toxicity, as these same concentrations of CsA did not inhibit cell proliferation, as measured by an MTT assay (Fig. 1b). As SVG-A cells are from an SV40 T-antigen transformed cell line, we also controlled for the possibility that CsA may be exerting its inhibitory effect by reducing SV40 T-antigen expression. CsA treatment did not result in a significant reduction of SV40 T-Ag expression, as determined by a Western blot assay (Fig. 1c).
FIG. 1.
NFAT activity is required for JCV infection. (a) SVG-A cells were treated with various concentrations of CsA (Sigma) 6 h prior to infection. The cells were infected with JCV for 1 h in the presence of CsA. Following infection, the cells were maintained in CsA for 72 h before fixing and staining them for VP1. The numbers of infected cells were counted, and the percentages of cells infected compared to those for untreated infected cells were calculated. Each experiment was repeated three times. (b) Cell proliferation assays (Promega) were carried out on SVG-A cells treated for 2 days in various concentrations of CsA. Each concentration was tested three times. (c) SVG-A cells were treated for 2 days in various concentrations of CsA. The expression of SV40 large T-Ag was then examined by Western blot analysis using either anti-SV40 T-Ag antibodies (Ab2; Oncogene) or an anti-tubulin (α-Tubulin) antibody (Santa Cruz Biotech Inc.). (d and e) Cells were treated with a cell-permeable NFAT inhibitor peptide (Calbiochem) 6 h prior to infection with JCV. Cells were infected for 1 h in the presence of the inhibitor. Following infection, the cells were maintained in the inhibitor. Due to the half-life of the peptide, at 24 h and 48 h p.i., cells were treated with fresh peptide. At 72 h p.i., cells were fixed and stained for either V-Ag (d) or T-Ag (e). The numbers of infected cells per slide in a minimum of 10 fields were counted per slide, and the percentages of cells infected were compared to those for the untreated control. The average number of cells infected in the untreated control was ∼60 cells per field at a magnification of ×200.
The main target of CsA is calcineurin. The primary target of calcineurin is the NFAT transcription factor family. Consequently, CsA treatment results in an inhibition of NFAT activity. As CsA has multiple effects on the cell, in addition to the inhibition of NFAT activity, SVG-A cells were treated with a highly specific peptide inhibitor of NFAT activation to determine whether NFAT is specifically required for JCV infection. The peptide mimics the calcineurin binding site of NFAT and can interfere with the activation of NFAT by competing for calcineurin binding (3). Cells were pretreated with peptide for 6 h and then maintained in media containing the peptide during and after infection. When SVG-A cells were treated with the NFAT inhibitor, there was a dose-dependent decrease in the number of cells infected with JCV, as detected by VP1 expression (Fig. 1d). This suggests that the effect of CsA on JCV infection is at least in part through its inhibition of NFAT activation. In addition to a reduction in VP1 staining, JCV infected cells were stained for the early viral protein T-Ag by using a JCV specific antibody (PAB962). The effect of CsA on the number of cells expressing T-Ag mirrored the results for VP1 (Fig. 1e), indicating a role for NFAT either at the stage of early transcription or during events prior to early transcription.
NFAT4 is functionally active in glial cells.
