Abstract
Polycomb response elements (PREs) are chromosomal elements, typically comprising thousands of base pairs of poorly defined sequences that confer the maintenance of gene expression patterns by Polycomb group (PcG) repressors and trithorax group (trxG) activators. Genetic studies have indicated a synergistic requirement for the trxG protein GAGA and the PcG protein Pleiohomeotic (PHO) in silencing at several PREs. However, the molecular basis of this cooperation remains unknown. Here, using DNaseI footprinting analysis, we provide a high-resolution map of sites for the sequence- specific DNA-binding PcG protein PHO, trxG proteins GAGA and Zeste and the gap protein Hunchback (HB) on the 1.6 kb Ultrabithorax (Ubx) PRE. Although these binding elements are present throughout the PRE, they display clear patterns of clustering, suggestive of functional collaboration at the level of PRE binding. We found that while GAGA could efficiently bind to a chromatinized PRE, PHO alone was incapable of binding to chromatin. However, PHO binding to chromatin, but not naked DNA, was strongly facilitated by GAGA, indicating interdependence between GAGA and PHO already at the level of PRE binding. These results provide a biochemical explanation for the in vivo cooperation between GAGA and PHO and suggest that PRE function involves the integrated activities of genetically antagonistic trxG and PcG proteins.
INTRODUCTION
The identity of body segments in Drosophila is determined by the spatially restricted expression patterns of the homeotic genes. Early in development, the concerted activities of the strictly localized products of the segmentation genes establish the expression domains of the homeotic genes. However, these early regulators are only transiently expressed and disappear after the first hours of embryogenesis. Subsequently, the ubiquitously expressed trithorax group (trxG) of activators and Polycomb group (PcG) of repressors perpetuate the differential transcription patterns of the homeotic genes throughout development (1–8). The function of these two genetically opposing groups is believed to involve the modulation of chromatin structure.
The PcG/trxG maintenance system acts through specialized cis-acting elements called Polycomb or Trithorax Response Elements (PREs or TREs), which are distinct from the segment-specific enhancers that mediate the initiation of restricted homeotic gene expression patterns by segmentation proteins (1,3,5,6). Although below we will use the name PRE, it is pertinent to note that, generally speaking, PREs and TREs appear to physically overlap or are closely integrated. PREs can be defined by a number of criteria. First, PREs maintain the silenced state of a linked gene in body segments where it was first silenced during embryogenesis (9–16). Second, PREs are recognized by PcG/trxG proteins. When present in a transposable element, PREs create a new chromosomal binding site for PcG proteins at the point of integration, as revealed by antibody staining of polytene chromosomes (11,12,17). Moreover, formaldehyde cross-linking chromatin immunoprecipitation experiments demonstrated direct binding to PREs by various PcG/trxG proteins (18–20). Third, PRE-mediated silencing of a linked mini-white transgene is enhanced when a fly is homozygous for the insertion. This ‘pairing-sensitive silencing’ suggests that paired PREs cooperate in trans to repress the transgene (11,13,16,21–24).
What are the structural features that make a PRE? PREs are much larger than typical enhancers, ranging from hundreds to a few thousand base pairs. PREs appear to lack clear-cut borders or a well-defined common core and multimerization of DNA elements with weak PRE activity can create a strong PRE. Thus, a picture emerges of a large, rather diffuse gene control element whose function depends on multiple distinct DNA elements that together function as an integrated unit (3,5,6). Although PcG/trxG proteins specifically associate with PREs, the majority seem to lack sequence-specific DNA-binding activity. It appears therefore that specialized sequence-specific DNA-binding proteins might function as tethers for the PcG/trxG factors.
A priori, there are two non-exclusive groups of candidate targeting factors. One possibility is that the early segmentation proteins directly recruit PcG/trxG complexes to specific target sites. These, in turn, may remain associated after the segmentation proteins themselves have disappeared, and in this way mediate the transition to PcG/trxG controlled maintenance. In support of this notion is the finding that the early gap repressor Hunchback (HB) binds dMi-2, a core subunit of the Drosophila NURD remodeling and histone deacetylase complex (25). Genetic experiments have implicated dMi-2 in Polycomb (PC) repression, suggesting that it may link HB to PC recruitment (25). This is an attractive possibility because it would provide a link between the temporally distinct establishment and maintenance of homeotic gene silencing.
The few sequence-specific DNA binding PcG/trxG that have been identified to date make up the second class of potential tethering factors. This group comprises PcG protein PHO and the trxG proteins GAGA and Zeste. The pho gene encodes a zinc finger DNA-binding protein, related to the mammalian transcription factor YY1 (26). PHO binding elements have been identified in a number of PREs (26,27) and several studies have shown that PHO is involved in the silencing activity of distinct PREs (26,28–31). Thus, PHO sites are required for the function of at least some PREs. Moreover, we recently obtained evidence for a direct interaction between PHO and a Polycomb (PC) containing complex (32), suggesting a direct role for PHO in PC recruitment. However, PHO sites by themselves do not suffice to direct PcG repression in vivo (26), pointing to the involvement of additional factors.
The trxG proteins GAGA and Zeste might be such accessory proteins required for PRE-function. GAGA, the product of the essential Trithorax-like (Trl) gene, is an activator of homeotic, as well as many other genes (reviewed in 13,33–35). Analysis of mutant flies has emphasized the in vivo importance of GAGA in the activation of several patterning genes including en, ubx and ftz (36,37). GAGA elements have recently been implicated in the tethering of the trithorax (TRX) protein to DNA (38). In addition, GAGA colocalizes with several PcG proteins at PREs (20,39). Somewhat paradoxically for a trxG protein, GAGA has been reported to also directly interact with PcG proteins (40) and is required for silencing function at several PREs (28,30,40,41).
Zeste can direct stimulation as well as repression of homeotic and other genes (7,42–44). Biochemical studies have demonstrated that Zeste can activate chromatin transcription through recruitment of the BRM complex (45), while Zeste has also been identified as a component of the PRC1 PcG complex (46). Moreover, Zeste shows positive as well as negative allele-specific genetic interactions with several PcG genes (47,48). Thus, Zeste and GAGA appear to perform dualistic regulatory functions during gene silencing as well as activation.
