Abstract
The homotypic fusion of yeast vacuoles occurs in an ordered cascade of priming, docking, and fusion. The linkage between these steps has so far remained unclear. We now report that Vam7p (the vacuolar SNAP-23/25 homolog) signals from the cis-SNARE complex to Ypt7p (the vacuolar Rab/Ypt) to initiate the docking process. After Vam7p has been released from the cis-SNARE complex by Sec18p-mediated priming, it is still required for Ypt7p-dependent docking and it needs Ypt7p to remain on the vacuole. Thus, after priming, Vam7p is released from the vacuole altogether if Ypt7p has been extracted by Gdi1p or inactivated by antibody but is not released if docking is blocked simply by vacuole dilution; it is therefore Ypt7p function, and not docking per se, that retains Vam7p. In accord with this finding, cells deleted for the gene encoding Ypt7 have normal amounts of Vam7p but have little Vam7p on their isolated vacuoles. Interaction of Vam7p and Ypt7p is further indicated by two-hybrid analysis [Uetz, P., Giot, L., Cagney, G., Mansfield, T. A., Judson, R. S., Knight, J. R., Lockshon, D., Narayan, V., Srinivasan, M., Pochart, P., et al. (2000) Nature (London) 403, 623–627] and by the effect of Vam7p on the association of the Rab/Ypt-effector HOPS complex (homotypic fusion and vacuole protein sorting; Vam2p and Vam6p plus four vacuole protein sorting class C proteins) with Ypt7p. Vam7p provides a functional link between the priming step, which releases it from the cis-SNARE complex, and docking.
Membrane trafficking requires a regulated cascade of vesicle budding from the donor membrane and fusion with the acceptor membrane (1, 2). Many proteins have been characterized that are essential for the fusion of vesicles with the target membrane. Among these are the NEM-sensitive protein (Sec18p/NSF) (3), soluble NSF attachment proteins (Sec17p/SNAPs) (4), a family of proteins termed SNAREs (5), GTPases of the Ypt/Rab family, and Ypt/Rab effectors or tethering factors (6, 7). SNAREs are initially found in cis complexes on membranes (8–10) and are dissociated by NSF and α-SNAP (11) before they function downstream in the docking reaction through associations in trans (10, 12, 13). Tethering factors together with Rab-proteins initiate the contact between the membranes (8). Tethering can precede (14, 15) or follow (16, 17) the dissociation of the cis-SNARE complex in the priming reaction. Finally, several factors coordinate the fusion reaction. SNAREs, calmodulin, synaptotagmin, and protein phosphatase 1 have all been implicated in this reaction stage (18–21). Although Rab/Ypt proteins and their effectors regulate the assembly of the trans-SNARE complex, we now report that the Ypt/Rab function can itself be regulated by a SNARE that has been released from the cis-SNARE complex.
For the fusion of yeast vacuoles, the disassembly of the preexisting cis-SNARE complex during priming is a prerequisite for docking (16, 17). Part of this signaling from priming to docking is performed by the homotypic fusion and vacuole protein sorting (HOPS) complex (formerly called Vam2/6p complex). The HOPS complex, which includes Vps11p, Vps16p, Vps18p, Vps33p, Vps39p/Vam6p, and Vps41p/Vam2p (22–24, 36), is initially in association with SNAREs on isolated vacuoles and is dissociated from the SNAREs during the priming reaction (23). After priming, HOPS is recovered in a complex with the GTP-form of Ypt7 (23, 36), defining it as a Rab effector complex (6). Dilution of vacuoles during priming, or removal of Ypt7p by Gdi1p, leads to a loss of the HOPS complex from the vacuole. Furthermore, HOPS is essential for docking (22). Thus, HOPS is one important element in signaling from priming to docking. We now report that the Vam7p SNARE also signals between priming and the Ypt7p-dependent stage of docking on the vacuole fusion pathway.
Materials and Methods
Materials and Strains.
All strains and reagents have been described previously (13, 24–26).
Biochemical Methods.