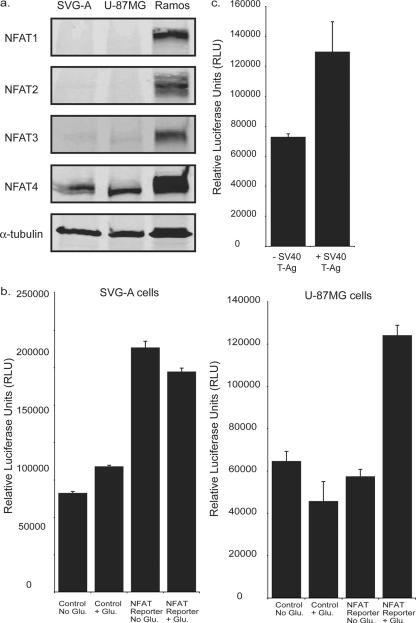
The NFAT family encompasses five proteins evolutionarily related to the Rel/NFκB family (6, 7). NFATs 1 to 4 are all regulated in response to Ca2+ and the Ca2+-calmodulin-dependent serine phosphatase calcineurin. NFAT4 has previously been demonstrated to be expressed in primary astrocytes and glioma cell lines (12). We probed for the presence of NFATs 1 to 4 in whole-cell lysates from SVG-A and U-87MG glioma cells (Fig. 2a). NFAT4 was detected in both cell lines. NFATs 1 to 3 were undetectable in SVG-A and U-87MG cells by Western blot analysis (Fig. 2a). Each antibody was capable of recognizing NFATs 1 to 4 isolated from the Burkitt's lymphoma cell line RAMOS (Fig. 2a).
FIG. 2.
NFAT4 is functionally active in glial cells. (a) NFAT expression in SVG-A and U-87MG whole-cell lysates. NFATs 1 to 4 were detected using antibodies (NFATc1 7A6, NFATc2 4G6-G5, NFATc3 F-1, and NFATc4 H-74; Santa Cruz Biotech Inc.) diluted 1:200. Ramos cell lysates were used as a positive control. (b) Luciferase reporter gene assays using an NFAT-responsive reporter construct were used to compare NFAT activity in SVG-A cells and U-87MG cells in the presence or absence of 292 mg/liter glutamate. A control construct that lacked the NFAT binding site was used to measure basal transcriptional activity. (c) The NFAT reporter construct was cotransfected with either a control construct (− SV40 T-Ag) or a construct expressing the SV40 large T-Ag (+ SV40 T-Ag).
To establish whether NFAT-mediated transcription was functional in SVG-A and U-87MG cells, both cell types were transfected with a luciferase reporter construct (pNFAT-luc) that contains an NFAT-responsive element obtained from the IL-2 promoter, and luciferase activity was measured in the presence or absence of glutamate, a compound that activates NFAT in glial cells (12). Comparison of the NFAT reporter with a control promoter (pTA-luc) that does not contain an NFAT-responsive element indicated that there is a basal level of NFAT activity in SVG-A cells even in the absence of glutamate stimulation (Fig. 2b). In contrast, the U-87MG cells show only NFAT activity in the presence of glutamate (Fig. 2b).
NFAT activity in the SVG-A cells indicates an inherent activation of calcium signaling. SVG-A cells express SV40 large T-Ag. To investigate the possibility that SV40 large T-Ag is capable of activating NFAT in glial cells, we cotransfected a construct expressing SV40 large T-Ag with the NFAT reporter construct into U-87MG cells, which lack SV40 large T-Ag. There was a twofold increase in the activity of NFAT in the presence of SV40 large T-Ag expression, suggesting that the presence of SV40 large T-Ag has resulted in NFAT activity in SVG-A cells (Fig. 2c).
NFAT4 binds directly to the JCV promoter and activates both early and late transcription.