The Ubx PRE (also known as bxd PRE) plays a critical role in the maintenance of the Ubx expression pattern, and is one of the best-studied silencer elements (11,29,49,50). A 1.6 kb Ubx PRE fragment can confer both the perpetuation of embryonic silencing and pairing sensitive repression (11,29). The Ubx PRE is recognized by GAGA and PHO, and genetic studies have implicated both proteins in PcG silencing mediated by this PRE (29,40,41,50). In addition, Zeste was recently shown to maintain repression of Ubx in a Polycomb dependent manner (42) and has been proposed to be involved in the regulation of the Ubx PRE (51). Furthermore, the gap protein HB, directly or indirectly, acts on the Ubx PRE to induce repression of the Ubx gene (49,51). To gain insight into the functional relationship between DNA-binding proteins involved in Ubx PRE function in vivo, we performed an extensive DNaseI footprinting analysis to map the binding elements for HB, GAGA, Zeste and PHO on this silencer. Although these elements are present throughout large portions of the PRE, they appear to be organized in overlapping clusters. Because previous studies demonstrated a genetic cooperation between GAGA and PHO during PRE-mediated silencing (28,30), we were intrigued by the interdigitation of GAGA and PHO elements on the 1.6 kb Ubx PRE. We found that GAGA strongly facilitates PHO binding to chromatin, thus providing a molecular explanation for the functional interdependence between these genetically antagonistic trxG and PcG proteins.
MATERIALS AND METHODS
DNA constructs
The 1.6 Kb Ubx PRE corresponds to 2212StR1.6 (11) and its sequence obtained from GenBank (accession no. L32205, spanning nucleotide positions 33106–34667). Plasmids PRE A, B, C and D were generated using the oligonucleotide primers listed in Table 1 to PCR amplify overlapping subfragments of the 1.6 kb Ubx PRE from Drosophila genomic DNA and clone into pBluescript. Forward primers contain an EcoRI site and reverse primers contain a BamHI site. The nucleotide positions in L32205 pertaining to the subfragments in each PRE construct are indicated in Table 1.
Table 1. Oligonucleotide primers used for the generation of PRE plasmids.
| Plasmid | Forward primer | Reverse primer |
|---|---|---|
| PRE A (33106–33541) | 5′-gtcgaattcaaaaagaattatgtttgtc-3′ | 5′-gaggatccgatatttttatcttgggttgcatatgc-3′ |
| PRE B (33502–33916) | 5′-gagaattccaggttttatttgttgcatatgcaacccc-3′ | 5′-gaggatccattttgagtgcgttcttccgccgcttctt-3′ |
| PRE C (33881–34299) | 5′-gagaattcaaagaagaagaagcggcggaa-3′ | 5′-gaggatccggcgaaagagagcaccaaacaatt-3′ |
| PRE D (34288–34667) | 5′-gagaattcgaatggtttgtctcaattgtttggtgc-3′ | 5′-gaggattcccttggcgctctctttcgtttt-3′ |
Figures in parentheses indicate the nucleotide positions in L32205 pertaining to the subfragments in each PRE construct.
The expression vectors for Flag-tagged Zeste (45) and PHO (32), HA-tagged GAGA (52) and HB (53) have been described previously.
Protein procedures
Recombinant proteins Zeste (containing a C-terminal Flag epitope), PHO and HB (each containing an N-terminal Flag epitope) and GAGA (containing an N-terminal HA-tag) were expressed in Sf9 cells using the baculovirus expression system and immunopurified from cell extracts essentially as described (54). Briefly, Sf9 cells were infected at an m.o.i. of ∼5 and harvested 48 h after infection. All protein procedures were carried out at 4°C or on ice using HEMG buffer [25 mM HEPES–KOH pH 7.6, 0.1 mM EDTA, 12.5 mM MgCl2, 10% glycerol, 1 mM DTT, 0.2 mM AEBSF, 1 µM pepstatin, 0.01% Nonidet P-40 (NP-40)] containing varying amounts of KCl. Whole-cell extracts were generated and cleared extracts were incubated with the appropriate anti-Flag M2 beads (Sigma) or protein A Sepharose beads (Pharmacia) covalently conjugated with anti-HA (12CA5) monoclonal antibodies. Bound proteins were eluted with peptides corresponding to either the FLAG or the HA epitope. The purified protein fractions were analysed by SDS–PAGE and visualized by Coomassie blue staining. The protein concentrations of the purified preparations were determined by Bradford assay (BioRad).
DNaseI footprinting
Plasmids PRE A, B, C and D were used either in the form of naked DNA or chromatin as templates for primer extension DNaseI footprinting. Either 100 ng of plasmid or an equimolar amount of chromatin template was incubated in binding buffer (80 mM KCl, 10% glycerol, 25 mM HEPES pH 7.6, 5 mM MgCl2, 0.1% NP-40, 5 µM ZnCl2, 50 ng/µl BSA) in either the absence or presence of recombinant proteins. Following a 45 min incubation at room temperature, samples were treated with DNaseI. For digestion of the chromatin template, a 150-fold higher amount of DNaseI was used than that used for naked DNA digestion. The DNaseI treated samples were phenol–chloroform extracted, ethanol precipitated and analysed by primer extension using a radiolabeled primer (T3) and Klenow, followed by separation on an 8% denaturing polyacrylamide gel and autoradiography. Dideoxy DNA sequencing reactions using the T3 primer were run in parallel in order to identify the protected sequences in the equivalent region of the DNA. Drosophila core histones were purified from fly embryo nuclear extracts essentially as described (55). The S-190 assembly extract was prepared and used for chromatin assembly as described (55,56). This 0–6 h Drosophila embryo cytoplasmic extract contains a host of factors including chromatin remodeling complexes.
