Abstract
The site-specific recombinase Cre has often been used for on/off regulation of expression of transgenes introduced into the mammalian chromosome. However, this method is only applicable to the regulation of a single gene and cannot be used to simultaneously regulate two genes, because site-specific recombination occurs from the target loxP sequence of one regulating unit to the loxP sequence of any other unit and would eventually disrupt the structure of both regulating units. We previously reported a mutant loxP sequence with a two base substitution called loxP V (previously called loxP 2272), with which wild-type loxP cannot recombine but with which the identical mutant loxP recombines efficiently. In the present study we isolated cell lines bearing two regulating units on a chromosome containing a pair of wild-type loxP sequences or mutant loxP V sequences. After infection with Cre-expressing recombinant adenovirus AxCANCre, expression of a gene in each regulating unit was simultaneously turned on and off. Southern analyses showed that both regulating units were processed simultaneously and independently, even after infection with a limited amount of AxCANCre. The results showed that simultaneous regulation of gene expression on a mammalian chromosome mediated by Cre can be achieved by using mutant loxP V and wild-type loxP. This method may be a useful approach for conditional transgenic/knockout animals and investigation of gene function involving two genes simultaneously. Another possible application is for preparation of a new packaging cell line of viral vectors through simultaneous production of toxic viral genes.
INTRODUCTION
Methods of regulating the genes introduced into mammalian cell chromosomes have recently been attracting greater attention in fields involved with elucidation of gene function by using established cell lines and the transgenic/knockout mouse approach as well as gene therapy. The site-specific recombinase Cre (1), derived from bacteriophage P1, has been widely applied to the activation and inactivation of transgenes located on mammalian chromosomes in cell lines (for reviews see 2–5). This strategy includes conditional activation or knockout of mouse genes mediated by a Cre-expressing recombinant adenovirus (rAd) (6,7). Cre-mediated gene regulation in these applications is affected by precise excision of ‘stuffer’ DNA or target cDNA flanked by a pair of 34 nt loxP target sequences in the regulation unit, and since it enables quite strict regulation with a very low level of background expression, it is sometimes referred to as ‘on/off switching’ rather than ‘induction’ of gene expression. Although this strategy allows any type of promoter to be used, for example an authentic promoter of the target gene in a conditional knockout strategy (7–11) or a very potent and constitutive CAG or CMV promoter (6,12–15), only a single regulation unit in each study has been used to date. A clear drawback of this strategy is that two or more regulation units cannot be used simultaneously. When two regulation units, each containing a pair of loxP sequences, are located on the same chromosome, Cre-mediated recombination between the loxP sequences will occur not only within a single unit but between the units, and as a result will eventually disrupt both regulation units. Even when the two units are located on different chromosomes, Cre-mediated recombination will cause chromosome rearrangement (16,17), making interpretation complex. However, experiments using two regulation units are sometimes desirable to simultaneously conditionally knockout two genes with related or homologous function.
‘Exclusive’ or ‘non-compatible’ loxP mutants have been identified as a means of solving this problem. Ideally, such loxP mutants would not recombine with wild-type loxP, but would exclusively and efficiently recombine with the identical loxP mutant. Thus, one regulation unit containing a pair of wild-type loxP sites and the other bearing a pair of the mutant loxP sites would be expected to function independently and simultaneously. loxP 511, which contains a one base substitution within the central eight base region of the 34 nt loxP sequence, was first described as a candidate for such an ‘exclusive’ loxP mutant (18–21), however, we reported finding that mutant loxP sequences with a one base substitution, including loxP 511, slightly but reproducibly recombined with wild-type loxP in our in vitro recombination (22). Most mutant loxP sites with a two base substitution exhibited high fidelity against wild-type loxP but low efficiency of recombination with the identical mutant loxP. A loxP mutant with high fidelity and efficiency, loxP 2272 (renamed loxP V in this paper; V denoting transversion mutation), was newly identified among mutant loxP sequences having a two base substitution (22), but simultaneous gene regulation using both a wild-type loxP unit and a mutant loxP unit on mammalian chromosomes has never been characterized. In addition, it was unknown whether the mutant loxP unit could be successfully processed in the same way as the wild-type loxP unit, because recombination efficiency between the mutant loxP sites with the two base substitution, even loxP V, has been reported to be lower than between wild-type loxP sites in vitro (22).
In this study we used an adenovirus vector expressing Cre (23) and demonstrated simultaneous regulation with the wild-type loxP unit and mutant loxP V unit on a mammalian chromosome.
MATERIALS AND METHODS
Cell lines and rAd
Human embryo kidney cell line 293 (24) and monkey kidney cell line CV1 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). The 293 cells constitutively express adenoviral E1 genes and support replication of E1-substituted rAds. After infection with rAds, the 293 cells were maintained in DMEM supplemented with 5% FCS. CV1 cell lines were isolated and maintained in DMEM supplemented with 10% FCS plus 10 and 7.5 mg/ml Geneticin, respectively. Typically, 4.5 × 106 cells of an established cell line were infected with a diluted virus solution of prescribed multiplicity of infection (MOI). The virus solution was removed after adsorption for 1 h and infected cells were maintained in DMEM supplemented with 5% FCS. The Cre-expressing rAd AxCANCre (23) was generated by the COS-TPC method (25). The Cre recombinase gene (1) used here has an initiation codon that complies with Kozak’s rule (26) and the coding sequence of the nuclear localization signal (NLS) from SV40 T antigen (23). The Cre gene is expressed under the control of a CAG promoter (27) consisting of the cytomegalovirus IE enhancer, chicken β-actin promoter and rabbit β-globin poly(A) signal (GpA).