Vacuoles were isolated by spheroplasting in the presence of oxalyticase, DEAE lysis, and Ficoll gradient centrifugation (13). For each Vam7p release reaction, 30 μg of vacuoles were incubated for the indicated times in the presence of cytosol under fusion assay conditions (13), then chilled on ice, diluted 5-fold with wash buffer (0.15 M KCl/200 mM sorbitol/10 mM Pipes/KOH, pH 6.8), and centrifuged (10 min, 8,000 × g, 4°C). The vacuole pellet was resuspended in 400 μl of wash buffer and was centrifuged again. The proteins in the pooled supernatant fraction were precipitated by addition of TCA to 13% (vol/vol), incubated on ice for 10 min, centrifuged (10 min, 16,000 × g, 4°C), and washed with ice-cold acetone. SDS/PAGE, immunoblotting using ECL (27), and purification of IgGs (28) were as described.
Results and Discussion
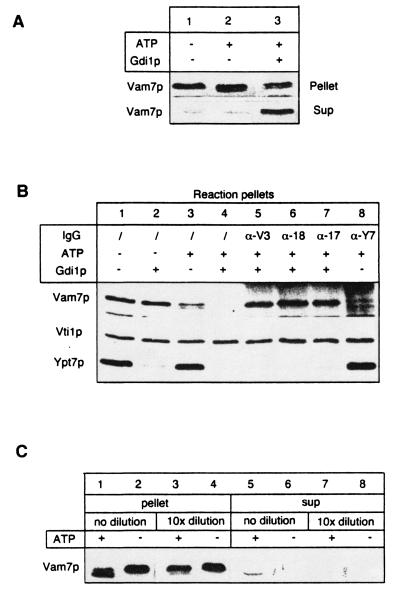
Wild-type vacuoles must be primed to undergo Ypt7p-dependent docking (16, 17). Addition of antibody to Ypt7p or the extraction of Ypt7p from the vacuole membrane by Gdi1p does not affect priming (13). However, we find that Vam7p, the SNAP-23/25 homolog on vacuoles (Fig. 1A, lane 1), is released upon incubation with ATP and Gdi1p (Fig. 1A, lane 3) and is recovered in the reaction supernatant (Fig. 1A, lane 3, bottom). Vam7p was also released when antibodies to Ypt7p were added (Fig. 1B, lane 8). Gdi1p mediated release (Fig. 1B, lane 4) is not seen without ATP (lane 2) and is blocked by antibody to Sec17p, Sec18p, or Vam3p (lanes 5–7), showing that it is strictly dependent on priming. When docking is blocked by dilution (16), Vam7p remains associated with the vacuoles (Fig. 1C, lane 3 vs. lane 7). This shows that it is Ypt7p per se rather than docking that is required to maintain the vacuole association of Vam7p. We note that a portion of the Vam7p undergoes covalent modification to a form of slightly greater mobility (Fig. 1 A, lane 3; B, lane 3; and C, lane 1). This modification requires ATP (Fig. 1B, lane 2), priming (lanes 5–7), and vacuole contact (Fig. 1C, lane 1 vs. lane 3). It can be reversed after priming by incubating vacuoles with apyrase without loss of subsequent fusion (data not shown); its molecular nature remains to be determined.
Figure 1.
Ypt7p is needed for the vacuole association of Vam7p after priming. (A) Vam7p is released from the vacuole upon extraction of Ypt7p. BJ3505 vacuoles (30 μg) were incubated for 60 min at 27°C in the presence or absence of Gdi1p and 0.5 mM Mg-ATP and an ATP regenerating system (13) where indicated and were reisolated (4 min, 8,000 × g, 4°C) as in Materials and Methods. The pooled supernatant of reaction and wash and the reisolated vacuoles were each mixed in sample buffer and were analyzed by SDS/PAGE and immunoblotting. Immunoblots were decorated with antibodies to Vam7p. (B) After priming, Ypt7p is required for Vam7p association with vacuoles. BJ3505 vacuoles (30 μg) were incubated for 60 min at 27°C. Gdi1p or antibodies to the indicated proteins were added to the reaction where indicated. Proteins from the pellet fraction were analyzed by SDS/PAGE and immunoblot with antibodies to Vam7p, Vti1p, and Ypt7p. (C) Vam7p release is not triggered by dilution. Vacuoles (30 μg) were incubated in 150 μl (lanes 1, 2, 5, and 6) or 1.5 ml (lanes 3, 4, 7, and 8) as in A, and the pellet and supernatant fractions were analyzed by immunoblot with antibodies to Vam7p.