RESULTS
Identification of binding elements for sequence-specific DNA binding PcG and trxG proteins within the Ubx PRE
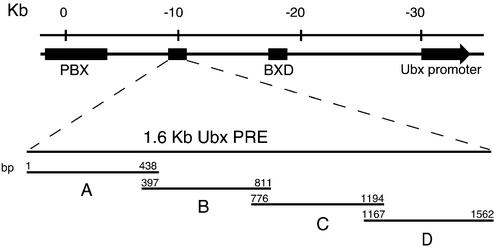
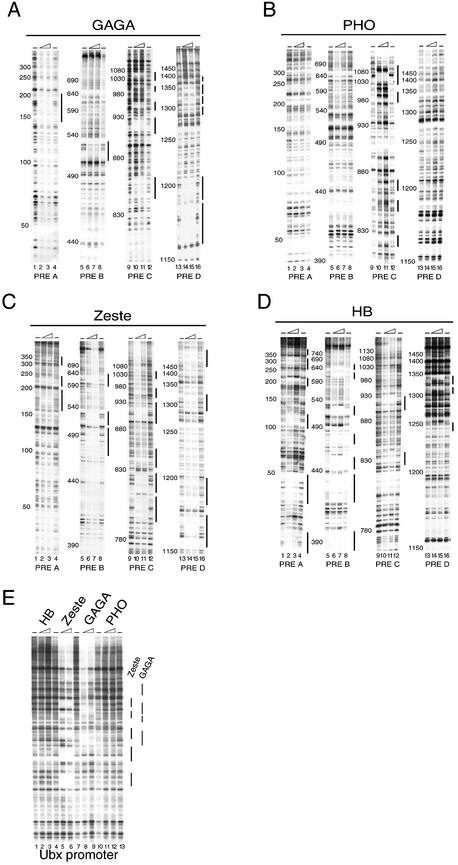
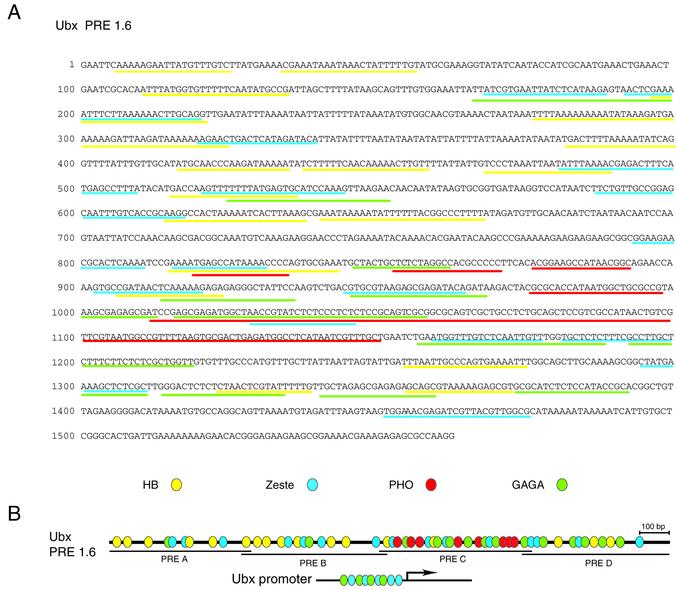
To precisely map the recognition elements of DNA-binding proteins involved in Ubx PRE function, we performed an extensive DNaseI footprinting analysis. As illustrated in Figure 1, the Ubx PRE is a 1.6 kb cis-regulatory sequence, located ∼25 kb upstream of the Ubx transcription start site. Furthermore, we have indicated the positions of the BXD and PBX enhancers, which act during embryogenesis and larval stages, respectively. To facilitate the analysis, we divided the 1.6 kb PRE into four partially overlapping subfragments, named A, B, C and D, and subcloned them into pBluescript (see Materials and Methods for details). We next used a directed approach to determine which of the protected elements corresponded to binding sites for GAGA, Zeste, PHO and HB, which are all involved in Ubx regulation. These proteins were expressed in Sf9 cells using the baculovirus expression system and subsequently immunopurified from cell extracts using either the HA-tag (GAGA) or the FLAG-tag (PHO, Zeste and HB). The protein preparations were analysed by SDS–PAGE followed by Coomassie blue staining (Fig. 2) and used in subsequent DNaseI footprinting experiments. As shown in Figure 3A, we observed multiple areas of protection by GAGA, mainly concentrated within the PRE C and D regions. The location of each of the binding sites was determined by sequencing reactions run in parallel with the DNaseI digestions. As expected, most protected areas harbor a GAGAG core consensus recognition sequence (Fig. 4A). In agreement with results of Fritsch et al. (29), footprinting experiments with PHO identified five areas of protection within the PRE C region (Figs 3B and 4A). One of the footprints corresponds to four binding elements and by sequence comparison we identified seven consensus PHO sites containing the core GCCAT, and one variant element, GCCAC, within the protected regions (Fig. 4A). As illustrated in Figure 4A and B, the PHO (shown in red) and GAGA sites (shown in green) are interdigitated, raising the possibility that these elements may function in a coordinate fashion.
Figure 1.
Schematic representation of the bithorax complex (BX-C) including the pbx/bxd regulatory regions, the Ubx PRE and Ubx promoter. The map coordinates are indicated above in kilobases (kb) and follow the numbering of Bender et al. (70). The Ubx PRE 1.6 is represented schematically as four overlapping fragments termed PRE A, B, C and D, which were separately cloned into pBluescript and used as templates in primer extension DNaseI footprinting experiments. The numbering indicated above each PRE fragment refers to positions (in bp) within the 1.6 kb PRE.
Figure 2.
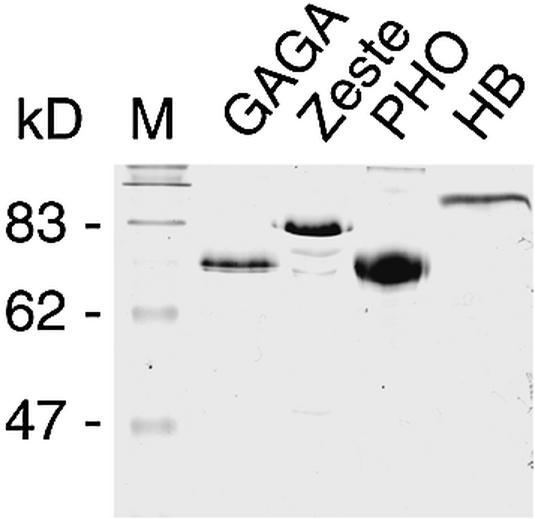
Expression and purification of recombinant GAGA, Zeste, PHO and HB. HA-tagged recombinant GAGA and FLAG-tagged Zeste, PHO and HB were expressed in Sf9 cells and immunopurified from cell extracts using anti-HA or anti-FLAG columns, respectively, and eluted with a peptide corresponding to either the HA or the FLAG epitope. Aliquots of eluted proteins were analysed by SDS–PAGE and visualized with Coomassie Blue staining. The positions and molecular weights (kDa) of the protein standards are indicated on the left.
Figure 3.