Construction of target plasmids
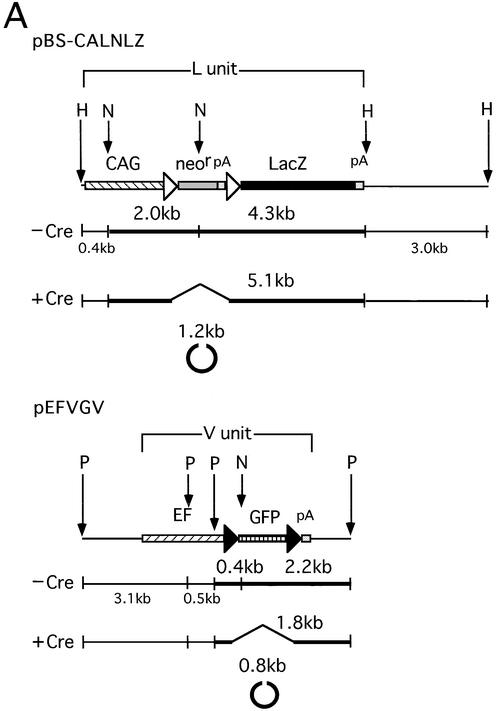
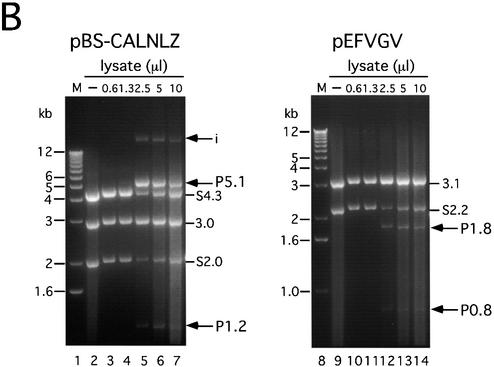
pBS-CALNLZ (Fig. 1A, upper) is a pBluescript II SK(–) (Stratagene) vector containing a wild-type loxP (referred to as the L sequence) unit CALNLZ derived from plasmid pCALNLZ (23). The L unit consists of the CAG promoter, first L sequence, neo resistance gene, SV40 early region poly(A) signal (SpA), second L sequence, a LacZ coding sequence and GpA, in that order. pEFVGV (Fig. 1A, lower) is a plasmid containing a mutant loxP V (referred to as the V sequence) unit EFVGV consisting of the human polypeptide elongation factor 1α (EF1α) promoter (28), first V sequence, green fluorescent protein (GFP) gene, second V sequence and SpA, in that order. The mutant loxP V is identical to loxP 2272 (22) and has two transversion mutations within the eight base spacer region ATGTATGC of wild-type loxP: T→A at the second base and G→C at the seventh base. The V sequence was derived from a synthesized DNA of 56 nt (5′-AAATcgtacg[BsiWI]ATAACTTCGTATAAAGTA TCCTATACGAAGTTATcttaag[AflII]AAAT-3′), where the 34 nt functional loxP V (underlined) was flanked by BsiWI and AflII sites (lower case letters). A head-to-tail dimer of this sequence was cloned at the SmaI site of pUC19 (named pUVwV) and used as a donor of the V sequence for the above plasmid construction. The sequence was verified by nucleotide sequencing. The GFP gene (29) was derived from Enhanced GFP (Clontech). pEFVGV-CALNLZ (Fig. 2A, upper) contains the V unit derived from pEFVGV and the L unit from pBS-CALNLZ, in that order. pVEFGV-CALNLZ (Fig. 2A, lower) contains another V unit consisting of the first V sequence, the EF1α promoter, the GFP gene, SpA and the second V sequence, followed by the L unit from pBS-CALNLZ, in that order. A 5 µg sample of these two plasmids was transfected into 1.5 × 106 CV1 cells after linearization with ScaI using CellPhect transfection kit (Amersham Biosciences).
Figure 1.
In vitro recombination assay for comparison between the L target unit and V target unit. (A) Structure of substrate plasmids. (Top) Plasmid pBS-CALNLZ, substrate for L-L recombination. The Cre-mediated excisional deletion between a pair of L sequences removes both a neo resistance gene (neor) and SV40 early region poly(A) signal (pA) in the form of a circular DNA molecule. As a result of Cre recombination, a 5.1 kb band derived from outside the pair of L sequences and a 1.2 kb band derived from the NcoI-linearized circular molecule were detected. (Bottom) Plasmid pEFVGV, substrate for V-V recombination. The Cre-mediated excisional deletion between a pair of V sequences removes GFP cDNA in the form of a circular DNA molecule. As a result of Cre recombination, a 1.8 kb band derived from outside the pair of V sequences and a 0.8 kb band derived from the NcoI-linearized circular molecule were detected. N, NcoI; H, HindIII; P, PstI. See Figure 2 for the other abbreviations on the line of plasmid DNA. (B) Quantitation of in vitro recombination products. M (lanes 1 and 8), 1 kb Ladder Marker (Gibco BRL). Above the gel, amounts of Cre lysate are shown, and – indicates the negative control. After adding 10 µl of the Cre lysate to the reaction mixture, phenol/chloroform extraction was carried out to inactivate the Cre and the substrate DNA was added. S, substrate; i, intermediate; P, product.
Figure 2.
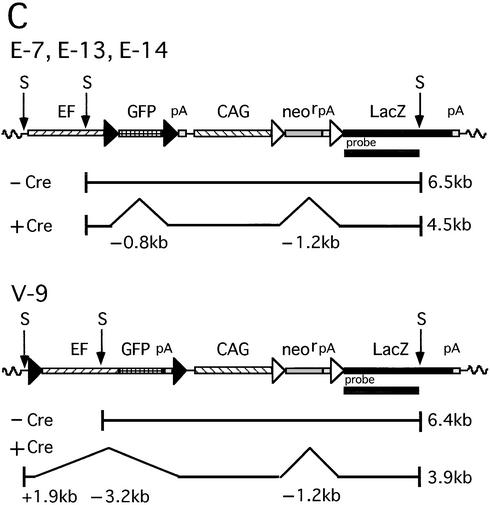
Strategy for simultaneous gene regulation of the GFP gene and LacZ gene on mammalian chromosomes. EF, EF1α promoter; GFP, GFP cDNA; pA, polyadenylation signal; CAG, CAG promoter; neor, neo resistance gene; LacZ, β-galactosidase gene of E.coli; V, mutant loxP V; L, wild-type loxP; Ap, ampicillin resistance gene. (A) Structure of plasmids for simultaneous gene regulation. Each plasmid contains two Cre recombinase target units, the V unit and the L unit, respectively. Two different plasmids contain the identical L target unit containing the CAG promoter, a stuffer sequence flanked by a pair of L sequences, the LacZ gene and pA, in that order, while pEFVGV-CALNLZ (top) has a V target unit containing the EF1α promoter, GFP cDNA flanked by a pair of V sequences and pA, in that order, pVEFGV-CALNLZ (bottom) has the other V target unit containing a GFP expression unit flanked by a pair of V sequences. (B) Structure of a cell line containing the EFVGV-CALNLZ unit and the strategy of simultaneous gene regulation on mammalian chromosomes mediated by AxCANCre. Wavy line, cell chromosomal DNA.