The need for Ypt7p to maintain the vacuole association of Vam7p after priming presumably reflects either a direct Vam7p:Ypt7p interaction or an interaction of Vam7p with a common element such as the HOPS complex. Although ypt7Δ cells have a normal content of Vam7p, almost none of it is localized to vacuoles (Fig. 2), showing that Ypt7p has a physiological role in maintaining Vam7p on the vacuole. Indeed, a recent two-hybrid analysis of protein-protein interactions of yeast proteins identified Vam7p as an interaction partner of Ypt7p (29). This concordance between the need for Vam7p for docking (25), the demonstration of Vam7p:Ypt7p interaction by two-hybrid analysis (29), the need for Ypt7p to keep Vam7p on the vacuole (Fig. 2), and our biochemical studies (Fig. 1) that show that Ypt7p only functions to anchor Vam7p after priming has released Vam7p from the cis-SNARE complex (25) establish this as a vital signaling event linking priming and docking.
Figure 2.
Localization of Vam7p to the vacuoles depends on Ypt7p. Whole cell extracts and vacuoles were prepared from BJ3505 wt, vam7Δ, and ypt7Δ strains (25) and were analyzed by SDS/PAGE and immunoblotting with antibodies to Vam7p and Vac8p.
Because both Vam7p (Fig. 2) and HOPS (23) require Ypt7p to remain on the vacuole after incubation with ATP, we asked whether these requirements might be related. Release of HOPS by dilution was enhanced in vam7Δ vacuoles as compared with the wild type (62% vs. 28%; Fig. 3, lane 4 vs. lane 8), whereas release of the HOPS complex in vam3Δ and nyv1Δ vacuoles was indistinguishable from the wild type (data not shown). These data suggest that Vam7p and HOPS may interact with each other as well as with Ypt7p. We conclude that Vam7p plays a central role in signaling from priming to docking: (i) Vam7p is initially essential for the integrity of cis-SNARE complexes (25), yet the need for Vam7p function cannot be fulfilled before vacuole docking (25). (ii) Sec18p is needed for Vam7p release, in vivo (30) and in vitro (Fig. 1B). (iii) Ypt7p is only essential for Vam7p to remain on the vacuole after priming has occurred (Fig. 1A). (iv) Although priming, which releases Vam7p from the cis-SNARE complex (25), is required for tethering of wild-type vacuoles (17), vacuoles without Vam3p, in which the Vam7p is not in complex with other SNAREs (25), do not need priming to tether (17). Conceivably, priming might only be needed for tethering because it specifically releases Vam7p and HOPS (22, 23, 36). (v) Vam7p participates in stabilizing HOPS association with the vacuole (Fig. 3).
Figure 3.
HOPS release is enhanced in vam7Δ vacuoles. BJ3505 wt or vam7Δ vacuoles (12 μg) were incubated in reaction buffer for 90 min in the absence or presence of ATP (24). Gdi1p (64 μg/ml) was added where indicated. One sample was incubated in a 20-fold diluted reaction volume. The reactions were then diluted 5-fold with 10 mM Pipes/KOH (pH 6.8) and 200 mM sorbitol, and the vacuoles were sedimented by centrifugation (14,000 × g, 5 min, 4°C). Proteins in the supernatant were precipitated as described in Fig. 1 and were analyzed by SDS/PAGE and immunoblotting with antibodies to Vam6p. Bands were quantified by laser densitometry.
Vam7p Regulates Docking Through Its Cycle of Associations.