(Opposite) Identification of binding sites for regulatory proteins within the Ubx PRE and promoter. The binding of GAGA, PHO, Zeste and HB to the PRE A, B, C and D templates were examined by primer extension DNaseI footprinting. The indicated plasmids harboring the distinct PRE fragments were incubated with increasing amounts of recombinant purified GAGA (A), PHO (B), Zeste (C) and HB (D) or in the absence of protein (– lanes), followed by partial DNaseI digestion. (E) Similar DNaseI footprinting analysis of the Ubx promoter region. The concentration of recombinant protein in these reactions ranged from ∼50 to 150 nM as indicated by the triangles above the lanes. Digestion products were analysed by primer extension, resolved on an 8% denaturing polyacrylamide gel followed by autoradiography. The positions within the PRE, as determined by sequencing reactions run in parallel to the DNaseI reactions, are indicated. Vertical bars indicate regions of protection against DNaseI digestion.
Figure 4.
Distribution of regulator binding sites on the Ubx PRE and promoter. (A) The various binding sites identified by DNaseI footprinting are indicated in the sequence of the Ubx PRE and highlighted in different colors: GAGA (green), Zeste (blue), PHO (red) and HB (yellow). (B) Schematic representation of protein binding sites within the Ubx PRE and promoter.
Footprinting with Zeste resulted in protection of many sites scattered throughout the PRE (Fig. 3C). Surprisingly, only two of the areas of protection harbored a Zeste (T/C)GAG(T/C)G consensus sequence. All other footprints, while containing a core GAG sequence, displayed significant deviations in their flanking sequences (Fig. 4A). While the isolated Zeste DNA-binding domain displays fairly strict sequence requirements (57), these results indicate that cooperative DNA-binding by Zeste oligomers allows binding to a variety of DNA elements. Moreover, we noted that many of the Zeste binding sites are flanked by stretches of A/T rich DNA (Fig. 4A). Although the functional relevance of this observation is unclear, the AT-rich sequences interspersed between Zeste sites may induce intrinsic DNA bending that facilitates binding by Zeste oligomers. We also observed a multitude of HB sites, particularly within the PRE A and B fragments (Fig. 3D). Several of the HB sites are neighboring Zeste recognition elements (Fig. 4A and B). Finally, as shown in Figure 3E, the Ubx promoter itself contains multiple binding sites for GAGA as well as Zeste (see also 58), while no binding was observed by PHO or HB.
In summary, these footprinting studies provide a high-resolution map of binding sites for sequence-specific DNA-binding proteins, which have previously been implicated in PRE function and Ubx regulation by in vivo studies. The binding sites for the distinct PRE-binding proteins are each clustered differently (Fig. 4A and B). Whereas the Zeste elements are found scattered throughout the 1.6 kb PRE, all PHO sites are grouped within the PRE C portion. Most GAGA elements are found within PRE fragments C and D whereas the HB sites are located mostly within PRE A and B. In general, the highest density of binding sites for GAGA as well as all PHO sites are found within PRE C. Interestingly, the PHO and GAGA sites are interdigitated and, albeit to a lesser extent, Zeste elements are found associated with HB and PHO sites. In addition, the presence of Zeste and GAGA elements in both the Ubx PRE and the Ubx promoter might be reflective of a role for these factors in long-range PRE-promoter interactions (see Discussion).
GAGA is required for PHO binding to chromatin
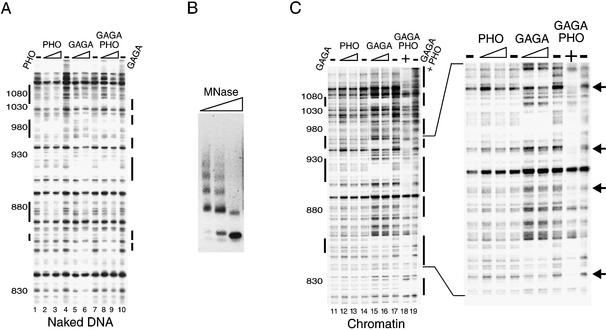
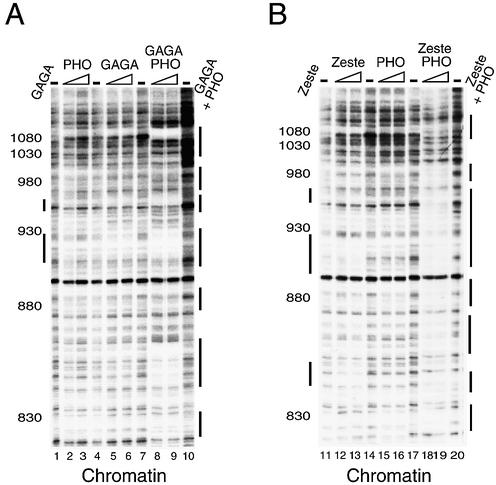
Previous genetic studies suggested that PHO and GAGA cooperate to mediate stable PRE-directed silencing during Drosophila development (28). Because the PHO and GAGA elements are interlaced within the Ubx PRE, we were interested in determining whether this collaboration might occur at the level of PRE binding. To test this hypothesis, we performed a series of DNaseI footprinting experiments either on naked DNA or on a chromatinized PRE C template, as this region of the Ubx PRE contains all PHO sites and the highest concentration of GAGA elements. As shown in Figure 5A, when added either alone or together, PHO (lanes 2–3) as well as GAGA (lanes 5–6) efficiently bind their cognate sites within PRE C in the form of naked DNA. Importantly, we did not observe any cooperation between GAGA and PHO during binding to naked DNA (compare lanes 1–10). The Drosophila embryo-derived S-190 assembly system (56) was used to assemble the PRE C plasmid into an array of regularly spaced nucleosomes, as shown by a partial micrococcal nuclease digestion of the chromatin (Fig. 5B). It is pertinent to note that chromatin footprinting is performed in the presence of much higher amounts of DNaseI than naked DNA footprinting. Consequently, any naked DNA that might be present in the reaction is completely digested under the conditions used (data not shown). We used this template to test the ability of GAGA and PHO to bind to a chromatinized PRE (Fig. 5C). Whereas GAGA was capable of efficiently binding a chromatinized PRE (lanes 15–16), PHO failed to bind to its sites when assembled into chromatin (lanes 12–13). Even in the presence of an almost 10-fold higher PHO concentration than needed to protect the PHO sites on naked DNA, no chromatin binding was detected. Thus, assembly of DNA into chromatin creates a barrier to PRE binding by PHO but not by GAGA. Strikingly, in the presence of GAGA, an amount of PHO incapable of binding chromatin by itself is now sufficient to completely occupy all PHO sites (Fig. 5C, compare lanes 13 and 18). Because the cooperation between GAGA and PHO was not observed on naked DNA (Fig. 5A), and we failed to detect a direct protein–protein interaction between GAGA and PHO (data not shown), we conclude that chromatin remodeling induced by GAGA binding allows subsequent DNA binding by PHO. Finally, the addition of suboptimal amounts of GAGA to the footprinting reactions revealed that the cooperation between PHO and GAGA was reciprocal (Fig. 6A). Under these conditions, not only was the binding of PHO enhanced, but also a higher occupancy at the GAGA sites was observed.