In vitro recombination assay
Cre lysate was prepared as described previously (22). Briefly, 2 × 107 293 cells were infected with AxCANCre at a MOI (plaque forming units/cell) of 5 and cells were collected 20 h later. The cell pellet was resuspended with 1 ml of Cre storage buffer (30) (50% glycerol, 20 mM Tris–HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA) and was then sonicated and immediately centrifuged. The supernatant was stored at –80°C. The recombination reaction was initiated by mixing either HindIII-linearized pBS-CALNLZ or PstI-linearized pEFVGV with the Cre lysate and incubation for 30 min at 37°C in 50 µl Cre reaction buffer (30) (50 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 100 µg/ml DNase-free bovine serum albumin, 1 mM phenylmethylsulfonyl fluoride, 5 µg/ml aprotinin). The reaction was terminated by phenol/chloroform extraction followed by two cycles of chloroform extraction and ethanol precipitation. The DNA recovered after RNase treatment was digested with NcoI and visualized by ethidium bromide staining after agarose gel electrophoresis. Recombination efficiency was calculated by quantifying the density of the DNA bands on the photograph based on the densities of the quantitation standard DNA digests on the same gel as described previously (22,29). The bands with high recombination efficiency were re-quantified precisely based on the band density of a one-fifth dilution of the sample.
Detection and quantitative determination of GFP and LacZ gene expression
A fluorescence microscope (IX70; Olympus) was used to observe GFP expression. Before infection and 7 days after infection, cells were washed twice with Hank’s balanced salt solution and the fluorescence intensity of GFP was quantified with Fluoroskan Ascent FL (Labsystems). Before infection and 3 days after infection, LacZ-expressing cells were stained by washing twice with Ca2+, Mg2+-free phosphate-buffer saline [PBS(–)], fixing with 0.25% glutaraldehyde and staining with 0.1% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) (31). To quantify β-galactosidase activity, 3 × 105 cells were disrupted by sonication and the lysate was subjected to a color reaction with chlorophenyl red β-d-galactopyranoside (CPRG) using a high sensitivity β-gal assay kit (Stratagene).
Southern blotting analyses
Before and 3 days after infection, total cell DNAs were prepared from a 6 cm dish according to the method described by Saito et al. (32). After SacI digestion, the DNA was electrophoresed on a 0.6% agarose gel at 35 V for 17 h. Before alkaline treatment, the gel was exposed to 0.1 N HCl for partial depurination (33) and the DNA was then transferred to a Hybond-N nylon membrane (Amersham Biosciences) by the capillary transfer method (34). Specific DNA was detected with a DIG DNA Labeling and Detection Kit (Roche Diagnostics). A 1.9 kb BamHI–SacI fragment of the LacZ gene was labeled with digoxigenin-UTP as a probe for the DNA and specific DNA was detected by autoradiography with chemiluminescence of CDP-Star (Roche Diagnostics).
Polymerase chain reaction (PCR) analysis
The 5′ primer used for the PCR analysis was the 30 nt sequence 5′-TCT CGA GCT TTT GGA GTA CGT CGT CTT TAG-3′, 243 nt upstream from the 3′ end of the EF1α promoter fragment, and the 3′ primer was the 30 nt sequence 5′-TAC GCC AGC TGG CGA AAG GGG GAT GTG CTG-3′, 78 nt downstream from the initiation codon of the LacZ gene. The following conditions were used for amplification: 95°C for 1.5 min, 58°C for 1.5 min and 72°C for 2 min. A 2 µg sample of total cell DNA used in the Southern blot analysis was amplified for 35 cycles with Taq DNA polymerase (Amersham Biosciences) in a 25 µl volume. A 10 µl sample of reaction mixture was subjected to electrophoresis on a 2% agarose gel containing ethidium bromide. The control reaction was carried out with pEFVLacZ (8.8 kb) containing the EF1α promoter, V sequence and the LacZ coding sequence, in that order, as a quantitation standard. The amount of pEFVLacZ DNA added was 1.05 pg, so the molar ratio would be equivalent to 1 copy/cell pEFVGV-CALNLZ (12.9 kb) present in 2 µg total cell DNA containing 1 copy EFVGV-CALNLZ/cell. The other quantitation standards used are 10-fold stepwise dilutions of the 1 copy/cell solution.
RESULTS
Recombination efficiency of loxP sites in vitro
To compare the Cre-mediated recombination efficiency of wild-type loxP (L) with mutant loxP V (V), an in vitro recombination assay was carried out as described previously (22) by using Cre recombinase produced by Cre-expressing rAd AxCANCre. HindIII-digested pBS-CALNLZ (Fig. 1A, upper) containing the L target unit (see below) was mixed with Cre lysate and incubated for 30 min at 37°C. When the DNA recovered from the reaction mixture was digested with NcoI, the substrate yielded fragments of 2.0 and 4.3 kb, whereas the recombined products were detected as 5.1 and 1.2 kb fragments [excised neor and poly(A) sequence] (Fig. 1A, top, –Cre and +Cre; Fig. 1B, lanes 4–7). In addition, PstI-digested pEFVGV (Fig. 1A, lower) containing the V target unit as substrate DNA was incubated with Cre lysate. After digestion with NcoI, the substrate yielded fragments of 0.4 kb (data not shown) and 2.2 kb, whereas the recombination products were detected as 1.8 and 0.8 kb fragments (excised GFP cDNA) (Fig. 1A, bottom, –Cre and +Cre; Fig. 1B, lanes 12–14). The recombination efficiency was calculated by quantifying the density of the DNA band based on densities of the quantitation standard DNA. The results showed that the maximum rate of recombination efficiency of both the L target unit and the V target unit was achieved with 2.5 µl of the lysate, and the efficiencies were ∼60% (Fig. 1B, lane 5) and ∼40% (lane 12). Although the efficiency may be influenced by the adjacent sequence or by the distance between the loxP sites, the result showed that V-V recombination was slightly less efficient than L-L recombination in these particular constructs.
