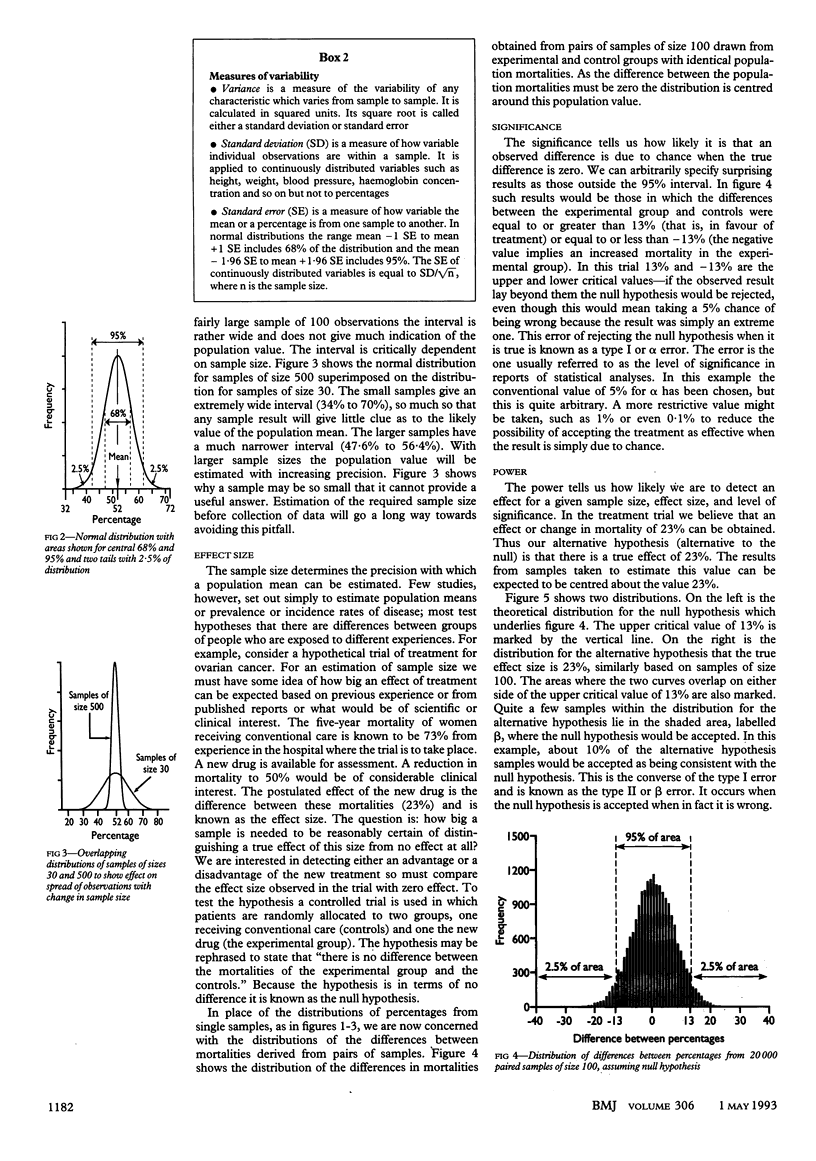
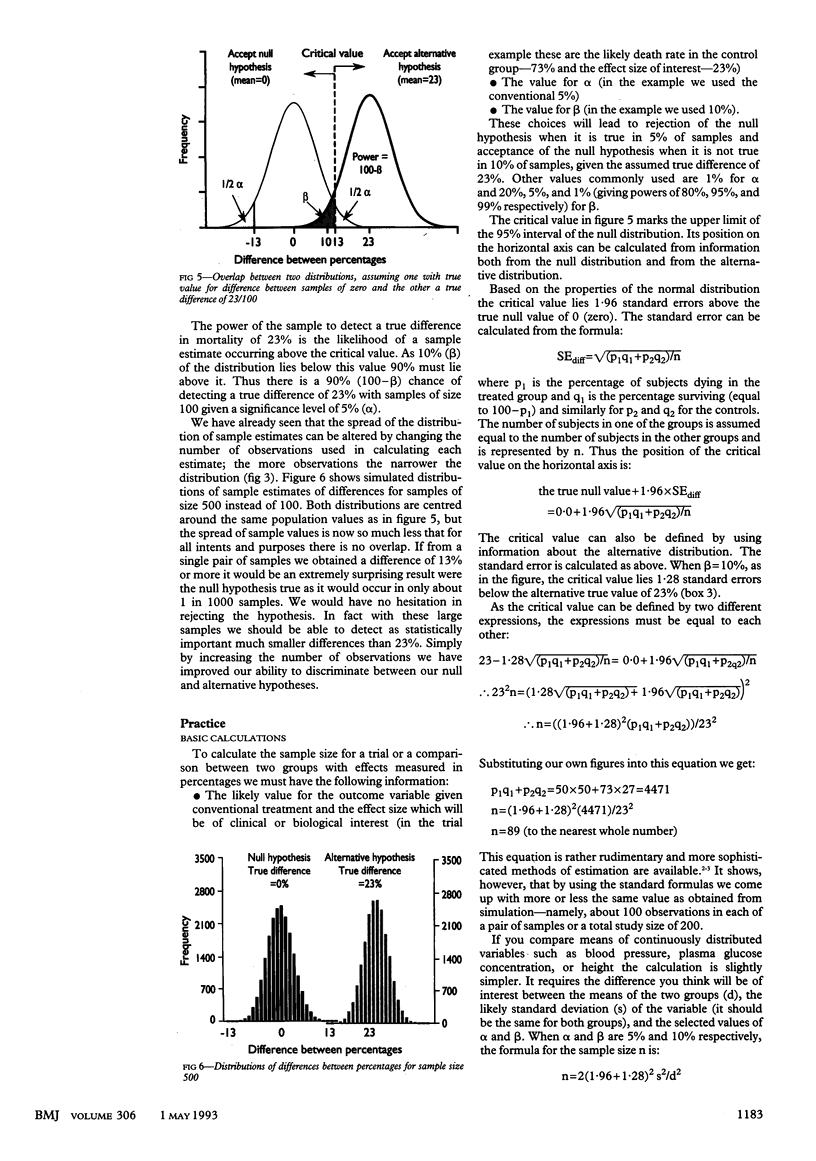
Abstract
The common failure to include an estimation of sample size in grant proposals imposes a major handicap on applicants, particularly for those proposing work in any aspect of research in the health services. Members of research committees need evidence that a study is of adequate size for there to be a reasonable chance of a clear answer at the end. A simple illustrated explanation of the concepts in determining sample size should encourage the faint hearted to pay more attention to this increasingly important aspect of grantsmanship.