Figure 5.
GAGA facilitates PHO binding to a chromatinized PRE. (A) GAGA and PHO do not cooperate during binding to naked DNA. The binding of GAGA and PHO to the PRE C template was examined by primer extension DNaseI footprinting. The PRE C plasmid was incubated with increasing amounts of either recombinant purified PHO alone, GAGA alone, both PHO and GAGA or in the absence of protein (– lanes), followed by partial DNaseI digestion. The concentrations of recombinant proteins in these reactions ranged from ∼50 to 150 nM as indicated by the triangles above the lanes. Digestion products were analysed by primer extension, resolved on an 8% denaturing polyacrylamide gel followed by autoradiography. The positions within the PRE, as determined by sequencing reactions run in parallel to the DNaseI reactions, are indicated. Vertical bars indicate regions of protection against DNaseI digestion. (B) The PRE C template was assembled into chromatin and visualized by MNase digestion. After completion of chromatin assembly using the Drosophila embryo S-190 assembly system, the template was digested with increasing amounts of MNase and the digestion patterns were visualized by ethidium bromide staining of agarose gel. (C) GAGA facilitates PHO binding to chromatin. DNaseI footprinting analysis of GAGA and PHO (at higher concentrations) binding to a chromatinized PRE C template. Following completion of assembly, chromatin was incubated for 45 min in either the absence (– lanes) or presence of increasing concentrations of GAGA and PHO (ranging from ∼200 to 400 nM) as indicated by the triangles above the lanes. The reaction shown in lane 18 contains 400 nM of each protein). Arrows indicate bands corresponding to regions bound by PHO. For DNaseI digestion of the chromatin template, a 150-fold higher amount of DNaseI was used than that used for digestion of naked DNA. The DNaseI digestion pattern was visualized by primer extension and separation on an 8% acrylamide gel followed by autoradiography. Footprints are indicated with bars located to the left and right of the autoradiograms.
Figure 6.
GAGA and Zeste facilitate PHO binding to a chromatinized PRE. DNaseI footprinting analysis of (A) GAGA and PHO and (B) Zeste and PHO binding to chromatin in the presence of either ∼50 or ∼150 nM of each protein as indicated by triangles above the lanes. The DNaseI digestion pattern was visualized by primer extension and separation on an 8% acrylamide gel followed by autoradiography. Footprints are indicated with bars located to the left and right of the autoradiograms.
Given the abundance of Zeste sites within PRE C, we also tested whether Zeste and PHO display cooperativity in binding to chromatin. Similar to GAGA, we found that the presence of sub-optimal amounts of Zeste and PHO, which alone do not efficiently bind chromatin, together result in binding to all PHO and Zeste sites within the chromatinized PRE C (Fig. 6B). In conclusion, these results suggest that the trxG proteins GAGA and Zeste are required for binding of the PcG protein PHO to a chromatin-assembled PRE. This finding provides a simple molecular explanation for the in vivo cooperation between PHO and GAGA during PRE-mediated silencing. Thus, already on the level of binding to the chromatinized PRE, there appears to be extensive cross-talk between genetically identified factors involved in positive as well as negative regulation of PRE activity.
DISCUSSION
Maintenance of the mitotically stable active or repressed state of homeotic genes in Drosophila requires the association of PcG and trxG proteins with cis-acting PREs. The 1.6 kb Ubx PRE is critical for the maintenance of correct expression of the Ubx gene. Here, we have determined the precise distribution within this PRE of the recognition elements for four sequence-specific DNA-binding proteins that have all been implicated in Ubx regulation in vivo: PcG protein PHO, gap protein HB and trxG proteins GAGA and Zeste. Our results indicate that, rather than a random collection, the binding site distribution within the Ubx PRE reflects a functional arrangement, allowing cooperation between distinct PRE binding proteins. Of particular interest is our observation that chromatin binding by the PcG protein PHO is strongly facilitated by the trxG protein GAGA. This finding provides a molecular mechanism for the requirement for both factors during PRE-directed silencing in vivo (28,30), and suggests that PHO and GAGA elements together may form a functional module.
Several independent genetic studies have pointed to a concurrent requirement for GAGA and PHO during gene silencing directed by distinct PREs. The PcG-dependent silencing conferred by a 230 bp fragment of the iab-7 PRE is dependent on both GAGA and PHO binding (30). Similarly, a 138 bp fragment of the MCP silencer, which was found to be sufficient for maintenance of embryonic silencing, contains PHO and GAGA sites (28). Mutations in either PHO or GAGA sites compromised silencing and revealed cooperation between both proteins. Particularly relevant for the current study are results that support a critical role in PcG silencing for GAGA and PHO sites within the Ubx PRE (29,40,41,50).
Functional dissection of the Ubx PRE by Fritsch et al. (29) revealed that a Pc-dependent PRE silencer is contained in the central 567 bp fragment from position 577 to 1143 (see Fig. 4A), which includes all PHO and the highest density of GAGA sites. Another study showed that an oligomerized subfragment, corresponding to positions 890–1079 within PRE C (Fig. 4A), harboring two PHO and five GAGA elements, was able to confer PcG silencing in vivo (40). Finally, deletion of a 160 bp region corresponding to positions 851–1011 within PRE C (Fig. 4A) impaired maintenance of silencing (40,50). The large extent of overlap between the DNA fragments identified in these independent studies strongly suggests that the common region within PRE C represents the critical core of the Ubx PRE. The most noticeable feature of this region is the many alternating GAGA and PHO binding elements. Moreover, it is of interest to note that our footprinting analysis revealed the presence of Zeste as well as HB sites within this region, which may also contribute to the in vivo maintenance of repression.