Simultaneous gene regulation on a mammalian chromosome
To determine whether gene expression of two independent units could be regulated simultaneously with two ‘exclusive’ loxP sites despite their different recombination efficiencies, two different plasmids containing both the L and V target units in a single molecule (Fig. 2A) were constructed and cell lines containing each of the plasmids were isolated by G418 selection. One plasmid, pEFVGV-CALNLZ (Fig. 2A, top), initially expresses both GFP under control of the EF1α promoter and the neo resistance gene under control of the CAG promoter (Fig. 2B, top). After Cre recombinase is supplied via an adenovirus vector, expression of the GFP and neo resistance genes is turned off by excisional deletion and expression of the LacZ gene is simultaneously switched on, as a result of independent recombination of L and V (Fig. 2B, lower). The other plasmid, pVEFGV-CALNLZ (Fig. 2A, lower), was constructed to contain the identical L target unit and a modified V target unit so that the entire GFP expression unit instead of GFP cDNA was flanked by a pair of V sites in order to determine whether a similar efficiency of V-V recombination would be obtained. Each of the two plasmids was transfected into CV1 cells, and 15 and 9 neo-resistant clones with GFP expression were selected, respectively. After infection of AxCANCre, β-galactosidase expression was detected by X-gal staining in five and two of the clones, respectively, whereas the other cell lines contained a truncated and deficient LacZ gene (data not shown). The cell lines containing the intact unit for simultaneous gene regulation were then screened by Southern blot analyses (data not shown) and cell lines E-13 and E-14, containing one copy of the EFVGV-CALNLZ unit, cell line V-9, containing one copy of the VEFGV-CALNLZ unit, and cell line E-7, containing about five intact copies (data not shown) of the EFVGV-CALNLZ unit, were identified.
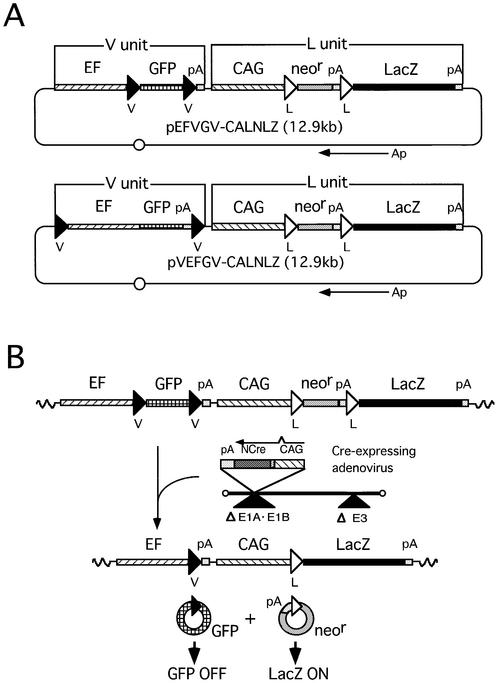
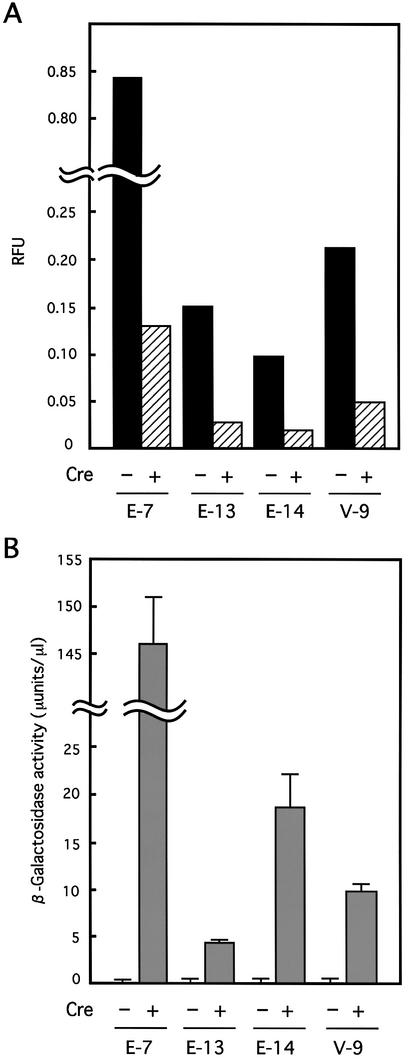
Simultaneous gene regulation was analyzed using the above four cell lines. Each clone was infected with AxCANCre at a MOI of 3 and expression of GFP and LacZ was detected by fluorescence microscopy (Fig. 3, top) and X-gal staining (Fig. 3, bottom), respectively. Almost 100% of the cells in cell line E-13 exhibited GFP fluorescence before infection (Fig. 3c, left), but the fluorescence gradually decreased thereafter and became undetectable by 7 days after infection (Fig. 3c, right). Although no X-gal staining was detected before infection (Fig. 3d, left), almost 100% of the cells stained 3 days after infection (Fig. 3d, right). Similar results were obtained for cell lines E-14 and V-9, although the GFP and LacZ expression levels varied from line to line (Fig. 3e–h). Cell line E-7, which contained about five copies of the simultaneous regulation unit, showed much higher GFP expression before infection (Fig. 3a, left) and some cells with faint GFP fluorescence remained after infection (Fig. 3a, right). This finding suggests that a MOI of 3 may not have been enough to recombine all loxP sites in cell line E-7 in this experiment, because no such cells in cell line E-7 exhibited GFP fluorescence after AxCANCre infection at a MOI of 10 (data not shown). The GFP and LacZ gene expression levels were quantified by measuring fluorescence intensity and β-galactosidase activity (Fig. 4). GFP fluorescence intensity in all clones was significantly decreased after infection, with the intensity in the cell lines containing one copy of the regulation unit diminishing to almost the background level (Fig. 4A). No β-galactosidase activity was detected in any of the cell lines before infection, but high level activity was detected after infection (Fig. 4B), suggesting that expression of both marker genes was correctly regulated in the cultured mammalian cells. However, it was noted that while the level of LacZ expression increased sharply, the level of GFP expression decreased only gradually (see Discussion).