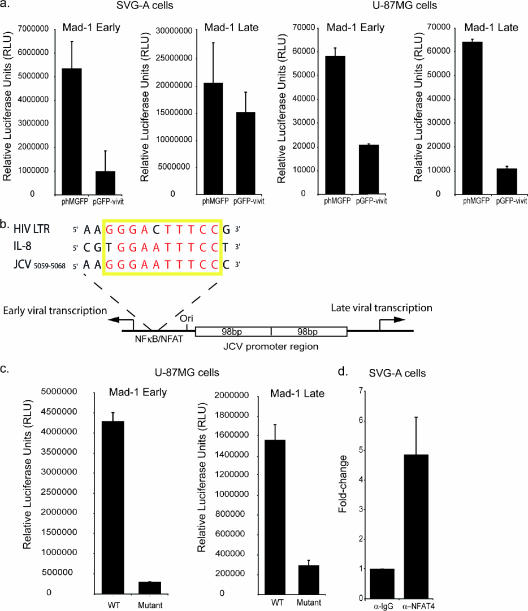
To determine whether NFAT4 acts on viral transcription, a plasmid expressing the NFAT peptide inhibitor (pGFP-VIVIT) was cotransfected with a reporter construct containing the viral promoter region in either the early or the late orientation. Inhibition of NFAT4 in SVG-A cells led to a significant reduction of both the early- and the late-promoter activities (Fig. 3a). The effect on the late promoter was less than that on the early promoter. SVG cells express SV40 T-Ag, and as T-Ag is a potent activator of the late promoter (19), this could explain the reduced effect of NFAT inhibition on the late promoter. U-87MG cells are from a glioblastoma cell line that does not express the SV40 T-Ag. Inhibition of NFAT4 significantly reduced both early- and late-viral-promoter activity in U-87MG cells (Fig. 3a), confirming that NFAT4 is important for both early and late JCV transcription in glial cells.
FIG. 3.
NFAT4 binds directly to the JCV promoter and activates both early and late viral transcription. (a) Luciferase reporter assays were used to monitor the effect of expressing the NFAT inhibitor on both the late and the early Mad-1 promoters in SVG-A and U-87MG glioblastoma cells. All experiments were conducted in the presence of 292 mg/liter glutamate. (b) Schematic of the JCV promoter region indicating the position of the NFκB/NFAT binding site. Highlighted is the binding site sequence aligned with similar sites within the HIV LTR and the IL-8 promoter. The yellow box indicates the site, and the nucleotides in red are conserved between all three sites. (c) The NFAT/NFκB sites in the early and late Mad-1 promoter reporter constructs were mutated from GGGAATTTCC to AAGAATTTCC and transfected into U-87MG cells. (d) ChIP was carried out on cells infected with JCV. Chromatin was immunoprecipitated using either a polyclonal anti-NFAT4 antibody (α-NFAT4; Santa Cruz Biotech Inc.) or a mouse IgG antibody (α-IgG; Santa Cruz Biotech Inc.). Quantitative PCR was used to analyze the subsequent DNA samples. WT, wild type.
Analysis of the JCV untranslated region reveals the presence of a potential NFAT binding site (Fig. 3b). This particular site binds a related set of transcription factors, the NFκB family (29), and is responsive to tumor necrosis factor alpha (TNF-α) and IL-2 (24), both of which are strong activators of NFAT. In the case of the IL-8 promoter, NFAT1 binds to a site that also binds NFκB (10). Alignment of the NFAT/NFκB site in the IL-8 promoter with the site in JCV shows that the two sites are homologous (Fig. 3b). This site also shares sequence homology with another NFAT/NFκB site located within the HIV long terminal repeat (LTR) region. In this case, both transcription factors are capable of binding to the site and activating transcription depending on the cellular context. Mutation of this site in either pMad-1Eluc or pMad-1Lluc resulted in a significant loss of promoter activity (Fig. 3c), confirming results from previous studies that show this site to be important for both early and late transcription (29).
ChIP assays using an anti-NFAT4 antibody on SVG-A cells infected with JCV demonstrated a fivefold enrichment for JCV promoter DNA over DNA immunoprecipitated with a nonspecific immunoglobulin G (IgG) control antibody (Fig. 3d). This indicates that during JCV infection of glial cells, NFAT4 does bind directly to the JCV untranslated region.
Expression of JCV early products results in NFAT activity.
As SV40 is a related polyomavirus and expression of SV40 large T-Ag activates NFAT in glial cells (Fig. 2c), we also tested the JCV early transcription products for the ability to activate NFAT. The NFAT reporter construct (pNFAT-luc) was cotransfected with a construct expressing the JCV early region under a constitutive promoter (pCMV-JCVE), and luciferase activity was measured 48 h following transfection. The expression of the entire JCV early region was also capable of activating NFAT-mediated transcription in U-87MG cells (Fig. 4). Both mouse polyomavirus and hamster polyomavirus have previously been shown to activate NFAT through the action of early gene products (5, 13). The use of such a mechanism by JCV and SV40 suggests that the activation of NFAT by early gene products and its use for viral transcription may be common features in the polyomavirus family designed to optimize transcription.