Although most of the Vam7p is in a cis-complex with the SNAREs on isolated vacuoles and is resistant to extraction by high pH (25), priming releases Vam7p from the other SNAREs (25) and its continued membrane association becomes more labile. Cell fractionation has shown that a portion of Vam7p is normally cytosolic, but the cytosolic Vam7p depends on functional Sec18p (30), consistent with our finding that Vam7p release requires priming (Fig. 1A). Roche and coworkers (31) have demonstrated a similar partitioning of SNAP-23 between the cytosol and the membrane. Surprisingly, the final membrane attachment of SNAP-23 is regulated by a kinase, SNAK (31). Although we see an ATP-dependent modification of Vam7p, we have not yet established whether this is phosphorylation.
The partitioning of Vam7p between the cytosol and the vacuole membrane may regulate vacuole fusion. Priming without tethering would release Vam7p and thereby avoid an unproductive activation of Ypt7p. Guo et al. (32) have shown that the SNAP-23 protein itself is relocated to the plasma membrane in regulated secretion as a prerequisite for regulated exocytosis. Some effector elements are thought to signal from Ypt7/Rab proteins to SNAREs. For example, Rab effectors interact physically with a syntaxin required for endosomal fusion (33), and the yeast Rab effector Vac1 acts downstream of the Vps21 Rab protein to regulate trans-SNARE complex assembly (34, 35). In contrast, Vam7p is initially associated with SNAREs and only associates with Ypt7p after it is released from this cis-SNARE complex. Effectors like Vam7p that move from the cis-SNARE complex to the Rab proteins may be distinct from those that move from the Rabs to the trans-SNARE complex.
Acknowledgments
We thank Dr. C. Barlowe and members of the Wickner and Ungermann labs for comments and advice. C.U. is grateful to Dr. Ed Hurt for his support. This work was supported by grants from the National Institute of General Medical Sciences to W.W. and the Deutsche Forschungsgemeinschaft to C.U.
Abbreviation
- HOPS
homotypic fusion and vacuole protein sorting
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160269997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160269997
References
- 1.Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Warren G. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 3.Block M R, Glick B S, Wilcox C A, Wieland F T, Rothman J E. Proc Natl Acad Sci USA. 1988;85:7852–7858. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clary D O, Griff I C, Rothman J E. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- 5.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 6.Novick P, Zerial M. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 7.Waters M G, Pfeffer S R. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/s0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- 8.Walch-Solimena C, Blasi J, Edelmann L, Chapman E R, Fischer von Mollard G, Jahn R. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto H, Hanson P I, Jahn R. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungermann C, Nichols B J, Pelham H R B, Wickner W. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–323. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee A, Barry V, DasGupta B R, Martin T F. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 13.Mayer A, Wickner W, Haas A. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Ballew N, Barlowe C. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer A, Wickner W. J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungermann C, Sato K, Wickner W. Nature (London) 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- 18.Peters C, Mayer A. Nature (London) 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 19.Weber T, Zemelman B V, McNew J A, Westermann B, Gmachl M, Parlati F, Söllner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 20.Peters C, Andrews P D, Stark M J, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A. Science. 1999;285:1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- 21.Davis A F, Bai J, Fasshauer D, Wolowick M J, Lewis J L, Chapman E R. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 22.Price A, Seals D, Wickner W, Ungermann C. J Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price A, Wickner W, Ungermann C. J Cell Biol. 2000;148:1223–1230. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieder S E, Emr S D. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungermann C, Wickner W. EMBO J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas A. Methods Cell Sci. 1995;17:283–294. [Google Scholar]
- 27.Xu Z, Wickner W. J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uetz P, Giot L, Cagney G, Mansfield T A, Judson R S, Knight J R, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. Nature (London) 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 30.Sato T K, Darsow T, Emr S D. Mol Cell Biol. 1998;9:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabaniols J-P, Ravichandran V, Roche P A. Mol Biol Cell. 1999;10:4033–4041. doi: 10.1091/mbc.10.12.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W, Turner C, Castle D. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 33.McBride H M, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 34.Tall G G, Hama H, DeWald D B, Horazdovsky B F. Mol Biol Cell. 1999;10:1873–1899. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson M R, Burd C G, Emr S D. Curr Biol. 1999;9:159–162. doi: 10.1016/s0960-9822(99)80071-2. [DOI] [PubMed] [Google Scholar]
- 36.Seals, D., Eitzen, G., Wickner, W. & Price, A. (2000) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]