Figure 3.
Cre-mediated simultaneous GFP and LacZ gene regulation on mammalian chromosomes. Analysis of cell lines E-7 (a and b), E-13 (c and d), E-14 (e and f) and V-9 (g and h). (Top) (a, c, e and g) GFP portraits obtained by fluorescence microscopy. (Bottom) (b, d, f and h) X-gal staining. Cre – and Cre + refer to pre- and post-infection with AxCANCre, respectively. GFP and X-gal staining was performed 7 and 3 days after infection, respectively.
Figure 4.
Quantitation of the expressed GFP fluorescence and β-galactosidase activity. (A) GFP expressed in each cell line was quantified before infection (–Cre) and 7 days post-infection with AxCANCre (+Cre). The fluorescence intensity (relative fluorescence units, RFU) of GFP was measured with Fluoroskan Ascent FL (Labsystems). (B) Expressed β-galactosidase activity was quantified before infection (–Cre) and 3 days post-infection with AxCANCre (+Cre). β-Galactosidase activity was quantified using CPRG and averages of three wells in a 24-well plate are shown. The lines on the graph are standard deviations.
Structure of the Cre-mediated mammalian chromosome based on Southern blot analysis
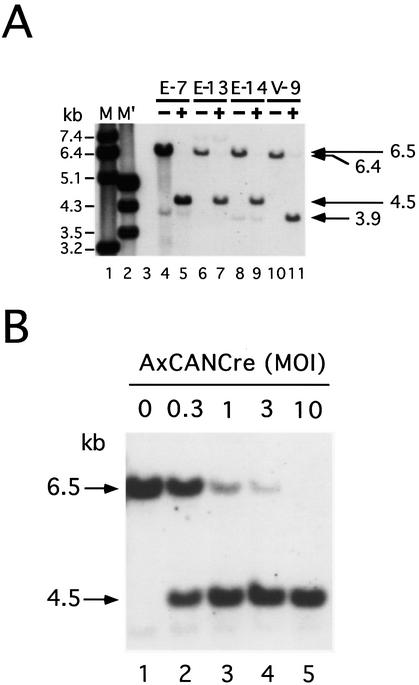
To obtain direct evidence that the expression of both marker genes was regulated by Cre-mediated recombination, as shown in Figure 2B, total DNA was prepared from the cell lines infected with AxCANCre at a MOI of 3 and the DNA structure was investigated by Southern blot analysis (Fig. 5). After SacI digestion, the 6.5 kb band extending from the 3′ side of the EF1α promoter to the 5′ side of the LacZ gene (Fig. 5A, lanes 4, 6 and 8; Fig. 5C, upper, –Cre) had shifted to the 4.5 kb band in cell lines E-7, E-13 and E-14 (Fig. 5A, lanes 5, 7 and 9; Fig. 5C, upper, +Cre) after AxCANCre infection. Also, the 6.4 kb band extending from the 3′ side of the EF1α promoter to the 5′ side of the LacZ gene (Fig. 5A, lane 10; Fig. 5C, lower, –Cre) had shifted to the 3.9 kb band in cell line V-9 (Fig. 5A, lane 11; Fig. 5C, lower, +Cre). These results strongly suggest that both GFP deletion by V-V recombination and 1.2 kb stuffer [neo gene + poly(A)] deletion by L-L recombination occurred accurately. They also show that both the V-V and L-L recombinations were complete 3 days after infection at a MOI of 3, even in cell line E-7, which contains multiple copies of the regulation units and yielded very dense bands. Not only were no unrecombined structures detected in this particular experiment, but no partially recombined structures were found either. Because the V-V recombination in cell line V-9 was complete, as in other cell lines, there seemed to be no difference in recombination efficiency due to the different positions of V.
Figure 5.
Simultaneous recombination at gene regulation units on mammalian chromosomes. (A) Southern blot analysis of four cell lines before infection (–) and 3 days post-infection with AxCANCre at a MOI of 3 (+). M (lane 1) and M′ (lane 2) represent λ phage DNA digested with MscI and EcoRI + HindIII, respectively, as size markers. A faint extra band below the 6.5 kb band in lane 4 was derived from the region outside the detection area of the transfected plasmid DNA and was attributable to DNA contamination when preparing the probe. (B) Autoradiogram of the E-7 genome containing about five copies of regulation unit infected with AxCANCre at the MOIs indicated at the top of the gel. (C) A map of simultaneous Cre-mediated recombination at gene regulation units. The 6.5 kb band in cell lines E-7, E-13 and E-14 (top) before infection (–Cre) had shifted to a 4.5 kb band after infection (+Cre) because of the 0.8 and 1.2 kb deletion by V-V and L-L recombination, respectively. In cell line V-9 (lower), the 6.4 kb band before infection (–Cre) had shifted to a 3.9 kb band after infection (+Cre), because of a 1.2 kb deletion as a result of L-L recombination, a 3.2 kb deletion as a result of V-V recombination and 1.9 kb annexation due to the skipped SacI site. The position of the probe is shown as a closed bar. S, SacI.
As described above, V-V recombination was less efficient than L-L recombination according to the result of assays in vitro (Fig. 1), suggesting that after AxCANCre infection a partial recombination product might arise having a deletion within the L unit while containing an unprocessed V unit. However, the putative 5.3 kb band expected from such a structure with only a 1.2 kb deletion was not detected (Fig. 5A). To investigate this in greater detail, cell line E-7, which contains multiple copies of the units, was infected with AxCANCre at MOIs of 0–10 and their cellular DNAs were analyzed (Fig. 5B). The results showed that the unprocessed 6.5 kb band had shifted to a fully processed 4.5 kb band at MOIs of 0.3–3 (lanes 2–4). Unexpectedly, however, the putative 5.3 kb band was not detected at any of the MOIs used, and this observation was reproducible in three independent experiments. These results showed that L-L recombination and V-V recombination occur equally on the mammalian chromosome when infected with AxCANCre, even at a very low MOI.