FIG. 4.
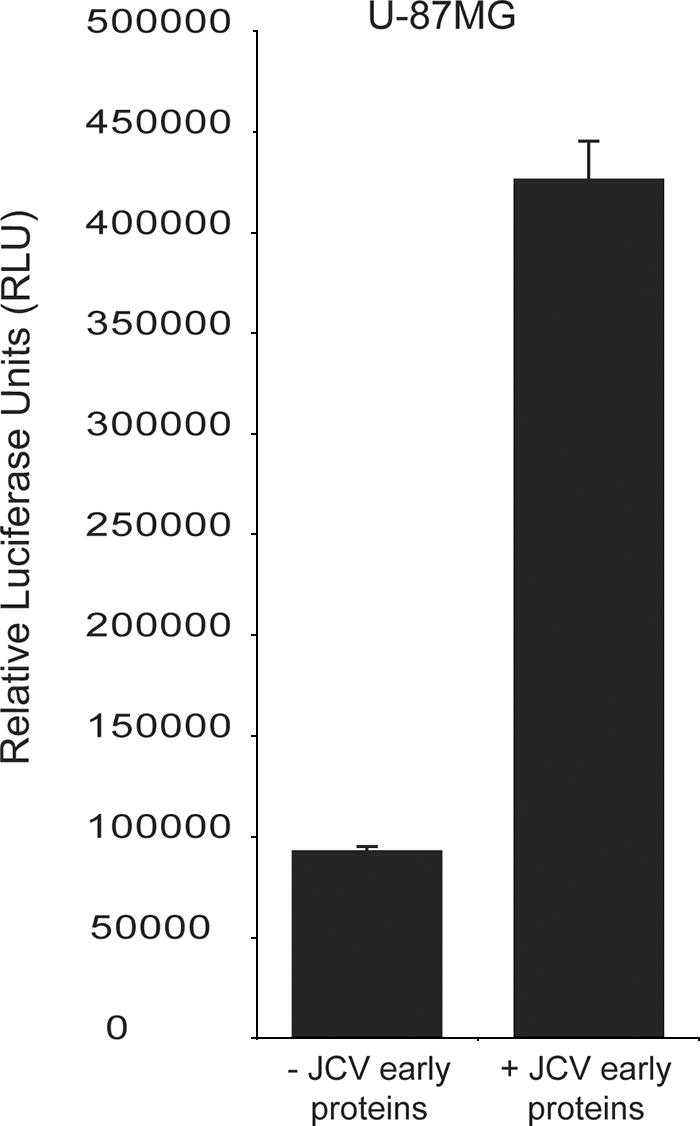
JCV early proteins activate NFAT. The NFAT reporter construct was cotransfected into U-87MG cells with either a control construct (− JCV early proteins) or a construct expressing the JCV early region (+ JCV early proteins) under the control of the CMV promoter. Cells were grown in the absence of glutamate, and the experiment was carried out in triplicate.
DISCUSSION
Our data show that NFAT4 directly upregulates both early and late transcription in glial cells, and the loss of NFAT activity results in the inhibition of viral infection. In addition to this, early viral gene products activate NFAT, suggesting a positive feedback loop involving the activation of calcium signaling.