The identification of Zeste as a component of the PRC1 PcG complex (46), suggests that it may play a direct role in PcG complex recruitment to the Ubx PRE. Further evidence for the involvement of Zeste in the maintenance of Ubx repression as well as activation has been provided by recent transgene experiments (42). Finally, the presence of HB sites within the Ubx PRE suggests a potential role for HB, not only during the initiation of Ubx repression, but also during the transition from establishment to maintenance. One attractive possibility is that this transition involves dMi-2 recruitment by HB (25). It should be noted that in the absence of initiating activation and repression elements, HB-independent PcG repression of the Ubx promoter could be established (59).
Although there is substantial evidence for the notion that the proteins discussed above are involved in PcG silencing of homeotic genes, it remains unclear whether they can be sufficient for targeting or whether additional factors are required. One way to determine a minimal set of protein recognition sequences that can mediate PcG silencing will be the generation of synthetic PREs, which should be tested in vivo. Our results suggest that, within such a PRE, PHO sites will need to be flanked by GAGA sites in order to facilitate chromatin binding. The proteins GAGA and Zeste may be particularly well adapted for such a purpose. Both GAGA and Zeste form large homo-oligomers that bind cooperatively to the multiple sites present in their natural response elements, such as the Ubx PRE and promoter (52,60,61). This cooperative mode of DNA-binding may allow these proteins to first bind an accessible site within a nucleosomal array and then progressively displace histones during binding to flanking sites (62). In addition, GAGA and Zeste have both been shown to recruit selective ATP-dependent chromatin remodeling factors (45,63,64). The process of targeting of remodelers to specific DNA elements may enable GAGA and Zeste to create nucleosome-free or remodeled areas, thus facilitating binding of other regulators. We consider it likely that the remodeling complexes present in the chromatin preparations used in our assays, are involved in the observed synergistic binding between PHO and either GAGA or Zeste.
GAGA oligomerization may also promote the communication between the Ubx PRE and promoter. Both elements, which are separated by ∼24 kb of intervening DNA, contain a preponderance of binding sites for GAGA (Fig. 4B). We have recently demonstrated that GAGA oligomerization through its POZ domain allows it to form a protein bridge that directs long-range enhancer–promoter association (65). In fact, GAGA could even mediate enhancer function in trans by simultaneous binding of two separate DNA fragments. Thus, it is tempting to speculate that GAGA may link the Ubx PRE to the Ubx promoter. It should be noted that both the chromatin remodeling and long-range bridging functions of GAGA might accommodate PRE-mediated activation as well as repression.
The interdependence between proteins belonging to antagonistic genetic groups for efficient chromatin binding described here will have to be taken into account when interpreting mutational analysis of PRE function. Thus, removal of recognition sequences for the trxG protein GAGA may block its activation function but could also affect binding of the PcG protein PHO. Moreover, recent results suggest additional opportunities for cross-talk during recruitment of non-DNA-binding PcG complexes. Although a clear consensus between different studies is still lacking, there is experimental evidence for PcG complex recruitment by PHO, GAGA and Zeste (32,40,46). Because binding sites for either one of these proteins alone do not confer PRE function, it appears likely that they work in a combinatorial fashion. Depending on their context, the multitude of distinct binding elements that constitute a PRE might be redundant, cooperative or antagonistic to each other. Furthermore, distinct PREs may require different sets of PRE-binding proteins, and additional recruiters may be involved in PcG-silencing. Attractive candidates are GAGA-related factors batman (66) and pipsqueak (67) and the PHO-related factor PHO-like (68).
In conclusion, current evidence suggests that PRE-directed maintenance of gene activation or repression is not achieved by a simple binary switch set by competing trxG and PcG proteins. Although their relative ratios vary considerably and correlate with transcription levels, they coexist at PREs during gene activation as well as repression (18–20,39,50,69). Likewise, genetic suppressor studies indicated extensive cross-talk between PcG and trxG proteins (reviewed in 2). Here we have shown that, already at the level of PRE binding, there is strong interdependence between trxG protein GAGA and PcG protein PHO. Our results demonstrate a direct biochemical mechanism for the cooperation between PcG and trxG proteins during PRE binding.
Acknowledgments
ACKNOWLEDGEMENTS
We thank J. Arredondo, E. Kalkhoven, I. Oruetxebarria, K. R. Katsani and R. Vries for comments on the manuscript. This work was supported in part by a grant from the Dutch Cancer society KWF.
REFERENCES
- 1.Bienz M. and Muller,J. (1995) Transcriptional silencing of homeotic genes in Drosophila. Bioessays, 17, 775–784. [DOI] [PubMed] [Google Scholar]
- 2.Brock H.W. and van Lohuizen,M. (2001) The Polycomb group–no longer an exclusive club? Curr. Opin. Genet. Dev., 11, 175–181. [DOI] [PubMed] [Google Scholar]
- 3.Francis N.J. and Kingston,R.E. (2001) Mechanisms of transcriptional memory. Nature Rev. Mol. Cell Biol., 2, 409–421. [DOI] [PubMed] [Google Scholar]
- 4.Kennison J.A. (1995) The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu. Rev. Genet., 29, 289–303. [DOI] [PubMed] [Google Scholar]
- 5.Lyko F. and Paro,R. (1999) Chromosomal elements conferring epigenetic inheritance. Bioessays, 21, 824–832. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudi T. and Verrijzer,C.P. (2001) Chromatin silencing and activation by Polycomb and trithorax group proteins. Oncogene, 20, 3055–3066. [DOI] [PubMed] [Google Scholar]
- 7.Pirrotta V. (1998) Polycombing the genome: PcG, trxG, and chromatin silencing. Cell, 93, 333–336. [DOI] [PubMed] [Google Scholar]
- 8.Simon J.A. and Tamkun,J.W. (2002) Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr. Opin. Genet. Dev., 12, 210–218. [DOI] [PubMed] [Google Scholar]