Analysis of putative recombination between L and V
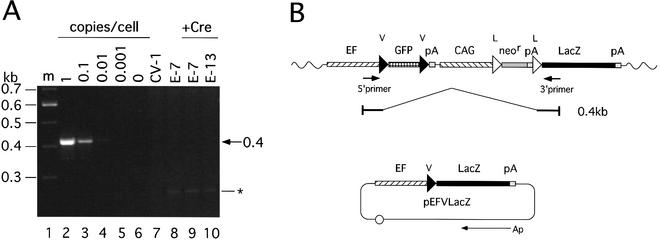
We previously reported that no recombination between loxP V and wild-type loxP was detected in an in vitro assay (22). To investigate whether L-V recombination occurred or not when located on a mammalian chromosome, we performed PCR analysis (Fig. 6) using cellular DNAs derived from cell lines E-7 and E-13 infected with AxCANCre and a pair of primers detecting the L-V recombination product as a 0.4 kb band. The result showed that no such band was detected with either cell line (Fig. 6A, lanes 9 and 10), even under conditions in which a positive control equivalent to 0.01 copies/cell was detected (lane 4). On the other hand, using a combination of a primer near the 3′ end of the CAG promoter and the 3′ primer of this experiment, a 0.3 kb fragment containing an L sequence was detected by parallel PCR analysis, even when a 1/1000 sample of the infected E-13 DNA used in lane 10 was analyzed (data not shown). Therefore, the efficiency of L-V recombination on the mammalian chromosome seems to be very low, no more than 1/100, if there is any at all, showing that the L and V units were processed independently in cultured mammalian cells.
Figure 6.
Analysis of L-V recombination on mammalian chromosomes. (A) Ethidium bromide staining after PCR using chromosomal DNA before infection and 3 days post-infection with AxCANCre at a MOI of 3. The copy marker as a positive control was derived from plasmid pEFVLacZ, which contains the V sequence between the EF1α promoter and the LacZ gene. Copy markers for 1, 0.1, 0.01, 0.001 and 0 copies/cell were also included (lanes 2–6) to measure numbers of copies of EFVGV-CALNLZ per cell. CV1 (lane 7) and uninfected E-7 (lane 8) are also included. m (lane 1), 100 bp Ladder Marker (Gibco BRL). The asterisk shows the bands derived from CV1. (B) Structure of a putative recombination between L and V. Arrows show the positions of the 5′ and 3′ primers. Wavy line, cell chromosomal DNA. If the putative recombination between L and V occurred, recombinant structure will be the EF1α promoter sequence, loxP sequence and LacZ gene, in that order, as a result of recombination between the 5′ end of L and the 3′ end of V. The structure would be detected as a 0.4 kb band with these primers.
DISCUSSION
The results of this study show that both the wild-type loxP unit and the loxP V unit can be processed simultaneously and independently in virtually 100% of cultured mammalian cells when mediated by Cre-expressing adenovirus. It is noteworthy that these two regulation units in our experiments are located very near each other, because that strongly suggests that independent processing will also occur when the two units are far apart or located on different chromosomes. Therefore, the approach described here is expected to be capable of being applied to conditional and simultaneous knockout of two functionally related genes.
Our in vitro recombination assay showed that the recombination efficiency between a pair of loxP V sequences was lower than between wild-type loxP sequences, although recombination reactions in vitro are known to not necessarily reflect the reactions in mammalian cells. For example, in vitro recombination reactions are apparently reversible and their efficiency reaches a plateau level of ∼50% in the later processing phase (22,35). In contrast, excision is overwhelmingly dominant (≥99%) on both mammalian chromosomes (36) and the adenovirus genome (23) when a sufficient amount of Cre is supplied to mammalian cells by adenovirus expression vector. Although a difference was evident in the later processing phase, the low efficiency of loxP V in in vitro recombination in the early processing phase suggested the possibility of incomplete or retarded processing of the loxP V unit in mammalian cells. Our results, however, showed no incomplete processing of the loxP V unit, even when a cell line containing about five copies of the unit per cell and a very limited amount of AxCANCre was infected (Fig. 5). Even under infection conditions of a MOI of 0.3, under which some cells do not receive any viral particles and the rest receive only one or a few, all of the DNAs detected were unprocessed or completely processed. One possible explanation for this is that the recombination efficiency of loxP V being equal to that of wild-type loxP in mammalian cells is a matter of chance. However, this does not seem plausible, because, if it were the case, a mixture of two different partial products with only one of the two units unprocessed should have resulted. A more plausible explanation is that infection of a cell by a single virus particle provides enough Cre enzyme to process all five copies of each mutant and wild-type unit in the cell within the sensitivity of Southern analysis. Our in vitro recombination assay showed that V-V recombination was only slightly less efficient than L-L recombination in our substrate. Processing by one AxCANCre particle was previously observed in which AxCANCre infection at a MOI of 0.1 mediated activation of the LacZ expression unit in 20% of the cultured cells (23). Thus, the results suggest that partial or incomplete processing does not cause a problem in simultaneous regulation as long as loxP V is used with AxCANCre, which expresses Cre tagged with a nuclear localization signal under the control of a very potent CAG promoter (27). However, it may be possible to observe partial recombination by using a promoter whose activity is weaker than that of the CAG promoter.
Fidelity is an important factor in addition to efficiency. A mutant loxP 511 has been reported as the first identified ‘exclusive’ loxP mutant (18). However, we and others observed that loxP 511 recombines with wild-type loxP with low but significant efficiency (22,37). loxP V, on the other hand, showed a recombination efficiency similar to that of loxP 511 without detectable recombination with wild-type loxP in both an in vitro assay (22) and in an E.coli system using M13 phagemid (37). In the present study, PCR assays did not detect any recombination product between loxP V and wild-type loxP at all, or at most 0.01 copy/cell (Fig. 6) or 0.001 copy/cell (S. Kondo, unpublished data), confirming the fidelity of loxP V even when located on a mammalian chromosome. The superior fidelity of loxP V was also noted when applied to the Cre recombinase-mediated cassette exchange (RMCE) method (38,39). Because we have found that loxP S (the new name for loxP 5171), which bears a two base transition substitution and is incompatible with either wild-type loxP or loxP V (22), up to three genes could be regulated simultaneously. However, the adjacent sequences of the mutant loxP sites including loxP V may have an influence on fidelity, because it is reported that the presence of an artificial palindromic configuration of DNA around loxP V influences the fidelity of loxP V and wild-type loxP (40).