The involvement of NFAT4 in JCV infection raises a number of questions regarding the activation of NFAT in glial cells as well as the relationship between the immune system and the development of PML in HIV-infected patients. As the activation of NFAT is important for the productive infection of glial cells, scenarios in which NFAT becomes activated may have a significant bearing on viral production in the CNS. HIV itself utilizes NFAT for its own gene expression (31) and is capable of activating NFAT by a number of mechanisms. One mechanism is the direct interaction between the calcium-signaling pathway and HIV accessory proteins, such as tat, nef, and vpr, which have all been demonstrated to upregulate NFAT activity (17, 22, 36). Another mechanism of HIV-induced NFAT activity is the indirect activation of calcium signaling via an increase in the levels of certain extracellular stimuli. Many HIV patients exhibit high levels of cytokine expression in the CNS (32). These include strong activators of calcium signaling, such as TNF-α and IL-2, both of which bind and activate immunoreceptors. Activation of NFAT4 in HIV-infected patients due to increasing levels of TNF-α and IL-2 may be conducive for JCV infection of the CNS. The correlation between increases in TNF-α expression and the occurrence of PML in HIV-infected patients supports this idea (25). The possible activation of NFAT by HIV may offer a mechanism for explaining the increased incidence of JCV-induced PML in HIV-infected patients.
JCV has a restricted tropism and infects only glial cells and, to a limited extent, B cells. The JCV promoter has been implicated as playing an important part in the neurotropism of JCV, and numerous transcription factors have been identified as contributing factors (1, 2, 8, 14, 30, 33). A role for NFAT4 in JCV tropism is possible, as this transcription factor is specifically found in a number of cell types implicated in productive viral infection and latency, including glial cells and bone marrow cells (11). Our data argue that NFAT4 is sufficient to drive JCV gene expression in glial cells, as these cells do not express NFATs 1 to 3. Our data do not rule out the possibility that NFATs 1 to 3 can functionally substitute for NFAT4 in driving JCV gene expression, so a specific role for NFAT4 is glial cell tropism is not proven here. A related question is how NFκB activation influences JCV transcription. The ChIP data presented here and previous gel shift analysis (29) indicate that the κB element in the JCV promoter can bind both NFAT and NFκB. The IL-8 promoter and the HIV LTR can also bind both NFAT and NFκB at the same site, but which one binds depends on the cell type and signaling context. Whether this is the case with JCV and what effect this has on JCV transcription and tropism remain to be seen.
Treatment of PML patients by targeting NFAT4 is unlikely due to the crucial involvement of NFAT in activating T-cell proliferation, but monitoring the activity of NFAT in HIV patients may be a useful indicator of an increased risk for developing PML. Gene expression profiling and cytokine array data indicate a correlation between the expression of a certain set of gene transcripts and JCV infection (our unpublished observations). Cytokine-profiling data on HIV patients show higher levels of cytokines such as MCP-1 and IL-6 in PML patients than in PML-negative HIV patients (23, 25). These data, when combined, may be useful in identifying a profile of genes expressed in response to NFAT4 activity in glial cells. These genes can be used as biomarkers for determining increased susceptibility to JCV-induced PML, allowing for prophylactic treatment, such as the use of serotonin receptor inhibitors.
Acknowledgments
We thank all members of the Atwood laboratory for critical discussions during the course of this work. We thank A. Rao, H. Sawa, R. Frisque, J. DeCaprio, R. Davis, S. Tevethia, and E. Harlow for providing constructs and antibodies. We also thank Amanda Robinson, Amy Bozek, Tammy Glass, and Lorie St. Pierre for administrative assistance.
Work in our laboratory was supported by a grant from the National Cancer Institute, R01 CA71878, and by a grant from the National Institute of Neurological Disorders and Stroke, R01 NS43097, to W.J.A.
