Abstract
A human papillomavirus (HPV) vaccine consisting of virus-like particles (VLPs) was recently approved for human use. It is generally assumed that VLP vaccines protect by inducing type-specific neutralizing antibodies. Preclinical animal models cannot be used to test for protection against HPV infections due to species restriction. We developed a model using chimeric HPV capsid/cottontail rabbit papillomavirus (CRPV) genome particles to permit the direct testing of HPV VLP vaccines in rabbits. Animals vaccinated with CRPV, HPV type 16 (HPV-16), or HPV-11 VLPs were challenged with both homologous (CRPV capsid) and chimeric (HPV-16 capsid) particles. Strong type-specific protection was observed, demonstrating the potential application of this approach.
Human papillomaviruses (HPVs) are the etiological agents of various anogenital lesions and cancers. Cervical cancer, which is induced by a subset of HPV genotypes termed “high risk,” is the third leading cause of cancer deaths among women worldwide (1, 7, 8). Recently, clinical trials have demonstrated that the vaccination of women with HPV virus-like particles (VLPs) can protect against type-specific natural infections (4, 6, 15). The results of these clinical trials, and of previous work with various animal models (2, 5, 14), have demonstrated that VLP-based vaccines are capable of eliciting a strong humoral response, which is presumed to be primarily responsible for protection against papillomavirus infections. Because the humoral response to VLP-based vaccines is highly type specific, and protection against multiple HPV types is required to prevent HPV-associated disease within a population, additional research has been recently focused on the development of cross-protective HPV vaccines (10, 12).
Papillomaviruses are species restricted with more than 100 different genotypes infecting humans. The efficacy of HPV VLP vaccines in laboratory animal models cannot be tested beyond the analysis of neutralizing antibodies in serum samples using virus-neutralization cell culture assays (9, 13). While such experiments offer valuable information regarding the magnitude and specificity of the antibody response in vaccinated animals, they do not directly test the ability of a vaccine to protect against challenge with infectious HPV particles.
Using pseudovirion technology (3, 11), we previously described the production of infectious particles composed of a heterologous cottontail rabbit papillomavirus (CRPV) genome encapsidated by HPV-16 or CRPV capsid proteins (3a). This CRPV genome contains the simian virus 40 origin of replication (337 nucleotides) cloned at the BglII site within the upstream regulatory region for improved CRPV genome amplification within the 293TT producer cells. Both homologous (CRPV capsid/CRPV genome) and chimeric (HPV type 16 [HPV-16] capsid/CRPV genome) papillomavirus particles are infectious in vitro, can be fully neutralized by L1-targeting specific monoclonal antibodies and, importantly, at low volumes (2 to 10 μl) can induce epidermal papillomas on New Zealand White rabbits characteristic of those produced by native CRPV virions.
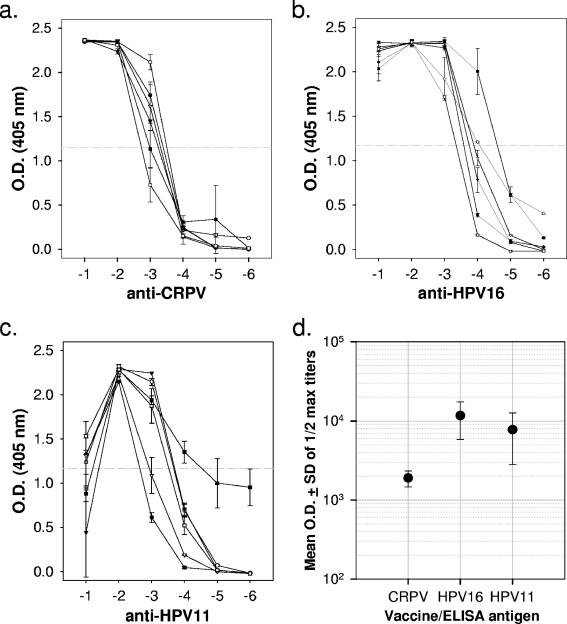
For direct in vivo testing of an HPV vaccine in this model, three groups of six rabbits each were vaccinated three times at 2-week intervals with either CRPV, HPV-11, or HPV-16 L1-only VLPs (50 μg) in phosphate-buffered saline (PBS) without additional adjuvants. Four days after the final booster immunization, blood samples were obtained for analysis of capsid-specific antibody by using an enzyme-linked immunosorbent assay (Fig. 1). Each serum was tested for its ability to bind CRPV, HPV-11, and HPV-16 L1-only VLPs adhered overnight to 96-well plates in PBS (pH 7.4) at 4°C. Half-maximal binding titers for sera exposed to vaccine-matched VLPs typically ranged from 1/103 to 1/104, indicating a strong humoral response to VLP vaccination. As expected, reactivity against antigenically unrelated VLP types was at background levels, showing no evidence for cross-reactivity of the antibodies generated in these animals (data not shown).