Full text
PDF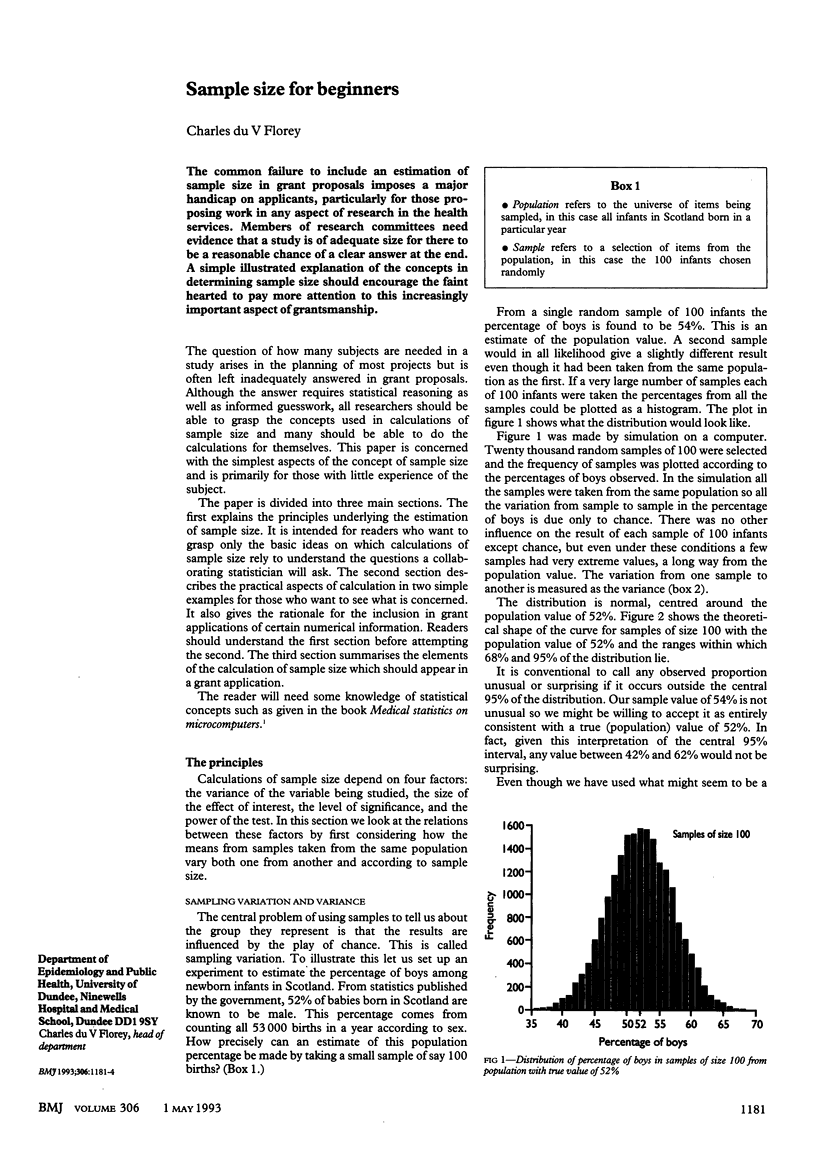

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwelder W. C. "Proving the null hypothesis" in clinical trials. Control Clin Trials. 1982 Dec;3(4):345–353. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Daly L. E. Confidence intervals and sample sizes: don't throw out all your old sample size tables. BMJ. 1991 Feb 9;302(6772):333–336. doi: 10.1136/bmj.302.6772.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. S. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982 Apr-Jun;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- Lachin J. M. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981 Jun;2(2):93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Homan S. M. Graphical aid for determining power of clinical trials involving two groups. BMJ. 1988 Sep 10;297(6649):672–676. doi: 10.1136/bmj.297.6649.672. [DOI] [PMC free article] [PubMed] [Google Scholar]