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Amemiya, K., R. Traub, L. Durham, and E. O. Major. 1992. Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC virus. A common characteristic of many brain-specific genes. J. Biol. Chem. 267:14204-14211. [PubMed] [Google Scholar]
- 2.Amemiya, K., R. Traub, L. Durham, and E. O. Major. 1989. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J. Biol. Chem. 264:7025-7032. [PubMed] [Google Scholar]
- 3.Aramburu, J., M. B. Yaffe, C. Lopez-Rodriguez, L. C. Cantley, P. G. Hogan, and A. Rao. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133. [DOI] [PubMed] [Google Scholar]
- 4.Atwood, W. J., L. Wang, L. C. Durham, K. Amemiya, R. G. Traub, and E. O. Major. 1995. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J. Neurovirol. 1:40-49. [DOI] [PubMed] [Google Scholar]
- 5.Brizuela, L., E. T. Ulug, M. A. Jones, and S. A. Courtneidge. 1995. Induction of interleukin-2 transcription by the hamster polyomavirus middle T antigen: a role for Fyn in T cell signal transduction. Eur. J. Immunol. 25:385-393. [DOI] [PubMed] [Google Scholar]
- 6.Chytil, M., and G. L. Verdine. 1996. The Rel family of eukaryotic transcription factors. Curr. Opin. Struct. Biol. 6:91-100. [DOI] [PubMed] [Google Scholar]
- 7.Graef, I. A., J. M. Gastier, U. Francke, and G. R. Crabtree. 2001. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc. Natl. Acad. Sci. USA 98:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson, J. W. 1994. Regulation of the glial-specific JC virus early promoter by the transcription factor Sp1. J. Biol. Chem. 269:1046-1050. [PubMed] [Google Scholar]
- 9.Ho, S., N. Clipstone, L. Timmermann, J. Northrop, I. Graef, D. Fiorentino, J. Nourse, and G. R. Crabtree. 1996. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 80:S40-S45. [DOI] [PubMed] [Google Scholar]
- 10.Hogan, P. G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205-2232. [DOI] [PubMed] [Google Scholar]
- 11.Ishida, N., K. Hayashi, M. Hoshijima, T. Ogawa, S. Koga, Y. Miyatake, M. Kumegawa, T. Kimura, and T. Takeya. 2002. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 277:41147-41156. [DOI] [PubMed] [Google Scholar]
- 12.Jones, E. A., D. Sun, L. Kobierski, and A. J. Symes. 2003. NFAT4 is expressed in primary astrocytes and activated by glutamate. J. Neurosci. Res. 72:191-197. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy, A. P., A. Sekulic, B. J. Irvin, A. E. Nilson, S. M. Dilworth, and R. T. Abraham. 1998. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J. Biol. Chem. 273:11505-11513. [DOI] [PubMed] [Google Scholar]
- 14.Khalili, K., J. Rappaport, and G. Khoury. 1988. Nuclear factors in human brain cells bind specifically to the JCV regulatory region. EMBO J. 7:1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschmidt-DeMasters, B. K., and K. L. Tyler. 2005. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 353:369-374. [DOI] [PubMed] [Google Scholar]
- 16.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115-123. [DOI] [PubMed] [Google Scholar]
- 17.Lahti, A. L., A. Manninen, and K. Saksela. 2003. Regulation of T cell activation by HIV-1 accessory proteins: Vpr acts via distinct mechanisms to cooperate with Nef in NFAT-directed gene expression and to promote transactivation by CREB. Virology 310:190-196. [DOI] [PubMed] [Google Scholar]
- 18.Langer-Gould, A., S. W. Atlas, A. J. Green, A. W. Bollen, and D. Pelletier. 2005. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353:375-381. [DOI] [PubMed] [Google Scholar]
- 19.Lashgari, M. S., H. Tada, S. Amini, and K. Khalili. 1989. Regulation of JCVL promoter function: transactivation of JCVL promoter by JCV and SV40 early proteins. Virology 170:292-295. [DOI] [PubMed] [Google Scholar]
- 20.Liu, C. K., and W. J. Atwood. 2001. Propagation and assay of the JC virus. Methods Mol. Biol. 165:9-17. [DOI] [PubMed] [Google Scholar]
- 21.Ludlow, J. W., J. A. DeCaprio, C. M. Huang, W. H. Lee, E. Paucha, and D. M. Livingston. 1989. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell 56:57-65. [DOI] [PubMed] [Google Scholar]