FIG. 1.
Titration of antibody binding to L1-VLPs by enzyme-linked immunosorbent assay. All sera were tested against CRPV (a), HPV-16 (b), and HPV-11 (c) VLPs. The data shown are means ± the standard deviation (SD) for serum dilutions from each of six rabbits in the antigen-matched group. No cross-reactivity was seen with sera from rabbits in the other two vaccine groups (data not shown). Tenfold dilutions of sera shown on the abscissa (log10) were tested in duplicate wells against VLPs preadhered to plates in PBS (pH 7.4) and blocked with 5% nonfat dry milk in PBS. Antibody binding was indicated by using an anti-rabbit secondary antibody conjugated to alkaline phosphatase and a standard colorimetric assay. (d) Mean optical density (O.D.) readings (± the SD) for each vaccine group against the specific antigen.
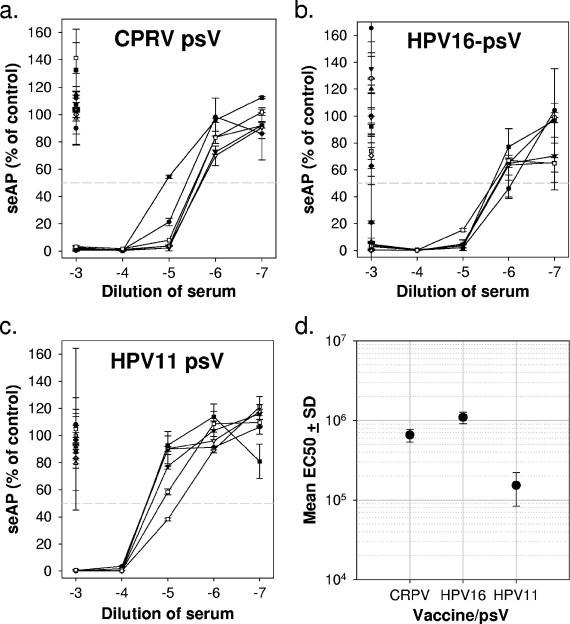
Sera were also tested for their abilities to neutralize CRPV, HPV-11, and HPV-16 L1/L2 pseudovirions delivering a secreted alkaline phosphatase (seAP) reporter gene into 293TT cells (Fig. 2) (9). These experiments yielded results consistent with the VLP-binding assays in that pseudovirion neutralization was exclusively and consistently seen with sera from vaccine-matched animals. Neutralization titers ranged from approximately 1/105 to 1/106, demonstrating the production of high titers of neutralizing antibodies in vaccinated animals.
FIG. 2.
Titration of pseudovirus neutralization by rabbit sera. Serial 10-fold dilutions of antigen-specific rabbit sera (and a single dilution of sera from rabbits in the other two vaccine groups) were preincubated with CRPV (a), HPV-16 (b), and HPV-11 (c) pseudovirions produced in 293TT cells using a protocol described previously (3). The abscissa shows serum concentrations (log10) during the 1-h preincubation with pseudovirions at 37°C, prior to infecting triplicate wells of 293TT cells seeded the previous day in 96-well plates at 3 × 104 cells/well. At 3 days postinfection, spent media were analyzed for seAP levels by using a colorimetric assay. The data are presented relative to seAP in wells receiving pseudovirions incubated in the absence of rabbit serum. The horizontal gray line represents 50% less seAP than control wells and is interpreted as the successful neutralization of 50% input pseudovirions. All line scatter plots show data from individual rabbits in the antigen-specific group, while the single points plotted at 10−3 represent the sera of individual animals from the other two vaccine groups. (d) Mean concentrations (± the SD) of antiserum required to neutralize 50% of input pseudovirions.