- 9.Busturia A. and Bienz,M. (1993) Silencers in abdominal-B, a homeotic Drosophila gene. EMBO J., 12, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busturia A., Wightman,C.D. and Sakonju,S. (1997) A silencer is required for maintenance of transcriptional repression throughout Drosophila development. Development, 124, 4343–4350. [DOI] [PubMed] [Google Scholar]
- 11.Chan C.S., Rastelli,L. and Pirrotta,V. (1994) A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J., 13, 2553–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang A., O’Connor,M.B., Paro,R., Simon,J. and Bender,W. (1995) Discrete Polycomb-binding sites in each parasegmental domain of the bithorax complex. Development, 121, 1681–1689. [DOI] [PubMed] [Google Scholar]
- 13.Hagstrom K., Muller,M. and Schedl,P. (1997) A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax complex. Genetics, 146, 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller J. and Bienz,M. (1991) Long range repression conferring boundaries of Ultrabithorax expression in the Drosophila embryo. EMBO J., 10, 3147–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon J., Peifer,M., Bender,W. and O’Connor,M. (1990) Regulatory elements of the bithorax complex that control expression along the anterior-posterior axis. EMBO J., 9, 3945–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gindhart J.G. Jr, and Kaufman,T.C. (1995) Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics, 139, 797–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zink D. and Paro,R. (1995) In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. EMBO J., 14, 5660–5671. [DOI] [PubMed] [Google Scholar]
- 18.Orlando V., Jane,E.P., Chinwalla,V., Harte,P.J. and Paro,R. (1998) Binding of trithorax and Polycomb proteins to the bithorax complex: dynamic changes during early Drosophila embryogenesis. EMBO J., 17, 5141–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlando V. and Paro,R. (1993) Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin. Cell, 75, 1187–1198. [DOI] [PubMed] [Google Scholar]
- 20.Strutt H., Cavalli,G. and Paro,R. (1997) Co-localization of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J., 16, 3621–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassis J.A. (1994) Unusual properties of regulatory DNA from the Drosophila engrailed gene: three ‘pairing-sensitive’ sites within a 1.6-kb region. Genetics, 136, 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassis J.A., VanSickle,E.P. and Sensabaugh,S.M. (1991) A fragment of engrailed regulatory DNA can mediate transvection of the white gene in Drosophila. Genetics, 128, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller M., Hagstrom,K., Gyurkovics,H., Pirrotta,V. and Schedl,P. (1999) The mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics, 153, 1333–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigrist C.J. and Pirrotta,V. (1997) Chromatin insulator elements block the silencing of a target gene by the Drosophila polycomb response element (PRE) but allow trans interactions between PREs on different chromosomes. Genetics, 147, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehle J., Beuchle,D., Treuheit,S., Christen,B., Kennison,J.A., Bienz,M. and Muller,J. (1998) dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science, 282, 1897–1900. [DOI] [PubMed] [Google Scholar]
- 26.Brown J.L., Mucci,D., Whiteley,M., Dirksen,M.L. and Kassis,J.A. (1998) The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol. Cell, 1, 1057–1064. [DOI] [PubMed] [Google Scholar]
- 27.Mihaly J., Mishra,R.K. and Karch,F. (1998) A conserved sequence motif in Polycomb-response elements. Mol. Cell, 1, 1065–1066. [DOI] [PubMed] [Google Scholar]
- 28.Busturia A., Lloyd,A., Bejarano,F., Zavortink,M., Xin,H. and Sakonju,S. (2001) The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development, 128, 2163–2173. [DOI] [PubMed] [Google Scholar]
- 29.Fritsch C., Brown,J.L., Kassis,J.A. and Muller,J. (1999) The DNA-binding polycomb group protein pleiohomeotic mediates silencing of a Drosophila homeotic gene. Development, 126, 3905–3913. [DOI] [PubMed] [Google Scholar]
- 30.Mishra R.K., Mihaly,J., Barges,S., Spierer,A., Karch,F., Hagstrom,K., Schweinsberg,S.E. and Schedl,P. (2001) The iab-7 polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol. Cell. Biol., 21, 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimell M.J., Peterson,A.J., Burr,J., Simon,J.A. and O’Connor,M.B. (2000) Functional analysis of repressor binding sites in the iab-2 regulatory region of the abdominal-A homeotic gene. Dev. Biol., 218, 38–52. [DOI] [PubMed] [Google Scholar]
- 32.Mohd-Sarip A., Venturini,F., Chalkley,G.E. and Verrijzer,C.P. (2002) Pleiohomeotic can link polycomb to DNA and mediate transcriptional repression. Mol. Cell. Biol., 22, 7473–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkas G., Leibovitch,B.A. and Elgin,S.C. (2000) Chromatin organization and transcriptional control of gene expression in Drosophila. Gene, 253, 117–136. [DOI] [PubMed] [Google Scholar]
- 34.Granok H., Leibovitch,B.A., Shaffer,C.D. and Elgin,S.C. (1995) Chromatin. Ga-ga over GAGA factor. Curr. Biol., 5, 238–241. [DOI] [PubMed] [Google Scholar]
- 35.Wilkins R.C. and Lis,J.T. (1997) Dynamics of potentiation and activation: GAGA factor and its role in heat shock gene regulation. Nucleic Acids Res., 25, 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhat K.M., Farkas,G., Karch,F., Gyurkovics,H., Gausz,J. and Schedl,P. (1996) The GAGA factor is required in the early Drosophila embryo not only for transcriptional regulation but also for nuclear division. Development, 122, 1113–1124. [DOI] [PubMed] [Google Scholar]
- 37.Farkas G., Gausz,J., Galloni,M., Reuter,G., Gyurkovics,H. and Karch,F. (1994) The Trithorax-like gene encodes the Drosophila GAGA factor. Nature, 371, 806–808. [DOI] [PubMed] [Google Scholar]
- 38.Poux S., Horard,B., Sigrist,C.J. and Pirrotta,V. (2002) The Drosophila Trithorax protein is a coactivator required to prevent re-establishment of Polycomb silencing. Development, 129, 2483–2493. [DOI] [PubMed] [Google Scholar]
- 39.Cavalli G. and Paro,R. (1998) Chromo-domain proteins: linking chromatin structure to epigenetic regulation. Curr. Opin. Cell Biol., 10, 354–360. [DOI] [PubMed] [Google Scholar]