- 22.Manninen, A., and K. Saksela. 2002. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 195:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzocchetti, A., A. Cingolani, S. D. Giambenedetto, A. Ammassari, M. L. Giancola, R. Cauda, A. Antinori, and A. D. Luca. 2005. Macrophage chemoattractant protein-1 levels in cerebrospinal fluid correlate with containment of JC virus and prognosis of acquired immunodeficiency syndrome—associated progressive multifocal leukoencephalopathy. J. Neurovirol. 11:219-224. [DOI] [PubMed] [Google Scholar]
- 24.Mayreddy, R. P., M. Safak, M. Razmara, P. Zoltick, and K. Khalili. 1996. Transcription of the JC virus archetype late genome: importance of the kappa B and the 23-base-pair motifs in late promoter activity in glial cells. J. Virol. 70:2387-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagano, I., S. Nakamura, M. Yoshioka, J. Onodera, K. Kogure, and Y. Itoyama. 1994. Expression of cytokines in brain lesions in subacute sclerosing panencephalitis. Neurology 44:710-715. [DOI] [PubMed] [Google Scholar]
- 26.Okada, Y., H. Sawa, S. Tanaka, A. Takada, S. Suzuki, H. Hasegawa, T. Umemura, J. Fujisawa, Y. Tanaka, W. W. Hall, and K. Nagashima. 2000. Transcriptional activation of JC virus by human T-lymphotropic virus type I Tax protein in human neuronal cell lines. J. Biol. Chem. 275:17016-17023. [DOI] [PubMed] [Google Scholar]
- 27.Padgett, B. L., and D. L. Walker. 1973. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127:467-470. [DOI] [PubMed] [Google Scholar]
- 28.Padgett, B. L., D. L. Walker, G. M. ZuRhein, R. J. Eckroade, and B. H. Dessel. 1971. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet i:1257-1260. [DOI] [PubMed] [Google Scholar]
- 29.Ranganathan, P. N., and K. Khalili. 1993. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 21:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renner, K., H. Leger, and M. Wegner. 1994. The POU domain protein Tst-1 and papovaviral large tumor antigen function synergistically to stimulate glia-specific gene expression of JC virus. Proc. Natl. Acad. Sci. USA 91:6433-6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanchikova, N., V. Ivanova, C. Scheller, E. Jankevics, C. Jassoy, and E. Serfling. 2003. NFAT transcription factors control HIV-1 expression through a binding site downstream of TAR region. Immunobiology 208:361-365. [DOI] [PubMed] [Google Scholar]
- 32.Speth, C., M. P. Dierich, and S. Sopper. 2005. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol. Immunol. 42:213-228. [DOI] [PubMed] [Google Scholar]
- 33.Tada, H., M. Lashgari, J. Rappaport, and K. Khalili. 1989. Cell type-specific expression of JC virus early promoter is determined by positive and negative regulation. J. Virol. 63:463-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tevethia, S. S., M. Epler, I. Georgoff, A. Teresky, M. Marlow, and A. J. Levine. 1992. Antibody response to human papovavirus JC (JCV) and simian virus 40 (SV40) T antigens in SV40 T antigen-transgenic mice. Virology 190:459-464. [DOI] [PubMed] [Google Scholar]
- 35.Tyagarajan, S. K., and R. J. Frisque. 2006. Stability and function of JC virus large T antigen and T′ proteins are altered by mutation of their phosphorylated threonine 125 residues. J. Virol. 80:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vacca, A., M. Farina, M. Maroder, E. Alesse, I. Screpanti, L. Frati, and A. Gulino. 1994. Human immunodeficiency virus type-1 tat enhances interleukin-2 promoter activity through synergism with phorbol ester and calcium-mediated activation of the NF-AT cis-regulatory motif. Biochem. Biophys. Res. Commun. 205:467-474. [DOI] [PubMed] [Google Scholar]
- 37.Van Assche, G., M. Van Ranst, R. Sciot, B. Dubois, S. Vermeire, M. Noman, J. Verbeeck, K. Geboes, W. Robberecht, and P. Rutgeerts. 2005. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353:362-368. [DOI] [PubMed] [Google Scholar]