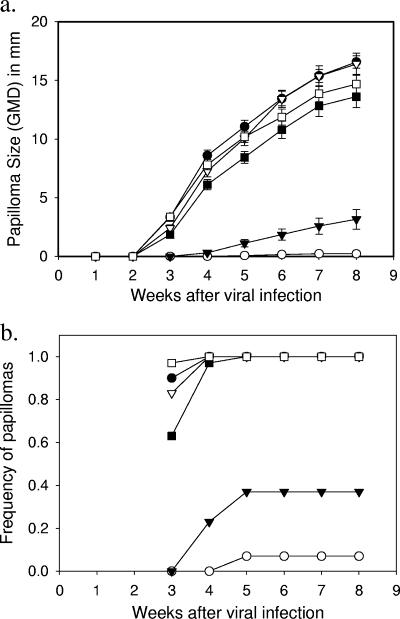
At 4 weeks after the final immunization with VLPs, animals were challenged with both homologous (CRPV capsid/CRPV genome) and chimeric (HPV-16 capsid/CRPV genome) infectious particles. All rabbits were inoculated with both particle types at five sites each on the dorsal skin. The ability of the VLP vaccinations to protect against type-specific infection was evaluated in vivo by observation of the appearance and growth of papillomas at the sites of challenge (Fig. 3). Consistent with the in vitro data, animals vaccinated with CRPV and HPV-16 VLPs showed evidence of significant protection against the vaccine-matched virus but not against the antigenically unrelated infectious particles. Since all animals were challenged with both homologous (CRPV/CRPV) and chimeric (HPV-16/CRPV) particles, the significantly faster appearance and growth rate of papillomas at sites inoculated with the vaccine-mismatched particles is strong evidence for both capsid-dependent infection and type-specific protection (Fig. 4). Both the homologous (CRPV/CRPV) and the chimeric (HPV-16/CRPV) infectious particles induced papillomas at equivalent growth rates on each of the rabbits immunized with HPV-11 VLPs, demonstrating that viral challenge was similar between the two types of infectious particles. These results provide no evidence of a cross-protective response in vivo between HPV-11 and HPV-16. The delayed appearance of small numbers of papillomas at sites inoculated with the antigenically matched particles (beginning at week 4) may be due to (i) residual escape from antibody-mediated neutralization of the high titer of input infectious particles, (ii) capsid-independent infection by CRPV DNA, and/or (iii) the lack of an assisting L1-specific cell-mediated response (in the animals vaccinated with HPV-16 VLPs). The model as described provides opportunities to examine each of these possible outcomes in more detailed future experiments. The growth rates, morphologies and histologies of papillomas produced by each type of infectious particle were indistinguishable and comparable to what we have previously seen with papillomas caused by native CRPV virions (data not shown).
FIG. 3.
Papilloma occurrence and growth at sites inoculated with papillomavirus particles. New Zealand White rabbits were vaccinated with CRPV (circles), HPV-16 (triangles), or HPV-11 (squares) L1 VLPs as described in the text. At 4 weeks after the third immunization, scarified sites on the dorsal skin of each rabbit were inoculated with 10 μl of a stock of homologous (CRPV capsid/CRPV genome [open symbols]) or chimeric (HPV-16 capsid/CRPV genome [solid symbols]) particles purified from DNase-treated 293TT cell lysates using an Optiprep density gradient (3, 3a). (a) The mean papilloma size (± the SD) is calculated by using the geometric mean diameters (GMD) for all sites receiving the inoculum. (b) The frequencies of papillomas induced at all sites inoculated with the homologous or chimeric infectious particles are shown separately for the three vaccine groups.
FIG. 4.