After infection with AxCANCre, LacZ expression sharply turned on, changing from an undetectable level to a very high level over a 3 day period (Fig. 4B) (23,36). However, GFP expression did not switch off sharply, but persisted over as much as a 7 day period (Fig. 4A). The prolonged expression after off regulation is probably attributable to the GFP protein synthesized before switching off being quite stable and persisting for a long period. This explanation is supported by the data showing that processing of both regulation units at the DNA level is simultaneously completed 3 days after infection. Therefore, it seems important to take into consideration the stability of the protein to be turned off and that it is better to choose a protein with a short half-life when quick switching off is desirable. The integrated DNA was amplified in cell line E-7 together with its flanking cellular DNA (data not shown). Although its detailed structure is unknown, the recombination processing and expression switching occurred as expected in E-7 cells, as in the one copy integrated cell lines.
The methods of simultaneous regulation of gene expression reported previously have involved the use of the internal ribosome entry site (IRES) of RNA viruses (41) and the tetracyclin (Tet)-induced gene activation/inactivation system (42,43). Since both genes are controlled under the same regulatable promoter in the IRES system, regulation of the two genes is not independent. With the Tet-induced system, a single Tet-regulating promoter (42) or both Tet-on and Tet-off promoters (43) are used for expression of two genes. The Tet system has been widely applied to conditional transgenic approaches because it is simple to use and reversible. The method involving Cre-expressing adenovirus and mutant loxP V described in this report has the following special features. (i) Any promoter can be chosen for either expression unit, including authentic, inducible or very potent promoters. (ii) The locations of the two regulation units are rather flexible, they can be very close to each other, far apart from each other or even on different chromosomes. (iii) The method allows not only simultaneous switching on or off of two genes but ‘alternative switching’, as described here, i.e. turning one gene off and at the same time turning the other gene on. Such regulation may be useful for functional examination and comparison of two structurally related genes. Because the adenovirus vector provides highly efficient gene expression, the method can be used in animal experiments as well as in cultured mammalian cells. The expression-off state before switching on is quite strict and, hence, the method is especially useful for functional studies of genes associated with apoptosis (44) and genes essential for cell growth. For example, this method will probably prove useful in establishing packaging cell lines that efficiently produce a viral vector, where two or more toxic viral genes should be expressed efficiently and simultaneously.
This method currently involves Cre-mediated excision, an irreversible reaction in mammalian cells without drug selection, and therefore can switch gene expression only once. However, we have recently established a gene regulation system that uses an adenovirus vector expressing FLP recombinase (45). Combination of the Cre and FLP systems will enable a methodology that provides not only simultaneous but also stepwise regulation. The study is currently underway. AxCANCre and plasmids containing two copies of wild-type loxP (pULwL), loxP V (pUVwV) and loxP S (pUSwS) will be available from the Riken DNA Bank (http://www.rtc.riken.go.jp). The sequences pEFVGV-CALNLZ and pVEFGV-CALNLZ will be available from our homepage (http://www.ims.u-tokyo.ac.jp/idenshi/hpmain.html) and the Riken DNA Bank (http://www.rtc.riken.go.jp).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr J. Miyazaki for the CAG promoter and Ms M. Kadota and Ms K. Nishijima for their excellent secretarial support. This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology to Y.K. and I.S.
REFERENCES
- 1.Sternberg N. and Hamilton,D. (1981) Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol., 150, 467–486. [DOI] [PubMed] [Google Scholar]
- 2.Barinaga M. (1994) Knockout mice: round two. Science, 265, 26–28. [DOI] [PubMed] [Google Scholar]
- 3.Rossant J. and Nagy,A. (1995) Genome engineering: the new mouse genetics. Nature Med., 1, 592–594. [DOI] [PubMed] [Google Scholar]
- 4.Rajewsky K., Gu,H., Kuhn,R., Betz,U.A., Muller,W., Roes,J. and Schwenk,F. (1996) Conditional gene targeting. J. Clin. Invest., 98, 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. [DOI] [PubMed] [Google Scholar]
- 6.Wakita T., Taya,C., Katsume,A., Kato,J., Yonekawa,H., Kanegae,Y., Saito,I., Hayashi,Y., Koike,M. and Kohara,M. (1998) Efficient conditional transgene expression in hepatitis C virus cDNA transgenic mice mediated by the Cre/loxP system. J. Biol. Chem., 273, 9001–9006. [DOI] [PubMed] [Google Scholar]
- 7.Shibata H., Toyama,K., Shioya,H., Ito,M., Hirota,M., Hasegawa,S., Matsumoto,H., Takano,H., Akiyama,T., Toyoshima,K., Kanamaru,R., Kanegae,Y., Saito,I., Nakamura,Y., Shiba,K. and Noda,T. (1997) Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science, 278, 120–123. [DOI] [PubMed] [Google Scholar]
- 8.Rohlmann A., Gotthardt,M., Willnow,T.E., Hammer,R.E. and Herz,J. (1996) Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol., 14, 1562–1565. [DOI] [PubMed] [Google Scholar]
- 9.Tarutani M., Itami,S., Okabe,M., Ikawa,M., Tezuka,T., Yoshikawa,K., Kinoshita,T. and Takeda,J. (1997) Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc. Natl Acad. Sci. USA, 94, 7400–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Wang,J., Wilhelmsson,H., Hansson,A., Thoren,P., Duffy,J., Rustin,P. and Larsson,N.G. (2000) Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl Acad. Sci. USA, 97, 3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backman S.A., Stambolic,V., Suzuki,A., Haight,J., Elia,A., Pretorius,J., Tsao,M.S., Shannon,P., Bolon,B., Ivy,G.O. and Mak,T.W. (2001) Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nature Genet., 29, 396–403. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto S., Niwa,H., Tashiro,F., Sano,S., Kondoh,G., Takeda,J., Tabayashi,K. and Miyazaki,J. (2000) A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett., 470, 263–268. [DOI] [PubMed] [Google Scholar]