- 40.Horard B., Tatout,C., Poux,S. and Pirrotta,V. (2000) Structure of a polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol. Cell. Biol., 20, 3187–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodgson J.W., Argiropoulos,B. and Brock,H.W. (2001) Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid polycomb group response element-dependent silencing. Mol. Cell. Biol., 21, 4528–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hur M.W., Laney,J.D., Jeon,S.H., Ali,J. and Biggin,M.D. (2002) Zeste maintains repression of Ubx transgenes: support for a new model of Polycomb repression. Development, 129, 1339–1343. [DOI] [PubMed] [Google Scholar]
- 43.Laney J.D. and Biggin,M.D. (1992) zeste, a nonessential gene, potently activates Ultrabithorax transcription in the Drosophila embryo. Genes Dev., 6, 1531–1541. [DOI] [PubMed] [Google Scholar]
- 44.Rastelli L., Chan,C.S. and Pirrotta,V. (1993) Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J., 12, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kal A.J., Mahmoudi,T., Zak,N.B. and Verrijzer,C.P. (2000) The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev., 14, 1058–1071. [PMC free article] [PubMed] [Google Scholar]
- 46.Saurin A.J., Shao,Z., Erdjument-Bromage,H., Tempst,P. and Kingston,R.E. (2001) A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature, 412, 655–660. [DOI] [PubMed] [Google Scholar]
- 47.Pelegri F. and Lehmann,R. (1994) A role of polycomb group genes in the regulation of gap gene expression in Drosophila. Genetics, 136, 1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips M.D. and Shearn,A. (1990) Mutations in polycombeotic, a Drosophila polycomb-group gene, cause a wide range of maternal and zygotic phenotypes. Genetics, 125, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christen B. and Bienz,M. (1994) Imaginal disc silencers from Ultrabithorax: evidence for Polycomb response elements. Mech. Dev., 48, 255–266. [DOI] [PubMed] [Google Scholar]
- 50.Tillib S., Petruk,S., Sedkov,Y., Kuzin,A., Fujioka,M., Goto,T. and Mazo,A. (1999) Trithorax- and Polycomb-group response elements within an Ultrabithorax transcription maintenance unit consist of closely situated but separable sequences. Mol. Cell. Biol., 19, 5189–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C.C. and Bienz,M. (1992) Segmental determination in Drosophila conferred by hunchback (hb), a repressor of the homeotic gene Ultrabithorax (Ubx). Proc. Natl Acad. Sci. USA, 89, 7511–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katsani K.R., Hajibagheri,M.A. and Verrijzer,C.P. (1999) Co-operative DNA binding by GAGA transcription factor requires the conserved BTB/POZ domain and reorganizes promoter topology. EMBO J., 18, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauer F., Hansen,S.K. and Tjian,R. (1995) DNA template and activator-coactivator requirements for transcriptional synergism by Drosophila bicoid. Science, 270, 1825–1828. [DOI] [PubMed] [Google Scholar]
- 54.Chen J.L. and Tjian,R. (1996) Reconstitution of TATA-binding protein-associated factor/TATA-binding protein complexes for in vitro transcription. Methods Enzymol., 273, 208–217. [DOI] [PubMed] [Google Scholar]
- 55.Bulger M. and Kadonaga,J.T. (1994) Biochemical reconstitution of chromatin with physiological nucleosome spacing. Methods Mol. Genet., 5, 241–262. [Google Scholar]
- 56.Kamakaka R.T., Bulger,M. and Kadonaga,J.T. (1993) Potentiation of RNA polymerase II transcription by Gal4-VP16 during but not after DNA replication and chromatin assembly. Genes Dev., 7, 1779–1795. [DOI] [PubMed] [Google Scholar]
- 57.Mohrmann L., Kal,A.J. and Verrijzer,C.P. (2002) Characterization of the Extended Myb-like DNA-binding Domain of Trithorax Group Protein Zeste. J. Biol. Chem., 277, 47385–47392. [DOI] [PubMed] [Google Scholar]
- 58.Biggin M.D., Bickel,S., Benson,M., Pirrotta,V. and Tjian,R. (1988) Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell, 53, 713–722. [DOI] [PubMed] [Google Scholar]
- 59.Poux S., Kostic,C. and Pirrotta,V. (1996) Hunchback-independent silencing of late Ubx enhancers by a Polycomb Group Response Element. EMBO J., 15, 4713–4722. [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J.D. and Pirrotta,V. (1993) Multimerization of the Drosophila zeste protein is required for efficient DNA binding. EMBO J., 12, 2075–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espinas M.L., Jimenez-Garcia,E., Vaquero,A., Canudas,S., Bernues,J. and Azorin,F. (1999) The N-terminal POZ domain of GAGA mediates the formation of oligomers that bind DNA with high affinity and specificity. J. Biol. Chem., 274, 16461–16469. [DOI] [PubMed] [Google Scholar]
- 62.Utley R.T., Cote,J., Owen-Hughes,T. and Workman,J.L. (1997) SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. J. Biol. Chem., 272, 12642–12649. [DOI] [PubMed] [Google Scholar]
- 63.Tsukiyama T., Becker,P.B. and Wu,C. (1994) ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature, 367, 525–532. [DOI] [PubMed] [Google Scholar]
- 64.Xiao H., Sandaltzopoulos,R., Wang,H.M., Hamiche,A., Ranallo,R., Lee,K.M., Fu,D. and Wu,C. (2001) Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol. Cell, 8, 531–543. [DOI] [PubMed] [Google Scholar]
- 65.Mahmoudi T., Katsani,K.R. and Verrijzer,C.P. (2002) GAGA can mediate enhancer function in trans by linking two separate DNA molecules. EMBO J., 21, 1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faucheux M., Roignant,J.Y., Netter,S., Charollais,J., Antoniewski,C. and Theodoer,L. (2003) batman interacts with polycomb and trithorax group genes and encodes a BTB/POZ protein that is included in a complex containing GAGA factor. Mol. Cell. Biol. 23, 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang D.H., Chang,Y.L., Yang,C.C., Pan,I.C. and King,B. (2002) pipsqueak encodes a factor essential for sequence-specific targeting of a polycomb group protein complex. Mol. Cell. Biol., 22, 6261–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown J.L., Fritsch,C., Mueller,J. and Kassis,J.A. (2003) The Drosophila pho-like gene encodes a YY1-related DNA binding protein that is redundant with pleiohomeotic in homeotic gene silencing. Development, 130, 285–294. [DOI] [PubMed] [Google Scholar]
- 69.Chinwalla V., Jane,E.P. and Harte,P.J. (1995) The Drosophila trithorax protein binds to specific chromosomal sites and is co-localized with Polycomb at many sites. EMBO J., 14, 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bender W., Akam,M., Karch,F., Beachy,P., Peifer,M., Spierer,P., Lewis,E. and Hogness,D. (1983) Molecular genetics of the bithorax complex in Drosophila melanogaster. Science, 221, 23–29. [DOI] [PubMed] [Google Scholar]