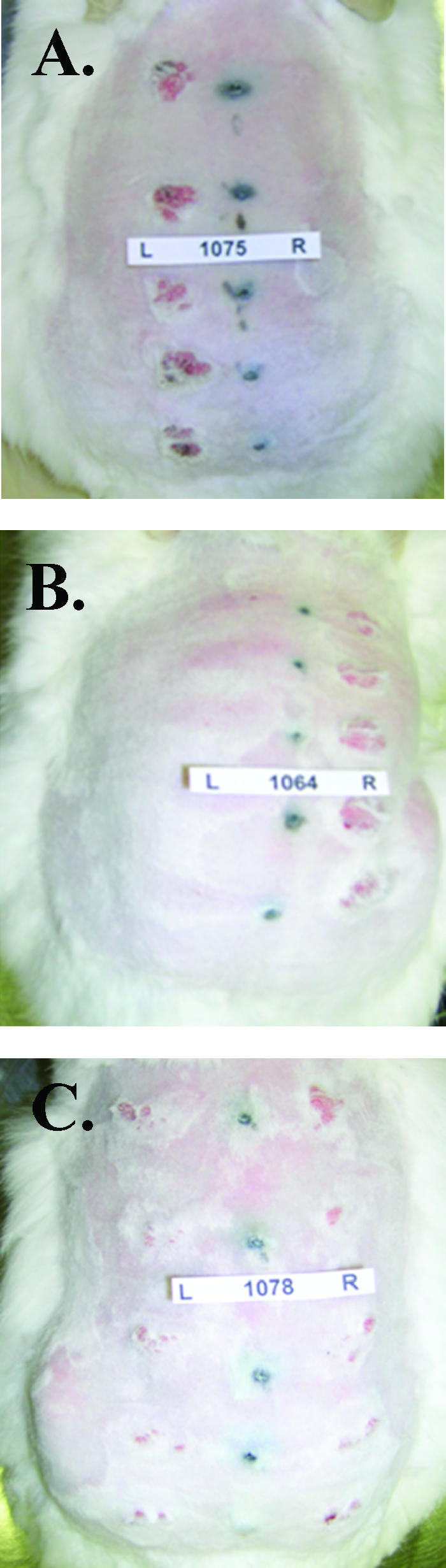
Papillomas on selected rabbits 4 weeks after challenge with infectious particles. Rabbits vaccinated with CRPV (a), HPV-16 (b), or HPV-11 (c) VLPs were inoculated at five sites each with homologous (CRPV capsid/CRPV genome) particles on the left side (L) and with chimeric (HPV-16 capsids/CRPV genomes) particles on the right side (R).
Our experiments demonstrate that chimeric HPV particles can be used in an animal model to test the efficacy of HPV L1 VLP and HPV L2-based vaccines to prevent lesions induced by papillomavirus infections. This in vivo approach provides a rigorous test of immunity since vaccinated animals can be challenged with low- or high-titer infectious particles under controlled conditions and be evaluated for the appearance and growth rates of papillomavirus-induced lesions. This model also provides opportunities to test for protection against a number of different HPV particle types on the same animal to address questions regarding potential cross-protection of related and unrelated HPV types and to determine what contributory role a cell-mediated immune response to HPV capsid proteins may play in the protection generated by these capsid vaccines.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Culp, T. D., N. M. Cladel, K. Balogh, L. R. Budgeon, A. Mejia, and N. D. Christensen. 2006. Papillomavirus particles assembled in 293TT cells are infectious in vivo. J. Virol. 80:11381-11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757-1765. [DOI] [PubMed] [Google Scholar]
- 5.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 6.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 7.Munoz, N., F. X. Bosch, X. Castellsague, M. Diaz, S. De Sanjose, D. Hammouda, K. V. Shah, and C. J. Meijer. 2004. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer 111:278-285. [DOI] [PubMed] [Google Scholar]
- 8.Parkin, D. M., F. Bray, J. Ferlay, and P. Pisani. 2005. Global cancer statistics, 2002. CA Cancer J. Clin. 55:74-108. [DOI] [PubMed] [Google Scholar]
- 9.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, K. S. Kruger, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216. [DOI] [PubMed] [Google Scholar]
- 10.Pastrana, D. V., R. Gambhira, C. B. Buck, Y. Y. Pang, C. D. Thompson, T. D. Culp, N. D. Christensen, D. R. Lowy, J. T. Schiller, and R. B. Roden. 2005. Cross-neutralization of cutaneous and mucosal papillomavirus types with antisera to the amino terminus of L2. Virology 337:365-372. [DOI] [PubMed] [Google Scholar]
- 11.Pyeon, D., P. F. Lambert, and P. Ahlquist. 2005. Production of infectious human papillomavirus independently of viral replication and epithelial cell differentiation. Proc. Natl. Acad. Sci. USA 102:9311-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden, R. B., W. H. Yutzy, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 13.Roden, R. B. S., N. L. Hubbert, R. Kirnbauer, N. D. Christensen, D. R. Lowy, and J. T. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, K. A. Ault, A. R. Giuliano, C. M. Wheeler, L. A. Koutsky, C. Malm, M. Lehtinen, F. E. Skjeldestad, S. E. Olsson, M. Steinwall, D. R. Brown, R. J. Kurman, B. M. Ronnett, M. H. Stoler, A. Ferenczy, D. M. Harper, G. M. Tamms, J. Yu, L. Lupinacci, R. Railkar, F. J. Taddeo, K. U. Jansen, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271-278. [DOI] [PubMed] [Google Scholar]