- 13.Sakai Y., Kaneko,S., Sato,Y., Kanegae,Y., Tamaoki,T., Saito,I. and Kobayashi,K. (2001) Gene therapy for hepatocellular carcinoma using two recombinant adenovirus vectors with alpha-fetoprotein promoter and Cre/lox P system. J. Virol. Methods, 92, 5–17. [DOI] [PubMed] [Google Scholar]
- 14.Braz J.M., Rico,B. and Basbaum,A.I. (2002) Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc. Natl Acad. Sci. USA, 99, 15148–15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed F., Wyckoff,J., Lin,E.Y., Wang,W., Wang,Y., Hennighausen,L., Miyazaki,J., Jones,J., Pollard,J.W., Condeelis,J.S. and Segall,J.E. (2002) GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res., 62, 7166–7169. [PubMed] [Google Scholar]
- 16.Smith A.J., De Sousa,M.A., Kwabi-Addo,B., Heppell-Parton,A., Impey,H. and Rabbitts,P. (1995) A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nature Genet., 9, 376–385. [DOI] [PubMed] [Google Scholar]
- 17.Van Deursen J., Fornerod,M., Van Rees,B. and Grosveld,G. (1995) Cre-mediated site-specific translocation between nonhomologous mouse chromosomes. Proc. Natl Acad. Sci. USA, 92, 7376–7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoess R.H., Wierzbicki,A. and Abremski,K. (1986) The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res., 14, 2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse P., Griffiths,A.D., Johnson,K.S. and Winter,G. (1993) Combinatorial infection and in vivo recombination: a strategy for making large phage antibody repertoires. Nucleic Acids Res., 21, 2265–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethke B. and Sauer,B. (1997) Segmental genomic replacement by Cre-mediated recombination: genotoxic stress activation of the p53 promoter in single-copy transformants. Nucleic Acids Res., 25, 2828–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sblattero D. and Bradbury,A. (2000) Exploiting recombination in single bacteria to make large phage antibody libraries. Nat. Biotechnol., 18, 75–80. [DOI] [PubMed] [Google Scholar]
- 22.Lee G. and Saito,I. (1998) Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene, 216, 55–65. [DOI] [PubMed] [Google Scholar]
- 23.Kanegae Y., Lee,G., Sato,Y., Tanaka,M., Nakai,M., Sakaki,T., Sugano,S. and Saito,I. (1995) Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res., 23, 3816–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham F.L., Smiley,J., Russell,W.C. and Nairn,R. (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol., 36, 59–74. [DOI] [PubMed] [Google Scholar]
- 25.Miyake S., Makimura,M., Kanegae,Y., Harada,S., Sato,Y., Takamori,K., Tokuda,C. and Saito,I. (1996) Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl Acad. Sci. USA, 93, 1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozak M. (1981) Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res., 9, 5233–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa H., Yamamura,K. and Miyazaki,J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 108, 193–199. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.W., Uetsuki,T., Kaziro,Y., Yamaguchi,N. and Sugano,S. (1990) Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene, 91, 217–223. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M., Odaka,K., Ishimura,M., Kondo,S., Tachikawa,N., Chiba,J., Kanegae,Y. and Saito,I. (2001) Efficient gene activation in cultured mammalian cells mediated by FLP recombinase-expressing recombinant adenovirus. Nucleic Acids Res., 29, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anton M. and Graham,F.L. (1995) Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J. Virol., 69, 4600–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholer H.R., Balling,R., Hatzopoulos,A.K., Suzuki,N. and Gruss,P. (1989) Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J., 8, 2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito I., Oya,Y., Yamamoto,K., Yuasa,T. and Shimojo,H. (1985) Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J. Virol., 54, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito I., Groves,R., Giulotto,E., Rolfe,M. and Stark,G.R. (1989) Evolution and stability of chromosomal DNA coamplified with the CAD gene. Mol. Cell. Biol., 9, 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J., Fritsh,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Abremski K., Hoess,R. and Sternberg,N. (1983) Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell, 32, 1301–1311. [DOI] [PubMed] [Google Scholar]
- 36.Kanegae Y., Takamori,K., Sato,Y., Lee,G., Nakai,M. and Saito,I. (1996) Efficient gene activation system on mammalian cell chromosomes using recombinant adenovirus producing Cre recombinase. Gene, 181, 207–212. [DOI] [PubMed] [Google Scholar]
- 37.Siegel R.W., Jain,R. and Bradbury,A. (2001) Using an in vivo phagemid system to identify non-compatible loxP sequences. FEBS Lett., 505, 467–473. [DOI] [PubMed] [Google Scholar]
- 38.Kolb A.F. (2001) Selection-marker-free modification of the murine beta-casein gene using a lox2272 [correction of lox2722] site. Anal. Biochem., 290, 260–271. [DOI] [PubMed] [Google Scholar]
- 39.Araki K., Araki,M. and Yamamura,K. (1997) Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res., 25, 868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mlynarova L., Libantova,J., Vrba,L. and Nap,J. (2002) The promiscuity of heterospecific lox sites increases dramatically in the presence of palindromic DNA. Gene, 296, 129–137. [DOI] [PubMed] [Google Scholar]
- 41.Vagner S., Galy,B. and Pyronnet,S. (2001) Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep., 2, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baron U., Freundlieb,S., Gossen,M. and Bujard,H. (1995) Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res., 23, 3605–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron U., Schnappinger,D., Helbl,V., Gossen,M., Hillen,W. and Bujard,H. (1999) Generation of conditional mutants in higher eukaryotes by switching between the expression of two genes. Proc. Natl Acad. Sci. USA, 96, 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuyama T., Fujino,M., Li,X.K., Funeshima,N., Kosuga,M., Saito,I., Suzuki,S. and Yamada,M. (1998) Efficient Fas-ligand gene expression in rodent liver after intravenous injection of a recombinant adenovirus by the use of a Cre-mediated switching system. Gene Ther., 5, 1047–1053. [DOI] [PubMed] [Google Scholar]
- 45.Nakano M., Ishimura,M., Chiba,J., Kanegae,Y. and Saito,I. (2001) DNA substrates influence the recombination efficiency mediated by FLP recombinase expressed in mammalian cells. Microbiol. Immunol., 45, 657–665. [DOI] [PubMed] [Google Scholar]